Abstract
In less than one year since the outbreak of the COVID-19 pandemic, two mRNA-based vaccines, BNT162b2 and mRNA-1273, were granted the first historic authorization for emergency use, while another mRNA vaccine, CVnCoV, progressed to phase 3 clinical testing. The COVID-19 mRNA vaccines represent a new class of vaccine products, which consist of synthetic mRNA strands encoding the SARS-CoV-2 Spike glycoprotein, packaged in lipid nanoparticles to deliver mRNA to cells. This review digs deeper into the scientific breakthroughs of the last decades that laid the foundations for the rapid rise of mRNA vaccines during the COVID-19 pandemic. As well as providing momentum for mRNA vaccines, SARS-CoV-2 represents an ideal case study allowing to compare design-activity differences between the different mRNA vaccine candidates. Therefore, a detailed overview of the composition and (pre)clinical performance of the three most advanced mRNA vaccines is provided and the influence of choices in their structural design on to their immunogenicity and reactogenicity profile is discussed in depth. In addition to the new fundamental insights in the mRNA vaccines' mode of action highlighted here, we also point out which unknowns remain that require further investigation and possibly, optimization in future mRNA vaccine development.
Graphical abstract
1. COVID-19 creates momentum for mRNA vaccines
Since Edward Jenner's fist successful vaccination studies in the late 1700's, vaccine development and large-scale immunization campaigns have been society's response to infectious disease outbreaks worldwide. The COVID-19 pandemic is in that sense no different, although it is remarkable that the global search for a vaccine against SARS-CoV-2 has introduced a completely new class of vaccine products: at least three of the candidates that are in the forefront of the vaccine development race are messenger RNA (mRNA) nanoparticles. This is quite a revolution in vaccinology. To exemplify, all of the basal vaccines recommended by the World Health Organization (WHO) trigger immunity by injecting weakened or whole inactivated pathogens. The more recently developed vaccines, such as the ones against the human papillomavirus (HPV), Hepatitis B or the seasonal flu no longer contain intact virus particles, but rather purified or recombinantly produced viral proteins, which need to be delivered in combination with immune adjuvants to improve the vaccine's immunogenicity. The mRNA vaccines for COVID-19 do not resemble any of these vaccine products whatsoever, as they do not contain protein compounds, let alone whole virus particles. By contrast, they consist of mRNA strands packaged in a neutrally charged, lipid-based nanoparticle. The speed at which these novel therapeutics have made it to phase III clinical testing is remarkable, especially since they have become the very first mRNA-based therapeutics to receive approval by FDA and EMA. This raises the question: are mRNA vaccines ready for prime time?
To address this, it is important to realize that research into the use of mRNA as a source of protein has been around for three decades [1] and although it has been employed for various therapeutic targets, its use as a source of antigen in vaccination approaches is by far the most popular [2]. During this investigational phase, two major discoveries clearly played their part in today's success story.
The first discovery originates from our increased understanding of the complexity of innate immunity. Indeed, the identification of Toll-like receptors (TLRs) by Jules Hoffman and Bruce Beutler elucidated how the mammalian immune system could recognize highly conserved patterns associated with the presence of pathogens or damage [3]. As an example, upon viral infection, the host cells can recognize the viral nucleic acids via specific TLRs (TRL3, 7 and 8 for RNA; TLR9 for DNA) and cellular RNA sensors (e.g., MDA-5 and RIG-I). The result of this innate immune sensing is the production of inflammatory cytokines, including type I interferons (IFNs), aiming to counteract the translation of these viral nucleic acids in an attempt to avoid the production of new viral particles. Katalin Karikó and Drew Weissman added an important new insight to this: not only viral RNA, but also endogenous mRNA associated with apoptotic cells as well as in vitro transcribed (IVT) mRNA could bind and activate TLR3, TLR7 and TLR8 [4,5]. Follow-up studies revealed further crucial insights that the many modifications observed in natural mRNA are in fact a way to avoid immune recognition, allowing the immune system to distinguish self from non-self RNA [5]. The strategy proved to be translatable to IVT mRNA: by incorporating naturally occurring modified nucleotides into the IVT mRNA, its innate immune activity could be drastically reduced [6,7]. Additional efforts to remove double-stranded (ds)RNA contaminants even further ablated IVT RNA's immunostimulatory activity [8,9]. As a result of avoiding innate immunity, modified and purified IVT mRNA enables much higher protein expression and has a more favorable safety profile, as it drastically reduces the production of pro-inflammatory type I IFNs [6]. In their final COVID-19 vaccines, both BioNTech and Moderna have made use of a highly-purified and N1-methyl-pseudouridine (1mΨ) modified mRNA. In contrast, CureVac has applied its “unmodified” mRNA vaccine technology, which employs sequence optimization and selected untranslated regions (UTRs) to enhance mRNA translation, while keeping a balanced type I IFN activity [10,11].
Secondly, substantial progress has been made with respect to the delivery vehicles used to package and protect the mRNA upon administration. All three major players in the mRNA vaccine field have previously optimized so-called lipid nanoparticles (LNPs), constructs that typically consist of an ionizable lipid and one or more helper lipids to improve LNP stability and promote endosomal escape upon cellular uptake [12,13]. These mRNA-loaded nanomedicines are formulated through microfluidic mixing, a scalable production system that can easily be applied in GMP facilities. During the mixing step, the pH of the formulation is kept low to protonate the ionizable lipid's amine function, enabling electrostatic complexation of the now positively charged lipids and the negatively charged mRNA. A dialysis or ultrafiltration is then performed to neutralize the pH, resulting in uncharged, solid-core LNPs that densely package the mRNA [14]. This carrier concept is very similar to the recently FDA-approved siRNA-based therapeutic Onpattro® (Alnylam® Pharmaceuticals) which was designed for hepatocyte targeting after intravenous administration [15]. Although these LNPs are not entirely new to the clinic, it should be mentioned that the approval of the COVID-19 vaccines heralds their first large-scale administration, as Onpattro® is registered as an orphan drug.
LNPs packaging IVT mRNA have already been evaluated in several phase 1/2 clinical trials for both cancer immunotherapy as well as prophylactic vaccination against infectious disease. As example, Moderna already reported on the good tolerability of an intramuscular/intradermal nucleoside-modified mRNA vaccine against two Influenza viruses (H10N8 and H7N9). Typical adverse events related to immune activation such as myalgia, fatigue and headache were reported which were mostly mild to moderate in severity and short-lived, not requiring medical interventions [16]. In the early days of the COVID-19 pandemic, CureVac announced positive interim results from a phase 1 trial evaluating the safety and immunogenicity of a prophylactic mRNA Rabies LNP vaccine [17]. Taken together, as a platform technology, mRNA-LNP vaccines already reached a substantial degree of maturity.
Another factor that contributes to the rapid progression of the SARS-CoV-2 vaccines is the ability to build on earlier research on coronaviruses that induced severe acute respiratory syndrome (SARS, first reported in 2003) and Middle East respiratory syndrome (MERS, first outbreak in 2012). Coronaviruses contain a single RNA genome that encodes four major viral proteins (Spike, Envelope, Membrane and Nucleocapsid) and a couple of accessory proteins. Among these, the Spike (S) protein sparked particular interest [18]. This protein mediates viral entry by first binding to its cellular receptor (i.e. angiotensin-converting enzyme 2, ACE2 for SARS-CoV-1 or dipeptidyl peptidase 4, DPP4/CD26 for MERS-CoV). Upon interaction, the S protein undergoes structural changes resulting in the fusion of viral and host cell membranes. Importantly, most of the MERS-CoV and SARS-CoV-1 neutralizing antibodies were directed to the S protein, in particular to its receptor-binding domain (RBD) [18,19]. When the new SARS-CoV-2 virus was identified, detailed investigations into the structure and dynamics of the SARS-CoV-2 S protein revealed large homology to the SARS-CoV-1 S protein, which allowed the major players in vaccine development to immediately turn to the S protein as vaccine target [20,21].
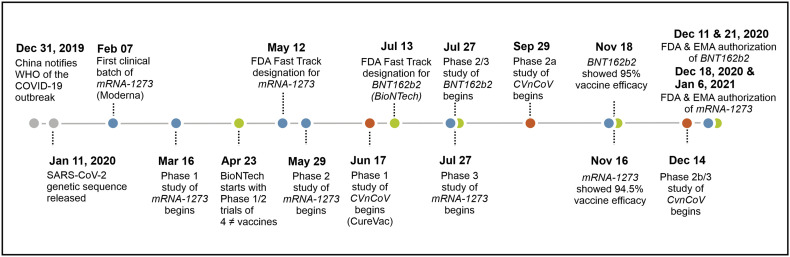
Taken together, the rapid development of the COVID-19 vaccines did not come out of the blue at all, the current COVID-19 pandemic merely provided momentum for mRNA vaccines to speed up their progress to approval. The flexibility and fast production of any mRNA undoubtedly provided a head-start in the vaccine race (Fig. 1 ). To exemplify, merely 42 days after sequence selection from the genetic sequence of the new coronavirus by the Chinese authorities, the first clinical-grade batch of Moderna's mRNA vaccine was ready [22]. The promising COVID-19 vaccines rapidly received Fast Track designation by the FDA and EMA, resulting in their approval by both regulatory agencies before the end of 2020 for emergency use [[23], [24], [25], [26]].
Fig. 1.
