Abstract
Genetics is the branch of biology concerned with study of individual genes and how they work whereas genomics is involved with the analysis of all genes and their interactions. Both of these approaches have been applied extensively to CF. Identification of the CFTR gene initiated the dissection of CF genetics at the molecular level. Subsequently, thousands of variants were found in the gene and the functional consequences of a subset have been studied in detail. The completion of the human genome ushered in a new phase of study where the role of genes beyond CFTR could be evaluated for their contribution to the severity of CF. This will be a brief overview of the contribution of these complementary methods to our understanding of CF pathogenesis.
Keywords: Cystic fibrosis, CFTR, Genetic modifiers, Linkage, Association
1. Background
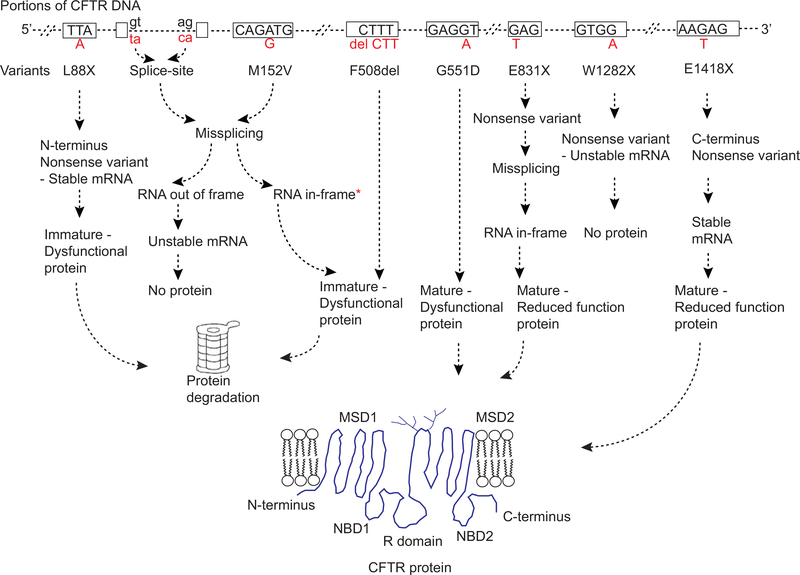
Since the discovery of Cystic Fibrosis Transmembrane Conductance Regulator ( CFTR ) gene in 1989 [1–3], the CF Mutation Database (CFMD available at http://www.genet.sickkids.on.ca/cftr/) has been a valuable repository for DNA variants. As of 2019, 2065 variants in the CFTR gene have been inventoried by the CFMD. These variants have a plethora of effects upon gene function (Fig. 1). Nearly 40% of the reported missense variants are predicted to alter a single amino acid and should therefore allow CFTR protein to be synthesized, although it may be misfolded and/or dysfunctional. Extensive studies of the consequences of missense variants have been performed that are summarized in reviews elsewhere [4–8]. It should be noted that a small fraction of predicted missense variants also affect splicing of the CFTR mRNA producing transcript [9–11]. In such situations, if CFTR protein is predicted to be synthesized, it may be altered in quantity or its sequence may be different than predicted from the single amino acid change. Another 35% of variants (nonsense, frameshift, canonical splice site) introduce a premature termination codon (PTC) that generally leads to mRNA degradation, and substantially reduced levels of unstable truncated CFTR protein that is rapidly degraded. Interestingly, there is considerable inter-individual and intra-individual variability in mRNA degradation of PTC variants [12]. Additionally, the introduced PTC may lead to the production of a stable truncated protein that has altered function [13,14]. The remaining variants (5%) consist of gain or loss of DNA sequence which involves the insertion or deletion of one or a few amino acids to many amino acids. Among these is F508del, a three nucleotide deletion that leads to the omission of a phenylalanine residue at codon 508. The F508del is the most common disease causing variant with a frequency of about 70% in CF population. Most of the remaining 20% of CFTR variants have either benign effects on CFTR functionor of unknown effect. It should be kept in mind that variants reported to CFMD were found in a variety of situations; diagnosis of individuals who have CF, testing of individuals who have features of CF (but may not have CF) and sequencing of CFTR for other reasons.
Fig. 1.
