Abstract
Background
COVID-19, a severe global pandemic caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has emerged as one of the most threatening transmissible disease. As a great threat to global public health, the development of treatment options has become vital, and a rush to find a cure has mobilized researchers globally from all areas.
Scope and approach
This review focuses on deciphering the potential of different secondary metabolites from medicinal plants as therapeutic options either as inhibitors of therapeutic targets of SARS-CoV-2 or as blockers of viral particles entry through host cell receptors. The use of medicinal plants containing specific phytomoieties could be seen in providing a safer and long-term solution for the population with lesser side effects.
Key Findings and Conclusions: Considering the high cost and time-consuming drug discovery process, therapeutic repositioning of existing drugs was explored as treatment option in COVID-19, however several molecules have been retracted as therapeutics either due to no positive outcomes or the severe side effects. These effects call for exploring the alternate treatment options which are therapeutically effective as well as safe. Keeping this in mind, phytopharmaceuticals derived from medicinal plants could be explored as important resources in the development of COVID-19 treatment, as their role in the past for treatment of viral diseases like HIV, MERS-CoV, and influenza has been well reported. Considering this fact, different phytoconstituents such as flavonoids, alkaloids, tannins and glycosides etc. Possessing antiviral properties against coronaviruses and possessing potential against SARS-CoV-2 have been reviewed in the present work.
Keywords: ACE-2, COVID-19, COVID-19 main protease, nCoV-2019, Phytoconstituents, TMPRSS2
Graphical abstract
1. Introduction
The ongoing global COVID-19 pandemic still remains a major challenge for scientific communities since its outbreak in December 2019 and has become one of the major causes of mortality and morbidity in large populations. As of June 19, 2020, the World Health Organization (WHO) reported that 213 countries and territories have been affected from this deadly infectious disease originated from Wuhan, China with 8,585,194 infected and 456,439 death cases confirmed worldwide Fig. 3 [1]. COVID-19 has not only affected the global health care system but also hampered the economic growth of the countries along with changes in the normal lifestyle and habits [2,3]. COVID-19, caused by the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), is recognized as a communicable and deadly respiratory illness, which begins with normal common cold symptoms and become adverse with course of time leading to fever, dry cough and shortness of breath [4,5]. Certain morbidity factors such as hypertension, cardiovascular diseases, diabetes mellitus, pollution and age increase the risk of COVID-19 [6,7].
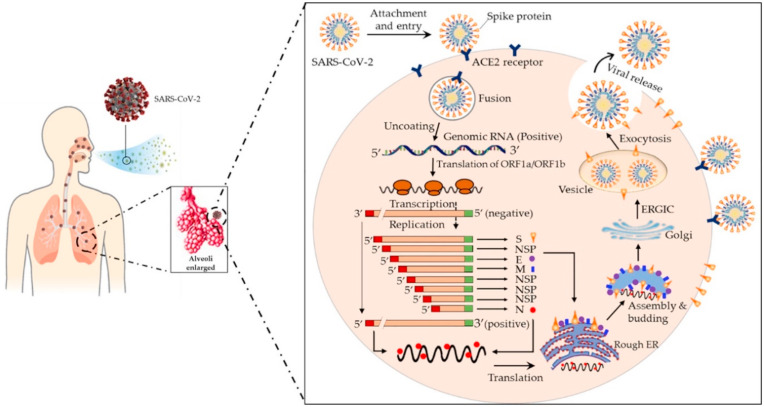
Fig. 3.
Schematic diagram of coronavirus infection. Reproduced from Ref. [12] with permission from Elsevier.
Coronavirus was first discovered in animals and birds commonly in bats and cats in mid-1960s and found to spread to human by zoonotic transmission [8,9]. The coronaviruses went unrecognised globally until the SARS-CoV pandemic emerged in 2002–03 in Guangdong China, which caused severe acute respiratory syndrome (SARS) infecting more than 8000 people with 800 deaths, followed by Middle east respiratory syndrome (MERS-CoV) which emerged in 2012 in Saudi Arabia infecting nearly 2300 people with 850 deaths [10,11]. At the end of December 2019, a novel coronavirus infected several numbers of people in Wuhan, China. On January 2020, this novel virus was named as 2019‐nCoV officially by WHO and in the same month, several countries became the victim of this deadly infectious virus [12]. Therefore, we can say that coronavirus mediated infections occur periodically in different areas of the world. The 2019 novel coronavirus shows 88% sequence similarity with bat derived SARS-CoV and 50% similarity with MERS-CoV [13]. WHO estimated the reproductive number (R0) for SARS-CoV-2 between 2 and 2.5 higher than the SARS-CoV which is 1.7–1.9 and MERS with <1, thus a higher potential of spreading with mortality rate of COVID-19 is 2.3% which is less than that of SARS (~9.5%) and MERS (34.4%). According to various genomic and in vitro analyses, it is clear that SARS-CoV-2 shares same host cell entry receptor as SARS-CoV, i.e., angiotensin converting enzyme-2 (ACE-2) while MERS-CoV uses dipeptidyl peptidase (DPP4). Similar to earlier SARS-CoV and MERS-CoV, the novel SARS-CoV-2 infects part of lungs and thus causes bilateral peripheral pneumonia with multiple organ failures and thus has been declared more contagious than earlier SARS and alveolar MERS [[14], [15], [16]]. Table 1 includes the comparative data between SARS-CoV, MERS-CoV and SARS-CoV-2 in terms of their origin, host, transmission, symptoms, incubation period, recovery, etc.
Table 1.
| SARS-CoV | MERS-CoV | SARS-CoV-2 | |
|---|---|---|---|
| Origin | Southern China | Saudi Arabia | Wuhan, China |
| Host | Palm civets | Camel | Not known |
| Transmission | Through close contacts between human to human | Touching infected camels or consuming their milk or meat | Human to human transmission through close contacts |
| Symptoms (major) | Fever, dry cough, headache, difficulty in breathing, muscle aches, loss of appetite, diarrhoea | Fever, chills, diarrhoea, nausea, vomiting, sneezing, congestion, sore throat | Fever, fatigue, cough, shortness of breath |
| Incubation Period | 2–7 days | 5–6 days | 2–14 days |
| Recovery | 5–6 weeks | 6–7 weeks | 2–8 weeks |
| Treatment | No vaccines available, antiviral medicines | No vaccines available, only drugs for symptoms are available | No vaccines available, only existing drugs are in use for symptoms |
| Countries effected | 29 | 26 | >140 |
| Fatality Rate | 9.5% | 34.4% | 2.3% |
1.1. Family of coronaviruses
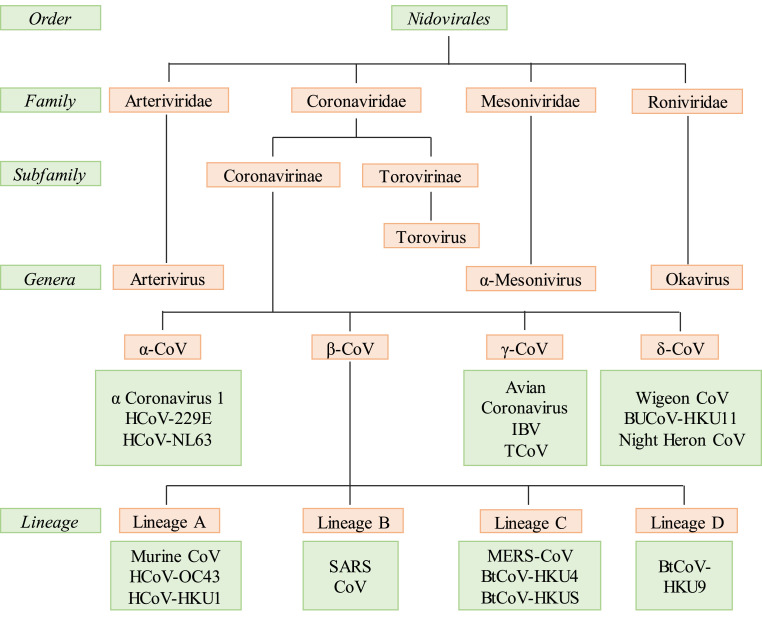
According to International Committee on Taxonomy of Viruses, coronaviruses come under subfamily of Coronavirinae from the family Coronaviridae and order Nidovirales (Fig. 1 ) [19]. The Coronavirinae subfamily is further divided into four genera, i.e., alpha-coronavirus, beta-coronavirus, gamma-coronavirus, and delta-coronavirus [20]. First two infect only mammals and rest infect birds and in some case mammals too. The alpha-coronaviruses include human coronavirus NL63 (HCoV-NL63), porcine transmissible gastroenteritis coronavirus (TGEV), PEDV, and porcine respiratory coronavirus (PRCV), whereas the beta-coronaviruses include SARS-CoV, MERS-CoV, bat coronavirus HKU4, mouse hepatitis coronavirus (MHV), bovine coronavirus (BCoV), and human coronavirus OC43. The gamma-and delta-coronaviruses include avian infectious bronchitis coronavirus (IBV) and porcine delta-coronavirus (PdCV), respectively [21]. The alpha-coronaviruses and beta-coronaviruses result into pulmonary illness in humans and cholera infantum in animals [22]. MERS-CoVs are deadly pathogenic viruses causing serious respiratory problem in humans, while other viruses like SARS-CoV and HCoV- NL63, HCoV-229E, HCoV-OC43 and HKU1 do not result into critical conditions compared with the earlier. Domestic animals are the sources for transmission of these viruses from natural host to humans [23]. Data collected from various databases show that all the human coronaviruses originated from animals such as SARS-CoV, MERS-CoV, HCoV-NL63 and HCoV-229E originated from bats [24]. These studies concluded that bats are mainly responsible for transmission of these. SARS-CoV-2 also has been found to be originated from bats and further spread to human by zoonotic transmission according to reports published [25].
Fig. 1.
Family of coronaviruses.
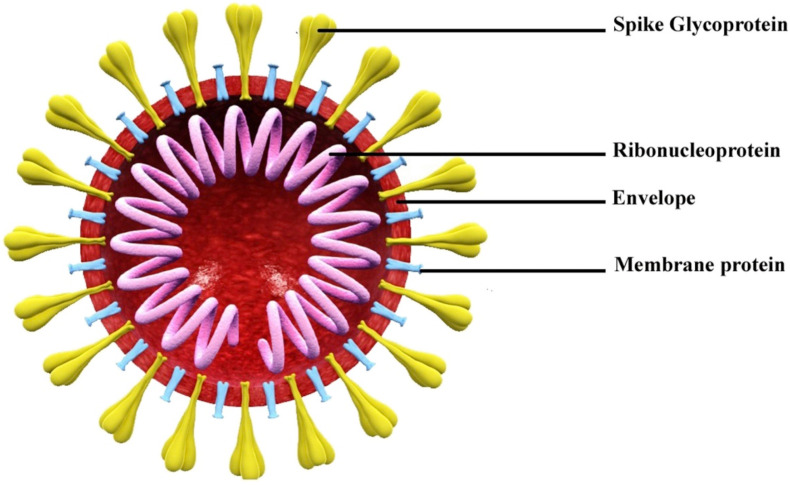
The SARS-CoV-2 is a single stranded, enveloped, large positive sense RNA virus with a size of nearly 125 nm and genome size of 27–32 kb [17]. The virus contains four structural proteins i.e., envelope protein (E), membrane protein (M), spike protein (S) and nucleocapsid protein (N) (Fig. 2 ). Viral genome is packed within nucleocapsid (N) with a shell of envelope outside [26]. This envelope is associated with other three structural proteins (M, S, and E) having different functions; the membrane protein is a transmembrane glycoprotein which helps the virus in developing capsid and structure assembly whereas envelope protein is involved in virus packaging and spike protein helps entry into host cells [27]. The spikes are responsible for the formation of large protrusion and thus gives them the shape of crown and hence the name corona meaning crown in Latin [28].
Fig. 2.
Schematic diagram of SARS-CoV-2 structure. Reproduced from Ref. [29] with permission from Elsevier.
Currently there is no specific treatment for this deadly infectious disease except quarantine and symptomatic treatment protocol for disease management [29]. As it is clear from different reports that the sequence homology of SARS-CoV and SARS-CoV-2 is nearly similar but the transmission efficiencies are different which are probably due to changes in the nucleotide sequences in spike protein (S) and receptor binding domain [30]. Medical practitioners are using existing drugs for controlling the symptoms and a community of researchers globally are involved in developing new drug modalities and vaccines since past 8 months, however no success has been met so far and it may still take months to years in developing the same [31]. Considering the high virulence of SARS-CoV-2, it is imperative to develop new preventive or therapeutic agents at the earliest to control the current pandemic situation. Bioactive molecules from plants have served as invaluable sources of different therapeutic drugs and are being used in the treatment of various diseases around the world. Medicinal plants are also being explored in management of COVID-19 pandemic in two ways, i.e., either as immunomodulators or viral target inhibitors based on their established roles in managing other viral diseases. The secondary metabolites and other chemical compounds from plants could prove as promising therapeutic agents as antiviral either by inhibiting the viral replication or viral cell entry and/or otherwise treating the underlying infection caused by SARS-CoV-2 and thus could prove a safer and innovative treatment for this viral infectious disease [[32], [33], [34], [35]].
This review has thus been planned to list the potential phytomoieties and their plant sources which can be used as inhibitor molecules targeting either the host cell or SARS-CoV-2 therapeutic receptors in providing a safer and long-term solution for the population with lesser side effects.
1.2. Coronavirus infection mechanism
The SARS-CoV-2 share some sequence homology with SARS-CoV but show different transmission potential and infection range [36]. The ongoing COVID-19 disease is seen as more treacherous with still no treatment and could be potentially due to certain functional mutations occurring in the SARS-CoV-2. The major differences observed in SARS-CoV-2 are absent 8a, longer 8b and shorter 3b segments and different Nsp-2 and -3 proteins. Along with these, the open reading frames are also different in some places like in orf8 and orf10 [17]. The process of infection is totally dependent on the interaction between virus and the host cell and begins when the viral particles get attached to the cell surface receptor of the host cell and it delivers the nucleocapsid inside the target cell membrane. The main function is provided by S-protein in binding and fusion with the host cell membrane. SARS-CoV-2 binds with the same receptor ACE-2 present in respiratory epithelium and alveoli of lungs, as SARS-CoV. Fusion takes place because of gain of conformational changes by the spike protein and the process depends upon two factors i.e., pH acidification and proteolytic activation [[36], [37], [38], [39]] (Fig. 3 ). The S-protein is homotrimeric with each subunit having two domains S1; the receptor binding domain and S2; the membrane fusion domain enabling the virus to enter the host cell. After fusion, the cleavage of fusion protein is the prime event for further process of infection and it is the main characteristic of class I viral fusion proteins [40].
