Abstract
BACKGROUND:
Despite strong evidence linking fibroblast growth factor 2 (FGF2) with anxiety and depression in both rodents and humans, the molecular mechanisms linking FGF2 with anxiety are not understood.
METHODS:
We compare 1) mice that lack a functional Fgf2 gene (Fgf2 knockout [KO]), 2) wild-type mice, and 3) Fgf2 KO with adult rescue by FGF2 administration on measures of anxiety, depression, and motor behavior, and further investigate the mechanisms of this behavior by cellular, molecular, and neuroendocrine studies.
RESULTS:
We demonstrate that Fgf2 KO mice have increased anxiety, decreased hippocampal glucocorticoid receptor (GR) expression, and increased hypothalamic-pituitary-adrenal axis activity. FGF2 administration in adulthood was sufficient to rescue the entire phenotype. Blockade of GR in adult mice treated with FGF2 precluded the therapeutic effects of FGF2 on anxiety behavior, suggesting that GR is necessary for FGF2 to regulate anxiety behavior. The level of Egr-1/NGFI-A was decreased in Fgf2 KO mice and was reestablished with FGF2 treatment. By chromatin immunoprecipitation studies, we found decreased binding of EGR-1 to the GR promoter region in Fgf2 KO mice. Finally, we examined anxiety behavior in FGF receptor (FGFR) KO mice; however, FGFR1, FGFR2, and FGFR3 KO mice did not mimic the phenotype of Fgf2 KO mice, suggesting a role for other receptor subtypes (i.e., FGFR5).
CONCLUSIONS:
These data suggest that FGF2 levels are critically related to anxiety behavior and hypothalamic-pituitary-adrenal axis activity, likely through modulation of hippocampal glucocorticoid receptor expression, an effect that is likely receptor mediated, albeit not by FGFR1, FGFR2, and FGFR3.
Keywords: Anxiety, FGF receptors, FGF2, Glucocorticoids, Hippocampus, Mouse, Stress
An important role for the regulation of the hypothalamic-pituitary-adrenal (HPA) axis by the hippocampus has been proposed in several developmental rodent models of adult emotional dysregulation caused by early life stress and manipulations of maternal care. Yet, how the hippocampal circuitry responds to local and systemic perturbations by modulating the HPA axis activity is not understood. One crucial mediator that may link responses to early life perturbations, including brain injury and stressful events, with neuroendocrine control of emotional behavior is basic fibroblast growth factor 2 (FGF2). FGF2 is a potent growth factor that regulates stem cell maintenance and neurogenesis both during embryonic development and in response to challenges such as stress or injury in the adult brain (1–6). The hippocampus expresses the highest levels of FGF2 and its receptors in the brain. FGF2 modulates hippocampal neurogenesis, neurite outgrowth, and synapse formation, and as such it affects learning and memory, long-term potentiation, and the reaction to injury (7–9). Importantly, this growth factor has also been correlated to emotional dysregulation, both in humans affected by mood disorders and in rodent models of anxiety behavior (10–15). Increased hippocampal FGF2 levels are correlated with reduced manifestations of anxiety (11) and with positive response to treatment with the antidepressant fluoxetine (12,16). In spite of this large body of circumstantial evidence, the causal link and mechanism by which FGF2 may regulate emotional behavior is not understood. Here we perform behavioral, cellular, and molecular studies in mice lacking the Fgf2 gene, which manifest increased levels of anxiety, and rescue their phenotype with FGF2 administration in adulthood. We establish a causal link between the anxiolytic effects of FGF2 and glucocorticoid receptors (GR) and suggest that FGF2 is required in adulthood for normal neuroendocrine HPA axis activity and emotional behavior. Finally, using mice lacking FGF receptors (FGFRs), we found that FGFR1, FGFR2, and FGFR3 did not mediate the effect of FGF2 on anxiety behavior, but paradoxically, Fgfr1 and Fgfr2 knockout (KO) mice showed decreased anxiety behavior.
METHODS AND MATERIALS
Mice
We used the following lines: Fgf2 exon 1 KO mice (Fgf2 KO) (3,4,17); conditional embryonic hGFAP-cre Fgfr2 KO (18); germline Fgfr3 KO (19); and embryonic Fgfr1/Fgfr2 conditional KO driven by Emx1-cre (20). For additional information related to selection of mice, number of mice cohort tested in independent experiments, and other details, see Supplemental Methods and Materials.
Drug Treatments
FGF2 Administration.
FGF2 ligand (10 ng/g) (R&D Systems, Minneapolis, MN) dissolved in 1 mol/L phosphate buffered saline with 0.1% bovine serum albumin or vehicle control was administered by daily subcutaneous injections. This dose of FGF2 was previously established to induce recovery from injury and neurogenesis with no documented side effects (21,22).
RU486 Administration.
RU486 (25 mg/g) (mifepristone; Sigma-Aldrich, St. Loius, MO Sigma) was dissolved in sunflower oil and administered subcutaneously 40 to 45 minutes before behavioral testing.
Behavioral Analysis
All behavioral tests took place under direct bright lights (about 300 lux) with the exception of the sucrose consumption test, which took place under normal, home cage conditions. Behavior took place at the same time during the day cycle, with control and experimental mice tested together each time, rotating between groups to counterbalance for order effects. The light/dark cycle was set at 7:00 AM/7:00 PM. For details on locomotor/open field, elevated plus maze, sucrose consumption test, forced swim test, and accelerating rotarod test, see Supplemental Methods and Materials.
Immunostaining, Cell Counting, and Microscopic Analysis
Unbiased stereological estimates of cell number were obtained via a Zeiss Axioskope 2 Mot Plus attached to a motorized stage and connected to a computer running the Stereo Investigator Software (MicroBrightfield, Colchester, VT). Images presented in figures were acquired on an ApoTome equipped Axiovert 200M with Axiovision 4.5 software (Zeiss, Oberkochen, Germany). For details on tissue processing, immunostaining, and stereological cell counting procedures, see Supplemental Methods and Materials.
Chromatin Immunoprecipitation
Chromatin immunoprecipitation (ChIP) was conducted with 10 μg/IP of Egr-1 antibody (SC-189) using the MAGnify ChIP system from Life Technologies (Carlsbad, CA) as per the instruction manual, with the following modifications: perfused tissue was used and thus the fixation step was omitted; primary antibody was incubated overnight at 4°C; and an increased efficiency protocol was used for de-cross linking: samples were incubated in ChIP de-cross-linking buffer at 65°C overnight and then 0.5 mol/L ethylenediaminetetraacetic acid, 1 mol/L Tris-HCl, and Proteinase K were added and incubated for 2 hours at 45°C.
Statistical Analyses
All data were analyzed using StatView 4.51 software (Abacus Concepts, Calabasas, CA). Analysis of variance was employed with genotype and time or treatment as independent variables, wherever appropriate. Probability values were considered significant when p ≤ .05. Fisher’s least significant difference post hoc tests were used with a Bonferroni correction for nonorthogonal comparisons.
RESULTS
Assessment of the FGF2 Knockout Phenotype
Behavioral Measures.
Results from the elevated plus maze in male wild-type and Fgf2 KO mice showed that knockout mice had a remarkably higher latency to enter the open arm (F = 80.013, p < .0001) and spent significantly less time on the open arms than their wild-type littermates (F = 14.659, p < .001) (Figure 1A). No significant differences were found between genotypes in tests associated with depressive behavior: the sucrose consumption test (Figure 1B) (F = 0.144, p > .05) and the forced swim test (F = 1.269, p > .05) (Figure 1C). Finally, no differences were observed on the rotarod test (Figure 1D), suggesting that Fgf2 KO mice do not show deficits in motor abilities and motor learning.
Figure 1.