Genetic variants in CFTR and their impact on RNA and protein production. Only a portion of CFTR gene is shown. Rectangular boxes are exons, dashed lines are either introns, 5′UTR or 3′UTR, and dashed hashes are multiple exon-introns within CFTR gene. Please note, only few nucleotides within each exon are shown. Dinucleotides, gt and ag, in black letters represent consensus splice site signals. Alphabets in red beneath each exon or intron represent nucleotide changes. Variant names are written beneath each nucleotide change. Nonsense and frameshift variants can have heterogeneous effects on mRNA stability depending upon their location [13]. Misfolded CFTR protein undergoes ER-associated degradation via the ubiquitin–proteasome system. Fully glycosylated mature protein can be dysfunctional due to impaired gating, conductance or reduced residence time at the cell surface [4]. Missense variants predicted to make mature protein can undergo mis-splicing resulting in no protein or immature dysfunctional CFTR protein [9–11]. *indicates in-frame mis-spliced mRNA may also generate mature but dysfunctional CFTR protein.
Thus, only a fraction of the CFMD variants cause CF and the status of many other variants is unclear.
2. CFTR2: assigning disease liability of CFTR variants
To define exactly which CFTR variants cause CF, a project was started in the late 1990s called the Clinical and Functional Translation of CFTR, or CFTR2. This effort was designed to collect all CFTR variants reported in individuals who have been diagnosed with CF by a medical professional and attend a CF clinic or are enrolled in a CF Patient Registry. The nearly 90,000 individuals recruited by the CFTR2 project world-wide carry 1640 different CFTR variants. Most of these variants are rare, occurring in only one or a few individuals. However, ~520 occur in three or more individuals worldwide of which 159 achieve a frequency greater than 0.01% among individuals enrolled in CFTR2. The 159 variants were characterized using a three-step process that evaluates 1) clinical features, 2) penetrance in CF carriers and 3) functional consequences and to assign disease liability [15]. Using this approach, the CFTR2 team is in the process of interpreting the next 361 variants to reach a goal of 520 characterized variants. As of July 2019, a total of 412 variants have been interpreted and posted to the CFTR2 website (https://cftr2.org/).
Since CFTR variants have been associated with a spectrum of conditions ranging from classic CF to ‘monosymptomatic’ conditions, it is essential that specific and reliable clinical metrics be used for the differential diagnosis [4,16]. The French CF database elected to distinguish CF from other disorders related to CFTR dysfunction thereby providing variant classifications that cover the phenotypic spectrum [17]. The potential for certain variants to give rise to several conditions is elegantly illustrated by this approach. An alternative, undertaken by the CFTR2 project, is to define CF in relatively narrow terms and limit interpretation to only those individuals determined to have CF. To implement this approach, the CFTR2 team defined CF as a lifelimiting disorder involving the lungs and pancreas that is accompanied by a sweat chloride concentration of 60 mM or higher. At least three individuals meeting this definition were required in order to qualify a variant as ‘CF-causing’ from a clinical perspective. Whenever possible, the CFTR2 team incorporates penetrance evidence from CF heterozygote studies. In practice, presence of a variant in the ‘healthy’ CFTR gene of a CF carrier (generally parents of individuals with CF) is taken as evidence that the variant does not cause CF. While this approach was used for the 159 most common variants [15], applying the same method is more challenging for rare variants due to the difficulty of locating carriers of rare variants.
Consequently, the CFTR2 project consults the genome aggregation (gnomAD) database of DNA variants occurring in healthy individuals (https://gnomad.broadinstitute.org/) toassess pathogenicityof rarevariants.
3. Assessment of chloride channel function in cell line models expressing CFTR variants
Functional assessment has been a gold standard for establish-ing pathogenicity of DNA variants. CFTR variants can be parsed into two broad groups; those that are predicted to allow protein synthesis and those that aren’t. Before the initiation of the CFTR2 project, only a few dozen CFTR variants had undergone testing due to the time and resource intensive aspects of functional assays. The discovery of a CFTR-specific inhibitor [18], standard-ization of primary cell culture [19], and the development of molecular systems that enable rapid selection of isogenic cell lines expressing single copies of cDNAs [8] facilitated functional testing using cell lines on all variants predicted to produce CFTR protein that have been annotated by CFTR2 [15,20–22]. Impor-tantly, epithelial cells obtained from the lungs, nasal passages and rectum of individuals bearing a subset of the CFTR2 variants have been assessed for CFTR-mediated chloride transport thereby providing correlation with variant forms of CFTR in anative setting [23].
