Abstract
Rationale: Prior studies investigating associations of rheumatoid factor (RF) and anti–citrullinated protein antibody (ACPA) seropositivity with risk for rheumatoid arthritis (RA)-associated interstitial lung disease (ILD) have mostly used cross-sectional or case–control designs.
Objectives: To determine whether combined autoantibody seropositivity and higher individual autoantibody concentrations were associated with increased risk for RA-ILD in a prospective RA cohort.
Methods: Within the Veterans Affairs Rheumatoid Arthritis prospective registry, we performed a cross-sectional study of prevalent ILD and a retrospective cohort study of incident ILD (diagnosed after at least 12 mo of longitudinal follow-up). We used logistic and Cox regression methods to determine whether combined RF/ACPA seropositivity and higher autoantibody concentrations were independently associated with greater risk for prevalent and incident ILD, respectively.
Results: Among 2,328 participants (median age 64 yr, 89.3% male), 100 (4.3%) subjects had prevalent ILD at enrollment. During 14,281 patient-years of follow-up, 83 (3.7%) of the remaining 2,228 were subsequently diagnosed with incident ILD (5.8 cases per 1,000 person-years). Patients with combined RF/ACPA seropositivity had a higher probability of prevalent ILD compared with seronegative subjects (odds ratio [OR], 2.90; 95% confidence interval [CI], 1.24–6.78). RF titers demonstrated a monotonic association with prevalent ILD (OR, 2.69; 95% CI, 1.11–6.51 for low-positive [15–45 IU/ml] titers; OR, 3.40; 95% CI, 1.61–7.18 for high-positive [>45 IU/ml] titers; P for trend 0.01). Patients with high-positive (>15 U/ml) ACPA titers were also at higher risk for prevalent ILD (OR, 1.91; 95% CI, 1.04–3.49) compared with ACPA-negative subjects. Combined RF/ACPA seropositivity was not associated with increased risk for incident ILD, nor were high- or low-positive RF or ACPA titers. In a piecewise linear spline model, however, RF titers greater than 90 IU/ml independently correlated with increased risk for incident ILD (hazard ratio, 1.68, 95% CI, 1.02–2.77).
Conclusions: Combined RF/ACPA seropositivity and individual autoantibody concentrations were strongly associated with prevalent but not incident RA-ILD. Only patients with RF concentrations >90 IU/ml were observed to be at higher risk of incident RA-ILD.
Keywords: rheumatoid arthritis–associated interstitial lung disease, rheumatoid factor, anti–citrullinated protein antibodies
Rheumatoid arthritis (RA) is a systemic autoimmune disease characterized by inflammatory synovial changes that eventually lead to symmetric arthritis and bony destruction (1). Extraarticular involvement of RA is common and frequently affects the respiratory system (2, 3). Interstitial lung disease (ILD) due to RA (hereafter, RA-ILD) is among the most severe pulmonary manifestations. Approximately 3–10% of patients with RA are diagnosed with clinically relevant ILD over the course of their disease (4–7). RA-ILD is often progressive and frequently leads to disabling symptoms and respiratory failure. Risk for mortality differs greatly for patients with RA with and without ILD. For example, one study described a threefold increase in risk of mortality in patients with ILD (8).
Few risk factors for RA-ILD have been consistently identified, including older age, male sex, articular disease severity, functional status, and tobacco exposure (9, 10). The presence of circulating rheumatoid factor (RF) and anti–citrullinated protein antibodies (ACPA), two autoantibodies routinely tested in clinical practice, have been associated with RA-ILD (10–17); however, these associations were demonstrated mostly in the context of relatively small cross-sectional and case–control studies that analyzed RF and ACPA as separate, binary (seropositive or seronegative) risk factors. Prior translational studies have observed higher concentrations of ACPA in bronchoalveolar lavage fluid from patients with RA with parenchymal lung abnormalities (18, 19), suggesting that RA autoantibodies may be produced within the lungs. Despite these findings, the implications of combined RF and ACPA seropositivity and their individual autoantibody concentrations on ILD risk remain largely unknown. An improved understanding of the relationship between these autoantibodies and RA-ILD could inform important underlying disease mechanisms and potentially strengthen their clinical utility.
We aimed to determine whether combined RF/ACPA seropositivity and higher individual autoantibody concentrations were associated with increased risk for RA-ILD. We hypothesized that dual autoantibody seropositivity would be associated with prevalent ILD and would confer greater risk for the development of incident ILD. We also hypothesized there would be a dose-dependent relationship between RF and ACPA titers and RA-ILD risk, with implications for both prevalent and incident disease.
Methods
Study Design and Patient Population
We performed both a cross-sectional study of prevalent ILD and a retrospective cohort study of incident ILD within the Veterans Affairs Rheumatoid Arthritis (VARA) prospective registry (20). The VARA registry, initiated in 2003, is an ongoing multicenter prospective observational study of U.S. veterans with RA meeting the 1987 American College of Rheumatology criteria (21). All patients provided informed consent before enrollment. In addition, all 13 participating sites obtained local institutional review board approval. The present study was approved by the VARA Scientific Ethics and Advisory Committee.
Data on patient sociodemographics, smoking history (current, former, or never), education level, RA disease onset, medications, and comorbidities were collected at enrollment, in addition to functional status and disease activity metrics such as the Multidimensional Health Assessment Questionnaire (MDHAQ) (22) and the Disease Activity Score in 28 Joints (DAS28) (23).