S protein is cleaved by proteases into two domain S1 and S2, of which S2 domain is responsible for entering fusion protein inside the membrane of host cell and ultimately facilitating the entry of virus into the cell [17,36]. Unlike SARS-CoV-2, some coronaviruses like MHV-2 and SARS-CoV do not need cleavage of spike protein for entering purpose, they can directly enter inside the cell with the presence of exogenous proteases and this process is 100–1000 fold efficient than the endosomal pathway. Proteases like transmembrane protease/serine family (TMPRSS) and human airway trypsin like protease (HAT) both induce the fusion of SARS-CoV-2 [41,42]. Once the fusion occurs and fusion protein enters the host cell, ACE-2 gets cleaved and it is covered by ADAM17 in extra membranal space which convert angiotensin I to angiotensin II, a negative regulator of renin angiotensin pathway and this reduced ACE-2 is responsible for respiratory injury and illness [43]. Once viral protein gets translated into the cell, various pathways gets activated and are associated with spreading of infection. An ORF3a protein code for Ca+2 ion channel and activates transcription of NF-kB pathway by interacting TRAF3. The ORF8b protein activates the inflammasome pathway through NLRP3. All these pathways result in overproduction of cytokines and thus cause respiratory problems. JNK pathway is also activated by ORF3a, ORF3b and ORF7a which is responsible for lung disorders [17,44,45]. The viral infection can be minimized if the virions do not bind to the cell and this can be done by inactivating the virus. SARS-CoV-2 binds more strongly to the receptor ACE-2 than the SARS-CoV and therefore infection transmission rate and virulence is much higher than the SARS-CoV [46]. TMPRSS2 associated with ACE-2 is responsible for spreading the infection at initial stages and thus could be another therapeutic target for the new drug discovery [47].
1.3. Therapeutic targets
Currently there are no approved specific treatments or drugs to treat COVID-19 except the clinical management such as infection prevention, control measures and life supportive care [48]. The prevailing conditions of current pandemic such as re-emergence of infection cases, uncontrolled transmission and morbidity associated with the disease are crippling the healthcare systems with grave effects on the global economies and posing a challenging situation for health practitioners and scientists to identify the therapeutic strategies to handle this problem [49]. Therapeutic repurposing of existing drugs like anti-malarials, antivirals, and NSAIDs etc., has been used as an effective approach in management of COVID-19 with drugs inhibiting either the different viral receptors/enzymes or the host cell receptors. Identification of drug targets (viral or host cell) remains one of the important criteria for the discovery of new molecules as well as to check the therapeutic efficacy of existing drug molecules [13,50]. The below section briefly enlists the potential therapeutic targets along with the current drugs repurposed towards these targets.
1.4. Angiotensin converting enzyme-2 (ACE-2)
ACE-2 is the functional receptor of SARS-CoV-2 and is a negative regulator of renin angiotensin system (RAS) [51]. The S-proteins of SARS-CoV-2 get attached with ACE-2 receptor of the host cell in a much stronger manner compared to the SARS-CoV thus producing higher pathogenicity [52]. Many reports have suggested that ACE-2 could be one of the possible COVID-19 targets as it can block the binding of the virus with the host cell and thus blocking the viral cell entry. Therapeutic options for blocking the viral entry utilizing neutralizing antibodies such as soluble receptor binding domain that could occupy ACE-2 and soluble ACE-2 are envisaged as possible viral trap and thus inactivators of SARS-CoV-2 [53]. Despite the initial controversy on use of ACE inhibitors and angiotensin receptor blockers (ARBs) in hypertensive COVID-19 patients, several clinical findings have recommended their continued use during infection treatment as these drugs possess anti-inflammatory activities as well as inhibit platelets aggregation and thus preventing clot formation seen in COVID-19. Besides synthetic products, some natural products such as amentoflavone, chrysanthemin, and biovobin have shown good binding affinity towards ACE-2 [54,55].
Choroquine (CQ), a well-known anti-malarial drug has been explored for prophylactic and therapeutic treatment in current pandemic due to multiple roles in inhibiting the viral life cycle [56]. CQ inhibits the viral infection by increasing the endosomal pH value required for the fusion of virus and host cell. Treatment with CQ has shown improved pulmonary abrasion with healthy outcomes. It is also reported that high dose of CQ will cause cardiac risks and its safety and effectiveness is not investigated till now [[57], [58], [59]]. Hydroxychloroquine (HCQ) shares the similar structure and antiviral activity with CQ and was looked upon as a better and safer treatment for COVID-19. Both are responsible for inhibiting the spike protein attachment with ACE-2 receptor in the host cell and also interfere with the glycosylation process of ACE-2 of host cell. CQ and HCQ decreased the cytokine storm, in COVID 19 patients. It disrupts the DNA/RNA interaction and reduces the expression of pro inflammatory genes [60,61]. However, WHO halted the use of combination of HCQ and lopinavir/ritonavir after their failure to reduce the mortality rate [62,63].
1.5. Transmembrane protease serine 2 (TMPRSS2)
Along with ACE-2, TMPRSS2 is also responsible for virus entry into the host cells by activating the viral S-proteins [36]. TMPRSS2 cuts the S-glycoprotein into two-part S1 and S2. Both subunits function differently; S1 binds with host cell surface receptor and S2 helps in fusion of host and viral cell. It is pertinent to note that spike protein activity is one of the main reasons for the viral pathogenicity and virulence [64]. Various studies have shown that inactivating the TMPRSS2 could facilitate inhibition of the viral infection. Camostat mesylate is an example of FDA approved serine TMPRSS2 inhibitor used in other diseases, and is under clinical trials for COVID-19 and will take several months for clinical validation [65].
1.6. RNA dependent RNA polymerase (RdRp)
Nsp12 or RdRp is the main conserved protein present in coronavirus which is responsible for viral replication and transcription. It is a complex machinery, where Nsp12 binds with nsp7 and nsp8 (cofactors) to produce viral genomic RNA [66]. SARS-CoV shares the same structure of Nsp12 with the SARS-CoV-2. It was observed that Nsp12 was the potential drug target with much safer profile and less toxicity for SARS-CoV and MERS-CoV [52]. Currently, remdesivir (antiviral drug) that targets RdRp activity is under clinical trial and also been used in severely ill-terminal COVID19 patients [67]. Docking-based screening studies have found some natural compounds targeting RdRp including nympholide A, viniferin and theaflavin [55,68].
1.7. 3CL protease
Main protease Nsp5, is also known as 3C like protease which first auto cleaved from the poly proteins and then thereafter cleaves the nsps at 11 sites, i.e., nsp4-nsp16, an essential step in the mechanism of viral infection [69]. This is the main protease hence sometimes called as Mpro and it shares almost 96% homology with SARS-CoV. Difference in structure arises in the dimer interface where polar amino group is replaced with nonpolar groups and hence catalytic activity of dimer increases [70]. MPro is responsible for viral replication and transcription and considered as an important therapeutic target for COVID-19 [71]. A combination strategy using lopinavir-ritonavir; the protease inhibitors was tested against COVID-19, however no benefits was observed and it was observed to cause several side effects like diarrhoea, nausea, asthenia etc., but in some regions of South Korea, this combination was found to be effective, however the testing was done only in one patient [72,73]. Thus, clinical study with large cohort of population is needed to establish the importance of this protease as drug target for COVID19.
1.8. Papain-like proteinase (PLpro)
PLpro is a 213 kDa multidomain polypeptide which cleaves the N-terminal region of polyprotein and releases nsp1, nsp2 and nsp3 required for correction of viral replication. Along with having role in viral replication, it has other important functions of stripping ubiquitin, deISGylation from proteins of host cell for viral immune evasion processes [74]. It is also considered as a promising therapeutic target that not only inhibits viral replication but also saves other cells from being infected by dysregulation of signalling cascades in the infected cells. There is a list of FDA approved drugs for various other diseases that specifically binds with viral PLpro effectively, as observed by virtual screening, however needs in vitro, in vivo and clinical validation to establish their effectiveness and clinical use [75,76].
1.9. Cathepsin
Like viral protein processing, cellular machinery of virus is also very important and could be a potential therapeutic target [77]. Keeping in view that SARS-CoV-2 shares similar viral entry mechanism with SARS-CoV, hence similarities in their cellular machinery such as endocytic mechanism for entering into cell could been visaged. Cathepsin is the lysosomal protease that has role in cellular protein turnover [78]. According to various studies, cathepsin B has important role in life cycles of coronavirus, Nipah virus, Ebola virus, influenza virus etc., and helps the virus to enter into cytoplasm through endosomes by fusing with viral envelope and endosomal membrane [79]. The most common pathway of viral internalization is clathrin mediated endocytosis as seen in the life cycles of various viruses including coronavirus [80,81]. Cathepsin L is also a promising target in coronavirus as proved by different studies, and therefore it could be a beneficial target for inhibiting SARS-CoV-2. Drug chlorpromazine is used to inhibit clathrin mediated endocytosis in Ebola virus and is being tested for SARS-CoV-2 too [82]. There are several other drugs available to target cathepsin but so far, all are under clinical trials [83].
Table 2 summarizes the various repurposed drugs alongwith their SARS-CoV-2 therapeutic targets.
Table 2.
List of repurposed drugs under clinical trial for COVID-19.
| Drugs | Type | Target | Status | Refs. |
|---|---|---|---|---|
| Camostat mesylate | Serine protease inhibitor | TMPRSS2 | Under phase 1 and 2 of clinical trials | [73] |
| Nafamostat mesylate | Serine protease inhibitor | TMPRSS2 | Under phase 2 and 3 of clinical trials | [73] |
| Chloroquine phosphate | Antimalarial | ACE-2 | Under phase 2 of clinical trials | [73] |
| Hydroxy-chloroquine | Antimalarial | Endosome, pH elevation | Still in Controversy | [73] |
| Remdesivir | Antiviral | RdRp | Under clinical trials | [73] |
| Umifenovir | Antiviral | Membrane fusion, clathrin-mediated endocytosis | Under phase 4 of clinical trials | [73] |
| Favipiravir | Antiviral | RdRp | Inconsistent results in clinical trials | [73] |
| Lopinavir | Protease inhibitor | 3clpro, plpro | Inconsistent results in clinical trials | [63] |
| Ribavirin | Antiviral | RdRp | Under phase 3 of clinical trials | [63] |
| Arbidol | Antiviral | ACE2 | Under clinical trials | [63] |
| Baricitinib | Rheumatoid arthritis | JAK kinase | Under clinical trials | [63] |
| Ritonavir | Antiviral | Protease inhibitor | Inconsistent results in completed clinical trials | [61] |
| EIDD-2801 | Antiviral | Viral replication | Prepared for clinical trials | [61] |
| Tociliczumab | mAb | IL-6 pathway | Under phase 3 of clinical trials | [61] |
| Sarilumab | mAb | IL-6 pathway | Under phase 3 of clinical trials | [61] |
| Bevacizumab | mAb | VEGF pathway | Under clinical trials | [61] |
| Vitamin C | Dietary supplements | Boost immunity | Under phase 2 of clinical trials | [61] |
| Vitamin D | Dietary supplements | Boost immunity | Under phase 2 of clinical trials | [61] |
| Azithromycin | Antibiotic | mRNA translation | Under phase 4 of clinical trials | [61] |
| Corticosteroids | Corticosteroids | Dampen pro-inflammatory cytokines and possess antifibrotic property | Still in controversy | [61] |
| Clevudine | Antiviral | Blocks DNA supply of virus to nucleus | Under phase 2 of clinical trials | [13] |
| Isotretinoin | Retinoid | Against PLPro | Under phase 3 of clinical trials | [13] |
| Ivermectin and Nitazoxanide | Antiparasitic | Inhibit import in alpha/beta receptor | Under phase 2 and 3 of clinical trials | [13] |
| Deferoxamine | Chelating agents | Inhibit IL6 synthesis through decreasing NF-Kb. | Under phase 1 and 2 of clinical trials | [13] |
| Dexamethasone | Steroid | Anti-inflammatory action | Under phase 4 of clinical trials | [13] |
| Piclidenoson | A3 adenosine receptor agonist | Inhibit cytokine storm | Under phase 2 of clinical trials | [13] |
| Tranexamic acid | Antifibrinolytics | Inhibit conversion of plasminogen to plasmin | Under phase 2 of clinical trials | [13] |
| BLD-2660 | Antiviral | Targets IL6 | Under phase 2 of clinical trials | [13] |
| Sildenafil citrate | Phospho-diesterase inhibitor | Dilates blood vessles | Under phase 3 of clinical trials | [13] |
| Losartan | Angiotensin II receptor antagonist | Targets ACE2 | Under phase 1 of clinical trials | [13] |
| Telmisartan | Antifibrotic | Angiotensin receptor blocker | Under phase 2 of clinical trials | [13] |
| Atorvastatin | Statin | Target NF-kB | Under phase 2 of clinical trials | [13] |
| Prazosin | Alpha-blockers | Prevent cytokine storm | Under phase 2 of clinical trials | [13] |
| Chlorpromazine | Antipsychotics | Inhibit viral replication | Under phase 3 of clinical trials | [13] |
| Lenalidomide | Antiangiogenic agent | For multiple myeloma patients | Under phase 4 of clinical trials | [13] |
| Ruxolitinib | Anti-inflammatory | Targets cytokine storm | Under phase 3 of clinical trials | [13] |
1.10. Therapeutic potential of phytoconstituents against coronaviruses
During this pervasive situation, world is facing unhealthy circumstances impeding the normal lifestyle. It is very much necessary to develop drugs or vaccine for this current deadly infectious disease at the earliest, but unfortunately there are no FDA approved drugs available in the market and development of new therapeutic moieties and vaccine remains a costly and time-consuming affair with high failure chances too [12]. Keeping that in mind it is very crucial to use alternate options to combat the current situation. Medicinal plants offer the most common treatment options since ancient times against various viral diseases because of their safer, cheaper and less toxicity profile [84]. The National Health Commission of China suggested traditional Chinese medicine as an alternative defence treatment option [85]. From literature studies it was seen that phytoconstituents such as polysaccharides, triterpenes, phenolic acids, alkaloids, proanthocyanidins and anthraquinones etc., possessed anti-viral activity against rabies virus, HIV, Chandipura virus, Japanese Encephalitis Virus, Enterovirus, Influenza A/H1N1 and other influenza viruses, and SARS [[86], [87], [88], [89]]. Table 3 encompasses a list of different phytoconstituents which have shown anti-viral activity against different types of coronaviruses.