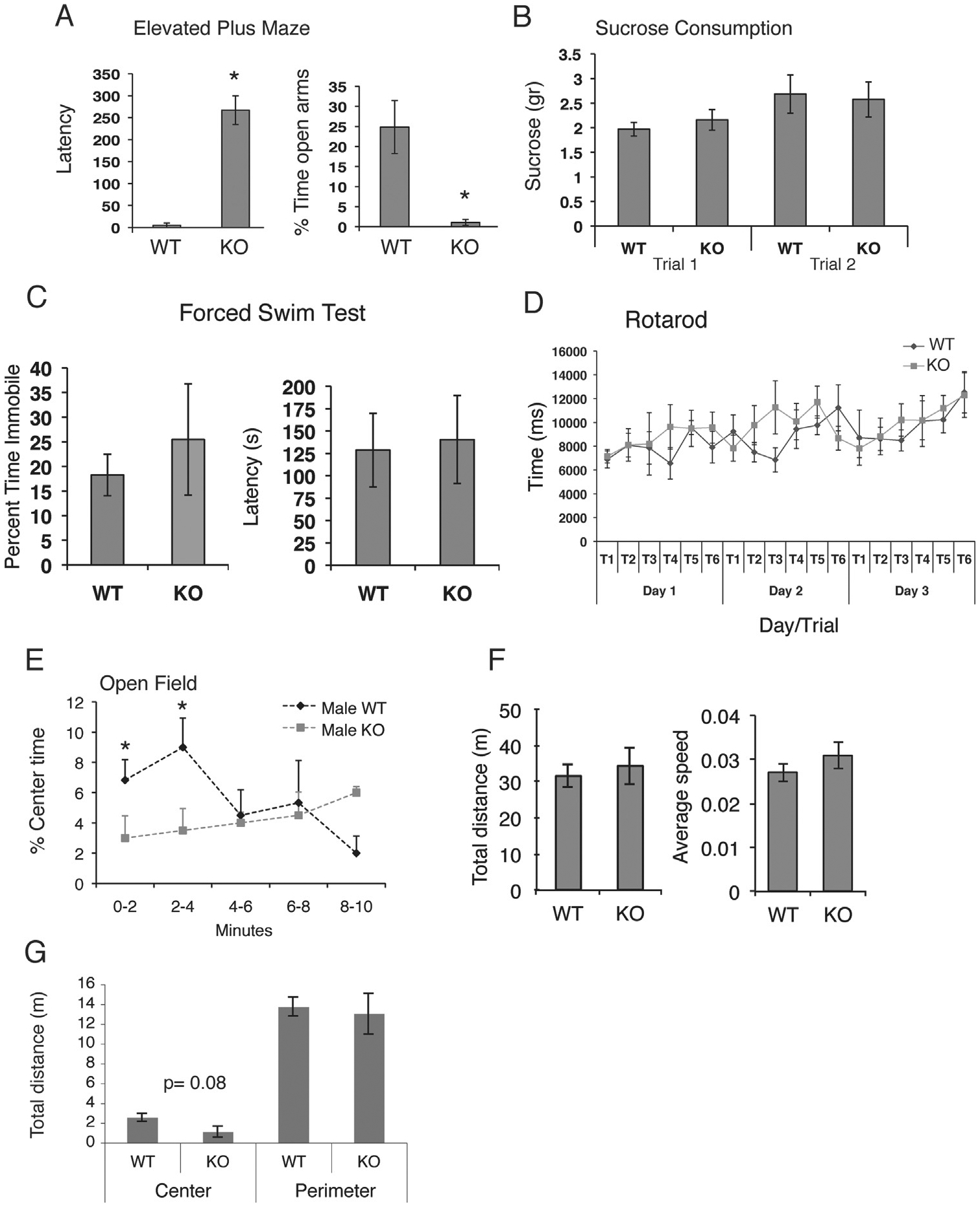
Increased anxiety in fibroblast growth factor 2 (Fgf2) knockout (KO) mice as compared to wild-type (WT) mice. Latency to enter the open arm and the percent time spent on the open arms of the elevated plus maze (A). KO male mice took longer to enter the open arms and spent significantly less time in the open arms. No significant differences were observed on the sucrose consumption test at either an early time point, trial (T) 1, or in trial 2, a time point at the end of all behavioral treatments (B). No significant group differences were found on the forced swim test on percent time immobile and the latency to immobility (C), and finally, there were no significant differences on the accelerating rotarod test (D). (E–G) Performance of WT and Fgf2 KO mice on the locomotor test. KO male mice spent significantly less time in the center of the open field during the first 4 minutes of the task (E), suggesting increased anxiety. No significant differences, however, were observed between WT and KO male mice on total distance and speed (F) or on total distance in the perimeter region over the entire duration of the test (20 minutes) (G). There was a trend (p = .08) for decreased distance travelled in the center region over the entire duration of the task (G). *Denotes significant differences (p < .05). Error bars denote SEM. Wild-type mice, n = 6; Fgf2 KO mice, n = 6.
The elevated plus maze data were further supported by results of the locomotor activity test in the open field. Mice showed no significant differences in baseline locomotor activity regardless of genotype, including total distance traveled in the field (F = 1.530, p > .05) or average speed (F = 1.661, p > .05) (Figure 1F). However, Fgf2 KO mice spent significantly less time in the center of the open field (F = 5.505, p < .05) (Figure 1E), another indication of increased anxiety. The difference in anxiety behavior in the open field test was observed predominantly during the first 4 minutes of the test; by 6 minutes, there were no significant differences in center time between groups (Figure 1E). In addition, there was a trend toward significance on the distance traveled in the center (p = .08), such that knockout animals traveled approximately half of the distance measured in wild-type control animals within the center; however, these two groups did not differ on distance traveled in the perimeter of the open field (F = 1.768, p > .05) (Figure 1G). Results of the open field were confirmed in a second group of mice (n = 6 Fgf2 KO and n = 6 wild-type mice) (Table 1).
Table 1.
Open Field Results From a Second Naïve Cohort, Percent Time Spent in the Center of the Open Field
| Trial Day | Genotype | Minutes 1–5 Mean ± SE | Minutes 6–10 Mean ± SE | Minutes 11–15 Mean ± SE |
|---|---|---|---|---|
| Day 1 | WT | 32.7 ± 2.3 | 30.3 ± 5.6 | 31.9 ± 8.8 |
| KO | 17.0 ± 4.9a | 18.1 ± 3.6a | 17.2 ± 4.5a | |
| Day 2 | WT | 18.1 ± 4.7 | 14.6 ± 1.9 | 19.1 ± 2.8 |
| KO | 10.1 ± 2.9 | 18.0 ± 4.8 | 17.1 ± 3.6 | |
| Day 3 | WT | 8.8 ± 2.1 | 11.1 ± 2.9 | 13.4 ± 3.2 |
| KO | 11.4 ± 4.6 | 13.2 ± 3.7 | 18.1 ± 6.6 |
KO, Fgf2 knockout; WT, wild-type.
Denotes significant difference (p < .05) from WT control mice.
Brain Anatomy and Cellular Phenotypes.
The hippocampus exhibits the highest levels of FGF2 and FGFR in the brain. This region also contains the highest concentration of GR in the brain, which makes it highly responsive to serum corticosterone, the rodent analog of glucocorticoids. In particular, the hippocampus is thought to exert an inhibitory tone on the HPA axis (23–25) (Figure 2A). To understand whether the increased anxiety behavior in Fgf2 KO mice was accompanied by a change in responsivity of the hippocampus and HPA axis to negative feedback by corticosterone (26,27), we examined GR immunoreactivity (ir) levels within the hippocampus in Fgf2 KO mice and wild-type control mice. GRir was present in granule neurons of the dentate gyrus (DG) and pyramidal cells of the hippocampal cornu ammonis (CA) regions. We found a significant decrease in GRir in both the DG and the CA1/CA3 of Fgf2 KO mice as compared with wild-type littermates (F = 10.686, p < .01) (Figure 2B–F). Previous work has shown that there is an age-related increase in localization of GR on glial fibrillary acidic protein (GFAP)+ cells instead of neurons (28); however, co-staining with GFAP showed no differences in the percentages of GFAP+ cells that were also GRir throughout the hippocampus (wild-type = 19.15% ± 5.3; Fgf2 knockout = 17.07% ± 3.9). Thus, the lack of a functional Fgf2 gene resulted in a strong decrease in GR expression in granule neurons and in CA1/CA3 neurons, without changing the expression of GR in GFAP+ cells.
Figure 2.
