Abstract
Rationale:
Psychostimulants are often used in close temporal proximity to nicotine and have been reported to enhance acutely nicotine’s desirability in humans.
Objective:
To investigate the acute associations between amphetamine and nicotine, we examined the potentiative interactions between clinically-relevant, low doses of these drugs on locomotor activity and dopamine overflow in the rat.
Methods:
Locomotor activity was measured by telemetry in the home cage environment, and dopamine overflow was evaluated in striatal slice preparations from female Holtzman rats.
Results:
When administered simultaneously, nicotine and amphetamine produced a predominantly additive effect on locomotor behavior. However amphetamine, when given 2-4 hours prior to nicotine, strongly potentiated nicotine-induced locomotor activity. Correspondingly, nicotine given 1-4 h prior to amphetamine, robustly enhanced amphetamine-stimulated locomotor activity when, even when the effects of the nicotine pretreatment dissipated. Acute nicotine pretreatment similarly potentiated the effects of dopamine transporter ligands, cocaine, nomifensine, and methamphetamine, but not a direct dopamine receptor agonist. Consistent with the behavioral studies, in vivo nicotine pretreatment exaggerated amphetamine-induced dopamine efflux from rat striatal slices. Likewise, in vivo pretreatment of rats with amphetamine potentiated nicotine-induced dopamine efflux from striatal slices. Direct pretreatment of striatal tissue by nicotine also potentiated subsequent amphetamine-stimulated dopamine overflow, further suggesting that the nicotine-amphetamine interaction occurs at the level of the dopamine terminal.
Conclusion:
Overall, the present data demonstrate that acute interactions of nicotine and other psychomotor stimulants produce potentiative effects and that these transient interactions may play a role in the frequent co-use and abuse of nicotine and other stimulants.
Keywords: amphetamine, nicotine, nicotinic acetylcholine receptors, rats, locomotor activity, dopamine
Introduction
Repeated nicotine exposure exaggerates amphetamine- and cocaine-related behaviors and neurobiological changes, a process referred to as behavioral cross-sensitization that has been suggested to play a role in the development and maintenance of addiction. In rats, repeated exposure to nicotine increased activity stimulated by amphetamine (Birrell and Balfour 1998; Schoffelmeer et al. 2002; Collins et al. 2004; Celik et al. 2006), cocaine (Collins and Izenwasser, 2004), and methamphetamine (Suemaru et al. 1993). Similarly, repeated nicotine pretreatment facilitated cocaine self-administration in rats (Horger et al. 1992; Bechtholt and Mark, 2002; McQuown et al. 2007). Consistent with behavioral studies, amphetamine-stimulated dopamine overflow in the accumbens (Birrell and Balfour 1998) and prefrontal cortex (Drew and Werling 2003) was enhanced in rats repeatedly exposed to nicotine. These data suggest that repeated nicotine produces persistent alterations in psychomotor and reward systems that are shared with other drugs of abuse such as amphetamine and cocaine.
Although repeated nicotine administration induces long-lasting sensitization, little is known about the acute interactions between nicotine and psychomotor stimulants. Acute interactions of these drugs are important to consider because humans frequently use these drugs in close temporal proximity, and nicotine use is typically higher in individuals who abuse methamphetamine, amphetamine, and cocaine (Budney et al. 1993; Burling et al. 1996; Barrett et al. 2006; Martínez-Ortega et al. 2006; Yen and Chong 2006). Correspondingly, acute exposure to stimulants enhances smoking. For example, acute doses of methylphenidate, amphetamine, bupropion, or cocaine increased smoking-related behaviors and subjective responses (Schuster et al. 1979; Henningfield and Griffiths, 1981; Roll et al. 1997; Tidey et al. 2000; Cousins et al. 2001; Sigmon et al. 2003; Rush et al. 2005; Vansickel et al. 2007). Conversely, nicotine exposure increased cocaine craving in crack cocaine-using smokers (Reid et al. 1998). Consistent with the clinical research, preclinical studies demonstrated that acute bupropion, methamphetamine, and methylphenidate increased nicotine self-administration in rats (Rahut et al. 2003; Wooters et al. 2007). Overall, these data suggest that there is a pharmacological basis for the interactions between nicotine and stimulants that influence or elevate their use (Vansickel et al. 2007). Further analysis regarding acute interactions of nicotine and other stimulants are needed to investigate neurobiological mechanisms involved in these drug interactions.
Therefore, in the present study we investigated the acute, transitory interactions between nicotine and amphetamine on locomotor behavior in rats and dopamine efflux in rat striatal tissue. Nicotine and amphetamine were administered simultaneously or as acute pretreatments prior (2-8 h) to challenge injections of either nicotine or amphetamine. Based on the clinical literature, we hypothesized that amphetamine pretreatments would enhance nicotine-stimulated activity and dopamine overflow in the striatum. Significantly, pretreatment with low doses of nicotine, equivalent to 1-3 cigarettes, potentiated amphetamine-stimulated locomotor activity and dopamine overflow. These findings suggest that either nicotine or amphetamine administration induces transient alterations in cellular mechanisms to enhance the effects of these drugs.
Methods
Subjects.
Female Holtzman rats (200-250g) were obtained from Harlan Sprague Dawley (Indianapolis, IN) and housed in groups of three rats per cage upon arrival, and food and water were freely available at all times. The housing and experimental rooms were maintained on a 12 h light/dark cycle with lights on at 7:00 AM and maintained at an average temperature of 21°C. The experimental protocols were approved by the University of Michigan University Committee on the Use and Care of Animals and conformed to the guidelines established by the NIH Guide for the Use of Laboratory Animals.
Surgical Implant and Telemetry System.
Rats were implanted with telemetry devices (model ER-4000 E-Mitter, Mini Mitter Co., Bend, OR) and singly-housed following the surgery. Under ketamine (90 mg/kg, i.p.) and xylazine (10 mg/kg, i.p.) anesthesia, a rostral-caudal incision (1-2 cm) was made in the skin and muscle of the abdomen. The telemetry device was placed inside the peritoneal cavity, and the muscle and skin were closed separately with suture. Implant surgeries were conducted at least 7-8 days prior to conducting an experiment. The implanted transmitters relayed changes in location relative to a receiver (model ER-4000 Receiver, Mini Mitter Co.) located directly beneath a rat’s home cage. Data were collected and processed simultaneously by the Vital View data acquisition system (Mini Mitter Co.), and recorded as locomotor activity counts.
Locomotor Activity Experiments.
Baseline locomotor activity was collected for at least 40 min prior to injections without disturbing the rats’ home cages. Data were collected during pretreatment and challenge injections. Locomotor activity counts were summed every 5 min for the duration of an experiment; the first data point on time course graphs represented activity counts 3 min post-injection. Mean locomotor activity counts were calculated for each experimental group and vertical lines from each data point represent standard errors of the mean (sem). Total activity counts also were calculated over the first 90 min following the challenge injection only. All rats were tested with one experimental condition only and then euthanized. Each experimental group is composed of 5-7 rats.
Experiment 1.
