Abstract
Rationale: The coronavirus disease (COVID-19) pandemic struck an immunologically naive, globally interconnected population. In the face of a new infectious agent causing acute respiratory failure for which there were no known effective therapies, rapid, often pragmatic trials were necessary to evaluate potential treatments, frequently starting with medications that are already marketed for other indications. Early in the pandemic, hydroxychloroquine and azithromycin were two such candidates.
Objectives: To assess the relative efficacy of hydroxychloroquine and azithromycin among hospitalized patients with COVID-19.
Methods: We performed a randomized clinical trial of hydroxychloroquine versus azithromycin among hospitalized patients with COVID-19. Treatment was 5 days of study medication. The primary endpoint was the COVID ordinal outcomes scale at Day 14. Secondary endpoints included hospital-, intensive care unit–, and ventilator-free days at Day 28. The trial was stopped early after the enrollment of 85 patients when a separate clinical trial concluded that a clinically important effect of hydroxychloroquine over placebo was definitively excluded. Comparisons were made a priori using a proportional odds model from a Bayesian perspective.
Results: We enrolled 85 patients at 13 hospitals over 11 weeks. Adherence to study medication was high. The estimated odds ratio for less favorable status on the ordinal scale for hydroxychloroquine versus azithromycin from the primary analysis was 1.07, with a 95% credible interval from 0.63 to 1.83 with a posterior probability of 60% that hydroxychloroquine was worse than azithromycin. Secondary outcomes displayed a similar slight preference for azithromycin over hydroxychloroquine. QTc prolongation was rare and did not differ between groups. The 20 safety outcomes were similar between arms, with the possible exception of postrandomization-onset acute kidney injury, which was more common with hydroxychloroquine (15% vs. 0%). Patients in the hydroxychloroquine arm received remdesivir more often than those in the azithromycin arm (19% vs. 2%). There was no apparent association between remdesivir use and acute kidney injury.
Conclusions: Although early termination limits the precision of our results, we found no suggestion of substantial efficacy for hydroxychloroquine over azithromycin. Acute kidney injury may be more common with hydroxychloroquine than azithromycin, although this may be due to the play of chance. Differential use of remdesivir may have biased our results in favor of hydroxychloroquine. Our results are consistent with conclusions from other trials that hydroxychloroquine cannot be recommended for inpatients with COVID-19; azithromycin may merit additional investigation.
Clinical trial registered with www.clinicaltrials.gov (NCT04329832).
Keywords: COVID-19, trials, hydroxychloroquine
Effective therapies for coronavirus disease (COVID-19) are urgently sought. The nucleotide analog remdesivir has suggested efficacy in patients with moderate or severe disease (1, 2). The corticosteroid dexamethasone has suggested efficacy in severe or critical disease (3). Other therapies are currently in trials or have been reported with mixed results (4, 5). Given the high morbidity and mortality of COVID-19, patients, families, and clinicians are eager for actionable knowledge regarding potential therapies. The first wave of therapeutic options under consideration included medications with known safety profiles and regulatory approvals for other indications but often minimal preclinical data to support potential efficacy. This approach was taken given the rapid spread of infection and the desire to identify agents that might be rapidly deployed for emergency use. It also offered important efficiencies across regulatory and logistical domains to allow trial launch at unprecedented speed.
In March–April 2020 considerable interest focused on the antimalarial agent hydroxychloroquine on the basis of in vitro data, experience in other diseases (including as an immune modulator) (6–10), and small trials that suggested no safety concerns and the possibility of efficacy (6, 11–16). Similar types of suggestive data were available in support of azithromycin as an immunomodulator and antiviral agent, complementing its familiar antibacterial effects (17–22).
As this trial was being prepared, public interest and local government proposals, including an attempt to provide hydroxychloroquine on demand, were driving ubiquitous off-label use of medications, including hydroxychloroquine and azithromycin, without a prescription. We were eager to determine the potential benefit of each of these agents, but in this atmosphere, we believed that a placebo-controlled trial might not be feasible, especially across the diverse study sites considered. We therefore developed an open-label, randomized clinical trial comparing hydroxychloroquine with azithromycin, with an eye to enrolling patients across the state of Utah in multiple types of hospitals (including academic, nonacademic, referral, and community). The 13 study hospitals were drawn from Intermountain Healthcare and the University of Utah, the major nonprofit systems in the state.
Methods
Design
We published detailed methods for the HAHPS (Hydroxychloroquine versus Azithromycin for Hospitalized Patients with COVID-19) trial previously (23). We enrolled patients from April 3 to June 19, 2020, at 13 hospitals in Utah. We enrolled hospitalized patients with symptomatic laboratory-confirmed COVID-19 within 10 days of a positive test for COVID-19. Patients were excluded for ethical reasons (e.g., prisoners) or for safety reasons (e.g., known long QT, seizure disorder, or renal or liver failure). Further details of eligibility criteria are provided in the trial protocol (appended to the online supplement) and the detailed methods publication (23).
Eligible patients were randomly assigned (permuted blocks with concealed allocation) in a 1:1 ratio to hydroxychloroquine or azithromycin. Randomization was stratified by study site. Hydroxychloroquine sulfate was administered orally as a loading dose of 400 mg twice on the first day, followed by 200 mg twice daily for the following 4 days (total dose, 2.4 gm) or until discharge or death. Azithromycin was administered orally as a loading dose of 500 mg on the first day, followed by 250 mg daily for the next 4 days (total dose, 1.5 gm) or until discharge or death. The study protocol was approved by the Intermountain Institutional Review Board and Data and Safety Monitoring Board, both of which oversaw the trial. Informed consent (according to pandemic procedures using no-touch procedures, as published previously) (23) was obtained from each patient (or legally authorized representative, as appropriate) before any study procedures were performed. The funder (the Heart and Lung Foundation of the Intermountain Research and Medical Foundation) had no input into the design or reporting of the trial.
