Abstract
Dyspnea in low-preload states is an underrecognized but growing diagnosis in patients with unexplained dyspnea. Patients can often experience debilitating symptoms at rest and with exertion, as low measured preload often leads to decreased cardiac output and ultimately dyspnea. In the present article, we performed a review of the literature and a multidisciplinary evaluation to understand the pathophysiology, diagnosis, and treatment of dyspnea in low-preload states. We explored selected etiologies and suggested an algorithm to approach unexplained dyspnea. The mainstay of diagnosis remains as invasive cardiopulmonary exercise testing. We concluded with a variety of nonpharmacological and pharmacological therapies, highlighting that a multifactorial approach may lead to the best results.
Keywords: dyspnea, preload failure, preload insufficiency, autonomic dysfunction, postural orthostatic tachycardia syndrome
Dyspnea is a common complaint among patients, affecting up to 25% of individuals in the outpatient setting (1, 2). Major causes of dyspnea stem from cardiac, pulmonary, vascular, neurological, and/or metabolic abnormalities. However, around 10–20% of patients with dyspnea who undergo a comprehensive diagnostic evaluation have no identifiable etiology (3). In general, patients with unexplained dyspnea undergo an extensive workup that commonly involves repeating several diagnostic tests (4). Hence, these patients incur risks and bear great financial burden to themselves and to the healthcare system overall (5).
Recently, Oldham and colleagues have implicated low preload as a cause of dyspnea in several patients, including those with postural orthostatic tachycardia syndrome (POTS) and other autonomic diseases (6). Preliminary data by our group described that inadequate cardiac preload may help explain the cause of dyspnea in a significant proportion of patients, many of whom receive a diagnosis after numerous diagnostic tests (7). Low preload can occur in association with a variety of clinical conditions that interfere with an adequate cardiovascular venous return, and it may play a larger role in patients with autonomic disease. The decrease in preload when sitting or standing or the lack of augmentation during exercise can lead to insufficient cardiac output () and decreased oxygen delivery, resulting in fatigue and dyspnea. Although symptoms, the orthostatic response, and results of some tests suggest the condition, low preload as a cause of dyspnea is diagnosed by using right heart catheterization during upright exercise (6).
Chronic low preload as a cause for dyspnea is frequently underrecognized. In view of advances in the diagnosis, management, and treatment, we sought to perform a review of the literature to summarize current knowledge and unmet needs. The focus of this paper is chronic or persistent low-preload states, as opposed to acute low-preload states (i.e., hemorrhagic shock or acute dehydration). Given the multidisciplinary nature of the disorder, we gathered knowledge from several specialties, including pulmonary, cardiovascular, and neurological specialties, to offer a comprehensive description and strategy for multispecialty management of this elusive condition.
Causes of Dyspnea
The definition of dyspnea is not fully established. However, in a recent article, dyspnea is described as an “unpleasant subjective experience of discomfort with breathing that generally worsens as underlying disease processes increase in severity; in its chronic and severe state, it can lead to significant disability, progressive inactivity, social isolation, and substantive suffering.” (2, 8–10) The symptom of dyspnea is common, as it affects nearly half of admitted patients and a quarter of patients seen in the ambulatory setting (2).
In a retrospective study conducted by Huang and colleagues, nearly 80% of patients with unexplained dyspnea had repeated testing, and 54% had already tried specific therapies without response (inhalers, diuretics, and antidepressive treatment). Frequent diagnoses in these patients include oxidative myopathy (25%), dysautonomia/preload failure (21%), heart failure with preserved ejection fraction (18%), primary hyperventilation (8%), and others (11%) (5). In our experience, 50% of patients with unexplained dyspnea had a chronic low-preload state as the most likely explanation for their symptom, understanding that this is a selected group of patients who underwent a large number of investigations to rule out other causes of dyspnea, including cardiopulmonary exercise testing (CPET) (7).
Preload Physiology
Preload is defined as the wall stress or force to stretch fibers at the end of diastole, also known as the maximal resting length of the cardiac sarcomere (11). Many definitions describe preload in terms of left ventricular end-diastolic volume or pressure; however, the term “end-diastolic wall stress” may be more appropriate, as it encompasses the numerous factors that determine preload: ventricular dimensions, wall thickness, transmural diastolic pressures, chamber compliance, venous return, intrathoracic and intrapericardial pressures, and ventricular interdependence (12).
Blood volume has a key role in determining preload. Gravity shifts 700–900 ml of blood volume to the lower parts of the body (13), primarily through venous pooling in the lower extremities (14) and splanchnic circulation (15). Indeed, the venous compartment serves as a volume reservoir where hydrostatic pressure interplays with vascular compliance to drive blood back to the right atrium (13). In healthy persons, preload is maintained in the upright position by a reduction in venous compliance with increasing hydrostatic pressure, an increase in interstitial fluid with further reduction in vein compliance, functional venous valves, muscle contraction, and the suctioning action of the respiratory pump (13).
Preload largely remains the main determinant for systolic performance. is determined by stroke volume (SV) and heart rate (HR). The determinants of SV are preload, afterload, and contractility. Based on the effects described by Frank-Starling, the ventricular output increases in relation to the stretch of myocardial fibers before contraction or preload (16), augmenting the heart to respond to hemodynamic changes (17). Many responses facilitate the increased preload to meet the demands, including the sympathetic nervous system (17), which is activated by the anticipation of exercise; vasodilation of the exercising muscles and a transient drop in arterial blood pressure (BP) that is sensed by baroreceptors in the common carotid and aortic arch; metabolites (i.e., lactate, IL-6, cathepsin B, and irisin) released by exercising muscles that directly signal the brainstem (18); and changes in the blood composition of oxygen and lactic acid, which activate chemoreceptors. These signals amount to a norepinephrine release, together with sympathetic nerve activation that increase actin–myosin interactions, preload, , HR, and contractility (19). Sympathetic nerve activity helps with vasodilation in the exercising muscles coupled with constriction in the nonworking muscles (20).
Measuring Preload
In vivo measurement of preload is challenging, as current technology limits measurement of sarcomeric stretch at end-diastole. Notably, most of our clinical estimations of preload are conducted while the patient is in the supine position, although the most physiologically pertinent measurements are performed while the patient is sitting or standing. Because sarcomeric length cannot be determined in vivo, preload is estimated either invasively by measuring the right atrial (RA) and pulmonary arterial wedge pressure (PAWP) during right heart catheterization or noninvasively by determining the ventricular end-diastolic volume.
Data from studies that tested fluid resuscitation revealed that central venous pressure/RA pressure may not always provide an accurate measure of preload (21). Errors can be technical, including zeroing and leveling of the pressure transducer or errors due to artificial increases in cases of high intrathoracic pressure (22). Other techniques include estimating ventricular end-diastolic volume via echocardiography (23) or cardiac magnetic resonance imaging (24) and the ultrasonographic determination of the inferior vena cava diameter and its respiratory variation (25). Further investigation of these tools is needed to comment on chronic low-preload states in which symptoms manifest in the upright position and during activities.