Characterization of Lung Disease within the VARA Registry
RA-ILD status was determined through standardized detailed medical record adjudication among patients who initially screened positive for one or more International Classification of Diseases, Ninth Revision (ICD-9) or International Classification of Diseases, Tenth Revision (ICD-10) codes previously proposed to ascertain ILD status or for one or more closely related codes, as described in prior studies (24, 25). Patients were classified as having RA-ILD if they had a provider diagnosis of ILD and either 1) chest computed tomographic (CT) scan features of ILD or 2) lung biopsy histopathological abnormalities consistent with ILD. The vast majority of RA-ILD cases (96%) were confirmed based on CT findings, whereas the remaining 4% without readily available CT data had pathology findings consistent with ILD. Imaging reports were reviewed by three rheumatologists with clinical expertise in RA-ILD who trained against each other in pilot abstraction. The reports were reviewed for impressions of ILD and pulmonary fibrosis accompanied by characteristic imaging findings (e.g., reticular opacities, honeycombing, interstitial thickening, traction bronchiectasis, ground-glass opacities). A random sample of all VARA registry subjects not identified via initial screening using ILD diagnostic codes (n = 243) was similarly reviewed via a standardized detailed medical record adjudication process. Among these subjects, only 7 out of 243 (2.9%) were ultimately classified as having ILD based on the aforementioned criteria (25). A prevalent case was defined as ILD diagnosed before or within 1 year of enrollment, whereas an incident case was defined as ILD diagnosed after at least 12 months of longitudinal follow-up within the VARA Registry. Follow-up occurred through December 31, 2018. Up until that date, we continued to query and review participating subjects’ medical records for RA-ILD evidence (diagnoses, testing, etc.). We also queried VA vital status records available through the end of the study period.
Rheumatoid Factor and Anti–Citrullinated Protein Antibody Measurements
RF and ACPA measurements were obtained from banked serum collected at the time of VARA registry enrollment. Low- and high-positive RF and ACPA titer thresholds were defined according to the 2010 American College of Rheumatology/European League Against Rheumatism updated RA classification criteria (26). ACPA (immunoglobulin G) titers were determined using a second-generation anti–cyclic citrullinated peptide antibody ELISA (Diastat; Axis-Shield Diagnostics), for which “low positivity” was defined as 5–15 U/ml and “high positivity” was defined as >15 U/ml. RF titers were determined by nephelometry (Siemens Healthcare Diagnostics), for which “low positivity” was defined as 15–45 IU/ml and “high positivity” was defined as >45 IU/ml (15). RF and ACPA concentrations were measured at regular intervals shortly after biospecimen collection (almost universally within 1 yr of subject enrollment).
Statistical Analysis
For describing differences in baseline clinical characteristics within our cross-sectional and cohort study populations, patients were categorized into three groups based on autoantibody status: 1) dual autoantibody seropositive (RF+/ACPA+); 2) single autoantibody seropositive (RF+/ACPA− or RF−/ACPA+); and 3) dual autoantibody seronegative (RF−/ACPA−). Differences in patient characteristics were analyzed using the Mann-Whitney U test, chi-squared test, or Fisher’s exact test, as appropriate. Individual RF and ACPA titers were logarithmically transformed given their skewed distributions.
We performed univariate and multivariable logistic regression analyses to assess the associations of autoantibody status as defined above, degree of RF and ACPA seropositivity (negative, low, and high) as defined by prespecified assay cutoffs, and log-transformed RF and ACPA titers with prevalent ILD. Locally weighted scatterplot smoothing was used to determine whether inclusion of spline terms improved model fit. The addition of splines allows for the evaluation of associations between the exposure (log-transformed autoantibody concentrations) and outcome (RA-ILD) over the range of the exposure to determine if there are thresholds beyond which an association is observed. The following covariates were selected for inclusion in multivariable models based on preexisting mechanistic and biological knowledge: age at enrollment, sex, race (white, African American, or other), smoking history, RA disease duration, and baseline severity of articular disease as measured by the DAS28. Other covariates such as MDHAQ scores and use of biologics, specifically anti–tumor necrosis factor therapies, were excluded given concerns for having collinearity with articular disease severity scores. Given the completeness of the data (only 2% of participants had missing data), a complete case analysis approach was performed.
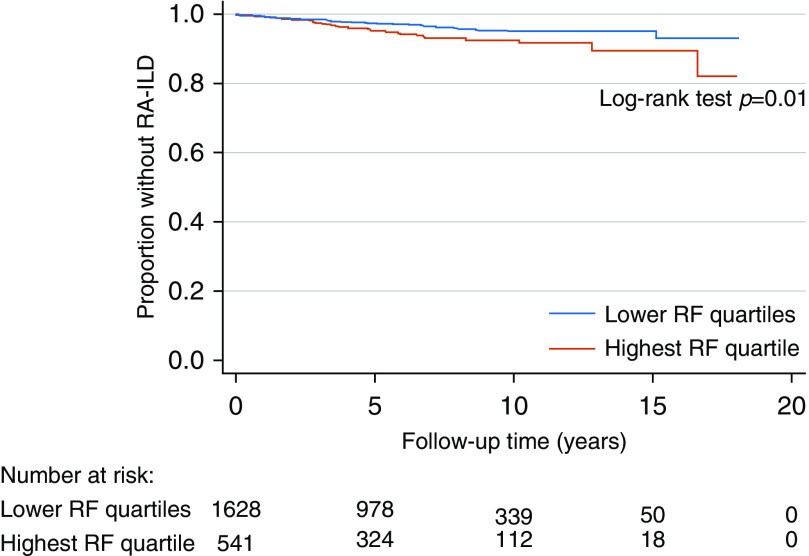
Univariate and multivariable Cox proportional hazards models were used to determine whether combined RF/ACPA seropositivity and higher individual RF and ACPA titers conferred greater risk for developing incident ILD in participants without ILD at baseline (or diagnosed within 1 yr of enrollment) (27). Based on our definition of incident ILD, subjects only began to contribute person-years to our Cox models 1 year following enrollment to mitigate risk for immortal time bias (28). Patients were followed until the development of incident ILD. Censoring occurred after December 31, 2018, or at the time of death. The proportional hazards assumption was assessed via weighted versions of Kaplan-Meier curves using log–log plots and tests and graphical displays of Schoenfeld and scaled Schoenfeld residuals. Unadjusted Kaplan-Meier survival curves were generated to demonstrate differences in incident ILD risk based on RF titers in the highest quartile (>295 IU/ml) relative to the lower three quartiles.
