Abstract
Short-term or acute temperature stress affect the immune responses and alters the gut microbiota of broilers, but the influences of long-term temperature stress on stress biomarkers and the intestinal microbiota remains largely unknown. Therefore, we examined the effect of three long-term ambient temperatures (high (HC), medium (MC), and low (LC) temperature groups) on the gene expression of broilers’ heat shock proteins (Hsps) and inflammation – related genes, as well as the caecal microbial composition. The results revealed that Hsp70 and Hsp90 levels in HC group significantly increased, and levels of Hsp70, Hsp90, IL-6, TNF-α, and NFKB1 in LC group were significantly higher than in MC group (p < 0.05). In comparison with the MC group, the proportion of Firmicutes increased in HC and LC groups, while that of Bacteroidetes decreased in LC group at phylum level (p < 0.05). At genus level, the proportion of Escherichia/Shigella, Phascolarctobacterium, Parabacteroides,and Enterococcus increased in HC group; the fraction of Faecalibacterium was higher in LC group; and the percentage of Barnesiella and Alistipes decreased in both HC and LC groups (p < 0.05). Functional analysis based on communities’ phylogenetic investigation revealed that the pathways involved in environmental information processing and metabolism were enriched in the HC group. Those involved in cellular processes and signaling, metabolism, and gene regulation were enriched in LC group. Hence, we conclude that the long-term temperature stress can greatly alter the intestinal microbial communities in broilers and may further affect the host’s immunity and health.
Keywords: broiler, temperature, 16S rRNA sequencing, caecal microbial composition, KEGG pathway
Introduction
Poultry provides an crucial protein source in the human diet and thus has enormous economic value. The broiler breeds were produced by selecting a high growth rate and feed conversion in intensive poultry farming, but they display a low tolerance to ambient temperature change (Lara and Rostagno 2013). Temperature stress may adversely affect poultry immunity, growth performance, physiology, and intestinal morphology and may further cause several health problems such as immunosuppression, microbial infection, and other diseases (Song et al. 2018; Rioja-Lang et al. 2019). Heat shock proteins (Hsps) and inflammatory proteins (such as NF-κB and TNF-α) were reported to be associated with the function of immune systems (Tsan and Gao 2009). Hsps have been identified as important modulators of adaptive immunity and can be induced during multiple types of cellular stresses (Santoro 2000). Besides, extracellular Hsps can interact with dendritic cells (DCs) and macrophages, subsequently activating NF-κB signaling pathway and enhancing the expression of the inflammatory cytokines IL-1, IL-6, and TNF-α (Appenheimer and Evans 2018).
The gastrointestinal compartments of chickens are densely populated with diverse and complex microbial communities, with the most densely populated microbiota found in the caecum (Mohd Shaufi et al. 2015; Shang et al. 2018). The close symbiosis between the host and its intestinal microbes is critical for maintaining the host’s health (Chow et al. 2010). Gut microbes produce various essential metabolites that play vital roles in nutrient digestion, metabolism, and immunity modulation (Hou et al. 2016). Furthermore, caecal microbiota and its modulation are closely associated with poultry health, productivity, and disease control (Montoro-Dasi et al. 2020). The environmental temperature could affect the composition of intestinal microbiota (Wang et al. 2018). For example, the composition of broilers’ gut microbiome varies seasonally (Oakley et al. 2018). The microbial community structure in the ileum and caecum altered with short-term heat stress (Burkholder et al. 2008), and exposure to low temperature led to remarkable changes in the gut microbiota composition (Chevalier et al. 2015).
Although much is known about the effect of acute or continuous heat and cold stresses on the host immune responses and gut microbiota of broilers, little evidence is available regarding how temperature stress that span the entire cycle of broilers (e.g. lasting over 40 days) impact the Hsps, the expression of inflammatory genes and caecal microbiota. Therefore, in this study, we investigated the effects of 42-day temperature stress on the expression of heat shock proteins and inflammation-related genes in the liver, and the composition and function of caecal microbiota in broilers. Our results may provide experimental evidence for designing optimal breeding temperature and developing useful probiotics.
Experimental
Materials and Methods
Ethics statement. All experiments performed in this study were approved by the International Animal Care and Use Committee of the Yunnan Agricultural University (permission code: YNAU20160016). The study complied with the guidelines of the Institutional Administrative Committee and Ethics Committee of Laboratory Animals.
Animal and management. A total of 36 one-day-old Avian chickens (Hunan Shuncheng Industrial Co. Ltd., Hunan, China) were randomly split into three open-circuit calorimetry chambers (Sun et al. 2017) in a commercial chicken farm. One chamber was for the high-temperature treatment (HC), the second one for the medium temperature treatment (MC), and the third one for the low temperature treatment (LC). The temperature of the MC group originated from the Avian 500 broiler feeding standard of Beijing Poultry Breeding Co., Ltd., while the temperatures for HC and LC groups were increased or decreased by 3°C, respectively. The temperature schedule was started at 36.5°C (HC), 33.5°C (MC), and 30.5°C (LC), and respectively reduced to 22°C, 19°C, and 16°C on day 42 as shown in Table SI. Chickens in each chamber were further subdivided into three replicates (four chickens for each) and provided with a bedding of rice husks, feed, and water ad libitum. Aside from the different temperatures, the chickens in all three groups received the same treatments, including the National Research Council (NRC) diet and environment (Table SII).
Samples collection. On day 42, four chickens from each replicate were euthanized by cervical dislocation. The caecal contents and liver samples from all 36 broilers were obtained and immediately stored in liquid nitrogen for further 16S rRNA amplicon sequencing and quantitative real-time polymerase chain reaction (qPCR).
Quantitative real-time PCR. qPCR analysis was performed to compare the relative expression level of Hsp70, Hsp90, tumor necrosis factor alpha (TNF-α), and NF-κB signaling pathway-related genes (NFKB1, NFKB2) in the three temperature groups. Total RNA from the 36 liver samples was extracted using RNA simple total RNA kit (Tiangen Co. Ltd., Cat. #DP419) according to the manufacturer’s recommendations. Primers were designed with Primer Premier 5.0 and shown in Table I. The cDNA was synthesized using FastKing RT kit with gDNAase (Tiangen Co. Ltd., Cat. #KR116). The SYBR Green SuperReal PreMix Plus kit (Tiangen Co. Ltd., Cat. #FP205) was used in the qPCR analysis (CFX96 Real-time system, Bio-Rad) to assess the mRNA expression levels. The data were analyzed by the 2−ΔΔCt method with normalization by the Ct of the housekeeping gene ACTB (β-Actin) (Livak and Schmittgen 2001).
