Abstract
Patients hospitalized for pneumonia are at high risk for mortality. Effective therapies are therefore needed. Recent randomized clinical trials suggest that systemic steroids can reduce the length of hospital stays among patients hospitalized for pneumonia. Furthermore, preliminary findings from a feasibility study demonstrated that early treatment with a combination of an inhaled corticosteroid and a bronchodilator can improve oxygenation and reduce risk of respiratory failure in patients at risk of acute respiratory distress syndrome. Whether such a combination administered early is effective in reducing acute respiratory failure (ARF) among patients hospitalized with pneumonia is unknown. Here we describe the ARREST Pneumonia (Arrest Respiratory Failure due to Pneumonia) trial designed to address this question. ARREST Pneumonia is a two-arm, randomized, double-blinded, placebo-controlled trial designed to test the efficacy of a combination of an inhaled corticosteroid and a β-agonist compared with placebo for the prevention of ARF in hospitalized participants with severe pneumonia. The primary outcome is ARF within 7 days of randomization, defined as a composite endpoint of intubation and mechanical ventilation; need for high-flow nasal cannula oxygen therapy or noninvasive ventilation for >36 hours (each alone or combined); or death within 36 hours of being placed on respiratory support. The planned enrollment is 600 adult participants at 10 academic medical centers. In addition, we will measure selected plasma biomarkers to better understand mechanisms of action. The trial is funded by the U.S. National Heart Lung and Blood Institute.
Clinical trial registered with www.clinicaltrials.gov (NCT 04193878).
Keywords: respiratory failure, pneumonia, aerosol drug therapy, prevention
Pneumonia is the leading infectious cause of hospitalization and death in the United States, with associated medical costs exceeding $10 billion annually (1–3). When patients require intensive care unit (ICU)-level care, mortality exceeds 30%, and survivors often require prolonged hospital stays and suffer from long-term disability (4, 5). The host response to pneumonia is characterized by release of inflammatory cytokines that activate the vascular endothelium and recruit neutrophils to help contain local infection. However, dysregulated inflammation can perpetuate injury, leading to loss of endothelial and epithelial integrity and flooding of alveoli with protein-rich edema fluid, which can lead to loss of lung compliance, refractory hypoxemia, and ultimately acute respiratory failure (ARF) and acute respiratory distress syndrome (ARDS) (6). Currently, other than antibiotics and supportive care, there are no established treatments directly targeting the underlying lung injury that occurs with severe pneumonia.
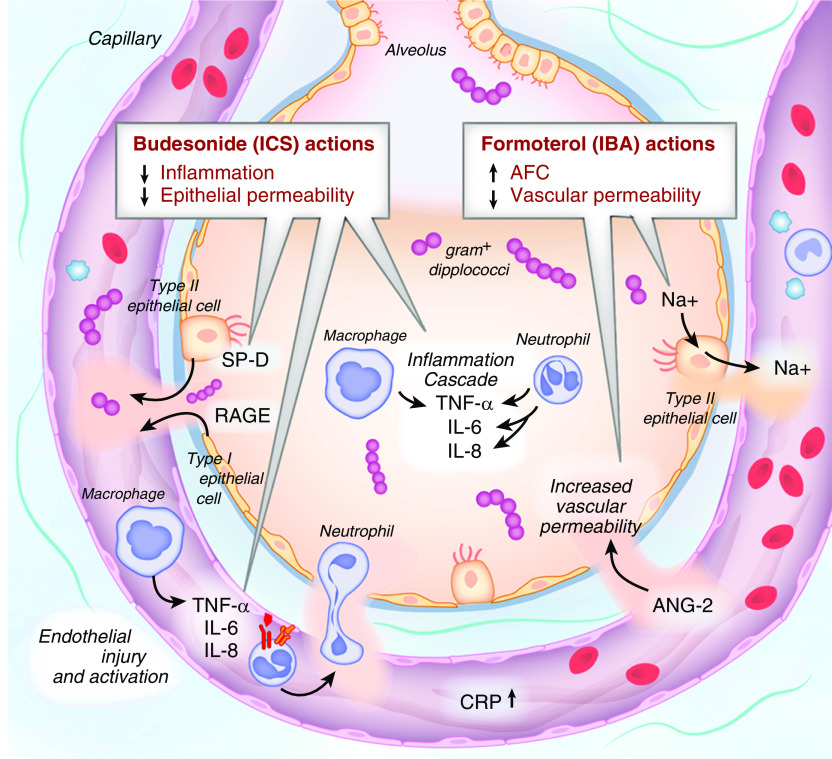
Two clinical trials in Europe recently found that systemic steroids reduced treatment failures and hospital length of stay in patients hospitalized with pneumonia (7, 8). However, systemic steroids were associated with more adverse effects (9), and a third trial (10) showed no clinical benefits. Inhaled corticosteroids have been shown to reduce inflammation and preserve alveolar barrier function, whereas β-agonists have been shown to increase alveolar fluid clearance and preserve vascular permeability (11, 12) (Figure 1). In our recent U.S. National Heart Lung and Blood Institute (NHLBI)-funded phase IIa, feasibility trial of 60 participants (LIPS-B [Lung Injury Prevention Study with Budesonide and a β-Agonist]), we demonstrated that early treatment (initiated within 12 h of meeting entry criteria) of participants at risk for ARDS with combined aerosolized budesonide and formoterol improved oxygenation (primary endpoint) compared with placebo (13). Rates of ARDS, mechanical ventilation, and hospital length of stay were also reduced. The improvement in oxygenation primarily occurred in the prespecified subgroup of participants with pneumonia. Our preliminary results suggested that early inhaled delivery of a corticosteroid combined with a β-agonist directly to the target organ may cause a reduction in inflammation (corticosteroids) and pulmonary edema (β-agonists), leading to more rapid improvement in oxygenation and prevention of related respiratory failure. As resolution of hypoxemia is an important discharge criterion, inhaled delivery may in addition result in a shorter hospital length of stay, thus improving efficacy while avoiding adverse effects observed with systemic corticosteroid use. Given the prevalence of pneumonia as an admitting diagnosis, even a 1-day reduction in the hospital stay would have important implications for cost and resource use globally.
Figure 1.
Key pathways in progression of lung injury. AFC = alveolar fluid clearance; ANG-2 = angiopoietin-2; CRP = C-reactive protein; IBA = inhaled β-agonist; ICS = inhaled corticosteroid; IL-6 = interleukin 6; IL-8 = interleukin 8; RAGE = receptor for advanced glycation end products; SP-D = surfactant protein D; TNF-α = tumor necrosis factor-α.
The phase III multicenter clinical trial, ARREST Pneumonia (Arrest Respiratory Failure due to Pneumonia), was designed to examine the efficacy and safety of a combination of an inhaled corticosteroid and a β-agonist compared with placebo for the prevention of ARF in hospitalized participants with pneumonia and hypoxemia. The trial is funded by a UG3/UH3 and U24 grant from the NHLBI and is registered with www.clinicaltrials.gov (NCT 04193878). It has been endorsed by the Discovery Network of the Society of Critical Care Medicine. The U.S. Food and Drug Administration provided an Investigational New Drug Exemption.
Methods
Objectives
We hypothesize that early inhaled delivery of therapy directly to the target organ may be particularly effective for early reversal of noncardiogenic pulmonary edema, leading to improved hypoxemia, and a reduced rate of progression to ARF. We investigate our hypotheses through the following objectives:
-
1.
Primary objective: To examine the efficacy of early treatment with an inhaled corticosteroid combined with a β-agonist versus placebo for the prevention of ARF in hospitalized participants with pneumonia and hypoxemia.
-
2.
Secondary objective: To identify baseline clinical and biologic characteristics impacting the risk of ARF due to pneumonia and impacting variable response to treatment.
Trial Design and Participants
The ARREST Pneumonia trial is a two-arm, parallel group, randomized controlled trial designed to test the efficacy of a combination of an inhaled corticosteroid (budesonide) and a β-agonist (formoterol) in treating pneumonia to prevent ARF. The primary endpoint is ARF within 7 days of randomization, defined as a composite endpoint of any combination of intubation for mechanical ventilation, need for high-flow nasal cannula (HFNC) oxygen therapy, or noninvasive ventilation (NIV) for >36 hours, or death within 36 hours of being placed on advanced respiratory support. The planned enrollment is 600 adult participants at 10 clinical sites. The trial organizational structure is shown in Figure 2.