We performed several sensitivity analyses, including one in which death before the development of ILD was modeled as a competing risk in our regression models (29). We also completed a series of analyses in which subjects were censored at the date of their last VARA registry visit to account for the possibility that a small proportion of study participants might have sought ongoing medical care permanently outside the VA system before the conclusion of our study. Finally, we performed a sensitivity analysis in which we restricted observations in our retrospective cohort study only to subjects who were diagnosed with RA within 5 years of VARA registry enrollment to account for a potential “depletion of susceptibles” bias given differences observed in RA disease duration depending on autoantibody status (30). Statistical significance was defined as P < 0.05. All analyses were performed using Stata/IC, version 15.1.
Results
Cross-Sectional Study Results
The cross-sectional study sample used for examining cases of prevalent RA-ILD comprised 2,328 participants (median age 64 yr, 89.3% male). Patients with combined RF/ACPA seropositivity were significantly more likely to be male and current or former cigarette smokers (Table 1). Significant differences were also noted in RA disease duration and baseline DAS28 and MDHAQ scores, as well as in the proportion receiving anti–tumor necrosis factor therapies. There were no racial or ethnic differences, nor were there differences in body mass index or the frequencies of prednisone or methotrexate use.
Table 1.
Baseline sociodemographic and clinical characteristics of cross-sectional study participants, stratified by combinations of RF and ACPA seropositivity
| Overall | RF−/ACPA− | RF+/ACPA−or RF−/ACPA+ | RF+/ACPA+ | |
|---|---|---|---|---|
|
N |
2,328 | 341 | 384 | 1,603 |
| Age, median (IQR) |
64 (58–71) | 65 (59–73) | 63 (56–70) | 64 (58–71) |
| Male, n (%) |
2,065 (89.3) | 298 (87.9) | 326 (85.6) | 1,441 (90.5) |
| Race, n (%) | ||||
| American Indian or Alaska Native | 18 (0.8) | 3 (0.9) | 4 (1.0) | 11 (0.7) |
| Asian | 7 (0.3) | 2 (0.6) | 1 (0.3) | 4 (0.2) |
| Black or African American | 345 (14.8) | 40 (11.7) | 67 (17.4) | 238 (14.9) |
| Multiracial | 18 (0.8) | 5 (1.5) | 1 (0.3) | 12 (0.7) |
| Native Hawaiian or Pacific Islander | 10 (0.4) | 1 (0.3) | 2 (0.5) | 7 (0.4) |
| White | 1,775 (76.3) | 274 (80.4) | 282 (73.4) | 1,219 (76.1) |
| Missing | 153 (6.6) | 16 (4.7) | 27 (7.0) | 110 (6.9) |
| Ethnicity, n (%) |
||||
| Hispanic or Latino | 100 (4.3) | 11 (3.2) | 20 (5.2) | 69 (4.3) |
| Not Hispanic or Latino | 2,140 (92.0) | 322 (94.4) | 346 (90.1) | 1,472 (91.9) |
| Missing | 86 (3.7) | 8 (2.3) | 18 (4.7) | 60 (3.7) |
| BMI, median (IQR) |
28.1 (24.8–32.0) | 28.5 (25.5–32.4) | 27.8 (24.9–32.0) | 28.1 (24.8–31.9) |
| Smoking history, n (%) |
||||
| Never | 478 (21.0) | 93 (27.7) | 104 (27.9) | 281 (17.9) |
| Former | 572 (25.1) | 61 (18.2) | 68 (18.2) | 443 (28.2) |
| Current | 1,231 (54.0) | 182 (54.2) | 201 (53.9) | 848 (53.9) |
| RA disease duration, years, median (IQR) |
8.0 (2.4–17.5) | 5.9 (1.9–12.9) | 7.0 (2.2–15.4) | 8.8 (2.8–19.0) |
| DAS28, median (IQR) |
3.6 (2.5–5.1) | 3.5 (2.5–5.0) | 3.3 (2.3–4.6) | 3.7 (2.5–5.2) |
| MDHAQ, median (IQR) |
0.9 (0.4–1.3) | 0.9 (0.4–1.3) | 0.7 (0.3–1.2) | 0.9 (0.4–1.3) |
| Prednisone use (%) |
1,133 (48.7) | 150 (44.0) | 192 (50.1) | 791 (49.3) |
| Methotrexate use (%) |
1,301 (55.9) | 175 (51.3) | 216 (56.4) | 910 (56.8) |
| Anti-TNF use (%) | 659 (28.3) | 75 (22.0) | 103 (26.9) | 481 (30.0) |
Definition of abbreviations: ACPA = anti–citrullinated protein antibody; BMI = body mass index; DAS28 = Disease Activity Score in 28 Joints; IQR = interquartile range; MDHAQ = Multidimensional Health Assessment Questionnaire; RA = rheumatoid arthritis; RF = rheumatoid factor; TNF = tumor necrosis factor.
At baseline, 100 subjects (4.3%; 95% confidence interval [CI], 3.5–5.2%) had prevalent ILD. After adjustment for prespecified baseline covariates, subjects with combined RF/ACPA seropositivity were significantly more likely to have prevalent ILD compared with combined seronegative subjects (odds ratio [OR], 2.90; 95% CI, 1.24–6.78; Table 2). In addition, patients with combined autoantibody seropositivity had a significantly increased odds of prevalent ILD compared with single autoantibody–seropositive subjects (OR, 2.03; 95% CI, 1.00–4.10). Single autoantibody seropositivity was not significantly associated with prevalent ILD relative to seronegative autoantibody status.