Given the large number of variants to be tested, the U.S. CF Foundation has sponsored a highthroughput approach to functional and modulator testing using the combined efforts of laboratories at UT Southwestern, Rosalind Franklin (Chicago) and the CF Therapeutics Lab in Boston. It should be noted that quite a few of the variants cause minimum to no effect on CFTR function, indicating that they are likely benign variants. There are also situations where CFTR variants alter other functions of CFTR like bicarbonate transport that are not routinely assayed [24]. Furthermore, the combination of two or more variants in the same CFTR gene (a.k.a. complex alleles) present a particular challenge [25–28]. There are over 120 complex alleles already recorded in CFTR and more are likely tobe discovered as sequencing of the entire CFTR gene becomes routine. Although these variants of ‘variable clinical consequence’ represent only 5% of all CFTR variants interpreted thus far, they can create substantial challenges when trying to establish a diagnosis of CF.
4. Evaluation of modulator response in cell line models expressing CFTR variants
Creation of cell lines expressing many of the CFTR2 variants has also facilitated testing their response to currently available and newly developed CFTR modulators. Studies of CFTR variants that permit synthesis of CFTR have indicated a correlation between the amount of residual function and response to modulators, but it should be noted there are a number of important exceptions [21]. Amongst the exceptions is the well-known variant G551D which was an outstanding first target given that it is particularly responsive to the effects of the potentiator, Ivacaftor. Correlation between modulator effect and residual function is also observed for the corrector Lumacaftor, and when the two modulators are combined [21]. If the recent successes with the triple combination of modulators are included [29,30], a promising picture emerges revealing that the vast majority of individuals with CF should benefit from modulator therapy. The triple combination appears to produce clinically relevant improvements in individuals who are homozygous of the common variant, F508del, and individuals who carry one copy of F508del. It is also likely that the triple combination will be effective for well-established residual function variants. A further 8% of individuals with CF have already demonstrated clinically relevant response to the Ivacaftor alone. Together, modulator therapy is expected to be clinically efficacious for 92% to 93% of individuals with CF worldwide. Of those remaining, isogenic cell and organoid studies reveal that ~300 variants are likely to be responsive to some degree to modulators, leaving ~500 mostly very rare variants that are undergoing testing as noted above. A final group of almost 20 0 0 individuals ( ~3%) carry variants ineach CFTR gene for which there are no treatments currently available. A minor fraction of this group carry variants that allow productionof truncated protein that may be amenable to modulator treatment [13]. The remainder carry variants that do not allow stable mRNA and protein production that makes them non-responsive to current modulator therapies. Individuals who carry these nonresponsive variants in each CFTR gene will require alternative therapies such as gene editing or gene replacement [31].
5. Correlation of CFTR function with clinical features to inform precision treatment in CF
With the unprecedented number of CFTR variants that have an assigned level of function, it has been possible to perform detailed correlations of CFTR function with the severity of the phenotype. Using function derived from 226 different combinations of CFTR variants and clinical data of 54,671 individuals revealed a non-linear relationship between increases of CFTR function and a response in the phenotype [22]. At very low levels of CFTR function, minor increases in function cause disproportionately large improvements in clinical status compared to the improvement observed at higher levels of function. The non-linear relationship has important implications for treatment as it indicates that even minor improvements in function for individuals with severe disease could produce a clinically relevant change in outcome. Correlations between CFTR function and CF phenotype derived from CFTR2 data can also aid in benchmarking efficacy of the modulator treatments. To date, increases in function estimated from the effect of modulators matches quite well with the clinical responses predicted from CFTR2 data [22]. Ongoing monitoring of these response should be able to determine the degree to which CF is reversible when CFTR is brought to a higher level of function by modulators.