Study Procedures
Patients were assessed according to a schedule of assessments through study Day 28, with daily monitoring through Day 5. Study drug was held if QTc was >500 ms, if the patient declined a dose, or if enteral access became unavailable. Concomitant medications were not restricted but were recorded both before and through 5 days after randomization. Although both study drugs are considered generally safe, a priority in the trial was optimization of safety. We employed the following four parallel mechanisms for safety monitoring: 1) exclusion of patients at increased risk of arrhythmia or other side effects, 2) an electrocardiogram performed 24–48 h after enrollment, 3) daily review of medications to assure no contraindicated or potentially contraindicated medications were initiated, and 4) daily review for potential adverse events. Further details of safety monitoring are available in the protocol.
Study Endpoints
The primary endpoint was the Day 14 COVID ordinal outcomes scale (Table E1 in the online supplement). This endpoint ranges from 1 (home without limitations on usual activities) to 8 (death). Secondary endpoints included hospital-free, ventilator-free, and intensive care unit (ICU)–free days, which were all censored at 28 days. Safety outcomes were assessed daily through Day 5, with a summary at the time of hospital discharge. We defined acute kidney injury (AKI) as Kidney Disease: Improving Global Outcomes stage 2 (a doubling of creatinine or any value >4 mg/dl) as acute onset if it was not present at randomization but was present within the first 5 days after randomization.
Statistical Considerations
The primary analysis was an ordinal regression of the Day-14 score on the COVID ordinal outcomes scale, with treatment group as the independent variable and patient age, comorbidities (dichotomized as either no comorbidities or any comorbidities, with the comorbidities drawn from hypertension, chronic obstructive pulmonary disease, and the Charlson constituent comorbidities) (24), and baseline level of the COVID ordinal outcomes scale as covariates. The statistical analysis plan was finalized by investigators/statisticians blinded to trial data. Secondary efficacy endpoints, including hospital-, ICU-, and ventilator-free days, were analyzed using the same framework (i.e., ordinal regression including the same prespecified covariates). In the context of a rapidly evolving pandemic, we used a Bayesian framework to accommodate frequent and flexible interim monitoring. For each ordinal efficacy endpoint, we used a moderately skeptical prior, which assigned a median odds ratio (OR) of 1.0 and a probability of 0.95 to an OR between 0.5 and 2.0. This shifted estimated ORs toward 1.0 to mitigate the anticipated variability observed with assessments with small sample sizes. The trial was intended to be interpreted in the context of other concurrent trials according to an inference grid published before data were reviewed (23).
Analyses were performed according to the intention-to-treat principle. The safety cohort included all patients who received at least one dose of study drug. The primary analysis was on patients in the intention-to-treat cohort who had no missing data for the relevant variables. A sensitivity analysis controlled for the amount of clinical azithromycin received before randomization. Recognizing the importance of differential treatment exposures after randomization in an open-label trial, we report concomitant medications through Day 5 by the class of drug administered. A post hoc exploratory analysis controlled for receipt of remdesivir, as described in the online supplement.
Power and Sample Size
Details of the sample size calculation are presented in the methods publication (23). Briefly, we estimated 80% power to detect an OR of 0.55 at 300 patients for the superiority of hydroxychloroquine over azithromycin. After 85 patients had been enrolled in the HAHPS trial, a large placebo-controlled trial (ORCHID [Outcomes Related to COVID-19 treated with Hydroxychloroquine among Inpatients with symptomatic Disease], NCT04332991) of hydroxychloroquine versus placebo was concluded on the grounds that an effect (positive or negative) was definitively excluded. The ORCHID results were made available in the context of similar reports from other trials. This cumulative evidence led investigators and the Data and Safety Monitoring Board to close the HAHPS trial to enrollment on June 19, 2020, on the basis of the totality of evidence. One interim analysis had been performed at 60 patients with 14-day follow-up. Investigators were blind to the results of the interim analysis at the time the trial closed. The sample size of 85 patients at the time the study was stopped was insufficient to provide adequate power to detect plausible but clinically important effects on the primary or secondary efficacy outcome. We use the Bayesian posterior probabilities to describe the implications of our data for efficacy outcomes while accounting for the uncertainty associated with the limited sample size.
Results
We assessed 829 patients (Figure 1) for eligibility, approached 211, and enrolled 85. Forty-two (49%) were randomized to hydroxychloroquine, whereas 43 (51%) were randomized to azithromycin.
Figure 1.