Figure 2.
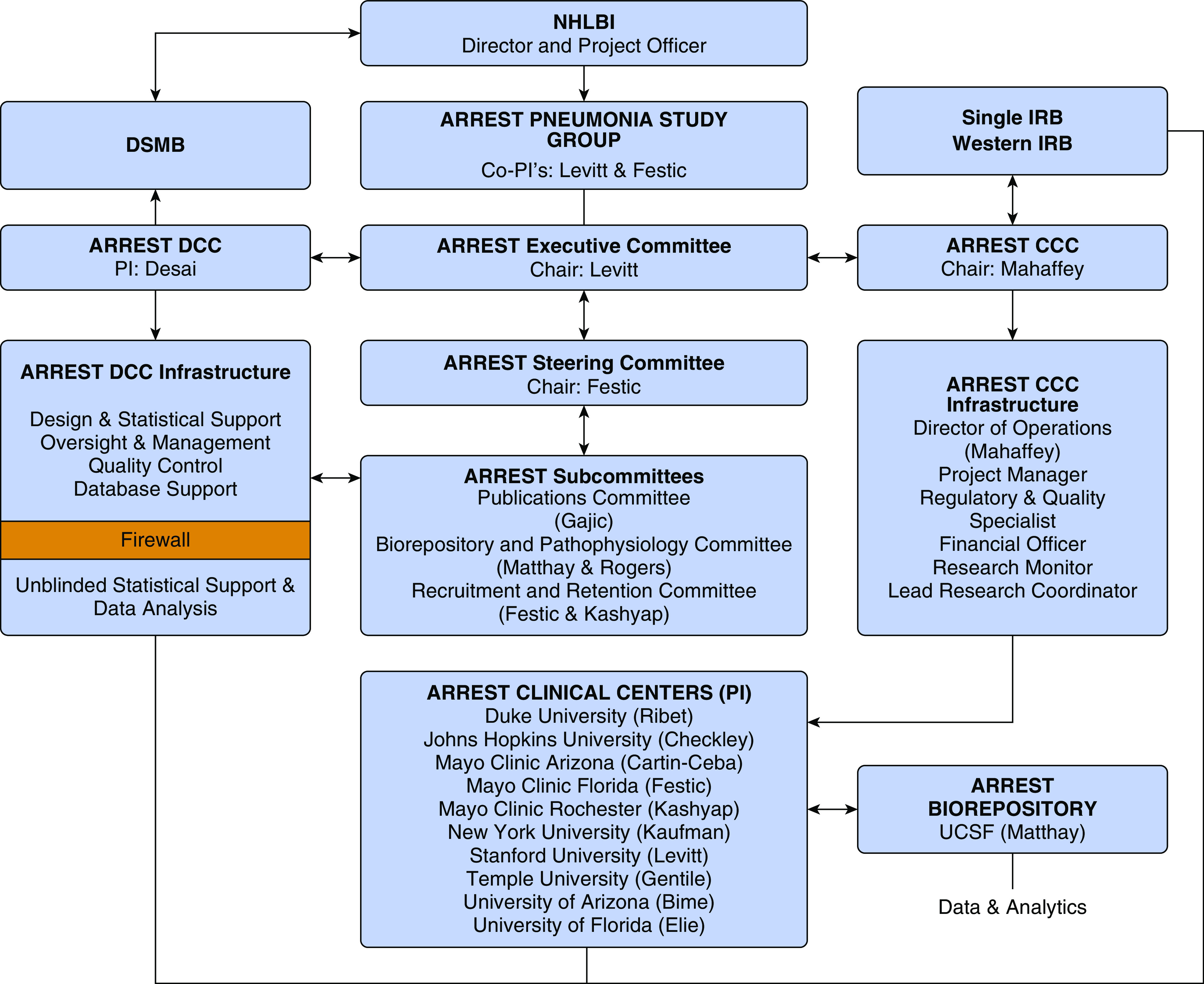
Organizational structure. ARREST Pneumonia = Arrest Respiratory Failure due to Pneumonia; CCC = Clinical Coordinating Center; DCC = Data Coordinating Center; DSMB = Data and Safety Monitoring Board; IRB = institutional review board; NHLBI = U.S. National Heart Lung and Blood Institute; PI = principal investigator; UCSF = University of California, San Francisco.
Eligibility Criteria and Exclusions
Adult patients with severe pneumonia or hypoxemia are eligible for inclusion. Such patients will be excluded, however, if they have a confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection, were receiving home oxygen support, have a condition that requires inhaled corticosteroids or β-agonists or a chronic systemic steroid therapy that cannot be discontinued, or have a “do-not-intubate” advanced directive. Full inclusion and exclusion criteria are listed in Table 1.
Table 1.
Inclusion and exclusion criteria
| Inclusion criteria | Patients 18 yr or older with |
| 1. Severe pneumonia defined as hospitalization for acute (<7 d) onset of symptoms (cough, sputum production, or dyspnea) and radiographic evidence of pneumonia by chest radiograph or CT scan AND evidence of systemic inflammation (temperature of <35°C or >38°C OR WBC < 4,000 or >11,000 OR procalcitonin > 0.5 μg/L) | |
| AND | |
| 2. Hypoxemia defined as new requirement for supplemental oxygen with SpO2 < 92% on room air, ≤96% on ≥2 L/min of oxygen, or >6 L/min or NIV regardless of SpO2 at enrollment. Patients admitted with pneumonia but not meeting criteria for hypoxemia will be followed for up to 24 h from emergency department admission to assess for development of qualifying hypoxemia. | |
| Exclusion criteria | • Confirmed COVID-19 infection |
| • Inability to obtain consent within 24 h of presentation to emergency room | |
| • Intubation (or impending intubation) before enrollment | |
| Patients receiving HFNC oxygen or NIV before enrollment are not excluded. | |
| • A condition requiring inhaled corticosteroids or β-agonists or chronic systemic steroid therapy equivalent to a dose >10 mg of prednisone (Patients receiving inhaled β-agonists in the emergency department without an established indication will be eligible if treating clinician is willing to discontinue subsequent treatments.) | |
| • Do-not-intubate order but does not include a “do-not-resuscitate” order | |
| • Chronic lung or neuromuscular disease requiring daytime oxygen or mechanical ventilation other than for OSA or obesity hypoventilation syndrome | |
| • Not anticipated to survive >48 h or not expected to require >48 h of hospitalization | |
| • Contraindication or allergy to corticosteroids or β-agonists | |
| • Patients with heart rate >130 bpm, ventricular tachycardia, or new SVT within last 4 h (potentially eligible for enrollment after the condition has resolved) | |
| • Patients with K+ < 3.0 mmol/L (potentially eligible for enrollment after the condition has resolved) | |
| • Pregnancy | |
| • Incarcerated individual | |
| • Physician refusal of consent to protocol | |
| • Patient/surrogate refusal of consent to protocol |
Definition of abbreviations: bpm = beats per minute; COVID-19 = coronavirus disease; CT = computed tomography; HFNC = high-flow nasal cannula; NIV = noninvasive ventilation; OSA = obstructive sleep apnea; SpO2 = oxygen saturation as measured by pulse oximetry; SVT = supraventricular tachycardia; WBC = white blood cell count.
The ARREST Pneumonia trial enrolled patients starting in May of 2020, including patients with coronavirus disease (COVID-19) pneumonia. After publication of preliminary results from the RECOVERY (Randomised Evaluation of COVID-19 Therapy) trial (14) in June 2020, which suggested a benefit of systemic dexamethasone for patients with COVID-19 requiring oxygen supplementation, we observed rapid changes in clinical practice at all enrolling sites related to early use of systemic steroids in patients with COVID-19, even in patients requiring minimal oxygen supplementation. Because of uncertainty regarding our power to detect an additional benefit of an inhaled steroids and β-agonist in addition to systemic steroids, we opted to specifically exclude further enrollment of patients with COVID-19 from ARREST Pneumonia after July 1, 2020, and reverted back to the original protocol with the exception of increasing the enrollment window to 24 hours from 12 hours to allow time for return of a negative SARS-CoV-2 test result.
