Abstract
Background
Severe acute respiratory syndrome coronavirus 2, the disease-causing pathogen of the coronavirus disease 2019 pandemic, has resulted in morbidity and mortality worldwide. Pregnant women are more susceptible to severe coronavirus disease 2019 and are at higher risk of preterm birth than uninfected pregnant women. Despite this evidence, the immunologic effects of severe acute respiratory syndrome coronavirus 2 infection during pregnancy remain understudied.
Objective
This study aimed to assess the impact of severe acute respiratory syndrome coronavirus 2 infection during pregnancy on inflammatory and humoral responses in maternal and fetal samples and compare antibody responses to severe acute respiratory syndrome coronavirus 2 among pregnant and nonpregnant women.
Study Design
Immune responses to severe acute respiratory syndrome coronavirus 2 were analyzed using samples from pregnant (n=33) and nonpregnant (n=17) women who tested either positive (pregnant, 22; nonpregnant, 17) or negative for severe acute respiratory syndrome coronavirus 2 (pregnant, 11) at Johns Hopkins Hospital. We measured proinflammatory and placental cytokine messenger RNAs, neonatal Fc receptor expression, and tetanus antibody transfer in maternal and cord blood samples. In addition, we evaluated antispike immunoglobulin G, antispike receptor-binding domain immunoglobulin G, and neutralizing antibody responses to severe acute respiratory syndrome coronavirus 2 in serum or plasma collected from nonpregnant women, pregnant women, and cord blood.
Results
Pregnant women with laboratory-confirmed severe acute respiratory syndrome coronavirus 2 infection expressed more interleukin-1 beta, but not interleukin 6, in blood samples collected within 14 days vs >14 days after performing severe acute respiratory syndrome coronavirus 2 test. Pregnant women with laboratory-confirmed severe acute respiratory syndrome coronavirus 2 infection also had reduced antispike receptor-binding domain immunoglobulin G titers and were less likely to have detectable neutralizing antibody than nonpregnant women. Although severe acute respiratory syndrome coronavirus 2 infection did not disrupt neonatal Fc receptor expression in the placenta, maternal transfer of severe acute respiratory syndrome coronavirus 2 neutralizing antibody was inhibited by infection during pregnancy.
Conclusion
Severe acute respiratory syndrome coronavirus 2 infection during pregnancy was characterized by placental inflammation and reduced antiviral antibody responses, which may impact the efficacy of coronavirus disease 2019 treatment in pregnancy. In addition, the long-term implications of placental inflammation for neonatal health require greater consideration.
Key words: antibody, COVID-19, cytokine, maternal infection, pregnancy, SARS-CoV-2
Introduction
The ongoing coronavirus disease 2019 (COVID-19) pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has resulted in over 75 million infections and over 1.5 million deaths worldwide, as of December 2020.1 Despite global efforts to characterize the pathogenesis of SARS-CoV-2 infection, the effects of infection on immunity during pregnancy remain undefined. Because of pregnancy-associated immune and endocrine fluctuations, pregnant women and their fetuses are at greater risk of severe complications caused by infectious diseases.2 Most pregnant women with COVID-19 are asymptomatic or experience mild disease. However, the US Centers for Disease Control and Prevention (CDC) reports that 1 in 4 women, aged 15 to 49 years, hospitalized for COVID-19 during March 1, 2020, to August 22, 2020, was pregnant and that pregnant women were more likely to require mechanical ventilation than nonpregnant women.3 In addition, pregnant women are at increased risk of mortality following SARS-CoV-2 infection,4 prompting the CDC to revise their guidelines and include pregnant women as an at-risk population for severe COVID-19. SARS-CoV-2 surveillance of pregnant women in Washington state further reveals greater morbidity and mortality in pregnant women with SARS-CoV-2 infection and suggests possible underreporting in nationwide surveillance data.5 In addition to maternal morbidity and mortality, the CDC reports that women infected with SARS-CoV-2 during pregnancy are at higher risk of preterm birth.6 Because maternal immune activation can be associated with adverse fetal outcomes, including preterm birth,7 , 8 it is possible that SARS-CoV-2 infection during pregnancy may have harmful effects on the developing fetus.
AJOG at a Glance.
Why was this study conducted?
Inflammatory and humoral responses during severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection of pregnant women have not been extensively evaluated.
Key findings
Pregnant women who delivered <14 days after a positive SARS-CoV-2 test expressed more interleukin-1 beta messenger RNA in their blood than pregnant women who were uninfected or delivered >14 days after a confirmed SARS-CoV-2 test. Pregnant women with a confirmed SARS-CoV-2 infection had lower antispike receptor-binding domain immunoglobulin G titers and were less likely to have detectable neutralizing antibodies than nonpregnant women. Protein concentrations of placental neonatal Fc receptor, a receptor essential for maternal transfer of antibodies to the fetus were not affected by SARS-CoV-2 infection during pregnancy.
What does this add to what is known?
Our results have demonstrated potential differences in the pathogenesis of SARS-CoV-2 between pregnant and nonpregnant women, including inflammatory and antibody responses to the virus.
During pregnancy, a typical inflammatory response to pathogens includes the secretion of proinflammatory cytokines, such as interleukin-1 beta (IL-1β) and IL-6, not only at the site of infection but also in the placenta; these cytokines can readily enter the amniotic cavity and interfere with normal fetal development.7, 8, 9 Thus, even in the absence of severe maternal symptoms or fetal viral infection, the maternal immune response to SARS-CoV-2 could lead to short- and long-term consequences in the fetus and neonate.2 , 10 , 11 Simultaneously, the maternal immune response can also have a protective effect on neonatal health, including the placental Fc receptor (FcRn)-mediated transfer of SARS-CoV-2–specific antibodies transplacentally.12 , 13
In this study, we investigated the inflammatory and humoral responses to SARS-CoV-2 using maternal blood, cord blood, and placenta samples collected from pregnant women who had tested either positive or negative for SARS-CoV-2 before admission and delivery at the Johns Hopkins Hospital (JHH). We measured maternal and cord blood serum or plasma antispike (S) and anti-S receptor-binding domain (RBD) IgG and neutralizing antibody (nAb) responses to SARS-CoV-2, whole blood proinflammatory cytokine mRNA expression, and placental cytokine and FcRn expression. Furthermore, we compared the antibody responses of outpatient nonpregnant women with laboratory-confirmed SARS-CoV-2 infection.