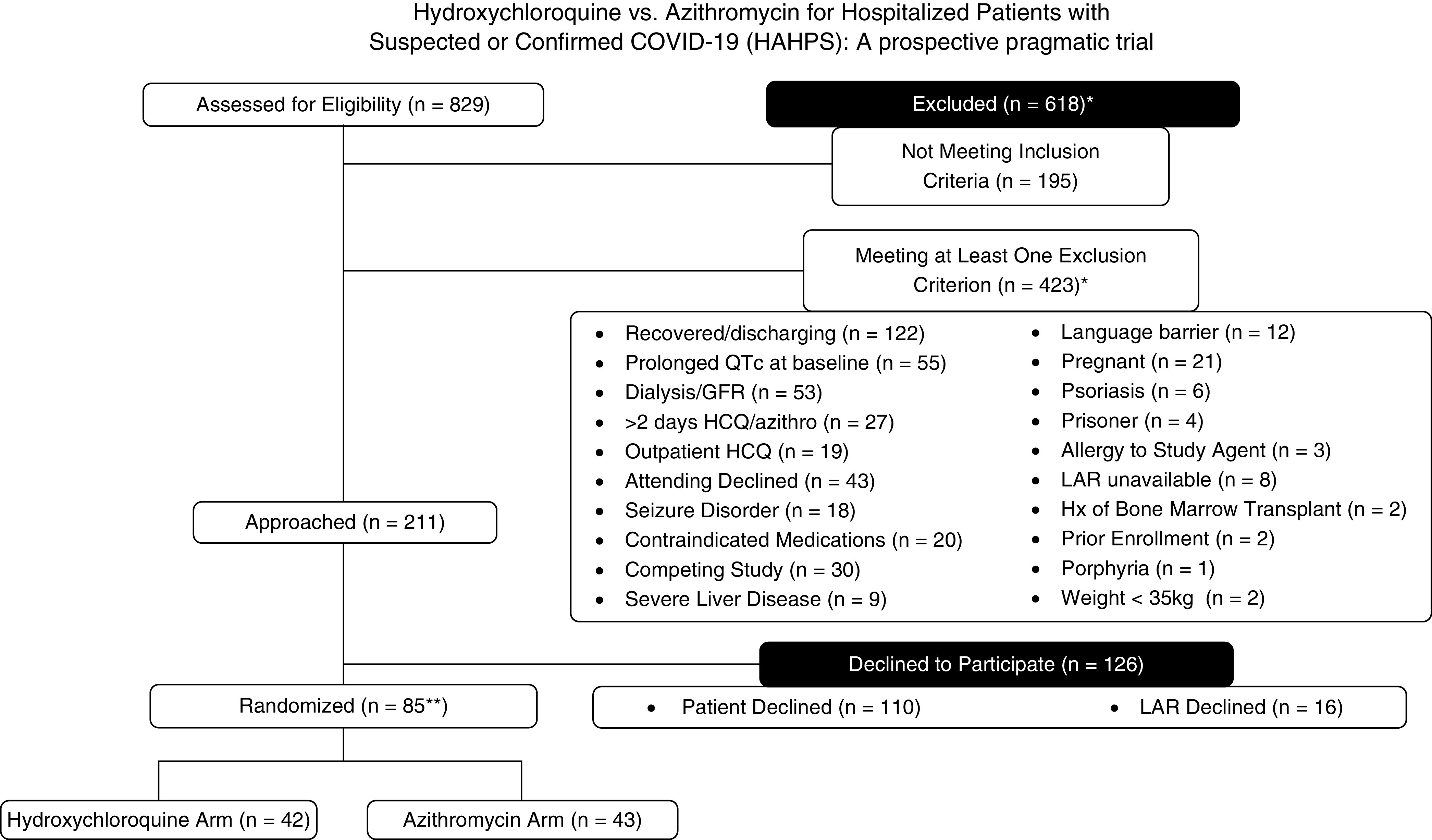
Flow of participants through the trial. *Adjusted for patients who met >1 exclusion criterion. **One patient in each arm (two patients total) did not receive study drug. azithro = azithromycin; COVID-19 = coronavirus disease; GFR = glomerular filtration rate; HAHPS = Hydroxychloroquine versus Azithromycin for Hospitalized Patients with COVID-19; HCQ = hydroxychloroquine; Hx = medical history; LAR = legally authorized representative; QT = measurement made on an electrocardiogram from the start of the Q wave to the end of the T wave; QTc = corrected QT.
Two patients were unexpectedly discharged after randomization, before the first dose could be administered (according to intention-to-treat principles, these patients were retained in the intention-to-treat cohort). The mean (standard deviation) duration of treatment course was 4.14 ± 1.41 days (additional details in Table E10). Sixty-six percent of patients completed all possible doses; in all but two cases (one patient refusal and one pharmacy error), failure to receive all doses was related to hospital discharge or death within the first 5 days. Compliance with safety monitoring was high; all patients had documented safety monitoring performed on all five of the first 5 days on study.
Baseline demographics and severity of illness measures, including the distribution of the COVID ordinal outcome scale at baseline, are displayed in Table 1. Additional baseline characteristics are displayed in the online supplement. Median (interquartile range [IQR]) age was 55 (42–65) years; 39% were female; 32 (38%) were of Latinx ethnicity; and 61% were of Latinx ethnicity and/or nonwhite race. Approximately half of patients had no comorbidities. Thirty-one percent of patients were receiving high-flow oxygen or greater intensity support (baseline ordinal scale ≥5); only 14% were hospitalized without supplemental oxygen. The median (IQR) duration from symptom onset to randomization was 8 (6–12) days. All enrolled patients were confirmed severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) positive via polymerase chain reaction testing.
Table 1.