Locomotor stimulation in the home cage environment was measured following nicotine and amphetamine administration. Rats were treated with a single dose of saline, 0.32 mg/kg nicotine, 0.32 mg/kg amphetamine, or a combination of 0.32 mg/kg nicotine with 0.32 mg/kg amphetamine simultaneously.
Experiment 2.
The effects of sequential administration of nicotine and/or amphetamine were measured in order to determine if nicotine or amphetamine pretreatments have persisting effects to alter challenge treatments. Rats were injected with saline or 0.32 mg/kg nicotine or 0.32 mg/kg amphetamine and 2 h later challenged with either nicotine (0.32 mg/kg) or amphetamine (0.32 mg/kg).
Experiment 3.
Rats received pretreatments either 4 or 8 h prior to challenge injections in order to determine the time course of the challenge enhancement. Saline or increasing doses of nicotine (0.1 or 0.32 mg/kg) were administered 4 h prior to amphetamine challenge (0.32 mg/kg). Also, saline or increasing doses of amphetamine (0.32 or 1.0 mg/kg) were administered 4 h prior to nicotine. A nicotine pretreatment dose of 0.32 mg/kg was administered 8 h prior to 0.32 mg/kg amphetamine challenge; and an amphetamine pretreatment dose of 1.0 mg/kg was administered 8 h prior to 0.32 mg/kg nicotine challenge.
Experiment 4.
To determine if the nicotinic acetylcholine receptor was involved in this behavioral effect, saline or mecamylamine (1.0 mg/kg) was administered subcutaneously 60 min prior to the pretreatment injection of either saline or 0.32 mg/kg nicotine. An amphetamine challenge (0.32 mg/kg) was administered 4 h after the nicotine pretreatment.
Experiment 5.
To determine the effect of nicotine pretreatment on other stimulants, saline or nicotine pretreatment (0.32 mg/kg) was administered 2 h prior to different challenge drugs, either 3.2 mg/kg cocaine, 1.0 mg/kg nomifensine, 0.56 mg/kg methamphetamine, or the direct dopamine D1 agonist SKF81297 at 0.32 or 1.0 mg/kg.
Striatal Slice Preparation.
Rats were sacrificed by decapitation and the brains were rapidly removed. Coronal sections (2 mm) containing the entire striatum were dissected using an ice-cold brain matrix (Heffner et al. 1980). For each rat, striatal tissue from both brain halves was sliced, mixed, divided into two equal portions on ice. Tissue was weighed and placed on Whatman GF/B filter disks in chamber on a Brandel perfusion apparatus (Brandel SF-12, Gaithersburg, MD).
Dopamine Efflux Experiments.
The chambers were perfused at 37°C with oxygenated Krebs-Ringer Buffer (KRB, final pH 7.4) containing 24.9 mM NaHCO3, 1.2 mM KH2PO4, 145 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 1.0 mM MgCl2, 10 mM glucose, 0.25 mM ascorbic acid, and 50 μM pargyline. After 75 min perfusion (wash period), perfusates were collected into vials containing an internal standard solution with a final concentration of 50 mM HClO4, 25 μM sodium bisulfate, 25 μM EDTA, and 10 nM dihydroxybenzylamine (DHBA) or 10 nM 2-AP as internal standards. Dopamine was measured by high performance liquid chromatography (HPLC) coupled with electrochemical detection (Waters, Milford, MA) and expressed as pmol dopamine per milligram wet tissue weight per fraction. For measuring amphetamine-stimulated dopamine overflow, KRB flowed at 100 μl/min in 5 min fractions. Amphetamine (final concentration: 10 μM) was added to the KRB during fraction 5 only to stimulate dopamine efflux. In experiments with in vitro nicotine exposure, nicotine (10 μM) was added to the final 15 min of the wash period only, and amphetamine alone was added at fraction 4. To measure nicotine-stimulated dopamine overflow, buffer flowed at 400 μl/min and fractions were collected every 2 min. Nicotine (final concentration: 30 μM) was added to the KRB during fraction 6 only to stimulate dopamine efflux.
Drugs.
(−)-Nicotine bitartrate, d-amphetamine sulfate, mecamylamine HCl, and nomifensine were purchased from Sigma-Aldrich (St. Louis, MO) and were prepared daily in saline. Cocaine and methamphetamine were obtained from NIDA and dissolved in saline. SKF81297 was obtained also from NIDA and dissolved in sterile water. Doses and concentration were calculated based on the salt form of each drug. All injections were administered i.p. in a volume of 1 ml/kg.
Statistical Analysis.
Time course of locomotor stimulation was analyzed by two-way ANOVA with Tukey’s post hoc tests (SigmaStat 3.5, Systat Software, San Jose, CA). Total locomotor activity counts for 90 min following the challenge injection were compared by one-way ANOVA with Tukey’s post hoc test (GraphPad Prism Software). Dopamine perfusion studies also were analyzed by two-way ANOVA with Bonferroni’s post hoc tests.
Results
Low doses of nicotine and amphetamine (0.32 mg/kg) stimulated slightly more locomotor activity as compared with saline in the home cage environment. Saline injection stimulated some activity increases due to handling and injection procedures. Simultaneous administration of 0.32 mg/kg nicotine and 0.32 mg/kg amphetamine produced greater activity levels than nicotine or amphetamine alone (Fig. 1). The nicotine and amphetamine produced total activity counts over 90 min of 397.83 (± 65) and 553.33 (± 148), respectively; whereas simultaneous administration of nicotine and amphetamine produced 916 (± 39.07). Simultaneous administration stimulated activity levels similar to that expected from an additive response; however, there might be time points when this effect was more than additive (i.e., 50-75 min).
Figure 1.
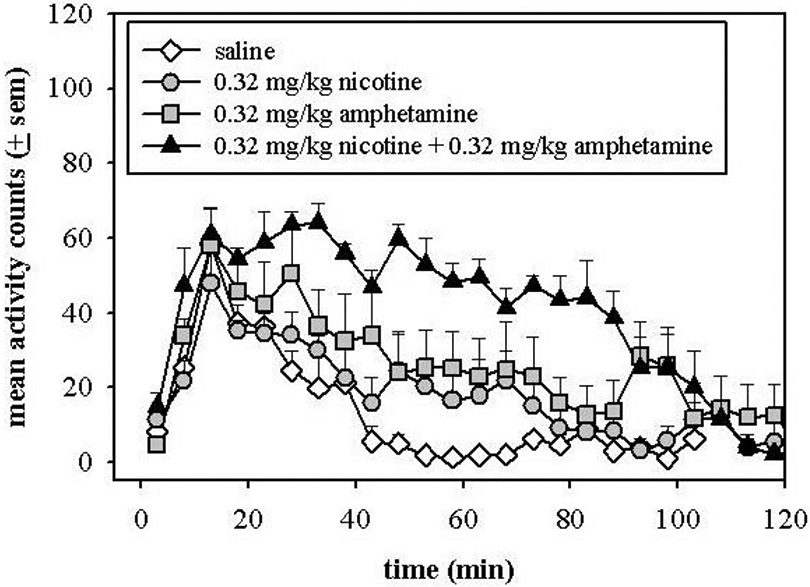
Simultaneous administration of amphetamine and nicotine. Rats were administered (i.p.) either saline, 0.32 mg/kg amphetamine, 0.32 mg/kg nicotine, or a combination of 0.32 mg/kg amphetamine with 0.32 mg/kg nicotine. The injections occurred at time 0.