Materials and Methods
Study participants, sample collection, and storage
Pregnancy cohort
Pregnant women were recruited by convenience sampling through JHH outpatient obstetrical clinics and the JHH labor and delivery unit before or after delivery of the patient. Here, we used discarded maternal blood, discarded neonatal cord blood, and a small placental sample collected during admission for delivery. Patients were contacted, informed of the study, and consented by phone to decrease face-to-face exposure owing to the concern of SARS-CoV-2 spread or infection. Basic demographic information and clinical data, including info on the history of SARS-CoV-2 testing (usually via nasopharyngeal swab), were collected from the patient’s medical record. Blood samples were collected in gold top serum separator tube (SST) tubes and purple top ethylenediaminetetraacetic acid tubes. The top SST tubes were inverted several times before being centrifuged for 10 minutes at 3000 rpm at 22°C. Furthermore, both maternal and cord whole blood and serum samples were aliquoted and stored at −80°C. Placental samples were collected after delivery and were not treated with any preservatives or reagents. Samples were processed using 2 methods for both the maternal and fetal sides; placental tissue was either frozen at −80°C immediately or was placed in RNA later for 48 hours before −80°C storage. To obtain tissue that was representative of the placental sample, half thickness samples using a disc tissue punch were taken from 2 locations on each side of the placenta. Thus, ultimately, each method of processing placental tissue had 2 tissue punches from different locations on a given side of the placenta.
Nonpregnant cohort
A convenience sample of nonhospitalized participants was recruited and provided informed consent by phone between April 21, 2020, and August 13, 2020, after receiving a positive SARS-CoV-2 reverse transcription-polymerase chain reaction (RT-PCR) test from an outpatient or emergency department facility within the Johns Hopkins Health System.14 Notably, 1 participant requested participation in the study via the Johns Hopkins HOPE (Hopkins Opportunities for Participant Engagement) COVID-19 registry. Samples from adult women of reproductive age, 18 to 49 years,15 with positive RT-PCR results for SARS-CoV-2 were included in this study. Basic demographic information and clinical data, including that regarding the history of SARS-CoV-2 testing, were collected from the patient and the patient’s medical records. Participants in this study attended a research clinic visit on average of 42.2 days after COVID-19 symptom onset (range 29–92 days), at which blood was drawn. Approximately 25 mL of whole blood was collected in acid citrate dextrose glass tubes. Peripheral blood mononuclear cells were separated, and the remaining plasma was stored in 1 mL aliquots at −80°C. Plasma was defrosted and then heat inactivated at 56°C for 30 minutes before serologic assays. The study was approved by the Johns Hopkins School of Medicine Institutional Review Board.
Gene expression analysis
Total RNA was extracted from placental tissue samples using the RNeasy Plus Mini Kit (Qiagen, Hilden, Germany) or from whole blood using NucleoSpin RNA Blood Kit (Macherey-Nagel, Düren, Germany). Complementary (c) DNA synthesis in a 40-μL reaction was performed using Bio-Rad iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA). TaqMan mRNA assays (Thermo Fisher Scientific, Waltham, MA) were run for analysis. The primers used were IL-1β (Integrated DNA Technologies, Coralville, IA) and IL-6 (Integrated DNA Technologies, Coralville, IA) (Table 1 ). mRNA expression was calculated relative to housekeeping genes: 18S (Hs99999901_s1; Applied Biosystems, Foster City, CA) and Actin (Integrated DNA Technologies, Coralville, IA) (Table 1).
Table 1.
Primer/Probe sequences
| IL-1β | |
|---|---|
| Forward | 5'-GAACAAGTCATCCTCATTGCC-3' |
| Reverse | 5'-CAGCCAATCTTCATTGCTCAAG-3' |
| Probe | 5'-/ /56-FAM/AGAAGTACC/ZEN/TGAGCTCGCCAGTGA/3IABkFQ//-3' |
| IL-6 | |
| Forward | 5'-GCAGATGAGTACAAAAGTCCTGA-3' |
| Reverse | 5'-TTCTGTGCCTGCAGCTTC-3' |
| Probe | 5'-/5Cy5/CAACCACAAATGCCAGCCTGCT/3IAbRQSp/-3' |
| Actin | |
| Forward | 5'-CCTTGCACATGCCGGAG-3' |
| Reverse | 5'-ACAGAGCCTCGCCTTTG-3' |
| Probe | 5'-/5Cy5/TCATCCATGGTGAGCTGGCGG/3IAbRQSp/-3' |
IL-1β, interleukin-1 beta; IL-6, interleukin 6.
Sherer et al. Coronavirus disease 2019 and pregnancy. Am J Obstet Gynecol 2021.
Indirect enzyme-linked immunosorbent assays
The protocol was adapted from a published protocol from Dr Florian Krammer’s laboratory,16 as described in Klein et al.17 Briefly, 96-well plates (Immulon 4HBX, Thermo Fisher Scientific, Waltham, MA) were coated with either full-length S protein or S-RBD at 4°C overnight. Coating buffer was removed, and plates were washed and then blocked for 1 hour at room temperature. All plasma samples were heat inactivated at 56°C on a heating block for 1 hour before use. Negative control samples were prepared at 1:10 dilutions and plated at a final concentration of 1:100. A monoclonal antibody against the SARS-CoV-2 S protein was used as a positive control (1:5000; catalog 40150-D001, Sino Biological, Beijing, China). For serial dilutions of plasma on either S- or S-RBD–coated plates, plasma samples were prepared in 3-fold serial dilutions starting at 1:20. Blocking solution was removed, and 10 μL diluted plasma was added in duplicate to the plates and incubated at room temperature for 2 hours. Plates were washed 3 times with PBST wash buffer, and 50 μL secondary antibody was added to the plates and incubated at room temperature for 1 hour (Fc-specific total IgG HRP 1:5000 dilution, catalog A18823, Invitrogen, Thermo Fisher Scientific, Waltham, MA). Plates were washed and all residual liquid removed before the addition of 100 μL SIGMAFAST OPD (o-phenylenediamine dihydrochloride) solution (MilliporeSigma, Burlington, MA) to each well, followed by incubation in darkness at room temperature for 10 minutes. To stop the reaction, 50 μL 3M HCl (Thermo Fisher Scientific, Waltham, MA) was added to each well. The optical density of each plate was read at 490 nm (OD490) on a SpectraMax i3 enzyme-linked immunosorbent assay (ELISA) Plate Reader (BioTek Instruments). The positive cutoff value for each plate was calculated by summing the average of the negative values and 3 times the standard deviation of the negatives. All values at or above the cutoff value were considered positive.
Microneutralization assay
The plasma nAb protocol was adapted from A.P.’s laboratory,18 as described in Klein et al.17 Briefly, an infectious virus (SARS-CoV-2/USA-WA1/2020) was added to 2-fold diluted plasma at a final concentration of 1×104 TCID50/mL (100 TCID50 per 100 μL). Samples were added to VeroE6-TMPRSS2 cells in sextuplet for 6 hours at 37°C. The inocula were removed, fresh infection media was added, and the plates were incubated at 37°C for 2 days. Cells were fixed by the addition of 150 μL of 4% formaldehyde per well, incubated for at least 4 hours at room temperature, and then stained with naphthol blue black (MilliporeSigma, Burlington, MA). The nAb titer was calculated as the highest serum dilution that eliminated the cytopathic effect in 50% of the wells.