Table I.
List of primer sequences for qPCR.
| Gene | Forward primer (5’→3’) | Reverse primer (5’→3’) | Gene accession number |
|---|---|---|---|
| ACTB | CTCGGCTGTGGTGGTGAA | CCATCTATGAAGGCTACGC | AB495648 |
| Hsp70 | TGGTGGGAATGGTGGTGTTAC | ATCTGCTCCTGTTGGATGTCA | MH422508.1 |
| Hsp90 | CAGCAGCAGTATCATCTTCATC | CCTGTCCTCTGGCTTTAGTTT | NM001109785.1 |
| NF-kB1 | AGTTCAGGATGCACCAAGAGT | AGTCAACGCAGGACCTAAAGA | GGAF000241 |
| NF-KB2 | TGACGGTGGGATAGGTCTTGT | CTGCCTGGATGGGATTGACTA | U00111 |
| IL-6 | CCTAGAAGGAAATGAGAATGCCTAT | CGTTTATGGAGAAGACCGTGAG | AJ309540 |
| TNF-α | GCTTACTTCCCTTCTTCTCC | TCTACATCTGACCCATCCC | XM015284187 |
Microbial DNA extraction and 16S rRNA sequencing. Genomic DNA of caecal contents samples was extracted with QIAamp® Fast DNA Stool Mini Kit (Qiagen, Cat No.19593, Dusseldorf, Germany) following the manufacturer’s recommendations. The amplification of the hypervariable V3-V4 region of the 16S rRNA gene was performed following the recommendations of the 16S Metagenomic Sequencing Library Preparation guide (Illumina, San Diego, CA, USA) using the primer pair 341 F 5’-CCTACGGGRSGCAGCAG-3’ and 806 R 5’-GGACTACVVGGGTATCTAATC-3’. Amplicons were electrophoresed in 2% agarose gels before they were extracted from the gel and purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, U.S.) according to the manufacturer’s instructions and quantified using Qubit®2.0 (Invitrogen, CA, U.S.). The prepared library was sequenced in the HiSeq 2500 platform (Illumina, San Diego, CA, USA) for paired-end reads of 250 bp. The reads were deposited in the NCBI sequence archive (SRA) under accession no. PRJNA573420.
Bioinformatics analyses. Bioinformatics analyses were performed as previously described (Li et al. 2019). In brief, paired-end reads in the fastaq files were assembled through PANDAseq software (https://github.com/neufeld/pandaseq/releases/tag/v2.8.1). The quality control and the filtering process were made pursuant as suggested (Caporaso et al., 2010). The clean reads were clustered and classified into the same operational taxonomic units (OTUs) with a similarity of 97% using UPARSE (http://drive5.com/uparse/), while the chimeric sequences were identified and removed using Userach (version 7.0). Each representative read was assigned to a taxon by RDP Classifier against the Ribosomal Database Project (RDP) database (http://rdp.cme.msu.edu/) with a threshold value of 0.8–1. According to the bacterial annotation, taxonomic information, and each sample’s community composition at various classification levels (Kingdom, Phylum, Class, Order, Family, Genus) were assessed. Alpha diversity of Chao1 values, Good’s Coverage values, Shannon indices, observed species indices, PD whole tree indices, Simpson indices, and beta diversity of principal component analysis (PCA) were calculated using QIIME (version 1.9.1) (Caporaso et al. 2010). The linear discriminant analysis (LDA) effect size (LEfSe) method was used to identify the different taxa between the three groups (Segata et al. 2011). Phylogenetic Investigation of Communities by Reconstruction of Unobserved States (PICRUSt) based on closed-reference OTUs was used to predict the abundance of functional categories in the Kyoto Encyclopedia of Genes and Genomes (KEGG) ortholog (KO) (Langille et al. 2013).
Statistical analysis. The SPSS 22.0 software (IBM SPSS Statistics for Windows, NY) was used to analyze the experimental data. The general linear model analysis with Duncan’s multiple comparison test was used to analyze the gene expression data. Microbial data were analyzed using the Kruskal-Wallis rank test by the SPSS software.
Results
Expression of the genes encoding for heat shock proteins and inflammation-related genes in the liver. Compared to the MC group, the Hsp70 and Hsp90 mRNA expression increased in HC and LC groups (p < 0.05). The NF-κB1 mRNA expression in LC group was upregulated when compared to MC and HC groups (p < 0.05), while higher mRNA expression of IL-6 and TNF-α was observed in the LC group than in the MC group (Fig. 1).
Fig. 1.
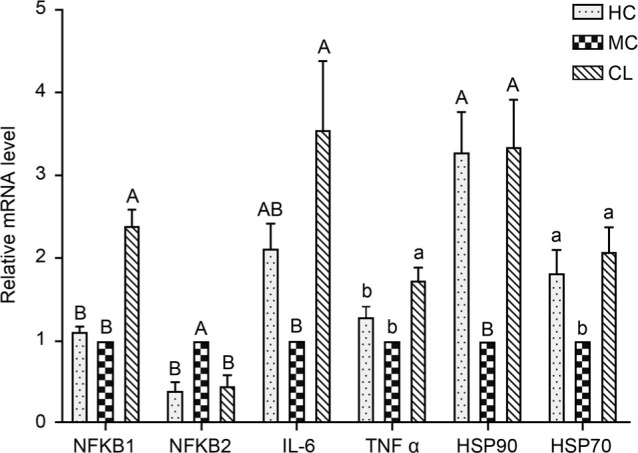
The relative mRNA expression level of heat shock proteins and inflammation-related genes in the liver of chickens. Different uppercase and lowercase letters indicate the significance of difference at p < 0.01 and p < 0.05, respectively. The same letters indicate no significant difference.