Randomization
The randomization module is automated within the database capture platform (Research Electronic Data Capture [Vanderbilt University]) for ease of use by the site coordinators. Randomization is performed in a 1:1 ratio to the two study arms. In addition to stratification by site, randomization factors also include HFNC oxygen therapy and/or NIV and shock at time of enrollment, performed through use of Efron’s biased coin-randomization tool (15).
Treatment Arms, Administration, and Standardization of Care
Participants will be enrolled within 24 hours of emergency department admission and will subsequently receive the experimental treatment, combined aerosolized doses of budesonide (1.0 mg/2 ml) and formoterol (20 μg/2 ml), or placebo (4 ml of aerosolized 0.9% saline) twice daily for up to 10 doses (or until hospital discharge), with the first dose administered within 4 hours after randomization. All study medications will be delivered via the Aerogen Ultra nebulizer, which is already available and had been used at all trial sites before the trial inception.
The study drug will be temporarily held if the heart rate exceeds 130 beats/min, if there is new ventricular or supraventricular tachycardia within the 4 hours before treatment, or if K+ is <3.0 mmol/L on the most recent chemistry panel. The pharmacy will provide either study drugs or placebos in identically appearing containers to ensure blinding. If a participant needs treatment with bronchodilators during the study period, treating physicians will be encouraged to use aerosolized ipratropium. If participants receive an open-label β-agonist, the study drug will continue as per the randomized protocol. If a participant requires systemic steroids after randomization (e.g., stress-dose steroids), the assigned study treatments will continue, and the participant will be considered adherent to the assigned protocol. Systemic steroids specifically as adjuvant treatment of pneumonia will not be allowed per protocol.
Clinical care that may affect development of ARF and secondary outcomes in participants with severe pneumonia will be standardized with a set of clinical interventions that reduce the risk of development or improve outcomes of lung injury. Standardization of such best practices will decrease the heterogeneity of the risk factors that may influence the development as well as subsequent management of ARF and has been used in previous LIPS (Lung Injury Prevention Study) trials (13) (Table 2). The decision to use HFNC oxygen therapy or NIV or to intubate will be left to the clinical team as per their routine practice. However, to minimize clinical-practice variation around the primary endpoint, we have developed specific advanced oxygen-support escalation and weaning protocols (see Appendices E1 and E2 in the online supplement). Briefly, patients failing standard oxygen support up to 10 L/min will be escalated to HFNC oxygen therapy. The flow of oxygen and fraction of inspired oxygen (FiO2) concentration will be titrated to patient tolerance as per allowed pairings modeled after the positive end-expiratory pressure and FiO2 tables for lung-protective mechanical ventilation (Appendix E1).
Table 2.
Elements of standardized care
| Pneumonia care set | Early microbiologic evaluation, early and adequate empiric antimicrobial treatment |
| Aspiration precautions | Elevated head of the bed, oral care/antiseptics |
| Adequate empiric antibiotics/source control | According to suspected site of infection, antibiogram, healthcare exposure, and immunosuppression |
| Limiting fluid overload | Modified ARDSNet FACTT protocol after resolution of early shock (beyond first 12 h) |
| Restrictive transfusion | Hemoglobin target > 7 g/dl in the absence of acute bleeding and/or ischemia. Plasma and platelet transfusion should be avoided before minimally invasive procedures unless there is active bleeding. |
Definition of abbreviations: ARDSNet = Acute Respiratory Distress Network; FACTT = Fluid and Catheter Treatment Trial.
Study Adherence and Monitoring
Given the relatively short duration of intervention (5 d) and monitoring for the primary endpoint (7 d) in hospitalized participants, no additional adherence strategies are expected to be necessary. However, the study staff will contact any participant discharged before 7 days to confirm the absence of ARF and to assess for any adverse events occurring within 7 days. During hospitalization, study staff will monitor participants’ clinical status before each dose of the study drug (twice daily). Although conditions for holding the study drug (severe hypokalemia, tachycardia, or arrhythmia) are standard clinical practice, respiratory therapists providing care for enrolled participants have been educated to hold treatment on the basis of the cardiac contraindications. An on-call member of the clinical coordinating center will be available on a 24-hour basis to discuss enrollment questions, adverse events, or protocol deviations.
Patient Timeline, Assessments, and Measures
The patient timeline is depicted in Figure 3. In addition to the primary outcome assessed within 7 days of randomization, serum for biomarker analysis will be collected at baseline and 48 hours after randomization. In addition, hospitalization variables such as length of stay and survival through Day 60 will be recorded.
Figure 3.
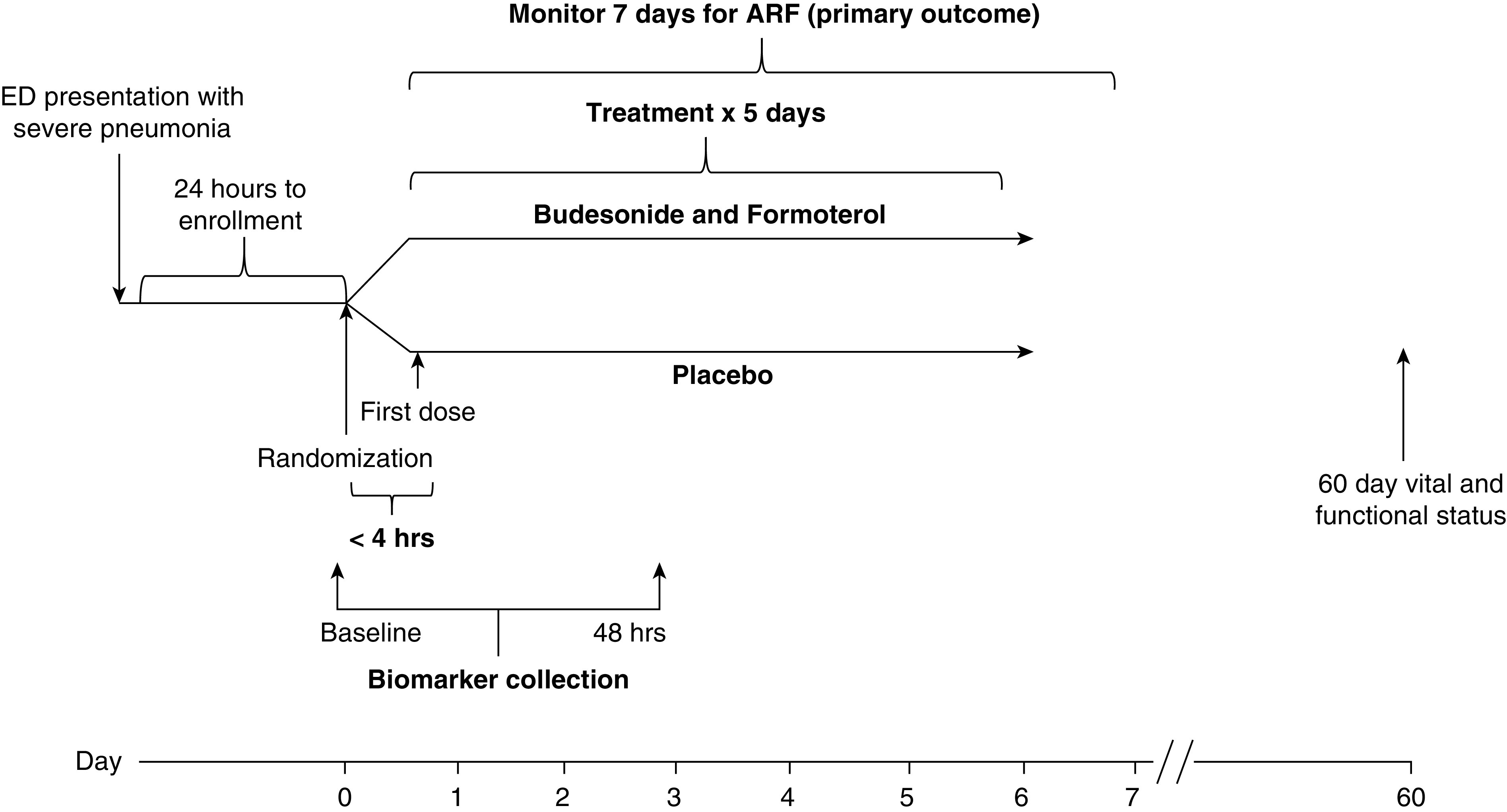
Study timeline. ARF = acute respiratory failure; ED = emergency department.