Baseline characteristics of enrolled patients
| Overall (N = 85) | Hydroxychloroquine (n = 42) | Azithromycin (n = 43) | |
|---|---|---|---|
| Age, yr | 55 (42–65) | 51 (42–60) | 58 (43–68) |
| Sex, F, n (%) | 33 (39) | 19 (44) | 14 (33) |
| Latinx ethnicity, n (%) | 32 (38) | 17 (40) | 15 (36) |
| Race, n (%) | |
||
| Black/African American | 1 (1) | 1 (2) | 0 (0) |
| Native Hawaiian/Pacific Islander | 10 (12) | 6 (14) | 4 (10) |
| American Indian/Alaska Native | 8 (9) | 3 (7) | 5 (12) |
| White | 54 (64) | 26 (60) | 28 (67) |
| Nonwhite race or Latinx ethnicity | 52 (61) | 29 (67) | 23 (55) |
| Other/multiple | 12 (14) | 7 (16) | 5 (12) |
| Admission SOFA score, median (IQR) | 3 (2–4) | 3 (2–3) | 3 (2–4) |
| Comorbidities | |||
| Total Charlson count, median (IQR) | 1 (0–2) | 1 (0–2) | 0 (0–2) |
| No comorbidities, n (%) | 41 (48) | 18 (42) | 23 (55) |
| Baseline ordinal scale, n (%) | |||
| 3 (hospitalized no oxygen) | 12 (14) | 6 (14) | 6 (14) |
| 4 (hospitalized, some oxygen) | 47 (55) | 24 (56) | 23 (55) |
| 5 (HFNC or NIV) | 13 (15) | 7 (16) | 6 (14) |
| 6 (mechanical ventilation) | 9 (11) | 4 (9) | 5 (12) |
| 7 (mechanical ventilation and other organ support) | 4 (5) | 2 (5) | 2 (5) |
| Duration of symptoms (d), median (IQR) | 8 (6–12) | 9 (7–11) | 8 (5–12) |
Definition of abbreviations: HFNC = high-flow nasal cannula oxygen; IQR = interquartile range; NIV = noninvasive ventilation; SOFA = Sequential Organ Failure Assessment.
No data were missing for the 14-day analyses; only one participant had a missing outcome for the 28-day analysis. That participant was excluded from the Day 28 analysis. Primary and key secondary outcomes are displayed in Table 2 (additional outcomes are presented in the online supplement). In the primary analysis, the posterior median OR (95% credible interval) for a less favorable COVID ordinal outcome was 1.07 (0.63–1.83) for the hydroxychloroquine arm compared with the azithromycin arm (see also Figure E2). The OR is consistent with a small benefit of azithromycin over hydroxychloroquine; the posterior probability that hydroxychloroquine is worse than azithromycin was 0.60. Our data are also consistent with approximately equal posterior probabilities that the treatment effect is negligible or nonnegligible.
Table 2.
Primary and key secondary outcomes for randomized patients
| COVID ordinal scale at 14 d | Results |
|---|---|
| Results of primary ordinal regression model | |
| OR for a less favorable COVID ordinal outcome at 14 d in hydroxychloroquine arm compared with the azithromycin arm (95% credible interval)* | 1.07 (0.63–1.83)* |
| Posterior probabilities from primary ordinal regression model for 14-d COVID ordinal outcome | |
| P1 = Pr(OR < 1) (any benefit of hydroxychloroquine over azithromycin) | 0.40 |
| P2 = Pr(OR < 1/1.25) (at least moderate benefit of hydroxychloroquine over azithromycin) | 0.14 |
| P3 = Pr(OR > 1) (any benefit of azithromycin over hydroxychloroquine) | 0.60 |
| P4 = Pr(OR > 1.25) (at least moderate benefit of azithromycin over hydroxychloroquine) | 0.29 |
| P5 = Pr(1/1.2 < OR <1.2) (negligible difference between the two agents) | 0.48 |
| Key secondary endpoints, OR (95% credible interval) | |
| COVID ordinal scale at 7 d | 1.16 (0.68–1.96)* |
| Hospital-free days at 28 d | 0.91 (0.54–1.54)† |
| ICU-free days at 28 d | 0.85 (0.50–1.46)† |
| 28-d mortality | Too few events |
Definition of abbreviations: COVID = coronavirus disease; ICU = intensive care unit; OR = odds ratio; Pr = probability.
An OR >1 favors azithromycin over hydroxychloroquine for this comparison.
An OR <1 favors azithromycin over hydroxychloroquine for this comparison.
The 28-day mortality was 8%, with one (2%) death in the azithromycin group and six (14%) deaths in the hydroxychloroquine group. There were too few deaths to support regression modeling; we do not report a P value to avoid misleading readers. Mortality and quality of life at 6 months will be reported in a subsequent manuscript to allow timely reporting of 28-day outcomes during the pandemic. Extrapulmonary complications were uncommon (Table E7) and did not differ substantially between treatment arms. The overall incidence of venous thromboembolism during the hospitalization was 6% among all patients, with no substantial difference between treatment arms.
Exploratory subgroup analyses are presented in the Table E6—the estimates in general differed little from each other. Among non-Latinx white patients (n = 33), the OR from the primary analysis was 1.20 (0.64–2.24), whereas for patients of Latinx ethnicity and/or nonwhite race (n = 52), the OR was 0.91 (0.50–1.64).
