To the Editor: Currently, two vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) that involve messenger RNA (mRNA) platform technology have been approved for emergency use by the Food and Drug Administration (FDA) (mRNA-1273, Moderna; and BNT162b2, Pfizer).1,2 Phase 3 trials of these vaccines showed greater than 90% efficacy at preventing symptomatic infection after two doses administered 3 to 4 weeks apart. These trials primarily involved participants without previous SARS-CoV-2 infection. More than 26 million cases of coronavirus disease 2019 (Covid-19) have been documented in the United States, and high rates of seropositivity have been observed in recent studies.3 Thus, the immune response to vaccination in persons with previous SARS-CoV-2 infection needs to be defined.
In this study, we determined antibody levels at baseline and 3 weeks after the first dose of the BNT162b2 SARS-CoV-2 mRNA vaccine in 36 health care workers who received laboratory confirmation of SARS-CoV-2 infection 30 to 60 days before they received the vaccine and 152 health care workers without a history of SARS-CoV-2 infection (Table S1 in the Supplementary Appendix, available with the full text of this letter at NEJM.org). The biospecimens from vaccine recipients were obtained in the context of a clinical study at Children’s Mercy Kansas City, and their use was reviewed and approved by the Children’s Mercy institutional review board. The requirement for written informed consent was waived, given that participants self-enrolled after they had reviewed a study information letter and were given the opportunity to ask questions.
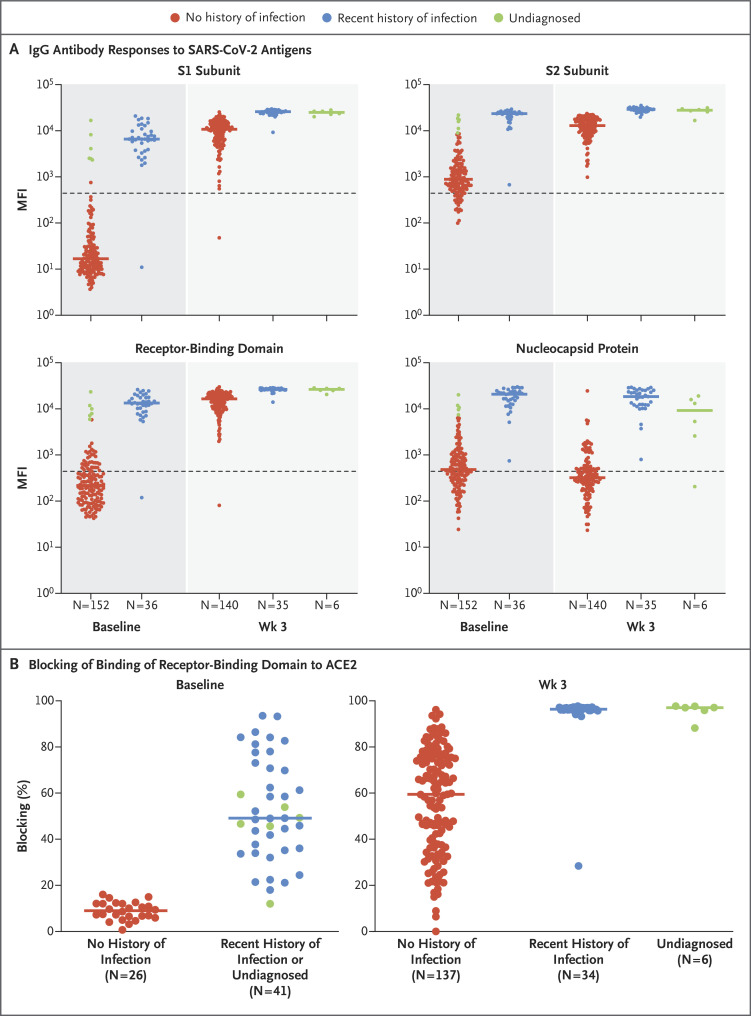
Using a multiplex bead-binding assay (Milliplex SARS-CoV-2 Antigen Panel 1 IgG, Millipore) that measures levels of IgG against SARS-CoV-2 spike protein subunits S1 and S2, the spike receptor-binding domain, and nucleocapsid protein, we found that after the first vaccine dose, antibody titers in both groups of participants were enhanced against all spike protein subunits but not against nucleocapsid protein, which is not a vaccine antigen (Figures 1A, S1, and S2). At baseline, 6 of the participants with no history of SARS-CoV-2 infection had antibody levels that matched those of participants with recent infection (≥1000 median fluorescence intensity units for subunit S1); these 6 participants may have had undiagnosed infection. We separated these participants into a different group (“undiagnosed”) for analysis and found that their week 3 serologic assay results resembled those of the participants with recent infection. After the first vaccine dose, recently infected participants had higher titers of antibody to S1, S2, and the receptor-binding domain than did those with no history of infection (Figure 1A and Table S2).