Primary and Secondary Endpoints
The primary endpoint is ARF ascertained within 7 days of randomization, defined as a composite of any combination of a sustained need for HFNC oxygen, NIV, or intubation and mechanical ventilation lasting 36 hours or longer. Initially, the primary outcome in the trial was ARF defined as need for intubation and mechanical ventilation for 24 hours. However, after publication of the FLORALI (Clinical Effect of the Association of NIV and High-Flow Nasal Oxygen Therapy in Resuscitation of Patients with Acute Lung Injury) trial (16), the frequency of use of high-flow oxygen increased and the frequency of the intubation and mechanical ventilation for pneumonia-induced ARF decreased. The three components of the composite outcome are standard of current practice and have been widely used as therapeutic options for the ARF. Besides mechanical ventilation, use of high-flow oxygen therapy versus NIV for ARF has been extensively studied (17). Development of our primary endpoint has been an iterative process with input from the extensive clinical and research expertise in ARDS and ARF among our Executive Committee members as well as the U.S. National Institutes of Health–appointed Data and Safety Monitoring Board (DSMB). We performed a review of the practice at our 10 sites in the months of December 2017 and January 2018 and found that the frequency of mechanical ventilation was about 10% across our network (likely reflecting change in practice with increased use of HFNC oxygen therapy since publication of the FLORALI trial), leaving us underpowered and necessitating change to a composite endpoint. Our initial definition included 24 hours of advanced respiratory support but was increased to 36 hours on advisement from the DSMB to limit the impact of variation in time of enrollment because, for safety and staffing reasons, our standardized respiratory-support weaning protocols will only be required during normal day-shift hours (0800–2000) and we wanted to ensure all patients would have been closely assessed for weaning parameters for at least an entire day shift before meeting our primary endpoint, regardless of time of enrollment. Patients initiating advanced respiratory support who die before 36 hours will be considered to have met the primary endpoint of ARF.
Therefore, in order for the trial to be adequately powered, we broadened the definition of ARF to include need for HFNC oxygen therapy and/or NIV, in addition to intubation and mechanical ventilation, lasting continuously for 36 hours or longer, subsequent to randomization. Participants will only have been considered to have met the primary endpoint of ARF if they continue to require HFNC oxygen or NIV beyond 36 hours from initiation (or from randomization if already receiving HFNC oxygen or NIV at enrollment). Participants requiring HFNC oxygen or NIV (or subsequent intubation) for >36 hours will be considered to have met the primary endpoint of ARF, and subsequent weaning will be as per the discretion of the clinical team. Participants who are weaned to standard oxygen therapy before 36 hours but who subsequently require HFNC oxygen or NIV (or intubation) within the next 24 hours will have met criteria for ARF. However, if a participant is successfully weaned off HFNC oxygen or NIV (unassisted breathing) in less than 36 hours and tolerates unassisted breathing for >24 hours but subsequently requires reinitiation of HFNC oxygen or NIV within 7 days from enrollment, the protocol will reinitiate as above with restarting of the 36-hour clock to define ARF.
Our composite primary outcome has not been previously validated; however, each of the three components have been studied in ARF (16). As these three “advanced respiratory” strategies require skilled personnel and close monitoring, the patients requiring advanced respiratory support are cared for in the ICUs or progressive care units. This in turn impacts the prognosis and length of stay and as such is pertinent to the patients and families. In addition, advanced respiratory-support strategies have been shown to reduce mobilization, have been shown to restrict effective communication and oral nutrition, and may cause gastric distension and patient discomfort (17). If the intervention in our trial is found to be effective, these results would likely lead to widespread adoption of this inexpensive and widely available therapy and would have meaningful impact for both patients and healthcare use. Although ARDS was a focus of earlier prevention studies, ARDS is not a patient-centered outcome, and adjudication of ARDS is subjective with poor interrater reliability (18).
Secondary endpoints include intubation for respiratory failure within 7 days of randomization, length of stay in the hospital, and the length of time supplemental oxygen is needed. All clinical endpoints are listed in Table 3.
Table 3.
Endpoints and laboratory evaluations
| Primary endpoint | ARF within 7 d of randomization defined as composite of: |
|
| • HFNC and/or NIV and/or invasive mechanical ventilation or any combination of these for >36 h OR | ||
| • Death in a patient placed on respiratory support (HFNC, NIV, ventilator) who dies before 36 h | ||
| Secondary endpoints | • Intubation for respiratory failure within 7 d of randomization |
|
| • Hospital length of stay | ||
| • Duration of need for supplemental oxygen | ||
| Exploratory endpoints | • Proportion of patients discharged on supplemental oxygen |
|
| • Change in SpO2/FiO2 ratio (defined as a continuous time-dependent variable) | ||
| • Incidence and severity (by berlin criteria) of ARDS in intubated patients assessed by local PI | ||
| • Development of shock (MAP < 60 mm Hg or need for vasopressors) not present at enrollment | ||
| • ICU admission and ICU length of stay | ||
| • SOFA at Days 5 and discharge (or Day 30 if earlier) | ||
| • Intubation through hospital discharge | ||
| • Hospital survival defined as being alive and spontaneously breathing at discharge home or place of residence before admission within 60 d from study enrollment | ||
| Exploratory biologic endpoints | • Change in plasma biomarkers of IL-6 and CRP (inflammation), Ang-2 (endothelial activation), RAGE (alveolar epithelial injury), and IL-18 (inflammasome activation) at baseline and 48 h after randomization |
|
| • Biologic endpoints and the impact of inhaled corticosteroids and β-agonist will be evaluated by pneumonia subtypes (bacterial vs. viral vs. aspiration) categorized by gene-expression signatures as well as clinically by the site PI before hospital discharge by inclusion of additional supporting clinical and microbiologic data. | ||
| Safety endpoints | Protocol-specified adverse events based on side-effect profiles of active drugs: |
|
| • Allergic reactions (both medications) | ||
| • Pharyngitis, viral URI (inhaled steroids) | ||
| • Oral candidiasis (inhaled steroids), | ||
| • Secondary upper or lower respiratory tract infections (inhaled steroids) | ||
| • New sinus tachycardia >130 bpm or atrial fibrillation or other SVT (inhaled β-agonists) | ||
| • Hypokalemia, K+ < 3.0 mmol/L (inhaled β-agonists) | ||
| Laboratory evaluations | Per clinical indication | White blood cells and differential, BUN, creatinine, lactate, CRP, sodium, potassium, bicarbonate, total bilirubin, albumin, and procalcitonin |
| Study mandated (if not obtained per clinical indication) | CRP and procalcitonin will be collected at baseline and 48 h | |
| Biomarkers | Epithelial injury (RAGE), inflammation (IL-6 and CRP), and inflammasome activation (IL-18) at baseline and 48 h | |
| Genetic analysis | 2.5-ml PAXgene tube at baseline and 48 h | |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; ARF = acute respiratory failure; bpm = beats per minute; BUN = blood urea nitrogen; CRP = C-reactive protein; FiO2 = fraction of inspired oxygen; HFNC = high-flow nasal cannula; ICU = intensive care unit; IL-6 = interleukin 6; IL-18 = interleukin 18; MAP = mean arterial pressure; NIV = noninvasive ventilation; PI = principal investigator; RAGE = receptor for advanced glycation end products; SOFA = Sequential Organ Failure Assessment; SpO2 = oxygen saturation as measured by pulse oximetry; SVT = supraventricular tachycardia; URI = upper respiratory infection.
Laboratory Evaluations
Laboratory studies to be obtained as per routine clinical care are listed in Table 3. Additional plasma samples will be collected at baseline and at 48 hours. We have selected established biomarkers of epithelial injury (RAGE), inflammation (IL-6 and CRP), and inflammasome activation (IL-18). Their association with the baseline risk of ARF and response to treatment will provide mechanistic insight by elucidating which biologic pathways important in lung injury and progression to ARF are modulated by the inhaled therapy. In addition, pneumonia etiology will be defined biologically by host gene-expression signature (19). Analysis of potential effect modification between baseline biomarker levels with treatment will allow better understanding of the risk of ARF and may identify biologically defined subgroups most likely to have both a clinical and biologic response to treatment.