Timeline of the development of three most advanced mRNA vaccines against COVID-19; BNT162b2 (Green dots), mRNA-1273 (Blue) and CVnCoV (Orange). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
2. COVID-19 mRNA vaccines: Differences and similarities
The particular journey of the first approved mRNA vaccines for COVID-19 took about a year, starting with the announcement of vaccine development programs to regulatory approval and the initiation of widespread vaccination campaigns. Within this short timeframe, an enormous amount of new information on mRNA vaccines was published, all focussing on the exact same disease target, thereby providing more insight in the relation between the vaccine's design features and the resulting immunogenicity and degree of protection. As such, this COVID-19 case might have been the best way so far to investigate and compare the potential of the different mRNA vaccine platforms.
A detailed comparison of the COVID-19 mRNA vaccines of the three vaccine developers, BNT162b2 (BioNTech/Pfizer), mRNA-1273 (Moderna) and CVnCoV (CureVac), including their differences and similarities in antigen choice, LNP design, and mRNA structure is provided in Table 1 .
Table 1.
Composition of the three most advanced COVID-19 mRNA vaccines.
| BNT162b2 – BioNTech/Pfizer | ||||
|---|---|---|---|---|
| mRNA | Lipid nanoparticle | Administration | Status | References |
| Full-length Spike with Proline substitutions (K986P, V987P) |
Ionizable cationic lipid
(50:10:38.5:1.5 mol%) RNA to lipid ratioa ~0.05 (wt/wt) |
Intramuscular 30 μg mRNA Two doses with 21-day interval | Emergency use in the U.K, U.S., Europe and other countries & approved in Switzerland, Bahrain and Saudi Arabia | Preclinical: [48] Phase 1/2: [49,50] Phase 3: [51] |
| ||||
| mRNA-1273 – Moderna | ||||
|---|---|---|---|---|
| mRNA | Lipid nanoparticle | Administration | Status | References |
| Full-length Spike with Proline substitutions (K986P, V987P) |
Ionizable cationic lipid
(50:10:38.5:1.5 mol%) RNA to lipid ratioa ~0.05 (wt/wt) |
Intramuscular 100 μg mRNA Two doses with 28-day interval | Emergency use in the U.K, U.S., Europe and other countries & approved in Switzerland | Preclinical: [27,52] Phase 1/2: [53] Phase 3: [54] |
| ||||
| CVnCoV – CureVac | ||||
|---|---|---|---|---|
| mRNA | Lipid nanoparticle | Administration | Status | References |
| Full-length Spike with Proline substitutions (K986P, V987P) |
Ionizable cationic lipid
(50:10:38.5:1.5 mol%) RNA to lipid ratioa ~0.05 (wt/wt) |
Intramuscular 12 μg mRNA Two doses with 28-day interval | Phase 2b/3 initiated mid-December | Preclinical: [55,56] Phase 1: [57] |
| ||||
Based on prior research of the developers, and not yet confirmed for the COVID-19 mRNA vaccines.
2.1. Antigen target
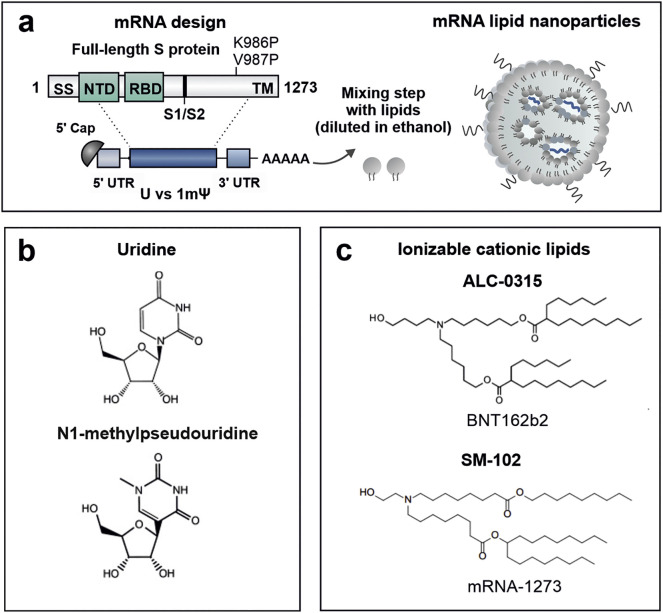
All mRNA vaccines target the same SARS-CoV-2 antigen and incorporate mRNA encoding the full-length, transmembrane anchored S protein. The genetic sequence is slightly altered to stabilize the prefusion conformation of the glycoprotein using two proline (2P) substitutions (K986P and V987P mutations) (Fig. 2a) [21]. A major advantage of the mRNA approach, is that the proteins are produced by the host cells as they would be in case of a natural infection with the virus. As a result, the produced proteins will undergo the same post-translational processing, including glycosylation, subunit cleavage and proper protein folding. As such, the S glycoprotein is eventually incorporated as a trimer in the membrane of mRNA-transfected cells, allowing it to be efficiently exposed in its antigenically native prefusion conformation to B cells. Precedent work by Moderna and the NIH National Institute for Allergy and Infectious Diseases (NIAID) demonstrated that the membrane-bound MERS-CoV 2P S mRNA elicited more potent neutralizing antibody responses as compared to secreted MERS-CoV 2P S, or wild-type S mRNA; fundamental insights that could immediately be transferred to the mRNA vaccine design for the SARS-CoV-2 pandemic [27]. Moreover, isolation of neutralizing antibodies from the serum of COVID-19 patients confirmed the strong immunogenicity of the S protein, but showed an equal immunogenicity of the RBD and the N-terminal domain (NTD). This implies that vaccinating against the entire S protein, rather than only one of its structural components, is expected to result in an improved response which will be less affected when the virus undergoes genetic drift [28].
Fig. 2.
COVID-19 mRNA vaccine design. (a) The COVID-19 mRNA vaccines contain a mRNA sequence encoding the full length S protein with two proline substitutions (K986P and V987P). The S protein's genetic code is flanked by structural elements to produce a mature mRNA. Each of these elements can be optimized in order to modulate mRNA stability, translation capacity and innate immune activity. (b) While the CVnCoV vaccine candidate make use of unmodified uridines, BNT162b2 and mRNA-1273 are nucleoside-modified with a substitution of N1-methylpseudouridine (1mψ) for uridine (U). (c) Chemical structures of the ionizable cationic lipids ALC-0315 (((4-Hydroxybutyl)azanediyl)bis(hexane-6,1-diyl)bis(2-hexyldecanoate)) and SM-102 (Heptadecan-9-yl 8-((2-hydroxyethyl)(8-(nonyloxy)-8-oxooctyl)amino)octanoate) used in the LNP formulation of BNT162b2 and mRNA-1273, respectively. The ionizable cationic lipid used in CVnCoV has not (yet) been disclosed. Abbreviations: SS; Signal sequence, NTD; N-terminal domain, S1/S2; native furine cleavage site, TM; transmembrane domain.
Of note, the S protein is also very suitable as a source of T cell epitopes. Grifoni et al. identified S-specific CD4+ T cells in 100% of the COVID-19 patients, and found their levels to correlate with the magnitude of anti-SARS-CoV-2 IgG and IgA titers. About 70% of these patients also exhibited disease-specific CD8+ T cells, but the immunodominance of the S protein for CD8+ T cell recognition was less pronounced: ~26% of the elicited anti-SARS-CoV-2 CD8+ T cells reacted to the S protein [29]. The cytoplasmic localization of the mRNA-encoded proteins allows for direct intracellular processing of the translated S proteins, thereby efficiently presenting peptide fragments in MHC-I complexes to CD8+ T cells. Finally, exposure of S proteins in the extracellular environment during turnover of mRNA-transfected cells, makes them accessible for MHC-II antigen processing by bystander cells, while also intracellular recycling mechanisms (e.g., autophagy) on the translated proteins can contribute to the antigen presentation to CD4+ T (helper) cells [30].
2.2. mRNA design and modifications
Several optimizations to the mRNA structure can drastically improve the final outcome. The design of (non-coding) structural elements of the mRNA such as the CAP structure, poly(A) tail and untranslated regions (UTRs) all have a major impact on the mRNA stability and translation capacity [31,32]. Codon optimization in the mRNA sequence to e.g., match host transfer (t)RNA abundances, or as a determinant of introducing secondary structures, can drastically impact the protein synthesis rate and ribosome dwell time (i.e. mRNA functional half-life) [33,34]. In this context, 1mΨ nucleotide-modifications were shown to provide additional base pair stability, giving rise to a high degree of secondary structure which significantly improves the mRNA translation [35]. Furthermore, the secondary structure design of mRNA can be optimized in order to improve mRNA stability against cleavage by endonucleases and chemical degradation processes, including hydrolysis [36].
BNT162b2 and mRNA-1273 implement a combination of modified nucleotide 1mΨ replacement and removal of dsRNA fragments in the mRNA production process, which strongly reduces the innate immune signaling in response to mRNA through decreased activation of TLR signaling and cytosolic RNA sensors. Moderna claimed that with such an approach, both local and systemic innate immune effects upon mRNA (vaccine) administration can be limited to a bare minimum in mice [37]. In contrast to BNT162b2 and mRNA-1273, the CVnCoV vaccine candidate contains an “unmodified” mRNA, which employs sequence engineering (e.g., reduction in uridine content), selected UTRs, and a stringent purification protocol to remove dsRNA fragments [11].