Nicotine and amphetamine were administered sequentially to further evaluate the acute interactions of these drugs (Fig. 2). The effect of pretreatment with nicotine or saline was evaluated on subsequent locomotor activity levels stimulated by nicotine or amphetamine (challenge drug) (Fig. 2a, 2c). Pretreatment with a low dose of nicotine (0.32 mg/kg i.p.) alone produced a small, if any, increase in activity depending on the level of activity stimulated by the saline injection. The nicotine challenge injection produced small, but not statistically significantly, increases in nicotine-pretreated rats as compared with saline-pretreated controls [pretreatment x time following challenge interaction: F(23,192)=1.44, p=0.31; pretreatment main effect: F(1,192)=2.53, p=0.11]. The total activity counts during the nicotine challenge were not significantly different between saline- and nicotine-pretreated rats (Fig. 2b; t=0.74, p=0.48). Although nicotine pretreatment did not significantly alter the effects of nicotine challenge, nicotine pretreatment strongly potentiated activity levels stimulated by a challenge of 0.32 mg/kg amphetamine (Fig. 2c). Analysis of the activity induced by amphetamine challenge revealed no significant interaction (pretreatment x time following challenge injection) because the stimulation patterns were parallel; however, there was a significant main effect for pretreatment [F(1,250)=40.78, p<0.0001]. Similarly, there was a significant difference in the total activity counts produced by amphetamine challenge between saline- and nicotine-pretreated rats (Fig. 2d; t=2.77, p=0.02). These data demonstrated that 2 h nicotine pretreatment significantly augmented the effects of amphetamine.
Figure 2.
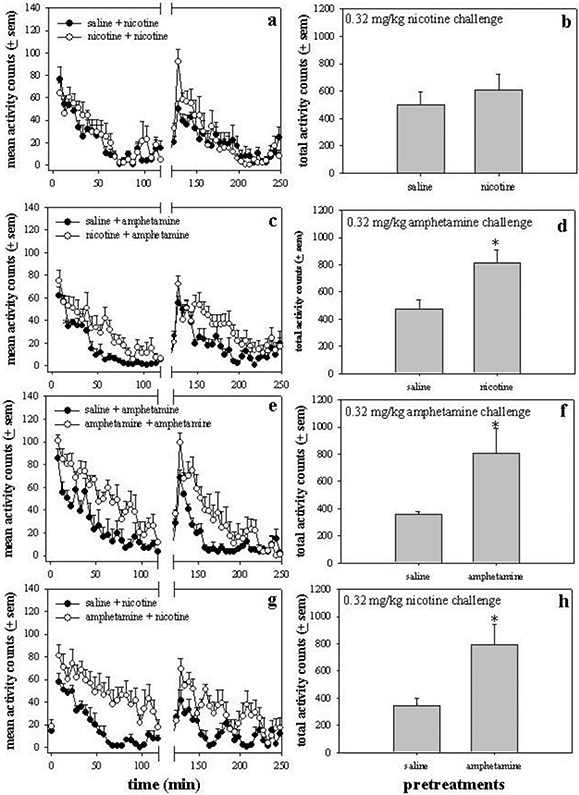
Acute interactions between amphetamine and nicotine. (a,c,e,f) Nicotine (0.32 mg/kg) or amphetamine (0.32 mg/kg) pretreatments were administered 2 h prior to an acute challenge with either nicotine (0.32 mg/kg) or amphetamine (0.32 mg/kg). The combination of pretreatment and challenge is designated by “pretreatment + challenge” on each graph. The pretreatment injection occurred at time 0, and the challenge injection occurred at 120 min as indicated by the break in the x-axis. (b,d,f,h) The locomotor activity counts were summed from 120 to 210 min in order to obtain the total activity counts produced by each challenge condition. * p<0.05 as compared with saline-pretreated rats.
The effect of a 2 h amphetamine pretreatment was evaluated on amphetamine- and nicotine-stimulated locomotor activity (Fig. 2e, 2g). During the pretreatment phase, amphetamine (0.32 mg/kg) elevated locomotor activity above that of saline for approximately 1.5-2 h. Amphetamine pretreatment significantly enhanced amphetamine-induced locomotor activity as indicated by a significant main effect for pretreatment [F(1,260)=48.6, p<0.0001] with a statistical increase at 143 min only (p<0.05) (Fig. 2e). In addition, the total activity counts during the amphetamine challenge were greater in amphetamine-pretreated rats than in saline-pretreated rats (Fig. 2f; t=2.41, p=0.04). As shown in fig. 2g, amphetamine pretreatment also augmented activity levels stimulated by nicotine challenge (0.32 mg/kg) [nearly significant pretreatment x time following challenge interaction: F(23,216)=1.49, p<0.08; pretreatment main effect: F(1,216)=78.87, p<0.0001] with a statistical increase at 163 (p<0.01), 168 (p<0.05), and 213 min (p<0.05). This is demonstrated further by a significant increase in nicotine-induced total activity counts in amphetamine-pretreated rats (Fig. 2h; t=2.7, p=0.02).
Longer pretreatments were evaluated prior to nicotine or amphetamine challenges. Increasing doses of nicotine pretreatment administered 4 h prior to amphetamine challenge (0.32 mg/kg) dose-dependently enhanced amphetamine-stimulated locomotor activity (Fig. 3a) (F(2,17)=17.14, p=0.0001). An 8 h nicotine pretreatment (0.32 mg/kg) failed to augment the locomotor stimulating effects of amphetamine challenge (Fig. 3b); however, the amphetamine-stimulated locomotor activity was greater in the 8 h challenge group as compared with the 4 h challenge group. These differences may be influenced by the time of day that the amphetamine was injected. For example, in the 8 h challenge group, amphetamine was administered later in the afternoon, closer to the dark cycle, in order to allow for the long pretreatment time.
Figure 3.