Safety Data
Assuring participant safety is an essential component of this protocol. The investigators will document daily whether any adverse events occur during the period from enrollment, which begins on the date of randomization through study Day 7 (2 d after completion of the study drug delivery) and will determine if such adverse events are reportable. If a participant is discharged before Day 7, investigator(s) or a designee will attempt to contact the participant by phone to confirm the absence of any adverse events through Day 7. The reportable adverse events will be collected in the adverse-event electronic case-report forms. The specific protocol for evaluation and management of adverse-effect evaluation and management is listed in Appendix E3 in the online supplement.
Data and Safety Monitoring
An independent DSMB, appointed by the NHLBI, consists of five members who will have access to unblinded data at the scheduled interim analysis, for safety reviews, and as per any potential safety-related request.
Statistical Considerations
Our primary objective will be tested using an intention-to-treat principle. As such, all patients randomized will be analyzed according to their randomized assignment for the length of time spanning randomization until the occurrence of outcome or censoring, whichever occurs first. Specifically, all events occurring within the specified time periods will be included in the analysis regardless of whether the participant adheres to the protocol. Information from participants who withdraw their consent to participate in the study will be included up to the time when they withdraw their consent.
The analysis of the primary endpoint of ARF occurrence at both the interim and final analyses will involve applying a generalized mixed-effect model with a logit-link function and a random effect for enrollment site to estimate the effect of inhaled therapy relative to placebo. All models will be adjusted for the randomization-stratification variables (site, HFNC/NIV use, and shock at enrollment).
To address the secondary objective that compares the intubation rate between study arms, we will similarly apply generalized mixed-effect regression techniques as described above. To compare the time-to-event or censored endpoints (e.g., duration of the need for supplemental oxygen, time to hospital discharge), we will derive a rank-based composite outcome of the event and death, taking into account the time until the event or death (20). We will compare differences in the distribution of the composite time-to-event outcomes with a heteroskedasticity-robust variant of the Wilcoxon Mann-Whitney test developed by Brunner and Munzel at the 0.05 significance level (21). The median rank and the interquartile range will be presented by arm for each composite outcome. For the length of stay, we will in addition present the proportion of patients who are discharged before 7 days in each arm. We expect loss to follow-up to be minimal.
Relative to heterogeneity of treatment-effect analysis, we will use prespecified factors (baseline CRP, hypoxemia by oxygen saturation as measured by pulse oximetry [SpO2]/FiO2, and shock) that we anticipate will contribute to the heterogeneity of treatment (see Table E2 in the online supplement). In the secondary analyses, we will not adjust for multiplicity. We will, however, report the findings as hypothesis generating, and the P values will therefore be purely descriptive. We will also report the total number of assessments included in the analysis for transparency.
Sample-Size Justification
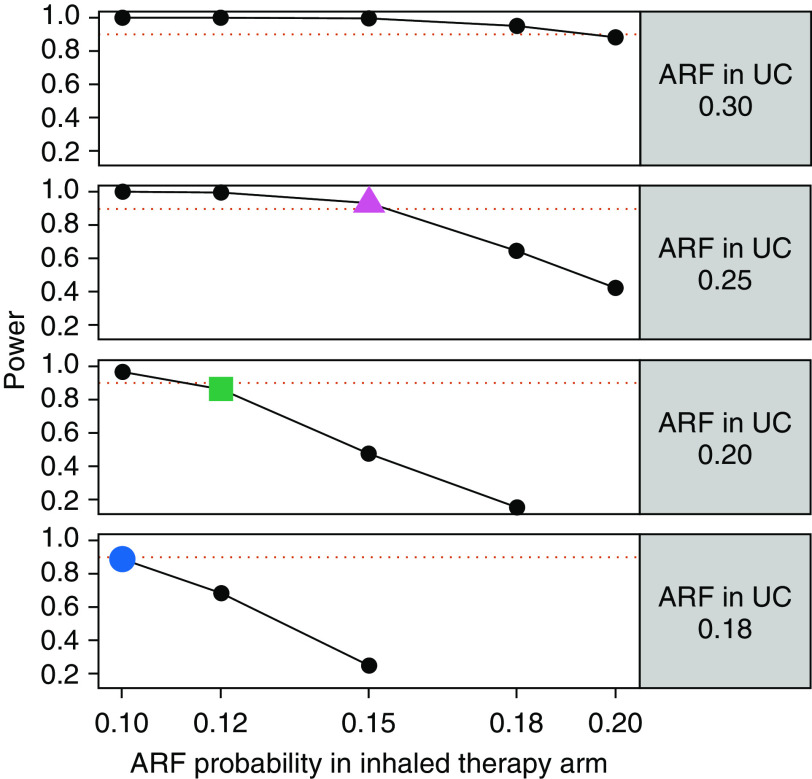
Total enrollment will target 600 participants in a group-sequential design. The study is designed with 93% power to detect a 10% absolute reduction with inhaled therapy in our primary endpoint, ARF, from a baseline of 25% with placebo (magenta triangle in Figure 4). This expected event rate is based on rates observed in LIPS-B and data on participants admitted for pneumonia at participating clinical sites. If the baseline ARF event rate is 20%, we will have 90% power to detect an 8% reduction in ARF from 20% to 12% and 90% power for a reduction from 18% to 10% (green square and blue circle, respectively, in Figure 4). The preplanned interim analysis consists of formally testing for overwhelming efficacy after 50% and 75% of participants have been accrued. The DSMB will recommend whether the study should be stopped early on the basis of the interim analysis for efficacy performed at the 0.00005 and 0.003 significance levels, respectively, leaving a two-sided α of 0.047 for the final analysis performed upon study completion. The actual significance levels used will reflect the actual number of participants included in the interim analyses, as determined by the α spending function obtained from the power family with the power parameter equal to 1025 (22–23).
Figure 4.
Power analysis. ARF = acute respiratory failure; UC = usual care.
Discussion
In hospitalized patients with pneumonia, an increased host inflammatory response is associated with higher rates of treatment failure, ARF, and mortality. As previously shown, admission levels of procalcitonin, TNF-α, IL-6, and CRP were higher in patients with pneumonia admitted to the ICU than in patients admitted to a standard ward or in ward patients who failed initial treatment and required subsequent ICU admission (24). Corticosteroids have well-established antiinflammatory properties; however, clinical trials of systemic corticosteroids for pneumonia have shown mixed results (7, 8, 10, 25). Two recent clinical trials demonstrated benefit in patients hospitalized with pneumonia. Torres and colleagues showed in a trial of 120 patients with severe pneumonia and high CRP that intravenous methylprednisolone reduced the composite endpoint of treatment failure, although this was primarily driven by less radiographic progression and was underpowered to detect a difference in ARF (8). Studying a less sick population, Blum and colleagues showed a 1-day reduction in hospital length of stay in 785 hospitalized patients treated with prednisone, although rates of hyperglycemia requiring insulin were higher (7). An earlier trial of 213 patients found no clinical benefit from treatment with prednisolone but raised concern for increased rates of late treatment failures and superinfections (10).
Corticosteroids have been shown to decrease permeability of ex vivo lung epithelial cells (26). A recent study demonstrated dissociation between the systemic and alveolar antiinflammatory effects of intravenous dexamethasone after local installation of endotoxin in the lungs of healthy volunteers. In contrast to the marked reductions in proinflammatory cytokine levels observed in peripheral blood, bronchoalveolar lavage (BAL)-fluid levels of IL-6 were only slightly decreased, and IL-8 and TNF-α in BAL fluid were not significantly reduced (27). Therefore, inhaled corticosteroids may offer additional benefit by direct delivery of the therapy to the alveolar epithelium. In a secondary analysis of the large LIPS cohort, prehospital use of an inhaled corticosteroids was associated with a lower rate of ARDS, particularly in patients with direct lung injury (primarily pneumonia) (28). Similarly, in a population-based study from Olmsted County, Minnesota, we found that patients had lower rates of progression to ARDS if they were receiving an inhaled corticosteroid and/or β-agonist before hospitalization, particularly patients with pneumonia (29). Two recent trials have demonstrated the benefit of inhaled budesonide. A preventative clinical trial found that pretreatment with inhaled budesonide before single-lung ventilation increased lung compliance and improved cytokine profiles after lobectomy (30), and a trial of 60 intubated patients with ARDS found improved arterial oxygen pressure/FiO2, lung mechanics, and cytokine profiles with 1 mg of nebulized budesonide twice daily for 3 days (31).