2.3. Delivery system
How much protein will be produced from the mRNA template will initially be determined by the amount of intact mRNA that reaches the cytosolic compartment. This is where the LNP delivery technology comes into play. Upon administration, proteins and other biological components present in the extracellular space can bind at the surface of the mRNA LNPs. The polyethylene glycol (PEG)-lipid stabilizing the mRNA LNP system against aggregation during manufacturing and storage contains short acyl chains. This design facilitates that the PEG-lipid rapidly dissociates from the LNP following injection, as an essential first step to allow cellular interactions [38,39]. Upon intravenous administration, Akinc et al. found that the surface of neutrally-charged LNPs is strongly enriched with apolipoprotein E (ApoE), which leads to enhanced uptake by hepatocytes through low density lipoprotein (LDL) receptor-mediated endocytosis [40]. There are good reasons to believe that ApoE binding also plays a critical role in the uptake of the mRNA LNP vaccines after intramuscular injection. Vaccine targeted cell types (see Mode of action), such as dendritic cells (DCs) and monocytes, highly express LDL receptors and other scavenger receptors [41]. Moreover, the transfection of human DCs with mRNA LNPs in an in vitro setting was also reported to be promoted in the presence of ApoE [42]. Upon internalization, mRNA LNPs are routed through the endo-lysosomal compartment, where most of the mRNA LNPs remain entrapped in endosomes and degrade over time. The intracellular trafficking and underlying mechanisms on how LNPs enable the escape of mRNA from the endosomes to reach the cytoplasm are still not fully understood [[43], [44], [45]]. The hypothesis stands that the ionizable lipid components of the LNPs (pKa < 7) become protonated due to the acidic pHs in the endosomes, and leads to lipid exchange with anionic phospholipids of the endosomal membrane. This lipid mixing also induces a non-bilayer structure conversion in the LNPs (i.e. lamellar-to-inverted hexagonal phase), which facilitates the release of mRNA from the LNPs [44]. These effects of membrane fusion and structural changes in the LNPs are suggested to drive the destabilization of the endosomal membrane and eventually the endosomal escape of mRNA. Interestingly, a recent study by Sebastiani et al. suggested that ApoE binding also affects the internal structure of LNPs, which might contribute to the endosomal escape and successful cytosolic delivery of mRNA [46]. Thus, depending on the fusogenic properties of the LNPs a fraction of the mRNA can be released into the cytosol, where the mRNA molecules need to be recruited in ribosomes in order to be translated into proteins.
The COVID-19 mRNA vaccines of (at least) BioNTech and Moderna employ a “next-generation” LNP delivery system composed of biodegradable ionizable lipids that introduces ester-linkages in the lipid tails. As an example, the usage of the SM-102 lipid in Moderna's mRNA-1273 vaccine was found to outperform Onpattro's MC3 LNPs for the intramuscular (i.m.) delivery of mRNA in rodents and non-human primates, given its improved tolerability and higher endosomal escape efficiency [12,13]. A strong chemical similarity can be found between the ionizable lipid ALC-0315 (Acuitas' proprietary lipid) and SM-102 (Moderna) used in the LNP formulations of BNT162b2 and mRNA-1273, respectively (Fig. 2c). Nevertheless, it should be noted that subtle structural differences in lipid structure and composition might strongly impact the delivery efficiency and immunogenicity of mRNA vaccines. Moreover, BioNTech's and Moderna's LNPs also contain (more or less) the same helper lipids; 1,2-distearoyl-snglycero-3-phosphocholine (DSPC), cholesterol and a diffusible PEG-lipid (2-[(polyethylene glycol)-2000]-N,N-ditetradecylacetamide, PEG2000-DMA in BNT162b2 or 1,2-dimyristoyl-rac-glycero3-methoxypolyethylene glycol-2000, PEG2000-DMG in mRNA-1273) [23,24]. The ratios at which the lipids are combined in the different COVID-19 mRNA LNP vaccines have not (yet) been confirmed. However, from prior research published by the developers, it can be speculated that all three mRNA LNPs contain a lipid formulation of ionizable lipid: DSPC: cholesterol: PEG-lipid at molar ratios of 50:10:38.5:1.5 mol%, and an mRNA-to-lipid ratio of 0.05 (wt/wt) [10,13,47].
3. The mode of action of mRNA vaccines
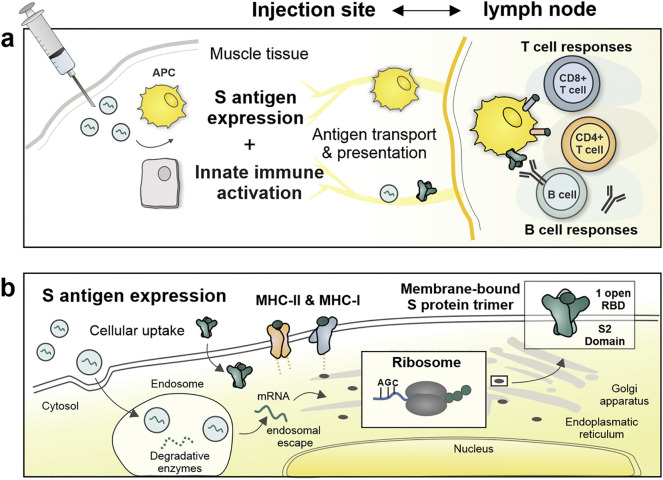
Similar to the more classical vaccine approaches, the COVID-19 mRNA vaccines are injected in the muscle, where they trigger localized and transient inflammation that recruits different immune cells to the injection site (Fig. 3 ) [11,41]. According to mechanistic studies on the fate of mRNA LNPs in rhesus monkeys, primarily monocytes and DC subsets translate the mRNA, likely involving ApoE dependent endocytosis [41]. These locally transfected antigen-presenting cells (APCs) subsequently migrate to the draining lymph nodes (LN) where they present the mRNA-encoded antigens to B cells and T cells (see Antigen target). Moreover, owing to their relatively small size (~100 nm), neutral surface charge and diffusible PEG lipid coating, mRNA LNPs might also enter the lymphatics to directly target LN-resident APCs and B cells [11,58]. Finally, although these cells are often overlooked in flow cytometry studies, it is very likely that cell types such as myocytes, epithelial cells and fibroblasts also contribute to local mRNA expression [59].
Fig. 3.
The mode of action of mRNA vaccines. (a –at the injection site) Upon endocytosis by muscle-resident cells, mRNA LNPs trigger a transient inflammatory response recruiting neutrophils, monocytes and DCs to the injection site. Local and recruited APC subsets transiently express the S protein mRNA and undergo maturation in response to innate immune sensing of the mRNA. The migration of targeted/activated APCs and direct lymphatic transport of mRNA LNPs and cell debris containing S proteins, brings the S antigen to B cells and T cells in draining lymph nodes. (b – at the cellular level) To avoid lysosomal degradation, mRNA must escape the endosomes and binds to ribosomes, known as a complex and rate-limiting process, which is facilitated by the ionizable LNP carrier. After translation and transport of S proteins through the endoplasmatic reticulum and Golgi apparatus, S proteins are exposed as prefusion-stabilized trimer constructs at the cell surface. This membrane-bound S antigen can efficiently be recognized and internalized by B cells, which leads to a series of events activating B cells responses towards neutralizing antibody generation against the S protein. Moreover, the expressed S antigens can gain access to the MHC class I antigen presentation pathway to prime CD8+ T cells that can eliminate infected cells, while recycling mechanisms allow the presentation of antigenic epitopes in MHC-II complexes to CD4+ helper T cells, especially needed to promote the antibody production by providing B cell help. Abbreviations: APC; Antigen presenting cell, RBD; Receptor binding domain, MHC; major histocompatibility complex.
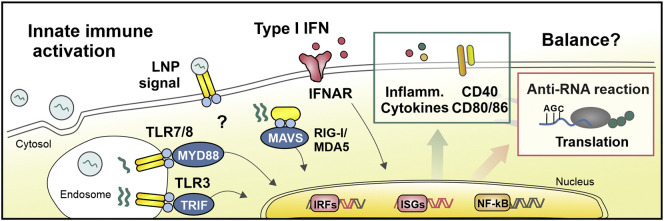
At the same time, mRNA vaccines also need to engage the innate immune system to improve their ability to induce, and tailor, antigen-specific immune responses (Fig. 4 ). Upon sensing inflammatory stimuli, lymphatic migration of innate immune cells is promoted, while APCs become activated (i.e. maturation), in their turn providing co-stimulatory signals and cytokine responses. As previously mentioned, mRNA can mediate type I IFN responses upon cellular uptake, which can vary greatly depending on their structural design. Among other effects on antiviral immunity, type I IFNs restrict viral replication in infected host cells and induce stimulatory-genes involved in the maturation process of DCs. Furthermore, IFN-α directly acts as a third cytokine signal during T cell priming. Although the role of type I IFNs in mRNA-based vaccines has been heavily debated, several research groups underlined that the type I IFN response can act as a driving force for mRNA vaccines to elicit cytotoxic T cell responses [1,60,61]. Several preclinical studies have indicated that an unmodified mRNA platform induces a more pronounced type-I IFN-polarized innate immune response [11,62]. In general, this has made the use of unmodified mRNA a preferred choice in the design of mRNA vaccines for cancer indications, where the main focus lies on cytotoxic T cells as key effector cells to eliminate tumor cells. However, the impact of type I IFNs on the ability of mRNA vaccines to elicit antibody responses has been less characterized. In this context, it should be taken in account that the type I IFN activity of mRNA vaccines can prematurely stop mRNA-translation, thereby reducing antigen availability and diminishing the vaccine's efficacy to obtain adaptive immune responses [63,64]. Moreover, the (local) induction of type IFNs and other inflammatory cytokines plays - in all likelihood - an important role in the reactogenicity of mRNA vaccines.