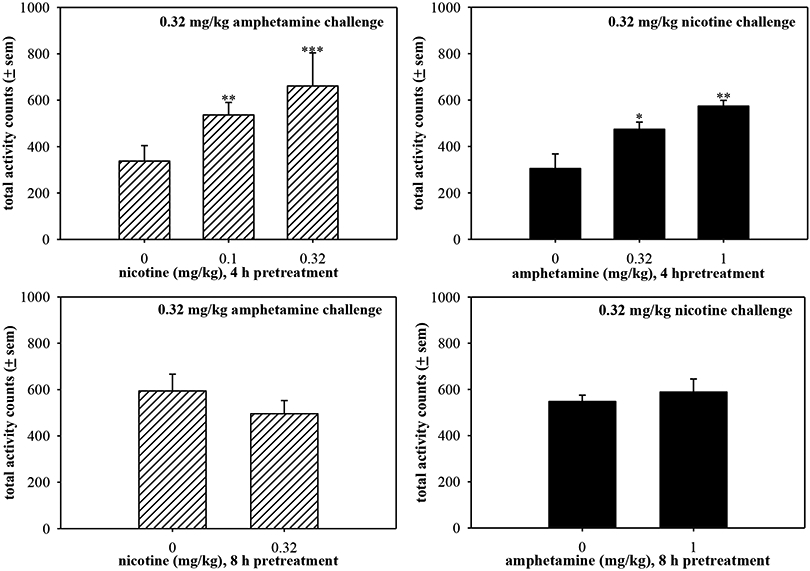
Activity counts stimulated by amphetamine challenge (0.32 mg/kg) following nicotine pretreatments (a) 4 h or (b) 8 h prior to challenge injection. Only one nicotine dose (0.32 mg/kg) was administered as a 8 h pretreatment to amphetamine challenge. Activity counts stimulated by nicotine challenge (0.32 mg/kg) following amphetamine pretreatments (c) 4 h or (d) 8 h prior to challenge injection. Only one amphetamine dose (0.32 mg/kg) was administered as an 8 h pretreatment to nicotine challenge.
Pretreatment with increasing doses of amphetamine 4 hours prior to nicotine dose-dependently enhanced nicotine-stimulated locomotor activity (F(2,15)=10.1, p=0.002) (Fig. 3c). The 8 h amphetamine pretreatment failed to alter locomotor activity stimulated by nicotine challenge (Fig. 3d). Similar to earlier observations, nicotine challenge following the 8 h pretreatment was larger than that produced following the 4 h pretreatment, further demonstrating that this effect is likely due to the time of day the challenge injection occurred. It is unlikely that the lack of effect observed with an 8 h pretreatment is due to a ceiling response because later data (Fig. 6) showed that 2 h nicotine pretreatment augmented the high activity levels stimulated by methamphetamine.
Figure 6.
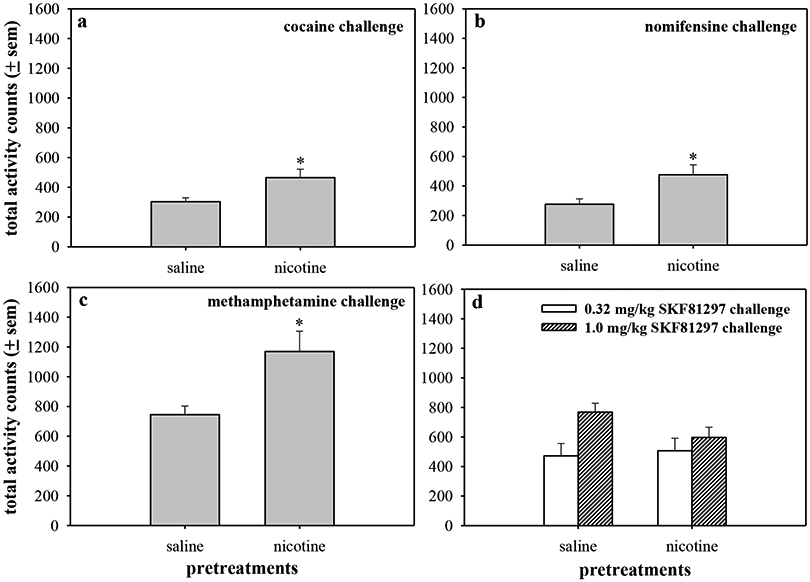
The effects of nicotine pretreatment on locomotor activity induced by other psychomotor stimulants: (a) cocaine, (b) nomifensine, (c) methamphetamine, or (d) the direct dopamine D1 agonist SKF81297. Saline or 0.32 mg/kg nicotine (i.p.) was administered 2 h prior to 3.2 mg/kg cocaine, 1.0 mg/kg nomifensine, 0.56 mg/kg methamphetamine, 0.32 mg/kg SKF81297, or 1.0 mg/kg SKF81297. For comparison, saline alone stimulates approximately 200 counts of activity. Data are expressed as total locomotor activity counts for 90 min following the challenge injection ± sem. * p<0.05 as compared with saline pretreatment.
Alterations in striatal dopamine overflow were examined in response to the nicotine and amphetamine to determine if the neurochemical changes were reflective of the potentiated behaviors. The effects of in vivo 4 h nicotine and amphetamine pretreatments were evaluated on challenge-induced dopamine overflow in rat striatal slices. In rats pretreated with 0.32 mg/kg nicotine, 10 μM amphetamine stimulated greater dopamine overflow as compared with saline-pretreated rats (Fig. 4a; pretreatment x fraction interaction, F(14,120)=2.26, p=0.009) with significant increases in dopamine levels at fractions 7 (p<0.001), 8 (p<0.05), and 9 (p<0.05). In order to determine if this nicotine-amphetamine interaction was occurring directly at the level of the striatum, the pretreatment-challenge paradigm was recapitulated in the striatal tissue from a naïve rat. In vitro nicotine pretreatment, as compared to buffer, produced a robust increase in amphetamine-stimulated dopamine overflow in striatal slices from naïve rats (pretreatment x fraction interaction, F(14,104)=1.92, p=0.03) with significant increases at fractions 6 (p<0.05), 7 (p<0.05), 8 (p<0.0001), and 9 (p<0.05) (Fig. 4b).
Figure 4.
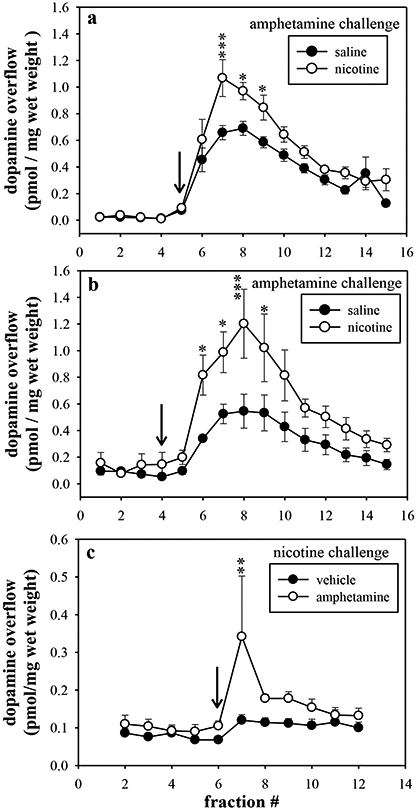
(a) The effects of in vivo saline (●) or nicotine (○) pretreatment on amphetamine-stimulated dopamine overflow from striatal slices. Rats were injected with saline or 0.32 mg/kg nicotine (i.p.) 4 h prior to sacrifice. Striatal slices were collected and washed for 75 min, then exposed to 10 μM amphetamine for 5 min at fraction 5 to stimulate dopamine release. (b) The effects of in vitro saline (●) or nicotine (○) pretreatment on amphetamine-stimulated dopamine overflow from striatal slices. Striatal slice from naïve rats were collected and washed for 60 min. Slices were exposed to 10 μM nicotine for 15 min prior to the start of fraction collection. Dopamine overflow was stimulated by 10 μM amphetamine. Dopamine overflow is reported as pmol/mg wet weight of striatum. (c) The effects of in vivo saline (●) or amphetamine (○) pretreatment on nicotine-stimulated dopamine overflow from rat striatal slices. Rats were injected with saline or 0.32 mg/kg amphetamine (i.p.) 4 h prior to sacrifice. Striatal slices were collected and washed for 75 min and then exposed to 30 μM nicotine for 5 min at fraction 6 only. In all graphs, the arrow indicates when the challenge was administered. * p<0.05, ** p<0.01, *** p<0.001 as determined by Bonferroni’s post hoc test.