Low Preload and Dyspnea
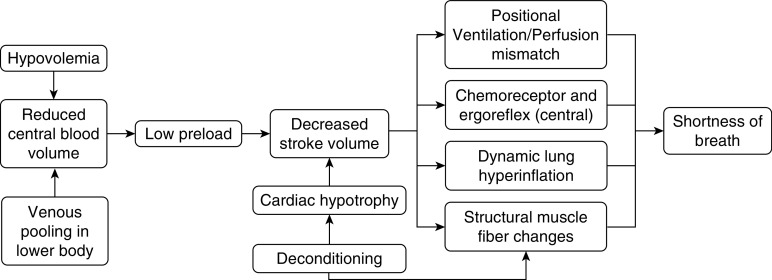
An overall persistent low blood volume or failure to return blood to the heart contributes to the low preload and dyspnea. The mechanism of dyspnea in these patients remains obscure, but a few hypotheses have been suggested (Figure 1). Ventilation–perfusion (/) mismatch is a driver of dyspnea in patients with platypnea orthodeoxia (26, 27). A lower venous return, and hence a lower SV, may generate lower apical pulmonary pressures that increase dead-space ventilation, potentially exacerbated by inadequate reflex vasoconstriction in these regions (28). Other investigators have highlighted a central cause of breathlessness, describing a mismatch between respiratory neural drives and afferent feedback in the respiratory system (29). For instance, in patients with heart failure, chemoreceptor activity and the ergoreflex (muscle receptors that sense exercise and work) are enhanced, yet the baroreflex and autonomic control may be depressed, limiting the chemoreceptor activity (chemoreflex) and enhancing the sensation of dyspnea (29, 30).
Figure 1.
Mechanisms of dyspnea in preload failure.
Other potential explanations for dyspnea related to low preload include air-trapping (marked by increases in residual volume due to impaired exhalation) constraining tidal volume and limiting minute ventilation (e), thus leading to dyspnea (31). Several studies have highlighted the structural effects of heart failure on the respiratory muscle fibers that have led to weakness and fatigue in these patients (32). These respiratory muscles may also be competing for blood flow with locomotor muscles, resulting in ischemia and dysfunction (31). A study by Oldham and colleagues suggests a decrease in peripheral oxygen extraction with peripheral limitation (skeletal-muscle dysfunction) in exercise performance (6, 33). Importantly, the difference in arterial minus mixed venous oxygen content (oxygen extraction) increases during exercise and is reduced in metabolic myopathies, in which the peripheral oxygen is affected. The Fick principle is based on the concept that the total uptake of a substance is equal to the product of the arteriovenous gradient and blood flow (34). In patients with a low-preload state, there is a reduction in SV, oxygen extraction, and oxygen consumption (o2) (6). These processes may further be exacerbated by deconditioning and structural changes such as cardiac hypotrophy, as seen in some patients with POTS (35).
Etiologies
Low preload is not an isolated entity but is rather a hemodynamic condition associated with a variety of diseases. Dyspnea in chronic low-preload states can be exacerbated by acute hypovolemia from reduced fluid intake, excessive perspiration, diarrhea, hemorrhage, and impairment of regulatory mechanisms (i.e., thirst and diuresis). In certain cases, chronic hypovolemia, as seen in chronic diarrhea, high-output ostomies, and/or chronic diuretic use, may lead to persistent dyspnea. A case report linked low-volume states with adrenocorticotropic hormone deficiency (36), and other endocrinopathies (i.e., carcinoid, pheochromocytoma, hypothyroidism, etc.) should also be considered. Medications can also exacerbate low-volume states, as seen in patients who receive diuretics, β-blockers, calcium channel blockers, serotonin–norepinephrine reuptake inhibitors, stimulants, etc. (37).
A few cases demonstrated low preload in the context of structural defects or obstructions of the venous system (14). Case reports described the improvement in exertional dyspnea after a recanalization of a thrombosed inferior vena cava filter (38) or a chronically obstructed azygos vein (39). Exercise intolerance was described in a series of patients with a congenital absence of venous valves (40) and in a patient with failed Fontan circulation and chronic preload limitation (41).
In many cases, low preload may result from a myriad of diseases that reduce venous tone directly. In these cases, increased venous capacitance and excessive blood pooling lead to reduced blood return and dyspnea. Studies have highlighted venous pooling in the upright posture of patients with POTS (42, 43) and orthostatic hypotension (44). The pathophysiology of POTS has been attributed to peripheral autonomic denervation, neuropathy of distal ganglionic fibers, inadequate postural changes in renin–aldosterone activity, hyperadrenergic states due to norepinephrine transport deficiency, deconditioning with reduced left ventricular mass, etc. (44–46). Small-fiber neuropathy (SFN) is a neurological condition characterized by a reduced nerve count leading to sensory disturbances and possible autonomic dysfunction (47). Table 1 shows the above etiologies by category.
Table 1.
Etiologies of low preload
| Decreased Intravascular Volume | Venous Obstruction/Restriction | Reduction of Venous Tone |
|---|---|---|
|
|
|
Description of Selected Etiologies
POTS
POTS is a heterogeneous syndrome that may be related to low preload (48). In this condition, patients suffer chronic symptoms of orthostatic intolerance for more than 3 months (48). The estimated prevalence of POTS is approximately 170 per 100,000 subjects, and it is more common in women (5:1) of childbearing age (49, 50). POTS is a clinical syndrome characterized by symptoms that occur with standing, an increase of HR ≥30 beats per minute when moving from a recumbent to a standing position (or ≥40 beats per min in individuals 12–19 yr of age) and absence of orthostatic hypotension (drop of systolic BP >20 mm Hg or diastolic BP >10 mm Hg) within 3 minutes of standing (37, 51). HR and BP must be verified in two separate measurements at least 1 minute apart. Up to 65–80% of patients may complain of dyspnea, and some describe dysfunctional breathing with hyperventilation (52).
Orthostatic Hypotension
Orthostatic hypotension is characterized by a fall in systolic BP ≥20 mm Hg or a fall in diastolic BP ≥10 mm Hg within 3 minutes of standing or during a head-up tilt-table test (53). Some patients may have progressive hypotension that may only be evident with a prolonged tilt-table test (54). Orthostatic hypotension becomes more common with increasing age, with prevalence around 18% in individuals ≥65 years (55). The autonomic nervous system plays a key role in these patients (56). Gibbons and colleagues suggest that 30% of patients with orthostatic hypotension may also present with fatigue and dyspnea with coincident BP fall, termed “orthostatic dyspnea” (26). In fact, during autonomic testing, these patients reported resolved symptoms when returning to the supine position (26).
SFN
SFN is a common sensory peripheral nerve disorder, marked by degeneration of small myelinated and unmyelinated (C) fibers (47). The myelinated and C fibers are involved in pain and temperature sensation, but C fibers also participate in autonomic function. Skin biopsy specimens of these individuals show significant loss of these epidermal fibers (57). Patients with SFN can present with any degree of pain and sensory loss, and half of patients may also have autonomic dysfunction due to decreased sympathetic tone (47, 58). The prevalence of SFN is unknown, given its underrecognition, with a minimum prevalence of 53 per 100,000 subjects (59).