Table 2.
Multivariable logistic regression analyses of the cross-sectional study sample
| No. Exposed | No. with ILD (%) | Adjusted OR* | 95% CI | P Value | ||
|---|---|---|---|---|---|---|
| RF/ACPA seropositivity | RF−/ACPA− | 341 | 6 (1.8) | Ref. | — | — |
| RF+/ACPA− or RF−/ACPA+ | 384 | 10 (2.6) | 1.48 | 0.52–4.24 | 0.46 | |
| RF+/ACPA+ | 1,603 | 84 (5.2) | 2.90 | 1.24–6.78 | 0.01 | |
| | ||||||
| RF titer analyses | ||||||
| RF titer category | Negative | 535 | 8 (1.5) | Ref. | — | — |
| Low positive (15–45 IU/ml) | 363 | 17 (4.7) | 2.69 | 1.11–6.51 | 0.03 | |
| High positive (>45 IU/ml) | 1,430 | 75 (5.2) | 3.40 | 1.61–7.18 | 0.001 | |
| RF titer quartiles | 1 | 583 | 9 (1.5) | Ref. | — | — |
| 2 | 582 | 22 (3.8) | 2.16 | 0.96–4.83 | 0.06 | |
| 3 | 581 | 21 (3.6) | 2.27 | 1.02–5.05 | 0.04 | |
| 4 | 582 | 48 (8.3) | 5.44 | 2.60–11.41 | <0.001 | |
| Log-transformed RF titer | Per 1 log-transformed unit | — | — | 1.40 | 1.22–1.59 | <0.001 |
| | ||||||
| ACPA titer analyses | ||||||
| ACPA titer category | Negative | 531 | 14 (2.6) | Ref. | — | — |
| Low positive (5–15 U/ml) | 94 | 1 (1.1) | 0.41 | 0.05–3.16 | 0.39 | |
| High positive (>15 U/ml) | 1,703 | 85 (5.0) | 1.91 | 1.04–3.49 | 0.04 | |
| ACPA titer quartiles | 1 | 582 | 15 (2.6) | Ref. | — | — |
| 2 | 582 | 18 (3.1) | 1.32 | 0.64–2.70 | 0.45 | |
| 3 | 582 | 29 (5.0) | 1.95 | 1.01–3.78 | 0.05 | |
| 4 | 582 | 38 (6.5) | 2.48 | 1.31–4.68 | 0.005 | |
| Log-transformed ACPA titer | Per 1 log-transformed unit | — | — | 1.17 | 1.05–1.30 | 0.004 |
Definition of abbreviations: ACPA = anti–citrullinated protein antibody; CI = confidence interval; DAS28 = Disease Activity Score in 28 Joints; ILD = interstitial lung disease; No. = number; OR = odds ratio; RA = rheumatoid arthritis; Ref. = reference; RF = rheumatoid factor.
Multivariable models are adjusted for age at enrollment, sex, race (white, African American, or other), smoking history, RA disease duration, and baseline articular disease severity (DAS28).
The highest values of RF were most strongly associated with ILD, with corresponding ORs of 2.69 (95% CI, 1.11–6.51) for low-positive RF titers and 3.40 (95% CI, 1.61–7.18) for high-positive RF titers (p for trend 0.01; Table 2). Similarly, higher concentrations of RF were associated with ILD when modeled in quartiles and continuous values. The highest quartile of RF concentrations was associated with greater than fivefold higher odds of ILD, and the odds for ILD increased by 40% per one log-transformed RF titer unit. Patients with high-positive ACPA titers had a higher prevalence of ILD compared with ACPA-negative patients (OR, 1.91; 95% CI, 1.04–3.49; Table 2). Higher quartiles of ACPA concentrations were also more closely associated with the presence of ILD. The odds for ILD were 17% higher per one log-transformed ACPA titer unit. Results from our univariate logistic regression analyses are provided in Table E2 in the online supplement.
Retrospective Cohort Study Results
Comparable sociodemographic and clinical differences were noted in our retrospective cohort study sample (n = 2,228, median age 64 yr, 88.9% male; Table E1). In addition, no differences were observed in median follow-up time across various autoantibody status subgroups. During 14,281 patient-years of follow-up, 83 (3.7%; 95% CI, 3.0–4.6%) of the remaining 2,228 participants without prevalent ILD at enrollment were diagnosed with incident ILD during follow-up, corresponding to 5.8 incident ILD cases per 1,000 person-years. In contrast to our cross-sectional analyses, combined RF/ACPA seropositivity was not associated with increased risk for ILD (hazard ratio, 1.05; 95% CI, 0.54–2.02; Table 3). In addition, high concentrations of RF by clinically established cutoffs and by quartiles were not significantly associated with incident ILD (Table 3). However, a multivariable-adjusted model in which subjects with an RF titer in the top quartile (>295 IU/ml) were compared with all other subjects demonstrated a significantly increased hazard of incident ILD (hazard ratio, 1.66; 95% CI, 1.04–2.66). Unadjusted Kaplan-Meier survival estimates for this analysis are shown in Figure 1 (log-rank test P = 0.01). This observation was further supported by a 15% relative increased hazard of incident ILD per one log-transformed RF titer unit (Table 3). This effect was more pronounced with inclusion of a spline term, such that there was an 68% relative increased hazard of incident ILD for every one log-transformed RF titer unit beyond an RF titer greater than 90 IU/ml. Higher ACPA concentrations were not significantly associated with incident ILD risk (Table 3). Results from our univariate Cox regression analyses are provided in Table E3.
Table 3.