Fig. 4.
Proposed innate immune signaling in response to mRNA vaccination. The internalization of mRNA LNPs can be detected by innate immune sensors that are localized in the endosomes and cytosol. The detection of mRNA, by the endosomal TLR/8, recruits the MYD88 signal transduction adaptor and leads to the expression of type I IFNs (IFN-α and IFN-β) through IFN regulatory factor 7, and to the secretion of other proinflammatory cytokines through nuclear factor κB (NF-κB). In addition, dsRNA contaminants and/or secondary structures in the mRNA product can interact with TLR3 in the endosomes, recruiting TRIF, as well as upon their arrival in the cytosol be detected by RIG-I and MDA5, binding MAVS. The activation of TRIF and MAVS is followed by molecular cascades that results in the expression of type I IFNs in control of IRF3 and IRF7. In turn, the type I IFN cytokines bind autocrine or paracrine receptors, which eventually regulates the gene expression of hundreds of proteins involved in antiviral immunity. This includes the expression of MHC-I and co-stimulatory molecules, needed for T cell responses, as well as antiviral proteins involved with undesirable anti-RNA responses. Methods such as the introduction of modified nucleotides, the removal of dsRNA fragments, and sequence-engineering, can be utilized to minimize or control the type I IFN activity of mRNA. However, it remains unclear how to strike the perfect balance between obtaining sufficient mRNA-encoded antigen expression and adequate immunostimulation in order to support adaptive immunity. In addition, more research is needed to investigate whether and how the recognition of lipid components in the LNP vehicle might contribute to the innate immune response to mRNA vaccines. Abbreviations: IRF; interferon regulatory factor, ISG; interferon-stimulated gene, NF-κB; nuclear factor-κB, MAVS; mitochondrial antiviral signaling protein, MDA5; melanoma differentiation-associated protein 5, MYD88; myeloid differentiation primary response protein 88, TRIF, Toll-IL-1 receptor domain-containing adapter protein inducing IFNβ.
To clearly understand innate immune dynamics following mRNA vaccination, it should be emphasized that both the mRNA and the LNP vehicle can have intrinsic adjuvant properties. Cationic lipids have been associated with the activation of several cellular pathways like pro-apoptotic and pro-inflammatory cascades [65]. Whether or not this also occurs for the proprietary ionizable lipids of the different companies, is yet to be determined. In fact, no research data on the general performance nor adjuvancy of the (Acuitas' proprietary) LNPs used in the CVnCoV and BNT162b2 have been made public. For Moderna's LNP formulation, Hassett et al. reported that the SM-102 lipid was selected based on its improved tolerability profile in non-human primates, as evidenced by a reduced local reactogenicity (e.g., edema and erythema) and lowest induction of systemic cytokine responses (e.g., IL-6) [13]. Taken together, it remains to be elucidated whether the mRNA or rather the LNP system is responsible for the mRNA vaccine's innate immune signature. Maybe they act in synergy? Maybe additional modalities can be developed to further reduce the innate immune activity/reactogenicity of mRNA vaccines, without affecting the vaccine potency?
4. Immune responses and protection induced by COVID-19 mRNA vaccines
The COVID-19 mRNA vaccines are primarily focused on triggering B cells to promote the induction of neutralizing antibodies, but there are also good reasons to believe that CD8+ T cell and CD4+ T cell responses may contribute to the protection against SARS-CoV-2 [66]. Memory T cells, particularly those residing at the upper airways might limit disease severity and shorten the duration of disease by rapidly eliminating infected cells and coordinating the production of antibodies [67]. In COVID-19 patients, a coordinated adaptive immunity of CD4+ T cells, CD8+ T cells, and antibody responses was correlated to milder disease, whereas an uncoordinated response frequently failed to control disease [68]. Moreover, previous experience with the closely related SARS-CoV-1, showed that CD8+ and CD4+ memory T cells were detectable as late as 17 years post-infection, while neutralizing antibody titers had waned substantially by 1 year after infection [69,70]. Although these reports clearly attribute beneficial roles to T cells in controlling COVID-19 and durability of immunity, T cells, on their own, will probably not be capable to prevent viral entry by providing sterilizing immunity against SARS-COV-2.
As for humoral immunity, two vaccine doses of CVnCoV (12 μg mRNA dose) induced SARS-CoV-2 neutralizing antibody titers in all participants at levels that were comparable to those found in individuals who had recovered from natural infection [57]. In comparison, the nucleoside-modified mRNA vaccines BNT162b2 (30 μg mRNA dose) and mRNA-1273 (100 μg mRNA dose) generally surpassed the titers from convalescent COVID-19 patient samples, even in the elderly trial group, hinting towards potential stronger induction of humoral immunity to the unmodified mRNA vaccine CVnCoV [49,53]. Since the type I IFN activity can be better controlled with 1mΨ-modified mRNA, resulting in higher maximal tolerable doses of the 1mΨ-modified mRNA vaccines, they might achieve a more durable protein expression and thus prolonged antigen availability. This is a particularly favorable feature to enhance germinal center (GC) responses. In GCs, B cells undergo affinity maturation and isotype switching. After rounds of clonal expansion, this gives rise to high affinity B cells and their differentiation into plasma cells and memory B cells, which eventually determines the quality and durability of the antibody response.
Moreover, several studies have shown that i.m. delivery of 1mΨ-modified mRNA vaccines results in the rapid and potent induction of follicular T helper cells (Tfh) [71,72]. This specialized T cell phenotype is essential for the proper regulation of GCs [73]. Indeed, Pardi and colleagues recently demonstrated that a single immunization of mice with 1mΨ-modified mRNA LNP vaccines could elicit potent S-specific GC B cells and Tfh cells, in which their absolute numbers correlated with the levels of neutralizing antibodies [74]. In humans, both BNT162b2 and mRNA-1273 elicited S-specific CD4+ T cell responses directed against the S1 (including RBD) and S2 regions of the S glycoprotein, which again highlights the benefit of delivering mRNA encoding the full-length S protein [50,53]. The magnitude of CD4+ T cell responses correlated with the levels of S-binding IgG antibodies, underlining their supporting role in humoral immunity, Moreover, the majority of the activated CD4+ T cells displayed a Th1 skewed profile (i.e. cells producing IFN-ɣ, TNF-α, and IL-2), which is believed to be very important to potentially avoid vaccine-associated enhanced respiratory disease, in particular the risk for antibody-dependent enhancement and/or lung eosinophilic immunopathology upon SARS-CoV-2 infection [75].
With regards to inducing CD8+ T cell responses, study results indicate that BNT162b2 outperforms mRNA-1273. Most of the study participants vaccinated with BNT162b2 mounted significant S-specific CD8+ T cell responses (91.9%) [50], as compared to low or undetectable levels in the clinical evaluation of mRNA-1273 [53]. From the preclinical evidence of CVnCoV in mice, it can be appreciated that high numbers of S-specific CD8+ T cells were detected after two rounds of vaccination (up to 10% of total splenic CD8+ T cells) [56]. However, this capacity of CVnCoV to elicit robust CD8+ T cell responses could not yet be confirmed in humans, nor were any details on T cell activation reported in the first clinical data of the phase 1 trial of this mRNA vaccine [57].
The interim analysis report on the ongoing phase 3 trial of BNT162b2 showed that a two dose vaccine regimen was very effective in preventing COVID-19 disease (up to 95% efficacy) [51]. The vaccine efficacy of mRNA-1273 is in line with the outcome of BNT162b2, conferring 94.1% protection against symptomatic COVID-19 disease [54]. Moreover, real-world data from Israel's immunization program with BNT162b2 demonstrate that two weeks after the second dose vaccine effectiveness was estimated at 97% in preventing symptomatic and severe COVID-19 disease, while the vaccine was 94% effective against asymptomatic SARS-CoV-2 infections [76]. Supported by evidence obtained in viral challenge experiments in non-human primates that both vaccines produced rapid viral control in the upper and lower airways, we can hope that the mRNA vaccines are also capable to avoid viral transmission and thus curtailing the pandemic spread. [48,52].
Whether these mRNA vaccines are also effective against new SARS-CoV-2 variants, including the emerging U.K variant (B1.1.7) and the South African variant (B.1.351), was recently assessed by measuring neutralizing antibody activities against pseudoviruses bearing the mutated B.1.1.7 or B1.351 spike protein [[77], [78], [79]]. The neutralization activity of sera collected from both mRNA-1273 and BNT162b2 vaccinated individuals was largely preserved against the B.1.1.7 variant relative to prior variants. In contrast, in a study by Moderna a 6.4-fold reduction in neutralization titers was detected against a pseudovirus with a full set of B.1.351 mutations, but remained above levels that are expected to be protective and sera of all individuals were capable to obtain full neutralization [78]. Moreover, in response to this emerging South Africa coronavirus variant, Moderna already announced that they are working on an adapted booster vaccine candidate (mRNA-1273.351) [80].