6. Exploring the genetic architecture of CF traits through a genomic lens
While variants in the CFTR gene are responsible for the development of CF, they only partially explain variation in the severity of disease. In general, variants that cause complete or near complete loss of CFTR function results in severe features in the organ systems primarily affected in CF (i.e., lungs, pancreas, intestine and sweat gland). However, there can be considerable intra- and inter- individual differences in the degree of organ dysfunction. Soon after the discovery of the CFTR gene, it was realized that individuals who were homozygous for the common F508del variant varied considerably in the severity of lung disease, as measured by pulmonary function tests [32]. Individuals of the same age were shown to have substantial variation in lung function, ranging from normal (i.e., 100% FEV1% predicted) to life-threating (i.e., 20% FEV1% predicted). Subsequently, it was shown that variants that allow residual CFTR function are associated with less severe lung disease, although considerable variability among individuals was observed. Similar observations were made regarding exocrine pancreatic disease although the degree of variability among individuals was less pronounced, likely a consequence of severe dysfunction (i.e., insufficiency) manifesting at birth or shortly thereafter. In contrast, variation in lung function manifests over decades. These observations indicated that the nature of the defect in the CFTR gene contributed to variation in severity, but other factors, notably genetic and environmental modifiers are important determinants of phenotype variability.
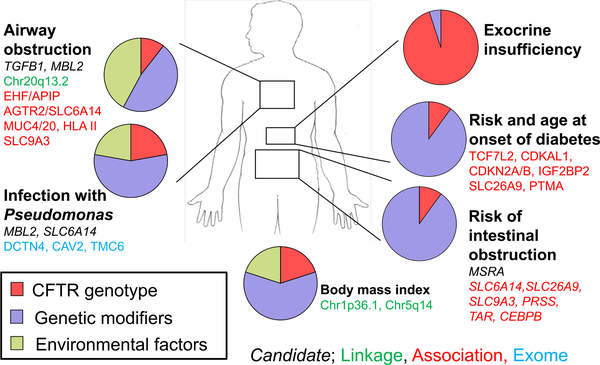
Estimating the effect of genetic and non-genetic factors to phenotype variability in humans can be achieved by analysis of related individuals. The classic approach is to compare the degree of similarity among twins who are monozygous (a.k.a., MZ; identical) to those who are dizygous (a.k.a. DZ; fraternal). MZ twins share all (or nearly all) variants while DZ twins share 50% of their variants onaverage. Thus, greater similarity of a trait among MZ twin pairs compared to DZ twin pairs indicates that genetic variants contribute to variability in the trait. This in turn translates into the concept of heritability; the degree to which variation in a trait can be attributed to genetic factors. In this manner, twins who are both affected with CF have been studied to deduce the degree to which genetic factors independent of CFTR (as the CFTR variants are already shared by the twins) contribute to organ disease severity. Studies of twins in Europe and the U.S. have elucidated the role and quantified the contribution of genetic and non-genetic (environmental and stochastic) factors to CF variability [33,34] (Fig. 2).
Fig. 2.
Relative contribution of genetic and non-genetic factors by organ system to variation in cystic fibrosis traits (adapted from Reference [33]). The magnitude of effect of CFTR, modifier genes and environment to variation in each trait were derived from CF twin and sibling analysis. Select modifier genes and loci implicated by candidate, linkage, association or exome sequencing methods are shown.
Establishing that modifier genes contribute substantially to variation of most features of CF provided a compelling rationale to pursue the responsible specific genes and loci. A variety of approaches have been used to decipher the contribution of variants in genes other than CFTR to variability in CF (Fig. 2). Five loci were associated with variation in lung function using genome-wide approaches [35,36]. These loci contain appealing biological candidates genes such an epithelial cell amino acid transporter ( SLC6A14 ) that facilitates sodium and chloride transport and can augment mutant CFTR function [37]. Other loci harbor tethered lung mucins (MUC4 and MUC20 ), an epithelial brush border sodium/hydrogen exchanger ( SLC9A3 ), and a transcription factor involved in lung epithelial maintenance ( EHF ) [38]. Genome-wide association studies have identified six loci conferring risk for meconium ileus harboring interesting candidates such the pancreatic serine protease 1 (PRSS1 ) and a hydrogen/potassium exchanger driven by ATP hydrolysis ( ATP12A ) [39,40]. Cystic fibrosis-related diabetes appears to be modified by genes that play a role only in CF (e.g. SLC26A9 ), and others that are risk factors for diabetes in CF as well as in the general population (e.g., TCF7L2) [41,42].