High-throughput 16S rDNA sequencing. HiSeq sequencing of the 16S rRNA gene amplicons generated 2,100,180 clean reads (mean length of 414 bp), yielding an average of 58,338 clean reads (52,667–64,809) per sample. Then we rarefied the library size to 32,296 tags per sample using the rarefy function to minimize the impact of the sequencing depth on microbial composition (Table SIII). With a 97% sequence similarity as a cutoff, we obtained 460, 537, and 547 OTUs from the HC, MC, and LC groups, in which the proportion of the core microbiome was 79%, 68%, and 67%, respectively (Fig. S1). Alpha diversity demonstrated different temperature treatments changes in terms of richness and evenness, as summarized in Table II. The Chao1 index in the MC group was significantly higher than that in the LC group (p < 0.05).
Table II.
Library diversity of the 16S rRNA genes from the chicken cecum1.
| Group ID | HC | MC | LC |
|---|---|---|---|
| OTUs | 292 ± 22.70 | 297 ± 42.57 | 271 ± 52.59 |
| Chao1 | 334.06 ± 22.72ab | 351.67 ± 44.98a | 316.75 ± 45.95b |
| Observed species | 289.16 ± 22.77 | 294.25 ± 42.11 | 267.66 ± 53.71 |
| Shannon | 4.93 ± 0.43 | 4.62 ± 0.64 | 4.83 ± 1.01 |
| Simpson | 0.91 ± 0.03 | 0.86 ± 0.05 | 0.89 ± 0.11 |
| PD whole tree | 17.32 ± 1.18 | 17.83 ± 2.11 | 16.64 ± 2.88 |
| Good’s Coverage | 0.9983 ± 0.0001 | 0.9981 ± 0.0002 | 0.9983 ± 0.0002 |
– Different superscripts in the same row indicate significant difference (p < 0.05)
– n = 12 per treatment group (Mean ± SD)
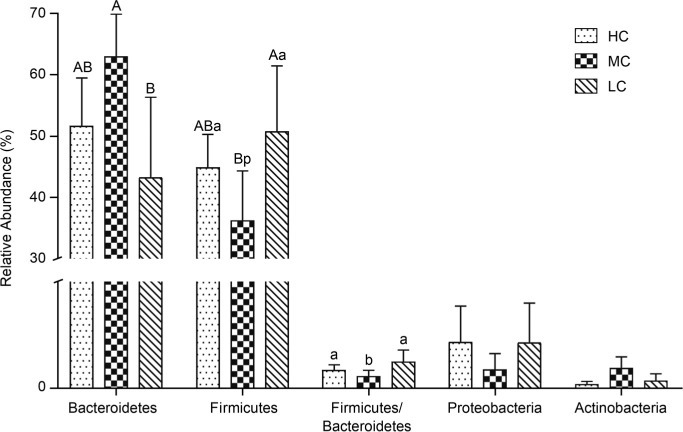
Effects of temperature stress on taxonomic composition. Out of the classifiable sequences, 10 phyla were identified (Fig. S2). Out of the classifiable sequences, ten phyla were identified (Fig. S2). The significant changes of dominant microbiota at the phylum level were determined between the HC, MC, and LC groups (Fig. 2). In the MC group, Bacteroidetes (60.70%) and Firmicutes (36.22%) were the dominant phyla, followed by Actinobacteria (1.00%), Proteobacteria (0.93%), and Tenericutes (0.23%). In comparison, the HC group displayed a 9.02% decrease in the relative abundance of Bacteroidetes (p>0.05), an 8.67% increase of Firmicutes (p< 0.05). The LC group also exhibited considerable phylum-level changes: 17.43% reduction in the relative abundance of Bacteroidetes (p< 0.01), 14.56% increase of Firmicutes (p< 0.01), and 1.65% increase of Proteobacteria (p> 0.05). Meanwhile, the ratio of Firmicutes/Bacteroidetes in LC group increased by 23.31% and 43.98% compared to the HC and MC (p< 0.05) groups, respectively.
Fig. 2.
Composition of the dominant microbiome at the phylum level. Different uppercase and lowercase letters indicate significant differences at p < 0.01 and p < 0.05, respectively. The same letters indicate no significant difference.
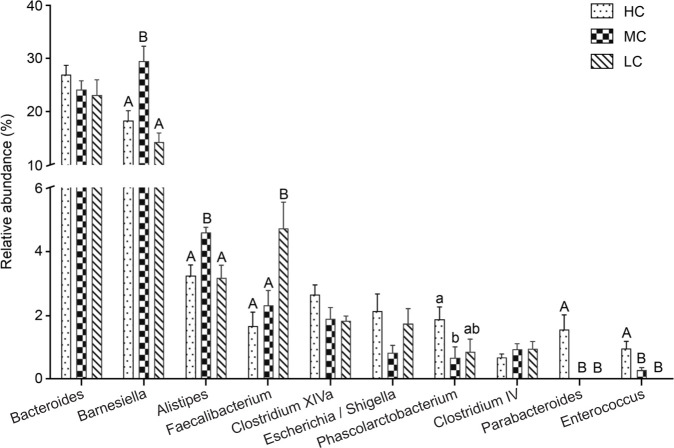
At the genus level, the most abundant phylotypes were Barnesiella, Bacteroides, Alistipes, Faecalibacterium, and Clostridium XlVa, which accounted for 29.48%, 24.19%, 4.59%, 2.33%, and 1.92% of the cecal microbiota of the MC group, respectively (Fig. S3). The remarkable alteration of dominant microbiota at the genus level was noticed between the the HC, MC, and LC groups (Fig. 3). In comparison to MC group, HC group manifested the decrease of 11.12% in the relative abundance of Barnesiella (p< 0.01), and Alistipes (1.34%, p< 0.01), as well as increase of Escherichia/Shigella by 1.28% (p<0.05), Phascolarctobacterium (1.21%, p< 0.05), Parabacteroides (1.58%, p< 0.001), and Enterococcus (0.67%, p< 0.01). The LC group also exhibited considerable changes, including a 15.11% decrease in the relative abundance of Barnesiella (p< 0.001), a 2.38% increase in that of Faecalibacterium (p<0.01), and a 1.39% decrease in that of Alistipes (p< 0.01), respectively.
Fig. 3.