Table 3.
Phytoconstituents and their plant sources having antiviral potential against different types of coronaviruses.
| Compound (source) | Structure | Strain | Targets | Refs. |
|---|---|---|---|---|
| Flavonoids | ||||
| Luteolin (Galla Chinensis) | 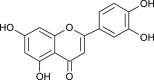 |
SARS-CoV BJ01 | Binds with S2 subunit | [90] |
| Myricetin | 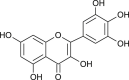 |
SARS-CoV | Inhibit ATPase activity | [91] |
| Procyanidin A2 (Cinnamomi sp.) | 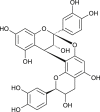 |
SARS-CoV PUMC01 F5 | Targets clathrin dependent endocytosis pathway | [92] |
| Scutellarein (Scutellaria barbata) | 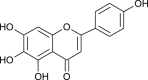 |
SARS-CoV | Inhibit ATPase activity | [11] |
| Amentoflavone (Torreya nucifera) | 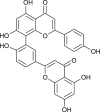 |
SARS-CoV | Targets CLPro | [93] |
| Bilobetin (Torreya nucifera) | 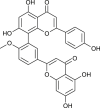 |
SARS-CoV | Targets CLPro | [93] |
| Ginkgetin (Torreya nucifera) | 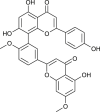 |
SARS-CoV | Targets CLPro | [93] |
| Bavachinin (Psoralea corylifolia) | 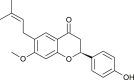 |
SARS-CoV | Targets PL Pro | [94] |
| Neobavaisoflavone (Psoralea corylifolia) | 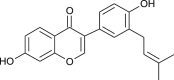 |
SARS-CoV | Targets PL Pro | [94] |
| Tomentin A (Paulownia tomentosa) | 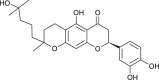 |
SARS-CoV | Inhibits PL Pro | [95] |
| Tomentin B (Paulownia tomentosa) | 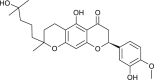 |
SARS-CoV | Inhibits PL Pro | [95] |
| Tomentin C (Paulownia tomentosa) | 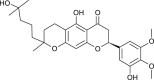 |
SARS-CoV | Inhibits PL Pro | [95] |
| Tomentin D (Paulownia tomentosa) | 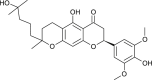 |
SARS-CoV | Inhibits PL Pro | [95] |
| Tomentin E (Paulownia tomentosa) | 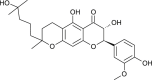 |
SARS-CoV | Inhibits PL Pro | [95] |
| Hesperetin |  |
SARS CoV | Against 3CL Pro | [96] |
| Phenolic compounds | ||||
| Kazinol F (Broussonetia papyrifera) |  |
MERS-CoV | Targets PL pro | [97] |
| Broussochalcone A (Broussonetia papyrifera) | 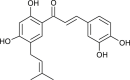 |
MERS-CoV | Targets PL pro | [97] |
| Caffeic acid (Sambucus formosana) | 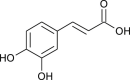 |
HCoV-NL63 | Inhibit cell docking | [11] |
| Isotheaflavin-3-gallate (Camellia sinensis) | 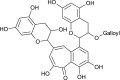 |
SARS-CoV1 | Against 3CL Pro | [98] |
| Dieckol (Ecklonia cava) | 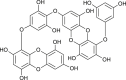 |
SARS CoV | Against 3CL Pro | [99] |
| Terrestrimine (Tribulus terrestris) | 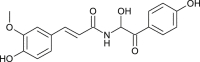 |
SARS CoV | Against PL Pro | [100] |
| Xanthones | ||||
| Blanco-xanthone (Calophyllum blancoi) | 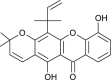 |
HCoV 229E | Against Protease | [101] |
| Pyrano-jacareubin (Calophyllum blancoi) | 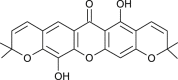 |
HCoV 229E | Against Protease | [101] |
| Anthraquinones | ||||
| Emodin (Rheum officinale) | 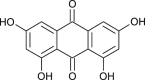 |
SARS-CoV | Inhibit binding of S-protein to ACE2 | [102] |
| Coumarins | ||||
| Psoralidin (Cullen corylifolium) | 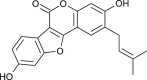 |
SARS CoV | Against PL Pro | [94] |
| Tannins | ||||
| Tannic acid (Camellia sinensis) | 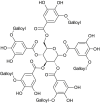 |
SARS- CoV1 | Against 3CL Pro | [16] |
| Terpenoids | ||||
| β-Ocimeni (Laurus nobilis) |  |
SARS-CoV FFM1 | Inhibit viral replication | [103] |
| β-Penene (Laurus nobilis) | 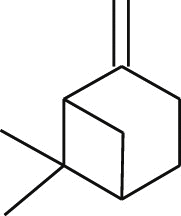 |
SARS-CoV FFM1 | Inhibit viral replication | [103] |
| Tanshinone I (Salvia miltiorrhiza) | 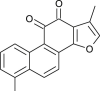 |
SARS-CoV | Targets Protease | [104] |
| Tanshinone IIA (Salvia miltiorrhiza) | 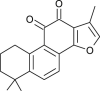 |
SARS-CoV | Targets Protease | [104] |
| Tanshinone IIB (Salvia miltiorrhiza) | 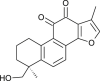 |
SARS-CoV | Targets Protease | [104] |
| Celastrol (Tripterygium regelii) | 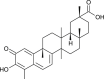 |
SARS-CoV | Targets protease | [93] |
| Leukamenin (Lactuca sativa) | 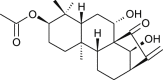 |
SARS-CoV1 | Protease inhibitor | [105] |
| Glaucocalyxin (Lactuca sativa) | 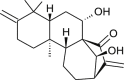 |
SARS-CoV1 | Protease inhibitor | [105] |
| Pseurata (Lactuca sativa) | 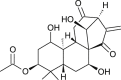 |
SARS-CoV1 | Protease inhibitor | [105] |
| Pristimerin (Celastrus orbiculatus) | 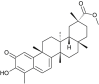 |
SARS-CoV1 | Targets Protease | [35] |
| Tingenone (Celastrus orbiculatus) | 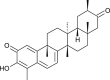 |
SARS-CoV1 | Targets 3CL Pro | [35] |
| Iguesterin (Celastrus orbiculatus) | 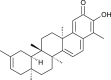 |
SARS-CoV1 | Targets 3CL Pro | [35] |
| Friedelanol (Euphorbia neriifolia) | 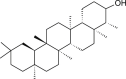 |
HCoV-229E | Antiviral activity | [106] |
| Friedelin (Euphorbia neriifolia) | 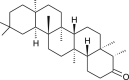 |
HCoV-229E | Antiviral activity | [106] |
| Epitaraxerol (Euphorbia neriifolia) | 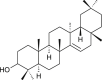 |
HCoV-229E | Antiviral activity | [106] |
| Tingenone (Tripterygium regeli) | 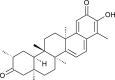 |
SARS CoV | Against 3CL Pro | [93] |
| Iguesterin (Tripterygium regeli) | 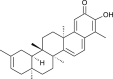 |
SARS CoV | Against 3CL Pro | [93] |
| Pristimererin (Tripterygium regeli) | 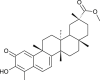 |
SARS CoV | Against 3CL Pro | [93] |
| Dihydrotanshinone I (Salvia miltiorrhiza) | 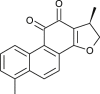 |
SARS CoV | Against 3 CL Pro and PL Pro | [107] |
| Cryptotanshinone (Salvia miltiorrhiza) | 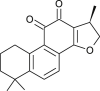 |
SARS CoV | Against 3 CL Pro and PL Pro | [107] |
| Alkaloids | ||||
| Tryptanthrin (Strobilanthes cusia) | 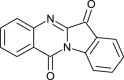 |
HCoV-NL63 | Targets papain like protease | [108] |
| Indigodole B (Strobilanthes cusia) | 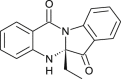 |
HCoV-NL63 | Targets papain like protease | [108] |
| Tetrandrine (Stephania tetrandra) | 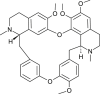 |
HCoV-OC43 | Inhibit viral replication | [109] |
| Fangchinoline (Stephania tetrandra) |  |
HCoV-OC43 | Inhibit viral replication | [109] |
| Cepharanthine (Stephania tetrandra) |  |
HCoV-OC43 | Inhibit viral replication | [109] |
| Fatty acids | ||||
| Isolinoleic acid (Mucuna pruriens) |  |
SARS-CoV1 | Targets Protease | [35] |
| Steroidal derivatives | ||||
| Saikosaponin B2 (Bupleurum sp.) |  |
HCoV-229E | Targets viral replication | [110] |
| β-Sitosterol (Isatis indigotica) | 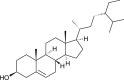 |
SARS CoV | Against 3CL Pro | [107] |
| Flavaglines | ||||
| Silvestrol (Algaria sp.) | 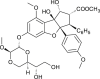 |
MERS-CoV | Targets RNA helicase | [111] |
| Glucosides | ||||
| Sinigrin | 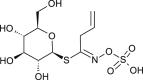 |
SARS CoV | Against 3CL Pro | [107] |
| Chalcones | ||||
| Xanthoangelol (Angelica keiskei) | 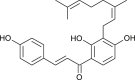 |
SARS CoV | Against 3 CL Pro and PL Pro | [112] |
| Diarylheptanoids | ||||
| Hirsutenone (Alnus japonica) |  |
SARS CoV | Against PL Pro | [113] |
| Lectins | ||||
| Agglutinin (Urtica dioica) | - | SARS-CoV | Targets viral replication | [114] |
| Griffithsin (Griffithsia sp.) | - | SARS-CoV | Against glycoprotein spike | [115] |
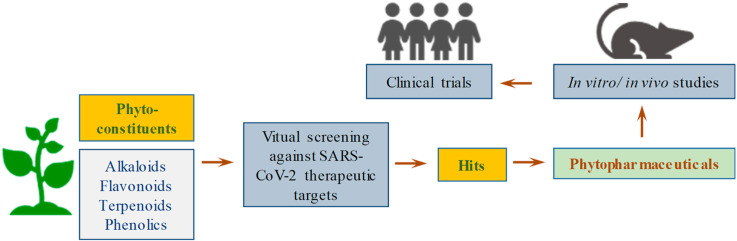
2. Various phytoconstituents that are being studied for the treatment of COVID-19 fall under the following classification
2.1. Flavonoids
Flavonoids are polyphenolic compounds present in plants and possess different biological functions [116]. Many flavonoids are seen having effective role against viral infections especially at the molecular level for inhibiting the growth of virus [117]. Flavonoids block the entry of virus into the cell by blocking the cellular receptors and also interfere with viral replication and translation [118]. Considering the important role played by Mpro enzyme in viral replication and translation, it can be used as one of the potential targets for COVID-19. Different virtual screening studies carried against Mpro enzyme have proposed several flavonoids like azithromycin, mangiferin, procyanidin-β-2,7-dimethoxyflavaN-4′-O-β-d-glucopyranoside, amentoflavone, hidrosmin, diosmin, gallocathechin gallate, elsamitrucin, pectolinaren, quercetin and isoquercetin having high binding affinities and thus could be used to combat the current situation. Along with the Mpro, ACE-2 is also a potential therapeutic target and flavonoids including hesperetin, myricetin, linebacker and caflanone have shown high binding affinity against ACE-2 making them important points of study against SARS-CoV-2 [119,120].
2.2. Alkaloids
Alkaloids are naturally occurring organic compounds containing basic nitrogen atoms [121]. Alkaloids were found to have key roles in inhibition of viral replication as it blocks the function of viral DNA polymerase [122]. As these plant's secondary metabolites have DNA intercalating properties, they may be effective candidates against viral infection and could lead to development of drug molecules. Resoquine, synthetic derivative of quinine (alkaloid), an antimalarial drug has been found as an effective therapeutic agent against COVID-19 [123]. Isoquinoline was effective against spike protein and nucleocapsid protein of SARS-CoV-OC43 in human lung cells suggesting that this could serve as a drug candidate against SARS-CoV-2. Some alkaloids which are having DNA intercalating activity are sanguinarine, quinine, cinchonine, hartmine, chelidonine, coptisine, berberine, palmatin, tetradine etc., and could be used for development of drug molecules [124,125]. Emetine isolated from ipecacuanha root for induction of vomiting in the poisoning management has been found as effective against COVID-19 [126].
2.3. Phenolics
Phenolics are naturally occurring plant polyphenolic compounds having one or more hydroxyl groups which bind with aromatic hydrocarbon group. They possess the chemical properties where hydroxyl group dissociates under various physiological conditions [127]. Phenolic compounds carrying five or more hydroxyl group along with methoxyl group have antiviral properties [128]. Plants like Euphorbia spindens and Bombax malabaricium were tested for anti-HSV and anti-RSV respectively and were effective like ribavirin (antiviral) synthetic drugs [129]. Phenols help in inhibiting the fusion of virus to the host cell by binding with the viral protein present in the viral envelope. Since most of the polyphenols are polar molecules and hence cannot be taken up by the host cells and thus may prove as good candidates against COVID-19 [123,130,131].