Western blot
Western blotting was used to measure the protein expression of FcRn in the placenta. To prepare tissue lysate, tissue was homogenized on ice in a RIPA lysis buffer (Sigma-Aldrich, St. Louis, MO) with proteinase inhibitor (Sigma-Aldrich, St. Louis, MO) and phosphatase inhibitor cocktail 2 (Sigma-Aldrich, St. Louis, MO). The homogenized specimens were then placed on ice for 15 minutes and centrifuged at 14,000 rpm for 20 minutes at 4°C. The resulting supernatants were collected for further experiments. Total protein was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (Bio-Rad, Hercules, CA) using 4% to 15% gels (Bio-Rad, Hercules, CA) and then transferred onto nitrocellulose membranes (Bio-Rad, Hercules, CA) using semi-dry transfer device (Trans-Blot Turbo, Bio-Rad, Hercules, CA). Membranes were blocked with 5% bovine serum albumin (BSA; Sigma-Aldrich, St. Louis, MO) in Tris-buffered saline (Corning) plus 0.1% of Tween-20 (TBST; Sigma-Aldrich, St. Louis, MO) for 15 min at room temperature and incubated with primary antibodies in 5% of BSA at 4°C overnight and then washed using TBST. FcRn antibody (1:1000, Santa Cruz) and glyceraldehyde 3-phosphate dehydrogenase (GAPDH; control marker, 1:1000, Abcam) were used for primary antibodies. ECL (GE Healthcare, Chicago, IL) was used for detection using the ImageQuant LAS 500 (GE Healthcare, Chicago, IL), and densitometric analysis was performed using ImageJ (National Institutes of Health; http://rsb.info.nih.gov/ij/).
Statistical analysis
Descriptive statistics stratified by pregnancy state (pregnant women with SARS-CoV-2 infection and nonpregnant women with SARS-CoV-2 infection) are presented as medians and interquartile ranges (IQRs). Comparisons of demographic characteristics were tested via exact Wilcoxon 2-sample test, the Pearson chi-square test, or the Fisher exact test, where appropriate, dependent on the variable structure as continuous, binary, or categorical and sample size within individual cells. Before conducting any inferential statistics, area under the curve (AUC) values for anti-S IgG and anti–S-RBD IgG titers were computed by plotting normalized optical density values against sample dilution for ELISA. The AUC for microneutralization assays used the exact number of wells protected from infection at each plasma dilution. For each assay, samples with titers below the limit of detection were assigned an AUC value of half of the lowest measured AUC value. Because of the nonnormal distribution of cytokine and antibody data, comparisons between pregnant women with SARS-CoV-2 infection and nonpregnant women with SARS-CoV-2 infection were examined via exact Wilcoxon 2-sample tests. Correlations between antibody isotypes and assays with days since the initial SARS-CoV-2–positive test or days since symptom onset were assessed using the Spearman correlation coefficient. The data were then log transformed for visualization. Finally, a generalized linear model was used to determine if the association between days since the initial SARS-CoV-2–positive test or days since symptom onset and antibody responses differed by pregnancy status (pregnant or nonpregnant). All analyses were 2-tailed tests with a significance threshold of P<.05.
Results
Cohorts
Our study included 2 cohorts: the pregnant cohort, consisting of 33 pregnant women who tested either positive (n=22) or negative (n=11) for SARS-CoV-2 before delivery (in inpatient or labor and delivery settings) at the JHH, and the nonpregnant cohort, consisting of women of reproductive age (18–48 years of age), as defined by World Health Organization15 (n=17), who tested positive for SARS-CoV-2 at an outpatient clinical testing site within the JHH Health System. Comparing demographic characteristics between pregnant women with and without SARS-CoV-2 infection revealed differences in maternal age at delivery, race, and ethnicity. Pregnant women with SARS-CoV-2 infection gave birth at a younger age (median, 27; IQR, 23–34) than pregnant women without SARS-CoV-2 infection (median, 32; IQR, 29–35) (P<.05) (Table 2 ), were more likely to identify as other (63.64%) or black or African American (22.73%) than pregnant women without SARS-CoV-2 infection (P<.001) (Table 2), and were more likely to identify as being of Hispanic or Latina ethnicity (50%) (P<.05) (Table 2). No significant difference was found between pregnant patients with and without SARS-CoV-2 infection according to prepregnancy body mass index (BMI), BMI at delivery, gestational age at birth, neonate late-onset sepsis, chorioamnionitis, gestational nicotine use, time between membrane rupture and delivery, preeclampsia, gestational diabetes mellitus, gestational hypertension, delivery type (cesarean vs vaginal delivery), size of neonate, sex of neonate, neonatal intensive care unit stay, or neonatal readmission (Table 3 ). In comparing pregnant and nonpregnant women with SARS-CoV-2 infection, pregnant women were younger (pregnant median age, 27; IQR, 23–34; nonpregnant median age, 34; IQR, 28–41; P<.05) (Table 2), less likely to identify as white (14% vs 47%; P<.05) (Table 2), and more likely to identify as Hispanic or Latina (50% vs 6%; P<.05) (Table 2) than nonpregnant women.
Table 2.
SARS-CoV-2 (+) and (-) pregnant women differed in age, race, and ethnicity
|
Variable |
Pregnant female cohort |
Pregnant vs nonpregnant female cohort |
|||||
|---|---|---|---|---|---|---|---|
| All | SARS-CoV-2 (+) | SARS-CoV-2 (−) | P value | Pregnant women with SARS-CoV-2 (+) | Nonpregnant women with SARS-CoV-2 (+) | P value | |
| n (%) | 33 | 22 (66.67) | 11 (33.33) | 22 | 17 | ||
| Median maternal age at delivery | 29 | 27 | 32 | .0421 | 27 | 34 | .0061 |
| Race, n (%) | |||||||
| Asian | 2 (6.06) | 0 (0) | 2 (18.8) | <.0001 | 0 (0) | 0 (0) | .0111 |
| Black or African American | 6 (18.18) | 5 (22.73) | 1 (9.09) | 5 (22.73) | 6 (35.29) | ||
| Other | 14 (42.42) | 14 (63.64) | 0 (0) | 14 (63.64) | 3 (17.65) | ||
| White | 11 (33.33) | 3 (13.64) | 8 (72.73) | 3 (13.64) | 8 (47.06) | ||
| Ethnicity, n (%) | |||||||
| Hispanic or Latina | 12 (36.36) | 11 (50) | 1 (9.09) | .0273 | 11 (50) | 1 (5.88) | .0031 |
| Not Hispanic or Latina | 21 (63.64) | 11 (50) | 10 (90.91) | 11 (50) | 16 (94.12) | ||
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Sherer et al. Coronavirus disease 2019 and pregnancy. Am J Obstet Gynecol 2021.
Table 3.
