To the Editor:
The coronavirus disease (COVID-19) pandemic has highlighted the critical need to understand the pathobiology of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in human nasal airway epithelial (AE) cells, the point of entry and initial growth of this virus (1–3). ACE2 (angiotensin-converting enzyme 2) is the primary receptor of SARS-CoV-2, and studies in adult human AE cells have found that IFN enhances ACE2 expression (4, 5). This shifts the paradigm for SARS-CoV-2 pathogenesis and therapy by identifying that this virus may enhance its spread by exploiting host IFN antiviral responses. However, IFN-induced expression of ACE2 has not been demonstrated in nasal AE cells of infants, an age group linked to milder COVID-19 (6) and reduced ACE2 airway expression (7). Examining this is needed to address the possibility that the immaturity of pediatric AE cells protects them from SARS-CoV-2 owing to lower IFN-induced expression of ACE2 or by mechanisms independent of IFN-stimulated ACE2 expression. Accordingly, in this study, we examine the ability of the human infant AE cells to upregulate ACE2 in response to IFN-γ, IFN-λ, and synthetic viral mimic and IFN inducer (poly (I:C)).
Methods
Primary nasal AE cells collected from human infants (n = 3; aged 8, 10, and 14 mo) were cultured in submerged monolayers as described (8). Two of the donors were males, two were Black/African American, and none had prior evidence of respiratory symptoms, atopy, or tobacco exposure. Human infant AE cells were exposed to IFN-γ (100 ng/ml), IFN-λ (100 ng/ml IL-29), or poly(I:C) (10 ng/μl) for 24 hours. Transcriptome profiling was performed via Clariom-S microarrays (ThermoFisher), and subsequent validation of ACE2 expression was conducted using qRT-PCR, Western blotting, and confocal microscopy. Gene expression array data have been deposited to the Gene Expression Omnibus (GSE153428). Approval for human subject research was granted by the Institutional Review Board of Children’s National Hospital in Washington, DC, and included parental informed consent.
Results
We found that IFN-γ, IFN-λ, and poly(I:C) exposure robustly increased the expression of IFN-stimulated genes (ISGs) in nasal AE cells from infants (Figure 1A). These treatments also robustly stimulated the expression of ACE2, which was among the top ISGs in each case (Figures 1A and 1B). Because administering exogenous IFN is being considered as therapy for SARS-CoV-2 infection (9–11), we next examined if administering IFN-γ or IFN-λ may alter ACE2 upregulation in AE cells after exposure to a synthetic viral mimic (poly(I:C)). These experiments demonstrated that poly(I:C) elicited greater ACE2 induction in infant AE cells than IFN treatments, and poly(I:C)-induced ACE2 expression was not increased further by exogenous IFN administration (Figure 1B).
Figure 1.

IFN-induced upregulation of ACE2 (angiotensin-converting enzyme 2) in human nasal infant airway epithelial cells (AECs). (A) Heatmaps of top differentially regulated genes (±1.5 logFC, adjusted P < 0.05) demonstrating that ACE2 is consistently coexpressed with IFN-stimulated genes in the human nasal AECs of infants (n = 3 donors). (B) Box plots represent RT-PCR validation results comparing the effect of IFN-λ, IFN-γ, and/or poly(I:C) in the expression of ACE2 (n = 3 donors). *P < 0.05 relative to untreated, **P < 0.01 relative to untreated, and †P < 0.05 relative to IFN-λ or IFN-γ alone. Poly(I:C) alone was not significantly different than the combination with IFN-λ (P = 0.9) or IFN-γ (P = 0.2). APOBEC3A = apolipoprotein B mRNA editing enzyme catalytic subunit 2; GBPS = guanylate-binding proteins; HLA-DRA = major histocompatibility complex, class II, DR alpha; IFI44L = interferon induced protein 44 like; IFIT1 = interferon induced protein With tetratricopeptide repeats 1; MX2 = MX dynamin like GTPase 2; RSAD2 = radical S-adenosyl methionine domain containing 2; USP41 = ubiquitin specific peptidase 41.
We next evaluated the effect of IFN-γ in ACE2 protein expression using Western blotting (Figure 2A). As was recently reported (11), we identified a large and small isoform of ACE2, with the smaller isoform being more abundant in these pediatric AE cells (Figure 2A). IFN-γ treatment increased expression of both these isoforms (Figure 2A). Using direct immunofluorescence labeling of ACE2, we next found that ACE2 localizes both at the surface and intracellularly, and IFN-γ stimulation increases both these pools of ACE2 protein and does so in all cells (Figures 2B and 2C). Together, these new results directly demonstrate IFN-stimulated upregulation of ACE2 transcript and protein.