How, and for how long the different vaccine-induced immune responses will contribute to the protection will need to be determined in long-term follow up studies. Moderna already reported that neutralizing antibodies continued to be detected in all the participants at 3 months post-vaccination [81]. Future studies should also try to investigate the generation of long-lived memory B cells and T cells, as it can be expected that neutralizing antibodies will wane over time, while these memory cells may be long-lived and provide rapid responses upon infection.
5. COVID-19 vaccines: safety first
With these highly novel vaccine approaches showing highly promising results in (pre)clinical studies, the key question was undoubtedly: are these safe in human? First and foremost, none of the mRNA vaccines for which clinical results are available, report any major safety concerns.
The mRNA vaccines that contain nucleoside-modified mRNA (BioNTech/Pfizer and Moderna) both report a clear dose-dependency in the occurrence of localized and systemic adverse events (AEs). Furthermore, AEs were more common after the boost vaccine compared to the prime dose. For example, fever was only reported after the second vaccination of Moderna's mRNA-1273 vaccine at the higher doses of 100 μg (fever in 40% of participants) and 250 μg (57% of participants, with 1/8 events graded as severe). No fever was reported for the 25 μg vaccine dose. Typical other systemic AEs that occurred in more than half of the dosed participants were fatigue, chills, myalgia and headache, which were all increasingly reported for higher dosages, particularly after the boost vaccine [53].
BioNTech/Pfizer kept their options wide as they started phase 1 clinical testing with different vaccine candidates. The BNT162b1 is a LNP packaging nucleoside-modified mRNA encoding the SARS-CoV-2 S protein's RBD, which is trimerized to increase its immunogenicity by multivalent display. Results of a phase 1/2 dose-escalation study (mRNA doses of 10–30-100 μg) revealed similar tolerability and safety. Both local and systemic AE occurred dose-dependently, were mostly mild to moderate and more common after boost vaccination. In line with the Moderna vaccine, events also peaked at day 2 after dosing and were resolved by day 7. Based on the reactogenicity to the 100 μg dose, no boost vaccination was performed at this dosage. Interestingly, despite the lower mRNA-doses used, AEs were reported more frequently with BNT162b1 as compared to mRNA-1273: fever was already reported after the first vaccination for all dose groups (8% for the 10 and 30 μg doses, 50% for the 100 μg dose), with up to 75% showing moderate fever after a 30 μg boost vaccine [82]. In a phase 1/2/3 comparative study, BNT162b1 was evaluated side-by-side to the BNT162b2 approach, where a nucleoside-modified mRNA now encodes the full-length, prefusion-stabilized S protein [49]. This study demonstrated that the systemic AEs were milder for the BNT162b2 (full length S protein mRNA) vaccine as compared to the BNT162b1 (RBD trimer mRNA). As such, merely 8% and 17% of participants aged 18–55 or 65–85, respectively, reported fever after a second 30 μg dose. Also local reactogenicity was less prominent for BNT162b2 and particularly in the older cohort, fewer severe systemic AEs were reported for this vaccine candidate. Because of its improved safety profile, together with the benefit of BNT162b2 to induce immunity against epitopes in S1 and S2 regions of the S antigen, while BNT162b1 is only restricted to epitopes inside the RBD region, made that BNT162b2 was selected as lead candidate for further advancement.
When LNPs package unmodified mRNA, this clearly affects the tolerability of the vaccine. The mRNA dose selected for phase 2b/3 clinical testing of CureVac's CVnCoV vaccine candidate was 12 μg, substantially lower than what is used in the nucleoside-modified mRNA vaccines. Even at this low dose, systemic AEs occurred more frequently in response to a prime dose compared to the modified mRNA vaccines. To exemplify, fever was reported by 57% of the participants after the first dose, but the incidence of this AE did not further increase after the boost vaccine [57]. Despite the high incidence of systemic AEs after a single dosage, no major toxicities were reported and all AEs resolved within 2 days after dosing. Although no studies were performed to investigate the cause of this stronger reactogenicity, one possibility is that it may be linked to the stronger type I IFN response to the unmodified mRNA in CureVac's vaccine candidate.
To put this in perspective, the frequency at which adverse events were reported upon administration of mRNA COVID-19 vaccines can be compared to the other COVID-19 vaccine candidates. Sinovac Life Sciences' CoronaVac is a more classical vaccine, as it consists of an β-propiolactone-inactivated SARS-CoV-2 adsorbed onto aluminum hydroxide. In the different prime-boost regimens tested, no more than 37% of the participants reported any (localized or systemic) AE after the second dose, with fever only reported by max. 5% of the participants (depending on the dosing and prime-boost schedule) [83]. Novavax developed a recombinant protein nanoparticle vaccine composed of trimeric SARS-CoV-2 spike glycoproteins (also stabilized in the prefusion conformation) and evaluated safety and tolerability with and without addition of a saponin-based adjuvant. As can be expected, AEs occurred more frequent when NVX-CoV2373 was combined with the adjuvant, and slightly more AEs were reported after the boost dose. However, only 1 out of the 82 participants who received the vaccine in a prime-boost schedule, developed a mild fever [84]. The ChAdOx1 nCoV-19 vaccine developed by the University of Oxford makes use of a replication-deficient simian adenoviral vector containing the full-length S protein of SARS-CoV-2, with a tissue plasminogen activator leader sequence [85]. The prime vaccine commonly caused fatigue and headache, but the incidence of these AEs could be significantly reduced by prophylactic paracetamol. In the limited subset of 10 participants who received a prime-boost vaccine, fever was exclusively reported after the prime dose in 40% of the subjects, but once again resolved after 2 days. Although it is hard to thoroughly compare these data due to differences in test populations, study designs, vaccination approaches, and potential differences in the efficacy of the different candidate vaccines, it is clear that all of the described vaccines are generally safe, commonly trigger local reactogenicity and that all AEs resolved within 2 days after dosing. It does, however, seem like mRNA vaccines more frequently elicit systemic AEs, particularly fever, compared to the other vaccine candidates.
Rare events of anaphylaxis have been reported after vaccination with mRNA-1273 and BNT162b2, with an incidence currently estimated at approximately 1 in 100,000 [86]. Of the confirmed cases, most of the individuals had a documented history of allergic reactions and/or anaphylaxis. It has not been elucidated which component of the vaccine is responsible for allergic reactions, but there might be a possible role for the PEGylated lipid and a pre-existence of anti-PEG antibodies (IgEs and/or IgGs), as recently discussed in other reviews [87,88].
6. Conclusions and perspectives
The speed at which the COVID-19 mRNA vaccines have made it to market authorization is truly historical. In less than one year, two mRNA vaccines were granted authorization for emergency use, as they were demonstrated to be generally safe and very efficacious to prevent against symptomatic COVID-19 disease. Although this rapid pace was possible due to substantial pre-existing knowledge, there are some remaining gaps in our understanding of the mRNA vaccines' mode of action. How does the innate immune response to mRNA LNPs impact on the translation capacity, immunogenicity, and reactogenicity of the vaccine? How long will immunity last? And is there still room for further improvement?
Although direct comparisons between the different vaccine candidates should be made with the necessary caution, this COVID-19 case seems to indicate that the nucleoside-modified mRNA approach allows higher maximal tolerable doses and might be better suited for the rapid generation of antibody responses. What is striking, is that not all of the differences in evoked immune responses to the different mRNA vaccine candidates, can be explained by the information provided by the manufacturers. As an example, the two seemingly similar (nucleoside-modified) mRNA vaccines elicited different S-specific CD8+ T cell responses. With the information at hand, it is not possible to pinpoint which vaccine components could be responsible for these differential effects. More insights on the performance of the different LNP technologies, as well as detailed information on the mRNA sequence design (e.g., UTR inclusion, codon optimizations) might reveal (sometimes subtle) differences that can affect the potency and reactogenicity of mRNA vaccines. It is beyond doubt, that more in-depth knowledge on the in vivo delivery efficiency and the particular innate immune effects of the different mRNA vaccines will contribute to the design of even safer and more effective mRNA vaccines in the future.
The approval of these first mRNA vaccines is possibly only the dawn of the mRNA-based vaccine's success story. In the coming months, we should look forward to the results of other clinically advanced mRNA vaccine platforms against COVID-19, including those that made use of self-amplifying mRNA [89,90]. While the SARS-CoV-2 pandemic captured all the attention in 2020, the technology readiness of mRNA-based therapeutics for other disease applications should also not be overlooked. On the contrary, all the very recent (clinical) information, regulatory steps and hurdles taken, efforts with respect to large-scale production of mRNA-LNPs are anticipated to accelerate the progress of mRNA vaccines for other infectious diseases, with phase 1/2 clinical trials ongoing to prevent Zika, Rabies, Influenza, Respiratory syncytial virus (RSV) and Cytomegalovirus. Furthermore, mRNA-based vaccines also has a future in oncology, triggering the immune system to eliminate cells expressing shared or patient-specific tumor antigens [91,92]. Taken together, mRNA vaccines gained momentum due to the COVID-19 pandemic. Their exceptionally rapid development and production as well as high efficiency to prevent SARS-CoV-2 infection, demonstrate that mRNA-based therapeutics are ready for their place in the sun.
Competing interests
The Ghent University and authors of this review have filed a patent application relating to mRNA vaccines (application no. EP18195181.5, Therapeutic nanoparticles and methods of use thereof).