Alveolar fluid clearance is driven by active transport of sodium and chloride ions across alveolar epithelial cells (32). In ex vivo human lungs, treatment with β-agonists doubled the rate of alveolar fluid clearance (11, 33). β-Agonists also have antiinflammatory properties and can preserve vascular-barrier function, potentially reducing edema formation (34). Importantly, aerosolized delivery of inhaled β-agonists attains therapeutic levels in undiluted pulmonary edema fluid suctioned from ventilated patients with ARF (35). In an early clinical trial of intubated patients with ARDS, treatment with an intravenous β-agonist reduced extravascular lung water (12). However, in two subsequent phase III clinical trials, neither inhaled nor systemic β-agonists improved clinical outcomes in intubated patients with established ARDS (36, 37), perhaps because severely injured alveolar epithelial cells in fully established ARDS may not adequately respond to β-agonists (38). Consistent with the theory that β-agonists confer the greatest benefit in spontaneously breathing patients before ARDS is established, a recent trial of inhaled salbutamol (a β-agonist) before esophagectomy found that perioperative treatment reduced the rate of postoperative pneumonia, the pulmonary vascular-permeability index, and serum levels of RAGE, TNF-α, and IL-1β (39).
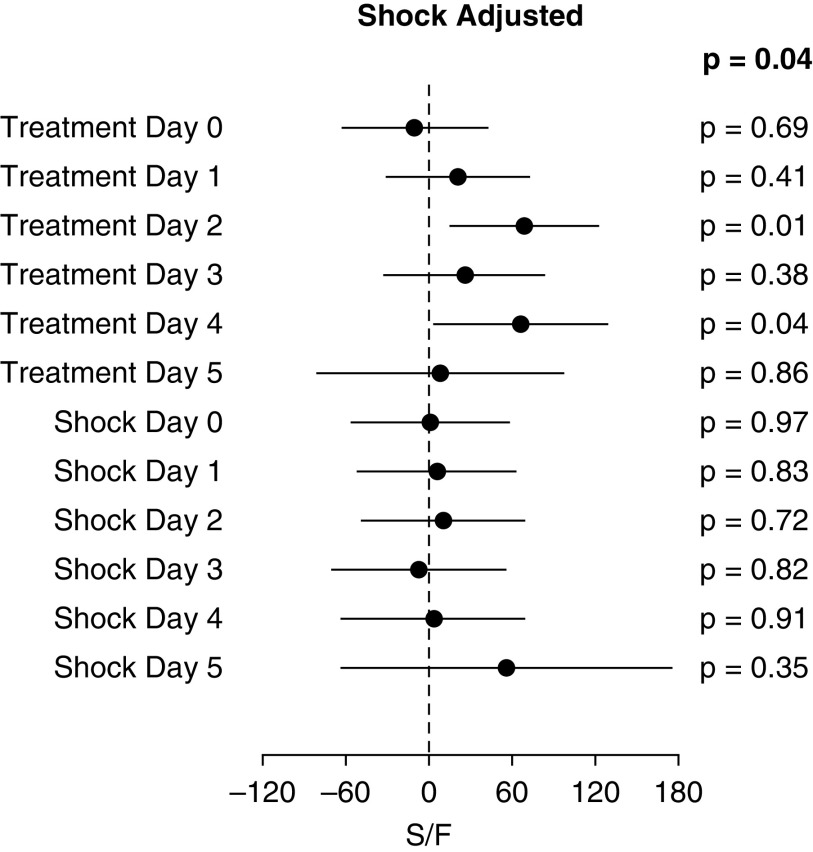
In the phase IIa LIPS-B trial, we randomized 60 emergency department patients at high risk for ARDS to early treatment with aerosolized budesonide and formoterol or placebo twice daily for 5 days (13). Despite the relatively small sample size, the primary endpoint of improved oxygenation, measured by a >20% change in the SpO2/FiO2 ratio from baseline to Day 5, was achieved (P = 0.01). This effect was most pronounced in the prespecified subset of 37 patients with pneumonia as their primary ARDS risk factor (P = 0.03). Patients in the treatment arm had lower rates of ARF requiring mechanical ventilation (53% vs. 21%, P = 0.01) and ARDS (23% vs. 0%, P = 0.01) (Table 4). Patients in the treatment arm also had a shorter duration of hospital and ICU lengths of stay. Importantly, although patients in the treatment arm had a higher rate of baseline shock, the treatment effect on the SpO2/FiO2 ratio (daily SpO2/FiO2 by treatment arm in mixed-effect model) was independent of shock (P = 0.04), and shock was not associated with change in SpO2/FiO2 ratios (Figure 5). These preliminary data provided a strong signal that early inhaled delivery of budesonide and formoterol improved oxygenation, presumably by reducing inflammation and increasing resorption of pulmonary edema, thus reducing the incidence of respiratory failure in patients with pneumonia.
Table 4.
LIPS-B outcomes
| Outcome | Placebo (N = 30) | Treatment (N = 29) | P Value |
|---|---|---|---|
| Categorical change in SpO2/FiO2, n (%) | 0.01 | ||
| Overall | |||
| >20% decrease | 8 (27) | 0 | |
| No change (within 20%) | 9 (30) | 11 (38) | |
| >20% increase | 13 (43) | 18 (62) | |
| Baseline pneumonia | N = 16 | N = 21 | 0.03 |
| >20% decrease | 5 (31) | 0 | |
| No change | 4 (25) | 7 (33) | |
| >20% increase | 7 (44) | 14 (67) | |
| No pneumonia | N = 14 | N = 8 | 0.51 |
| >20% decrease | 3 (21) | 0 | |
| No change | 5 (35) | 4 (50) | |
| >20% increase | 6 (43) | 4 (50) | |
| Baseline shock | N = 14 | N = 3 | 0.37 |
| >20% decrease | 4 (29) | 0 | |
| No change | 4 (29) | 0 | |
| >20% increase | 6 (44) | 3 (100) | |
| No shock | N = 16 | N = 26 | 0.04 |
| >20% decrease | 4 (25) | 0 | |
| No change | 5 (31) | 11 (42) | |
| >20% increase | 7 (44) | 15 (57) | |
| ARDS, n (%) | 7 (23) | 0 | 0.01 |
| Mechanical ventilation, n (%) | 16 (53) | 6 (20) | 0.01 |
| Hospital length of stay | N = 30 | N = 30 | 0.02 |
| Median (IQR) | 6.5 (3–14) | 3.5 (2–7) | |
| ICU length of stay | N = 21 | N = 13 | 0.01 |
| Median (IQR) | 6 (4–14) | 3 (2–4) |
Definition of abbreviations: ARDS = acute respiratory distress syndrome; FiO2 = fraction of inspired oxygen; ICU = intensive care unit; IQR = interquartile range; LIBS-B = Lung Injury Prevention Study with Budesonide and a β-Agonist; SpO2 = oxygen saturation as measured by pulse oximetry.
Figure 5.
Shock-adjusted change in S/F per treatment day in LIPS-B (Lung Injury Prevention Study with Budesonide and a β-Agonist). S/F = oxygen saturation as measured by pulse oximetry/fraction of inspired oxygen.
The ARREST Pneumonia trial will focus on the same early intervention used in the LIPS-B trial (in which drug delivery occurred a median of <9 h from emergency department presentation) in patients at high risk for ARF to prevent disease progression. Past trials have failed to show the benefit of β-agonists and systemic steroids for ARDS after onset of ARF, as discussed above; however, clinical benefit may require earlier intervention. Early inhaled delivery, directly to the target organ, of treatment with the potential to reduce inflammation and pulmonary edema is likely to amplify the clinical benefit by preventing ARF.