2.4. Essential oils
Essential oils are the volatile concentrated hydrophobic liquids extracted from plants. Essential oils are extensive used in phytomedicine and aromatherapy and also in pharmaceuticals. They possess different biological functions like antibacterial, antifungal, antiviral, antioxidant, anti-inflammatory, anticancer etc. [132]. Several reports described the effective antiviral activity of essential oils on influenza virus, herpes simplex virus etc. A mixture of essential oil and oleoresins from medicinal plants was also tested for coronavirus, bronchitis virus etc. [133]. Essential oil easily interacts with phospholipid bilayer of the cell membrane and it disrupt the viral envelope, thus starting their activity prior to host cell attachment.
Lypophillic essential oils work on membrane viral proteins required for fusion but its activity stops once the virus enters inside the cell [[134], [135], [136], [137]]. Recently a team of scientists tested cannabis terpenes for antiviral activity against COVID-19 and the terpenes were found to reduce the severity of SARS virus by interruption with RNA replication proteins thus interfering with viral penetration in healthy cells [124]. Various essential oils have proved to be effective against various types of viruses which includes isoborneol, a monoterpenene, having antiviral effect against HSV1. Similarly, eugenol delayed the growth of Herpes virus and also interferes with HSV, while beta-caryophyllene was found to be effective against dengue virus [138,139]. Vanguard Scientific recently launched an effective hand sanitizer having plant derived terpenes [140]. Essential oils thus constitute a major group of phytochemicals which are needed to be investigated thoroughly for the finding of new effective treatment against the COVID-19 severity.
2.5. Stilbenes
Stilbenes are naturally occurring defensive phenolic compounds found in grapes, berries, bark waste etc., also classified as phytoalexins as they are synthesized by plants in response to UV radiations. Stilbene have many biological functions like antioxidant, anticancer, anti-inflammatory, antileukemia, anti-HIV, anti-Herpes simplex virus, etc. [141,142]. Different virtual screening studies suggested the use of stilbenes in COVID-19 as they inhibit the complex formed between spike protein and ACE-2 receptor by disrupting the interface of S-ACE-2 complex and thus blocking the entry to the host cell. Stilbenes having high binding affinity to this complex include resveratrol and piceatannol. Some other derivatives of stilbenes like trans-resveratrol, pinosylvin, pterostilbene also have antiviral properties but with low binding capacity than mentioned previously [[143], [144], [145]]. Resveratrol has been already tested for MERS-CoV and was found effective as it decreases the cell death [146]. Based on these studies, it is suggested that stilbenes derivatives, especially resveratrol could be very important drug candidate for COVID-19.
2.6. Glycosides
Glycosides are simple sugar molecules having different functional groups. Different drugs and poisons extracted from plants are glycosides and thus useful in various treatments [147]. Antiviral effects of glycosides have been observed in various studies like the cardiac glycosides were seen effective against both DNA and RNA viruses including cytomegalo virus, herpes simplex virus, influenza virus and coronavirus [148]. It targets the cell host protein and thus makes them promising strategy against human viral infections [149]. It was already seen that glycosides are effective for influenza virus as it interferes with the virus attachment and disturbs the release of new viruses by inhibiting the hemagglutinin and sialidase enzymes [150,151]. Iridoid glycosides from Fructus gardeniae are very much effective against influenza A as it reduced the cell death by suppressing influenza virus replication [152]. According to a recent report, antiviral activity of Ginsenoside Rb1 class of steroid glycosides was tested and found to be effective against SARS-CoV [153]. Thus, glycosides derivatives need detailed investigation as drug targets for minimising the current pandemic scenario.
2.7. Saponins
Saponins are non-ionic detergents which possess antifungal, cytotoxic, antibacterial and antiviral properties [154]. Saponins are used in the development of steroidal drugs and thus used in modern medicine [155]. Saponins carries antiviral activity as it can interact with viral envelope and capsid protein which results into disruption of viral particles, also it can interact with host cell membrane which inhibit the viral particle attachment to the host cells thus prevent fusion by coating the cell surface which minimizes the spreading of infections. Some study found that Quilaja saponaria extract containing triterpenoid saponins having very strong activity in both human and animal's vaccines. This extract was very effective against HIV-1 and -2 [156,157]. Similarly, triterpene saponin from Anagallis arvensis showed antiviral activity against HSV1 and poliovirus 2 [158]. Saikosaponins are triterpene saponin glycosides that have been reported having effective antiviral activity against coronavirus HCoV-229E. Saikosaponins interfere with the early stage of viral replication including adsorption and penetration. Some studies demonstrated the antiviral activity of saikosaponins on other viruses like herpes simplex virus, hepatitis B virus and cytomegalovirus and HIV [110,159]. Thus, this approach of saponins needs attention of scientists for investigation in detail for the development of effective therapeutic candidate against COVID-19.
2.8. Tannins
Tannins are naturally occurring compounds found in plants, seeds, bark, wood and fruits etc. and possess various biological properties and medical uses. The viral infections lead to the overproduction of free radicals and thus oxidative stress triggering the production of reactive oxygen and nitrogen molecules and tannins extracted from plants are very much effective against oxidative stress [160,161]. Several studies confirmed the antioxidant effect as a potential therapy against various viral infections [162]. Antiviral activity of tannins against various viruses like enterovirus, calicioviruses, rotavirus, HIV, herpes simplex virus, and coronaviruses, etc., has been well established [163]. Tannins like pedunculagin, tercatainj and punicalin can binds effectively with SARS-CoV-2 which interferes with viral binding site and destroy the protease enzymes such as His41 and Cys145 and this was studied recently by molecular docking methods [164]. A recent study from India suggested the use of tea and haritaki having therapeutic potential against COVID19 as gallotannin present in tea can interfere with 3CLPro protease activity of coronavirus, but yet clinical trials are not done [165].
2.9. Anthraquinones
Anthraquinones are naturally occurring aromatic compounds having wide range of medicinal applications such as in constipation, arthritis, multiple sclerosis, cancer and others and demonstrate low toxicity with high activity [166,167]. Currently molecular docking studies showed the effect of anthraquinones against SARS-CoV-2. Derivatives such as emodin, aloin A and B, rubiadin, aloe-emodin, pseudohypericin, damnacanthal, and chryosphanic etc., were found as inhibitors of Mpro of SARS-CoV-2 using docking studies. But the detailed in vivo and invitro studies are required to be done to develop promising drug candidate against COVID19 [[168], [169], [170]]. Emodin extracted from Rheum officinale and Polygonum multiflorum was also able to block the S-protein binding with ACE-2 receptor in SARS coronavirus [102]. Anthraquinone derivatives such as hypericin are effective against various other viruses like herpes simplex virus, vaccinia virus and parainfluenza virus [171,172].
Various computational tools showed the data of phytoconstituents from different medicinal plants against various therapeutic targets of COVID-19 and thus could be effective against this viral disease. Various types of phytoconstituents investigated through in silico docking studies in the SARS-CoV-2 are listed in Table 4 .
Table 4.
List of phytoconstituents screened via in silico studies against targets of SARS-CoV-2.
| Compound |
Structure |
Source |
Mechanism |
Refs. |
|---|---|---|---|---|
| Flavonoids | ||||
| Myricitrin |  |
Myrica cerifera | interacts with 3CLPro | [173] |
| 3,5,7,3′,4′,5′-hexahydroxy flavanone-3-O-beta-D-gluco-pyranoside |  |
Phaseolus vulgaris | protease inhibition | [173] |
| (2S)-Eriodictyol 7-O-(6″-O-galloyl)-beta-D-glucopyranoside |  |
Phyllanthus emblica | protease inhibition | [173] |
| Calceolarioside B | 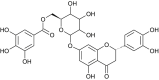 |
Fraxinus sieboldiana | interact with HIS41 and Cys145 |
[173] |
| Kaempferol | 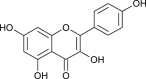 |
Securigera securidaca | PLpro & 3CLpro | [87] |
| Quercetin | 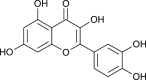 |
Withania somnifera | PLpro & 3CLpro | [35, 87] |
| Myricetin 3-O-beta-D-glucopyranoside | 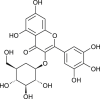 |
Camellia sinensis | 3CL Pro inhibition | [173] |
| Licoleafol | 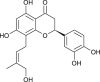 |
Glycyrrhiza uralensis | 3CL Pro inhibition | [173] |
| Taiwan-homoflavone A | 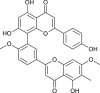 |
Cephalotaxus wilsoniana | Binds with 3CL pro and ACE2 | [55] |
| Afzelin | 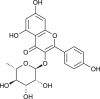 |
3CL Pro inhibition and ACE2 | [174] | |
| Isoquercitrin | 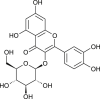 |
3CL Pro inhibition and ACE2 | [174] | |
| Amentoflavone | 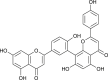 |
3CL Pro inhibition and ACE-2 | [174] | |
| Nympholide A | 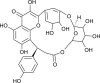 |
Aquatic plant | Mpro and RdRp | [55] |
| Biorobin | 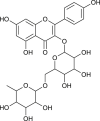 |
Acalypha indica | RdRp and hACE-2 | [55] |
| Luteolin-7-glucoside | 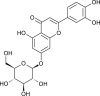 |
Olea europaea L | Mpro inhibitor | [87] |
| Naringenin | 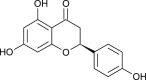 |
Citrus sinensis | Mpro inhibitor | [87] |
| Apigenine-7-glucoside | 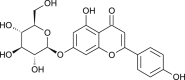 |
Averrhoa belimbi | Mpro inhibitor | [87] |
| Silybin | 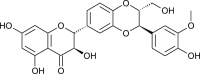 |
Silybum marianum | spike protein inhibition (TMPRSS2) | [175] |
| 5,7-Dimethoxy flavan-4-O-β-D-glucopyranoside | 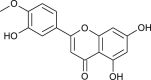 |
inhibit Mpro | [33] | |
| Baicalin | 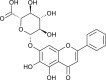 |
binds with TMPRSS2 | [174] | |
| Isoflavonoids | ||||
| 5,7,3',4'-Tetrahydroxy-2'-(3,3-dimethylallyl) isoflavone | 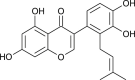 |
Psorothamnus sp. | interacts with 3CLPro | [173] |
| Daidzein | 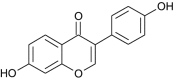 |
Cicer arietinum | Blocks HSPA5 | [68] |
| Genistein | 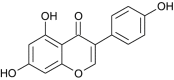 |
Cicer arietinum | Blocks HSPA5 | [68] |
| Formononetin | 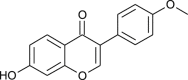 |
Cicer arietinum | Blocks HSPA5 | [68] |
| Biochanin A | 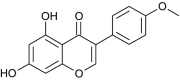 |
Cicer arietinum | Blocks HSPA5 | [68] |
| Nictoflorin | 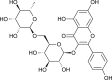 |
Nyctanthes arbortristis | interact with protease | [125] |
| Terpenoids | ||||
| Betulinic acid | 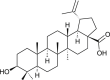 |
Chinese medicinal plant | replication &3CLpro | [11] |
| Crypto-tanshinone | 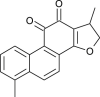 |
Chinese medicinal Plant | PLpro & 3CLpro | [11] |
| Dihydro-tanshinone | 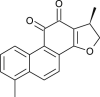 |
Chinese medicinal Plant | entry & spike protein | [11] |
| Sugiol | 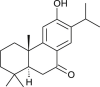 |
Chinese medicinal Plant | Replication & 3CLpro |
[11] |
| Tanshinone IIA | 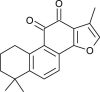 |
Chinese medicinal plant | PLpro & 3CLpro | [11] |
| Nimolicinol | 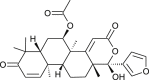 |
Azadirachta indica | protease inhibition | [35] |
| Lactucopicrin | 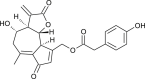 |
Lactuca virosa | 3CL Pro inhibition and ACE2 | [55] |
| Cordioside | 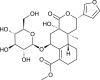 |
Tinospora cordifolia | binds with main protease | [175] |
| Vindolinine | 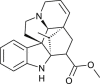 |
Catharanthus roseus | binds with NSP15 | [35] |
| Camphorating D |  |
Cinnamomum verum | blocking signalling pathway | [169] |
| Bonducellpin D |  |
inhibit Mpro | Gurung et al., 2020 | |
| Nimbin |  |
Azadirachta indica | Interact with protease | [33] |
| Alkaloids | ||||
| Desmethoxy-reserpine |  |
Chinese medicinal Plant | Replication, 3CLpro & entry | [11] |
| Moupinamide | 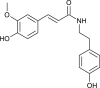 |
Piper nigru | PLpro | [11] |
| Amaranthin | 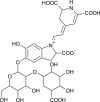 |
Amaranthus tricolor | 3CL Pro inhibition | [173] |
| Oriciacridone F | 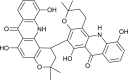 |
binds with 3CL and ACE2 | [174] | |
| Somniferine | 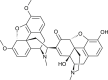 |
Withania somnifera | binds with NSP15 | [35] |
| 2,3-Dehydro-somnifericin | 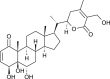 |
Withania somnifera | binds with NSP3 | [35] |