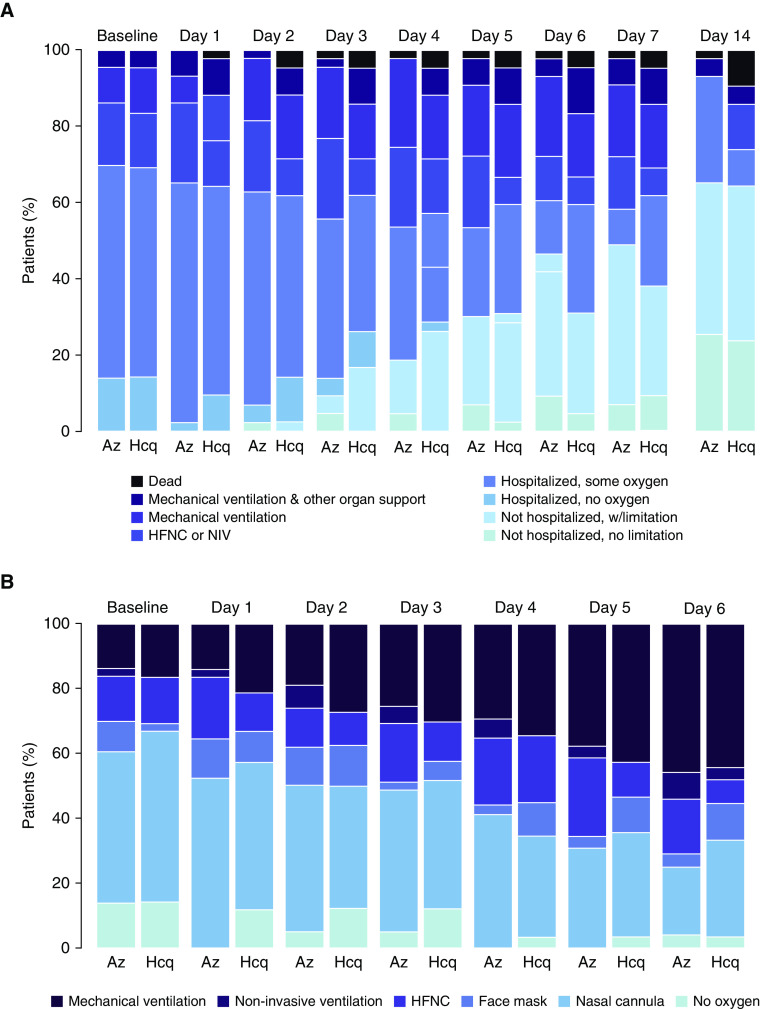
Disease progression over time is presented in Figures 2A and 2B. The proportion of patients dead or on mechanical ventilation increased through Days 4–5; by Day 14, the majority of patients had been discharged home.
Figure 2.
(A) COVID ordinal scale over time. (B) Level of oxygen support over time, among patients alive and in the hospital. Az = azithromycin; COVID = coronavirus disease; Hcq = hydroxychloroquine; HFNC = high-flow nasal cannula oxygen; NIV = noninvasive ventilation; w = with.
During the hospitalization, 29 (34%) patients were ever mechanically ventilated, 18 (21%) received vasopressors, no patients were treated with renal replacement therapy, and one patient was treated with extracorporeal membrane oxygenation, which was initiated >5 days after randomization.
Overall, 49% of patients received at least one COVID-19–targeted therapy after randomization. The distribution of those concomitant medications is displayed in the online supplement. Postrandomization concomitant medications did not differ between treatment groups (Table E16), with one possible exception. Remdesivir (given under an emergency use authorization) may have been used significantly more often in the hydroxychloroquine than in the azithromycin arm (19% vs. 2%). In the post hoc analysis controlling for the expected effect of remdesivir, the estimated OR was 1.26 (95% credible interval, 0.82–1.93). In the hydroxychloroquine arm, six patients (14%) received nonrandomized azithromycin after study enrollment (generally as clinician-directed treatment for suspected pneumonia). No patients randomized to the azithromycin arm received any hydroxychloroquine.
Safety Outcomes
The safety cohort included 83 patients (two patients were unexpectedly discharged home after randomization but before the first dose of drug was administered). Safety results are displayed in the Tables E17–E22. Briefly, adverse events and safety outcomes within the first 5 days were very similar between groups, with the possible exception among 20 nonmortality safety outcomes of postrandomization-onset AKI. AKI through Day 5 was present in 6/41 (15%) patients in the hydroxychloroquine arm and 0/42 (0%) patients in the azithromycin arm (unadjusted P = 0.01 from Barnard’s exact test); no patients in either arm underwent renal replacement. One additional patient in the hydroxychloroquine group (and no additional patients in the azithromycin group) experienced AKI between Day 5 and hospital discharge. QTc did not differ between treatment arms, including among hydroxychloroquine patients who received azithromycin before or after enrollment.
Study drug was permanently discontinued in three patients in the hydroxychloroquine arm and in two patients in the azithromycin arm for QTc >500 ms. Contraindicated or potentially contraindicated medications triggered a discussion between treating clinician and pharmacist in 13 (16%) patients through study Day 5. Safety outcomes through Day 5 did not in general differ between those who received both hydroxychloroquine and azithromycin versus those who received either hydroxychloroquine or azithromycin (see Table E23), although mechanical ventilation and vasopressor use were numerically more common in the azithromycin plus hydroxychloroquine group. This difference may be related to clinician preference to use azithromycin for severe community-acquired pneumonia therapy in patients requiring mechanical ventilation.
Discussion
We report here the results of a randomized, pragmatic, open-label, active comparator trial of two commonly available and much-discussed therapies early in the COVID-19 pandemic. We observed no compelling evidence for a difference in our primary outcome between the hydroxychloroquine and azithromycin treatments. This result must be interpreted in the context of the limitations resulting from our sample size and the fact that our data provide similar posterior probabilities that a treatment effect was negligible (0.48) versus nonnegligible (0.52). In the safety analysis, AKI was more common in the hydroxychloroquine arm, although this may reflect the number of safety outcomes we analyzed rather than a true finding. Differential use of remdesivir in the hydroxychloroquine arm may have masked a relative advantage of azithromycin over hydroxychloroquine, a possibility consistent with a post hoc sensitivity analysis.
In terms of the context of this trial, we note that in a large, open-label pragmatic trial in the United Kingdom with limited safety monitoring and a higher dose of hydroxychloroquine, there was a suggestion of modest (1–2% absolute risk increase) harm associated with hydroxychloroquine as opposed to usual care (see supplemental discussion in online supplement) (25). High doses of chloroquine with limited safety monitoring were associated with increased mortality in one randomized trial of high- versus low-dose chloroquine (26). Clinical cohorts described high rates of QTc prolongation or ventricular arrythmias (27, 28), although these have not been reproduced in controlled trials (25). An open-label Brazilian trial suggested no benefit for hydroxychloroquine among moderately ill patients (29). The gold-standard ORCHID trial, with rigorous eligibility criteria and safety monitoring, suggested neither benefit nor harm. Our results may thus be compatible with a benefit associated with azithromycin, as indicated in the inference grid we published previously (23). The comparison of the hydroxychloroquine arm with hydroxychloroquine + azithromycin arm (OR, 0.82) in the Brazilian COVID-19 trial may also be compatible with this observation (29). In terms of safety, we saw no Clostridioides difficile infection in the efficacy cohort, but we observed prolonged QTc occasionally in the azithromycin arm. In any case, our trial results are not sufficiently robust to advocate for clinical use of azithromycin for COVID-19.