Figure 2.
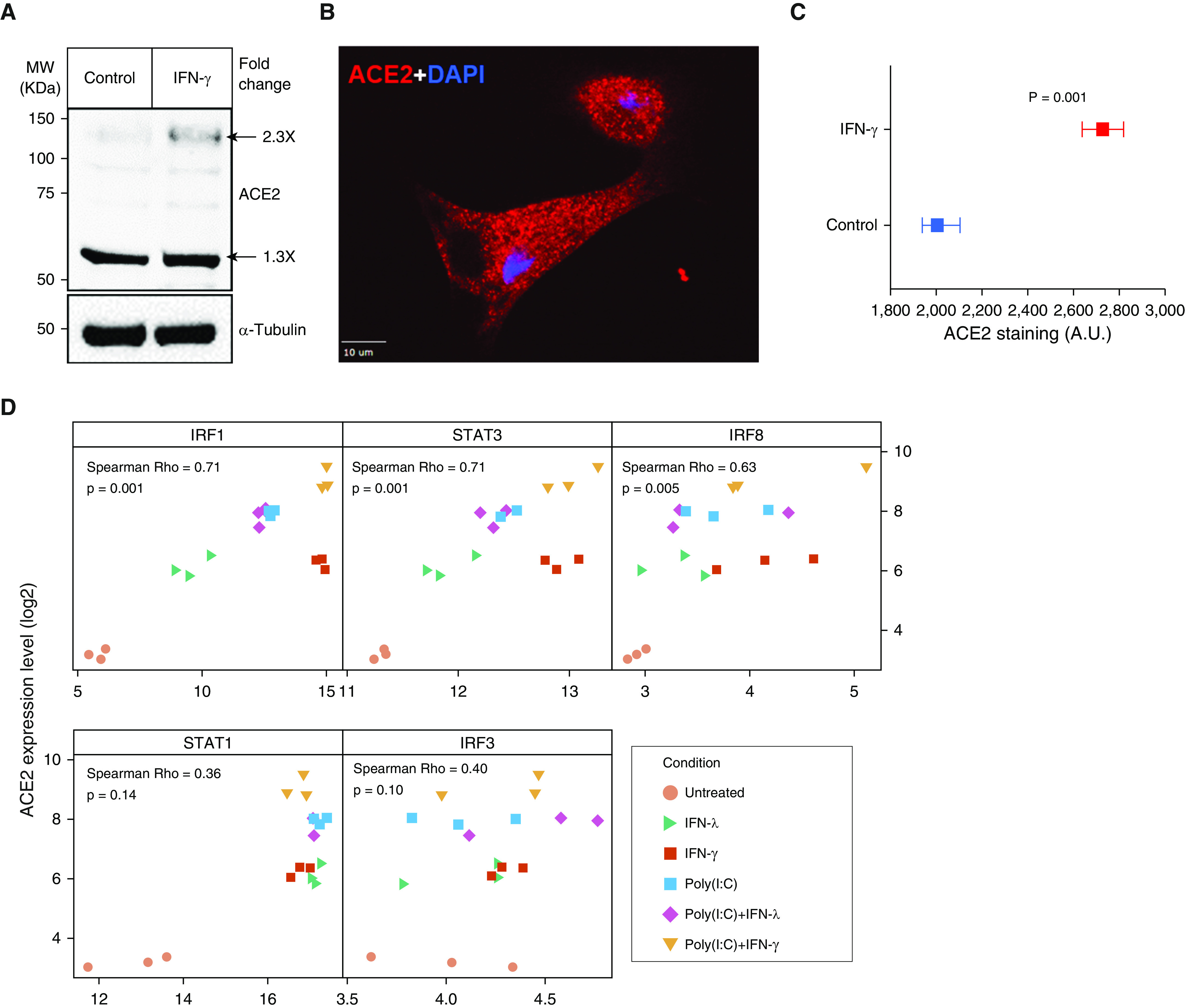
ACE2 protein expression in human nasal infant AECs and correlation of IFN-induced ACE2 upregulation with IFN-stimulated gene transcription factors. (A) AECs were left untreated or treated for 24 hours with IFN-γ (100 ng/ml), and cell lysates were probed for ACE2 (antibody catalog number AF933; R&D) or α-Tubulin (antibody catalog number sc-5286; Santa Cruz) proteins. The numbers adjacent to the large and the small ACE2 bands represent the normalized fold change in ACE2 level in the IFN-γ–treated samples compared with control. (B) Image showing ACE2 location in an untreated cell in an untreated cell and (C) plot showing mean value with 95% confidence interval for ACE2 immunostaining after IFN-γ treatment (n = 600 cells each). Scale bar, 10 μm. (D) Scatter plots showing the correlation (Spearman Rho) of ACE2 expression and transcription factors with binding sites at the ACE2 locus after exposure to IFN-λ, IFN-γ, and/or poly(I:C) (n = 3 donors). A.U. = arbitrary units; IRF = interferon regulatory factor; MW = molecular weight; STAT = signal transducer and activator of transcription.