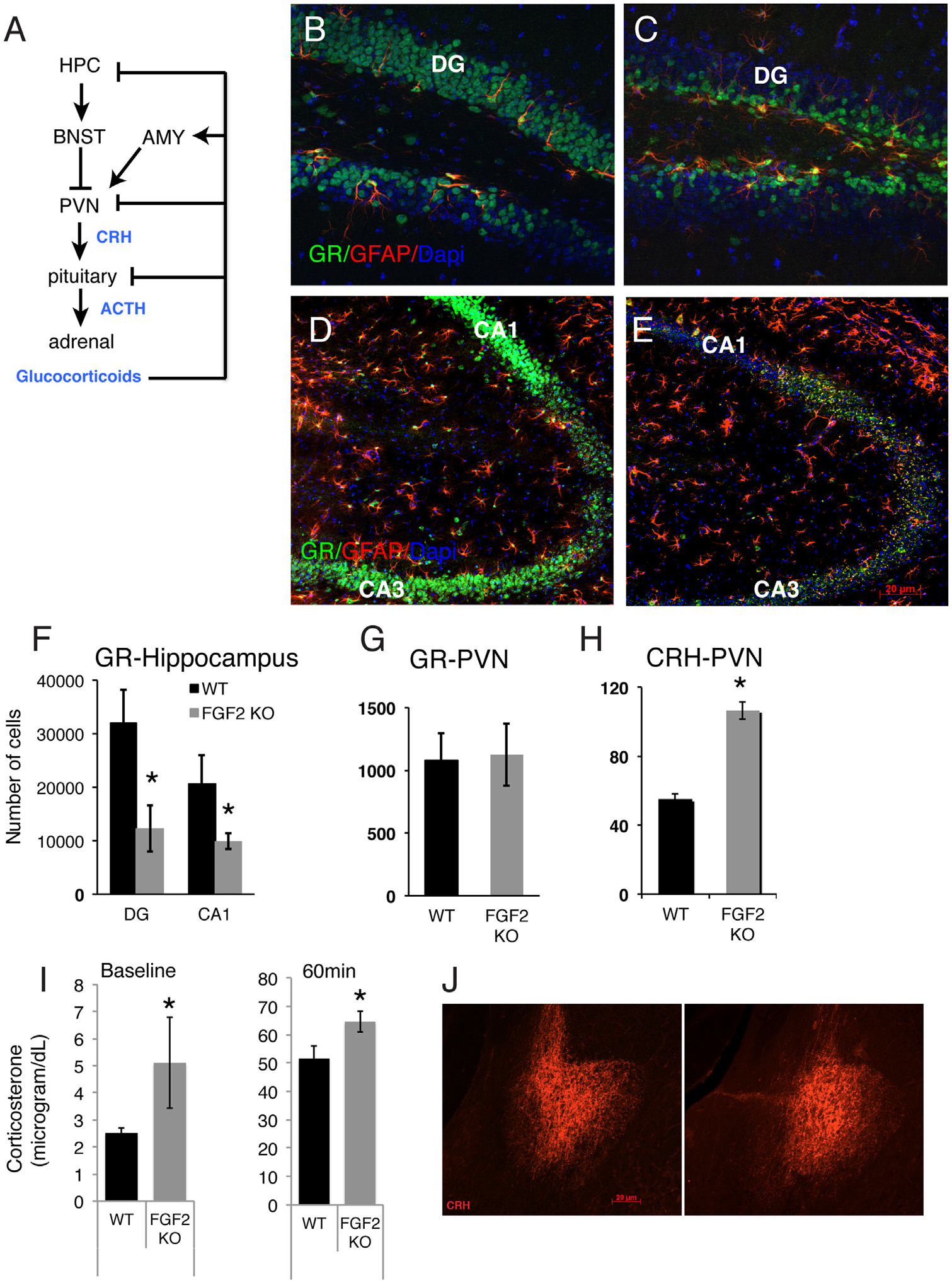
Dysregulated hypothalamic-pituitary-adrenal axis in fibroblast growth factor 2 (Fgf2) knockout (FGF2 KO) mice. (A) Schematic outline of hypothalamic-pituitary-adrenal axis and feedback loops. (B–E) Representative images of glucocorticoid receptor (GR) and glial fibrillary acidic protein (GFAP) co-immunostaining within the dentate gyrus (DG) (B, C) and cornu ammonis (CA) fields (D, E) of wild-type (WT) (B, D) and Fgf2 KO mice (C, E). Scale bar = 40 μm in (B) and (C) and 20 μm in (D) and (E). (F–H) Quantitative analyses of the immunocytochemical data carried out by unbiased stereology. Total number of GR+ cells in the DG, CA1 (F), and in the paraventricular nucleus of the hypothalamus (PVN) (G), and total number of corticotropin-releasing hormone (CRH)+ cells in the PVN (H) (WT, n = 4; Fgf2 KO, n = 3). (I) Mean corticosterone blood concentration at baseline and after 60 minutes of restraint stress. Significantly higher levels were observed in KO mice at the baseline and poststress time points (WT, n = 5; Fgf2 KO, n = 6). No differences in CRH staining intensity were observed in the amygdala (AMY) (J). *Denotes significant differences between groups (p < .05). Error bars denote SEM. ACTH, adrenocorticotropic hormone; BNST, bed nucleus of the stria terminalis; Dapi, 4′,6-diamidino-2-phenylindole dihydrochloride; HPC, hippocampus. [Adapted with permission from Brown et al. (34).]
Because we conducted our behavioral analysis and histology in the same animals, we were also able to correlate the number of hippocampal GRir cells with time spent on the open arms of the plus maze and the center of the open field. Total numbers of hippocampal GRir cells were significantly and positively correlated to both of these anxiety measures across all animals (Table 2), suggesting that decreased levels of hippocampal GR are associated with the increased anxiety behavior in mice.
Table 2.
Correlation Matrix for Anxiety Behavior and Hippocampal Glucocorticoid Receptors
| Open Field | EPM | |
|---|---|---|
| Open Field | 1 | – |
| EPM | 0.412a | 1 |
| GR-Total Hippocampus | 0.727b | 0.403a |
| GR-DG | 0.569b | 0.353c |
| GR-CA1 | 0.853b | 0.420a |
CA1, cornu ammonis 1; DG, dentate gyrus; EPM, elevated plus maze; GR, glucocorticoid receptor.
p ≤ .05.
p ≤ .01.
p = .09.
To better characterize HPA axis activity in the Fgf2 KO animals, we examined GR and corticotropin releasing hormone (CRH) ir cells within the paraventricular nucleus of the hypothalamus (PVN), which contains the CRH cell bodies important for the hypothalamic regulation of the HPA axis. No group differences were observed in GRir in this region (F = 0.019, p > .05) (Figure 2G). Interestingly, however, we found an increased number of CRHir neurons within the PVN of Fgf2 KO mice (F = 14.554, p < .01) (Figure 2H). Finally, because the central nucleus of the amygdala contains CRH cell bodies and regulates the HPA axis, we examined amygdalar CRHir. There was no difference in density of CRH protein level between wild-type and Fgf2 KO mice (mean optical density/area in arbitrary units, wild-type: 60.1 ± 8.04; Fgf2 KO: 54.61 ± 16.2; F = 0.110, p > .05). Together, the data suggest that FGF2 is crucial for maintenance of GR expression in differentiated neurons of the hippocampus, which, in turn, affects CRH protein expression within neurons of the PVN but not in the amygdala, consistent with the inhibitory role of the hippocampus on the HPA axis.
Corticosterone Responsivity.
Previous studies have shown that the profile observed in the current phenotype of Fgf2 KO mice, i.e., decreased hippocampal GR expression together with increases in PVN CRH, is associated with increased corticosterone response to an anxiogenic stimulus (29). Therefore, we also compared corticosterone blood levels in wild-type control mice and Fgf2 KO male mice in response to restraint stress. Corticosterone levels were assessed by enzyme-linked immunosorbent assay at baseline (2 weeks before) and at the end of a 60-minute restraint stressor. Results showed a main effect of genotype and time, such that Fgf2 KOs had higher corticosterone levels at baseline and 60-minute (peak) postrestraint stress (main effect of genotype at these time points: F = 6.02, p < .05; main effect of time: F = 362.83, p < .00001) (Figure 2I). Together, the data demonstrate abnormal HPA axis response to stress in Fgf2 KO mice.