The possible difference in stage 2 AKI merits comment even though it was not associated with renal replacement therapy. The observed difference is compatible with chance variation given comparisons of multiple safety outcomes between groups. Theoretical concerns identify hydroxychloroquine as having potential for worsening kidney injury in COVID-19 (30) despite suggestion of renal protection in chronic autoimmune disease (31–33). The RECOVERY trial did not collect data necessary to inform this observation (25), nor have most other prior trials. Given that AKI during hospitalization is often associated with worse outcomes over the intermediate to long term, this may be an important safety signal to evaluate in larger cohorts (34, 35).
The granularity of available data distinguishes this from other pragmatic trials, which makes this trial useful as a cohort study as well as a trial population. We observe that outcomes were remarkably good for enrolled patients regardless of study group; overall 28-day mortality was 8%, and mortality in the azithromycin group was 2%. Over two-thirds of patients were discharged home within 2 weeks. Extrapulmonary complications, including clinically diagnosed deep venous thrombosis, were uncommon or rare. COVID-19–specific therapies are provided to half of patients.
Although we are unable to make firm statistical claims on this point, our experience placed in context of both pragmatic and robust trials suggests that limiting hydroxychloroquine administration to a rigorous clinical trial environment increased population-level safety within the state of Utah. We thus draw attention to the important sociocultural and safety role that clinical trials—including open-label pragmatic trials—can play in a pandemic situation. In a setting where extreme local pressure existed to administer hydroxychloroquine “off label,” our decision to provide hydroxychloroquine in a clinical trial setting—with associated focus on patient autonomy and informed consent, appropriate safety monitoring, and the possibility of contributing to generalizable knowledge—played an important public health role.
Three key limitations of this clinical trial are the sample size, open-label treatment assignment, and use of an active comparator design. The sample size limits possible inferences. Crucially, we anticipate contributing data to patient-level network meta-analyses and are careful to avoid speculative claims. In terms of the lack of blinding, we assessed for differential distribution of concomitant medications and found that in fact, remdesivir was differentially prescribed to patients in the hydroxychloroquine arm. The differential use of remdesivir among hydroxychloroquine patients, which has expected efficacy in this patient population (1, 2), likely biases our estimates in favor of hydroxychloroquine. This trial may thus underestimate harms of hydroxychloroquine and/or benefits of azithromycin. Because remdesivir was prescribed in study hospitals using a standard treatment algorithm without regard to trial enrollment, we do not believe that the lack of blinding contributed to this imbalance. In terms of the active comparator design, we intended this trial to be complementary to larger placebo-controlled trials, and our nonuse of placebo allowed us to provide access and enrollments in hospitals that would not traditionally have been able to support a trial. We did not employ a “usual care” control arm, given longstanding controversies about the integrity and inferential validity of such groups (36–39). We acknowledge that other trials have used such control arms despite controversy about their interpretability. We are unable to comment on whether outcomes in our treatment arms would differ from a contemporaneous usual care control arm.
In summary, we find no suggestion of a large clinical benefit or harm associated with hydroxychloroquine as opposed to azithromycin among hospitalized patients with COVID-19, although AKI may be more common with hydroxychloroquine. Azithromycin may merit further investigation in focused trials but should not be implemented in clinical care without additional evidence.
Acknowledgments
Collaborators: Rilee Smith, Brent Armbruster, Katie Brown, Valerie Aston, Mardee Merrill, Amanda Nelson, Diana Grant, Bri Crook, Heather Maestas, and Jacki Anderson, Intermountain Healthcare, Murray, UT; Prathyusha Kodakandla, Lisa Weaver, Jorden Greer, and Macy Barrios, University of Utah, Salt Lake City, UT.
Footnotes
Supported by the Heart and Lung Research Foundation and the Intermountain Research and Medical Foundation, with additional support from the Office of the Associate Vice President for Research, University of Utah Health Sciences. This investigation was supported by the University of Utah Population Health Research Foundation, with funding in part from the National Center for Research Resources and the National Center for Advancing Translational Sciences, U.S. National Institutes of Health, through grant 5UL1TR001067-05 (formerly 8 UL1TR000105 and UL1RR025764). S.M.B. is supported by the National Heart, Lung, and Blood Institute (3U01HL123009-06S1 and 5U01HL123018) and the National Center for Advancing Translational Sciences (1UL1TR002538). R.S. is supported by the National Heart, Lung, and Blood Institute (U01HL143505) and the National Center for Advancing Translational Sciences (UL1TR002538). R.P. is supported by a Merit Award from the Department of Veterans Affairs.
Author Contributions: S.M.B., I.P., B.J.W., R.S., B.H., R.P., and T.G. conceived and designed the study. S.M.B., I.P., N.K., L.L., B.J.W., W.R.B., E.H., and S.J. acquired the data. N.K., N.S., C.K.G., A.M.B., D.G., and J.Y. participated in conception and design, analytical plan, and data analysis. All authors were involved in critical revision of the manuscript for important intellectual content and approval of the final version to be published.