Acknowledgements
R.V. and H.D. are funded by a UGent BOF postdoc grant. This work was supported by funding from the European Union's Horizon 2020 research and innovation programme under grant agreement No 810685 (DelNam project), and by grants from the Research Foundation-Flanders, Belgium (FWO-Vlaanderen; grant No. G040319N and grant No. G016221N), Ghent University (F2020/IOF-StarTT/039) and the Research Foundation “Kom op Tegen Kanker” (FAF-C/2018/1213).
References
- 1.Verbeke R., Lentacker I., De Smedt S.C., Dewitte H. Three decades of messenger RNA vaccine development. Nano Today. 2019;28:100766. [Google Scholar]
- 2.Pardi N., Hogan M.J., Porter F.W., Weissman D. mRNA vaccines - a new era in vaccinology. Nat. Rev. Drug Discov. 2018;17:261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.O’Neill L.A.J., Golenbock D., Bowie A.G. The history of toll-like receptors - redefining innate immunity. Nat. Rev. Immunol. 2013;13:453–460. doi: 10.1038/nri3446. [DOI] [PubMed] [Google Scholar]
- 4.Kariko K., Ni H.P., Capodici J., Lamphier M., Weissman D. mRNA is an endogenous ligand for toll-like receptor 3. J. Biol. Chem. 2004;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- 5.Kariko K., Buckstein M., Ni H.P., Weissman D. Suppression of RNA recognition by toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Andries O., Mc Cafferty S., De Smedt S.C., Weiss R., Sanders N.N., Kitada T. N-1-methylpseudouridine-incorporated mRNA outperforms pseudouridine-incorporated mRNA by providing enhanced protein expression and reduced immunogenicity in mammalian cell lines and mice. J. Control. Release. 2015;217:337–344. doi: 10.1016/j.jconrel.2015.08.051. [DOI] [PubMed] [Google Scholar]
- 7.Durbin A.F., Wang C., Marcotrigiano J., Gehrke L. RNAs containing modified nucleotides fail to trigger RIG-I conformational changes for innate immune signaling. Mbio. 2016;7 doi: 10.1128/mBio.00833-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baiersdorfer M., Boros G., Muramatsu H., Mahiny A., Vlatkovic I., Sahin U., Kariko K. A facile method for the removal of dsRNA contaminant from in vitro-transcribed mRNA. Mol. Ther. Nulceic Acids. 2019;15:26–35. doi: 10.1016/j.omtn.2019.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kariko K., Muramatsu H., Ludwig J., Weissman D. Generating the optimal mRNA for therapy: HPLC purification eliminates immune activation and improves translation of nucleoside-modified, protein-encoding mRNA. Nucleic Acids Res. 2011;39 doi: 10.1093/nar/gkr695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thess A., Grund S., Mui B.L., Hope M.J., Baumhof P., Fotin-Mleczek M., Schlake T. Sequence-engineered mRNA without chemical nucleoside modifications enables an effective protein therapy in large animals. Mol. Ther. 2015;23:1456–1464. doi: 10.1038/mt.2015.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lutz J., Lazzaro S., Habbeddine M., Schmidt K.E., Baumhof P., Mui B.L., Tam Y.K., Madden T.D., Hope M.J., Heidenreich R., et al. Unmodified mRNA in LNPs constitutes a competitive technology for prophylactic vaccines. npj Vaccines. 2017;2 doi: 10.1038/s41541-017-0032-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabnis S., Kumarasinghe E.S., Salerno T., Mihai C., Ketova T., Senn J.J., Lynn A., Bulychev A., McFadyen I., Chan J., et al. A novel amino lipid series for mRNA delivery: improved endosomal escape and sustained pharmacology and safety in non-human primates. Mol. Ther. 2018;26:1509–1519. doi: 10.1016/j.ymthe.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hassett K.J., Benenato K.E., Jacquinet E., Lee A., Woods A., Yuzhakov O., Himansu S., Deterling J., Geilich B.M., Ketova T., et al. Optimization of lipid nanoparticles forintramuscular administration of mRNA vaccines. Mol. Ther. Nucleic Acids. 2019;15:1–11. doi: 10.1016/j.omtn.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kulkarni J.A., Witzigmann D., Leung J., van der Meel R., Zaifman J., Darjuan M.M., Grisch-Chan H.M., Thöny B., Tam Y.Y.C., Cullis P.R. Fusion-dependent formation of lipid nanoparticles containing macromolecular payloads. Nanoscale. 2019;11:9023–9031. doi: 10.1039/c9nr02004g. [DOI] [PubMed] [Google Scholar]
- 15.Akinc A., Maier M.A., Manoharan M., Fitzgerald K., Jayaraman M., Barros S., Ansell S., Du X.Y., Hope M.J., Madden T.D., et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019;14:1084–1087. doi: 10.1038/s41565-019-0591-y. [DOI] [PubMed] [Google Scholar]
- 16.Feldman R.A., Fuhr R., Smolenov I., Ribeiro A., Panther L., Watson M., Senn J.J., Smith M., Almarsson O., Pujar H.S., et al. mRNA vaccines against H10N8 and H7N9 influenza viruses of pandemic potential are immunogenic and well tolerated in healthy adults in phase 1 randomized clinical trials. Vaccine. 2019;37:3326–3334. doi: 10.1016/j.vaccine.2019.04.074. [DOI] [PubMed] [Google Scholar]
- 17.CureVac Announces Positive Results in Low Dose – 1 μg – Rabies Vaccine Clinical Phase 1 Study. 2020. https://www.curevac.com/en/2020/01/07/curevac-announces-positive-results-in-low-dose-1-%C2%B5g-rabies-vaccine-clinical-phase-1-study/
- 18.Du L.Y., Yang Y., Zhou Y.S., Lu L., Li F., Jiang S.B. MERS-CoV spike protein: a key target for antivirals. Expert. Opin. Ther. Tar. 2017;21:131–143. doi: 10.1080/14728222.2017.1271415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tang X.C., Agnihothram S.S., Jiao Y.J., Stanhope J., Graham R.L., Peterson E.C., Avnir Y., Tallarico A.S., Sheehan J., Zhu Q., et al. Identification of human neutralizing antibodies against MERS-CoV and their role in virus adaptive evolution. Proc. Natl. Acad. Sci. USA. 2014;111 doi: 10.1073/pnas.1402074111. (pp. 6863–6863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cai Y.F., Zhang J., Xiao T.S., Peng H.Q., Sterling S.M., Walsh R.M., Rawson S., Rits-Volloch S., Chen B. Distinct conformational states of SARS-CoV-2 spike protein. Science. 2020;369:1586–1592. doi: 10.1126/science.abd4251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wrapp D., Wang N.S., Corbett K.S., Goldsmith J.A., Hsieh C.L., Abiona O., Graham B.S., McLellan J.S. Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science. 2020;367:1260–1263. doi: 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Moderna Ships mRNA Vaccine Against Novel Coronavirus (mRNA-1273) for Phase 1 Study. 2020. https://investors.modernatx.com/news-releases/news-release-details/moderna-ships-mrna-vaccine-against-novel-coronavirus-mrna-1273
- 23.European Medicines Agency EMA Recommends First COVID-19 Vaccine for Authorisation in the EU. 2020. https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-authorisation-eu
- 24.EMA Recommends COVID-19 Vaccine Moderna for Authorisation in the EU. 2021. https://www.ema.europa.eu/en/news/ema-recommends-covid-19-vaccine-moderna-authorisation-eu
- 25.FDA Briefing Document - BNT162b2. 2020. https://www.fda.gov/media/144245/download
- 26.FDA Briefing Document - mRNA-1273. 2020. https://www.fda.gov/media/144434/download
- 27.Corbett K.S., Edwards D.K., Leist S.R., Abiona O.M., Boyoglu-Barnum S., Gillespie R.A., Himansu S., Schäfer A., Ziwawo C.T., DiPiazza A.T., et al. SARS-CoV-2 mRNA vaccine design enabled by prototype pathogen preparedness. Nature. 2020;586:567–571. doi: 10.1038/s41586-020-2622-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liu L., Wang P., Nair M.S., Yu J., Rapp M., Wang Q., Luo Y., Chan J.F.W., Sahi V., Figueroa A., et al. Potent neutralizing antibodies against multiple epitopes on SARS-CoV-2 spike. Nature. 2020;584:450–456. doi: 10.1038/s41586-020-2571-7. [DOI] [PubMed] [Google Scholar]
- 29.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R., Rawlings S.A., Sutherland A., Premkumar L., Jadi R.S., et al. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181:1489–1501. doi: 10.1016/j.cell.2020.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roche P.A., Furuta K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat. Rev. Immunol. 2015;15:203–216. doi: 10.1038/nri3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Orlandini von Niessen A.G., Poleganov M.A., Rechner C., Plaschke A., Kranz L.M., Fesser S., Diken M., Lower M., Vallazza B., Beissert T., et al. Improving mRNA-based therapeutic gene delivery by expression-augmenting 3' UTRs identified by cellular library screening. Mol. Ther. 2019;27:824–836. doi: 10.1016/j.ymthe.2018.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Holtkamp S., Kreiter S., Selmi A., Simon P., Koslowski M., Huber C., Türeci O., Sahin U. Modification of antigen-encoding RNA increases stability, translational efficacy, and T-cell stimulatory capacity of dendritic cells. Blood. 2006;108:4009–4017. doi: 10.1182/blood-2006-04-015024. [DOI] [PubMed] [Google Scholar]