Multivariable Cox regression analyses of the retrospective cohort study sample
| No. Exposed | No. of ILD Cases | Total Person-Years | ILD Event Rate* | Adjusted HR† | 95% CI | P Value | ||
|---|---|---|---|---|---|---|---|---|
| RF/ACPA seropositivity | RF−/ACPA− | 335 | 12 | 2,107 | 5.7 | Ref. | — | — |
| RF+/ACPA− or RF−/ACPA+ | 374 | 13 | 2,313 | 5.6 | 1.12 | 0.50–2.52 | 0.78 | |
| RF+/ACPA+ | 1,519 | 58 | 9,861 | 5.9 | 1.05 | 0.54–2.02 | 0.90 | |
| RF titer analyses | ||||||||
| RF titer category | Negative | 527 | 18 | 3,243 | 5.6 | Ref. | — | — |
| Low positive (15–45 IU/ml) | 346 | 9 | 2,277 | 4.0 | 0.77 | 0.34–1.74 | 0.53 | |
| High positive (>45 IU/ml) | 1,355 | 56 | 8,761 | 6.4 | 1.08 | 0.62–1.89 | 0.79 | |
| RF titer quartiles | 1 | 557 | 19 | 3,395 | 5.6 | Ref. | — | — |
| 2 | 557 | 13 | 3,638 | 3.6 | 0.70 | 0.34–1.44 | 0.34 | |
| 3 | 557 | 20 | 3,670 | 5.4 | 1.01 | 0.53–1.92 | 0.99 | |
| 4 | 557 | 31 | 3,578 | 8.7 | 1.43 | 0.77–2.65 | 0.25 | |
| Log-transformed RF titer | Per 1 log-transformed unit | — | — | — | — | 1.15 | 1.00–1.32 | 0.05 |
| Log-transformed RF titer with spline term | Per 1 log-transformed unit | — | — | — | — | 0.85 | 0.62–1.17 | 0.31 |
| Spline term at 4.5 | — | — | — | — | 1.68 | 1.02–2.77 | 0.04 | |
| ACPA titer analyses | ||||||||
| ACPA titer category‡ | Negative | 517 | 19 | 3,284 | 5.8 | Ref. | — | — |
| Low positive (5–15 U/ml) | 93 | 0 | 497 | 0.0 | — | — | — | |
| High positive (>15 U/ml) | 1,618 | 64 | 10,500 | 6.1 | 1.05 | 0.62–1.79 | 0.85 | |
| ACPA titer quartiles | 1 | 557 | 19 | 3,475 | 5.5 | Ref. | — | — |
| 2 | 557 | 20 | 3,458 | 5.8 | 1.04 | 0.54–2.02 | 0.90 | |
| 3 | 557 | 15 | 3,704 | 4.0 | 0.78 | 0.39–1.56 | 0.48 | |
| 4 | 557 | 29 | 3,644 | 8.0 | 1.45 | 0.80–2.65 | 0.22 | |
| Log-transformed ACPA titer | Per 1 log-transformed unit | — | — | — | — | 1.04 | 0.94–1.15 | 0.50 |
Definition of abbreviations: ACPA = anti–citrullinated protein antibody; CI = confidence interval; DAS28 = Disease Activity Score in 28 Joints; HR = hazard ratio; ILD = interstitial lung disease; No. = number; RA = rheumatoid arthritis; RF = rheumatoid factor; Ref. = reference.
Event rate is per 1,000 person-years.
Multivariable models are adjusted for age at enrollment, sex, race (white, African American, or other), smoking history, RA disease duration, and baseline articular disease severity (DAS28).
No subjects with incident ILD had a low-positive ACPA titer, whereas 19 had a negative titer and 64 had a high-positive titer.
Figure 1.
The figure depicts graphical representations of unadjusted Kaplan-Meier survival functions stratified by RF titers in the highest quartile (>295 IU/ml) and lower three quartiles. RA-ILD = rheumatoid arthritis–associated interstitial lung disease; RF = rheumatoid factor.
Across all variables of interest, there were no significant differences in point estimates of sub–hazard ratios when death before the development of ILD was modeled as a competing risk (Table E4), nor were there differences noted when subjects were censored at the date of their last VARA registry visit. Finally, significant associations of incident ILD with the RF titer top quartile and with the log-transformed RF titer spline term persisted when we restricted our observations only to subjects who were diagnosed with RA within 5 years of VARA registry enrollment, whereas other independent variables of interest remained unassociated with incident ILD.
Discussion
In our study, patients with RA with combined RF/ACPA seropositivity were more likely to have prevalent ILD at the time of serologic testing compared with patients with only one or no detectable autoantibodies. Similarly, higher concentrations of RF and ACPA were independently associated with the presence of ILD. However, dual autoantibody seropositivity was not associated with the subsequent development of incident ILD. Although RF concentrations greater than 90 IU/ml were associated with a higher risk of developing ILD, elevated ACPA titers did not confer increased risk for incident ILD.
Patients with RA are roughly nine times more likely to develop ILD in comparison with the general population (9, 31). Previous studies have estimated that 3–10% of patients with RA have clinically significant ILD (4–7). In addition, mild interstitial abnormalities on chest CT scans have been described in more than 30% of patients with RA (32), although it is unclear what proportion of these patients subsequently progress to more advanced disease. The development of ILD has important implications for both morbidity and mortality, with patients with RA-ILD thought to have a threefold increased risk of mortality compared with patients with RA without ILD (8). A recent study reported dramatic differences in 1- and 5-year mortality rates between subjects with RA-ILD and those with RA without ILD (14% vs. 4% and 39% vs. 18%, respectively) (33). Thus, ongoing efforts to identify patients with RA who are most at risk for ILD remain crucial.
