Vaccination campaigns with the recently approved SARS-CoV-2 mRNA vaccines (BNT162b2 and mRNA-1273) have been recently launched worldwide to prevent COVID-19. Registration trials have been limited to SARS-CoV-2 naïve adults [1], [2], and both vaccine schedules recommend 2 doses 3 or 4 weeks apart, respectively. Nevertheless, most countries are invariably vaccinating convalescents and naïve subjects with the same schedule.
In the current manufacturing bottleneck, most health systems are suffering shortages of vaccines, so that usage optimization of the existing stockpiles has been hypothesized [3].
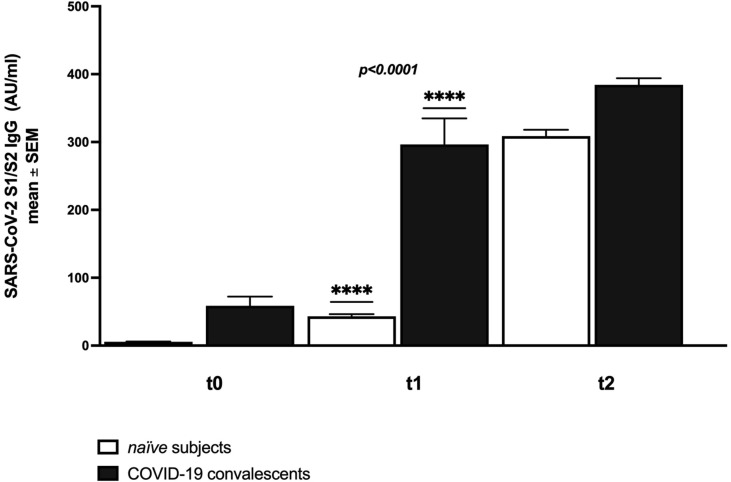
To investigate whether vaccine administration could be prioritized based on the serostatus, we compared the IgG responses 14 days after administration of the first and second dose of the BNT162b2 vaccine in 12 COVID-19 convalescents (defined as having had a previous nasopharyngeal swab positive for SARS-CoV-2 RNA, followed by a negative swab) and in 54 naïve subjects matched for age and sex (ethical protocol number 165/2020). Immune responses were measured using the Liaison® SARS-CoV-2 S1/S2 IgG chemiluminescent immunoassay the day of vaccination (t0), 14 days after the first dose (t1), and 14 days after the second dose (t2) (Fig. 1 ). Fig. 1 shows that COVID-19 convalescents are already positive for IgG when the vaccine is administered and that 14 days after the first dose they show higher absolute anti-S1/S2 IgG levels (mean ± standard error: 220 ± 42 vs. 37 ± 3 AU/ml; Mann-Whitney test, p < 0.0001) compared to naïve vaccinees. Moreover, IgG levels of COVID-19 convalescents 14 days after the first dose are very similar to that of naïve vaccinees after the second dose. At regression analysis, the increment in IgG levels correlated with past SARS-CoV-2 positivity (p < 0.0001) but not with the recipient’s age and gender.
Fig. 1.
Anti-S1/S2 IgG levels at the day of vaccination (t0), 14 days after the first dose (t1), and 14 days after the second dose (t2) of BNT162b2 vaccine in convalescent versus naïve vaccinees.
In this study we didn’t evaluate the quality of antibody response, but Liaison® SARS-CoV-2 S1/S2 IgG levels higher than 80 AU/ml have been previously shown to predict neutralizing antibody titers in 92% of cases [4]. Only 2% of naïve vaccinees, but 75% of COVID-19 convalescents showed IgG levels higher than 80 after the first dose of vaccine (mean ± standard error 355 ± 93).
Our findings suggest that a single dose of BNT162b2 could be enough for convalescents to achieve anti-S1/S2 IgG levels comparable to those achieved in naïve subjects after the regular 2-dose schedule. While defining the serostatus in candidate vaccinees would slow vaccine deployment, the convalescent status can be easily defined by consulting electronic health records. The saved doses could be retargeted to the waiting list, [5] contributing to reaching herd immunity faster.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. New Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Widge A.T., Rouphael N.G., Jackson L.A., et al. Durability of responses after SARS-CoV-2 mRNA-1273 vaccination. New Engl J Med. 2020;384(1):80–82. doi: 10.1056/NEJMc2032195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ayoub HH, Chemaitelly H, Makhoul M, et al. Epidemiological impact of prioritizing SARS-CoV-2 vaccination by antibody status: mathematical modeling analyses. 2021; 2021.01.10.21249382.
- 4.Bonelli F., Sarasini A., Zierold C., et al. Clinical and analytical performance of an automated serological test that identifies S1/S2-neutralizing IgG in COVID-19 patients semiquantitatively. J Clin Microbiol. 2020;58(9):e01224–e1320. doi: 10.1128/JCM.01224-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. SAGE roadmap for prioritizing uses of COVID19 vaccines in the context of limited supply. Version 1.1.; 2020.