| Anaferine | 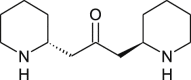 |
Withania somnifera | binds with NSP10, NSP16 | [35] |
| Berberine |  |
Tinospora cordifolia | interact with protease | [125] |
| Phenolic compounds | ||||
| Theaflavin |  |
Camellia sinensis | binds with RdRp | [11] |
| Methyl rosmarinate | 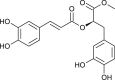 |
Hyptis atrorubens | interacts with 3CLPro | [173] |
| Coumaroyl-tyramine | 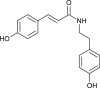 |
Chinese medicinal Plant | PLpro & 3CLpro | [11] |
| Lignan | 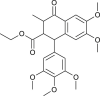 |
Chinese medicinal Plant | replication & 3CLpro | [11] |
| Demethoxy-curcumin | 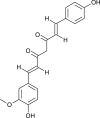 |
Curcuma longa | Mpro inhibitor | [87] |
| Aloenin | 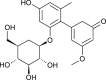 |
Aloe Vera | interact with protease | [125] |
| Gingerol | 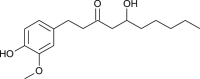 |
Zingiber officinale | Interact with protease | [125] |
| Steroidal compounds | ||||
| 27-Deoxy-14- hydroxy withaferin A | 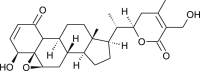 |
Withania somnifera | protease inhibition | [35] |
| 27-Hydroxy withanone | 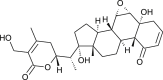 |
Withania somnifera | interferes with Spike protein | [35] |
| 12-Deoxy witha-stramonolide | 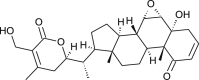 |
Withania somnifera | interferes with Spike protein and binds with NSP9 | [35] |
| 27-Deoxy withaferin A | 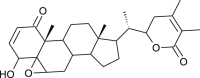 |
Withania somnifera | interferes with Spike protein | [35] |
| 2,3-Dihydro withaferin A | 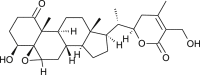 |
Withania somnifera | interfers with Spike | [35] |
| 27-Hydroxy withanolide B | 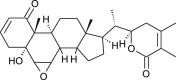 |
Withania somnifera | binds with NSP10 | [35] |
| Witha-stramonolide | 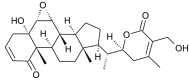 |
Withania somnifera | binds with NSP12 D2 | [35] |
| Withanolide B | 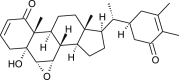 |
Withania somnifera | binds with NSP12 D2 and binds with NSP3 | [35] |
| Withanolide R | 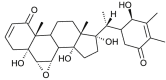 |
Withania somnifera | binds with NSP12 D2 | [35] |
| Withaferin A | 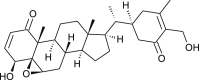 |
Withania somnifera | binds with NSP12 D2 | [35] |
| 27-Hydroxy withanolide B |  |
Withania somnifera | binds with NSP9 | [35] |
| Aza-diradionolide |  |
Azadirachta indica | binds with NSP9 | [35] |
| 27-Deoxy-14-hydroxyl withaferin A |  |
Withania somnifera | binds with NSP3 | [35] |
| Anthraquinones | ||||
| Microcarpin |  |
binds with TMPRSS2 | [174] | |
| Anthocyanins | ||||
| Chry-santhemin | 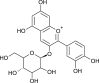 |
Black grapes | RdRp and hACE-2 | [55] |
| Stilbenes | ||||
| δ-Viniferin | 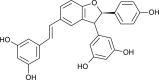 |
Grapevine leaves | MPro, RdRp and hACE-2 | [55] |
| Iso-gemichalcone | 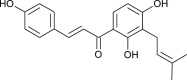 |
binds with TMPRSS2 | [174] | |
| Lactones | ||||
| Limonin | 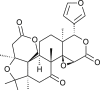 |
Nigella sativa | binds with NSP16 | [35] |
| Durumolide K | 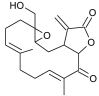 |
binds with TMPRSS2 | [174] | |
| Fatty acids | ||||
| Dihomo-gamma-linolenic acid | 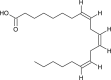 |
Chinese medicinal plant | 3CLpro | [11] |
| Phthalates | ||||
| Solvanol | 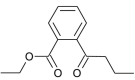 |
Solanum nigrum | binds with NSP16 | [35] |
3. Conclusions
In current adverse conditions of COVID-19 across the world, it is the urgent need of time to develop drugs or therapeutic options at the earliest. Developing new drugs and identifying novel drug targets are indeed time consuming and validation of such novel drugs/targets in clinical trials are mandatory to check their efficacy and effectiveness. Unavailability of the drugs and specific treatments for COVID-19 till date motivate the researchers to look for alternatives for successfully combating the current disease scenario. In this regard, medicinal plants containing specific phytomoieties could provide a wide scope as therapeutic drugs against COVID-19. Furthermore, the low toxicity of herbal medicines and an easy development process provide additional advantages in their fast and wide usage. This study has compiled a data of different types of phytoconstituents possessing antiviral activity against Coronaviruses as well as phytoconstituents showing affinities against therapeutic targets of SARS-CoV-2 like RdRP, 3CLpro, PLpro and the host cell targets like ACE-2 based on the computational screening methods. However, further in vivo and in vitro studies need to be done to confirm the bioactivity of these compounds against COVID-19. Overall, the development of phytopharmaceuticals as an alternative approach could be seen as a viable treatment option against SARS-CoV-2 in current COVID-19 pandemic.
Funding information
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.cbi.2021.109449.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organization . World Health Organization; Geneva, Switzerland: 2020. WHO coronavirus disease (COVID-19) dashboard.https://covid19.who.int/ [Google Scholar]
- 2.Ratten V. Coronavirus (covid-19) and entrepreneurship: changing life and work landscape. J. Small Bus. Enterpren. 2020;32:503–516. doi: 10.1080/08276331.2020.1790167. [DOI] [Google Scholar]
- 3.Barua S. SSRN; 2020. Understanding Coronanomics: the economic implications of the coronavirus (COVID-19) pandemic. [DOI] [Google Scholar]
- 4.Wu C., Liu Y., Yang Y., Zhang P., Zhong W., Wang Y., Wang Q., Xu Y., Li M., Li X., Zheng M., Chen L., Li H. Analysis of therapeutic targets for SARS-CoV-2 and discovery of potential drugs by computational methods. Acta Pharm. Sin. B. 2020;10:766–788. doi: 10.1016/j.apsb.2020.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang Y., Wang Y., Chen Y., Qin Q. Unique epidemiological and clinical features of the emerging 2019 novel coronavirus pneumonia (COVID‐19) implicate special control measures. J. Med. Virol. 2020;92:568–576. doi: 10.1002/jmv.25748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ogen Y. Assessing nitrogen dioxide (NO2) levels as a contributing factor to the coronavirus (COVID-19) fatality rate. Sci. Total Environ. 2020;726:138605. doi: 10.1016/j.scitotenv.2020.138605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Onder G., Rezza G., Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. J. Am. Med. Assoc. 2020;323(18):1775–1776. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 8.Decaro N., Lorusso A. Novel human coronavirus (SARS-CoV-2): a lesson from animal coronaviruses. Vet. Microbiol. 2020;244:108693. doi: 10.1016/j.vetmic.2020.108693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Helmy Y.A., Fawzy M., Elaswad A., Sobieh A., Kenney S.P., Shehata A.A. The COVID-19 pandemic: a comprehensive review of taxonomy, genetics, epidemiology, diagnosis, treatment, and control. J. Clin. Med. 2020;9(4):1225. doi: 10.3390/jcm9041225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Graham R.L., Donaldson E.F., Baric R.S. A decade after SARS: strategies for controlling emerging coronaviruses. Nat. Rev. Microbiol. 2013;11(12):836–848. doi: 10.1038/nrmicro3143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mani J.S., Johnson J.B., Steel J.C., Broszczak D.A., Neilsen P.M., Walsh K.B., Naiker M. Natural product-derived phytochemicals as potential agents against coronaviruses: a review. Virus Res. 2020;284:197989. doi: 10.1016/j.virusres.2020.197989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Abd El-Aziz T.M., Stockand J.D. Recent progress and challenges in drug development against COVID-19 coronavirus (SARS-CoV-2)-an update on the status. Infect. Genet. Evol. 2020;83:104327. doi: 10.1016/j.meegid.2020.104327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pandey A., Nikam A.N., Shreya A.B., Mutalik S.P., Gopalan D., Kulkarni S., Padya B.S., Fernandes G., Mutalik S., Prassl R. Potential therapeutic targets for combating SARS-CoV-2: drug repurposing, clinical trials and recent advancements. Life Sci. 2020;256:117883. doi: 10.1016/j.lfs.2020.117883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Petrosillo N., Viceconte G., Ergonul O., Ippolito G., Petersen E. COVID-19, SARS and MERS: are they closely related? Clin. Microbiol. Infect. 2020;26(6):729–734. doi: 10.1016/j.cmi.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang Y.Z., Holmes E.C. A genomic perspective on the origin and emergence of SARS-CoV-2. Cell. 2020;181:223–227. doi: 10.1016/j.cell.2020.03.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen N., Zhou M., Dong X., Qu J., Gong F., Han Y., Qiu Y., Wang J., Liu Y., Wei Y., Xia J., Yu T., Zhang X., Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507–513. doi: 10.1016/S0140-6736(20)30211-7. 10223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vellingiri B., Jayaramayya K., Iyer M., Narayanasamy A., Govindasamy V., Giridharan B., Ganesan S., Venugopal A., Venkatesan D., Ganesan H., Rajagopalan K., Rahman P., Cho S., Kumar N., Subramaniam M. COVID-19: a promising cure for the global panic. Sci. Total Environ. 2020;725:138277. doi: 10.1016/j.scitotenv.2020.138277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ashour H.M., Elkhatib W.F., Rahman M.M., Elshabrawy H.A. Insights into the recent 2019 novel coronavirus (SARS-CoV-2) in light of past human coronavirus outbreaks. Pathogens. 2020;9(3):186. doi: 10.3390/pathogens9030186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Carstens E.B. Ratification vote on taxonomic proposals to the international committee on taxonomy of viruses (2009) Arch. Virol. 2010;155(1):133–146. doi: 10.1007/s00705-009-0547-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cui J., Li F., Shi Z.L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019;17(3):181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Woo P.C., Lau S.K., Huang Y., Yuen K.Y. Coronavirus diversity, phylogeny and interspecies jumping. Exp. Biol. Med. 2009;234(10):1117–1127. doi: 10.3181/0903-MR-94. [DOI] [PubMed] [Google Scholar]
- 22.Qi X., Cui L., Jiao Y., Pan Y., Li X., Zu R., Huo X., Wu B., Tang F., Song Y., Zhou M., Wang H., Cardona C.J., Xing Z. Antigenic and genetic characterization of a European avian-like H1N1 swine influenza virus from a boy in China in 2011. Arch. Virol. 2013;158(1):39–53. doi: 10.1007/s00705-012-1423-7. [DOI] [PubMed] [Google Scholar]
- 23.Corman V.M., Muth D., Niemeyer D., Drosten C. Hosts and sources of endemic human coronaviruses. Adv. Virus Res. 2018;100:163–188. doi: 10.1016/bs.aivir.2018.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.To K.K., Hung I.F., Chan J.F., Yuen K.Y. From SARS coronavirus to novel animal and human coronaviruses. J. Thorac. Dis. 2013;5(Suppl2):S103–S108. doi: 10.3978/j.issn.2072-1439.2013.06.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ye Z.W., Yuan S., Yuen K.S., Fung S.Y., Chan C.P., Jin D.Y. Zoonotic origins of human coronaviruses. Int. J. Biol. Sci. 2020;16(10):1686–1697. doi: 10.7150/ijbs.45472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cheng R.H., Kuhn R.J., Olson N.H., Rossmann M.G., Choi H.K., Smith T.J., Baker T.S. Nucleocapsid and glycoprotein organization in an enveloped virus. Cell. 1995;80(4):621–630. doi: 10.1016/0092-8674(95)90516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Naqvi A.A.T., Fatima K., Mohammad T., Fatima U., Singh I.K., Singh A., Atif S.M., Hariprasad G., Hasan G.M., Hassan M.I. Insights into SARS-CoV-2 genome, structure, evolution, pathogenesis and therapies: structural genomics approach. Biochim. Biophys. Acta (BBA) - Mol. Basis Dis. 2020;1866(10):165878. doi: 10.1016/j.bbadis.2020.165878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li F. Structure, function, and evolution of coronavirus spike proteins. Annual Review of Virology. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abduljalil J.M., Abduljalil B.M. Epidemiology, genome, and clinical features of the pandemic SARS-CoV-2: a recent view. New Microbes and New Infections. 2020;35:100672. doi: 10.1016/j.nmni.2020.100672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tang X., Wu C., Li X., Song Y., Yao X., Wu X., Duan Y., Zhang H., Wang Y., Qian Z., Cui J., Lu J. National Science Review; 2020. On the origin and continuing evolution of SARS-CoV-2. 2020, nwaa036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chhikara B.S., Rathi B., Singh J., Poonam Corona virus SARS-CoV-2 disease COVID-19: infection, prevention and clinical advances of the prospective chemical drug therapeutics. Chemical Biology Letters. 2020;7(1):63–72. [Google Scholar]
- 32.Gyawali R., Paudel P.N., Basyal D., Setzer W.N., Lamichhane S., Paudel M.N., Gyawali S., Khanal P. A review on ayurvedic medicinal herbs as remedial perspective for COVID-19. JKAHS. 2020;3:1–21. doi: 10.3126/jkahs.v3i0.29116. [DOI] [Google Scholar]
- 33.Gurung A.B., Ali M.A., Lee J., Farah M.A., MashayAl-Anazi K. Unravelling lead antiviral phytochemicals for the inhibition of SARS-CoV-2 Mpro enzyme through in silico approach. Life Sci. 2020;255:117831. doi: 10.1016/j.lfs.2020.117831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kabera J.N., Semana E., Mussa A.R., He X. Plant secondary metabolites: biosynthesis, classification, function and pharmacological properties. J. Pharm. Pharmacol. 2014;2:377–392. [Google Scholar]