Several themes have emerged from modifier studies to date. First, risk variants for common and rare diseases modify similar features in CF. Examples include CF-related diabetes and type 2 diabetes, CF hepatic cirrhosis and AAT [43–45]. Second, gene-gene interactions have been documented among modifier variants and CFTR variants [46,47], modifier variants in different genes [48], and modifier variants and environment [49]. Third,gene sand their variants have been found to modify multiple features of CF (a.k.a. pleiotropy). Examples include SLC26A9 associated with risk of neonatal intestinal obstruction and diabetes [40,50],and SLC6A14 that is a candidate for modifying lung function severity, age at first infection with Pseudomonas aeruginosa and neonatal intestinal obstruction [51,52]. More recenty, investigation of the genetic architecture, cellular distribution and regulatory regions of SLC26A9 reveals that variation in the level of this gene modifies the age at onset of diabetes in individuals with CF [53].
7. What may the future hold for CF from a genetic and genomic perspective?
Complete assessment of variants that are responsible for CF and matching to efficacious drug combinations should qualify the vast majority of individuals with CF for modulator treatment. Intense effort s are underway to develop new approaches for those that carry variants that produce CFTR recalcitrant to modulators or variants that do not permit the synthesis of CFTR. A more complete understanding of the genetic architecture of CF traits will maximize the utility of genomic approaches in disease screening, diagnosis, prognosis and therapy. At this time, we do not know exactly which genes are affected by the associated variants and their mechanism of action. Whole genome sequencing (WGS) is one approach currently being used to explore loci identified by association analysis to identify DNA variants that modify CF. An increased understanding of the mechanism underlying modifier loci should facilitate the development of drugs that work for every individual with CF. Future treatment may combine customized CFTR modulator therapy with drugs targeting modifier loci to optimize outcomes for every individual with CF.
8. Summary
As of 2019, nearly 90,000 individuals recruited by the CFTR2 project world-wide carry 1640 different CFTR variants. The goal of the project is to assess the disease-liability of all CFTR variants and, as of late 2019, this has been completed for 412 of the most common variants (https://cftr2.org/).Thenon-linearrelationshipbe-tween increases of CFTR function and a response in the phenotype indicates that even minor improvements in function for individuals with severe disease could produce a clinically relevant change in outcome. The triple combination modulator therapy is expected to be clinically efficacious for ~ 93% of individuals with CF worldwide. Individuals who carry non-responsive variants in each CFTR gene will require alternative therapies. Gene modifier studies have estimated the effect of genetic and non-genetic factor st opheno-ty pevariabilit y.Modifie rs oflung functio n,meconi umile ussuscep-tibili tygene s,and modifiers of CFrelat eddiabet esha vebe eneluci-dat edusi nggeno mewi deapproache s.Targeti ng ofmodifie rscould augme ntmodulat ortherapy a ndprovi detreatme ntf orindividuals w hocar ryvarian tsthat a reunresponsi ve toCF TRmodulators.
Acknowledgements
The author’s work is supported by grants CF Foundation SHARMA19I0 to NS and R01DK44003 and CF Foundation CUTTIN13A1 to GRC.
Footnotes
This paper is part of a Supplement supported by The European Cystic Fibrosis Society (ECFS).
Declaration of Competing Interest
None.
