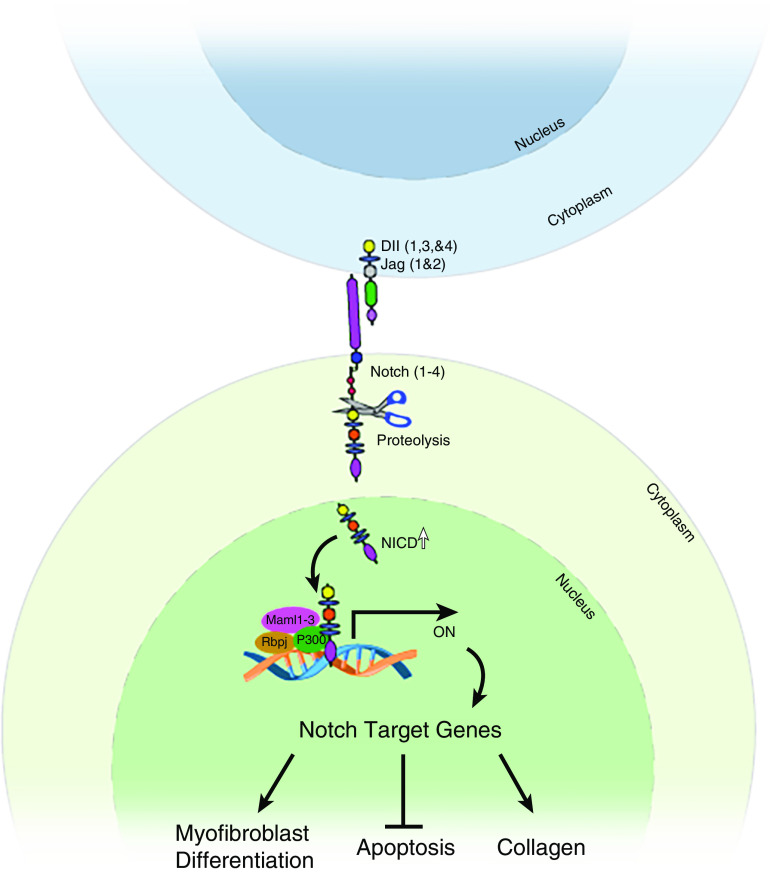
The respiratory system is designed for efficient gas exchange in the distal region of the lung. It consists of conducting airways that include the trachea, bronchi, and bronchioles that link to distal alveoli. Multiple pathways have been identified that play a pivotal role in the maintenance of progenitor-cell populations and in rebuilding damaged lungs deep within the parenchyma. In this regard, the Notch pathway has been studied extensively with respect to its role in lung development and regeneration and has been found to play a role specifically in proximal–distal patterning and cell differentiation, proliferation, and apoptosis (1). Notch signaling is a highly conserved pathway that mediates cell–cell contacts; in an ideal setting, it facilitates communication between cells within a short radius of the activated cell. The canonical pathway consists of four receptors (Notch 1, 2, 3, and 4) and five ligands (Jagged 1 and 2, Dll 1 (Delta-like ligand 1), Dll 3, and Dll 4), which are membrane-bound on adjacent cells. Upon ligand binding, the NICD (Notch intracellular domain) of the receptor is cleaved by the γ-secretase enzyme and is translocated to the nucleus, where it acts as a transcription factor (Figure 1). There, it interacts with other inactive transcriptional complexes (Rbpj and Maml1 [mastermind‐like 1], Maml2, and Maml3) and triggers the expression of Notch-mediated genes such as Hes (Hes family BHLH transcription factor 1) and Hey (2, 3). The Notch signaling pathway is known to play a prominent role during lung development, in maintaining homeostasis, responding to injury, and regeneration. During development, the Notch pathway is involved in proximal–distal patterning, cell-fate decisions in the conducting airways, and alveolar and vascular development (4). As Notch signaling plays a pivotal role in homeostasis and regeneration, dysregulation in Notch signaling can lead to respiratory disorders including lung cancer, pulmonary arterial hypertension, chronic obstructive pulmonary disease, and idiopathic pulmonary fibrosis (IPF) (5–9). IPF is a fatal fibrotic lung disease marked by key cellular events such as fibroblast proliferation, migration, myofibroblast differentiation, and resistance to apoptosis (10, 11). The lack of effective medical treatment to reduce mortality in IPF highlights the importance of identifying novel therapeutic targets that may be useful in preventing or delaying the progression of pulmonary fibrosis.