Clinical Presentation
From a respiratory standpoint, patients with inadequate preload hemodynamics demonstrate dyspnea with exertion that may progress with time, leading to limited functionality. In our experience, given the reduced preload, patients frequently have lightheadedness, presyncope, or syncope during or immediately after activities, rapid changes in positions, Valsalva maneuvers, etc. These symptoms are often seen in patients with autonomic conditions such as POTS (37, 58). The symptom burden for patients with chronic low preload can be incredibly debilitating, directly impacting health-related quality of life.
Diagnosis
The diagnosis of low preload as a cause of dyspnea is challenging, and patients are often underrecognized in the clinical setting. Evaluation of these patients should include a complete clinical history, thorough review of systems including autonomic involvement (sudomotor, secretomotor, cardiovascular, gastrointestinal, and genitourinary abnormalities), and physical examination with assessment of orthostatic vital signs. Other initial tests include blood work (i.e., complete blood count and electrolyte, renal-function, ferritin, thyroid-stimulating hormone, morning cortisol, vitamin B12, and fasting-lipid panels) and additional special studies, when clinically indicated (antiphospholipid antibody, C3 and C4, Sjogren syndrome antigen A/Sjogren syndrome antigen B, antinuclear antibody, and tissue transglutaminase/antigliadin antibody studies; a paraneoplastic antibody panel; copper, serum, and urine metanephrine studies; and serum and urinary monoclonal protein analysis).
It is important to rule out structural heart disease and arrhythmias (through electrocardiograms, echocardiograms, use of a Holter monitor, and stress tests), neurological conditions (through electromyograms and electroencephalograms) (60), and autonomic diseases (through tilt-table tests [61], quantitative sudomotor axon reflex tests, catecholamine determination [62], testing of HR variability, testing of the response to the Valsava maneuver, and thermoregulatory sweat tests). A tilt-table test can be useful to detect delayed changes in BP and HR while in the upright position that are not elucidated by orthostatic vital signs (54). If concern for POTS exists, 24-hour urine sodium, blood-volume measurement, and specific antibodies (i.e., ganglionic A3 acetylcholine, β1 and β2 adrenergic, M2/3 muscarinic, and N-type acetylcholine antibodies) need to be considered (37, 49, 63). Skin biopsy is the gold standard for the diagnosis of SFN (57).
The mainstay of the diagnosis of low preload lies with invasive CPET (iCPET) (see Figure 2). CPET results are not sufficient to establish the diagnosis but may provide clues such as the reduction in the peak o2, reduction in peak , early anaerobic threshold, increase in e/CO2 production, flattening of the O2 pulse (o2/HR), lack of increase of the arterial BP during exercise, and reduced HR reserve (6, 64). Parameters like a reduced O2 pulse (o2/HR) may be due to a low SV, lower arterial oxygen content, or limitation of maximal oxygen extraction due to abnormal blood-flow distribution to muscles or a defect in oxidative capacity. iCPET adds pulmonary and radial artery catheterization, allowing a comprehensive evaluation of ventilation, gas exchange, and cardiac function at rest and during incremental exercise (65). Body position during exercise testing is critical, as RA pressure, pulmonary arterial pressure, and are noted to decrease from a supine to an upright posture (66, 67). In fact, pulmonary hemodynamic determinations are normal in the supine position (68), but RA pressure drops significantly and may not fully correct with a normal saline infusion (69). As the preload is insufficient, SV rapidly reaches a plateau, and further increases through a rise in HR (35).
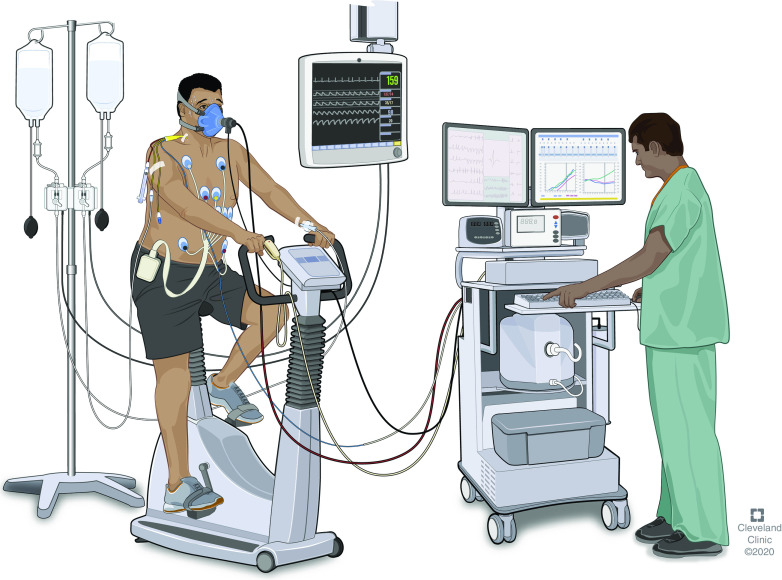
Figure 2.
Invasive cardiopulmonary exercise testing. Invasive hemodynamic measurements are done via pulmonary arterial and radial arterial catheters. These measurements are monitored while a patient is at rest and during incremental upright exercise. Reprinted by permission from the Cleveland Clinic Center for Medical Art and Photography, 2020. All Rights Reserved. Illustration by David R. Schumick, B.S., C.M.I.
Variability exists in the literature regarding the hemodynamic definition of preload insufficiency, even over time by the same working groups, augmented by differences in testing methodologies (right heart catheterization with exercise vs. iCPET) and the conditions on how the exercise is performed (e.g., normal saline administration before the test). The most recent criteria to define preload limitation to exercise include an unexplained abnormal cardiac limitation to exercise (peak o2 < 80% predicted and peak < 80% predicted) plus reduced ventricular preload (peak RA pressure < 9 mm Hg, peak PAWP < 14 mm Hg, and peak pulmonary arterial pressure < 30 mm Hg) (70). Prior attempts included only the hemodynamic component (5, 65) and/or proposed slightly different RA-pressure and PAWP cutoffs (peak RA < 6.5 mm Hg and peak PAWP < 12.5 mm Hg, with a change in RA pressure or PAWP between baseline and peak exercise <5.5 mm Hg or <6.75 mm Hg, respectively) (6). Of note, in the study by Oldham and colleagues, patients were given normal saline to optimize preload, and although this would have made the test more specific, it could have come at the expense of reducing sensitivity (6). A suggested algorithm to evaluate these patients is shown in Figure 3.