Prior cross-sectional and case–control studies have demonstrated an association between ILD and positive serologies for either RF or ACPA (11–17). However, this association has not been observed among cohort studies. For example, a UK cohort study failed to demonstrate a higher risk of ILD in patients with RA with RF seropositivity (34). A U.S. population–based cohort study found trends toward an association between RF seropositivity and ILD that did not achieve statistical significance (8). Neither study evaluated the association between ACPA seropositivity and ILD risk. In addition, there are no prior studies to our knowledge that have investigated the impact of combined RF/ACPA seropositivity or the dose-dependent effects of individual RF and ACPA concentrations on ILD risk. Thus, novel findings from our study included a higher prevalence of ILD in patients with RA with combined RF/ACPA seropositivity, the identification of dose-dependent relationships between RF and ACPA with prevalent ILD, and a higher risk of incident ILD only among those with the highest values of RF.
Results from our cross-sectional and cohort studies, examining risks of prevalent and incident disease, respectively, differed. This difference was most striking for dual autoantibody seropositivity and for high-titer ACPA concentrations, both of which were associated with prevalent disease but not with incident disease. Reverse causality is one possible explanation, in which ACPAs are produced locally (i.e., within the lungs) in the setting of active pulmonary parenchymal inflammation and fibrosis and thus are only detectable in high serum concentrations in the context of established ILD. This is consistent with recent reports demonstrating the presence of inducible bronchial-associated lymphoid tissue in patients with RA-ILD, a finding that correlated with higher ACPA concentrations in both serum and bronchoalveolar lavage fluid (18). Similar observations were noted in a study comparing patients with ACPA-positive and ACPA-negative RA with and without parenchymal lung abnormalities (19). Alternatively, there may be biologic differences between patients with prevalent ILD and those without ILD at baseline who develop pulmonary fibrosis over time. One final potential explanation for the discrepancies observed between our cross-sectional and retrospective cohort analyses is a “depletion of susceptibles” phenomenon given that combined RF/ACPA–seropositive subjects had a longer median duration of RA before VARA registry enrollment (8.7 yr vs. 7.1 yr for single autoantibody–positive subjects and 5.9 yr for combined seronegative subjects). However, we attempted to account for this by generating additional Cox models that restricted observations only to subjects diagnosed with RA within 5 years of VARA registry enrollment. In these models, RF titers >90 IU/ml remained significantly associated with incident ILD whereas other independent variables remained unassociated with incident ILD.
Beyond the established RA autoantibodies we studied, other novel biomarkers for RA-ILD risk such as matrix metalloproteinase 7 and surfactant protein D (11), antibodies to malondialdehyde-acetaldehyde adducts (24), and proinflammatory/profibrotic cytokines (35) are being investigated but are not yet clinically available. RF and ACPA titers are almost universally obtained in the evaluation of patients with suspected RA. Although our results demonstrated that individuals with combined RF/ACPA seropositivity were significantly more likely to have prevalent RA-ILD, 16% of prevalent ILD cases were among those who were single antibody positive or dual antibody negative and 95% of patients with dual antibody–positive RA did not have ILD. In our cohort analyses, only those patients with the highest RF concentrations appeared to be at higher risk for incident ILD. Thus, RA autoantibodies alone do not appear to be sufficient for ILD risk stratification. Whether consideration of these RA autoantibodies in combination with screening for clinical symptoms of ILD (e.g., exertional dyspnea and cough) and an assessment of other established risk factors will aid in the earlier identification of ILD warrants further investigation.
Limitations
Our approach provided us with a larger sample size in comparison with existing studies and allowed for standardization of autoantibody assessments. However, our study has several limitations. The male predominance, as well as military veteran status and lower prevalence of biologic therapies, within our cohort may affect generalizability. Thus, external validation of our findings is an important future direction. Conversely, our study population uniquely leveraged our ability to investigate associations between RA autoantibodies and ILD for a number of reasons. Because RA-ILD is more common in men than in women (4, 8) and given that almost 70% of patients with RA-ILD have smoked (14), our study population is significantly enriched for ILD risk factors. In addition, men with RA are historically underrepresented in epidemiologic studies, although they make up up to one-third of all affected cases (and half of RA-ILD cases) (36). Furthermore, men with RA tend to have more aggressive disease than women, characterized by more severe articular damage, a higher prevalence of extraarticular manifestations, and greater disease-related mortality (37, 38). Finally, the VA represents the largest integrated healthcare system in the United States and thus provides an opportunity to study a uniquely vulnerable RA population where socioeconomic barriers to healthcare access are limited.
There are other important limitations of our study. ILD data were collected retrospectively, and not all data were available within medical records. Thus, we may have underestimated the prevalence and incidence of ILD within our study population. We expect any misclassification of ILD to bias our results toward the null. We also could not definitively distinguish between clinical and subclinical ILD in all cases based on the retrospective classification performed. However, we believe the majority of ILD cases were clinically significant given that ILD status required a physician diagnosis in the medical records in addition to supportive findings on diagnostic testing. Given the low frequency with which the specific ILD phenotypic pattern (usual interstitial pneumonia vs. nonspecific interstitial pneumonia vs. other) was reported, we were not able to compare RF/ACPA seropositivity and individual concentrations by RA-ILD pattern. Furthermore, we were unable to determine the predictive value of RA autoantibodies for ILD earlier in the RA disease course as this was not an RA inception cohort, although we performed secondary analyses in which only patients with more recently diagnosed RA (diagnosed within 5 yr) were included. Finally, total smoking pack-years is not captured within the VARA registry, which limited our ability to adjust for smoking history as a continuous variable.
Conclusions
We found RF and ACPA to be associated with prevalent RA-ILD, whereas only RF concentrations >90 IU/ml were associated with incident RA-ILD. Our investigation of combined autoantibody status and individual autoantibody concentrations advances our understanding of the role of autoantibodies in RA-ILD pathogenesis and demonstrates the need for the development and validation of RA-ILD risk models to enhance its identification.