- 33.Presnyak V., Alhusaini N., Chen Y.H., Martin S., Morris N., Kline N., Olson S., Weinberg D., Baker K.E., Graveley B.R., et al. Codon optimality is a major determinant of mRNA stability. Cell. 2015;160:1111–1124. doi: 10.1016/j.cell.2015.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mao Y., Liu H., Liu Y., Tao S. Deciphering the rules by which dynamics of mRNA secondary structure affect translation efficiency in Saccharomyces cerevisiae. Nucleic Acids Res. 2014;42:4813–4822. doi: 10.1093/nar/gku159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mauger D.M., Cabral B.J., Presnyak V., Su S.V., Reid D.W., Goodman B., Link K., Khatwani N., Reynders J., Moore M.J., et al. mRNA structure regulates protein expression through changes in functional half-life. Proc. Natl. Acad. Sci. U. S. A. 2019;116:24075. doi: 10.1073/pnas.1908052116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wayment-Steele H.K., Kim D.S., Choe C.A., Nicol J.J., Wellington-Oguri R., Watkins A.M., Sperberg R.A.P., Huang P.-S., Participants E., Das R. Theoretical basis for stabilizing messenger RNA through secondary structure design. bioRxiv. 2021 doi: 10.1093/nar/gkab764. (Preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nelson J., Sorensen E.W., Mintri S., Rabideau A.E., Zheng W., Besin G., Khatwani N., Su S.V., Miracco E.J., Issa W.J., et al. Impact of mRNA chemistry and manufacturing process on innate immune activation. Sci. Adv. 2020;6 doi: 10.1126/sciadv.aaz6893. (eaaz6893) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harvie P., Wong F.M.P., Bally M.B. Use of poly(ethylene glycol)–lipid conjugates to regulate the surface attributes and transfection activity of lipid–DNA particles. J. Pharm. Sci. 2000;89:652–663. doi: 10.1002/(SICI)1520-6017(200005)89:5<652::AID-JPS11>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 39.Chen S., Tam Y.Y.C., Lin P.J.C., Sung M.M.H., Tam Y.K., Cullis P.R. Influence of particle size on the in vivo potency of lipid nanoparticle formulations of siRNA. J. Control. Release. 2016;235:236–244. doi: 10.1016/j.jconrel.2016.05.059. [DOI] [PubMed] [Google Scholar]
- 40.Akinc A., Querbes W., De S., Qin J., Frank-Kamenetsky M., Jayaprakash K.N., Jayaraman M., Rajeev K.G., Cantley W.L., Dorkin J.R., et al. Targeted delivery of RNAi therapeutics with endogenous and exogenous ligand-based mechanisms. Mol. Ther. 2010;18:1357–1364. doi: 10.1038/mt.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Liang F., Lindgren G., Lin A., Thompson E.A., Ols S., Röhss J., John S., Hassett K., Yuzhakov O., Bahl K., et al. Efficient targeting and activation of antigen-presenting cells in vivo after modified mRNA vaccine administration in rhesus macaques. Mol. Ther. 2017;25:2635–2647. doi: 10.1016/j.ymthe.2017.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pardi N., Tuyishime S., Muramatsu H., Kariko K., Mui B.L., Tam Y.K., Madden T.D., Hope M.J., Weissman D. Expression kinetics of nucleoside-modified mRNA delivered in lipid nanoparticles to mice by various routes. J. Control. Release. 2015;217:345–351. doi: 10.1016/j.jconrel.2015.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Juliano R.L. Intracellular trafficking and endosomal release of oligonucleotides: what we know and what we don’t. Nucleic Acid Ther. 2018;28:166–177. doi: 10.1089/nat.2018.0727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sayers E.J., Peel S.E., Schantz A., England R.M., Beano M., Bates S.M., Desai A.S., Puri S., Ashford M.B., Jones A.T. Endocytic profiling of cancer cell models reveals critical factors influencing LNP-mediated mRNA delivery and protein expression. Mol. Ther. 2019;27:1950–1962. doi: 10.1016/j.ymthe.2019.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patel S., Ashwanikumar N., Robinson E., Duross A., Sun C., Murphy-Benenato K.E., Mihai C., Almarsson Ö., Sahay G. Boosting intracellular delivery of lipid nanoparticle-encapsulated mRNA. Nano Lett. 2017;17:5711–5718. doi: 10.1021/acs.nanolett.7b02664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sebastiani F., Yanez Arteta M., Lerche M., Porcar L., Lang C., Bragg R.A., Elmore C.S., Krishnamurthy V.R., Russell R.A., Darwish T., et al. Apolipoprotein E binding drives structural and compositional rearrangement of mRNA-containing lipid nanoparticles. ACS Nano. 2021 doi: 10.1021/acsnano.0c10064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pardi N., Hogan M.J., Pelc R.S., Muramatsu H., Andersen H., DeMaso C.R., Dowd K.A., Sutherland L.L., Scearce R.M., Parks R., et al. Zika virus protection by a single low-dose nucleoside-modified mRNA vaccination. Nature. 2017;543:248–251. doi: 10.1038/nature21428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Vogel A.B., Kanevsky I., Che Y., Swanson K.A., Muik A., Vormehr M., Kranz L.M., Walzer K.C., Hein S., Güler A., et al. Immunogenic BNT162b vaccines protect rhesus macaques from SARS-CoV-2. Nature. 2021 doi: 10.1038/s41586-021-03275-y. [DOI] [PubMed] [Google Scholar]
- 49.Walsh E.E., Frenck R.W., Falsey A.R., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Mulligan M.J., Bailey R., et al. Safety and immunogenicity of two RNA-based COVID-19 vaccine candidates. N. Engl. J. Med. 2020;383:2439–2450. doi: 10.1056/NEJMoa2027906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sahin U., Muik A., Vogler I., Derhovanessian E., Kranz L.M., Vormehr M., Quandt J., Bidmon N., Ulges A., Baum A., et al. BNT162b2 induces SARS-CoV-2-neutralising antibodies and T cells in humans. medRxiv. 2020 (Preprint) [Google Scholar]
- 51.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Pérez Marc G., Moreira E.D., Zerbini C., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Corbett K.S., Flynn B., Foulds K.E., Francica J.R., Boyoglu-Barnum S., Werner A.P., Flach B., O'Connell S., Bock K.W., Minai M., et al. Evaluation of the mRNA-1273 vaccine against SARS-CoV-2 in nonhuman primates. N. Engl. J. Med. 2020;383:1544–1555. doi: 10.1056/NEJMoa2024671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jackson L.A., Anderson E.J., Rouphael N.G., Roberts P.C., Makhene M., Coler R.N., McCullough M.P., Chappell J.D., Denison M.R., Stevens L.J., et al. An mRNA vaccine against SARS-CoV-2 - preliminary report. N. Engl. J. Med. 2020;383:1920–1931. doi: 10.1056/NEJMoa2022483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Diemert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rauch S., Gooch K., Hall Y., Salguero F.J., Dennis M.J., Gleeson F.V., Harris D., Ho C., Humphries H.E., Longet S., et al. mRNA vaccine CVnCoV protects non-human primates from SARS-CoV-2 challenge infection. bioRxiv. 2020 (Preprint) [Google Scholar]
- 56.Rauch S., Roth N., Schwendt K., Fotin-Mleczek M., Mueller S.O., Petsch B. mRNA based SARS-CoV-2 vaccine candidate CVnCoV induces high levels of virus neutralizing antibodies and mediates protection in rodents. bioRxiv. 2020 doi: 10.1038/s41541-021-00311-w. (Preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kremsner P., Mann P., Bosch J., Fendel R., Gabor J.J., Kreidenweiss A., Kroidl A., Leroux-Roels I., Leroux-Roels G., Schindler C., et al. Phase 1 assessment of the safety and immunogenicity of an mRNA-lipid nanoparticle vaccine candidate against SARS-CoV-2 in human volunteers. medRxiv. 2020 doi: 10.1007/s00508-021-01922-y. (Preprint) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lindsay K.E., Bhosle S.M., Zurla C., Beyersdorf J., Rogers K.A., Vanover D., Xiao P., Araínga M., Shirreff L.M., Pitard B., et al. Visualization of early events in mRNA vaccine delivery in non-human primates via PET–CT and near-infrared imaging. Nat. Biomed. Eng. 2019;3:371–380. doi: 10.1038/s41551-019-0378-3. [DOI] [PubMed] [Google Scholar]
- 59.Blakney A.K., Deletic P., McKay P.F., Bouton C.R., Ashford M., Shattock R.J., Sabirsh A. Effect of complexing lipids on cellular uptake and expression of messenger RNA in human skin explants. J. Control. Release. 2021;330:1250–1261. doi: 10.1016/j.jconrel.2020.11.033. [DOI] [PubMed] [Google Scholar]
- 60.Kranz L.M., Diken M., Haas H., Kreiter S., Loquai C., Reuter K.C., Meng M., Fritz D., Vascotto F., Hefesha H., et al. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature. 2016;534:396–401. doi: 10.1038/nature18300. [DOI] [PubMed] [Google Scholar]
- 61.Broos K., Van der Jeught K., Puttemans J., Goyvaerts C., Heirman C., Dewitte H., Verbeke R., Lentacker I., Thielemans K., Breckpot K. Particle-mediated intravenous delivery of antigen mRNA results in strong antigen-specific T-cell responses despite the induction of type I interferon. Mol. Ther. Nucleic Acids. 2016;5 doi: 10.1038/mtna.2016.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verbeke R., Lentacker I., Wayteck L., Breckpot K., Van Bockstal M., Descamps B., Vanhove C., De Smedt S.C., Dewitte H. Co-delivery of nucleoside-modified mRNA and TLR agonists for cancer immunotherapy: restoring the immunogenicity of immunosilent mRNA. J. Control. Release. 2017;266:287–300. doi: 10.1016/j.jconrel.2017.09.041. [DOI] [PubMed] [Google Scholar]