- 35.Parida P.K., Paul D., Chakravorty D. ChemRxiv; 2020. Nature to nurture-identifying phytochemicals from Indian medicinal plants as prophylactic medicine by rational screening to be potent against multiple drug targets of SARS-CoV-2. 2020, (Pre-print) [DOI] [Google Scholar]
- 36.Belouzard S., Millet J.K., Licitra B.N., Whittaker G.R. Mechanisms of coronavirus cell entry mediated by the viral spike protein. Viruses. 2012;4(6):1011–1033. doi: 10.3390/v4061011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bosch B.J., van der Zee R., de Haan C., Rottier P. The coronavirus spike protein is a class I virus fusion protein: structural and functional characterization of the fusion core complex. J. Virol. 2013;77(16):8801–8811. doi: 10.1128/jvi.77.16.8801-8811.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hamming I., Timens W., Bulthuis M.L., Lely A.T., Navis G., van Goor H. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J. Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Heald-Sargent T., Gallagher T. Ready, set, fuse! the coronavirus spike protein and acquisition of fusion competence. Viruses. 2012;4:557–580. doi: 10.3390/v4040557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.White J.M., Delos S.E., Brecher M., Schornberg K. Structures and mechanisms of viral membrane fusion proteins: multiple variations on a common theme. Crit. Rev. Biochem. Mol. Biol. 2008;43(3):189–219. doi: 10.1080/10409230802058320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bertram S., Glowacka I., Müller M.A., Lavender H., Gnirss K., Nehlmeier I., Niemeyer D., He Y., Simmons G., Drosten C., Soilleux E.J., Jahn O., Steffen I., Pöhlmann S. Cleavage and activation of the severe acute respiratory syndrome coronavirus spike protein by human airway trypsin-like protease. J. Virol. 2011;85(24):13363–13372. doi: 10.1128/JVI.05300-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shulla A., Heald-Sargent T., Subramanya G., Zhao J., Perlman S., Gallagher T. A transmembrane serine protease is linked to the severe acute respiratory syndrome coronavirus receptor and activates virus entry. J. Virol. 2011;85(2):873–882. doi: 10.1128/JVI.02062-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li G., De Clercq E. Therapeutic options for the 2019 novel coronavirus (2019-nCoV) Nat. Rev. Drug Discov. 2020;19(3):149–150. doi: 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- 44.Siu Y.L., Teoh K.T., Lo J., Chan C.M., Kien F., Escriou N., Tsao S.W., Nicholls J.M., Altmeyer R., Peiris J.S., Bruzzone R., Nal B. The M, E, and N structural proteins of the severe acute respiratory syndrome coronavirus are required for efficient assembly, trafficking, and release of virus-like particles. J. Virol. 2008;82(22):11318–11330. doi: 10.1128/JVI.01052-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nieto-Torres J.L., Verdiá-Báguena C., Jimenez-Guardeño J.M., Regla-Nava J.A., Castaño-Rodriguez C., Fernandez-Delgado R., Torres J., Aguilella V.M., Enjuanes L. Severe acute respiratory syndrome coronavirus E protein transports calcium ions and activates the NLRP3 inflammasome. Virology. 2015;485:330–339. doi: 10.1016/j.virol.2015.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lukassen S., Chua R.L., Trefzer T., Kahn N.C., Schneider M.A., Muley T., Winter H., Meister M., Veith C., Boots A.W., Hennig B.P., Kreuter M., Conrad C., Eils R. SARS‐CoV‐2 receptor ACE 2 and TMPRSS 2 are primarily expressed in bronchial transient secretory cells. EMBO J. 2020;39(10) doi: 10.15252/embj.20105114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bourgonje A.R., Abdulle A.E., Timens W., Hillebrands J.L., Navis G.J., Gordijn S.J., Bolling M.C., Dijkstra G., Voors A.A., Osterhaus A.D., van der Voort P.H., Mulder D.J., van Goor H. Angiotensin‐converting enzyme‐2 (ACE2), SARS‐CoV‐2 and pathophysiology of coronavirus disease 2019 (COVID‐19) J. Pathol. 2020;251(3):228–248. doi: 10.1002/path.5471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Karthik A.R.A., Muthusamy V.S., Ramanathan M. Phytomolecules having flavone and napthofuran nucleus exhibited better binding G-score against protease and SPIKE protein of novel corona virus COVID-19. Research Square. 2020. 2020, (Pre-print) [DOI]
- 49.Chakraborty I., Maity P. COVID-19 outbreak: migration, effects on society, global environment and prevention. Sci. Total Environ. 2020;728:138882. doi: 10.1016/j.scitotenv.2020.138882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pang J., Wang M.X., Ang I.Y.H., Tan S.H.X., Lewis R.F., Chen J.I., Gutierrez R.A., Gwee S.X.W., Chua P.E.Y., Yang Q., Ng X.Y., Yap R.K., Tan H.Y., Teo Y.Y., Tan C.C., Cook A.R., Yap J.C., Hsu L.Y. Potential rapid diagnostics, vaccine and therapeutics for 2019 novel coronavirus (2019-nCoV): a systematic review. J. Clin. Med. 2020;9(3):623. doi: 10.3390/jcm9030623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gheblawi M., Wang K., Viveiros A., Nguyen Q., Zhong J.C., Turner A.J., Raizada M.K., Grant M.B., Oudit G.Y. Angiotensin-converting enzyme 2: SARS-CoV-2 receptor and regulator of the renin-angiotensin system: celebrating the 20th anniversary of the discovery of ACE2. Circ. Res. 2020;126(10):1456–1474. doi: 10.1161/CIRCRESAHA.120.317015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Y., Hou Y., Shen J., Huang Y., Martin W., Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discovery. 2020;6:14. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang H., Penninger J.M., Li Y., Zhong N., Slutsky A.S. Angiotensin-converting enzyme 2 (ACE2) as a SARS-CoV-2 receptor: molecular mechanisms and potential therapeutic target. Intensive Care Med. 2020;46(4):586–590. doi: 10.1007/s00134-020-05985-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Poduri R., Joshi G., Jagadeesh G. Drugs targeting various stages of the SARS-CoV-2 life cycle: exploring promising drugs for the treatment of Covid-19. Cell. Signal. 2020;74:109721. doi: 10.1016/j.cellsig.2020.109721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Joshi R.S., Jagdale S.S., Bansode S.B., Shankar S.S., Tellis M.B., Pandya V.K., Chugh A., Giri A.P., Kulkarni M.J. Discovery of potential multi-target-directed ligands by targeting host-specific SARS-CoV-2 structurally conserved main protease. J. Biomol. Struct. Dyn. 2020:1–16. doi: 10.1080/07391102.2020.1760137. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Abena P.M., Decloedt E.H., Bottieau E., Suleman F., Adejumo P., Sam-Agudu N.A., Muyembe TamFum J.J., Seydi M., Eholie S.P., Mills E.J., Kallay O., Zumla A., Nachega J.B. Chloroquine and hydroxychloroquine for the prevention or treatment of COVID-19 in Africa: caution for inappropriate off-label use in healthcare settings. Am. J. Trop. Med. Hyg. 2020;102:1184–1188. doi: 10.4269/ajtmh.20-0290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Devaux C., Rolain J., Colson P., Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int. J. Antimicrob. Agents. 2020;55(5):105938. doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Trani L., Savarino A., Campitelli L., Norelli S., Puzelli S., D'Ostilio D., Vignolo E., Donatelli I., Cassone A. Different pH requirements are associated with divergent inhibitory effects of chloroquine on human and avian influenza A viruses. Virol. J. 2007;4(1):39. doi: 10.1186/1743-422X-4-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Chatre C., Roubille F., Vernhet H., Jorgensen C., Pers Y.M. Cardiac complications attributed to chloroquine and hydroxychloroquine: a systematic review of the literature. Drug Saf. 2018;41(10):919–931. doi: 10.1007/s40264-018-0689-4. [DOI] [PubMed] [Google Scholar]
- 60.Liu J., Cao R., Xu M., Wang X., Zhang H., Hu H., Li Y., Hu Z., Zhong W., Wang M. Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discovery. 2020;6:16. doi: 10.1038/s41421-020-0156-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang J., Xie B., Hashimoto K. Current status of potential therapeutic candidates for the COVID-19 crisis. Brain Behav. Immun. 2020;87:59–73. doi: 10.1016/j.bbi.2020.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Geleris J., Sun Y., Platt J., Zucker J., Baldwin M., Hripcsak G., Labella A., Manson D.K., Kubin C., Barr R.G., Sobieszczyk M.E., Schluger N.W. Observational study of hydroxychloroquine in hospitalized patients with Covid-19. N. Engl. J. Med. 2020;382:2411–2418. doi: 10.1056/NEJMoa2012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Liu C., Zhou Q., Li Y., Garner L.V., Watkins S.P., Carter L.J., Smoot J., Gregg A.C., Daniels A.D., Jervey S., Albaiu D. Research and development on therapeutic agents and vaccines for COVID-19 and related human coronavirus diseases. ACS Cent. Sci. 2020;6:315–331. doi: 10.1021/acscentsci.0c00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gallagher T.M., Buchmeier M.J. Coronavirus spike proteins in viral entry and pathogenesis. Virology. 2001;279(2):371–374. doi: 10.1006/viro.2000.0757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hoffmann M., Kleine-Weber H., Schroeder S., Krüger N., Herrler T., Erichsen S., Schiergens T.S., Herrler G., Wu N.H., Nitsche A., Müller M.A., Drosten C., Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181(2):271–280. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kirchdoerfer R.N., Ward A.B. Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors. Nat. Commun. 2019;10(1):2342. doi: 10.1038/s41467-019-10280-3. 1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gordon C.J., Tchesnokov E.P., Feng J.Y., Porter D.P., Götte M. The antiviral compound remdesivir potently inhibits RNA-dependent RNA polymerase from Middle East respiratory syndrome coronavirus. J. Biol. Chem. 2020;295:4773–4779. doi: 10.1074/jbc.AC120.013056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Elfiky A.A. Natural products may interfere with SARS-CoV-2 attachment to the host cell. J. Biomol. Struct. Dyn. 2020;(2020):1–10. doi: 10.1080/07391102.2020.1761881. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Snijder E.J., Decroly E., Ziebuhr J. The nonstructural proteins directing coronavirus RNA synthesis and processing. Adv. Virus Res. 2016;96:59–126. doi: 10.1016/bs.aivir.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Anand K., Ziebuhr J., Wadhwani P., Mesters J.R., Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300(5626):1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- 71.Khodadadi E., Maroufi P., Khodadadi E., Esposito I., Ganbarov K., Espsoito S., Yousefi M., Zeinalzadeh E., Kafil H.S. Study of combining virtual screening and antiviral treatments of the Sars-CoV-2 (Covid-19) Microb. Pathog. 2020;146:104241. doi: 10.1016/j.micpath.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Stower H. Lopinavir–ritonavir in severe COVID-19. Nat. Med. 2020;26(4) doi: 10.1038/s41591-020-0849-9. 465-465. [DOI] [PubMed] [Google Scholar]
- 73.McKee D.L., Sternberg A., Stange U., Laufer S., Naujokat C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol. Res. 2020;157:104859. doi: 10.1016/j.phrs.2020.104859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Wu R., Wang L., Kuo H.D., Shannar A., Peter R., Chou P.J., Li S., Hudlikar R., Liu X., Liu Z., Poiani G.J., Amorosa L., Brunetti L., Kong A.N. An update on current therapeutic drugs treating COVID-19. Current Pharmacology Reports. 2020;2020:1–15. doi: 10.1007/s40495-020-00216-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Shin D., Mukherjee R., Grewe D., Bojkova D., Baek K., Bhattacharya A., Schulz L., Widera M., Mehdipour A.R., Tascher G., Geurink P.P., Wilhelm A., van der Heden van Noort G.J., Ovaa H., Müller S., Knobeloch K.P., Rajalingam K., Schulman B.A., Cinatl J., Hummer G., Ciesek S., Dikic I. Inhibition of papain-like protease PLpro blocks SARS-CoV-2 spread and promotes anti-viral immunity. Research Square. 2020 doi: 10.1038/s41586-020-2601-5. 2020, (Pre-print) [DOI] [Google Scholar]