Adult Rescue of Fgf2 KO Mouse Anxiety Phenotype
While we have observed a strong anxiety phenotype in response to the Fgf2 gene deletion, it remains to be determined whether this is due to irreversible developmental events consequential to the absence of Fgf2 or whether the FGF2 ligand itself is involved in the expression of anxiety behavior. Therefore, we performed a rescue experiment by administering either vehicle or FGF2 ligand daily for 1 week to adult Fgf2 KO or wild-type control mice and then assessing anxiety behavior, hippocampal GR levels, and circulating baseline corticosterone levels.
Behavior.
Adult male wild-type mice and Fgf2 KO mice were injected daily for 7 days with vehicle or FGF2 (see timeline, Figure 3A) and then tested on the elevated plus maze, open field test, and forced swim test. Results showed that again, vehicle-injected Fgf2 KO mice had similar increased levels of anxiety behavior on both the open field and the elevated plus maze; however, FGF2 treatment reinstated normal anxiety behavior such that there were no differences between wild-type control mice and FGF2-treated, Fgf2 KO mice (elevated plus maze: F = 0.075, p > .05; open field: F = 0.073, p > .05) (Figure 3B, C). No effect of FGF2 treatment on anxiety behavior was seen in control wild-type mice (EPM: F = 0.003, p > .05; open field: F = 6.849E-5, p > .05) (Figure 3B, C). Interestingly, as in our earlier experiments, no significant differences were observed between wild-type and Fgf2 KO mice on the forced swim test; however, a small decrease in the percentage of time spent immobile and increase in latency to become immobile were observed in the FGF2-injected versus vehicle-injected wild-type control mice, suggesting a mild antidepressant effect in wild-type mice (Figure 3D) (omnibus analysis of variance interaction F = 1.9, p ≥ .05; planned comparison in wild-type mice (t test p = .03).
Figure 3.
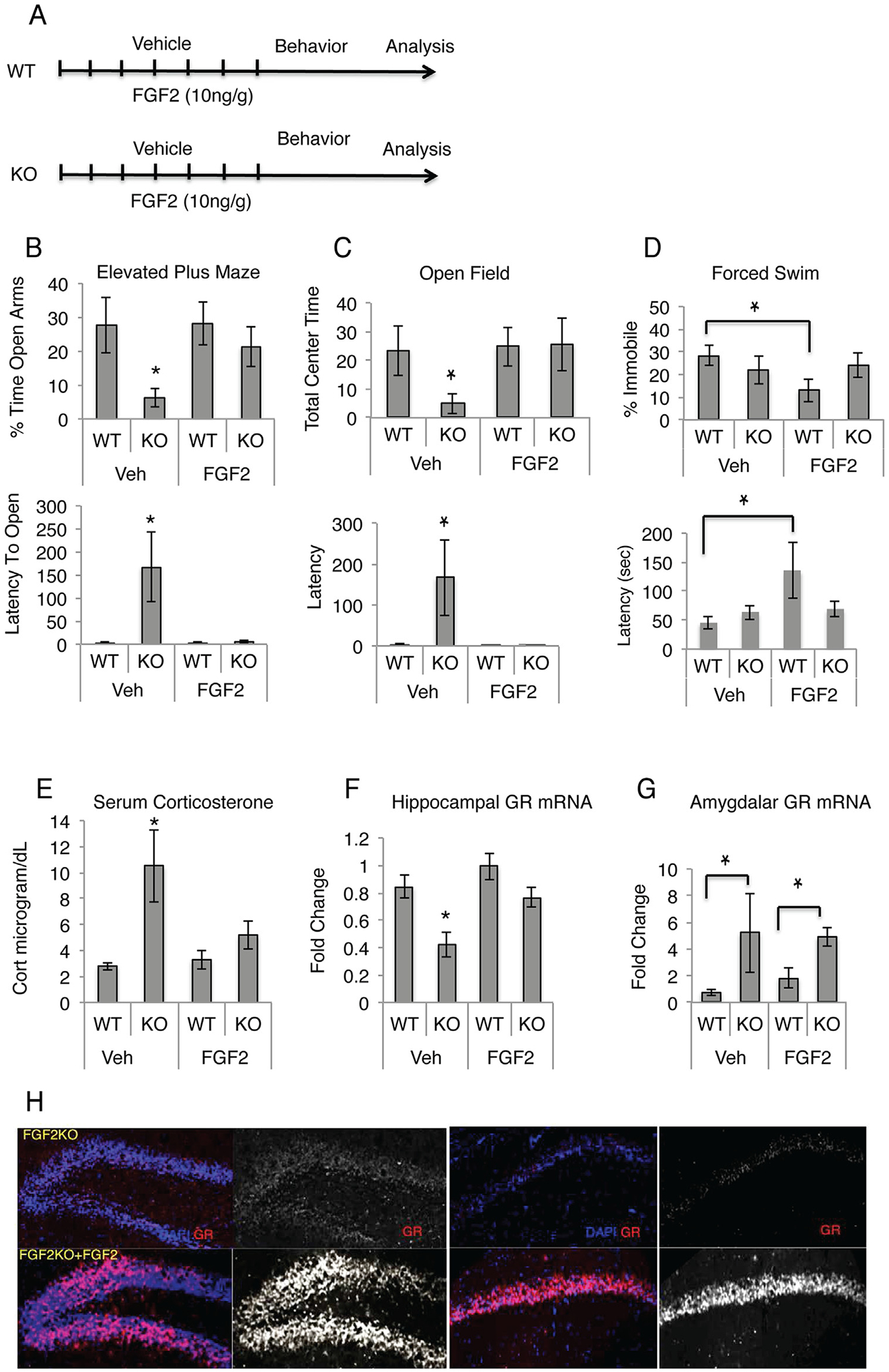
Adult fibroblast growth factor 2 (FGF2) administration rescues the anxiety phenotype. (A) Timeline of FGF2 rescue experiment. (B, C) Fgf2 knockout (FGF2 KO) mice show increased anxiety on the elevated plus maze (B) and the open field (5 minutes) (C), and FGF2 ligand administration returns anxiety behavior to that observed in wild-type (WT) mice. No differences were observed on the forced swim task between genotypes; however, a small antidepressant effect of FGF2 was observed in WT mice (D) (WT, vehicle [Veh], n = 8; Fgf2 KO, Veh, n = 8; WT, FGF2, n = 10; Fgf2 KO, FGF2, n = 8). (E) FGF2 administration restored the increased basal corticosterone level to that of WT mice (WT, Veh, n = 4; Fgf2 KO, Veh, n = 4; WT, FGF2, n = 3; Fgf2 KO, FGF2, n = 3). (F) FGF2 administration restored decreased hippocampal glucocorticoid receptor (GR) messenger RNA (mRNA) found in Fgf2 KO mice to levels observed in WT mice. (G) Fgf2 KO mice showed a significant increase in amygdalar GR mRNA, regardless of FGF2 administration (WT, Veh, n = 4; Fgf2 KO, Veh, n = 4; WT, FGF2, n = 3; Fgf2 KO, FGF2, n = 3). (H) Fgf2 KO also showed an increase in GR protein in both the cornu ammonis and dentate gyrus regions of the hippocampus (WT, Veh, n = 4; Fgf2 KO, Veh, n = 4; WT, FGF2, n = 4; Fgf2 KO, FGF2, n = 4). Asterisks denote significant differences from all other groups, p < .05. Lines indicate significant differences between two groups, p < .05. Error bars denote SEM. Cort, corticosterone; DAPI, 4′,6-diamidino-2-phenylindole dihydrochloride.
HPA Axis Phenotype.