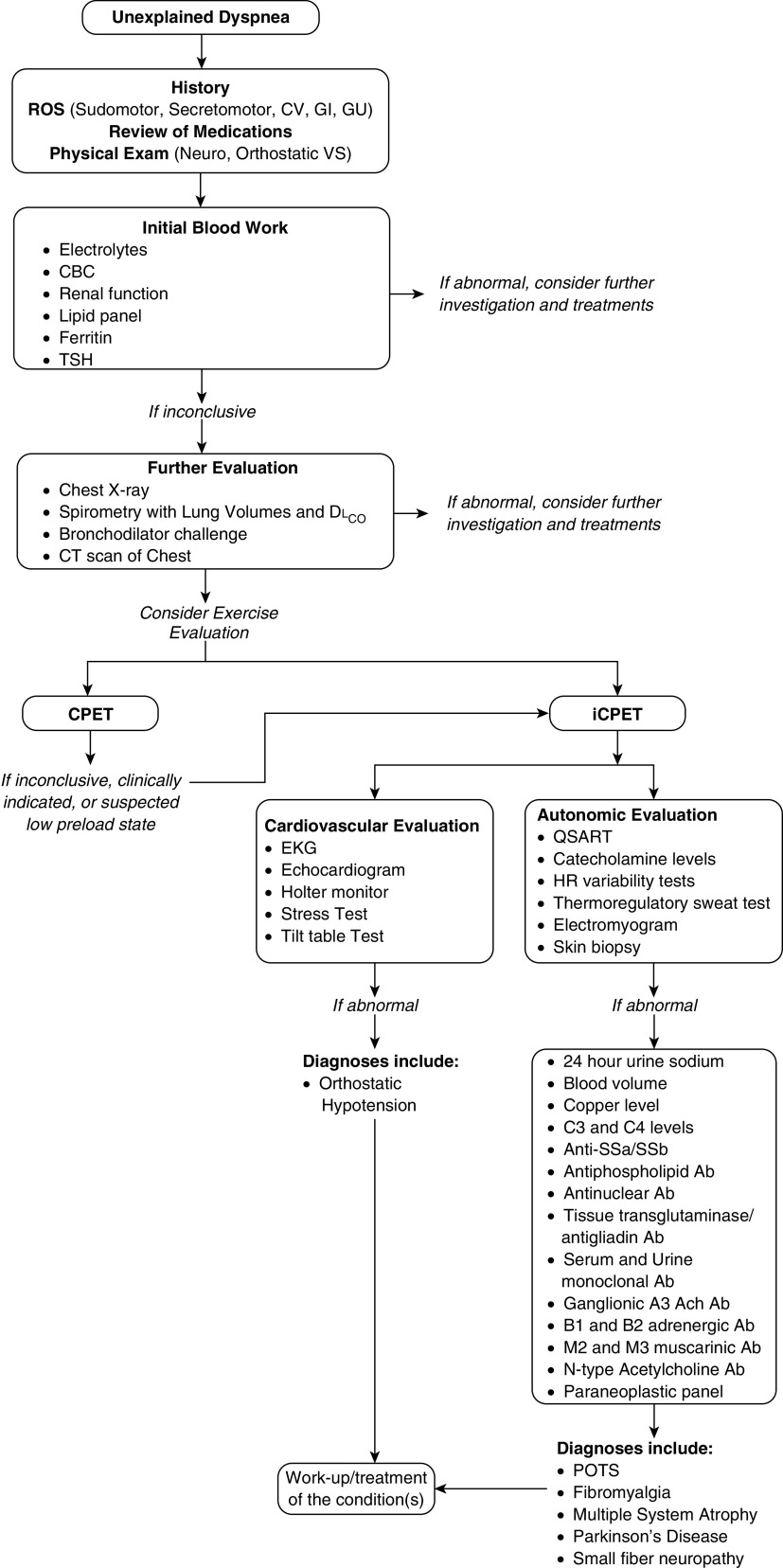
Figure 3.
Diagnostic algorithm of unexplained dyspnea. Ab = antibody; Ach = acetylcholine; CBC = complete blood count; CPET = cardiopulmonary exercise testing; CT = computed tomography; CV = cardiovascular; DlCO = diffusing capacity of the lung for carbon monoxide; EKG = electrocardiogram; GI = gastrointestinal; GU = genitourinary; HR = heart rate; iCPET = invasive CPET; POTS = postural orthostatic tachycardia syndrome; QSART = quantitative sudomotor axon reflex test; ROS = review of systems; SSa = Sjogren syndrome antigen A; SSb = Sjogren syndrome antigen B; TSH = thyroid-stimulating hormone; VS = vital signs.
Management
There is currently no cure for dyspnea in low-preload states. Treatment is provided to improve quality of life, optimize physical conditioning, and reduce symptoms. Nonpharmacological and pharmacological interventions should be used in combination, given that various conditions may be at play and a multidisciplinary approach is ideal (37). Broadly, preload can be increased by increasing thoracic blood volume (or venous return), increasing ventricular compliance, increasing atrial contraction, reducing the HR (to improve ventricular filling), and decreasing venous compliance (i.e., sympathetic activation of smooth muscles in the veins).
The mainstay of treatment of low preload is to optimize the volume status (71). A thorough review of the patient’s medication list is critical to identifying those that may affect cardiac preload (i.e., nitrates, diuretics, α-adrenergic agonists, calcium channel blockers, tricyclic antidepressants) (37). Classical nonpharmacological interventions include increasing salt intake (10 g daily or target 24-h urine sodium > 170 mmol) and hydration (up to 3–4 L/d), elevating the head of the bed at night to promote volume expansion and reduced nocturnal diuresis, minimizing sudden transitions from supine to sitting to standing positions (71), and avoiding prolonged standing and warm environments (see Table 2) (72). Over-the-knee or, ideally, waist-high compression of the lower extremities (starting at 30–40 mm Hg) helps decrease venous pooling, thereby attenuating the reduction in SV and when adopting an upright position (73). Tightly fitted abdominal binders are more efficacious, as the lower abdomen and pelvis contain 20–30% of total blood volume (74).
Table 2.
Therapies for exercise intolerance in low preload states and associated conditions
| Nonpharmacological | Pharmacological |
|---|---|
|
|
Definition of abbreviation: IVIG = intravenous immunoglobulin.
Most of the presented treatments have been adapted from the management of patients with autonomic dysfunction.
Exercise training may also be of great benefit, as seen in studies with patients with vasovagal syncope (75) and POTS (76). A supervised exercise program with endurance (aerobic) and resistance (strength) training with a focus on the core and lower body may confer the most benefits (76, 77). Some studies have shown that physical fitness may maximize aerobic capacity and increase SV over time (78). It is essential to start with a horizontal mode of training (i.e., swimming, recumbent cycles, or rowing or weight-lifting in the sitting position) that is consistent through the week, without more than 2 days off from exercising (79). Benefits may be noted within 4–6 weeks of a structured exercise program (79). Fu and Levine showed that after 3 months of exercise training, patients with POTS had increased oxygen uptake, cardiac mass, and blood volume as well as subjective well-being with improved quality of life (77). Reilly and colleagues also studied physiotherapy in POTS patients, showing that educational and breathing retraining exercises improve the breathing pattern and symptom burden (52).
In the setting of acute symptoms and orthostatic intolerance, several measures may be used for alleviation. Physical countermeasures that quickly increase venous return include leg crossing, muscle tensing, muscle pumping (sway-and-shift or tiptoe walking), bending forward, sitting/squatting/laying supine, hand squeezing a rubber ball with contraction of leg and abdominal muscles, negative-pressure breathing maneuvers, buttock clenching, sitting with the head between the knees, and skin-surface cooling (80). Rapid ingestion of water may also increase BP. In patients with neurogenic orthostatic hypotension or autonomic failure, rapidly ingesting 500 ml of fluid (in 2–3 min) can increase BP by 40 mm Hg in 5–10 minutes; however, effects may disappear after 1 hour (81). This response is likely related to the hypoosmolarity of water, causing an osmopressor response and an increase in plasma norepinephrine (82). In the clinical setting, patients with orthostatic hypotension improved with intravenous-fluid administration, as it may play a role in optimizing / mismatch (28). Exercise capacity and hemodynamics (i.e., SV and ) also improve with intravenous hydration in patients with other forms of low preload (6, 69).