Factors that were not significant between infection groups
| Pregnant female cohort | All | SARS-CoV-2 (+) | SARS-CoV-2 (−) | P value |
|---|---|---|---|---|
| n (%) | 33 | 22 (66.67) | 11 (33.33) | |
| Median BMI (prepregnancy) | 24.99 | 26.30 | 23.67 | .2713 |
| Median BMI (at delivery) | 29.48 | 29.80 | 28.08 | .144 |
| Median gestational age at delivery | 39.10 | 38.85 | 39.50 | .0725 |
| Median neonate late-onset sepsis | 2 | 2 | 2 | .4451 |
| Chorioamnionitis, n (%) | ||||
| Yes | 2 (6.06) | 2 (9.09) | 0 (0) | .5417 |
| No | 31 (93.94) | 20 (90.91) | 11 (100.00) | |
| Gestational nicotine use, n (%) | ||||
| Yes | 2 (6.06) | 1 (4.55) | 1 (9.09) | 1 |
| No | 31 (93.94) | 21 (95.45) | 10 (90.91) | |
| Membrane rupture >18 h before delivery, n(%) | 4 | 4 | 0 | .132 |
| Yes | 4 (12.12) | 4 (18.18) | 0 (0) | .2755 |
| No | 29 (87.88) | 18 (81.82) | 11 (100.00) | |
| Preeclampsia, n (%) | ||||
| Yes | 2 (6.06) | 2 (9.09) | 0 (0) | .5417 |
| No | 31 (93.94) | 20 (90.91) | 11 (100.00) | |
| Gestational diabetes, n (%) | ||||
| Yes | 2 (6.06) | 2 (9.09) | 0 (0) | .5417 |
| No | 31 (93.94) | 20 (90.91) | 11 (100.00) | |
| Gestational hypertension, n (%) | ||||
| Yes | 3 (9.09) | 2 (9.09) | 1 (9.09) | 1 |
| No | 30 (90.91) | 20 (90.91) | 10 (90.91) | |
| Delivery type, n (%) | ||||
| Cesarean delivery | 12 (36.36) | 7 (31.82) | 5 (45.45) | .4713 |
| Vaginal delivery | 21 (63.64) | 15 (68.18) | 6 (54.55) | |
| Size of neonate, n (%) | ||||
| AGA | 26 (78.79) | 17 (77.27) | 9 (81.82) | 1 |
| LGA | 5 (15.15) | 3 (13.64) | 2 (18.18) | |
| SGA | 2 (6.06) | 2 (9.09) | 0 (0) | |
| Sex of neonate, n (%) | ||||
| Female | 18 (54.55) | 13 (59.09) | 5 (45.45) | .4583 |
| Male | 15 (45.45) | 9 (40.91) | 6 (54.55) | |
| NICU stay, n (%) | ||||
| Yes | 4 (12.12) | 4 (18.18) | 0 (0) | .2755 |
| No | 29 (87.88) | 18 (81.82) | 11 (100.00) | |
| Neonate readmission, n (%) | ||||
| Yes | 1 (3.03) | 0 (0) | 1 (9.09) | .3333 |
| No | 32 (96.97) | 22 (100.00) | 10 (90.91) |
AGA, appropriate for gestational age; BMI, body mass index; LGA, large for gestational age; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SGA, small for gestational age.
Sherer et al. Coronavirus disease 2019 and pregnancy. Am J Obstet Gynecol 2021.
Cytokine expression after severe acute respiratory syndrome coronavirus 2 infection during pregnancy
Increased inflammation caused by infection during pregnancy can be detrimental to long-term fetal and neonatal outcomes.2 , 10 , 11 We assayed cytokine mRNA expression during SARS-CoV-2 infection as a biomarker for inflammation. Because IL-1β activation during pregnancy can cause adverse fetal outcomes,2 , 19 , 20 we measured IL-1β mRNA expression in maternal blood (total, 27; positive, 18; negative, 9), cord blood (total, 29; positive, 20; negative, 9), and the maternal (total, 11; positive, 8; negative, 1) and fetal (total, 26; positive, 19; negative, 7) sides of the placentas, which did not differ between pregnant women with and without SARS-CoV-2 infection (Figure 1 , A–D). To assess whether the expression of IL-1β differed depending on the number of days between a pregnant woman’s PCR test and blood sample collection, maternal blood IL-1β mRNA expression was compared on the basis of the time window between diagnosis and blood collection (total, 27; positive, 18; negative, 9). Day 14 was chosen for analysis based on the incubation period of SARS-CoV-2, which extends to 14 days after symptom onset.21 IL-1β expression in maternal blood was higher in samples collected within 14 days of a positive SARS-CoV-2 test than samples collected >14 days after the test, representative of an acute, as opposed to chronic, inflammatory response (P<.05) (Figure 1, E).
Figure 1.
IL-1β expression in maternal and fetal samples
Maternal and fetal blood and placentas were used to detect IL-1β gene expression relative to the HKGs, 18S and ACTB. A–D, Maternal blood, cord blood, and maternal and fetal side placental IL-1β expression between pregnant patients with SARS-CoV-2 infection (P[+]) and pregnant patients without SARS-CoV-2 infection (P[−]). E, Maternal blood IL-1β expression analyzed as a function of symptom expression and days between PCR tests positive for SARS-CoV-2 and blood sample collection; the dashed line indicates onset of symptoms at day 14; significance denotes comparison of samples collected within 14 days of a positive SARS-CoV-2 test with samples collected >14 days after test. Maternal blood (27); cord blood (29); maternal side placenta (11); and fetal side placenta (26). The single asterisk represents P<.05 using the Kruskal-Wallis, Dunn multiple comparison, or Mann-Whitney test.
IL-1β, interleukin-1 beta; HKG, housekeeping gene; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Sherer et al. Coronavirus disease 2019 and pregnancy. Am J Obstet Gynecol 2021.
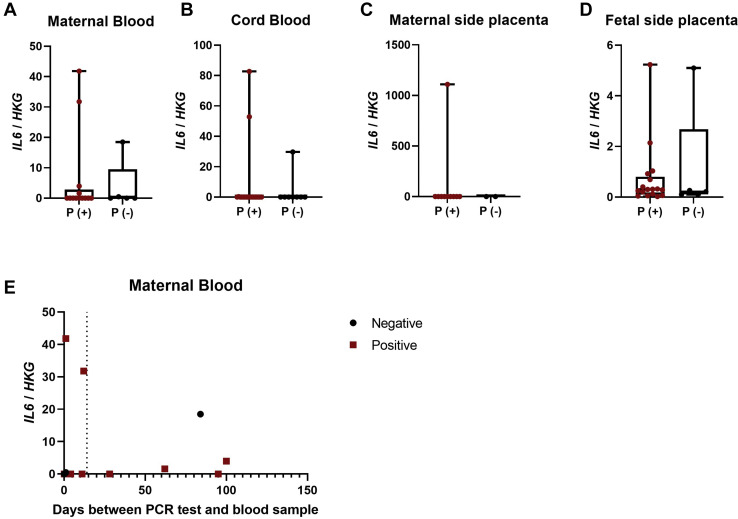
We measured IL-6 mRNA expression in maternal and fetal blood and tissue from our pregnant cohort with and without SARS-CoV-2 infection. It is important to note that all women with SARS-CoV-2 infection experience mild to moderate symptoms of COVID-19. In contrast to the elevation observed among severe COVID-19 cases in nonpregnant individuals,22, 23, 24 there was no change in the expression of IL-6 in blood or placentas based on SARS-CoV-2 infection status ( Figure 2 , A–D) or duration between a positive SARS-CoV-2 test and sample collection (Figure 2, E). These data provide evidence that IL-1β mRNA, in particular, is up-regulated early after infection and on the fetal side of the placenta in nonseverely ill pregnant women with SARS-CoV-2 infection.