To assess whether FGF2 treatment had a corresponding corrective effect on the neuroendocrine measures of anxiety, basal serum corticosterone levels at sacrifice were assessed by enzyme-linked immunosorbent assay. Fgf2 KO mice again showed a significant increase in basal corticosterone levels, with FGF2 treatment inducing a complete reversal of the chronically elevated corticosterone levels in Fgf2 KO mice (Figure 3E). Hippocampal GR messenger RNA (mRNA) levels were assessed by quantitative polymerase chain reaction (qPCR), and a decrease in GR mRNA was observed in Fgf2 KO mice, with a reversal to baseline levels following FGF2 administration (Figure 3F). No significant effect of FGF2 was observed in either corticosterone or GR levels in wild-type mice. Immunostaining for GR showed that FGF2 ligand administration increased GR in both the CA and DG regions of the hippocampus (Figure 3H).
Because the amygdala plays a crucial role in the positive modulation of HPA axis activity, we also examined GR mRNA levels of the amygdala and found a significant GR mRNA increase in Fgf2 KO mice, regardless of adult FGF2 administration (Figure 3G), suggesting a different role of FGF2 in the amygdala and that only those changes in the hippocampus are consistent with the behavioral phenotype we have observed in the adult rescue.
FGF2 Modulates GR Expression Through Egr-1
A potent regulator of Gr expression in the hippocampus is the immediate early gene and transcription factor EGR-1 (also known as zif-268 and NGF1A). GR expression increases in response to increased binding of EGR-1 to the exon 17 promoter region of the Gr gene, and increased EGR-1 activity has been associated with decreased anxiety behavior and decreased corticosterone release (30). To understand if EGR-1 could mediate the effect of FGF2 on Gr expression, we used qPCR to examine levels of Egr-1 mRNA expression in the hippocampus of wild-type and Fgf2 KO mice with or without FGF2 rescue treatment. Consistent with prior data suggesting that Egr-1 is downstream of FGF signaling (31), Egr-1 mRNA was significantly decreased in the hippocampus of Fgf2 KO mice and was reinstated to normal level with FGF2 administration (Figure 4A). Therefore, we hypothesized that FGF2 positively modulates Egr-1 transcription, leading to changes in binding activity of EGR-1 to the exon 17 promoter region of the Gr. To test this, we performed ChIP using an EGR-1 antibody in wild-type and Fgf2 knockout mice and then used qPCR to amplify the EGR-1 binding domain of the Gr exon 17 promoter region of both the input and IP samples. We analyzed the data by comparing the absolute difference between the cycle thresholds (ΔCT) of the input and the IP samples (Figure 4B), and additionally, we ran the qPCR product on a southern gel and analyzed the band intensities using National Institutes of Health ImageJ software (Bethesda, MD) (Figure 4C). Results showed a greater difference in ΔCT between input and IP samples in the hippocampus of Fgf2 KO mice as compared with wild-type control mice, indicating that there was less binding of EGR-1 to the exon 17 promoter region of the Gr in the absence of FGF2 (Figure 4B). This was verified by a decrease in the amount of quantifiable product using gel band analysis (F = 8.706, p < .05) (Figure 4C). The data suggest that FGF2, presumably acting through its receptor-mediated tyrosine kinase activity, regulates EGR-1 levels and binding activity at the Gr promoter region.
Figure 4.
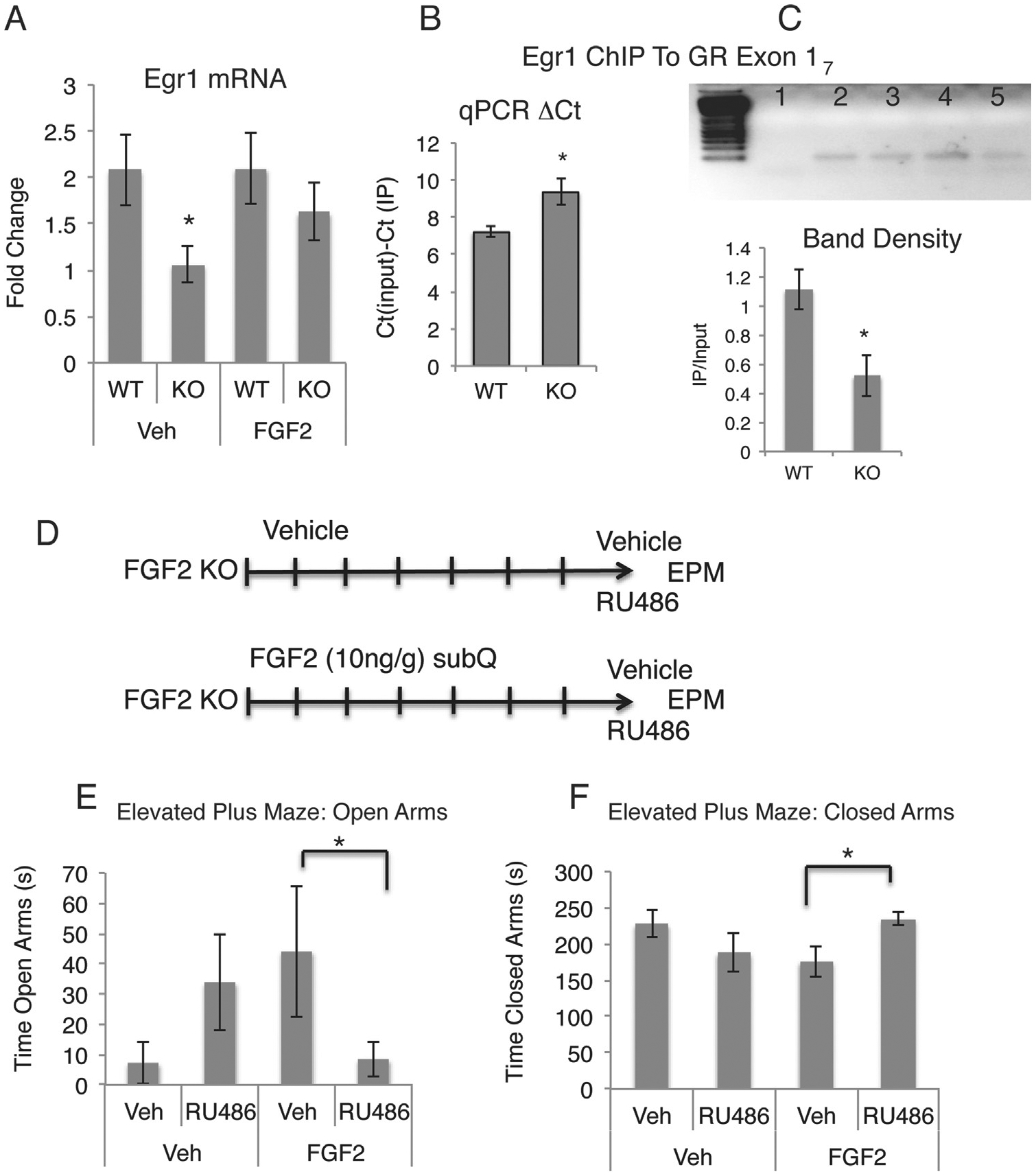
Mechanisms of fibroblast growth factor 2 (FGF2)-glucocorticoid receptor (GR) interactions. (A) Hippocampal Egr-1 messenger RNA (mRNA) is downregulated in Fgf2 knockout (KO) mice and reinstated with FGF2 administration as assessed by quantitative polymerase chain reaction (qPCR) (wild-type [WT], vehicle [Veh], n = 4; FGF2 KO, Veh, n = 4; WT, FGF2, n = 3; FGF2 KO, FGF2, n = 3). (B, C) Results of hippocampal chromatin immunoprecipitation (ChIP) with EGR-1 antibody followed by qPCR amplification of the Gr exon17 promoter region. Y axis in (B): cycle threshold (CT) (input) 2 CT (IP) indicates the difference in cycle thresholds (ΔCT) between the input and IP samples. Increased ΔCT in FGF2 KO mice suggests decreased EGR-1 binding. (C) Gel electrophoresis of qPCR amplicons. Lane 1: negative control; Lane 2: WT input; Lane 3: WT IP; Lane 4: Fgf2 KO input; Lane 5: Fgf2 KO IP. Below the gel, results of band densitometry showing decreased EGR-1 binding, quantified as IP/input. (D) Timeline of the RU486 experiment. (E, F) RU486 blocks the effect of FGF2 rescue administration on anxiety behavior in Fgf2 KO mice. RU486 decreased time in open arms of the elevated plus maze (EPM) (E) and increased the time spent in the closed arms (F) in FGF2-treated Fgf2 KO mice (Veh, Veh, n = 6; Veh, FGF2, n = 7; RU486, Veh, n = 6; RU486, FGF2, n = 11). Error bars denote SEM. Asterisks denote significant differences from all other groups, p < .05. Lines indicate significant differences between two groups, p < .05. subQ, subcutaneously.