Medications are helpful in patients who find limited benefit from nonpharmacological measures. Patients may try salt tablets of 1–2 g three times a day to help increase the intravascular volume, although, for some, this may induce an osmotic load and cause nausea, vomiting, and further dehydration. Other pharmacological interventions focus on reducing disproportionate tachycardia that contribute to symptom burden (51), improving blood volumes, and enhancing vasoconstriction. Tachycardia can be managed by propranolol (10–40 mg three times a day) and ivabradine (2.5–7.5 mg twice a day). Side effects of propranolol include bradycardia, and side effects for ivabradine include bradycardia and visual phosphenes. Fludrocortisone can be used to increase blood-plasma volume at a dosage of 0.1 mg to up to 1 mg daily (43, 83). Common side effects of this drug include edema, hypokalemia, and a risk of osteoporosis in young women (83). Midodrine is a prodrug that is metabolized to an α-1 adrenergic agonist that results in an increase in the arteriolar and venous tone and therefore may enhance venous return, preload, and SV (83). The dosage is 2.5–10 mg three times a day, and common side effects include supine hypertension, piloerection, and pruritis (83). Pyridostigmine inhibits peripheral acetylcholinesterase to increase synaptic acetylcholine at autonomic ganglia and peripheral muscarinic receptors (45, 83). The dosage can begin at 30 mg twice a day, titrating up to 60 mg three times a day if needed, with common side effects of loose stools, bladder irritability, diaphoresis, hypersalivation, and fasciculations (83). Hyperadrenergic agents such as droxidopa at 100–600 mg three times a day, methyldopa at 125 mg daily to 250 mg twice a day, and clonidine at 0.1–0.2 mg up to three times a day may also offer benefits (37, 56). The main side effect of central sympatholytic medications like droxidopa include supine hypertension (56). Additional off-label agents (atomoxetine, octreotide, yohimbine, ergotamine, pseudoephedrine, caffeine, and recombinant erythropoietin) may offer some promising avenues, but further research is warranted (Table 2) (56).
If a patient’s underlying pathology is an autoimmune disorder, other treatments may be of benefit. Patients may qualify for intravenous immunoglobulin at 400 mg/kg per day for 5 days, yet dosing recommendations vary, and symptoms may not improve for 2–3 months (84). Studies have shown that rituximab at 1 g/kg for two infusions 2 weeks apart may have a role in halting the immune system and allowing nerves to regenerate (84). Depending on the degree of disease, patients may also qualify for plasmapheresis to remove autoantibodies through several sessions (84). Certain studies have also recommended corticosteroids, mycophenolate, azathioprine, and cyclophosphamide to be used in conjunction with the above therapies for a better response (84). It is important to emphasize that the treatments presented here are derived from observational reports in patients with conditions associated with low-preload states, predominantly autonomic dysfunction.
Prognosis
The prognosis for patients with low preload as a cause for dyspnea remains unknown. Frequent delays in diagnosis and treatment can lead to significant morbidity and poor quality of life, as seen in other respiratory conditions (85). Importantly, studies have shown that with proper diagnosis of autonomic dysfunction, treatment, and adequate follow-up, symptom improvement may be seen, as described in patients with POTS (86).
Conclusions
Dyspnea of unknown origin may comprise nearly a fifth of clinical cases in both the outpatient and inpatient setting. Chronic low preload as a cause for dyspnea is underrecognized and has recently gained prominence through exercise testing. These patients may have a variety of clinical conditions that lead to insufficient cardiovascular venous return, low preload, and, thereby, insufficient cardiac output and oxygen delivery. iCPET in the upright position is the mainstay in diagnosing chronic low preload in these patients. Although different mechanisms can result in inadequate preload, there are effective supportive therapies and medications that can offer significant improvement and symptom relief. Interdisciplinary management of the chronic low-preload state is likely to provide the greatest benefit.
Acknowledgments
Acknowledgment
The authors thank the Cleveland Clinic Center for Medical Art and Photography for their assistance with Figure 2.
Footnotes
Supported by U.S. National Institutes of Health grant R01HL130307 (A.R.T.).
Author Contributions: R.T. participated in the literature search and review, writing the manuscript, and subsequent revisions. R.T. coordinated the efforts of all authors for this study. K.A.M. participated in the writing and critical revision of the manuscript. R.W. participated in the writing and critical revision of the manuscript. A.R.T. participated in the literature search and review, writing and critical revision of the manuscript for important intellectual content, and final approval of the version to be published. A.R.T. is the guarantor of the paper, taking responsibility for the integrity of the work, from inception to the published article.
CME will be available for this article at www.atsjournals.org.
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Kroenke K, Arrington ME, Mangelsdorff AD. The prevalence of symptoms in medical outpatients and the adequacy of therapy. Arch Intern Med. 1990;150:1685–1689. doi: 10.1001/archinte.150.8.1685. [DOI] [PubMed] [Google Scholar]
- 2.Parshall MB, Schwartzstein RM, Adams L, Banzett RB, Manning HL, Bourbeau J, et al. American Thoracic Society Committee on Dyspnea. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med. 2012;185:435–452. doi: 10.1164/rccm.201111-2042ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.DePaso WJ, Winterbauer RH, Lusk JA, Dreis DF, Springmeyer SC. Chronic dyspnea unexplained by history, physical examination, chest roentgenogram, and spirometry: analysis of a seven-year experience. Chest. 1991;100:1293–1299. doi: 10.1378/chest.100.5.1293. [DOI] [PubMed] [Google Scholar]
- 4.Karnani NG, Reisfield GM, Wilson GR. Evaluation of chronic dyspnea. Am Fam Physician. 2005;71:1529–1537. [PubMed] [Google Scholar]