In vivo amphetamine pretreatment similarly enhanced nicotine-stimulated dopamine overflow in rat striatal slices (Fig. 4c). In rats pretreated (4 h) with 0.32 mg/kg amphetamine, 30 μM nicotine stimulated greater overflow of endogenous dopamine in rats pretreated with amphetamine as compared with saline-pretreated rats (treatment main effect: F(1,85)=9.63, p=0.003) with a significant increase in dopamine at fraction 7 (p<0.01).
In behavioral studies, the 4 h nicotine pretreatment followed by amphetamine challenge was further analyzed to determine whether the effects of nicotine pretreatment were mediated through nicotinic receptors (Fig. 5). In rats that received a saline injection prior to the nicotine pretreatment, amphetamine challenge stimulated more locomotor activity as compared with saline-pretreated rats. Mecamylamine (1.0 mg/kg) administered prior to nicotine pretreatment reduced the ability of nicotine to potentiate the amphetamine response (Fig. 5a). This was additionally reflected in terms of total activity counts (F(3,28)=9.0, p=0.0003) (Fig. 5b). Nicotine pretreatment significantly increased the total activity counts stimulated by amphetamine challenge (p<0.01), and this enhancement was blocked by mecamylamine administration (p<0.01). Furthermore, mecamylamine alone had no effect on the amphetamine challenge administered 5 h later (p>0.05).
Figure 5.
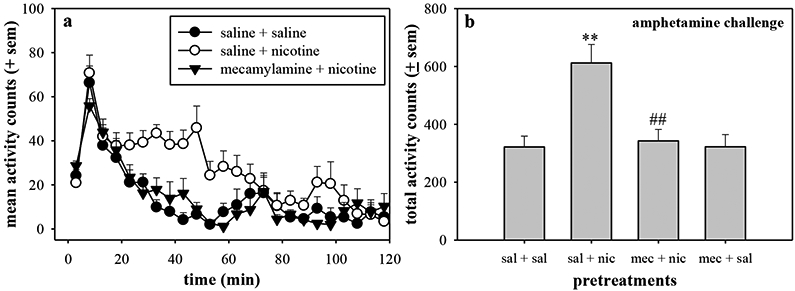
The nonselective nicotinic receptor antagonist mecamylamine was administered before nicotine pretreatment in order to determine of the nicotinic receptor mediated the nicotine-induced enhancement of amphetamine. Rats were injected with saline (●, ○) or mecamylamine (▾) 1 h prior to the pretreatment (saline (●) or 0.32 mg/kg nicotine (○)), and the amphetamine challenge (0.32 mg/kg) was administered 4 h later. These data show the data collected following the amphetamine challenge in terms of (a) time course or (b) total activity counts for 90 min following challenge injection. ** p<0.01 as compared with sal + sal pretreatment, ## p<0.01 as compared with sal + nic pretreatment.
Nicotine pretreatments were tested with different stimulant compounds to consider the diversity of this response and to better understand the site of interaction. Nicotine (0.32 mg/kg) was administered as a 2 h pretreatment to 3.2 mg/kg cocaine (Fig. 6a), 1.0 mg/kg nomifensine (Fig. 6b), 0.56 mg/kg methamphetamine, 0.32 mg/kg SKF81297 (Fig. 6d), or 1.0 mg/kg SKF81297 (Fig. 6d). Nicotine pretreatment significantly enhanced cocaine-, nomifensine-, and methamphetamine-stimulated activity (t=2.62, p=0.03; t=2.64, p=0.03; t=2.9, p=0.02, respectively). Cocaine, nomifensine, and methamphetamine all act at presynaptic transporters; however, stimulants can also increase locomotion by enhancing dopamine activation of post-synaptic dopamine receptors. Therefore, nicotine was administered as a pretreatment to a low and high challenge dose of the direct dopamine D1 receptor agonist SKF81297. Nicotine pretreatment did not alter activity levels stimulated by the low dose (0.32 mg/kg, t=0.29, p=0.78) or the high challenge dose (1 mg/kg) of SKF81297. In fact, nicotine pretreatment produced a trend to decrease SKF81297-stimulated activity at the high challenge dose (t=1.87, p=0.09).
Discussion
Nicotine and other drugs of abuse are used often in close temporal proximity to each other and have been reported to enhance each others’ effects. These experiments investigated sequential administration of nicotine and amphetamine in order to explore these acute interactions in terms of behavioral and neurochemical changes. This study reported the novel observation that an acute dose of nicotine, acting through the nicotinic cholinergic receptor, augmented the locomotor activity of psychostimulants, but not of direct-acting dopamine agonist. Moreover, these findings revealed that a temporal separation of stimulant administration enhanced this potentiated effect. Although co-administration of nicotine and amphetamine produced additive increases in activity levels, sequential administration of nicotine and amphetamine injections resulted in highly potentiated locomotor responses. These interactions were apparent when the drugs were spaced 2 to 4 hours apart, but not 8 h, suggesting that the altered synaptic effects may not involve protein synthesis and are a reversible, transient response. Notably, potentiated interactions were detected at low doses of the stimulants. As little as 0.1 mg/kg nicotine bitartrate, equivalent to 1-3 cigarettes (Matta et al. 2007), was able to enhance amphetamine-stimulated locomotor activity. Similarly, the dose of d-amphetamine used was within the range of therapeutic doses used for narcolepsy and attention deficit disorder (Ahmann et al. 2001). Although the nicotine pretreatment ‘primed’ the striatum through activation of nicotinic receptors, it is not known which downstream effectors might play a role in the enhanced amphetamine challenge response. Amphetamine has been shown to increase catecholamine release via nicotine receptor activation in bovine chromaffin cells, but this occurred at high μM concentrations of amphetamine which would not be achieved by our in vivo doses (Liu et al. 2003). Future studies will examine the molecular mechanism of these potentiated interactions.
Interestingly, pretreatment with amphetamine two hours prior potentiated locomotor behavior induced by a subsequent challenge of amphetamine. A single dose of amphetamine was reported to induce an enhanced (sensitized) behavioral response to amphetamine 24 hours later (Warburton et al. 1996) and 1-3 wks later (Vanderschuren et al. 1999). Our results demonstrate that a sensitized response to a low dose of amphetamine (0.32 mg/kg) could be induced within hours. On the other hand, pretreatment with nicotine two hours prior did not result in an enhanced response to a challenge dose of nicotine, possibly because nicotine did not elicit as strong a behavioral activation as amphetamine, or perhaps because nicotine receptors were desensitized by the nicotine pretreatment (Giniatullin et al. 2005). Although these experiments were performed using female rats, the ‘priming’ activity of nicotine and amphetamine is also demonstrable in male rats (Jutkiewicz and Gnegy, preliminary results).