Composition of the dominant microbiome at the genus level. Different uppercase and lowercase letters indicate significant differences at p < 0.01 and p < 0.05, respectively. The same letters indicate no significant difference.
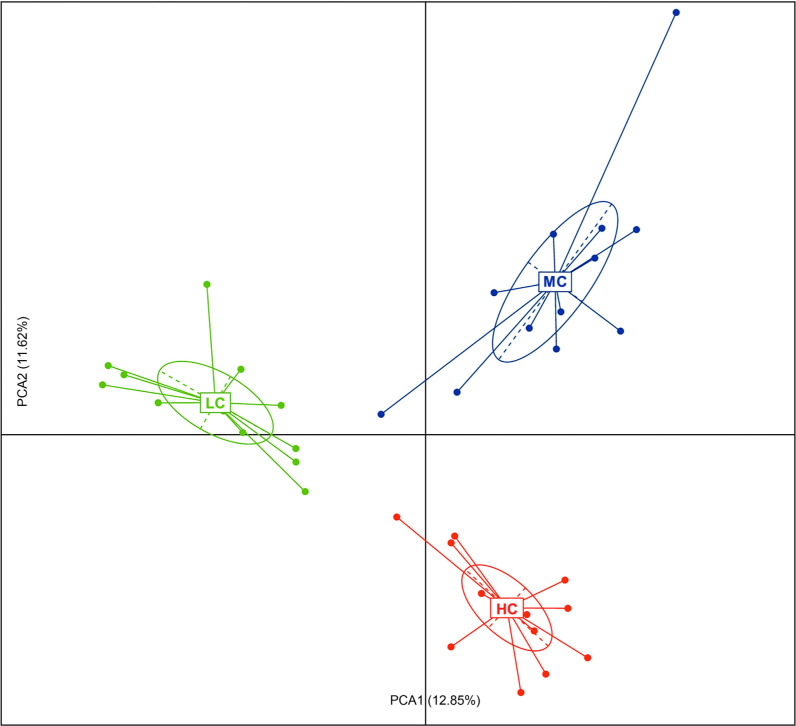
The effects of temperature stress on the caecal microbial communities. PCA analysis revealed a clear separation of the community structure of caecal microbiota in the three groups (Fig. 4), indicating that the high- and low-temperature treatments principally affected the microbial composition.
Fig. 4.
The effects of ambient temperatures on the caecal microbial communities. Principal component analysis (PCA) of the abundance profiling of microbes based on OTUs.
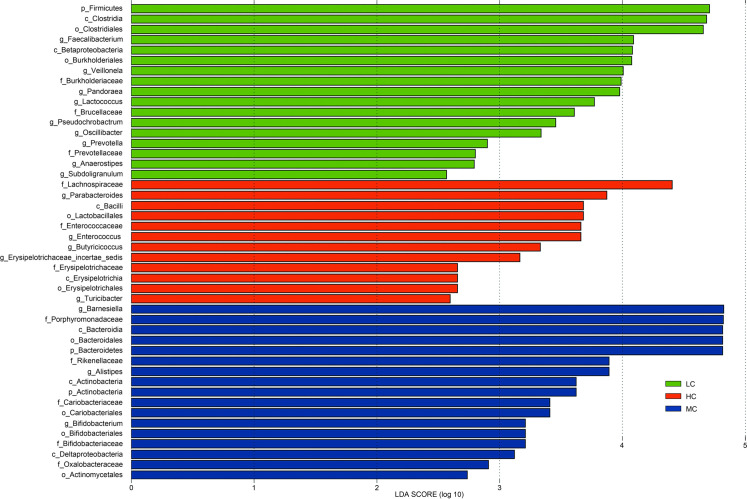
LefSe revealed that the thee phyla (Bacteroidetes, Actinobacteria, and Proteobacteria) enriched in the MC group included three classes (Bacteroidia, Actinobacteria, and Deltaproteobacteria), four orders (Bacteroidales, Coriobacteriales, Bifidobacteriales, and Actinomycetales), five families (Porphyromonadaceae, Rikenellaceae, Coriobacteriaceae, Bifidobacteriaceae, and Oxalobacteraceae) and three genera (Barnesiella, Alistipes, and Bifidobacterium). Then, three major phyla (Bacteroidetes, Firmicutes, and Proteobacteria) from the LC group consisted of two classes (Clostridia and Betaproteobacteria), two orders (Clostridiales and Burkholderiales), three families (Burkholderiaceae, Brucellaceae, and Prevotellaceae), nine genera (Faecalibacterium, Veillonella, Pandoraea, Lactococcus, Pseudochrobactrum, Oscillibacter, Prevotella, Anaerostipes, and Subdoligranulum). Lastly, two dominant phyla (Bacteroidetes and Firmicutes) were found in the HC group, which were comprised of two classes (Bacilli and Erysipelotrichia), two orders (Lactobacillales and Erysipelotrichales), two families (Enterococcaceae and Erysipelotrichaceae), and five genera (Parabacteroides, Enterococcus, Erysipelotrichaceae incertae sedis, Butyricicoccus, and Turicibacter) (Fig. 5). We suggested that the temperature stress greatly influences the composition of the broilers’ caecal microbiota.
Fig. 5.
LEfSe identified the most differentially abundant microbiota between HC, MC, and LC groups.