- 76.Barretto N., Jukneliene D., Ratia K., Chen Z., Mesecar A.D., Baker S.C. The papain-like protease of severe acute respiratory syndrome coronavirus has deubiquitinating activity. J. Virol. 2005;79(24):15189–15198. doi: 10.1128/JVI.79.24.15189-15198.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ha D.P., Van Krieken R., Carlos A.J., Lee A.S. The stress-inducible molecular chaperone GRP78 as potential therapeutic target for coronavirus infection. J. Infect. 2020;81(3):452–482. doi: 10.1016/j.jinf.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zhou N., Pan T., Zhang J., Li Q., Zhang X., Bai C., Huang F., Peng T., Zhang J., Liu C., Tao L., Zhang H. Glycopeptide antibiotics potently inhibit cathepsin L in the late endosome/lysosome and block the entry of Ebola virus, middle east respiratory syndrome coronavirus (MERS-CoV), and severe acute respiratory syndrome coronavirus (SARS-CoV) J. Biol. Chem. 2016;291(17):9218–9232. doi: 10.1074/jbc.M116.716100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Coleman M.D., Ha S.D., Haeryfar S.M.M., Barr S.D., Kim S.O. Cathepsin B plays a key role in optimal production of the influenza A virus. Journal of Virology and Antiviral Research. 2018:1–20. doi: 10.4172/2324-8955.1000178. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Inoue Y., Tanaka N., Tanaka Y., Inoue S., Morita K., Zhuang M., Hattori T., Sugamura K. Clathrin-dependent entry of severe acute respiratory syndrome coronavirus into target cells expressing ACE2 with the cytoplasmic tail deleted. J. Virol. 2007;81(16):8722–8729. doi: 10.1128/JVI.00253-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wang H., Yang P., Liu K., Guo F., Zhang Y., Zhang G., Jiang C. SARS coronavirus entry into host cells through a novel clathrin-and caveolae-independent endocytic pathway. Cell Res. 2008;18(2):290–301. doi: 10.1038/cr.2008.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stancioiu F., Papadakis G.Z., Kteniadakis S., Izotov B.N., Coleman M.D., Spandidos D.A., Tsatsakis A. A dissection of SARS CoV2 with clinical implications. Int. J. Mol. Med. 2020;46(2):489–508. doi: 10.3892/ijmm.2020.4636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Liu T., Luo S., Libby P., Shi G.P. Cathepsin L-selective inhibitors: a potentially promising treatment for COVID-19 patients. Pharmacol. Ther. 2020;213:107587. doi: 10.1016/j.pharmthera.2020.107587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Khan Z., Karatas Y., Ceylan A.F., Rahman H. Le Pharmacien Hospitalier et Clinicien; 2020. COVID-19 and therapeutic drugs repurposing in hand: the need for collaborative efforts. 2020, [E-pub ahead of print] [DOI] [Google Scholar]
- 85.Yang Y., Islam M.S., Wang J., Li Y., Chen X. Traditional Chinese medicine in the treatment of patients infected with 2019-new coronavirus (SARS-CoV-2): a review and perspective. Int. J. Biol. Sci. 2020;16(10):1708–1717. doi: 10.7150/ijbs.45538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mirzaie A., Halaji M., Dehkordi F.S., Ranjbar R., Noorbazargand H. A narrative literature review on traditional medicine options for treatment of corona virus disease 2019 (COVID-19) Compl. Ther. Clin. Pract. 2020;40:101214. doi: 10.1016/j.ctcp.2020.101214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Khaerunnisa S., Kurniawan H., Awaluddin R., Suhartati S., Soetjipto S. Potential Inhibitor of COVID-19 main protease (Mpro) from several medicinal plant compounds by molecular docking study. Preprints. 2020 doi: 10.20944/preprints202003.0226.v1. 2020, 2020030226. [DOI] [Google Scholar]
- 88.Ganjhu R.K., Mudgal P.P., Maity H., Dowarha D., Devadiga S., Nag S., Arunkumar G. Herbal plants and plant preparations as remedial approach for viral diseases. Virusdisease. 2015;26(4):225–236. doi: 10.1007/s13337-015-0276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Pillaiyar T., Meenakshisundaram S., Manickam M. Recent discovery and development of inhibitors targeting coronaviruses. Drug Discov. Today. 2020;25(4):668–688. doi: 10.1016/j.drudis.2020.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yi L., Li Z., Yuan K., Qu X., Chen J., Wang G., Zhang H., Luo H., Zhu L., Jiang P., Chen L., Shen Y., Luo M., Zuo G., Hu J., Duan D., Nie Y., Shi X., Wang W., Han Y., Li T., Liu Y., Ding M., Deng H., Xu X. Small molecules blocking the entry of severe acute respiratory syndrome coronavirus into host cells. J. Virol. 2004;78(20):11334–11339. doi: 10.1128/JVI.78.20.11334-11339.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yu M.S., Lee J., Lee J.M., Kim Y., Chin Y.W., Jee J.G., Keum Y.S., Jeong Y.J. Identification of myricetin and scutellarein as novel chemical inhibitors of the SARS coronavirus helicase, nsP13. Bioorg. Med. Chem. Lett. 2012;22(12):4049–4054. doi: 10.1016/j.bmcl.2012.04.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhuang M., Jiang H., Suzuki Y., Li X., Xiao P., Tanaka T., Ling H., Yang B., Saitoh H., Zhang L., Qin C., Sugamura K., Hattori T. Procyanidins and butanol extract of Cinnamomi cortex inhibit SARS-CoV infection. Antivir. Res. 2009;82(1):73–81. doi: 10.1016/j.antiviral.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ryu Y.B., Jeong H.J., Kim J.H., Kim Y.M., Park J.Y., Kim D., Nguyen T.T., Park S.J., Chang J.S., Park K.H., Rho M.C., Lee W.S. Biflavonoids from Torreya nucifera displaying SARS-CoV 3CLpro inhibition. Bioorg. Med. Chem. 2010;18(22):7940–7947. doi: 10.1016/j.bmc.2010.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kim D.W., Seo K.H., Curtis-Long M.J., Oh K.Y., Oh J.W., Cho J.K., Lee K.H., Park K.H. Phenolic phytochemical displaying SARS-CoV papain-like protease inhibition from the seeds of Psoralea corylifolia. J. Enzym. Inhib. Med. Chem. 2014;29(1):59–63. doi: 10.3109/14756366.2012.753591. [DOI] [PubMed] [Google Scholar]
- 95.Cho J.K., Curtis-Long M.J., Lee K.H., Kim D.W., Ryu H.W., Yuk H.J., Park K.H. Geranylated flavonoids displaying SARS-CoV papain-like protease inhibition from the fruits of Paulownia tomentosa. Bioorg. Med. Chem. 2013;21(11):3051–3057. doi: 10.1016/j.bmc.2013.03.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lin C.W., Tsai F.J., Tsai C.H., Lai C.C., Wan L., Ho T.Y., Hsieh C.C., Chao P.D. Anti-SARS coronavirus 3C-like protease effects of Isatis indigotica root and plant-derived phenolic compounds. Antivir. Res. 2005;68(1):36–42. doi: 10.1016/j.antiviral.2005.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Park J.Y., Yuk H.J., Ryu H.W., Lim S.H., Kim K.S., Park K.H., Ryu Y.B., Lee W.S. Evaluation of polyphenols from Broussonetia papyrifera as coronavirus protease inhibitors. J. Enzym. Inhib. Med. Chem. 2017;32(1):504–515. doi: 10.1080/14756366.2016.1265519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Chen C.N., Lin C.P., Huang K.K., Chen W.C., Hsieh H.P., Liang P.H., Hsu J.T. Inhibition of SARS-CoV 3C-like protease activity by theaflavin-3, 3'-digallate (TF3) Evid. base Compl. Alternative Med. 2005;2(2):209–215. doi: 10.1093/ecam/neh081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park J.Y., Kim J.H., Kwon J.M., Kwon H.J., Jeong H.J., Kim Y.M., Kim D., Lee W.S., Ryu Y.B. Dieckol, a SARS-CoV 3CLpro inhibitor, isolated from the edible brown algae Ecklonia cava. Bioorg. Med. Chem. 2013;21(13):3730–3737. doi: 10.1016/j.bmc.2013.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Song Y.H., Kim D.W., Curtis-Long M.J., Yuk H.J., Wang Y., Zhuang N., Lee K.H., Jeon K.S., Park K.H. Papain-like protease (PLpro) inhibitory effects of cinnamic amides from Tribulus terrestris fruits. Biol. Pharm. Bull. 2014;37(6):1021–1028. doi: 10.1248/bpb.b14-00026. [DOI] [PubMed] [Google Scholar]
- 101.Shen Y.C., Wang L.T., Khalil A.T., Chiang L.C., Cheng P.W. Bioactive pyranoxanthones from the roots of Calophyllum blancoi. Chem. Pharm. Bull. 2005;53(2):244–247. doi: 10.1248/cpb.53.244. [DOI] [PubMed] [Google Scholar]
- 102.Ho T.Y., Wu S.L., Chen J.C., Li C.C., Hsiang C.Y. Emodin blocks the SARS coronavirus spike protein and angiotensin-converting enzyme 2 interaction. Antivir. Res. 2007;74(2):92–101. doi: 10.1016/j.antiviral.2006.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Loizzo M.R., Saab A.M., Tundis R., Statti G.A., Menichini F., Lampronti I., Gambari R., Cinatl J., Doerr H.W. Phytochemical analysis and in vitro antiviral activities of the essential oils of seven Lebanon species. Chem. Biodivers. 2008;5(3):461–470. doi: 10.1002/cbdv.200890045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Park J.Y., Kim J.H., Kim Y.M., Jeong H.J., Kim D.W., Park K.H., Kwon H.J., Park S.J., Lee W.S., Ryu Y.B. Tanshinones as selective and slow-binding inhibitors for SARS-CoV cysteine proteases. Bioorg. Med. Chem. 2012;20(19):5928–5935. doi: 10.1016/j.bmc.2012.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kumar V., Jung Y.S., Liang P.H. Anti-SARS coronavirus agents: a patent review (2008–present) Expert Opin. Ther. Pat. 2013;23(10):1337–1348. doi: 10.1517/13543776.2013.823159. [DOI] [PubMed] [Google Scholar]
- 106.Chang F.R., Yen C.T., Ei-Shazly M., Lin W.H., Yen M.H., Lin K.H., Wu Y.C. Anti-human coronavirus (anti-HCoV) triterpenoids from the leaves of Euphorbia neriifolia. Natural Product Communications. 2012;7(11):1415–1417. 2012. [PubMed] [Google Scholar]
- 107.Orhan I.E., Senol Deniz F.S. Natural products as potential leads against coronaviruses: could they be encouraging structural models against SARS-CoV-2? Natural Products and Bioprospecting. 2020;10(4):1–16. doi: 10.1007/s13659-020-00250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tsai Y.C., Lee C.L., Yen H.R., Chang Y.S., Lin Y.P., Huang S.H., Lin C.W. Antiviral action of Tryptanthrin isolated from Strobilanthes cusia leaf against human coronavirus NL63. Biomolecules. 2020;10(3):366. doi: 10.3390/biom10030366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim D.E., Min J.S., Jang M.S., Lee J.Y., Shin Y.S., Song J.H., Kim H.R., Kim S., Jin Y.H., Kwon S. Natural bis-benzylisoquinoline alkaloids-tetrandrine, fangchinoline, and cepharanthine, inhibit human coronavirus OC43 infection of MRC-5 human lung cells. Biomolecules. 2019;9(11):696. doi: 10.3390/biom9110696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Cheng P.W., Ng L.T., Chiang L.C., Lin C.C. Antiviral effects of saikosaponins on human coronavirus 229E in vitro. Clin. Exp. Pharmacol. Physiol. 2006;33(7):612–616. doi: 10.1111/j.1440-1681.2006.04415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Muller C., Schulte F.W., Lange-Grünweller K., Obermann W., Madhugiri R., Pleschka S., Ziebuhr J., Hartmann R.K., Grünweller A. Broad-spectrum antiviral activity of the eIF4A inhibitor silvestrol against corona-and picornaviruses. Antivir. Res. 2018;150:123–129. doi: 10.1016/j.antiviral.2017.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Park J.Y., Ko J.A., Kim D.W., Kim Y.M., Kwon H.J., Jeong H.J., Kim C.Y., Park K.H., Lee W.S., Ryu Y.B. Chalcones isolated from Angelica keiskei inhibit cysteine proteases of SARS-CoV. J. Enzym. Inhib. Med. Chem. 2016;31(1):23–30. doi: 10.3109/14756366.2014.1003215. [DOI] [PubMed] [Google Scholar]
- 113.Park J.Y., Jeong H.J., Kim J.H., Kim Y.M., Park S.J., Kim D., Park K.H., Lee W.S., Ryu Y.B. Diarylheptanoids from Alnus japonica inhibit papain-like protease of severe acute respiratory syndrome coronavirus. Biol. Pharm. Bull. 2012;35(11):2036–2042. doi: 10.1248/bpb.b12-00623. [DOI] [PubMed] [Google Scholar]
- 114.Kumaki Y., Wandersee M.K., Smith A.J., Zhou Y., Simmons G., Nelson N.M., Bailey K.W., Vest Z.G., Li J.K., Chan P.K., Smee D.F., Barnard D.L. Inhibition of severe acute respiratory syndrome coronavirus replication in a lethal SARS-CoV BALB/c mouse model by stinging nettle lectin, Urtica dioica agglutinin. Antivir. Res. 2011;90(1):22–32. doi: 10.1016/j.antiviral.2011.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.O'Keefe B.R., Giomarelli B., Barnard D.L., Shenoy S.R., Chan P.K., McMahon J.B., Palmer K.E., Barnett B.W., Meyerholz D.K., Wohlford-Lenane C.L., McCray P.B., Jr. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. J. Virol. 2020;84(5):2511–2521. doi: 10.1128/JVI.02322-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Karak P. Biological activities of flavonoids: an overview. Int. J. Pharmaceut. Sci. Res. 2019;10(4):1567–1574. doi: 10.13040/IJPSR.0975-8232.10(4). 1567-74. [DOI] [Google Scholar]
- 117.Jo S., Kim S., Shin D.H., Kim M.S. Inhibition of SARS-CoV 3CL protease by flavonoids. J. Enzym. Inhib. Med. Chem. 2020;35(1):145–151. doi: 10.1080/14756366.2019.1690480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ahmad A., Kaleem M., Ahmed Z., Shafiq H. Therapeutic potential of flavonoids and their mechanism of action against microbial and viral infections—a review. Food Res. Int. 2015;77(2):221–235. doi: 10.1016/j.foodres.2015.06.021. [DOI] [Google Scholar]
- 119.Chauhan A., Kalra S. Identification of potent COVID-19 main protease (MPRO) inhibitors from flavonoids. Research Square. 2020:1–11. doi: 10.21203/rs.3.rs-34497/v1. 2020. [DOI] [Google Scholar]
- 120.Ngwa W., Kumar R., Thompson D., Lyerly W., Moore R., Reid T.E., Lowe H., Toyang N. Potential of flavonoid-inspired phytomedicines against COVID-19. Molecules. 2020;25(11):2707. doi: 10.3390/molecules25112707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Laskin A., Smith J.S., Laskin J. Molecular characterization of nitrogen-containing organic compounds in biomass burning aerosols using high-resolution mass spectrometry. Environ. Sci. Technol. 2009;43(10):3764–3771. doi: 10.1021/es803456n. [DOI] [PubMed] [Google Scholar]
- 122.McMahon J.B., Currens M.J., Gulakowski R.J., Buckheit R.W., Jr., Lackman-Smith C., Hallock Y.F., Boyd M.R. Michellamine B, a novel plant alkaloid, inhibits human immunodeficiency virus-induced cell killing by at least two distinct mechanisms. Antimicrob. Agents Chemother. 1995;39:484–488. doi: 10.1128/aac.39.2.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Wink M. Potential of DNA intercalating alkaloids and other plant secondary metabolites against SARS-CoV-2 causing COVID-19. Diversity. 2020;12(5):175. doi: 10.3390/d12050175. [DOI] [Google Scholar]