Our second objective is to explore the role of biologic pathways of lung injury, together with pneumonia severity and etiology (e.g., viral vs. bacterial) and comorbidities in predicting the risk of ARF. We anticipate that some variables, such as biologic markers of inflammation and hypoxemia, will increase the risk of ARF and response to treatment, whereas others, such as shock and comorbidities, may blunt treatment effects because of the increasing treatment-independent risk of ARF. Better understanding of risk factors contributing to ARF from pneumonia and their contributions to heterogeneity of the treatment effect will enhance interpretation of trial results and inform future efforts in the early treatment of pneumonia.
If ARREST Pneumonia trial results demonstrate that inhaled budesonide combined with formoterol reduces the occurrence of ARF and hospital lengths of stay, rapid adoption of early treatment with these safe, inexpensive, and readily available therapies would be highly feasible. A trial that establishes improved outcomes for hospitalized participants with severe pneumonia by targeting the host response is both timely and of critical clinical relevance. Inflammation (marked by inflammatory cytokines IL-6 and IL-8, CRP, and TNF-α) promotes epithelial injury (marked by increased levels of RAGE and SP-D) and lung vascular permeability (marked by increased Ang-2). Better understanding of such risk factors contributing to ARF from pneumonia and their contributions to heterogeneity of the treatment effect will enhance interpretation of trial results and inform future efforts in the early treatment of pneumonia.
Acknowledgments
Acknowledgment
The authors thank the clinical investigators, coordinators, and contributors who have participated in at least one of the following: critical review of the study proposal, data collection, care of study patients, and writing and/or technical editing of the manuscript.
ARREST Pneumonia Clinical Trial Investigators: Lora Reineck and Karen Bienstock, U.S. National Heart, Lung, and Blood Institute, U.S. National Institutes of Health; Ian Welsby, Daniel Gilstrap, and Jacob Ribet, Duke University; William Checkley, Laura Nicolaou, Katie Mattare, and Shakir Hossein, Johns Hopkins University; Emir Festic, Augustine S. Lee, Neal M. Patel, Kaitlin M. Moran, Jenna E. Murray, Jose L. Alonso, and Arjana Halilovi, Mayo Clinic, Jacksonville, FL; Ognjen Gajic, Rahul Kashyap, Aysun Tekin, Vikas Bansal, Lindsay A. Fogelson, and Amy L. Amsbaugh, Mayo Clinic, Rochester, MN; Rodrigo Cartin-Ceba, Ayan Sen, Emily Frank, and Leena Abraham, Mayo Clinic, Scottsdale, AZ; David A. Kaufman and Ashley Witzl, New York University; Joseph E. Levitt, Kenneth W. Mahaffey, Angela J. Rogers, Jennifer Wilson, Rosemary Vojnik, and Joe Yee Fung, Stanford University; Nina T. Gentile and Sarah Loughran, Temple University; Marie-Carmelle Elie, Torben Becker, Rohit Patel, Travis Murphy, Matthew Shaw, and Rebecca Murray, University of Florida; and Christian Bime, Jarrod Mosier, Cathleen Wilson, and Heidi Erickson, University of Arizona.
Footnotes
Supported by the U.S. National Heart, Lung, and Blood Institute, U.S. National Institutes of Health, Clinical Coordinating Center and Data Coordinating Center grants HL141722.
A complete list of the ARREST Pneumonia Clinical Trial Investigators may be found before the beginning of the References.
Author Contributions: J.E.L., E.F., M.D., H.H., K.W.M., A.J.R., O.G., and M.A.M. have provided substantial contributions to the conception of the work, drafted and revised work critically, provided final approval of the version to be published, and agreed to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: on behalf of ARREST Pneumonia Clinical Trial Investigators, Lora Reineck, Karen Bienstock, Ian Welsby, Daniel Gilstrap, Jacob Ribet, William Checkley, Laura Nicolau, Katie Mattare, Shakir Hossein, Emir Festic, Augustine S. Lee, Neal M. Patel, Kaitlin M. Moran, Jenna E. Murray, Jose L. Alonso, Arjana Halilovic, Ognjen Gajic, Rahul Kashyap, Aysun Tekin, Vikas Bansal, Lindsay A. Fogelson, Amy L. Amsbaugh, Rodrigo Cartin-Ceba, Ayan Sen, Emily Frank, Leena Abraham, David A. Kaufman, Ashley Witzl, Joseph E. Levitt, Kenneth W. Mahaffey, Angela J . Rogers, Jennifer Wilson, Rosemary Vojnik, Joe Yee Fung, Nina T. Gentile, Sarah Loughran, Marie-Carmelle Elie, Torben Becker, Rohit Patel, Travis Murphy, Matthew Shaw, Rebecca Murray, Christian Bime, Jarrod Mosier, Cathleen Wilson, and Heidi Erickson
References
- 1.Pfuntner A, Wier LM, Steiner C. Healthcare Cost and Utilization Project (HCUP) statistical briefs. Rockville, MD: U.S. Agency for Healthcare Research and Quality; 2006. Costs for hospital stays in the United States, 2011: statistical brief #168. [PubMed] [Google Scholar]
- 2.Pfuntner A, Wier LM, Stocks C. Healthcare Cost and Utilization Project (HCUP) statistical briefs. Rockville, MD: Agency for Healthcare Research and Quality (US); 2006. Most frequent conditions in US hospitals, 2011: statistical brief #162. [Google Scholar]
- 3.Heron M. Deaths: leading causes for 2011. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics. National Vital Statistics System. 2015;64:1–96. [PubMed] [Google Scholar]
- 4.Ewig S, Ruiz M, Mensa J, Marcos MA, Martinez JA, Arancibia F, et al. Severe community-acquired pneumonia: assessment of severity criteria. Am J Respir Crit Care Med. 1998;158:1102–1108. doi: 10.1164/ajrccm.158.4.9803114. [DOI] [PubMed] [Google Scholar]
- 5.Valencia M, Badia JR, Cavalcanti M, Ferrer M, Agustí C, Angrill J, et al. Pneumonia severity index class V patients with community-acquired pneumonia: characteristics, outcomes, and value of severity scores. Chest. 2007;132:515–522. doi: 10.1378/chest.07-0306. [DOI] [PubMed] [Google Scholar]
- 6.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med. 2000;342:1334–1349. doi: 10.1056/NEJM200005043421806. [DOI] [PubMed] [Google Scholar]
- 7.Blum CA, Nigro N, Briel M, Schuetz P, Ullmer E, Suter-Widmer I, et al. Adjunct prednisone therapy for patients with community-acquired pneumonia: a multicentre, double-blind, randomised, placebo-controlled trial. Lancet. 2015;385:1511–1518. doi: 10.1016/S0140-6736(14)62447-8. [DOI] [PubMed] [Google Scholar]
- 8.Torres A, Sibila O, Ferrer M, Polverino E, Menendez R, Mensa J, et al. Effect of corticosteroids on treatment failure among hospitalized patients with severe community-acquired pneumonia and high inflammatory response: a randomized clinical trial. JAMA. 2015;313:677–686. doi: 10.1001/jama.2015.88. [DOI] [PubMed] [Google Scholar]
- 9.Siemieniuk RA, Meade MO, Alonso-Coello P, Briel M, Evaniew N, Prasad M, et al. Corticosteroid therapy for patients hospitalized with community-acquired pneumonia: a systematic review and meta-analysis. Ann Intern Med. 2015;163:519–528. doi: 10.7326/M15-0715. [DOI] [PubMed] [Google Scholar]
- 10.Snijders D, Daniels JM, de Graaff CS, van der Werf TS, Boersma WG. Efficacy of corticosteroids in community-acquired pneumonia: a randomized double-blinded clinical trial. Am J Respir Crit Care Med. 2010;181:975–982. doi: 10.1164/rccm.200905-0808OC. [DOI] [PubMed] [Google Scholar]
- 11.Sakuma T, Okaniwa G, Nakada T, Nishimura T, Fujimura S, Matthay MA. Alveolar fluid clearance in the resected human lung. Am J Respir Crit Care Med. 1994;150:305–310. doi: 10.1164/ajrccm.150.2.8049807. [DOI] [PubMed] [Google Scholar]
- 12.Perkins GD, McAuley DF, Thickett DR, Gao F. The Beta-Agonist Lung Injury Trial (BALTI): a randomized placebo-controlled clinical trial. Am J Respir Crit Care Med. 2006;173:281–287. doi: 10.1164/rccm.200508-1302OC. [DOI] [PubMed] [Google Scholar]
- 13.Festic E, Carr GE, Cartin-Ceba R, Hinds RF, Banner-Goodspeed V, Bansal V, et al. Randomized clinical trial of a combination of an inhaled corticosteroid and beta agonist in patients at risk of developing the acute respiratory distress syndrome. Crit Care Med. 2017;45:798–805. doi: 10.1097/CCM.0000000000002284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, et al. RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with COVID-19: preliminary report. N Engl J Med. [online ahead of print] 17 Jul 2020; DOI: 10.1056/NEJMoa2021436. [Google Scholar]
- 15.Efron B. Forcing a sequential experiment to be balanced. Biometrika. 1971;58:403–417. [Google Scholar]
- 16.Frat JP, Thille AW, Mercat A, Girault C, Ragot S, Perbet S, et al. FLORALI Study Group; REVA Network. High-flow oxygen through nasal cannula in acute hypoxemic respiratory failure. N Engl J Med. 2015;372:2185–2196. doi: 10.1056/NEJMoa1503326. [DOI] [PubMed] [Google Scholar]
- 17.Corley A, Rickard CM, Aitken LM, Johnston A, Barnett A, Fraser JF, et al. High-flow nasal cannulae for respiratory support in adult intensive care patients. Cochrane Database Syst Rev. 2017;5:CD010172. doi: 10.1002/14651858.CD010172.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matthay MA, Zemans RL, Zimmerman GA, Arabi YM, Beitler JR, Mercat A, et al. Acute respiratory distress syndrome. Nat Rev Dis Primers. 2019;5:18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sweeney TE, Wong HR, Khatri P. Robust classification of bacterial and viral infections via integrated host gene expression diagnostics. Sci Transl Med. 2016;8:346ra91. doi: 10.1126/scitranslmed.aaf7165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin W, Halpern SD, Prasad Kerlin M, Small DSA. A “placement of death” approach for studies of treatment effects on ICU length of stay. Stat Methods Med Res. 2017;26:292–311. doi: 10.1177/0962280214545121. [DOI] [PubMed] [Google Scholar]
- 21.Brunner E, Munzel U. The nonparametric Behrens‐Fisher problem: asymptotic theory and a small‐sample approximation. Biometrical J. 2000;42:17–25. [Google Scholar]
- 22.Casper C, Perez OA. ldbounds: Lan-DeMets method for group sequential boundaries. Version 1. Vienna, Austria: R Foundation for Statistical Computing; 2014. Available from: https://CRAN.R-project.org/package=ldbounds.