Figure 2.
IL-6 expression in maternal and fetal samples
Maternal and fetal blood and placentas were used to detect IL-6 gene expression relative to the HKGs, 18S and ACTB. A–D, Maternal blood, cord blood, and maternal and fetal side placental IL-6 expression between pregnant patients with SARS-CoV-2 infection (P[+]) and pregnant patients without SARS-CoV-2 infection (P[−]). E–H, Maternal blood, cord blood, and maternal and fetal side placental IL-6 expression in pregnant women who were asymptomatic (P-A), symptomatic (P-S), or SARS-CoV-2 negative (P-N). I, Maternal blood IL-6 expression analyzed as a function of symptom expression and days between PCR tests positive for SARS-CoV-2 and blood sample collection; the dashed line indicates onset of symptoms at day 14. Maternal blood (27); cord blood (29); maternal side placenta (11); and fetal side placenta (26).
IL-6, interleukin 6; HKG, housekeeping gene; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Sherer et al. Coronavirus disease 2019 and pregnancy. Am J Obstet Gynecol 2021.
Antibody responses to sever acute respiratory syndrome coronavirus 2 in pregnant and nonpregnant women
To evaluate the impact of pregnancy on humoral responses to SARS-CoV-2, antibody responses measured in serum or plasma samples were collected at a median of 34 days (IQR, 31.5–40) since confirmed infection, from pregnant (18.91±29.57 days postconfirmed infection) (n=17) and nonpregnant (37.29±12.66 days postconfirmed infection) (n=17) women who tested positive for SARS-CoV-2. Pregnant and nonpregnant women showed similar titration of IgG (ie, AUC) recognizing the full-length SARS-CoV-2 spike (S) protein (Figure 3 , A). In contrast, pregnant women had significantly lower anti–S-RBD IgG titers than nonpregnant women (P<.05) (Figure 3, B). However, titers of nAb, which correlate with anti–S-RBD antibodies,25 were measured and were not significantly different between pregnant and nonpregnant women (Figure 3, C). Furthermore, we observed that significantly fewer pregnant women (8 of 17) had detectable nAb titers (ie, ≥1:20 titer) than nonpregnant women (16 of 17) (P<.05) (Figure 3, C), indicating reduced production of neutralizing antibodies in a subset of pregnant women.
Figure 3.
Anti–SARS-CoV-2 antibody in samples from pregnant and nonpregnant women
Peripheral serum or plasma was used to titer IgG antibodies against SARS-CoV-2 full-length spike (S), S receptor-binding domain, and whole virus nAbs. A, Anti-S IgG; B, anti–S-RBD IgG; and C, nAb AUC titrations in serum or plasma from pregnant (P) (n=17) and nonpregnant (NP) (n=17) women. The dashed line denotes the median AUC for SARS-CoV-2–negative samples. Above each boxplot is the proportion of samples with detectable antibody; the single asterisk represents P<.05 using the Kruskal-Wallis, Dunn multiple comparisons, Wilcoxon exact, or chi-square tests.
AUC, area under curve; IgG, immunoglobulin G; nAb, neutralizing antibody; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Sherer et al. Coronavirus disease 2019 and pregnancy. Am J Obstet Gynecol 2021.
To further explore how pregnancy altered the relationship between anti–S-RBD IgG and nAb, titers were directly compared and revealed that anti–S-RBD IgG titers were higher than nAb titers in both pregnant and nonpregnant women (P<.001) (Figure 4 , A and B). Among pregnant women only, a dichotomy in nAb titers was evident. Consistent with this observation, pregnant women with low nAb titers of <1:20 (ie, no detectable nAb) also had lower anti–S-RBD IgG titers (r=0.9023; P<.001). Furthermore, pregnant women with <1:20 nAb titers had significantly lower anti–S-RBD IgG responses than pregnant women with nAb titers >1:20 (P<.05) (Figure 4, A). To determine whether time since a SARS-CoV-2–positive test or time since symptom onset could predict antibody responses, we analyzed responses over time. Variation in anti–S-RBD IgG or nAb responses among pregnant women with nondetectable vs detectable nAb titers could not be explained by the length of time since a positive SARS-CoV-2 test (Figure 4, C and D). Furthermore, time since symptom onset did not explain the variation in anti–S-RBD IgG or nAb responses among pregnant women with nondetectable nAb titers compared with pregnant women with detectable nAb titers (Figure 4, E and F). Differences in the number of days between a PCR+ test or symptom onset and sample collection also did not statistically explain variation in either anti–S-RBD IgG or nAb responses between pregnant and nonpregnant women (Figure 4, C–F). These data suggest that, independent of time, pregnancy reduces the quality of antiviral antibodies against SARS-CoV-2 (pregnant, 17; nonpregnant, 17).
Figure 4.
Association between antispike-RBD IgG and nAb in pregnant and nonpregnant women
A, Comparison between anti–S-RBD IgG and nAb AUC in pregnant women, with additional comparison of anti–S-RBD IgG and nAb responses between pregnant with (nAb titer ≥1:20) and without (nAb titers <1:20) detectable nAb. B, Comparison between anti–S-RBD IgG and nAb AUC in nonpregnant women. C and D, Anti–S-RBD IgG AUC and nAb analyzed as a function of detectability of nAb and days between PRC tests positive for SARS-CoV-2 and blood sample. E and F, Anti–S-RBD IgG AUC and nAb analyzed as a function of detectability of nAb and days since symptom onset and blood collection; there are missing data points because of unknown symptom onset date (n=4). Single asterisk represents P<.05 using the Wilcoxon exact test.
AUC, area under curve; IgG, immunoglobulin G; nAb, neutralizing antibody; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Sherer et al. Coronavirus disease 2019 and pregnancy. Am J Obstet Gynecol 2021.