- 124.Jahan I., Onay A. Potentials of plant-based substance to inhabit and probable cure for the COVID-19. Turk. J. Biol. 2020;44(3):228–241. doi: 10.3906/biy-2005-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Srivastava A.K., Kumar A., Misra N. On the inhibition of COVID-19 protease by Indian herbal plants: an in silico investigation. 2020. https://arxiv.org/abs/2004.03411v1 arXiv (preprint) arXiv: 2004.03411 (2020)
- 126.Bleasel M.D., Peterson G.M. Emetine, ipecac, ipecac alkaloids and analogues as potential antiviral agents for coronaviruses. Pharmaceuticals. 2020;13(3):51. doi: 10.3390/ph13030051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Vermerris W., Nicholson R. Springer Science & Business Media; 2007. Phenolic Compound Biochemistry. [DOI] [Google Scholar]
- 128.Cai Y.Z., Sun M., Xing J., Luo Q., Corke H. Structure–radical scavenging activity relationships of phenolic compounds from traditional Chinese medicinal plants. Life Sci. 2006;78(25):2872–2888. doi: 10.1016/j.lfs.2005.11.004. [DOI] [PubMed] [Google Scholar]
- 129.Manivannan R., Punniyamoorthy A., Tamilselvan C. Plant secondary metabolites of antiviral properties a rich medicinal source for drug discovery: a mini review. J. Drug Deliv. Therapeut. 2019;9(5):161–167. doi: 10.22270/jddt.v9i5.3471. [DOI] [Google Scholar]
- 130.Rebensburg S., Helfer M., Schneider M., Koppensteiner H., Eberle J., Schindler M., Gürtler L., Brack-Werner R. Potent in vitro antiviral activity of Cistus incanus extract against HIV and Filoviruses targets viral envelope proteins. Sci. Rep. 2016;6:20394. doi: 10.1038/srep20394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Annunziata G., Sanduzzi Zamparelli M., Santoro C., Ciampaglia R., Stornaiuolo M., Tenore G.C., Sanduzzi A., Novellino E. May polyphenols have a role against coronavirus infection? An overview of in vitro evidence. Front. Med. 2020;7:240. doi: 10.3389/fmed.2020.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Edris A.E. Pharmaceutical and therapeutic potentials of essential oils and their individual volatile constituents: a review. Phytother Res. 2007;21(4):308–323. doi: 10.1002/ptr.2072. [DOI] [PubMed] [Google Scholar]
- 133.Singh G., Kapoor I.P.S., Singh P., de Heluani C.S., de Lampasona M.P., Catalan C.A.N. Chemistry, antioxidant and antimicrobial investigations on essential oil and oleoresins of Zingiber officinale. Food Chem. Toxicol. 2008;46(10):3295–3302. doi: 10.1016/j.fct.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 134.Nadjib B.M. Effective antiviral activity of essential oils and their characteristic terpenes against coronaviruses: an update. Journal of Pharmacology & Clinical Toxicology. 2020;8(1):1138. [Google Scholar]
- 135.Sharma A.D., Kaur I. Eucalyptol (1, 8 cineole) from eucalyptus essential oil a potential inhibitor of COVID 19 corona virus infection by molecular docking studies. Preprints. 2020 doi: 10.20944/preprints202003.0455.v1. 2020, 2020030455. [DOI] [Google Scholar]
- 136.Carson C.F., Hammer K.A. In: Lipids and Essential Oils as Antimicrobial Agents. Thormar H., editor. John Wiley & Sons Ltd; Chichester: 2011. Chemistry and bioactivity of essential oils; pp. 203–238. [DOI] [Google Scholar]
- 137.Meneses R., Ocazionez R.E., Martínez J.R., Stashenko E.E. Inhibitory effect of essential oils obtained from plants grown in Colombia on yellow fever virus replication in vitro. Ann. Clin. Microbiol. Antimicrob. 2019;8(1):8. doi: 10.1186/1476-0711-8-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Khan M.T., Ather A., Thompson K.D., Gambari R. Extracts and molecules from medicinal plants against herpes simplex viruses. Antivir. Res. 2005;67(2):107–119. doi: 10.1016/j.antiviral.2005.05.002. [DOI] [PubMed] [Google Scholar]
- 139.Govindarajan M., Rajeswary M., Hoti S.L., Bhattacharyya A., Benelli G. Eugenol, α-pinene and β-caryophyllene from Plectranthus barbatus essential oil as eco-friendly larvicides against malaria, dengue and Japanese encephalitis mosquito vectors. Parasitol. Res. 2016;115(2):807–815. doi: 10.1007/s00436-015-4809-0. [DOI] [PubMed] [Google Scholar]
- 140.Swamy M.K., Akhtar M.S., Sinniah U.R. Antimicrobial properties of plant essential oils against human pathogens and their mode of action: an updated review. Evid. base Compl. Alternative Med. 2016:3012462. doi: 10.1155/2016/3012462. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Khan Z.A., Iqbal A., Shahzad S.A. Synthetic approaches toward stilbenes and their related structures. Mol. Divers. 2017;21(2):483–509. doi: 10.1007/s11030-017-9736-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Reinisalo M., Kaelung A., Koskela A., Kaarniranta K., Karjalainen R.O. Polyphenol stilbenes: molecular mechanisms of defence against oxidative stress and aging-related diseases. Oxidative Medicine and Cellular Longevity. 2015:340520. doi: 10.1155/2015/340520. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Pandey P., Rane J.S., Chatterjee A., Kumar A., Khan R., Prakash A., Ray S. Targeting SARS-CoV-2 spike protein of COVID-19 with naturally occurring phytochemicals: an in silico study for drug development. J. Biomol. Struct. Dyn. 2020;(2020):1–11. doi: 10.1080/07391102.2020.1796811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Wahedi H.M., Ahmad S., Abbasi S.W. Stilbene-based natural compounds as promising drug candidates against COVID-19. J. Biomol. Struct. Dyn. 2020;2020:1–10. doi: 10.1080/07391102.2020.1762743. [DOI] [PubMed] [Google Scholar]
- 145.Li Y.Q., Li Z.L., Zhao W.J., Wen R.X., Meng Q.W., Zeng Y. Synthesis of stilbene derivatives with inhibition of SARS coronavirus replication. Eur. J. Med. Chem. 2006;41(9):1084–1089. doi: 10.1016/j.ejmech.2006.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lin S.C., Ho C.T., Chuo W.H., Li S., Wang T.T., Lin C.C. Effective inhibition of MERS-CoV infection by resveratrol. BMC Infect. Dis. 2017;17(1):1–10. doi: 10.1186/s12879-017-2253-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Kren V., Martínková L. Glycosides in medicine: “the role of glycosidic residue in biological activity.”. Curr. Med. Chem. 2001;8(11):1303–1328. doi: 10.2174/0929867013372193. [DOI] [PubMed] [Google Scholar]
- 148.Emamzadeh-Yazdi S. Dissertation, University of Pretoria; 2013. Antiviral, antibacterial and cytotoxic activities of South African plants containing cardiac glycosides.http://hdl.handle.net/2263/33163 2013. [Google Scholar]
- 149.Wong R.W., Lingwood C.A., Ostrowski M.A., Cabral T., Cochrane A. Cardiac glycoside/aglycones inhibit HIV-1 gene expression by a mechanism requiring MEK1/2-ERK1/2 signaling. Sci. Rep. 2018;8(1):850. doi: 10.1038/s41598-018-19298-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Loregian A., Mercorelli B., Nannetti G., Compagnin C., Palù G. Antiviral strategies against influenza virus: towards new therapeutic approaches. Cell. Mol. Life Sci. 2014;71(19):3659–3683. doi: 10.1007/s00018-014-1615-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vanderlinden E., Naesens L. Emerging antiviral strategies to interfere with influenza virus entry. Med. Res. Rev. 2014;34(2):301–339. doi: 10.1002/med.21289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Guo S., Bao L., Li C., Sun J., Zhao R., Cui X. Antiviral activity of iridoid glycosides extracted from Fructus Gardeniae against influenza A virus by PACT-dependent suppression of viral RNA replication. Sci. Rep. 2020;10(1):1897. doi: 10.1038/s41598-020-58443-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Islam M.T., Sarkar C., El-Kersh D.M., Jamaddar S., Uddin S.J., Shilpi J.A., Mubarak M.S. Phytotherapy Research; 2020. Natural products and their derivatives against coronavirus: a review of the non‐clinical and pre‐clinical data; p. 2020. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 154.Kregiel D., Berlowska J., Witonska I., Antolak H., Proestos C., Babic M., Babic L., Zhang B. In: Application and Characterization of Surfactants. Najjar R., editor. IntechOpen; London: 2017. Saponin-based, biological-active surfactants from plants; pp. 183–205. [DOI] [Google Scholar]
- 155.Waller G.R., Yamasaki K. Springer Science & Business Media; 2013. Saponins used in traditional and modern medicine. [DOI] [Google Scholar]
- 156.Roner M.R., Sprayberry J., Spinks M., Dhanji S. Antiviral activity obtained from aqueous extracts of the Chilean soapbark tree (Quillaja saponaria Molina) J. Gen. Virol. 2007;88(1):275–285. doi: 10.1099/vir.0.82321-0. [DOI] [PubMed] [Google Scholar]
- 157.Qin X.J., Zhang L.J., Zhang Y., Ni W., Yang X.Z., Yu Q., Yan H., An L.K., Lui H.Y. Polyphyllosides A–F, six new spirostanol saponins from the stems and leaves of Paris polyphylla var. chinensis. Bioorg. Chem. 2020;99:103788. doi: 10.1016/j.bioorg.2020.103788. [DOI] [PubMed] [Google Scholar]
- 158.Amoros M., Fauconnier B., Girre R.L. In vitro antiviral activity of a saponin from Anagallis arvensis, Primulaceae, against herpes simplex virus and poliovirus. Antivir. Res. 1987;8(1):13–25. doi: 10.1016/0166-3542(87)90084-2. [DOI] [PubMed] [Google Scholar]
- 159.Bahbah E.I., Negida A., Nabet M.S. Purposing saikosaponins for the treatment of COVID-19. Med. Hypotheses. 2020;140:109782. doi: 10.1016/j.mehy.2020.109782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Buzzini P., Arapitsas P., Goretti M., Branda E., Turchetti B., Pinelli P., Ieri F., Romani A. Antimicrobial and antiviral activity of hydrolysable tannins. Mini Rev. Med. Chem. 2008;8(12):1179–1187. doi: 10.2174/138955708786140990. [DOI] [PubMed] [Google Scholar]
- 161.Sen S., Chakraborty R., Sridhar C., Reddy Y.S.R., De B. Free radicals, antioxidants, diseases and phytomedicines: current status and future prospect. Int. J. Pharmaceut. Sci. Rev. Res. 2010;3(1):91–100. [Google Scholar]
- 162.Uchide N., Toyoda H. Antioxidant therapy as a potential approach to severe influenza-associated complications. Molecules. 2011;16(3):2032–2052. doi: 10.3390/molecules16032032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Khalifa I., Zhu W., Nafie M.S., Dutta K., Li C. Anti-COVID-19 effects of ten structurally different hydrolysable tannins through binding with the catalytic-closed sites of COVID-19 main protease: an in-silico approach. Preprints. 2020 doi: 10.20944/preprints202003.0277.v1. 2020, 2020030277. [DOI] [Google Scholar]
- 164.Zhang R., Wei D.Q., Du Q.S., Chou K.C. Molecular modeling studies of peptide drug candidates against SARS. Med. Chem. 2006;2(3):309–314. doi: 10.2174/157340606776930736. [DOI] [PubMed] [Google Scholar]
- 165.Pandit R.D., Singh R.K. COVID-19 Ayurveda treatment protocol of governments of Nepal and India: a review and perspective. Applied Science and Technology Annals. 2020;1(1):72–80. doi: 10.3126/asta.v1i1.30276. [DOI] [Google Scholar]
- 166.Malik E.M., Müller C.E. Anthraquinones as pharmacological tools and drugs. Med. Res. Rev. 2016;36(4):705–748. doi: 10.1002/med.21391. [DOI] [PubMed] [Google Scholar]
- 167.Li Y., Jiang J.-G. Health functions and structure–activity relationships of natural anthraquinones from plants. Food & Function. 2018;9(12):6063–6080. doi: 10.1039/c8fo01569d. [DOI] [PubMed] [Google Scholar]
- 168.Schinazi R.F., Chu C.K., Babu J.R., Oswald B.J., Saalmann V., Cannon D.L., Eriksson B.F., Nasr M. Anthraquinones as a new class of antiviral agents against human immunodeficiency virus. Antivir. Res. 1990;13(5):265–272. doi: 10.1016/0166-3542(90)90071-e. [DOI] [PubMed] [Google Scholar]
- 169.Khanal P., Patil B.M., Chand J., Naaz Y. Anthraquinone derivatives as an immune booster and their therapeutic option against COVID-19. Natural Products and Bioprospecting. 2020;1–11 doi: 10.1007/s13659-020-00260-2. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Das S., Roy A.S. Naturally occurring anthraquinones as potential inhibitors of SARS-CoV-2 main protease: a molecular docking study. ChemRxiv. 2020;2020:1–24. doi: 10.26434/chemrxiv.12245270.v1. (Pre-print) [DOI] [Google Scholar]
- 171.Naithani R., Huma L.C., Holland L.E., Shukla D., McCormick D., Mehta R., Moriarty R.M. Antiviral activity of phytochemicals: a comprehensive review. Mini Rev. Med. Chem. 2008;8(11):1106–1133. doi: 10.2174/138955708785909943. [DOI] [PubMed] [Google Scholar]
- 172.Sydiskis R.J., Owen D.G., Lohr J.L., Rosler K.H., Blomster R.N. Inactivation of enveloped viruses by anthraquinones extracted from plants. Antimicrob. Agents Chemother. 1991;35(12):2463–2466. doi: 10.1128/aac.35.12.2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ul Qamar M.T., Alqahtani S.M., Alamri M.A., Chen L.L. Structural basis of SARS-CoV-2 3CLpro and anti-COVID-19 drug discovery from medicinal plants. Journal of Pharmaceutical Analysis. 2020;10(4):313–319. doi: 10.1016/j.jpha.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Da Silva Antonio A., Moreira Wiedemann L.S., Veiga-Junior V.F. Natural products' role against COVID-19. RSC Adv. 2020;10(39):23379–23393. doi: 10.1039/D0RA03774E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Pandit M., Latha N. In silico studies reveal potential antiviral activity of phytochemicals from medicinal plants for the treatment of COVID-19 infection. Research Square. 2020;2020 doi: 10.21203/rs.3.rs-22687/v1. (Pre-print) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.