- 63.Pepini T., Pulichino A.M., Carsillo T., Carlson A.L., Sari-Sarraf F., Ramsauer K., Debasitis J.C., Maruggi G., Otten G.R., Geall A.J., et al. Induction of an IFN-mediated antiviral response by a self-amplifying RNA vaccine: implications for vaccine design. J. Immunol. 2017;198:4012–4024. doi: 10.4049/jimmunol.1601877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Van Hoecke L., Roose K., Ballegeer M., Zhong Z., Sanders N.N., De Koker S., Saelens X., Van Lint S. The opposing effect of type I IFN on the T cell response by non-modified mRNA-lipoplex vaccines is determined by the route of administration. Mol. Ther. Nucleic Acids. 2020;22:373–381. doi: 10.1016/j.omtn.2020.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lonez C., Vandenbranden M., Ruysschaert J.M. Cationic lipids activate intracellular signaling pathways. Adv. Drug Deliv. Rev. 2012;64:1749–1758. doi: 10.1016/j.addr.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 66.Karlsson A.C., Humbert M., Buggert M. The known unknowns of T cell immunity to COVID-19. Sci. Immunol. 2020;5 doi: 10.1126/sciimmunol.abe8063. (eabe8063) [DOI] [PubMed] [Google Scholar]
- 67.Channappanavar R., Fett C., Zhao J., Meyerholz D.K., Perlman S. Virus-specific memory CD8 T cells provide substantial protection from lethal severe acute respiratory syndrome coronavirus infection. J. Virol. 2014;88:11034–11044. doi: 10.1128/JVI.01505-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rydyznski Moderbacher C., Ramirez S.I., Dan J.M., Grifoni A., Hastie K.M., Weiskopf D., Belanger S., Abbott R.K., Kim C., Choi J., et al. Antigen-specific adaptive immunity to SARS-CoV-2 in acute COVID-19 and associations with age and disease severity. Cell. 2020;183:996–1012. doi: 10.1016/j.cell.2020.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Sariol A., Perlman S. Lessons for COVID-19 immunity from other coronavirus infections. Immunity. 2020;53:248–263. doi: 10.1016/j.immuni.2020.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Le Bert N., Tan A.T., Kunasegaran K., Tham C.Y.L., Hafezi M., Chia A., Chng M.H.Y., Lin M., Tan N., Linster M., et al. SARS-CoV-2-specific T cell immunity in cases of COVID-19 and SARS, and uninfected controls. Nature. 2020;584:457–462. doi: 10.1038/s41586-020-2550-z. [DOI] [PubMed] [Google Scholar]
- 71.Lindgren G., Ols S., Liang F., Thompson E.A., Lin A., Hellgren F., Bahl K., John S., Yuzhakov O., Hassett K.J., et al. Induction of robust B cell responses after influenza mRNA vaccination Is accompanied by circulating hemagglutinin-specific ICOS+ PD-1+ CXCR3+ T follicular helper cells. Front. Immunol. 2017;8 doi: 10.3389/fimmu.2017.01539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pardi N., Hogan M.J., Naradikian M.S., Parkhouse K., Cain D.W., Jones L., Moody M.A., Verkerke H.P., Myles A., Willis E., et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 2018;215:1571–1588. doi: 10.1084/jem.20171450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Crotty S. T follicular helper cell biology: a decade of discovery and diseases. Immunity. 2019;50:1132–1148. doi: 10.1016/j.immuni.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lederer K., Castaño D., Gómez Atria D., Oguin T.H., 3rd, Wang S., Manzoni T.B., Muramatsu H., Hogan M.J., Amanat F., Cherubin P., et al. SARS-CoV-2 mRNA vaccines foster potent antigen-specific germinal center responses associated with neutralizing antibody generation. Immunity. 2020;53:1281–1295. doi: 10.1016/j.immuni.2020.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee W.S., Wheatley A.K., Kent S.J., DeKosky B.J. Antibody-dependent enhancement and SARS-CoV-2 vaccines and therapies. Nat. Microbiol. 2020;5:1185–1191. doi: 10.1038/s41564-020-00789-5. [DOI] [PubMed] [Google Scholar]
- 76.BioNTech Real-World Evidence Confirms High Effectiveness of Pfizer-BioNTech COVID-19 Vaccine. 2021. https://investors.biontech.de/news-releases/news-release-details/real-world-evidence-confirms-high-effectiveness-pfizer-biontech
- 77.Muik A., Wallisch A.-K., Sänger B., Swanson K.A., Mühl J., Chen W., Cai H., Maurus D., Sarkar R., Türeci Ö., et al. Neutralization of SARS-CoV-2 lineage B.1.1.7 pseudovirus by BNT162b2 vaccine–elicited human sera. Science. 2021;371(6534):1152–1153. doi: 10.1126/science.abg6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wu K., Werner A.P., Moliva J.I., Koch M., Choi A., Stewart-Jones G.B.E., Bennett H., Boyoglu-Barnum S., Shi W., Graham B.S., et al. mRNA-1273 vaccine induces neutralizing antibodies against spike mutants from global SARS-CoV-2 variants. bioRxiv. 2021 (Preprint) [Google Scholar]
- 79.Liu Y., Liu J., Xia H., Zhang X., Fontes-Garfias C.R., Swanson K.A., Cai H., Sarkar R., Chen W., Cutler M., et al. Neutralizing activity of BNT162b2-elicited serum. N. Engl. J. Med. 2021 doi: 10.1056/NEJMc2102017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Moderna Moderna COVID-19 Vaccine Retains Neutralizing Activity Against Emergingvariants First Identified in the U.K. and the Republic of South Africa. 2021. https://investors.modernatx.com/news-releases/news-release-details/moderna-covid-19-vaccine-retains-neutralizing-activity-against
- 81.Widge A.T., Rouphael N.G., Jackson L.A., Anderson E.J., Roberts P.C., Makhene M., Chappell J.D., Denison M.R., Stevens L.J., Pruijssers A.J., et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. N. Engl. J. Med. 2021;384:80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mulligan M.J., Lyke K.E., Kitchin N., Absalon J., Gurtman A., Lockhart S., Neuzil K., Raabe V., Bailey R., Swanson K.A., et al. Phase I/II study of COVID-19 RNA vaccine BNT162b1 in adults. Nature. 2020;586:589–593. doi: 10.1038/s41586-020-2639-4. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y., Zeng G., Pan H., Li C., Hu Y., Chu K., Han W., Chen Z., Tang R., Yin W., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: a randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021;21(2):181–192. doi: 10.1016/S1473-3099(20)30843-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Keech C., Albert A., Cho I., Robertson A., Reed P., Neal S., Plested J.S., Zhu M., Cloney-Clark S., Zhou H., et al. Phase 1–2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020;383:2320–2332. doi: 10.1056/NEJMoa2026920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Folegatti P.M., Ewer K.J., Aley P.K., Angus B., Becker S., Belij-Rammerstorfer S., Bellamy D., Bibi S., Bittaye M., Clutterbuck E.A., et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: a preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet. 2020;396:467–478. doi: 10.1016/S0140-6736(20)31604-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Shimabukuro T.T., Cole M., Su J.R. Reports of anaphylaxis after receipt of mRNA COVID-19 vaccines in the US—December 14, 2020-January 18, 2021. JAMA. 2021;325:1101–1102. doi: 10.1001/jama.2021.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Castells M.C., Phillips E.J. Maintaining safety with SARS-CoV-2 vaccines. N. Engl. J. Med. 2020;384:643–649. doi: 10.1056/NEJMra2035343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Moghimi S.M. Allergic reactions and anaphylaxis to LNP-based COVID-19 vaccines. Mol. Ther. 2021;29:898–900. doi: 10.1016/j.ymthe.2021.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fuller D.H., Berglund P. Amplifying RNA vaccine development. N. Engl. J. Med. 2020;382:2469–2471. doi: 10.1056/NEJMcibr2009737. [DOI] [PubMed] [Google Scholar]
- 90.McKay P.F., Hu K., Blakney A.K., Samnuan K., Brown J.C., Penn R., Zhou J., Bouton C.R., Rogers P., Polra K., et al. Self-amplifying RNA SARS-CoV-2 lipid nanoparticle vaccine candidate induces high neutralizing antibody titers in mice. Nat. Commun. 2020;11:3523. doi: 10.1038/s41467-020-17409-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sahin U., Oehm P., Derhovanessian E., Jabulowsky R.A., Vormehr M., Gold M., Maurus D., Schwarck-Kokarakis D., Kuhn A.N., Omokoko T., et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. 2020;585:107–112. doi: 10.1038/s41586-020-2537-9. [DOI] [PubMed] [Google Scholar]
- 92.Esprit A., de Mey W., Bahadur Shahi R., Thielemans K., Franceschini L., Breckpot K. Neo-antigen mRNA vaccines. Vaccines. 2020;8:776. doi: 10.3390/vaccines8040776. [DOI] [PMC free article] [PubMed] [Google Scholar]