Acknowledgments
Acknowledgment
The authors thank all of the patients for their military service and for their contributions to this work. They also thank the Veterans Affairs Rheumatoid Arthritis registry site coordinators, as well as the University of Nebraska Medical Center laboratory personnel.
Footnotes
Supported by the National Heart, Lung, and Blood Institute, grant numbers T32HL007891 (J.G.N.) and K24HL103844 (S.M.K.), the VA Clinical Science Research and Development Merit Award, grant number CX001703 (J.F.B.), a Rheumatology Research Foundation Scientist Development Award (B.R.E.), and the National Institute of General Medical Sciences, grant number U54GM115458 (T.R.M. and B.R.E.).
Author Contributions: J.G.N., J.F.B., S.M.K., and B.R.E. contributed to the conception and design, the acquisition of data, and the analysis and interpretation of the data. C.R.J. contributed to the analysis and interpretation of the data. N.S., T.D.M., P.R., G.M.T., B.C.S., and T.R.M. contributed to the acquisition of the data. All authors revised the manuscript for important intellectual content and have provided final approval of the version to be published.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365:2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 2.Turesson C, O’Fallon WM, Crowson CS, Gabriel SE, Matteson EL. Extra-articular disease manifestations in rheumatoid arthritis: incidence trends and risk factors over 46 years. Ann Rheum Dis. 2003;62:722–727. doi: 10.1136/ard.62.8.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tanoue LT. Pulmonary manifestations of rheumatoid arthritis. Clin Chest Med. 1998;19:667–685, viii. doi: 10.1016/s0272-5231(05)70109-x. [DOI] [PubMed] [Google Scholar]
- 4.Olson AL, Swigris JJ, Sprunger DB, Fischer A, Fernandez-Perez ER, Solomon J, et al. Rheumatoid arthritis-interstitial lung disease-associated mortality. Am J Respir Crit Care Med. 2011;183:372–378. doi: 10.1164/rccm.201004-0622OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Duarte AC, Porter JC, Leandro MJ. The lung in a cohort of rheumatoid arthritis patients-an overview of different types of involvement and treatment. Rheumatology (Oxford) 2019;58:2031–2038. doi: 10.1093/rheumatology/kez177. [DOI] [PubMed] [Google Scholar]
- 6.Raimundo K, Solomon JJ, Olson AL, Kong AM, Cole AL, Fischer A, et al. Rheumatoid arthritis-interstitial lung disease in the United States: prevalence, incidence, and healthcare costs and mortality. J Rheumatol. 2019;46:360–369. doi: 10.3899/jrheum.171315. [DOI] [PubMed] [Google Scholar]
- 7.Bartels CM, Bell CL, Shinki K, Rosenthal A, Bridges AJ. Changing trends in serious extra-articular manifestations of rheumatoid arthritis among United State veterans over 20 years. Rheumatology (Oxford) 2010;49:1670–1675. doi: 10.1093/rheumatology/keq135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bongartz T, Nannini C, Medina-Velasquez YF, Achenbach SJ, Crowson CS, Ryu JH, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62:1583–1591. doi: 10.1002/art.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esposito AJ, Chu SG, Madan R, Doyle TJ, Dellaripa PF. Thoracic manifestations of rheumatoid arthritis. Clin Chest Med. 2019;40:545–560. doi: 10.1016/j.ccm.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sparks JA, He X, Huang J, Fletcher EA, Zaccardelli A, Friedlander HM, et al. Rheumatoid arthritis disease activity predicting incident clinically apparent rheumatoid arthritis-associated interstitial lung disease: a prospective cohort study. Arthritis Rheumatol. 2019;71:1472–1482. doi: 10.1002/art.40904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doyle TJ, Patel AS, Hatabu H, Nishino M, Wu G, Osorio JC, et al. Detection of rheumatoid arthritis-interstitial lung disease is enhanced by serum biomarkers. Am J Respir Crit Care Med. 2015;191:1403–1412. doi: 10.1164/rccm.201411-1950OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mori S, Koga Y, Sugimoto M. Different risk factors between interstitial lung disease and airway disease in rheumatoid arthritis. Respir Med. 2012;106:1591–1599. doi: 10.1016/j.rmed.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 13.Giles JT, Danoff SK, Sokolove J, Wagner CA, Winchester R, Pappas DA, et al. Association of fine specificity and repertoire expansion of anticitrullinated peptide antibodies with rheumatoid arthritis associated interstitial lung disease. Ann Rheum Dis. 2014;73:1487–1494. doi: 10.1136/annrheumdis-2012-203160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kelly CA, Saravanan V, Nisar M, Arthanari S, Woodhead FA, Price-Forbes AN, et al. British Rheumatoid Interstitial Lung (BRILL) Network. Rheumatoid arthritis-related interstitial lung disease: associations, prognostic factors and physiological and radiological characteristics: a large multicentre UK study. Rheumatology (Oxford) 2014;53:1676–1682. doi: 10.1093/rheumatology/keu165. [DOI] [PubMed] [Google Scholar]
- 15.Miriovsky BJ, Michaud K, Thiele GM, O’Dell JR, Cannon GW, Kerr G, et al. Anti-CCP antibody and rheumatoid factor concentrations predict greater disease activity in men with rheumatoid arthritis. Ann Rheum Dis. 2010;69:1292–1297. doi: 10.1136/ard.2009.122739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bernstein EJ, Barr RG, Austin JHM, Kawut SM, Raghu G, Sell JL, et al. Rheumatoid arthritis-associated autoantibodies and subclinical interstitial lung disease: the Multi-Ethnic Study of Atherosclerosis. Thorax. 2016;71:1082–1090. doi: 10.1136/thoraxjnl-2016-208932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Restrepo JF, del Rincón I, Battafarano DF, Haas RW, Doria M, Escalante A. Clinical and laboratory factors associated with interstitial lung disease in rheumatoid arthritis. Clin Rheumatol. 2015;34:1529–1536. doi: 10.1007/s10067-015-3025-8. [DOI] [PubMed] [Google Scholar]