A complete list of collaborators may be found before the beginning of the References.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
Contributor Information
Collaborators: Rilee Smith, Brent Armbruster, Katie Brown, Valerie Aston, Mardee Merrill, Amanda Nelson, Diana Grant, Bri Crook, Heather Maestas, Jacki Anderson, Prathyusha Kodakandla, Lisa Weaver, Jorden Greer, and Macy Barrios
References
- 1.Beigel JH, Tomashek KM, Dodd LE, Mehta AK, Zingman BS, Kalil AC, et al. Remdesivir for the treatment of Covid-19—preliminary report. N Engl J Med. doi: 10.1056/NEJMc2022236. [online ahead of print] 22 May 2020; DOI: 10.1056/NEJMoa2007764. [DOI] [PubMed] [Google Scholar]
- 2.Goldman JD, Lye DCB, Hui DS, Marks KM, Bruno R, Montejano R, et al. GS-US-540-5773 Investigators. Remdesivir for 5 or 10 days in patients with severe Covid-19. N Engl J Med. doi: 10.1056/NEJMoa2015301. [online ahead of print] 27 May 2020; DOI: 10.1056/NEJMoa2015301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.The RECOVERY Collaborative Group. Dexamethasone in hospitalized patients with covid-19—preliminary report. N Engl J Med. [online ahead of print] 17 Jul 2020; DOI: 10.1056/NEJMoa2021436. [Google Scholar]
- 4.Matthay MA, Aldrich JM, Gotts JE. Treatment for severe acute respiratory distress syndrome from COVID-19. Lancet Respir Med. 2020;8:433–434. doi: 10.1016/S2213-2600(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao B, Wang Y, Wen D, Liu W, Wang J, Fan G, et al. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Savarino A, Boelaert JR, Cassone A, Majori G, Cauda R. Effects of chloroquine on viral infections: an old drug against today’s diseases? Lancet Infect Dis. 2003;3:722–727. doi: 10.1016/S1473-3099(03)00806-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.van den Borne BE, Dijkmans BA, de Rooij HH, le Cessie S, Verweij CL. Chloroquine and hydroxychloroquine equally affect tumor necrosis factor-alpha, interleukin 6, and interferon-gamma production by peripheral blood mononuclear cells. J Rheumatol. 1997;24:55–60. [PubMed] [Google Scholar]
- 8.Picot S, Peyron F, Vuillez J-P, Polack B, Ambroise-Thomas P. Chloroquine inhibits tumor necrosis factor production by human macrophages in vitro. J Infect Dis. 1991;164:830. doi: 10.1093/infdis/164.4.830. [DOI] [PubMed] [Google Scholar]
- 9.Jang C-H, Choi J-H, Byun M-S, Jue D-M. Chloroquine inhibits production of TNF-α, IL-1β and IL-6 from lipopolysaccharide-stimulated human monocytes/macrophages by different modes. Rheumatology (Oxford) 2006;45:703–710. doi: 10.1093/rheumatology/kei282. [DOI] [PubMed] [Google Scholar]
- 10.Sperber K, Quraishi H, Kalb TH, Panja A, Stecher V, Mayer L. Selective regulation of cytokine secretion by hydroxychloroquine: inhibition of interleukin 1 alpha (IL-1-alpha) and IL-6 in human monocytes and T cells. J Rheumatol. 1993;20:803–808. [PubMed] [Google Scholar]
- 11.Jun C, Danping L, Liu L, Liu P, Xu Q, Xia L, et al. Hydroxychloroquine sulfate for the treatment of common 2019 coronavirus disease (COVID-19) Preliminary study of patients Journal of Zhejiang University (Medical Edition) 2020 [accessed 2020 Jun 1] Available from: http://kns.cnki.net/kcms/detail/33.1248.R.20200309.1507.006.html.
- 12.Vincent MJ, Bergeron E, Benjannet S, Erickson BR, Rollin PE, Ksiazek TG, et al. Chloroquine is a potent inhibitor of SARS coronavirus infection and spread. Virol J. 2005;2:69. doi: 10.1186/1743-422X-2-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P, et al. In Vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang M, Cao R, Zhang L, Yang X, Liu J, Xu M, et al. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-nCoV) in vitro. Cell Res. 2020;30:269–271. doi: 10.1038/s41422-020-0282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Biot C, Daher W, Chavain N, Fandeur T, Khalife J, Dive D, et al. Design and synthesis of hydroxyferroquine derivatives with antimalarial and antiviral activities. J Med Chem. 2006;49:2845–2849. doi: 10.1021/jm0601856. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Hu J, Zhang Z, Jiang S, Han S, Yan D, et al. Efficacy of hydroxychloroquine in patients with COVID-19: results of a randomized clinical trial [preprint]medRxiv2020[accessed 2019 Mar 1]. Available from: https://www.medrxiv.org/content/10.1101/2020.03.22.20040758v3
- 17.Gielen V, Johnston SL, Edwards MR. Azithromycin induces anti-viral responses in bronchial epithelial cells. Eur Respir J. 2010;36:646–654. doi: 10.1183/09031936.00095809. [DOI] [PubMed] [Google Scholar]
- 18.Menzel M, Akbarshahi H, Bjermer L, Uller L. Azithromycin induces anti-viral effects in cultured bronchial epithelial cells from COPD patients. Sci Rep. 2016;6:28698. doi: 10.1038/srep28698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walkey AJ, Wiener RS. Macrolide antibiotics and survival in patients with acute lung injury. Chest. 2012;141:1153–1159. doi: 10.1378/chest.11-1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kawamura K, Ichikado K, Takaki M, Sakata Y, Yasuda Y, Shingu N, et al. Efficacy of azithromycin in sepsis-associated acute respiratory distress syndrome: a retrospective study and propensity score analysis. Springerplus. 2016;5:1193. doi: 10.1186/s40064-016-2866-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simonis FD, de Iudicibus G, Cremer OL, Ong DSY, van der Poll T, Bos LD, et al. MARS consortium. Macrolide therapy is associated with reduced mortality in acute respiratory distress syndrome (ARDS) patients. Ann Transl Med. 2018;6:24. doi: 10.21037/atm.2017.12.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gibson PG, Yang IA, Upham JW, Reynolds PN, Hodge S, James AL, et al. Effect of azithromycin on asthma exacerbations and quality of life in adults with persistent uncontrolled asthma (AMAZES): a randomised, double-blind, placebo-controlled trial. Lancet. 2017;390:659–668. doi: 10.1016/S0140-6736(17)31281-3. [DOI] [PubMed] [Google Scholar]