References
- [1].Kerem B, Rommens JM, Buchanan JA, Markiewicz D, Cox TK, Chakravarti A, et al. Identification of the cystic fibrosis gene: genetic analysis. Science 1989;245:1073–80. [DOI] [PubMed] [Google Scholar]
- [2].Riordan JR, Rommens JM, Kerem B, Alon N, Rozmahel R, Grzelczak Z, et al. Identification of the cystic fibrosis gene: cloning and characterization of complementary DNA. Science 1989;245(4922):1066–73. [DOI] [PubMed] [Google Scholar]
- [3].Rommens JM, Iannuzzi MC, Kerem B, Drumm ML, Melmer G, Dean M, et al. Identification of the cystic fibrosis gene: chromosome walking and jumping. Science 1989;245:1059–65. [DOI] [PubMed] [Google Scholar]
- [4].Amaral MD. Novel personalized therapies for cystic fibrosis: treating the basic defect in all patients. J Intern Med 2015;277(2):155–66. [DOI] [PubMed] [Google Scholar]
- [5].De Boeck K, Amaral MD. Progress in therapies for cystic fibrosis. Lancet Respir Med 2016;4(8):662–74. [DOI] [PubMed] [Google Scholar]
- [6].Veit G, Avramescu RG, Chiang AN, Houck SA, Cai Z, Peters KW, et al. From CFTR biology toward combinatorial pharmacotherapy: expanded classification of cystic fibrosis mutations. Mol Biol Cell 2016;27(3):424–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Oliver KE, Han ST, Sorscher EJ, Cutting GR. Transformative therapies for rare CFTR missense alleles. Curr Opin Pharmacol 2017;34:76–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Clancy JP, Cotton CU, Donaldson SH, Solomon GM, VanDevanter DR, Boyle MP, et al. CFTR modulator theratyping: current status, gaps and future directions. J Cyst Fibros 2019;18(1):22–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].MolinskiS V, Gonska T, Huan LJ, Baskin B, JanahiI A, Ray PN, et al. Genetic, cell biological, and clinical interrogation of the CFTR mutation c.3700 a>g (p.Ile1234Val) informs strategies for future medical intervention. Genet Med 2014;16(8):625–32. [DOI] [PubMed] [Google Scholar]
- [10].Lee M, Roos P, Sharma N, Atalar M, Evans TA, Pellicore MJ, et al. Systematic computational identification of variants that activate exonic and intronic cryptic splice sites. Am J Hum Genet 2017;100(5):751–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Amato F, Scudieri P, Musante I, Tomati V, Caci E, Comegna M, et al. Two CFTR mutations within codon 970 differently im pact on the chloride channel functionality. Hum Mutat 2019;40(6):742–8. [DOI] [PubMed] [Google Scholar]
- [12].Linde L, Boelz S, Neu-Yilik G, Kulozik AE, Kerem B. The efficiency of nonsense-mediated mRNA decay is an inherent character and varies among different cells. Eur J Hum Genet 2007;15(11):1156–62. [DOI] [PubMed] [Google Scholar]
- [13].Sharma N, Evans TA, Pellicore MJ, Davis E, Aksit MA, McCague AF, et al. Capitalizing on the heterogeneous effects of CFTR nonsense and frameshift variants to inform therapeutic strategy for cystic fibrosis. PLoS Genet 2018;14(11):e1007723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Clarke LA, Awatade NT, Felicio VM, Silva IA, Calucho M, Pereira L, et al. The effect of premature termination codon mutations on CFTR mRNA abundance in human nasal epithelium and intestinal organoids: a basis for read-through therapies in cystic fibrosis. Hum Mutat 2019;40(3):326–34. [DOI] [PubMed] [Google Scholar]
- [15].Sosnay PR, Siklosi KR, VanGoor F, Kaniecki K, Yu H, Sharma N, et al. Defining the disease liability of variants in the cystic fibrosis transmembrane conductance regulator gene. Nat Genet 2013;45(10):1160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet 2015;16(1):45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Claustres M, Thèze C, des Georges M, Baux D, Girodon E, Bienvenu T, et al. CFTRFrance, a national relational patient database for sharing genetic and phenotypic data associated with rare CFTR variants. Hum Mutat 2017;38(10):1297–315. [DOI] [PubMed] [Google Scholar]
- [18].Thiagarajah JR, Song Y, Haggie PM, Verkman AS. A small molecule CFTR inhibitor produces cystic fibrosis-like submucosal gland fluid secretions in normal airways. FASEB J 2004;18(7):875–7. [DOI] [PubMed] [Google Scholar]