GR Is Necessary for Anxiolytic Effects of FGF2.
To ascertain whether the upregulation of hippocampal GR in response to FGF2 treatment is necessary to exert FGF2’s anxiolytic effects, we treated Fgf2 KO mice with vehicle or FGF2 and then injected them with RU486 (mifepristone), a glucocorticoid receptor antagonist, 40 minutes before testing them on the elevated plus maze (Figure 4D). GR blockade completely blocked the anxiolytic effects of FGF2 (open arms: F = 5.923, p < .05; closed arms: F = 7.877, p < .01) (Figure 4E, F). RU486 also showed a paradoxical anxiolytic effect in Fgf2 KO, vehicle-injected mice, although this was variable and did not reach statistical significance (F = 2.379, p > .15) (Figure 4E, F).
Role of Fgfr in Anxiety Behavior.
To ascertain if FGFRs are necessary for changes in anxiety behavior, we examined mice that conditionally lacked Fgfr1, Fgfr2, or both receptors in the dorsal forebrain. The conditional knockout of Fgfr1 and the double knockout of Fgfr1 and Fgfr2 were driven by the Emx1-cre line and the conditional knockout of Fgfr2 by the hGFAP-cre line. We also examined mice lacking Fgfr3 constitutively. Baseline behavioral tests on the elevated plus maze showed no changes in anxiety behavior in Fgfr3 knockout mice (Figure 5A). Interestingly, we observed a decrease in anxiety behavior in both Fgfr1 and Fgfr2 conditional knockout mice when compared with their respective control mice (F2,30 = 11.8546, p < .001) (Figure 5A, B), which is the opposite effect than what we observed with Fgf2 KO mice. We then administered FGF2 ligand to double Fgfr1/2 conditional knockout mice (driven by Emx1-cre) using the same protocol as in previous experiments and found that both FGF@ administration and Fgfr knockout showed a main effect of decreased anxiety behavior.
Figure 5.
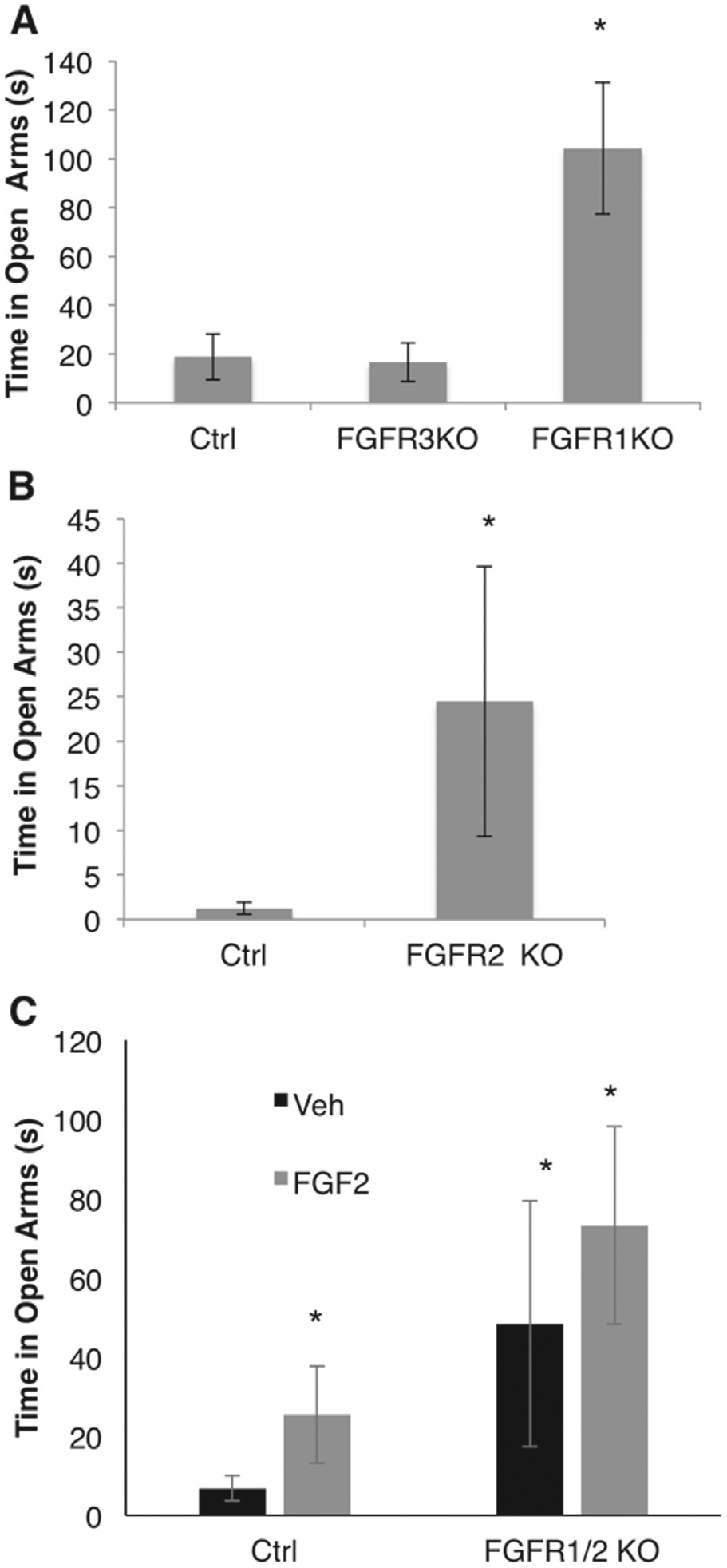
The role of fibroblast growth factor receptor (Fgfr)1, 2, and 3 in anxiety behavior. Panel (A) shows time on the open arms in control (Ctrl) (cre-negative or wild type), Fgfr3 constitutive knockout (FGFR3 KO), and Fgfr1 conditional KO mice (FGFR1 KO) driven by Emx1-cre (Ctrl, n 5 12; Fgfr3 KO, n = 13; Fgfr1 KO, n = 8). Panel (B) shows time on the open arms of the elevated plus maze in Ctrl (cre-negative) and Fgfr2 conditional KO mice (FGFR2 KO) driven by the hGFAP-cre line (Ctrl, n = 9; Fgfr2 KO, n = 9). Panel (C) shows time on the open arms in Ctrl (cre-negative) and FGFR1/2 conditional KO mice driven by Emx1-cre in response to vehicle (Veh) or Fgfr2 administration (Ctrl, Veh, n = 8; Fgfr1/2 KO, Veh, n = 6; Ctrl, FGF2, n = 10; Fgfr1/2 KO, FGF2, n = 6). *Denotes significant differences from Ctrl. Error bars denote SEM.
DISCUSSION
In the current study, we established for the first time that a primary role of FGF2 is to maintain appropriate levels of hippocampal (but not amygdalar) Gr gene expression, which, in turn, regulates HPA axis activity. These results tie together a body of literature that has demonstrated hippocampal regulation of HPA axis function with emerging data correlating FGF2 to mood and anxiety behavior. There were two domains where neuroendocrine function was altered in Fgf2 KO mice. First, decreased Gr expression was observed in both the DG and CA fields of the hippocampus of Fgf2 KO mice, and this correlated with increased levels of anxiety in both the open field and elevated plus maze. Secondly, there was overactivity of the HPA axis, as shown by increased corticosterone levels and increased CRH levels in the PVN.