- 23.Brown SM, Peltan ID, Webb B, Kumar N, Starr N, Grissom C, et al. Hydroxychloroquine versus Azithromycin for hospitalized patients with suspected or confirmed COVID-19 (HAHPS): protocol for a pragmatic, open-label, active comparator trial. Ann Am Thorac Soc. 2020;17:1008–1015. doi: 10.1513/AnnalsATS.202004-309SD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 25.Horby P, Mafham M, Linsell L, et al. Effect of hydroxychloroquine in hospitalized patients with COVID-19: preliminary results from a multi-centre, randomized, controlled trial [preprint]medRxiv2020[accessed 2020 Jul 1]. Available from: https://www.medrxiv.org/content/10.1101/2020.07.15.20151852v1
- 26.Borba MGS, Val FFA, Sampaio VS, Alexandre MAA, Melo GC, Brito M, et al. CloroCovid-19 Team. Effect of high vs low doses of chloroquine diphosphate as adjunctive therapy for patients hospitalized with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection: a randomized clinical trial. JAMA Netw Open. 2020;3:e208857. doi: 10.1001/jamanetworkopen.2020.8857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mercuro NJ, Yen CF, Shim DJ, Maher TR, McCoy CM, Zimetbaum PJ, et al. Risk of QT interval prolongation associated with use of hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:1036–1041. doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bessière F, Roccia H, Delinière A, Charrière R, Chevalier P, Argaud L, et al. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol. 2020;5:1067–1069. doi: 10.1001/jamacardio.2020.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cavalcanti AB, Zampieri FG, Rosa RG, Azevedo LCP, Veiga VC, Avezum A, et al. Coalition Covid-19 Brazil I Investigators. Hydroxychloroquine with or without azithromycin in mild-to-moderate covid-19. N Engl J Med. [online ahead of print] 23 Jul 2020; DOI: 10.1056/NEJMoa2019014. [Google Scholar]
- 30.Edelstein CL, Venkatachalam MA, Dong Z. Autophagy inhibition by chloroquine and hydroxychloroquine could adversely affect acute kidney injury and other organ injury in critically ill patients with COVID-19. Kidney Int. 2020;98:234–235. doi: 10.1016/j.kint.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu LJ, Yang YZ, Shi SF, Bao YF, Yang C, Zhu SN, et al. Effects of hydroxychloroquine on proteinuria in IgA nephropathy: a randomized controlled trial. Am J Kidney Dis. 2019;74:15–22. doi: 10.1053/j.ajkd.2019.01.026. [DOI] [PubMed] [Google Scholar]
- 32.Tang TT, Lv LL, Pan MM, Wen Y, Wang B, Li ZL, et al. Hydroxychloroquine attenuates renal ischemia/reperfusion injury by inhibiting cathepsin mediated NLRP3 inflammasome activation. Cell Death Dis. 2018;9:351. doi: 10.1038/s41419-018-0378-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gómez-Guzmán M, Jiménez R, Romero M, Sánchez M, Zarzuelo MJ, Gómez-Morales M, et al. Chronic hydroxychloroquine improves endothelial dysfunction and protects kidney in a mouse model of systemic lupus erythematosus. Hypertension. 2014;64:330–337. doi: 10.1161/HYPERTENSIONAHA.114.03587. [DOI] [PubMed] [Google Scholar]
- 34.Akram AR, Singanayagam A, Choudhury G, Mandal P, Chalmers JD, Hill AT. Incidence and prognostic implications of acute kidney injury on admission in patients with community-acquired pneumonia. Chest. 2010;138:825–832. doi: 10.1378/chest.09-3071. [DOI] [PubMed] [Google Scholar]
- 35.Coca SG, Peixoto AJ, Garg AX, Krumholz HM, Parikh CR. The prognostic importance of a small acute decrement in kidney function in hospitalized patients: a systematic review and meta-analysis. Am J Kidney Dis. 2007;50:712–720. doi: 10.1053/j.ajkd.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 36.Smelt AF, van der Weele GM, Blom JW, Gussekloo J, Assendelft WJ. How usual is usual care in pragmatic intervention studies in primary care? An overview of recent trials. Br J Gen Pract. 2010;60:e305–e318. doi: 10.3399/bjgp10X514819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson BT, Schoenfeld D. Usual care as the control group in clinical trials of nonpharmacologic interventions. Proc Am Thorac Soc. 2007;4:577–582. doi: 10.1513/pats.200706-072JK. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McCambridge J, Sorhaindo A, Quirk A, Nanchahal K. Patient preferences and performance bias in a weight loss trial with a usual care arm. Patient Educ Couns. 2014;95:243–247. doi: 10.1016/j.pec.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Castro M. Placebo versus best-available-therapy control group in clinical trials for pharmacologic therapies: which is better? Proc Am Thorac Soc. 2007;4:570–573. doi: 10.1513/pats.200706-073JK. [DOI] [PMC free article] [PubMed] [Google Scholar]