- 5.Huang W, Resch S, Oliveira RK, Cockrill BA, Systrom DM, Waxman AB. Invasive cardiopulmonary exercise testing in the evaluation of unexplained dyspnea: insights from a multidisciplinary dyspnea center. Eur J Prev Cardiol. 2017;24:1190–1199. doi: 10.1177/2047487317709605. [DOI] [PubMed] [Google Scholar]
- 6.Oldham WM, Lewis GD, Opotowsky AR, Waxman AB, Systrom DM. Unexplained exertional dyspnea caused by low ventricular filling pressures: results from clinical invasive cardiopulmonary exercise testing. Pulm Circ. 2016;6:55–62. doi: 10.1086/685054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tooba R, Al Abdi S, Melillo CA, Lane J, Mayuga K, Tonelli AR. Inadequate cardiac preload is a common but under-recognized reason for unexplained dyspnea [abstract] Am J Respir Crit Care Med. 2020;201:A3245. [Google Scholar]
- 8.Mularski RA. Advancing a common understanding and approach to dyspnea management: consensus proposal for the chronic breathlessness syndrome. Ann Am Thorac Soc. 2017;14:1108–1110. doi: 10.1513/AnnalsATS.201704-285ED. [DOI] [PubMed] [Google Scholar]
- 9.Lanken PN, Terry PB, Delisser HM, Fahy BF, Hansen-Flaschen J, Heffner JE, et al. ATS End-of-Life Care Task Force. An official American Thoracic Society clinical policy statement: palliative care for patients with respiratory diseases and critical illnesses. Am J Respir Crit Care Med. 2008;177:912–927. doi: 10.1164/rccm.200605-587ST. [DOI] [PubMed] [Google Scholar]
- 10.Bausewein C, Booth S, Gysels M, Kühnbach R, Haberland B, Higginson IJ. Understanding breathlessness: cross-sectional comparison of symptom burden and palliative care needs in chronic obstructive pulmonary disease and cancer. J Palliat Med. 2010;13:1109–1118. doi: 10.1089/jpm.2010.0068. [DOI] [PubMed] [Google Scholar]
- 11.Opie LH. Heart physiology: from cell to circulation. 4th ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2004. [Google Scholar]
- 12.Jeremias A, Brown DL. Cardiac intensive care. 2nd ed. Philadelphia, PA: Saunders Elsevier; 2010. [Google Scholar]
- 13.Rowell LB. Human cardiovascular control. New York, NY: Oxford University Press; 1993. [Google Scholar]
- 14.Gaw CE, Shields RW, Jr, Mayuga KA, Gornik HL, Fouad-Tarazi F. POTS due to excessive venous pooling in an enlarged inferior vena cava. Clin Auton Res. 2012;22:197–198. doi: 10.1007/s10286-012-0157-7. [DOI] [PubMed] [Google Scholar]
- 15.Tani H, Singer W, McPhee BR, Opfer-Gehrking TL, Haruma K, Kajiyama G, et al. Splanchnic-mesenteric capacitance bed in the postural tachycardia syndrome (POTS) Auton Neurosci. 2000;86:107–113. doi: 10.1016/S1566-0702(00)00205-8. [DOI] [PubMed] [Google Scholar]
- 16.Chatterjee NA, Fifer MA.Chapter 9: Heart FailureLilly LS, editorPathophysiology of heart disease: a collaborative project of medical students and faculty 5th edBaltimore, MD: Lippincott Williams & Wilkins; 2011 [Google Scholar]
- 17.Katz AM. Physiology of the heart. 3rd ed. Philadelphia, PA: Lippincott Williams & Wilkins; 2001. [Google Scholar]
- 18.Delezie J, Handschin C. Endocrine crosstalk between skeletal muscle and the brain. Front Neurol. 2018;9:698. doi: 10.3389/fneur.2018.00698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Higginbotham MB, Morris KG, Williams RS, McHale PA, Coleman RE, Cobb FR. Regulation of stroke volume during submaximal and maximal upright exercise in normal man. Circ Res. 1986;58:281–291. doi: 10.1161/01.res.58.2.281. [DOI] [PubMed] [Google Scholar]
- 20.Saltin B, Rådegran G, Koskolou MD, Roach RC. Skeletal muscle blood flow in humans and its regulation during exercise. Acta Physiol Scand. 1998;162:421–436. doi: 10.1046/j.1365-201X.1998.0293e.x. [DOI] [PubMed] [Google Scholar]
- 21.Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134:172–178. doi: 10.1378/chest.07-2331. [DOI] [PubMed] [Google Scholar]
- 22.Magder S. Understanding central venous pressure: not a preload index? Curr Opin Crit Care. 2015;21:369–375. doi: 10.1097/MCC.0000000000000238. [DOI] [PubMed] [Google Scholar]
- 23.Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39, e14. doi: 10.1016/j.echo.2014.10.003. [DOI] [PubMed] [Google Scholar]
- 24.Sechtem U, Pflugfelder PW, Gould RG, Cassidy MM, Higgins CB. Measurement of right and left ventricular volumes in healthy individuals with cine MR imaging. Radiology. 1987;163:697–702. doi: 10.1148/radiology.163.3.3575717. [DOI] [PubMed] [Google Scholar]
- 25.De Vecchis R, Baldi C, Giandomenico G, Di Maio M, Giasi A, Cioppa C. Estimating right atrial pressure using ultrasounds: an old issue revisited with new methods. J Clin Med Res. 2016;8:569–574. doi: 10.14740/jocmr2617w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gibbons CH, Freeman R. Orthostatic dyspnea: a neglected symptom of orthostatic hypotension. Clin Auton Res. 2005;15:40–44. doi: 10.1007/s10286-005-0227-1. [DOI] [PubMed] [Google Scholar]
- 27.Seward JB, Hayes DL, Smith HC, Williams DE, Rosenow EC, III, Reeder GS, et al. Platypnea-orthodeoxia: clinical profile, diagnostic workup, management, and report of seven cases. Mayo Clin Proc. 1984;59:221–231. doi: 10.1016/s0025-6196(12)61253-1. [DOI] [PubMed] [Google Scholar]
- 28.Fox JL, Brown E, Harrison JK, Williams J, Terry PB. Platypnea-orthodeoxia and progressive autonomic failure. Am Rev Respir Dis. 1989;140:1802–1804. doi: 10.1164/ajrccm/140.6.1802. [DOI] [PubMed] [Google Scholar]
- 29.Johnson MJ, Clark AL. The mechanisms of breathlessness in heart failure as the basis of therapy. Curr Opin Support Palliat Care. 2016;10:32–35. doi: 10.1097/SPC.0000000000000181. [DOI] [PubMed] [Google Scholar]
- 30.Ponikowski P, Chua TP, Piepoli M, Ondusova D, Webb-Peploe K, Harrington D, et al. Augmented peripheral chemosensitivity as a potential input to baroreflex impairment and autonomic imbalance in chronic heart failure. Circulation. 1997;96:2586–2594. doi: 10.1161/01.cir.96.8.2586. [DOI] [PubMed] [Google Scholar]
- 31.Dubé BP, Agostoni P, Laveneziana P. Exertional dyspnoea in chronic heart failure: the role of the lung and respiratory mechanical factors. Eur Respir Rev. 2016;25:317–332. doi: 10.1183/16000617.0048-2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tikunov B, Levine S, Mancini D. Chronic congestive heart failure elicits adaptations of endurance exercise in diaphragmatic muscle. Circulation. 1997;95:910–916. doi: 10.1161/01.cir.95.4.910. [DOI] [PubMed] [Google Scholar]
- 33.Wranne B, Tolagen K. Platypnoea after pneumonectomy caused by a combination of intracardiac right-to-left shunt and hypovolaemia: relief of symptoms on restitution of blood volume. Scand J Thorac Cardiovasc Surg. 1978;12:129–131. doi: 10.3109/14017437809100362. [DOI] [PubMed] [Google Scholar]