These data suggest that acute administration of nicotine or amphetamine amplify the other stimulant’s effects even after the primary action of the pretreatment is diminished and the drug is no longer at peak concentration in the brain. Both nicotine (Ghosheh et al. 1999) and amphetamine (Fuller et al. 1977; Kuhn and Schanberg, 1978) have short half-lives in the rat (52 min and ~60 min, respectively) following peripheral administration. In addition, it is unlikely that nicotine pretreatment inhibited the metabolism of amphetamine because these drugs interact with different metabolizing enzymes (von Weymarn et al. 2006; Law and Moody, 1994; Law et al. 2000); however, this has not been conclusively demonstrated. A long-lasting nicotine metabolite might be involved in the enhancement of amphetamine-stimulated activity; for example, nornicotine and cotinine had longer half-lives in rats (Ghosheh et al. 1999). Nornicotine was shown to be an agonist at neuronal nicotine receptors and to inhibit dopamine clearance in rat striatum, but the effective dose, 8 mg/kg, was significantly higher than the dose used in our study (Middleton et al. 2007b). On the other hand, nornicotine and cotinine directly increased [3H]dopamine overflow (Teng et al. 1997). Although the half lives of these metabolites were longer than that of nicotine, the concentrations of these metabolites achieved in the brain in response to the doses of nicotine used in this study would not have significant effects on dopamine overflow (Ghosheh et al. 1999; Teng et al. 1997; Dwoskin et al. 1999).
Our data demonstrated that pretreatment-induced potentiation of amphetamine and nicotine challenge responses corresponded with changes in dopaminergic systems to enhance dopamine release. Previous studies demonstrated that co-administration of 0.1 mg/kg nicotine free base with cocaine produced additive effects on dopamine overflow in microdialysis studies in ventral striatum (Zernig et al. 1997). Similar to our results, pretreatment of a rat with 2 mg/kg nomifensine i.p. 30 min before 0.5 mg/kg nicotine free base s.c. enhanced dopamine release in microdialysis in the dorsal striatum (Janhunen et al. 2005). Our results further demonstrated that nicotine pretreatment directly altered function in dopamine terminal areas because in vitro nicotine perfusion in striatal tissue enhanced subsequent amphetamine-stimulated dopamine overflow. Drew et al. (2000), however, did not detect a potentiative effect of nicotine on amphetamine-stimulated [3H]dopamine efflux in rat striatum. There were significant differences in our protocols. Drew et al. (2000) added the nicotine directly with a second stimulus of amphetamine in a S1/S2 paradigm whereas we washed the slices for 20 min in between nicotine and amphetamine. Secondly, we measured endogenous DA and had calcium in our Ringers buffer, and heightened responses to amphetamine are shown to involve calcium (Pierce and Kalivas, 1997; Kantor et al. 1999).
Nicotine has been reported to alter dopamine transporter function in the striatum. Nicotine increased dopamine clearance in the striatum (Hart and Ksir, 1996; Middleton 2004, 2007a), and Middleton (2007a) further reported that nicotine increased the Vmax of rat striatal dopamine uptake in a manner independent of transporter trafficking. This effect on transporter function was transient, in that it lasted less than one hour. It is difficult to reconcile this finding with the present data because decreased synaptic dopamine would not be expected to exaggerate amphetamine response, unless it made more dopamine available for amphetamine-stimulated release or assisted the transport of amphetamine into the terminal. In addition, the potentiated interaction observed in the present data lasted beyond one hour. In contrast to previous reports, Izenwasser (1991) reported that 1 nM nicotine indirectly inhibited dopamine uptake in the rat striatal slices. This effect might contribute to the in vitro synergistic interaction; however, the prolonged interactions in vivo observed in the current finding are unlikely to be explained by nicotine itself directly altering dopamine uptake, considering the half-life of nicotine.
Instead of alterations in dopamine terminals, nicotine pretreatment might have altered some aspect of the neural circuitry involved in amphetamine-stimulated activity to produce an exaggerated amphetamine effect. The neural circuitry could be directly in the striatum, as suggested by our in vitro experiments, or could more widely involve the dopamine cell (Dani and Harris, 2001). Acute nicotine administration can produce changes in the mesolimbic dopamine pathway that last for hours and increase the probability for synaptic plasticity (Fung and Lau, 1986; Pidoplichko et al. 1997; Ferrari et al. 2001). However, based on the present in vitro studies, these data suggest that the neural activity contributing to the potentiative actions of the stimulants is likely to reside within the striatum. A more likely scenario is that amphetamine and nicotine are eliciting a form of presynaptic facilitation, perhaps by altering calcium. Both nicotinic receptor activation (Fucile 2004) and amphetamine (Gnegy et al. 2004) increase intracellular Ca2+, which could affect the sensitivity of dopamine release pathways. Further studies will be needed to elucidate the mechanism of nicotine pretreatment-induced enhancement of amphetamine responses. The fact that, in the present study, nicotine pretreatment augmented locomotor activity stimulated by cocaine, nomifensine, and methamphetamine, but not the direct dopamine D1 receptor agonist SKF81297, further suggests that alterations of dopamine transporters or dopamine terminals are crucial for the potentiated interaction.
In summary, this study demonstrated that low, clinically-relevant doses of nicotine or amphetamine produced transitory changes in dopamine-related behavioral and neurochemical effects. Overall, these data suggest that small doses of nicotine may interact with stimulants, such as amphetamine, even within a couple of hours of use, to produce exaggerated drug responses. Thus, drugs of abuse might induce transitory alterations in psychomotor and reward systems that persist longer than the drug action itself. These effects could contribute to the frequent co-use of nicotine and other drugs of abuse in humans. These transient processes could also play a role in the development of drug dependence and long-term behavioral sensitization.
Acknowledgements
The authors declare that, except for income received from my primary employer, no financial support or compensation has been received from any individual or corporate entity over the past three years for research or professional service, and there are no personal financial holdings that could be perceived as constituting a potential conflict of interest. The authors also have full control access to all data examined in the present manuscript.
Funding Acknowledgement: These studies were supported by the MICHR Pilot and Collaborative Grant Program and the Unites States Public Health Service Grant T32 DA007268.