- 18.Rangel-Moreno J, Hartson L, Navarro C, Gaxiola M, Selman M, Randall TD. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest. 2006;116:3183–3194. doi: 10.1172/JCI28756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Reynisdottir G, Karimi R, Joshua V, Olsen H, Hensvold AH, Harju A, et al. Structural changes and antibody enrichment in the lungs are early features of anti-citrullinated protein antibody-positive rheumatoid arthritis. Arthritis Rheumatol. 2014;66:31–39. doi: 10.1002/art.38201. [DOI] [PubMed] [Google Scholar]
- 20.Mikuls TR, Reimold A, Kerr GS, Cannon GW. Insights and implications of the VA rheumatoid arthritis registry. Fed Pract. 2015;32:24–29. [PMC free article] [PubMed] [Google Scholar]
- 21.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–324. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 22.Pincus T, Swearingen C, Wolfe F. Toward a multidimensional Health Assessment Questionnaire (MDHAQ): assessment of advanced activities of daily living and psychological status in the patient-friendly health assessment questionnaire format. Arthritis Rheum. 1999;42:2220–2230. doi: 10.1002/1529-0131(199910)42:10<2220::AID-ANR26>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 23.van der Heijde DM, van ’t Hof M, van Riel PL, van de Putte LB. Development of a disease activity score based on judgment in clinical practice by rheumatologists. J Rheumatol. 1993;20:579–581. [PubMed] [Google Scholar]
- 24.England BR, Duryee MJ, Roul P, Mahajan TD, Singh N, Poole JA, et al. Malondialdehyde-acetaldehyde adducts and antibody responses in rheumatoid arthritis-associated interstitial lung disease. Arthritis Rheumatol. 2019;71:1483–1493. doi: 10.1002/art.40900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.England BR, Roul P, Mahajan TD, Singh N, Yu F, Sayles H, et al. Performance of administrative algorithms to identify interstitial lung disease in rheumatoid arthritis. Arthritis Care Res (Hoboken) 2020;72:1392–1403. doi: 10.1002/acr.24043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, III, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 27.George B, Seals S, Aban I. Survival analysis and regression models. J Nucl Cardiol. 2014;21:686–694. doi: 10.1007/s12350-014-9908-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jones M, Fowler R. Immortal time bias in observational studies of time-to-event outcomes. J Crit Care. 2016;36:195–199. doi: 10.1016/j.jcrc.2016.07.017. [DOI] [PubMed] [Google Scholar]
- 29.Chiang CL. Competing risks in mortality analysis. Annu Rev Public Health. 1991;12:281–307. doi: 10.1146/annurev.pu.12.050191.001433. [DOI] [PubMed] [Google Scholar]
- 30.Stovitz SD, Banack HR, Kaufman JS. ‘Depletion of the susceptibles’ taught through a story, a table and basic arithmetic. BMJ Evid Based Med. 2018;23:199. doi: 10.1136/bmjebm-2018-110972. [DOI] [PubMed] [Google Scholar]
- 31.Saag KG, Teng GG, Patkar NM, Anuntiyo J, Finney C, Curtis JR, et al. American College of Rheumatology. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008;59:762–784. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 32.Gabbay E, Tarala R, Will R, Carroll G, Adler B, Cameron D, et al. Interstitial lung disease in recent onset rheumatoid arthritis. Am J Respir Crit Care Med. 1997;156:528–535. doi: 10.1164/ajrccm.156.2.9609016. [DOI] [PubMed] [Google Scholar]
- 33.Hyldgaard C, Hilberg O, Pedersen AB, Ulrichsen SP, Løkke A, Bendstrup E, et al. A population-based cohort study of rheumatoid arthritis-associated interstitial lung disease: comorbidity and mortality. Ann Rheum Dis. 2017;76:1700–1706. doi: 10.1136/annrheumdis-2017-211138. [DOI] [PubMed] [Google Scholar]
- 34.Koduri G, Norton S, Young A, Cox N, Davies P, Devlin J, et al. ERAS (Early Rheumatoid Arthritis Study) Interstitial lung disease has a poor prognosis in rheumatoid arthritis: results from an inception cohort. Rheumatology (Oxford) 2010;49:1483–1489. doi: 10.1093/rheumatology/keq035. [DOI] [PubMed] [Google Scholar]
- 35.Kass DJ, Nouraie M, Glassberg MK, Ramreddy N, Fernandez K, Harlow L, et al. Comparative profiling of serum protein biomarkers in rheumatoid arthritis-associated interstitial lung disease and idiopathic pulmonary fibrosis. Arthritis Rheumatol. 2020;72:409–419. doi: 10.1002/art.41123. [DOI] [PubMed] [Google Scholar]
- 36.Crowson CS, Matteson EL, Myasoedova E, Michet CJ, Ernste FC, Warrington KJ, et al. The lifetime risk of adult-onset rheumatoid arthritis and other inflammatory autoimmune rheumatic diseases. Arthritis Rheum. 2011;63:633–639. doi: 10.1002/art.30155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Weyand CM, Schmidt D, Wagner U, Goronzy JJ. The influence of sex on the phenotype of rheumatoid arthritis. Arthritis Rheum. 1998;41:817–822. doi: 10.1002/1529-0131(199805)41:5<817::AID-ART7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 38.Jawaheer D, Lum RF, Gregersen PK, Criswell LA. Influence of male sex on disease phenotype in familial rheumatoid arthritis. Arthritis Rheum. 2006;54:3087–3094. doi: 10.1002/art.22120. [DOI] [PubMed] [Google Scholar]