The hippocampus, which contains high concentrations of GR, is thought to be a crucial link in the negative feedback regulation of the HPA axis (24,25,32) (Figure 2A). Transgenic mice that overexpress Gr in the hippocampus show enhancement of such feedback inhibition, and mice that lack one copy of the Gr alleles show, similar to Fgf2 KO mice, HPA axis hyperactivity (33). Those reports suggest that a primary function of Fgf2 gene expression is to increase Gr gene expression in the hippocampus. Conversely, the absence of FGF2 drives hyperactivity of the HPA axis and increased anxiety behavior. In support of this hypothesis, we were able to rescue both the hippocampal GR levels and the anxiety behavior in Fgf2 KO mice by administering FGF2. Pharmacologic antagonism of GR was sufficient to prevent the effects of FGF2, which together suggest that ongoing acute modulation of hippocampal GR expression by FGF2 is critically involved in this anxiety phenotype.
While the hippocampus regulates HPA axis function through negative feedback, the amygdala exerts positive feedback on the HPA axis through amygdalar GR receptors (34). In the current study, Gr mRNA levels were significantly increased within the amygdala of Fgf2 KO mice, consistent with Fgf2 KO mice having an upregulation of the HPA axis and increased baseline corticosterone levels. However, while increases in amygdalar GR may also play a contributory role to the increased HPA axis activity observed in Fgf2 KO mice, amygdalar GR changes were not affected by adult FGF2 rescue administration, suggesting that GR changes in the amygdala are not crucial for the anxiolytic action of FGF2 in adulthood.
Interestingly, Fgf2 KO mice injected with the GR antagonist RU486 that were not FGF2 treated showed mild anxiolytic responses to RU486 on the elevated plus maze, which may be a consequence of antagonizing the increased GR levels within the amygdala of Fgf2 KO mice. The dose of RU486 used in the current study typically does not have effects on anxiety behavior in rodents unless an animal has undergone repeated stress before its administration (35,36). Therefore, it could be that the elevated baseline anxiety levels in Fgf2 KO mice may, in part, recapitulate an animal that has undergone repeated stress, i.e., stress might induce increased GR expression in the amygdala, suggesting that the mild anxiolytic response of RU486 might be attributable to a decrease of amygdalar GR–mediated positive feedback on the HPA axis. It is important to note that RU486 can also act as a progesterone receptor blocker, and therefore the effects of RU486 on anxiety behavior reported may also be mediated through progesterone receptors. However, because the current study was conducted in male mice, progesterone levels are typically low throughout early adulthood and similar to the lowest levels observed in female mice across the estrous cycle. Nevertheless, further studies using different methods to attenuate GR receptor activity (i.e., using small hairpin RNAs) would be needed to assess the contribution of progesterone receptors, if any, to the anxiolytic effects of FGF2.
While, indeed, there may well be developmental effects of FGF2 on the amygdala, these data suggest that only the hippocampal changes are consistent with the behavioral phenotype we have observed in the adult rescue. The expression of FGF2 ligand and its receptors (FGFR1–3) in adulthood is highest in the hippocampus, a region that also exhibits a neuronal expression of these molecules (37,38), which might explain why neuronal GR expression may be uniquely modulated in neurons of the hippocampus by FGF2. In addition, Gr exon 17 activity, which is modulated by the immediate early gene and transcription factor EGR-1, which binds to the exon 17 promoter region of the Gr gene, is high within hippocampal neurons and relatively low in other regions (39,40), therefore placing the hippocampus in a position where glucocorticoid receptor expression may be uniquely sensitive to changes in FGF2 and EGR-1 levels.
A strikingly similar phenotype associating hippocampal GR levels and HPA axis function has been reported in the offspring of models of early adversity and high- and low-licking and grooming mothers (29,41–43). For example, mice raised by low-licking and grooming mothers show decreases in hippocampal GR levels, increased stress responsivity, and interestingly, lower levels of FGF2 expression within the hippocampus (29,44). This parallel suggests that hippocampal FGF2 levels may be perturbed by maternal behavior during the early postnatal period and may be responsible for the development of the abnormal HPA axis in animals reared under maternal deprivation. While the current studies demonstrate that acute restoration of FGF2 levels in adulthood is sufficient to normalize anxiety behavior, these experiments do not rule out additional roles for FGF2 in anxiety behavior and HPA axis reactivity during development.
While a role for FGF2 in anxiety behavior has been previously suggested, the FGFR involved in modulating anxiety behavior has not been previously assessed. The current study found no role of FGFR3 in modulating anxiety and a paradoxical decrease in anxiety in Fgfr1 and Fgfr2 knockout mice. Fgfr KO mice (45–47) have extensive behavioral deficits, including hyperactivity and cognitive alterations. Because FGFRs bind all 23 FGF ligands in a promiscuous way, their knockouts are likely disrupting a much larger system than FGF2 alone, making it a difficult model to use in this line of investigation. Furthermore, Fgfr KO mice are lacking FGFR throughout development. For all of these reasons, it is possible that the current results reflect specific interactions of FGFRs with any of the other 20 or so FGF ligands that could be modulating development and behavior. For example, FGF9 has recently been suggested to increase both anxiety- and depression-like behavior (48), and unlike FGF2, FGF9 is upregulated in the hippocampus of patients with major depression (48), implicating an action opposite to that of FGF2. Further studies are needed to understand the role of different FGFRs in anxiety behavior, including exploring the role of the newly discovered decoy receptor FGFR5.
While the current studies show that knockout of Fgf2 conduces to an anxiety phenotype and apparently not to depressive-like behaviors, it is important to note that we have only examined tests related to learned helplessness, and FGF2 may be involved in other aspects of depressive behaviors such as social, sexual, or other motivated behaviors.
Anxiety disorders are currently the most prevalent psychiatric illnesses in the United States (49); despite these alarmingly high rates, the available pharmacotherapy is far from optimal. The current study lends further support for necessary investigation of FGF2 (or a downstream target) as a potential HPA axis modulator and anxiolytic treatment in human populations.
Supplementary Material
ACKNOWLEDGMENTS AND DISCLOSURES
This work was supported by Grant No. R01 MH0677 (to FMV); a Natural Sciences and Engineering Research Council of Canada Undergraduate Student Research Award (to JM); and postdoctoral fellowships (to NS): Fonds de Recherches en Sante du Quebec (2009–2012) and the Canadian Institute of Health Research (2012–2014), as well as a Brain and Behavior Research Foundation (National Alliance for Research on Schizophrenia and Depression) Young Investigator Award (to NS) and National Alliance for Research on Schizophrenia and Depression Established and Senior Investigator Awards (to FMV).
We thank Shawna Ellis for her technical assistance with the retroorbital blood draw; Dr. Hymie Anisman and Dr. Shawn Hayley for insightful discussions of data interpretation; Dr. Anahita Amiri, Dr. Louis Charles Levros, and Dr. Jessica Mariani for technical discussions; and finally to Dr. Ian Weaver and Dr. Patrick McGowan for useful information on the glucocorticoid receptor Egr-1 binding domains. We also thank the late Dr. Wylie Vale for his generous gift of corticotropin releasing factor antibody.
Footnotes
The authors declare no biomedical financial interests or potential conflicts of interest.
Supplementary material cited in this article is available online at http://dx.doi.org/10.1016/j.biopsych.2016.02.026.