Antibody transfer in severe acute respiratory syndrome coronavirus 2 infection
To assess whether antibody transfer from mother to fetus was broadly affected by SARS-CoV-2 infection, SARS-CoV-2–specific antibody levels in maternal (n=17) and cord blood (n=17) serum, FcRn expression, and antitetanus IgG titers were assessed in women with SARS-CoV-2 infection (n=22) and women without SARS-CoV-2 infection (n=11). Anti-S and anti–S-RBD IgG titers did not differ between maternal and cord blood serum samples (Figure 5 , A and B); however, titers of nAb in maternal serum were significantly greater than in cord blood serum (P<.05) (Figure 5, C). Semiquantitative protein concentrations of placental FcRn, used as a biomarker of IgG transfer, were not affected by SARS-CoV-2 infection during pregnancy (Figure 5, D). To further evaluate whether SARS-CoV-2 infection altered the transfer of other antibodies from mother to fetus, maternal and cord blood serum antitetanus IgG titers were measured and were not inhibited by SARS-CoV-2 infection during pregnancy (Figure 5, E and F). These data suggest that although maternal transfer of SARS-CoV-2–specific nAb may be reduced, SARS-CoV-2 infection does not impact semiquantitative protein concentrations of placental FcRn or maternal transfer of antitetanus IgG.
Figure 5.
Effects of SARS-CoV-2 infection on antibody transfer from mother to fetus
A, Anti–S IgG; (B) anti–S-RBD IgG; and (C) nAb AUC titrations in maternal serum and cord blood serum in pregnant women with SARS-CoV-2 infection. D, Western blot analysis for the neonatal Fc receptor (FcRn) protein in placentas from women with SARS-CoV-2 (+) and without SARS-CoV-2 (−) infection (n=35). E, Quantification of FcRn western blot analysis relative to GAPDH was analyzed in placentas from women with SARS-CoV-2 (P[+]) and without SARS-CoV-2 (P[−]) infection (n=35). F and G, Maternal and cord blood serum antitetanus IgG titers in SARS-CoV-2–positive and SARS-CoV-2–negative samples in the pregnant cohort; maternal serum (35) and cord blood serum (21).
AUC, area under curve; FcRn, neonatal Fc receptor; IgG, immunoglobulin G; nAb, neutralizing antibody; RBD, receptor-binding domain; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Sherer et al. Coronavirus disease 2019 and pregnancy. Am J Obstet Gynecol 2021.
Discussion
Principal findings
Our study provides preliminary evidence that pregnant women exhibit an inflammatory response in maternal blood within 14 days of a PCR+ test, exhibit lower anti–S-RBD IgG titers, and are less likely to have detectable nAb than nonpregnant women. Protein concentrations of placental FcRn, a receptor essential for maternal transfer of antibodies to the fetus, were not affected by SARS-CoV-2 infection during pregnancy; however, reduced nAb responses against SARS-CoV-2 were detected in cord blood. These results suggested that, during pregnancy, there is an acute increase in IL-1β mRNA expression and reduced antiviral antibody responses during SARS-CoV-2 infection.
Results
The inflammatory response of pregnant women who experienced mild to moderate COVID-19 symptoms was characterized by greater IL-1β, but not IL-6, mRNA expression as has been reported in severe male and nonpregnant female with COVID-19.23 , 24 Current studies highlight differences in clinical manifestations between pregnant and nonpregnant women with SARS-CoV-2 infection, with some studies reporting differences in presenting symptoms, such as lower incidence of fever and cough in pregnant women.26 , 27 There is growing evidence that pregnant women with SARS-CoV-2 infection face greater risk of hospitalization, intensive care unit admission, invasive ventilation, and death than nonpregnant women with SARS-CoV-2 infection.4 , 5 , 28 Studies in SARS-CoV-2 infection report higher frequencies of neutrophils and D-dimer concentrations and lower percentages of lymphocytes, CD4+ and CD8+ ratios, and IgG levels in pregnant women than nonpregnant women.29, 30, 31, 32 Thus, our study adds to the growing literature demonstrating enhanced inflammatory responses and reduced humoral responses during SARS-CoV-2 infection of pregnant and nonpregnant women.
The antiviral response to SARS-CoV-2 includes development of antibodies that recognize the S-RBD and neutralize virus.33 Detection of anti–SARS-CoV-2 IgG antibodies in maternal and neonatal blood following infection has been reported34, 35, 36, 37, 38; however, how pregnancy status affect detection (qualitative) and titers (quantitative) of both anti–SARS-CoV-2 IgG and nAb responses has not been previously investigated. Here, we demonstrated that pregnant women infected with SARS-CoV-2 had lower titers of anti–S-RBD IgG than nonpregnant women infected with SARS-CoV-2. Although nAb titers were similar between pregnant and nonpregnant women, pregnant women were significantly less likely to have detectable nAb responses. Furthermore, pregnant women infected with SARS-CoV-2 who had nondetectable nAb responses had significantly lower anti–S-RBD IgG titers. Reduced antiviral antibody responses in pregnant women infected with SARS-CoV-2 were independent of time since infection. Other longitudinal studies evaluating antibody responses across gestational time points illustrate that neutralizing antibody is detectable in only 52.9% of pregnant women with SARS-CoV-2 infection, with no change over gestation; thus, reduced nAb titers in a subset of pregnant women are independent of time since infection.38 Furthermore, pregnant women with low antibody titers do not present with worse symptoms or experience worse disease outcomes, similar to studies in nonpregnant adults.39 , 40 We hypothesized that reduced antiviral antibody titers could increase the potential for reinfection following pregnancy, especially to variant viruses. Although we observed reduced titers of anti–S-RBD IgG in pregnant women compared with nonpregnant women, other studies report no difference in anti–S-RBD IgG titers between pregnant and nonpregnant women.37 Without complete details about how assays are standardized, it is difficult to compare results. The serologic assays used in this study have been well characterized and validated.16 , 17 , 41 It is well established that nAb titers are correlated with anti–S-RBD titers in nonpregnant individuals.17 Our observation that nAb titers and anti–S-RBD titers are correlated not only in nonpregnant women but also in pregnant women is clinically novel and adds to the growing literature in this field.
Despite reduced SARS-CoV-2 nAb titers in cord blood, semiquantitative protein concentrations of placental FcRn, responsible for placental IgG transfer, were not affected by SARS-CoV-2 infection during pregnancy. Similar results have been found in other cohorts, in which reduced SARS-CoV-2–specific placental antibody transfer is observed in pregnant women with infection, without differences in overall placental FcRn expression between women with and without SARS-CoV-2 infection being reported.36 , 37 The Fc-glycosylation in the third trimester of pregnancy of women with SARS-CoV-2 infection was also perturbed, which could impact the transfer of SARS-CoV-2–specific antibodies.37 In addition to tetanus-specific antibodies, influenza and pertussis-specific antibody transfer was not affected by SARS-CoV-2 infection.37 Overall, these results reiterate that non-SARS-CoV-2–specific antibody transfer is intact in women with SARS-CoV-2 infection but that SARS-CoV-2–specific antibody transfer mechanisms may be compromised by infection.