- [19].Gentzsch M, Boyles SE, Cheluvaraju C, Chaudhry IG, Quinney NL, Cho C, et al. Pharmacological rescue of conditionally reprogrammed cystic fibrosis bronchial epithelial cells. Am J Respir Cell Mol Biol 2017;56(5):568–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Raraigh KS, Han ST, Davis E, Evans TA, Pellicore MJ, McCague AF, et al. Functional assays are essential for interpretation of missense variants associated with variable expressivity. Am J Hum Genet 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Han ST, Rab A, Pellicore MJ, Davis EF, McCague AF, Evans TA, et al. Residual function of cystic fibrosis mutants predicts response to small molecule CFTR modulators. JCI Insight 2018;3(14). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].McCague AF, Raraigh KS, Pellicore MJ, Davis-Marcisak EF, Evans TA, Han ST, et al. Correlating cystic fibrosis transmembrane conductance regulator function with clinical features to inform precision treatment of cystic fibrosis. Am J Respir Crit Care Med 2019;199(9):1116–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Awatade NT, Wong SL, Hewson CK, Fawcett LK, Kicic A, Jaffe A, et al. Human primary epithelial cell models: promising tools in the era of cystic fibrosis personalized medicine. Front Pharmacol 2018;9:1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Larusch J, Jung J, IJ G, Lewis MD, Park HW, Brand RE, et al. Mecha-nisms of cftr functional variants that impair regulated bicarbonate permeation and increase risk for pancreatitis but not for cystic fibrosis. PLoS Genet 2014;10(7):e1004376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Kiesewetter S, Macek M, Davis C, Curristin SM, Chu CS, Graham C, et al. A mutation in cftr produces different phenotypes depending on chromosomal background. Nat Genet 1993;5(3):274–8. [DOI] [PubMed] [Google Scholar]
- [26].Massie RJ, Poplawski N, Wilcken B, Goldblatt J, Byrnes C, Robertson C. Intron-8 polythymidine sequence in Australasian individuals with CF mutations R117H and R117C. Eur Respir J 2001;17(6):1195–200. [DOI] [PubMed] [Google Scholar]
- [27].Claustres M, Altieri JP, Guittard C, Templin C, Chevalier-Porst F, DesGeorges M. Are p.I148T, p.R74W and p.D1270N cystic fibrosis causing mutations? BMC Med Genet 2004;5:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Rohlfs EM, Zhou ZQ, Sugarman EA, Heim RA, Pace RG, Knowles MR, et al. The I148T CFTR allele occurs on multiple haplotypes: a complex allele is associated with cystic fibrosis. Genet Med 2002;4(5):319–23. [DOI] [PubMed] [Google Scholar]
- [29].Keating D, Marigowda G, Burr L, Daines C, Mall MA, McKone EF, et al. VX-445-Tezacaftor-Ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med 2018;379(17):1612–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Davies JC, Moskowitz SM, Brown C, Horsley A, Mall MA, McKone EF, et al. VX-659Tezacaftor-Ivacaftor in patients with cystic fibrosis and one or two Phe508del alleles. N Engl J Med 2018;379(17):1599–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Harrison PT, Sanz DJ, Hollywood JA. Impact ofgene editing onthestudy of cystic fibrosis. Hum Genet 2016;135(9):983–92. [DOI] [PubMed] [Google Scholar]
- [32].Kerem E, Corey M, Kerem B-S, Rommens J, Markiewicz D, Levison H, et al. The relation between genotype and phenotype in cystic fibrosis–analysis of the most common mutation (deltaF508). N Engl J Med 1990;323:1517–22. [DOI] [PubMed] [Google Scholar]
- [33].Cutting GR. Cystic fibrosis genetics: from molecular understanding to clinical application. Nat Rev Genet 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].O’Neal WK, Knowles MR. Cystic fibrosis disease modifiers: complex genetics defines the phenotypic diversity in a monogenic disease. Annu Rev Genom Hum Genet 2018;19:201–22. [DOI] [PubMed] [Google Scholar]
- [35].Wright FA, Strug LJ, Doshi VK, Commander CW, Blackman SM, Sun L, et al. Genome-wide association and link age identify modifier loci of lung disease severity in cystic fibrosis at 11p13 and 20q13.2. Nat Genet 2011;43(6):539–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Corvol H, Blackman SM, Boelle PY, Gallins PJ, Pace RG, Stonebraker JR, et al. Genome-wide association meta-analysis identifies five modifier loci of lung disease severity in cystic fibrosis. Nat Commun 2015;6:8382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ahmadi S, Wu YS, Li M, Ip W, Lloyd-Kuzik A, DiPaola M, et al. Augmentation of CFTR function in human bronchial epithelial cells via SLC6A14-dependent amino acid uptake: implications for treatment of cystic fibrosis. Am J Respir Cell Mol Biol 2019. [DOI] [PubMed] [Google Scholar]