Figure 1. Antibody Response to SARS-CoV-2 mRNA Vaccine.
Panel A shows a multiplex bead-based antibody-binding assay that measures the IgG antibody response to four SARS-CoV-2 viral antigens (spike protein subunits S1 and S2, spike receptor-binding domain, and nucleocapsid protein). The median fluorescence intensity (MFI) is shown; background subtraction has been used to remove nonspecific signal. Participants designated as “undiagnosed” were those in the group with no history of SARS-CoV-2 infection who had antibody levels that matched those of participants with recent infection. The dashed line indicates a threshold determined by the sum of the mean and standard deviation for the negative control (i.e., beads without antigen). Panel B shows the results of a neutralization antibody proxy assay that determines the level of antibodies that block binding of the spike protein receptor-binding domain to the human host receptor angiotensin-converting enzyme 2 (ACE2), expressed as the percentage of binding that was blocked relative to a control with no plasma (representing maximum binding). The assay threshold for positivity was 30%. In both panels, each point represents a participant at baseline before receiving the vaccine or 3 weeks after receiving the first dose of vaccine and bars represent the group median. The numbers of participants in each group are shown below the graphs.
As a proxy for measuring virus-neutralizing antibodies, we used an FDA-approved in vitro assay that allows indirect detection of potential SARS-CoV-2–neutralizing antibodies in the blood through determination of antibody blocking of the binding of the SARS-CoV-2 receptor-binding domain to the human host receptor angiotensin-converting enzyme 2 (ACE2) (SARS-CoV-2 Surrogate Virus Neutralization Test Kit, Genscript) (Figure 1B). As expected, at baseline, blocking antibodies were undetectable in the group with no history of SARS-CoV-2 infection and were detectable at various levels in the recently infected group and the undiagnosed group. We found that after primary immunization, levels of blocking antibodies were higher among seropositive participants (i.e., the recently infected and undiagnosed groups) than among seronegative participants (the group with no history of infection) (95% confidence interval of the percentage of binding that was blocked: no history of infection, 49.6 to 66.2%; recent infection, 96.0 to 97.0%) (Figure 1B).
We found that 3 weeks after a single vaccination, persons with recent SARS-CoV-2 infection or seropositive status had higher levels of antibody to four SARS-CoV-2 antigens and higher levels of antibodies with neutralizing characteristics than did those without a history of infection. However, the duration of antibody responses and other potential measures of protective immunity need further investigation. Without an immune correlate of protection for SARS-CoV-2 vaccines in humans, protective immunity after vaccination cannot be precisely measured and variations in effective immunization programs cannot be confidently recommended.
Supplementary Appendix
Disclosure Forms
This letter was published on March 23, 2021, at NEJM.org.
Footnotes
Disclosure forms provided by the authors are available with the full text of this letter at NEJM.org.
References
- 1.Baden LR, El Sahly HM, Essink B, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 2020;384:403-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Polack FP, Thomas SJ, Kitchin N, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 2020;383:2603-2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lumley SF, O’Donnell D, Stoesser NE, et al. Antibody status and incidence of SARS-CoV-2 infection in health care workers. N Engl J Med 2020;384:533-540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.