Previous studies have identified transcription factor (TF) binding sites for STAT1, STAT3, IRF8, and IRF1 upstream of the human ACE2 transcription start site (5). To examine the potential role of these TFs in the IFN-driven induction of ACE2 in human infant AE cells, we next correlated ACE2 levels across different treatments with the expression of these TFs as well as with the expression of an unrelated TF induced by IFN (IRF3). As shown in Figure 2D, we found that the expression of ACE2 was significantly correlated with the expression of IRF1, IRF8, and STAT3, whereas little or no correlation was observed with IRF3 and STAT1.
Discussion
By using IFNs and a synthetic IFN inducer (poly(I:C)), our results identify that similar to other known ISGs, ACE2 is robustly activated by IFN-γ and IFN-λ in human infant AE cells. This establishes that ACE2 is an ISG in human infant AE cells, as was recently found in adults (4, 5). Although lower ACE2 expression has been reported in nasal AE cells from children (7), our findings indicate that lack of IFN-stimulated ACE2 expression is unlikely to be the basis for the protective advantage observed in these immature pediatric AE cells. Thus, mechanisms independent of IFN-stimulated ACE2 expression may be implicated in the reduced clinical severity observed in young children during SARS-CoV-2 infections (6).
Our combinatorial analyses show that neither IFN-γ nor IFN-λ further enhance ACE2 expression after inducing antiviral responses with poly(I:C) in nasal infant AE cells. Although antiviral AE responses involve multiple pathways (e.g., TLR7, TLR-4, RIG-I, or cGAS-STING), our findings related to potential TLR3 activation (by poly(I:C)) suggest that exogenous IFN treatment may extend their potential antiviral benefits without significantly enhancing viral entry. The latter is further supported by studies demonstrating that IFN type I (α/β) and type III (λ) can restrict SARS-CoV-2 infection (11, 12). Given that individuals with severe SARS-CoV-2 infections present milder IFN responses (10, 13), several IFN-based therapies are currently being tested for COVID-19 (9–11). However, there is also recent evidence that prolonged type I and type III IFN signaling may disrupt lung epithelial integrity upon virus recognition and during recovery from viral infection (14, 15). Accordingly, additional studies may still be needed to ensure the safety of IFN-based therapies in patients with COVID-19.
Future research is needed to define the mechanism mediating IFN-induced ACE2 expression in the AE. Our initial studies identified significant correlation between IFN-induced ACE2 expression and the ISG-regulatory TFs IRF1, IRF8, and STAT3. However, additional mechanistic experiments are required to appropriately address the role of each of these IRFs on the expression of ACE2. Identifying the precise molecular targets for IFN-driven induction of ACE2 and other ISGs that provide the beneficial antiviral function will help in more targeted design of therapies that can maintain this delicate balance between beneficial and harmful effects of IFNs during SARS-CoV-2 infection in both younger and older patients.
Supplementary Material
Footnotes
Supported by U.S. National Institutes of Health (NIH). Partially funded by NIH Grants HL145669, AI130502, HL141237, and U54HD090257.
Author Contributions: Study design: K.S., K.A., E.C., G.T., J.K.J., and G.N. Data collection: all authors. Analysis: K.S., K.A., G.T., J.L.G., M.J.G., and G.N. Manuscript drafting, editing, approval: all authors.
Originally Published in Press as DOI: 10.1165/rcmb.2020-0352LE on February 5, 2021
Author disclosures are available with the text of this letter at www.atsjournals.org.