- 34.Fick A. Sitzungsberichte für 1870. Verhandl d phys-med Ges. 1871;2:I–XXVIII. [Google Scholar]
- 35.Fu Q, Vangundy TB, Galbreath MM, Shibata S, Jain M, Hastings JL, et al. Cardiac origins of the postural orthostatic tachycardia syndrome. J Am Coll Cardiol. 2010;55:2858–2868. doi: 10.1016/j.jacc.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ito K, Uchida T, Manabe Y, Miyazaki Y, Itoh H, Mishina M, et al. A case of nivolumab-induced isolated adrenocorticotropic hormone deficiency presenting dyspnea [in Japanese] Hinyokika Kiyo. 2018;64:391–395. doi: 10.14989/ActaUrolJap_64_10_391. [DOI] [PubMed] [Google Scholar]
- 37.Raj SR, Guzman JC, Harvey P, Richer L, Schondorf R, Seifer C, et al. Canadian cardiovascular society position statement on postural orthostatic tachycardia syndrome (POTS) and related disorders of chronic orthostatic intolerance. Can J Cardiol. 2020;36:357–372. doi: 10.1016/j.cjca.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 38.Christiansen D, Baillie T, Mak S, Granton J. Dyspnea after pulmonary embolism. Ann Am Thorac Soc. 2019;16:914–919. doi: 10.1513/AnnalsATS.201811-818CC. [DOI] [PubMed] [Google Scholar]
- 39.Chloros T, Burt C, Dunning J. The role of intraoperative transesophageal echocardiography in identifying a fenestrated occlusion of the inferior vena cava during pulmonary thromboendarterectomy. J Cardiothorac Vasc Anesth. 2018;32:1329–1332. doi: 10.1053/j.jvca.2017.09.004. [DOI] [PubMed] [Google Scholar]
- 40.Bevegard S, Lodin A. Postural circulatory changes at rest and during exercise in five patients with congenital absence of valves in the deep veins of the legs. Acta Med Scand. 1962;172:21–29. doi: 10.1111/j.0954-6820.1962.tb07124.x. [DOI] [PubMed] [Google Scholar]
- 41.Opotowsky AR, Halpern D, Kulik TJ, Systrom DM, Wu F. Inadequate venous return as a primary cause for Fontan circulatory limitation. J Heart Lung Transplant. 2014;33:1194–1196. doi: 10.1016/j.healun.2014.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Stewart JM, Weldon A. Vascular perturbations in the chronic orthostatic intolerance of the postural orthostatic tachycardia syndrome. J Appl Physiol (1985) 2000;89:1505–1512. doi: 10.1152/jappl.2000.89.4.1505. [DOI] [PubMed] [Google Scholar]
- 43.Fouad FM, Tadena-Thome L, Bravo EL, Tarazi RC. Idiopathic hypovolemia. Ann Intern Med. 1986;104:298–303. doi: 10.7326/0003-4819-104-3-298. [DOI] [PubMed] [Google Scholar]
- 44.Streeten DH, Anderson GH, Jr, Richardson R, Thomas FD. Abnormal orthostatic changes in blood pressure and heart rate in subjects with intact sympathetic nervous function: evidence for excessive venous pooling. J Lab Clin Med. 1988;111:326–335. [PubMed] [Google Scholar]
- 45.Raj SR, Biaggioni I, Yamhure PC, Black BK, Paranjape SY, Byrne DW, et al. Renin-aldosterone paradox and perturbed blood volume regulation underlying postural tachycardia syndrome. Circulation. 2005;111:1574–1582. doi: 10.1161/01.CIR.0000160356.97313.5D. [DOI] [PubMed] [Google Scholar]
- 46.Garland EM, Raj SR, Black BK, Harris PA, Robertson D. The hemodynamic and neurohumoral phenotype of postural tachycardia syndrome. Neurology. 2007;69:790–798. doi: 10.1212/01.wnl.0000267663.05398.40. [DOI] [PubMed] [Google Scholar]
- 47.Zhou L. Small fiber neuropathy. Semin Neurol. 2019;39:570–577. doi: 10.1055/s-0039-1688977. [DOI] [PubMed] [Google Scholar]
- 48.Benarroch EE. Postural tachycardia syndrome: a heterogeneous and multifactorial disorder. Mayo Clin Proc. 2012;87:1214–1225. doi: 10.1016/j.mayocp.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Thieben MJ, Sandroni P, Sletten DM, Benrud-Larson LM, Fealey RD, Vernino S, et al. Postural orthostatic tachycardia syndrome: the Mayo clinic experience. Mayo Clin Proc. 2007;82:308–313. doi: 10.4065/82.3.308. [DOI] [PubMed] [Google Scholar]
- 50.Schondorf R, Benoit J, Wein T, Phaneuf D. Orthostatic intolerance in the chronic fatigue syndrome. J Auton Nerv Syst. 1999;75:192–201. doi: 10.1016/s0165-1838(98)00177-5. [DOI] [PubMed] [Google Scholar]
- 51.Sheldon RS, Grubb BP, II, Olshansky B, Shen WK, Calkins H, Brignole M, et al. 2015 heart rhythm society expert consensus statement on the diagnosis and treatment of postural tachycardia syndrome, inappropriate sinus tachycardia, and vasovagal syncope. Heart Rhythm. 2015;12:e41–e63. doi: 10.1016/j.hrthm.2015.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reilly CC, Floyd SV, Lee K, Warwick G, James S, Gall N, et al. Breathlessness and dysfunctional breathing in patients with postural orthostatic tachycardia syndrome (POTS): the impact of a physiotherapy intervention. Auton Neurosci. 2020;223:102601. doi: 10.1016/j.autneu.2019.102601. [DOI] [PubMed] [Google Scholar]
- 53.Freeman R, Wieling W, Axelrod FB, Benditt DG, Benarroch E, Biaggioni I, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Auton Neurosci. 2011;161:46–48. doi: 10.1016/j.autneu.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 54.Mayuga K, Tonelli AR, Goyanes AM. Dyspnea in disguise: a case of hidden orthostatic hypotension and preload insufficiency [abstract] Am J Respir Crit Care Med. 2020;201:A3219. [Google Scholar]
- 55.Rutan GH, Hermanson B, Bild DE, Kittner SJ, LaBaw F, Tell GS CHS Collaborative Research Group. Orthostatic hypotension in older adults: the cardiovascular health study. Hypertension. 1992;19:508–519. doi: 10.1161/01.hyp.19.6.508. [DOI] [PubMed] [Google Scholar]
- 56.Jones PK, Shaw BH, Raj SR. Orthostatic hypotension: managing a difficult problem. Expert Rev Cardiovasc Ther. 2015;13:1263–1276. doi: 10.1586/14779072.2015.1095090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lauria G, Hsieh ST, Johansson O, Kennedy WR, Leger JM, Mellgren SI, et al. European Federation of Neurological Societies; Peripheral Nerve Society. European Federation of Neurological Societies/Peripheral Nerve Society Guideline on the use of skin biopsy in the diagnosis of small fiber neuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Eur J Neurol. 2010;17:903–912, e44–e49. doi: 10.1111/j.1468-1331.2010.03023.x. [DOI] [PubMed] [Google Scholar]
- 58.Sène D. Small fiber neuropathy: diagnosis, causes, and treatment. Joint Bone Spine. 2018;85:553–559. doi: 10.1016/j.jbspin.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 59.Peters MJ, Bakkers M, Merkies IS, Hoeijmakers JG, van Raak EP, Faber CG. Incidence and prevalence of small-fiber neuropathy: a survey in the Netherlands. Neurology. 2013;81:1356–1360. doi: 10.1212/WNL.0b013e3182a8236e. [DOI] [PubMed] [Google Scholar]