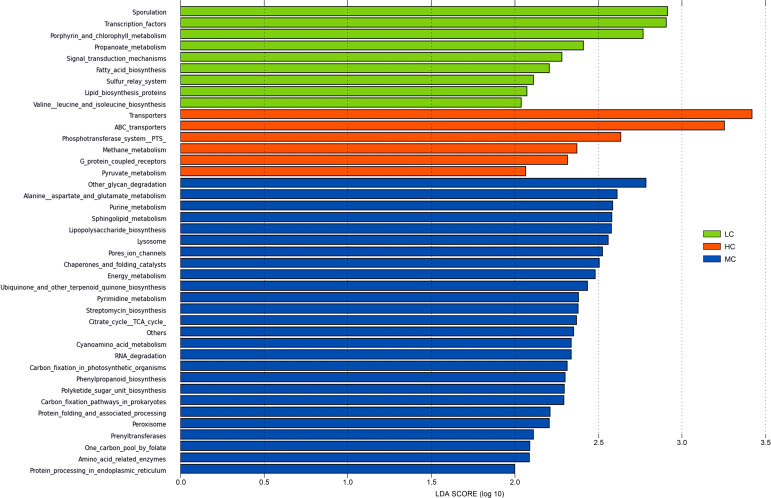
The effects of temperature stress on the microbial functions of caecal microbiota. We observed a striking difference in the KEGG Orthologs (KO) composition of the caecal microbiota. There were 328 differentially enriched KEGG pathways at the L3 hierarchy between the three groups, among which 41 pathways had LDA > 2 (Fig. 6). The pathways enriched in the LC group were related to cellular processes and signaling (e.g. sporulation, signal transduction mechanisms), metabolism (e.g., porphyrin and chlorophyll metabolism, propanoate metabolism, valine, leucine, and isoleucine biosynthesis, fatty acid biosynthesis, and lipid biosynthesis), and gene regulation (e.g., sulfur relay system, transcription factors). The pathways enriched in the HC group were related to environmental information processing (e.g., ABC transporters, G protein-coupled receptors, phosphotransferase system, and transporters) and metabolism (e.g. methane metabolism, and pyruvate metabolism). The pathways enriched in the MC group were related to metabolism (e.g. other glycan degradation, alanine, aspartate, and glutamate metabolism, purine metabolism, sphingolipid metabolism, lipopolysaccharide biosynthesis, ubiquinone and other terpenoid-quinone biosynthesis, pyrimidine metabolism, streptomycin biosynthesis, citrate acid cycle, cyanoamino acid metabolism, carbon fixation in photosynthetic organisms, phenylpropanoid biosynthesis, polyketide sugar unit biosynthesis, carbon fixation pathways in prokaryotes, prenyltransferases, one carbon pool by folate, and amino acid related enzymes), cellular processes (e.g. lysosome, peroxisome), and gene regulation (e.g. chaperones and folding catalysts, RNA degradation, and protein processing in the endoplasmic reticulum).
Fig. 6.
KEGG enrichment analyses of the caecal microbial taxa from the HC, MC, and LC groups at the L3 hierarchy.
Discussion
Temperature stress causes huge losses to poultry producers (Attia et al. 2011), as stresses from low or high temperatures exerted wide-ranging effects on host physiochemical and immune responses (Thaxton 1978). On the one hand, long-term exposure of poultry to high and low environmental temperatures had markedly increased feed consumption and the ratio of feed gain, while low temperature decreased chickens’ final body weight (Yang et al. 2020). On the other hand, both heat and cold stresses could influence the immune responses, such as depression of immune and endocrine functions, elevated expression of heat shock proteins (Hsps), and upregulation of inflammatory genes (such as NF-κB and TNF-α) (Hangalapura et al. 2004; Zhao et al. 2014). Also, increased gene expression of Hsps and NF-κB was reported to be associated with inflammation activation (Vidal Martins et al. 2016; Chebotareva et al. 2020). It was previously reported that cold stress could enhance the cytokine expression levels of IL-6 and TNF-α in humans (Rhind et al. 2001). The Hsp70 mRNA level in the pigs’ muscle increased after exposure to continuous high environmental temperature (35°C ± 1°C) (Abdelnour et al. 2019). In the present study, the Hsp70 and Hsp90 mRNA expression increased in HC and LC groups, while higher NFκB 1, IL-6, and TNF-α mRNA expression in the LC group was observed in the MC group. Consequently, our results were consistent with previous findings that both long-term heat and cold stress increased liver inflammation of broilers.
Firmicutes, Bacteroidetes, and Proteobacteria are the most dominant phyla in chickens’ caecal microbiota (Luo et al. 2013; Oakley et al. 2018), which was corroborated by our data from the MC group. Firmicutes is one of the most abundant phyla of the broilers’ gut microbiome (Corrigan et al. 2011; Hume et al. 2011). Many Firmicutes species are involved in nutrient absorption (Komaroff 2017), and some microbes can survive under extreme conditions (Filippidou et al. 2016). A mouse study revealed that fecal microbial composition changed when the animals were exposed to a cold environment (6°C), during which the relative abundance of Firmicutes increased from 19% to 61%, whereas that of Bacteroidetes decreased from 73% to 35% (Chevalier et al. 2015). In this study, Firmicutes was significantly increased by 9% and 15% in the HC and LC groups, respectively, in comparison with the MC group. These findings suggest that the increase in the proportion of Firmicutes is probably a consequence of the chickens’ physiological adaptations to the temperature stress.
Bacteroides, which belong to Bacteroidetes are generally associated with the degradation of polysaccharides, especially starch and glucans, and the production of short-chain fatty acids (Beckmann et al. 2006). It has been shown that Bacteroides was the most abundant genus in the caecum (Saxena et al. 2016), in line with the findings from our present study. Faecalibacterium prausnitzii in the genus Faecalibacterium is one of the most abundant and important symbiotic bacteria of the human intestinal microflora producing butyrate and other short-chain fatty acids through the fermentation of dietary fiber, and thus improve the intestinal tract immune function (Ferreira-Halder et al. 2017). The abundances of Faecalibacterium in the LC group increased compared with that in the MC group. Therefore, changes in the relative abundances Faecalibacterium may help utilize intestinal nutrients, maintain immune function, and further alleviate the adverse effects of long-term low environmental temperature on broilers.
Barnesiella is a genus in the family of Porphyromonadaceae, and is associated with hyperglycemia, insulin resistance, hepatic steatosis, and inflammation in rodents (Le Roy et al. 2013). Barnesiella is also one of the most abundant genera in human feces and can be used to treat vancomycin-resistant Streptococcus faecalis colonization (Wylie et al. 2012). In addition, it has been reported that Barnesiella intestinihominis from Barnesiella is an intestinal symbiotic bacteria, which can change the tumor microenvironment, reduce regulatory T cells, stimulate the anti-cancer immunomodulator cyclophosphamide (CTL) reaction, and further promote the antitumor efficacy of cyclophosphamide (CTX) (Daillère et al. 2016). In the present study, the relative abundances of Barnesiella in HC and LC groups were significantly lower than in the MC group, indicating Barnesiella as one of the most abundant genera in broilers, with its abundance influenced by both heat and cold stresses.