- 23.Reboussin DM, DeMets DL, Kim KM, Lan KK. Computations for group sequential boundaries using the Lan-DeMets spending function method. Control Clin Trials. 2000;21:190–207. doi: 10.1016/s0197-2456(00)00057-x. [DOI] [PubMed] [Google Scholar]
- 24.Menéndez R, Martínez R, Reyes S, Mensa J, Filella X, Marcos MA, et al. Biomarkers improve mortality prediction by prognostic scales in community-acquired pneumonia. Thorax. 2009;64:587–591. doi: 10.1136/thx.2008.105312. [DOI] [PubMed] [Google Scholar]
- 25.Salluh JI, Soares M, Coelho LM, Bozza FA, Verdeal JC, Castro-Faria-Neto HC, et al. Impact of systemic corticosteroids on the clinical course and outcomes of patients with severe community-acquired pneumonia: a cohort study. J Crit Care. 2011;26:193–200. doi: 10.1016/j.jcrc.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 26.Kielgast F, Schmidt H, Braubach P, Winkelmann VE, Thompson KE, Frick M, et al. Glucocorticoids regulate tight junction permeability of lung epithelia by modulating claudin 8. Am J Respir Cell Mol Biol. 2016;54:707–717. doi: 10.1165/rcmb.2015-0071OC. [DOI] [PubMed] [Google Scholar]
- 27.Bartko J, Stiebellehner L, Derhaschnig U, Schoergenhofer C, Schwameis M, Prosch H, et al. Dissociation between systemic and pulmonary anti-inflammatory effects of dexamethasone in humans. Br J Clin Pharmacol. 2016;81:865–877. doi: 10.1111/bcp.12857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Festic E, Bansal V, Gajic O, Lee AS U.S. Critical Illness and Injury Trials Group: Lung Injury Prevention Study Investigators (USCIITG-LIPS) Prehospital use of inhaled corticosteroids and point prevalence of pneumonia at the time of hospital admission: secondary analysis of a multicenter cohort study. Mayo Clin Proc. 2014;89:154–162. doi: 10.1016/j.mayocp.2013.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mangi AM, Bansal V, Li G, Pieper MS, Gajic O, Festic E. Pre-hospital use of inhaled corticosteroids and inhaled beta agonists and incidence of ARDS: a population-based study. Acta Med Acad. 2015;44:109–116. doi: 10.5644/ama2006-124.138. [DOI] [PubMed] [Google Scholar]
- 30.Ju NY, Gao H, Huang W, Niu FF, Lan WX, Li F, et al. Therapeutic effect of inhaled budesonide (Pulmicort® Turbuhaler) on the inflammatory response to one-lung ventilation. Anaesthesia. 2014;69:14–23. doi: 10.1111/anae.12479. [DOI] [PubMed] [Google Scholar]
- 31.Mohamed HS, Meguid MM. Effect of nebulized budesonide on respiratory mechanics and oxygenation in acute lung injury/acute respiratory distress syndrome: randomized controlled study. Saudi J Anaesth. 2017;11:9–14. doi: 10.4103/1658-354X.197369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matthay MA, Folkesson HG, Clerici C. Lung epithelial fluid transport and the resolution of pulmonary edema. Physiol Rev. 2002;82:569–600. doi: 10.1152/physrev.00003.2002. [DOI] [PubMed] [Google Scholar]
- 33.Frank JA, Briot R, Lee JW, Ishizaka A, Uchida T, Matthay MA. Physiological and biochemical markers of alveolar epithelial barrier dysfunction in perfused human lungs. Am J Physiol Lung Cell Mol Physiol. 2007;293:L52–L59. doi: 10.1152/ajplung.00256.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Maris NA, de Vos AF, Dessing MC, Spek CA, Lutter R, Jansen HM, et al. Antiinflammatory effects of salmeterol after inhalation of lipopolysaccharide by healthy volunteers. Am J Respir Crit Care Med. 2005;172:878–884. doi: 10.1164/rccm.200503-451OC. [DOI] [PubMed] [Google Scholar]
- 35.Atabai K, Ware LB, Snider ME, Koch P, Daniel B, Nuckton TJ, et al. Aerosolized β2-adrenergic agonists achieve therapeutic levels in the pulmonary edema fluid of ventilated patients with acute respiratory failure. Intensive Care Med. 2002;28:705–711. doi: 10.1007/s00134-002-1282-x. [DOI] [PubMed] [Google Scholar]
- 36.Gao Smith F, Perkins GD, Gates S, Young D, McAuley DF, Tunnicliffe W, et al. BALTI-2 study investigators. Effect of intravenous β-2 agonist treatment on clinical outcomes in acute respiratory distress syndrome (BALTI-2): a multicentre, randomised controlled trial. Lancet. 2012;379:229–235. doi: 10.1016/S0140-6736(11)61623-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matthay MA, Brower RG, Carson S, Douglas IS, Eisner M, Hite D, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Randomized, placebo-controlled clinical trial of an aerosolized β2-agonist for treatment of acute lung injury. Am J Respir Crit Care Med. 2011;184:561–568. doi: 10.1164/rccm.201012-2090OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Albertine KH, Soulier MF, Wang Z, Ishizaka A, Hashimoto S, Zimmerman GA, et al. Fas and Fas ligand are up-regulated in pulmonary edema fluid and lung tissue of patients with acute lung injury and the acute respiratory distress syndrome. Am J Pathol. 2002;161:1783–1796. doi: 10.1016/S0002-9440(10)64455-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perkins GD, Gates S, Park D, Gao F, Knox C, Holloway B, et al. BALTI-Prevention Collaborators. The beta agonist lung injury trial prevention: a randomized controlled trial. Am J Respir Crit Care Med. 2014;189:674–683. doi: 10.1164/rccm.201308-1549OC. [DOI] [PMC free article] [PubMed] [Google Scholar]