Figure 1.
A general scheme illustrating the potential mechanistic pathway of Notch signaling in the pathogenesis of pulmonary fibrosis. The current study demonstrates that Notch3 is upregulated in fibroblasts and promotes myofibroblast transformation and survival, which is involved in excessive collagen deposition in the lung parenchyma. Black arrows highlight positive regulation. Perpendicular line highlights negative regulation. Dll = Delta-like ligand; Jag = Jagged ligand; Maml = mastermind-like protein; NICD = the intracellular domain of the Notch protein; ON = transcriptional activation; P300 = histone acetyltransferase p300; Rbp = recombination signal binding protein.
In this issue of the Journal, Vera and colleagues (pp. 465–476) describe studies uncovering a pathogenic role for Notch3 in fibroblast activation and pulmonary fibrosis (12). They demonstrate that Notch3 amounts are elevated during bleomycin-induced pulmonary fibrosis and that active forms of Notch3 are coexpressed by αSMA-positive myofibroblasts in both mice and humans. They employed Notch3 knockout mice to delineate the role of Notch3 in pulmonary fibrosis. In the absence of Notch3, the lungs were protected from bleomycin-induced pulmonary fibrosis, and very few myofibroblasts were observed in comparison with control fibrotic lungs. In addition, they show that Notch3 deficiency in fibrotic fibroblasts leads to apoptotic events in vitro; this suggests Notch3 has a role in the pathogenesis of IPF. In another independent study, elevated levels of Notch1 were identified in IPF tissues that promoted myofibroblast transformation (13). The importance of the Notch pathway in myofibroblast differentiation was shown in a study by Xu and colleagues in which the canonical Notch pathway was disrupted. After this disruption, the expression of αSMA in mesenchymal cells, which is required for alveogenesis during lung development, was inhibited (14). Similarly, in vitro and in vivo studies have suggested that Notch signaling augments the differentiation of fibroblasts to myofibroblasts (15). Notably, mesenchymal-specific deletion of Notch1 attenuated pulmonary fibrosis in vivo that was associated with decreased myofibroblast transformation and a decrease in collagen production in the lungs (5). Likewise, in response to injury, Notch3 levels were shown to increase in PDGFRβ+ fibroblasts that are distinct from pericytes, as shown by single-cell analysis (16). However, more preclinical studies should be performed to delineate the mechanisms underlying the upregulation of the active form of Notch receptors, and their target genes, with respect to myofibroblast differentiation. Nevertheless, a growing body of evidence has emerged suggesting that a Notch signaling cascade, including Notch 1 and 3, positively regulates fibroblast activation and pulmonary fibrosis in vivo (Figure 1). Thus, the members of the Notch signaling pathway are legitimate therapeutic targets that, when blocked, may inhibit fibroblast activation and pulmonary fibrosis.
Supplementary Material
Footnotes
Supported by National Heart, Lung, and Blood Institute (NHLBI) grant 1R01HL134801.