Clinical implications
These preliminary observations have suggested that pregnant women who are infected with SARS-CoV-2 may have an altered cytokine and humoral response compared with nonpregnant women with SARS-CoV-2 infection, which must be verified in a larger clinical cohort. Specifically, we reported reduced anti–S-RBD IgG responses and a reduction of nAb production in a subset of pregnant women, suggesting that humoral immunity to SARS-CoV-2 infection during pregnancy is reduced in pregnant women compared with nonpregnant individuals. Increased cytokine activation at the maternal-fetal interface can have adverse implications for the developing fetus42; therefore, children born to mothers infected with SARS-CoV-2 during pregnancy should be longitudinally observed to assess long-term outcomes. With mRNA-based vaccines against SARS-CoV-2 now available, the unique biologic state of pregnancy needs be considered.43 None of the SARS-CoV-2 vaccine candidates included pregnant women in their phase III trials. The CDC acknowledges the lack of data for vaccine efficacy in pregnant women and urges women to consult with their healthcare provider before vaccination.44 This is a real burden to place on pregnant women. We urge greater use of animal models to assess the immunogenicity and reactogenicity of the approved SARS-CoV-2 vaccine platforms to provide some indication of how pregnancy may or may not alter responses, adverse reactions, and protection from infection and disease.43
Limitations
The limitations of this study included the small sample size and significant differences in age, race, and ethnicity between pregnant and nonpregnant women with SARS-CoV-2 infection. These differences were attributable to our reliance on convenience sampling and were a result of differences in participant recruitment, in which sample collection from pregnant women was based on time of delivery and sample collection from nonpregnant women was based on symptom presentation. Although there was a significant difference in age between the cohorts, all women in this study were within reproductive ages.15 Because of our inability to know precisely when each participant was infected with SARS-CoV-2, we used the number of days between a SARS-CoV-2 PCR test and blood collection as the metric to assess cytokine responses and in addition used the number of days since symptom onset to evaluate humoral responses over time. These metrics may not accurately represent the time since initial infection, as symptom onset is self-reported and studies have reported PCR positivity for extended periods past the initial infection.45 , 46 Furthermore, differences in blood volume among individuals and throughout gestation in pregnant women could lead to variability in antibody titers.
Conclusions
Our results demonstrated potential differences in the pathogenesis of SARS-CoV-2, including inflammatory and antibody responses to the virus, between pregnant and nonpregnant women. It is well established that immune responses change dramatically during pregnancy to accommodate the developing fetus.47 Therefore, understanding the impact of SARS-CoV-2 infection during pregnancy on the maternal immune system and how these changes alter maternal and fetal susceptibility to disease are crucial for developing vaccines and other therapeutics for COVID-19. In addition to further investigations of short- and long-term consequences of SARS-CoV-2 infection in pregnancy, the safety, immunogenicity, and efficacy of SARS-CoV-2 vaccines in pregnant women must be considered.
Acknowledgments
The authors thank patients who enrolled and participated in this research and the nurses and staff at the Johns Hopkins Hospitals for assistance with recruitment and sample collection from patients. We thank the National Institute of Infectious Diseases, Japan, for providing VeroE6TMPRSS2 cells and acknowledge the Centers for Disease Control and Prevention, Biodefense and Emerging Infections Research Resources Repository, National Institute of Allergy and Infectious Diseases, and National Institute of Health for severe acute respiratory syndrome–related coronavirus 2, isolate USA-WA1/2020, NR-5228. The authors would also like to thank Janna Shapiro for assistance in figure development.
Footnotes
The authors report no conflict of interest.
This work was supported by grants from the National Institutes of Health: the Eunice Kennedy Shriver National Institute of Child Health and Development (grant numbers R01HD097608 [I.B. and S.L.K.] and R21HD099000 [I.B.]), the National Cancer Institute (grant number U54CA260492 [S.L.K.]), and the National Institute of Allergy and Infectious Diseases (grant numbers HHSN272201400007C [A.P.] and T32AI007417 [M.L.S. and R.L.U.]).
Cite this article as: Sherer ML, Lei J, Creisher PS, et al. Pregnancy alters interleukin-1 beta expression and antiviral antibody responses during severe acute respiratory syndrome coronavirus 2 infection. Am J Obstet Gynecol 2021;225:301.e1-14.
Supplementary Data
References
- 1.Johns Hopkins Coronavirus Resource Center COVID-19 data in motion: Wednesday, March 31, 2021. 2021. https://coronavirus.jhu.edu/ Available at: Accessed Jan. 3, 2021.
- 2.Prochaska E., Jang M., Burd I. COVID-19 in pregnancy: placental and neonatal involvement. Am J Reprod Immunol. 2020;84:e13306. doi: 10.1111/aji.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Delahoy MJ, Whitaker M, Chai SJ, et al. Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory-confirmed COVID-19 - COVID-NET, 13 states, March 1-August 22, 2020. 2020;69:1347–1354. [DOI] [PMC free article] [PubMed]
- 4.Zambrano L.D., Ellington S., Strid P., et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, January 22-October 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lokken E.M., Huebner E.M., Gray Taylor G., et al. Disease severity, pregnancy outcomes, and maternal deaths among pregnant patients with severe acute respiratory syndrome coronavirus 2 infection in Washington State. Am J Obstet Gynecol. 2021 doi: 10.1016/j.ajog.2020.12.1221. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Woodworth K.R., Olsen E.O., Neelam V., et al. Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy - SET-NET, 16 jurisdictions, March 29-October 14, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1635–1640. doi: 10.15585/mmwr.mm6944e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yockey L.J., Iwasaki A. Interferons and proinflammatory cytokines in pregnancy and fetal development. Immunity. 2018;49:397–412. doi: 10.1016/j.immuni.2018.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Racicot K., Mor G. Risks associated with viral infections during pregnancy. J Clin Invest. 2017;127:1591–1599. doi: 10.1172/JCI87490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chudnovets A., Liu J., Narasimhan H., Liu Y., Burd I. Role of inflammation in virus pathogenesis during pregnancy. J Virol. 2020;95 doi: 10.1128/JVI.01381-19. e01381–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Estes M.L., McAllister A.K. Maternal immune activation: implications for neuropsychiatric disorders. Science. 2016;353:772–777. doi: 10.1126/science.aag3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mor G., Cardenas I. The immune system in pregnancy: a unique complexity. Am J Reprod Immunol. 2010;63:425–433. doi: 10.1111/j.1600-0897.2010.00836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Albrecht M., Arck P.C. Vertically transferred immunity in neonates: mothers, mechanisms and mediators. Front Immunol. 2020;11:555. doi: 10.3389/fimmu.2020.00555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flannery D.D., Gouma S., Dhudasia M.B., et al. Assessment of maternal and neonatal cord blood SARS-CoV-2 antibodies and placental transfer ratios. JAMA Pediatr. 2021 doi: 10.1001/jamapediatrics.2021.0038. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blair P.W., Brown D., Jang M., et al. The clinical course of COVID-19 in the outpatient setting: a prospective cohort study. Open Forum Infect Dis. 2021;8:ofab007. doi: 10.1093/ofid/ofab007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.World Health Organization . World Health Organization; Geneva: 2006. Reproductive health indicators: guidelines for their generation, interpretation and analysis for global monitoring; p. 9. [Google Scholar]