References
- 1.Wölfel R, Corman VM, Guggemos W, Seilmaier M, Zange S, Müller MA, et al. Virological assessment of hospitalized patients with COVID-2019. Nature. 2020;581:465–469. doi: 10.1038/s41586-020-2196-x. [Published erratum appears in Nature 588:E35.] [DOI] [PubMed] [Google Scholar]
- 2.Sungnak W, Huang N, Bécavin C, Berg M, Queen R, Litvinukova M, et al. HCA Lung Biological Network. SARS-CoV-2 entry factors are highly expressed in nasal epithelial cells together with innate immune genes. Nat Med. 2020;26:681–687. doi: 10.1038/s41591-020-0868-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, et al. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280, e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhuang MW, Cheng Y, Zhang J, Jiang XM, Wang L, Deng J, et al. Increasing host cellular receptor-angiotensin-converting enzyme 2 expression by coronavirus may facilitate 2019-nCoV (or SARS-CoV-2) infection. J Med Virol. 2020;92:2693–2701. doi: 10.1002/jmv.26139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ziegler CGK, Allon SJ, Nyquist SK, Mbano IM, Miao VN, Tzouanas CN, et al. HCA Lung Biological Network. Electronic address:; lung-network@humancellatlas.orgHCA Lung Biological Network. SARS-CoV-2 receptor ACE2 is an interferon-stimulated gene in human airway epithelial cells and is detected in specific cell subsets across tissues. Cell. 2020;181:1016–1035, e19. doi: 10.1016/j.cell.2020.04.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu X, Zhang L, Du H, Zhang J, Li YY, Qu J, et al. Chinese Pediatric Novel Coronavirus Study Team. SARS-CoV-2 infection in children. N Engl J Med. 2020;382:1663–1665. doi: 10.1056/NEJMc2005073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bunyavanich S, Do A, Vicencio A. Nasal gene expression of angiotensin-converting enzyme 2 in children and adults. JAMA. 2020;323:2427–2429. doi: 10.1001/jama.2020.8707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salka K, Arroyo M, Chorvinsky E, Abutaleb K, Perez GF, Wolf S, et al. Innate IFN-lambda responses to dsRNA in the human infant airway epithelium and clinical regulatory factors during viral respiratory infections in early life. Clin Exp Allergy. 2020;50:1044–1054. doi: 10.1111/cea.13701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.O’Brien TR, Thomas DL, Jackson SS, Prokunina-Olsson L, Donnelly RP, Hartmann R. Weak induction of interferon expression by severe acute respiratory syndrome coronavirus 2 supports clinical trials of interferon-λ to treat early coronavirus disease 2019. Clin Infect Dis. 2020;71:1410–1412. doi: 10.1093/cid/ciaa453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park A, Iwasaki A. Type I and type III interferons - induction, signaling, evasion, and application to combat COVID-19. Cell Host Microbe. 2020;27:870–878. doi: 10.1016/j.chom.2020.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Onabajo OO, Banday AR, Stanifer ML, Yan W, Obajemu A, Santer DM, et al. Interferons and viruses induce a novel truncated ACE2 isoform and not the full-length SARS-CoV-2 receptor. Nat Genet. 2020;52:1283–1293. doi: 10.1038/s41588-020-00731-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vanderheiden A, Ralfs P, Chirkova T, Upadhyay AA, Zimmerman MG, Bedoya S, et al. Type I and type III interferons restrict SARS-CoV-2 infection of human airway epithelial cultures. J Virol. 2020;94:e00985-20. doi: 10.1128/JVI.00985-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blanco-Melo D, Nilsson-Payant BE, Liu WC, Uhl S, Hoagland D, Møller R, et al. Imbalanced host response to SARS-CoV-2 drives development of COVID-19. Cell. 2020;181:1036–1045, e9. doi: 10.1016/j.cell.2020.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Broggi A, Ghosh S, Sposito B, Spreafico R, Balzarini F, Lo Cascio A, et al. Type III interferons disrupt the lung epithelial barrier upon viral recognition. Science. 2020;369:706–712. doi: 10.1126/science.abc3545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Major J, Crotta S, Llorian M, McCabe TM, Gad HH, Priestnall SL, et al. Type I and III interferons disrupt lung epithelial repair during recovery from viral infection. Science. 2020;369:712–717. doi: 10.1126/science.abc2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