Originally Published in Press as DOI: 10.1165/rcmb.2021-0024ED on February 10, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Whitsett JA, Kalin TV, Xu Y, Kalinichenko VV. Building and regenerating the lung cell by cell. Physiol Rev. 2019;99:513–554. doi: 10.1152/physrev.00001.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kovall RA, Gebelein B, Sprinzak D, Kopan R. The canonical Notch signaling pathway: structural and biochemical insights into shape, sugar, and force. Dev Cell. 2017;41:228–241. doi: 10.1016/j.devcel.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seymour PA, Collin CA, Egeskov-Madsen AR, Jørgensen MC, Shimojo H, Imayoshi I, et al. Jag1 modulates an oscillatory Dll1-Notch-Hes1 signaling module to coordinate growth and fate of pancreatic progenitors. Dev Cell. 2020;52:731–747, e8. doi: 10.1016/j.devcel.2020.01.015. [DOI] [PubMed] [Google Scholar]
- 4.Kiyokawa H, Morimoto M. Notch signaling in the mammalian respiratory system, specifically the trachea and lungs, in development, homeostasis, regeneration, and disease. Dev Growth Differ. 2020;62:67–79. doi: 10.1111/dgd.12628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hu B, Wu Z, Bai D, Liu T, Ullenbruch MR, Phan SH. Mesenchymal deficiency of Notch1 attenuates bleomycin-induced pulmonary fibrosis. Am J Pathol. 2015;185:3066–3075. doi: 10.1016/j.ajpath.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morris HE, Neves KB, Montezano AC, MacLean MR, Touyz RM. Notch3 signalling and vascular remodelling in pulmonary arterial hypertension. Clin Sci (Lond) 2019;133:2481–2498. doi: 10.1042/CS20190835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li X, Zhang X, Leathers R, Makino A, Huang C, Parsa P, et al. Notch3 signaling promotes the development of pulmonary arterial hypertension. Nat Med. 2009;15:1289–1297. doi: 10.1038/nm.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ye YZ, Zhang ZH, Fan XY, Xu XL, Chen ML, Chang BW, et al. Notch3 overexpression associates with poor prognosis in human non-small-cell lung cancer. Med Oncol. 2013;30:595. doi: 10.1007/s12032-013-0595-7. [DOI] [PubMed] [Google Scholar]
- 9.Zong D, Li J, Cai S, He S, Liu Q, Jiang J, et al. Notch1 regulates endothelial apoptosis via the ERK pathway in chronic obstructive pulmonary disease. Am J Physiol Cell Physiol. 2018;315:C330–C340. doi: 10.1152/ajpcell.00182.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sontake V, Gajjala PR, Kasam RK, Madala SK. New therapeutics based on emerging concepts in pulmonary fibrosis. Expert Opin Ther Targets. 2019;23:69–81. doi: 10.1080/14728222.2019.1552262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kasam RK, Ghandikota S, Soundararajan D, Reddy GB, Huang SK, Jegga AG, et al. Inhibition of aurora kinase B attenuates fibroblast activation and pulmonary fibrosis. EMBO Mol Med. 2020;12:e12131. doi: 10.15252/emmm.202012131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vera L, Garcia-Olloqui P, Petri E, Viñado AC, Valera PS, Blasco-Iturri Z, et al. Notch3 deficiency attenuates pulmonary fibrosis and impedes lung-function decline. Am J Respir Cell Mol Biol. 2021;64:465–476. doi: 10.1165/rcmb.2020-0516OC. [DOI] [PubMed] [Google Scholar]
- 13.Wang YC, Chen Q, Luo JM, Nie J, Meng QH, Shuai W, et al. Notch1 promotes the pericyte-myofibroblast transition in idiopathic pulmonary fibrosis through the PDGFR/ROCK1 signal pathway. Exp Mol Med. 2019;51:1–11. doi: 10.1038/s12276-019-0228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu K, Nieuwenhuis E, Cohen BL, Wang W, Canty AJ, Danska JS, et al. Lunatic fringe-mediated Notch signaling is required for lung alveogenesis. Am J Physiol Lung Cell Mol Physiol. 2010;298:L45–L56. doi: 10.1152/ajplung.90550.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu T, Hu B, Choi YY, Chung M, Ullenbruch M, Yu H, et al. Notch1 signaling in FIZZ1 induction of myofibroblast differentiation. Am J Pathol. 2009;174:1745–1755. doi: 10.2353/ajpath.2009.080618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xie T, Wang Y, Deng N, Huang G, Taghavifar F, Geng Y, et al. Single-cell deconvolution of fibroblast heterogeneity in mouse pulmonary fibrosis. Cell Rep. 2018;22:3625–3640. doi: 10.1016/j.celrep.2018.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.