- 16.Stadlbauer D., Amanat F., Chromikova V., et al. SARS-CoV-2 seroconversion in humans: a detailed protocol for a serological assay, antigen production, and test setup. Curr Protoc Microbiol. 2020;57:e100. doi: 10.1002/cpmc.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Klein S.L., Pekosz A., Park H.S., et al. Sex, age, and hospitalization drive antibody responses in a COVID-19 convalescent plasma donor population. J Clin Invest. 2020;130:6141–6150. doi: 10.1172/JCI142004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schaecher S.R., Stabenow J., Oberle C., et al. An immunosuppressed Syrian golden hamster model for SARS-CoV infection. Virology. 2008;380:312–321. doi: 10.1016/j.virol.2008.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chudnovets A., Lei J., Na Q., et al. Dose-dependent structural and immunological changes in the placenta and fetal brain in response to systemic inflammation during pregnancy. Am J Reprod Immunol. 2020;84:e13248. doi: 10.1111/aji.13248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Basu S., Agarwal P., Anupurba S., Shukla R., Kumar A. Elevated plasma and cerebrospinal fluid interleukin-1 beta and tumor necrosis factor-alpha concentration and combined outcome of death or abnormal neuroimaging in preterm neonates with early-onset clinical sepsis. J Perinatol. 2015;35:855–861. doi: 10.1038/jp.2015.86. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention Symptoms of coronavirus. 2021. https://www.cdc.gov/coronavirus/2019-ncov/symptoms-testing/symptoms.html Available at:
- 22.Del Valle D.M., Kim-Schulze S., Huang H.H., et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhu J., Pang J., Ji P., et al. Elevated interleukin-6 is associated with severity of COVID-19: a meta-analysis. J Med Virol. 2021;93:35–37. doi: 10.1002/jmv.26085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zeng F., Huang Y., Guo Y., et al. Association of inflammatory markers with the severity of COVID-19: a meta-analysis. Int J Infect Dis. 2020;96:467–474. doi: 10.1016/j.ijid.2020.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wajnberg A., Amanat F., Firpo A., et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science. 2020;370:1227–1230. doi: 10.1126/science.abd7728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu F., Liu H., Hou L., et al. Clinico-radiological features and outcomes in pregnant women with COVID-19 pneumonia compared with age-matched non-pregnant women. Infect Drug Resist. 2020;13:2845–2854. doi: 10.2147/IDR.S264541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gao Y.J., Ye L., Zhang J.S., et al. Clinical features and outcomes of pregnant women with COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 2020;20:564. doi: 10.1186/s12879-020-05274-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delahoy M.J., Whitaker M., O’Halloran A., et al. Characteristics and maternal and birth outcomes of hospitalized pregnant women with laboratory-confirmed COVID-19 - COVID-NET, 13 states, March 1-August 22, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1347–1354. doi: 10.15585/mmwr.mm6938e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu S., Shao F., Bao B., et al. Clinical manifestation and neonatal outcomes of pregnant patients with coronavirus disease 2019 pneumonia in Wuhan, China. Open Forum Infect Dis. 2020;7:ofaa283. doi: 10.1093/ofid/ofaa283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei L., Gao X., Chen S., et al. Clinical characteristics and outcomes of childbearing-age women with COVID-19 in Wuhan: retrospective, single-center study. J Med Internet Res. 2020;22:e19642. doi: 10.2196/19642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cheng B., Jiang T., Zhang L., et al. Clinical characteristics of pregnant women with coronavirus disease 2019 in Wuhan, China. Open Forum Infect Dis. 2020;7:ofaa294. doi: 10.1093/ofid/ofaa294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mohr-Sasson A., Chayo J., Bart Y., et al. Laboratory characteristics of pregnant compared to non-pregnant women infected with SARS-CoV-2. Arch Gynecol Obstet. 2020;302:629–634. doi: 10.1007/s00404-020-05655-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amanat F., Krammer F. SARS-CoV-2 vaccines: status report. Immunity. 2020;52:583–589. doi: 10.1016/j.immuni.2020.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zeng H., Xu C., Fan J., et al. Antibodies in infants born to mothers with COVID-19 pneumonia. JAMA. 2020;323:1848–1849. doi: 10.1001/jama.2020.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Flannery D.D., Gouma S., Dhudasia M.B., et al. SARS-CoV-2 seroprevalence among parturient women in Philadelphia. Sci Immunol. 2020;5 doi: 10.1126/sciimmunol.abd5709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Edlow A.G., Li J.Z., Collier A.Y., et al. Assessment of maternal and neonatal SARS-CoV-2 viral load, transplacental antibody transfer, and placental pathology in pregnancies during the COVID-19 pandemic. JAMA Netw Open. 2020;3 doi: 10.1001/jamanetworkopen.2020.30455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atyeo C., Pullen K.M., Bordt E.A., et al. Compromised SARS-CoV-2-specific placental antibody transfer. Cell. 2021;184:628–642.e10. doi: 10.1016/j.cell.2020.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cosma S., Carosso A.R., Corcione S., et al. Longitudinal analysis of antibody response following SARS-CoV-2 infection in pregnancy: from the first trimester to delivery. J Reprod Immunol. 2021;144:103285. doi: 10.1016/j.jri.2021.103285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zohar T., Alter G. Dissecting antibody-mediated protection against SARS-CoV-2. Nat Rev Immunol. 2020;20:392–394. doi: 10.1038/s41577-020-0359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu F, Wang A, Liu M, et al. Neutralizing antibody responses to SARS-CoV-2 in a COVID-19 recovered patient cohort and their implications. medRxiv 10.1101/2020.03.30.20047365 [DOI]
- 41.Klumpp-Thomas C., Kalish H., Drew M., et al. Standardization of ELISA protocols for serosurveys of the SARS-CoV-2 pandemic using clinical and at-home blood sampling. Nat Commun. 2021;12:113. doi: 10.1038/s41467-020-20383-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Smith S.E.P., Li J., Garbett K., Mirnics K., Patterson P.H. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Klein S.L., Creisher P.S., Burd I. COVID-19 vaccine testing in pregnant females is necessary. J Clin Invest. 2021;131:e147553. doi: 10.1172/JCI147553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Centers for Disease Control and Prevention Information about COVID-19 vaccines for people who are pregnant or breastfeeding. 2021. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations/pregnancy.html Available at:
- 45.Wajnberg A., Mansour M., Leven E., et al. Humoral response and PCR positivity in patients with COVID-19 in the New York City region, USA: an observational study. Lancet Microbe. 2020;1:e283–e289. doi: 10.1016/S2666-5247(20)30120-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Suri T., Mittal S., Tiwari P., et al. COVID-19 real-time RT-PCR: does positivity on follow-up RT-PCR always imply infectivity? Am J Respir Crit Care Med. 2020;202:147. doi: 10.1164/rccm.202004-1287LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sherer M.L., Posillico C.K., Schwarz J.M. The psychoneuroimmunology of pregnancy. Front Neuroendocrinol. 2018;51:25–35. doi: 10.1016/j.yfrne.2017.10.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.