References
- Ahmann PA, Theye FW, Berg R, Linquist AJ, Van Erem AJ, Campbell LR (2001) Placebo-controlled evaluation of amphetamine mixture-dextroamphetamine salts and amphetamine salts (Adderall): efficacy rate and side effects. Pediatrics 107:E10. [DOI] [PubMed] [Google Scholar]
- Barrett SP, Darredeau C, Pihl RO (2006) Patterns of simultaneous polysubstance use in drug using university students. Hum Psychopharmacol Clin Exp 21:255–263. [DOI] [PubMed] [Google Scholar]
- Bechtholt AJ, Mark GP (2002) Enhancement of cocaine-seeking behavior by repeated nicotine exposure in rats. Psychopharmacology 162:178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell CE, Balfour DJK (1998) The influence of nicotine pretreatment on mesoaccumbens dopamine overflow and locomotor responses to d-amphetamine. Psychopharmacology 140:142–149. [DOI] [PubMed] [Google Scholar]
- Budney AJ, Higgins ST, Hughes JR, Bickel WK (1993) Nicotine and caffeine use in cocaine-dependent individuals. J Subst Abuse 5:117–130. [DOI] [PubMed] [Google Scholar]
- Burling TA, Salvio MA, Seidner AL, Ramsey TG (1996) Cigarette smoking in alcohol and cocaine abusers. J Subst Abuse 8:445–452. [DOI] [PubMed] [Google Scholar]
- Celik E, Uzbay IT, Karakas S (2006) Caffeine and amphetamine produce cross-sensitization to nicotine-induced locomotor activity in mice. Prog Neuro-Psychopharmacol Biol Psychiatry 30:50–55. [DOI] [PubMed] [Google Scholar]
- Collins SL, Izenwasser S (2004) Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacology 46:349–362. [DOI] [PubMed] [Google Scholar]
- Collins SL, Montano R, Izenwasser S (2004) Nicotine treatment produces persistent increases in amphetamine-stimulated locomotor activity in periadolescent male but not female or adult male rats. Dev Brain Res 153:175–187. [DOI] [PubMed] [Google Scholar]
- Cousins MS, Stamat HM, de Wit H (2001) Acute doses of d-amphetamine and bupropion increase cigarette smoking. Psychopharmacology 157:243–253. [DOI] [PubMed] [Google Scholar]
- Dani JA, Harris RA (2005) Nicotine addiction and comorbidity with alcohol abuse and mental illness. Nat Neurosci 8:1465–1470. [DOI] [PubMed] [Google Scholar]
- Drew AE, Derbez AE, Werling LL (2000) Nicotinic receptor-mediated regulation of dopamine transporter activity in rat prefrontal cortex. Synapse 38:10–16. [DOI] [PubMed] [Google Scholar]
- Drew AE, Werling LL (2003) Nicotinic receptor-mediated regulation of the dopamine transporter in rat prefrontocortical slices following chronic in vivo administration of nicotine. Schizophrenia Res 65:47–55. [DOI] [PubMed] [Google Scholar]
- Dwoskin LP, Teng L, Buxton ST, Crooks PA (1999) (S)-(-)-Cotinine, the major metabolite of nicotine, stimulates nicotinic receptors to evoke [3H]dopamine release from rat striatal slices in a calcium-dependent manner. J Pharmacol Exp Ther 288:905–911. [PubMed] [Google Scholar]
- Ferrari R, Le Novère N, Picciotto MR, Changeux JP, Zoli M (2001) Acute and long-term changes in the mesolimbic dopamine pathway after systemic or local single nicotine injections. Eur J Neurosci 15:1810–1818. [DOI] [PubMed] [Google Scholar]
- Fucile S (2004) Ca2+ permeability of nicotinic acetylcholine receptors. Cell Calcium 35:1–8. [DOI] [PubMed] [Google Scholar]
- Fuller RW, Baker JC, Molly BB (1977) Biological disposition of rigid analogs of amphetamine. J Pharm Sci 66:271–272. [DOI] [PubMed] [Google Scholar]
- Fung YK, Lau YS (1986) Acute effect of nicotine on the striatal dopaminergic system in the rat. J Pharm Pharmacol 38:920–922. [DOI] [PubMed] [Google Scholar]
- Ghosheh O, Dwoskin LP, Li WK, Crooks PA (1999) Residence times and half-lives of nicotine metabolites in rat brain after acute peripheral administration of [2’-14C]nicotine. Drug Metab Dispos 27:1448–1455. [PubMed] [Google Scholar]
- Giniatullin R, Nistri A, Yakel JL (2005) Desensitization of nicotinic ACh receptors: shaping cholinergic signaling. Trends Neurosci 28:371–378. [DOI] [PubMed] [Google Scholar]
- Gnegy ME, Khoshbouei H, Berg KA, Javitch JA, Clarke WP, Zhang M, Galli A (2004). Intracellular Ca2+ regulates amphetamine-induced dopamine efflux and currents mediated by the human dopamine transporter. Mol Pharmacol 66:137–143. [DOI] [PubMed] [Google Scholar]
- Hart C, Ksir C (1996) Nicotine effects on dopamine clearance in rat nucleus accumbens. J Neurochem 66:216–221. [DOI] [PubMed] [Google Scholar]
- Heffner TG, Hartman JA, Seiden LS (1980) A rapid method for the regional dissection of the rat brain. Pharmacol Biochem Behav 13:452–456. [DOI] [PubMed] [Google Scholar]
- Henningfield JE, Griffiths RR (1981) Cigarette smoking and subjective responses: effects of d-amphetamine. Clin Pharmacol Ther 30:497–505. [DOI] [PubMed] [Google Scholar]
- Horger BA, Giles MK, Schenk S (1992) Preexposure to amphetamine and nicotine predisposes rats to self-administer a low dose of cocaine. Psychopharmacology 107:271–276. [DOI] [PubMed] [Google Scholar]
- Izenwasser S, Jacocks HM, Rosenberger JG, Cox BM (1991) Nicotine indirectly inhibits [3H]dopamine uptake at concentrations that do not promote [3H]dopamine release. J Neurochem 56:603–610. [DOI] [PubMed] [Google Scholar]
- Janhunen S, Mielikäinen P, Paldänius P, Tuominen RK, Ahtee L, Kaakkola (2005) The effect of nicotine in combination with various dopaminergic drugs on nigrostriatal dopamine in rats. Naunyn-Schmiedeberg’s Arch Pharmacol 371:480–491. [DOI] [PubMed] [Google Scholar]
- Kantor L, Hewlett GHK, Gnegy ME (1999) Enhanced amphetamine- and K+-mediated dopamine release in rat striatum after repeated amphetamine: differential requirements for Ca2+- and calmodulin-dependent phosphorylation and synaptic vesicles. J Neurosci 19:3801–3808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuhn CM, Schanberg SM (1978) Metabolism of amphetamine after acute and chronic administration to the rat. J Pharmacol Expt Ther 207:544–554. [PubMed] [Google Scholar]
- Kuo DY, Liu CN, Tsay JC, Chang CL, Cheng JT (1998) Effects of nicotine on spontaneous and amphetamine-induced motor behaviors: the differences between nicotine tolerant and nontolerant rats. Chin J Physiology 41:93–99. [PubMed] [Google Scholar]