- [38].Fossum SL, Mutolo MJ, Tugores A, Ghosh S, Randell SH, Jones LC, et al. Ets homologous factor (EHF) has critical roles in epithelial dysfunction in airway disease. J Biol Chem 2017;292(26):10938–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Strug LJ, Gonska T, He G, Keenan K, Ip W, Boelle PY, et al. Cystic fibrosisgene modifier SLC26A9 modulates airway response to CFTR-directed therapeutics. Hum mol Genet 2016;25(20):4590–600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Gong J, Wang F, Xiao B, Panjwani N, Lin F, Keenan K, et al. Genetic association and transcriptome integration identify contributing genes and tissues at cystic fibrosis modifier loci. PLoS Genet 2019;15(2):e1008007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Blackman SM, Hsu S, Vanscoy LL, Collaco JM, Ritter SE, Naughton K, et al. Genetic modifiers play a substantial role in diabetes complicating cystic fibrosis. J Clin Endo crinol Metab 2009;94(4):1302–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Blackman SM, Hsu S, Ritter SE, Naughton KM, Wright FA, Drumm ML, et al. A susceptibility gene for type 2 diabetes confers substantial risk for diabetes complicating cystic fibrosis. Diabetologia 2009;52(9):1858–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Bartlett JR, Friedman KJ, Ling SC, Pace RG, Bell SC, Bourke B, et al. Genetic modifiers of liver disease in cystic fibrosis. JAMA 2009;302(10):1076–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Boelle PY, Debray D, Guillot L, Corvol HFrench CFMGSI. SERPINA1 Z allele is associated with cystic fibrosis liver disease. Genet Med 2019;21(9):2151–5. [DOI] [PubMed] [Google Scholar]
- [45].Blackman SM, Commander CW, Watson C, Arcara KM, Strug LJ, Stonebraker JR, et al. Genetic modifiers of cystic fibrosis-related diabetes. Diabetes 2013;62(10):3627–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Drumm ML, Konstan MW, Schluchter MD, Handler A, Pace R, Zou F, et al. Gene modifiers of lung disease in cystic fibrosis. N Engl J Med 2005;353(14):1443–53. [DOI] [PubMed] [Google Scholar]
- [47].Bremer LA, Blackman SM, Vanscoy LL, McDougal KE, Bowers A, Naughton KM, et al. Interaction between a novel TGFB1 haplotype and CFTR genotype is associated with improved lung function in cystic fibrosis. Hum Mol Genet 2008;17(14):2228–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Dorfman R, Sandford A, Taylor C, Huang B, Frangolias D, Wang Y, et al. Complex twogene modulation of lung disease severity in children with cystic fibrosis. J Clin Invest 2008;118(3):1040–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Collaco JM, Vanscoy L, Bremer L, McDougal K, Blackman SM, Bowers A, et al. Interactions between secondhand smoke and genes that affect cystic fibrosis lung disease. JAMA 2008;299(4):417–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sun L, Rommens JM, Corvol H, Li W, Li X, Chiang TA, et al. Multiple apical plasma membrane constituents are associated with susceptibility to meconium ileus in individuals with cystic fibrosis. Nat Genet 2012;44(5):562–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Li W, Soave D, Miller MR, Keenan K, Lin F, Gong J, et al. Unraveling the complex genetic model for cystic fibrosis: pleiotropic effects of modifier genes on early cystic fibrosisrelated morbidities. Hum Genet 2014;133(2):151–61. [DOI] [PubMed] [Google Scholar]
- [52].Di Paola M, Park AJ, Ahmadi S, Roach EJ, Wu YS, Struder-Kypke M, et al. SLC6A14 is a genetic modifier of cystic fibrosis that regulates pseu-domonas aeruginosa attachment to human bronchial epithelial cells. MBio 2017;8(6). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Lam AN, Aksit MA, Vecchio-Pagan B, Shelton CA, Osorio DL, Anzmann AF, et al. Increased expression of anion transporter SLC26A9 delays diabetes onset in cystic fibrosis. J Clin Invest 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]