- 60.Basantsova NY, Starshinova AA, Dori A, Zinchenko YS, Yablonskiy PK, Shoenfeld Y. Small-fiber neuropathy definition, diagnosis, and treatment. Neurol Sci. 2019;40:1343–1350. doi: 10.1007/s10072-019-03871-x. [DOI] [PubMed] [Google Scholar]
- 61.Novak P. Cerebral blood flow, heart rate, and blood pressure patterns during the tilt test in common orthostatic syndromes. Neurosci J. 2016;2016:6127340. doi: 10.1155/2016/6127340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Goldstein DS, Cheshire WP. Roles of catechol neurochemistry in autonomic function testing. Clin Auton Res. 2018;28:273–288. doi: 10.1007/s10286-018-0528-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shannon JR, Flattem NL, Jordan J, Jacob G, Black BK, Biaggioni I, et al. Orthostatic intolerance and tachycardia associated with norepinephrine-transporter deficiency. N Engl J Med. 2000;342:541–549. doi: 10.1056/NEJM200002243420803. [DOI] [PubMed] [Google Scholar]
- 64.Guazzi M, Bandera F, Ozemek C, Systrom D, Arena R. Cardiopulmonary exercise testing: what is its value? J Am Coll Cardiol. 2017;70:1618–1636. doi: 10.1016/j.jacc.2017.08.012. [DOI] [PubMed] [Google Scholar]
- 65.Maron BA, Cockrill BA, Waxman AB, Systrom DM. The invasive cardiopulmonary exercise test. Circulation. 2013;127:1157–1164. doi: 10.1161/CIRCULATIONAHA.112.104463. [DOI] [PubMed] [Google Scholar]
- 66.Kovacs G, Berghold A, Scheidl S, Olschewski H. Pulmonary arterial pressure during rest and exercise in healthy subjects: a systematic review. Eur Respir J. 2009;34:888–894. doi: 10.1183/09031936.00145608. [DOI] [PubMed] [Google Scholar]
- 67.Thadani U, Parker JO. Hemodynamics at rest and during supine and sitting bicycle exercise in normal subjects. Am J Cardiol. 1978;41:52–59. doi: 10.1016/0002-9149(78)90131-5. [DOI] [PubMed] [Google Scholar]
- 68.O’Donnell DE, Milne KM, Vincent SG, Neder JA. Unraveling the causes of unexplained dyspnea: the value of exercise testing. Clin Chest Med. 2019;40:471–499. doi: 10.1016/j.ccm.2019.02.014. [DOI] [PubMed] [Google Scholar]
- 69.Figueroa RA, Arnold AC, Nwazue VC, Okamoto LE, Paranjape SY, Black BK, et al. Acute volume loading and exercise capacity in postural tachycardia syndrome. J Appl Physiol (1985) 2014;117:663–668. doi: 10.1152/japplphysiol.00367.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Oldham WM, Oliveira RKF, Wang RS, Opotowsky AR, Rubins DM, Hainer J, et al. Network analysis to risk stratify patients with exercise intolerance. Circ Res. 2018;122:864–876. doi: 10.1161/CIRCRESAHA.117.312482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raj SR, Coffin ST. Medical therapy and physical maneuvers in the treatment of the vasovagal syncope and orthostatic hypotension. Prog Cardiovasc Dis. 2013;55:425–433. doi: 10.1016/j.pcad.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.El-Sayed H, Hainsworth R. Salt supplement increases plasma volume and orthostatic tolerance in patients with unexplained syncope. Heart. 1996;75:134–140. doi: 10.1136/hrt.75.2.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Podoleanu C, Maggi R, Brignole M, Croci F, Incze A, Solano A, et al. Lower limb and abdominal compression bandages prevent progressive orthostatic hypotension in elderly persons: a randomized single-blind controlled study. J Am Coll Cardiol. 2006;48:1425–1432. doi: 10.1016/j.jacc.2006.06.052. [DOI] [PubMed] [Google Scholar]
- 74.Smit AA, Wieling W, Fujimura J, Denq JC, Opfer-Gehrking TL, Akarriou M, et al. Use of lower abdominal compression to combat orthostatic hypotension in patients with autonomic dysfunction. Clin Auton Res. 2004;14:167–175. doi: 10.1007/s10286-004-0187-x. [DOI] [PubMed] [Google Scholar]
- 75.Gardenghi G, Rondon MU, Braga AM, Scanavacca MI, Negrão CE, Sosa E, et al. The effects of exercise training on arterial baroreflex sensitivity in neurally mediated syncope patients. Eur Heart J. 2007;28:2749–2755. doi: 10.1093/eurheartj/ehm208. [DOI] [PubMed] [Google Scholar]
- 76.Fu Q, Levine BD. Exercise in the postural orthostatic tachycardia syndrome. Auton Neurosci. 2015;188:86–89. doi: 10.1016/j.autneu.2014.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fu Q, Levine BD. Exercise and non-pharmacological treatment of POTS. Auton Neurosci. 2018;215:20–27. doi: 10.1016/j.autneu.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saltin B, Blomqvist G, Mitchell JH, Johnson RL, Jr, Wildenthal K, Chapman CB. Response to exercise after bed rest and after training. Circulation. 1968;38(Suppl):VII1–VII78. [PubMed] [Google Scholar]
- 79.Raj SR. Row, row, row your way to treating postural tachycardia syndrome. Heart Rhythm. 2016;13:951–952. doi: 10.1016/j.hrthm.2015.12.039. [DOI] [PubMed] [Google Scholar]
- 80.Wieling W, van Dijk N, Thijs RD, de Lange FJ, Krediet CT, Halliwill JR. Physical countermeasures to increase orthostatic tolerance. J Intern Med. 2015;277:69–82. doi: 10.1111/joim.12249. [DOI] [PubMed] [Google Scholar]
- 81.Jordan J, Shannon JR, Black BK, Ali Y, Farley M, Costa F, et al. The pressor response to water drinking in humans : a sympathetic reflex? Circulation. 2000;101:504–509. doi: 10.1161/01.cir.101.5.504. [DOI] [PubMed] [Google Scholar]
- 82.McHugh J, Keller NR, Appalsamy M, Thomas SA, Raj SR, Diedrich A, et al. Portal osmopressor mechanism linked to transient receptor potential vanilloid 4 and blood pressure control. Hypertension. 2010;55:1438–1443. doi: 10.1161/HYPERTENSIONAHA.110.151860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lanier JB, Mote MB, Clay EC. Evaluation and management of orthostatic hypotension. Am Fam Physician. 2011;84:527–536. [PubMed] [Google Scholar]
- 84.Iodice V, Kimpinski K, Vernino S, Sandroni P, Low PA. Immunotherapy for autoimmune autonomic ganglionopathy. Auton Neurosci. 2009;146:22–25. doi: 10.1016/j.autneu.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 85.Hole B, Salem J. How long do patients with chronic disease expect to live? A systematic review of the literature. BMJ Open. 2016;6:e012248. doi: 10.1136/bmjopen-2016-012248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kimpinski K, Figueroa JJ, Singer W, Sletten DM, Iodice V, Sandroni P, et al. A prospective, 1-year follow-up study of postural tachycardia syndrome. Mayo Clin Proc. 2012;87:746–752. doi: 10.1016/j.mayocp.2012.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]