In the present study, the proportion of Escherichia/Shigella and Enterococcus increased in HC group compared to that in the MC group. Both Enterococcus and Escherichia/Shigella are potentially harmful bacteria from Enterobacteriaceae family (Kong et al. 2019). Enterococcus faecalis is a common commensal organism in humans’ intestines, which can damage eukaryotic cellular DNA in colonic epithelial cells by producing extracellular superoxides and hydroperoxides (Jones et al. 2008). Also, the percentage of Salmonella in Enterobacteriaceae was reported higher in heat-stressed birds than that in the control group (Alhenaky et al. 2017), and the increases of Enterobacteriaceae may be related to mice diarrhea (Yuan et al. 2018). Therefore, Escherichia/Shigella and Enterococcus may trigger inflammation through elevated Hsps and inflammatory genes.
PICRUSt analysis revealed that 41 pathways were differentially enriched between the three groups. Many of the enriched pathways in the HC group were potentially related to environmental information processing and metabolism, such as ABC transporters (LDA = 3.26), G protein-coupled receptors (GPCRs, LDA = 2.32), the phosphotransferase system (PTS, LDA=2.64) and transporters (LDA = 3.42). ABC transporters were involved in transfering a variety of substrates, including sugars, amino acids, glycans, cholesterol, phospholipids, peptides, proteins, toxins, antibiotics, and xenobiotics (Gottesman and Ambudkar 2001). GPCRs were the receptors for hormones, neurotransmitters, ions, photons, and other stimuli, comprising the essential components of communications between the intracellular and extracellular environments (Rosenbaum et al. 2009). The phosphotransferase system (PTS) catalyzed the uptake of carbohydrates and subsequent their conversion into phosphodiester (Deutscher et al. 2006). Therefore, the enhancement of environmental information processing related signal pathways in the HC group may help regulate the intestinal mucosal function and further meet broilers’ metabolic needs.
Many KEGG pathways enriched in the LC group were potentially related to cellular processes and signaling, metabolism, and gene regulation. The enriched metabolism pathways in the LC group included fatty acid biosynthesis (LDA = 2.21), lipid biosynthesis proteins (LDA = 2.07), porphyrin and chlorophyll metabolism (LDA = 2.78), propanoate metabolism (LDA = 2.41), and valine, leucine, and isoleucine biosynthesis (LDA = 2.04). Previous studies suggested that cells prefer glycolysis as a rapid compensatory mechanism to meet energy requirements for adaptive thermogenic responses under cold stress (Sajjanar et al. 2019), and that maintenance of body temperature in cold-exposed animals require increased energy intake and expenditure (Iossa et al. 2001). Our previous study demonstrated that long-term low temperature increased feed consumption but reduced body weight gain (Yang et al. 2020). Altogether, these results indicated that the microbiota in long-term cold stress might maintain the normal body temperature by elevating broilers’ energy and lipid metabolism with a tradeoff of decreased growth performance.
In conclusion, our data revealed that long-term high and low temperatures elevated immune-inflammatory responses in the liver. Stresses from high temperature led to increased potentially harmful bacteria proportion (such as Escherichia/Shigella and Enterococcus) in the caecum. Also, long-term cold stress may upregulate energy and lipid metabolism to maintain broilers’ normal body temperature. Therefore, avoiding unfavorable temperature is a critical factor in the maintainance of broiler birds’ production, welfare, and immune status.
Supplementary Material
Acknowledgments
This study was supported by the National Key R&D Program of China (2016YFD0500501).
Footnotes
Conflict of interest
The authors do not report any financial or personal connections with other persons or organizations, which might negatively affect the contents of this publication and/or claim authorship rights to this publication.
Literature
- Abdelnour SA, Abd El-Hack ME, Khafaga AF, Arif M, Taha AE, Noreldin AE. Stress biomarkers and proteomics alteration to thermal stress in ruminants: A review. J Therm Biol. 2019;79:120–134. 10.1016/j.jtherbio.2018.12.013 [DOI] [PubMed] [Google Scholar]
- Alhenaky A, Abdelqader A, Abuajamieh M, Al-Fataftah AR. The effect of heat stress on intestinal integrity and Salmonella invasion in broiler birds. J Therm Biol. 2017;70(Pt B):9–14. 10.1016/j.jtherbio.2017.10.015 [DOI] [PubMed] [Google Scholar]
- Appenheimer MM, Evans SS. Temperature and adaptive immunity. Handb Clin Neurol. 2018;156:397–415. 10.1016/B978-0-444-63912-7.00024-2 [DOI] [PubMed] [Google Scholar]
- Attia YA, Hassan RA, Tag El-Din AE, Abou-Shehema BM. Effect of ascorbic acid or increasing metabolizable energy level with or without supplementation of some essential amino acids on productive and physiological traits of slow-growing chicks exposed to chronic heat stress. J Anim Physiol Anim Nutr (Berl). 2011;95(6):744–755. 10.1111/j.1439-0396.2010.01104.x [DOI] [PubMed] [Google Scholar]
- Beckmann L, Simon O, Vahjen W. Isolation and identification of mixed linked beta-glucan degrading bacteria in the intestine of broiler chickens and partial characterization of respective 1,3-1,4-beta--glucanase activities. J Basic Microbiol. 2006;46(3):175–185. 10.1002/jobm.200510107 [DOI] [PubMed] [Google Scholar]
- Burkholder KM, Thompson KL, Einstein ME, Applegate TJ, Patterson JA. Influence of stressors on normal intestinal microbiota, intestinal morphology, and susceptibility to Salmonella enteritidis colonization in broilers. Poult Sci. 2008;87(9):1734–1741. 10.3382/ps.2008-00107 [DOI] [PubMed] [Google Scholar]
- Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK, Fierer N, Peña AG, Goodrich JK, Gordon JI, et al. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010;7(5):335–336. 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chebotareva N, Vinogradov A, Gindis A, Tao E, Moiseev S. Heat shock protein 90 and NFkB levels in serum and urine in patients with chronic glomerulonephritis. Cell Stress Chaperones. 2020;25(3):495–501. 10.1007/s12192-020-01089-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevalier C, Stojanović O, Colin DJ, Suarez-Zamorano N, Tarallo V, Veyrat-Durebex C, Rigo D, Fabbiano S, Stevanović A, Hagemann S, et al. Gut microbiota orchestrates energy homeostasis during cold. Cell. 2015;163(6):1360–1374. 10.1016/j.cell.2015.11.004 [DOI] [PubMed] [Google Scholar]
- Chow J, Lee SM, Shen Y, Khosravi A, Mazmanian SK. Host-bacterial symbiosis in health and disease. Adv Immunol. 2010;107: 243–274. 10.1016/B978-0-12-381300-8.00008-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corrigan A, Horgan K, Clipson N, Murphy RA. Effect of dietary supplementation with a Saccharomyces cerevisiae mannan oligosaccharide on the bacterial community structure of broiler cecal contents. Appl Environ Microbiol. 2011;77(18):6653–6662. 10.1128/AEM.05028-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daillère R, Vétizou M, Waldschmitt N, Yamazaki T, Isnard C, Poirier-Colame V, Duong CPM, Flament C, Lepage P, Roberti MP, et al. Enterococcus hirae and Barnesiella intestinihominis facilitate cyclophosphamide-induced therapeutic immunomodulatory effects. Immunity. 2016;45(4):931–943. 10.1016/j.immuni.2016.09.009 [DOI] [PubMed] [Google Scholar]
- Deutscher J, Francke C, Postma PW. How phosphotransferase system-related protein phosphorylation regulates carbohydrate metabolism in bacteria. Microbiol Mol Biol Rev. 2006;70(4):939–1031. 10.1128/MMBR.00024-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira-Halder CV, Faria AVS, Andrade SS. Action and function of Faecalibacterium prausnitzii in health and disease. Best Pract Res Clin Gastroenterol. 2017;31(6):643–648. 10.1016/j.bpg.2017.09.011 [DOI] [PubMed] [Google Scholar]
- Filippidou S, Wunderlin T, Junier T, Jeanneret N, Dorador C, Molina V, Johnson DR, Junier P. A combination of extreme environmental conditions favor the prevalence of endospore-forming Firmicutes. Front Microbiol. 2016;7:1707. 10.3389/fmicb.2016.01707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottesman MM, Ambudkar SV. Overview: ABC transporters and human disease. J Bioenerg Biomembr. 2001;33(6):453–458. 10.1023/a:1012866803188 [DOI] [PubMed] [Google Scholar]
- Hangalapura BN, Nieuwland MG, Buyse J, Kemp B, Parmentier HK. Effect of duration of cold stress on plasma adrenal and thyroid hormone levels and immune responses in chicken lines divergently selected for antibody responses. Poult Sci. 2004;83(10): 1644–1649. 10.1093/ps/83.10.1644 [DOI] [PubMed] [Google Scholar]
- Hou Q, Kwok LY, Zheng Y, Wang L, Guo Z, Zhang J, Huang W, Wang Y, Leng L, Li H, et al. Differential fecal microbiota are retained in broiler chicken lines divergently selected for fatness traits. Sci Rep. 2016;6:37376. 10.1038/srep37376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hume ME, Barbosa NA, Dowd SE, Sakomura NK, Nalian AG, Martynova-Van Kley A, Oviedo-Rondón EO. Use of pyrosequencing and denaturing gradient gel electrophoresis to examine the effects of probiotics and essential oil blends on digestive microflora in broilers under mixed Eimeria infection. Foodborne Pathog Dis. 2011;8(11):1159–1167. 10.1089/fpd.2011.0863 [DOI] [PubMed] [Google Scholar]
- Iossa S, Lionetti L, Mollica MP, Crescenzo R, Barletta A, Liverini G. Effect of cold exposure on energy balance and liver respiratory capacity in post-weaning rats fed a high-fat diet. Br J Nutr. 2001;85(1):89–96. 10.1079/bjn2000219 [DOI] [PubMed] [Google Scholar]
- Jones BV, Begley M, Hill C, Gahan CG, Marchesi JR. Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci USA, 2008; 105(36):13580–13585. 10.1073/pnas.0804437105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komaroff AL. The microbiome and risk for obesity and diabetes. JAMA. 2017;317(4):355–356. 10.1001/jama.2016.20099 [DOI] [PubMed] [Google Scholar]
- Kong C, Gao R, Yan X, Huang L, Qin H. Probiotics improve gut microbiota dysbiosis in obese mice fed a high-fat or high-sucrose diet. Nutrition. 2019;60:175–184. 10.1016/j.nut.2018.10.002 [DOI] [PubMed] [Google Scholar]
- Langille MG, Zaneveld J, Caporaso JG, McDonald D, Knights D, Reyes JA, Clemente JC, Burkepile DE, Vega Thurber RL, Knight R, et al. Predictive functional profiling of microbial communities using 16S rRNA marker gene sequences. Nat Biotechnol. 2013;31(9): 814–821. 10.1038/nbt.2676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lara LJ, Rostagno MH. Impact of heat stress on poultry production. Animals (Basel). 2013;3(2):356–369. 10.3390/ani3020356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Roy T, Llopis M, Lepage P, Bruneau A, Rabot S, Bevilacqua C, Martin P, Philippe C, Walker F, Bado A, et al. Intestinal microbiota determines development of non-alcoholic fatty liver disease in mice. Gut. 2013;62(12):1787–1794. 10.1136/gutjnl-2012-303816 [DOI] [PubMed] [Google Scholar]