- Law MY, Moody DE (1994) Urinary excretion of amphetamine and 4’-hydroxyamphetamine by Sprague Dawley and dark Agouti rats. Life Sci 54:1073–1079. [DOI] [PubMed] [Google Scholar]
- Law MY, Slawson MH, Moody DE (2000) Selective involvement of cytochrome P450 2D subfamily in in vivo 4-hydroxylation of amphetamine in rat. Drug Metab Dispos 28:348–353. [PubMed] [Google Scholar]
- Liu PS, Liaw CT, Lin MK, Shin SH, Kao LS, Lin LF (2003). Amphetamine enhances Ca2+ entry and catecholamine release via nicotinic receptor activation in bovine adrenal chromaffin cells. Eur J Pharmacol 460:9–17. [DOI] [PubMed] [Google Scholar]
- Martínez-Ortega JM, Jurado D, Martinez-González MA, Gurpegui M (2006) Nicotine dependence, use of illegal drugs and psychiatric morbidity. Addict Behav 31:1722–1729. [DOI] [PubMed] [Google Scholar]
- Matta SG, Balfour DJ, Benowitz NL, Boyd RT, Buccafusco JJ, Caggiula AR, Craig CR, Collins AC, Damaj MI, Donny EC, Gardiner PS, Grady SR, Heberlein U, Leonard SS, Levin ED, Lukas RJ, Markou A, Marks MJ, McCallum SE, Parameswaran N, Perkins KA, Picciotto MR, Quik M, Rose JE, Rothenfluh A, Schafer WR, Stolerman IP, Tyndale RF, Wehner JM, Zirger JM (2007) Guidelines on nicotine dose selection for in vivo research. Psychopharmacology 190:269–319. [DOI] [PubMed] [Google Scholar]
- McQuown SC, Belluzzi JD, Leslie FM (2007) Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotox Teratology 29:66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton LS, Cass WA, Dwoskin LP (2004) Nicotinic receptor modulation of dopamine transporter function in rat striatum and medial prefrontal cortex. J Pharmacol Exp Ther 308:367–377. [DOI] [PubMed] [Google Scholar]
- Middleton LS, Apparsundaram S, King-Pospisil KA, Dwoskin LP (2007a) Nicotine increases dopamine transporter function in rat striatum through a trafficking-independent mechanism. Eur J Pharmacol 554:128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton LS, Crooks PA, Wedlund PJ, Cass WA, Dwoskin LP (2007b) Nornicotine inhibition of dopamine transporter function in striatum via nicotinic receptor activation. Synapse 61:157–165. [DOI] [PubMed] [Google Scholar]
- Pidoplichko VI, DeBiasi M, Williams JT, Dani JA (1997) Nicotine activates and desensitizes midbrain dopamine neurons. Nature 390:401–404. [DOI] [PubMed] [Google Scholar]
- Pierce RC, Kalivas PW (1997) Repeated cocaine modifies the mechanism by which amphetamine releases dopamine. J Neurosci 17:3254–3261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauhut AS, Neugebauer N, Dwoskin LP, Bardo MT (2003) Effect of bupropion on nicotine self-administration in rats. Psychopharmacology 169:1–9. [DOI] [PubMed] [Google Scholar]
- Reid MS, Mickalian JD, Delucchi KL, Hall SM, Berger SP (1998) An acute dose of nicotine enhances cue-induced cocaine craving. Drug Alcohol Dep 49:95–104. [DOI] [PubMed] [Google Scholar]
- Roll JM, Higgins ST, Tidey J (1997) Cocaine use can increase cigarette smoking: evidence from laboratory and naturalistic settings. Exp Clin Psychopharmacol 5:263–268. [DOI] [PubMed] [Google Scholar]
- Rush CR, Higgins ST, Vansickel AR, Stoops WW, Lile JA, Glaser PEA (2005) Methylphenidate increases cigarette smoking. Psychopharmacology 181:781–789. [DOI] [PubMed] [Google Scholar]
- Schoffelmeer ANM, De Vries TJ, Wardeh G, van de Ven HWM, Vanderschuren LJMJ (2002) Psychostimulant-induced behavioral sensitization depends on nicotinic receptor activation. J Neurosci 22:3269–3276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuster CR, Lucchesi BR, Emley GS (1979) The effects of d-amphetamine, meprobamate, and lobeline on the cigarette smoking behavior of normal human subjects. NIDA Res Monogr 23:91–99. [PubMed] [Google Scholar]
- Sigmon SC, Tidey JW, Badger GJ, Higgins ST (2003) Acute effects of d-amphetamine on progressive-ratio performance maintained by cigarette smoking and money. Psychopharmacology 167:393–402. [DOI] [PubMed] [Google Scholar]
- Suemaru K, Gomita Y, Furuno K, Araki Y (1993) Chronic nicotine treatment potentiates behavioral responses to dopaminergic drugs in rats. Pharmacol Biochem Behav 46:135–139. [DOI] [PubMed] [Google Scholar]
- Teng L, Crooks PA, Buxton ST, Dwoskin LP (1997) Nicotinic- receptor mediation of S(-)Nornicotine-evoked [3H]overflow from rat striatal slices preloaded with [3H]dopamine. J Pharmacol Exp Ther 283:778–787. [PubMed] [Google Scholar]
- Tidey JW, O’Niell SC, Higgins ST (2000) d-Amphetamine increases choice of cigarette smoking over monetary reinforcement. Psychopharmacology 153:85–92. [DOI] [PubMed] [Google Scholar]
- Vanderschuren LJMJ, Schmidt ED, De Vries TJ, Van Moorsel CAP, Tilders FJH, Schoffelmeer ANM (1999) A single exposure to amphetamine is sufficient to induce long-term behavioral, neuroendocrine, and neurochemical sensitization in rats. J Neuroscience 19:9579–9586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vansickel AR, Stoops WW, Glaser PEA, Rush CR (2007) A pharmacological analysis of stimulant-induced increases in smoking. Psychopharmacology 193:305–313. [DOI] [PubMed] [Google Scholar]
- Von Weymarn LB, Brown KM, Murphy SE (2006) Inactivation of CYP2A6 and CYP2A13 during nicotine metabolism. J Pharmacol Exp Ther 316:295–303. [DOI] [PubMed] [Google Scholar]
- Warburton EC, Mitchell SN, Joseph MH (1996) Calcium dependence of sensitized dopamine release in rat nucleus accumbens following amphetamine challenge: implications for the disruption of latent inhibition. Behav Pharmacol 7:119–129. [PubMed] [Google Scholar]
- Wooters TE, Neugebauer NM, Rush CR, Bardo MT (2007) Methylphenidate enhances the abuse-related behavioral effects of nicotine in rats: intravenous self-administration, drug discrimination, and locomotor cross-sensitization. Neuropsychopharmacology E pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen CF, Chong MY (2006) Comorbid psychiatric disorders, sex, and methamphetamine use in adolescents: a case-control study. Comp Psychiatry 47:215–220. [DOI] [PubMed] [Google Scholar]
- Zernig G, O’Laughlin IA, Fibiger HC (1997) Nicotine and heroin augment cocaine-induced overflow in nucleus accumbens. Eur J Pharmacology 337:1–10. [DOI] [PubMed] [Google Scholar]