- Li X, Cao Z, Yang Y, Chen L, Liu J, Lin Q, Qiao Y, Zhao Z, An Q, Zhang C, et al. Correlation between jejunal microbial diversity and muscle fatty acids deposition in broilers reared at different ambient temperatures. Sci Rep. 2019;9(1):11022. 10.1038/s41598-019-47323-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25(4):402–408. 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- Luo YH, Peng HW, Wright AD, Bai SP, Ding XM, Zeng QF, Li H, Zheng P, Su ZW, Cui R, et al. Broilers fed dietary vitamins harbor higher diversity of cecal bacteria and higher ratio of Clostridium, Faecalibacterium, and Lactobacillus than broilers with no dietary vitamins revealed by 16S rRNA gene clone libraries. Poult Sci. 2013;92(9):2358–2366. 10.3382/ps.2012-02935 [DOI] [PubMed] [Google Scholar]
- Mohd Shaufi MA, Sieo CC, Chong CW, Gan HM, Ho YW. Deciphering chicken gut microbial dynamics based on high-throughput 16S rRNA metagenomics analyses. Gut Pathog. 2015;7:4. 10.1186/s13099-015-0051-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoro-Dasi L, Villagra A, de Toro M, Pérez-Gracia MT, Vega S, Marin C. Fast and slow-growing management systems: characterisation of broiler caecal microbiota development throughout the growing period. Animals. 2020;10(8):1401. 10.3390/ani10081401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley BB, Vasconcelos EJR, Diniz PPVP, Calloway KN, Richardson E, Meinersmann RJ, Cox NA, Berrang ME. The cecal microbiome of commercial broiler chickens varies significantly by season. Poult Sci. 2018;97(10):3635–3644. 10.3382/ps/pey214 [DOI] [PubMed] [Google Scholar]
- Rhind SG, Castellani JW, Brenner IK, Shephard RJ, Zamecnik J, Montain SJ, Young AJ, Shek PN. Intracellular monocyte and serum cytokine expression is modulated by exhausting exercise and cold exposure. Am J Physiol Regul Integr Comp Physiol. 2001; 281(1):R66–R75. 10.1152/ajpregu.2001.281.1.R66 [DOI] [PubMed] [Google Scholar]
- Rioja-Lang FC, Brown JA, Brockhoff EJ, Faucitano L. A review of swine transportation research on priority welfare issues: A Canadian perspective. Front Vet Sci. 2019;6:36. 10.3389/fvets.2019.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum DM, Rasmussen SG, Kobilka BK. The structure and function of G-protein-coupled receptors. Nature. 2009;459(7245): 356–363. 10.1038/nature08144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sajjanar B, Siengdee P, Trakooljul N, Liu X, Kalbe C, Wimmers K, Ponsuksili S. Cross-talk between energy metabolism and epigenetics during temperature stress response in C2C12 myoblasts. Int J Hyperthermia. 2019;36(1):776–784. 10.1080/02656736.2019.1639834 [DOI] [PubMed] [Google Scholar]
- Santoro MG. Heat shock factors and the control of the stress response. Biochem Pharmacol. 2000;59(1):55–63. 10.1016/s0006-2952(99)00299-3 [DOI] [PubMed] [Google Scholar]
- Saxena S, Saxena VK, Tomar S, Sapcota D, Gonmei G. Characterisation of caecum and crop microbiota of Indian indigenous chicken targeting multiple hypervariable regions within 16S rRNA gene. Br Poult Sci. 2016;57(3):381–389. 10.1080/00071668.2016.1161728 [DOI] [PubMed] [Google Scholar]
- Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, Huttenhower C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. 10.1186/gb-2011-12-6-r60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shang Y, Kumar S, Oakley B, Kim WK. Chicken gut microbiota: importance and detection technology. Front Vet Sci. 2018;5:254. 10.3389/fvets.2018.00254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song ZH, Cheng K, Zheng XC, Ahmad H, Zhang LL, Wang T. Effects of dietary supplementation with enzymatically treated Artemisia annua on growth performance, intestinal morphology, digestive enzyme activities, immunity, and antioxidant capacity of heat-stressed broilers. Poult Sci. 2018;97(2):430–437. 10.3382/ps/pex312 [DOI] [PubMed] [Google Scholar]
- Sun YK, Yan XG, Ban ZB, Yang HM, Hegarty RS, Zhao YM. The effect of cysteamine hydrochloride and nitrate supplementation on in-vitro and in-vivo methane production and productivity of cattle. Anim Feed Sci Tech. 2017;232:49–56. 10.1016/j.anifeedsci.2017.03.016 [DOI] [Google Scholar]
- Thaxton P. Influence of temperature on the immune response of birds. Poult Sci. 1978;57(5):1430–1440. 10.3382/ps.0571430 [DOI] [PubMed] [Google Scholar]
- Tsan MF, Gao B. Heat shock proteins and immune system. J Leukoc Biol. 2009;85(6):905–910. 10.1189/jlb.0109005 [DOI] [PubMed] [Google Scholar]
- Vidal Martins M, Montezano de Carvalho IM, Magalhães Caetano MM, Lopes Toledo RC, Avelar Xavier A, De Queiroz JH. Neuroprotective effect of Sapucaia nuts (Lecythis pisonis) on rats fed with high-fat diet. Nutr Hosp. 2016;33(6):1424–1429. 10.20960/nh.805 [DOI] [PubMed] [Google Scholar]
- Wang XJ, Feng JH, Zhang MH, Li XM, Ma DD, Chang SS. Effects of high ambient temperature on the community structure and composition of ileal microbiome of broilers. Poult Sci. 2018;97(6): 2153–2158. 10.3382/ps/pey032 [DOI] [PubMed] [Google Scholar]
- Wylie KM, Truty RM, Sharpton TJ, Mihindukulasuriya KA, Zhou Y, Gao H, Sodergren E, Weinstock GM, Pollard KS. Novel bacterial taxa in the human microbiome. Plos One 2012;7(6):e35294. 10.1371/journal.pone.0035294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Gao H, Li X, Cao Z, Li M, Liu J, Qiao Y, Ma L, Zhao Z, Pan H. Correlation analysis of muscle amino acid deposition and gut microbiota profile of broilers reared at different ambient temperatures. Anim Biosci. 2021. Jan;34(1):93–101. 10.5713/ajas.20.0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Zhang S, Li H, Yang F, Mushtaq N, Ullah S, Shi Y, An C, Xu J. The influence of gut microbiota dysbiosis to the efficacy of 5-fluorouracil treatment on colorectal cancer. Biomed Pharmacother. 2018;108:184–193. 10.1016/j.biopha.2018.08.165 [DOI] [PubMed] [Google Scholar]
- Zhao FQ, Zhang ZW, Qu JP, Yao HD, Li M, Li S, Xu SW. Cold stress induces antioxidants and Hsps in chicken immune organs. Cell Stress Chaperones. 2014;19(5):635–648. 10.1007/s12192-013-0489-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.