REFERENCES
- 1.Woodbury ME, Ikezu T (2014): Fibroblast growth factor-2 signaling in neurogenesis and neurodegeneration. J Neuroimmune Pharmacol 9: 92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vaccarino FM, Grigorenko EL, Smith KM, Stevens HE (2009): Regulation of cerebral cortical size and neuron number by fibroblast growth factors: Implications for autism. J Autism Dev Disord 39:511–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vaccarino FM, Schwartz ML, Raballo R, Nilsen J, Rhee J, Zhou M, et al. (1999): Changes in cerebral cortex size are governed by fibroblast growth factor during embryogenesis. Nat Neurosci 2: 246–253. [DOI] [PubMed] [Google Scholar]
- 4.Raballo R, Rhee J, Lyn-Cook R, Leckman JF, Schwartz ML, Vaccarino FM (2000): Basic fibroblast growth factor (Fgf2) is necessary for cell proliferation and neurogenesis in the developing cerebral cortex. J Neurosci 20:5012–5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Korada S, Zheng W, Basilico C, Schwartz ML, Vaccarino FM (2002): Fgf2 is necessary for the growth of glutamate projection neurons in the anterior neocortex. J Neurosci 22:863–875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng W, Nowakowski RS, Vaccarino FM (2004): Fibroblast growth factor 2 is required for maintaining the neural stem cell pool in the mouse brain subventricular zone. Dev Neurosci 26:181–196. [DOI] [PubMed] [Google Scholar]
- 7.Flores C, Stewart J, Salmaso N, Zhang Y, Boksa P (2002): Astrocytic basic fibroblast growth factor expression in dopaminergic regions after perinatal anoxia. Biol Psychiatry 52:362. [DOI] [PubMed] [Google Scholar]
- 8.Ganat Y, Soni S, Chacon M, Schwartz ML, Vaccarino FM (2002): Chronic hypoxia up-regulates fibroblast growth factor ligands in the perinatal brain and induces fibroblast growth factor -responsive radial glial cells in the sub-ependymal zone. Neuroscience 112: 977–991. [DOI] [PubMed] [Google Scholar]
- 9.Rai KS, Hattiangady B, Shetty AK (2007): Enhanced production and dendritic growth of new dentate granule cells in the middle-aged hippocampus following intracerebroventricular FGF-2 infusions. Eur J Neurosci 26:1765–1779. [DOI] [PubMed] [Google Scholar]
- 10.Akil H, Evans SJ, Turner CA, Perez J, Myers RM, Bunney WE, et al. (2008): The fibroblast growth factor family and mood disorders. Novartis Found Symp 289:94–96; discussion 97–100, 193–105. [DOI] [PubMed] [Google Scholar]
- 11.Eren-Kocak E, Turner CA, Watson SJ, Akil H (2011): Short-hairpin RNA silencing of endogenous fibroblast growth factor 2 in rat hippocampus increases anxiety behavior. Biol Psychiatry 69:534–540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans SJ, Choudary PV, Neal CR, Li JZ, Vawter MP, Tomita H, et al. (2004): Dysregulation of the fibroblast growth factor system in major depression. Proc Natl Acad Sci U S A 101:15506–15511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perez JA, Clinton SM, Turner CA, Watson SJ, Akil H (2009): A new role for FGF2 as an endogenous inhibitor of anxiety. J Neurosci 29: 6379–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Turner CA, Gula EL, Taylor LP, Watson SJ, Akil H (2008): Antidepressant-like effects of intracerebroventricular FGF2 in rats. Brain Res 1224:63–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salmaso N, Vaccarino FM (2011): Toward a novel endogenous anxiolytic factor, fibroblast growth factor 2. Biol Psychiatry 69:508–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bachis A, Mallei A, Cruz MI, Wellstein A, Mocchetti I (2008): Chronic antidepressant treatments increase basic fibroblast growth factor and fibroblast growth factor-binding protein in neurons. Neuropharmacology 55:1114–1120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou M, Sutliff RL, Paul RJ, Lorenz JN, Hoying JB, Haudenschild CC, et al. (1998): Fibroblast growth factor 2 controls vascular tone. Nat Med 4:201–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stevens HE, Smith KM, Maragnoli ME, Fagel D, Borok E, Shanab-rough M, et al. (2010): Fgfr2 is required for the development of the medial prefrontal cortex and its connections with limbic circuits. J Neurosci 30:5590–5602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng C, Wynshaw-Boris A, Zhou F, Kuo A, Leder P (1996): Fibroblast growth factor receptor 3 is a negative regulator of bone growth. Cell 84:911–921. [DOI] [PubMed] [Google Scholar]
- 20.Rash BG, Lim HD, Breunig JJ, Vaccarino FM (2011): FGF signaling expands embryonic cortical surface area by regulating notch-dependent neurogenesis. J Neurosci 31:15604–15617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Comeau W, Gibb R, Hastings E, Cioe J, Kolb B (2008): Therapeutic effects of complex rearing or bFGF after perinatal frontal lesions. Dev Psychobiol 50:134–146. [DOI] [PubMed] [Google Scholar]
- 22.Wagner JP, Black IB, DiCicco-Bloom E (1999): Stimulation of neonatal and adult brain neurogenesis by subcutaneous injection of basic fibroblast growth factor. J Neurosci 19:6006–6016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Kloet ER, Oitzl MS, Joels M (1993): Functional implications of brain corticosteroid receptor diversity. Cell Mol Neurobiol 13:433–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.De Kloet ER, Vreugdenhil E, Oitzl MS, Joels M (1998): Brain corticosteroid receptor balance in health and disease. Endocr Rev 19: 269–301. [DOI] [PubMed] [Google Scholar]
- 25.Sapolsky RM, Krey LC, McEwen BS (1984): Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc Natl Acad Sci U S A 81:6174–6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McEwen BS (2001): Plasticity of the hippocampus: Adaptation to chronic stress and allostatic load. Ann N Y Acad Sci 933:265–277. [DOI] [PubMed] [Google Scholar]
- 27.Sapolsky RM, Krey LC, McEwen BS (1986): The neuroendocrinology of stress and aging: The glucocorticoid cascade hypothesis. Endocr Rev 7:284–301. [DOI] [PubMed] [Google Scholar]
- 28.Garcia A, Steiner B, Kronenberg G, Bick-Sander A, Kempermann G (2004): Age-dependent expression of glucocorticoid- and mineralocorticoid receptors on neural precursor cell populations in the adult murine hippocampus. Aging Cell 3:363–371. [DOI] [PubMed] [Google Scholar]
- 29.Liu D, Diorio J, Tannenbaum B, Caldji C, Francis D, Freedman A, et al. (1997): Maternal care, hippocampal glucocorticoid receptors, and hypothalamic-pituitary-adrenal responses to stress. Science 277: 1659–1662. [DOI] [PubMed] [Google Scholar]
- 30.Weaver IC, Cervoni N, Champagne FA, D’Alessio AC, Sharma S, Seckl JR, et al. (2004): Epigenetic programming by maternal behavior. Nat Neurosci 7:847–854. [DOI] [PubMed] [Google Scholar]
- 31.Jiang C, Salton SR (2013): The role of neurotrophins in major depressive disorder. Transl Neurosci 4:46–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sapolsky RM, Meaney MJ, McEwen BS (1985): The development of the glucocorticoid receptor system in the rat limbic brain. III. Negative-feedback regulation. Brain Res 350:169–173. [DOI] [PubMed] [Google Scholar]
- 33.Ridder S, Chourbaji S, Hellweg R, Urani A, Zacher C, Schmid W, et al. (2005): Mice with genetically altered glucocorticoid receptor expression show altered sensitivity for stress-induced depressive reactions. J Neurosci 25:6243–6250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brown ES, Rush AJ, McEwen BS (1999): Hippocampal remodeling and damage by corticosteroids: Implications for mood disorders. Neuropsychopharmacology 21:474–484. [DOI] [PubMed] [Google Scholar]
- 35.Calvo N, Volosin M (2001): Glucocorticoid and mineralocorticoid receptors are involved in the facilitation of anxiety-like response induced by restraint. Neuroendocrinology 73:261–271. [DOI] [PubMed] [Google Scholar]
- 36.Korte SM, de Boer SF, de Kloet ER, Bohus B (1995): Anxiolytic-like effects of selective mineralocorticoid and glucocorticoid antagonists on fear-enhanced behavior in the elevated plus-maze. Psychoneur-oendocrinology 20:385–394. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez A-M, Berry M, Maher PA, Logan A, Baird A (1995): A comprehensive analysis of the distribution of FGF-2 and FGF-1 in the rat brain. Brain Res 701:201–226. [DOI] [PubMed] [Google Scholar]
- 38.Belluardo N, Wu G-Y, Mudo G, Hansson AC, Petterson R, Fuxe K (1997): Comparative localization of fibroblast growth factor receptor-1, −2, and −3 mRNAs in the rat brain: In situ hybridization analysis. J Comp Neurol 379:226–246. [PubMed] [Google Scholar]
- 39.McCormick JA, Lyons V, Jacobson MD, Noble J, Diorio J, Nyirenda M, et al. (2000): 5’-heterogeneity of glucocorticoid receptor messenger RNA is tissue specific: Differential regulation of variant transcripts by early-life events. Mol Endocrinol 14:506–517. [DOI] [PubMed] [Google Scholar]
- 40.Zhang TY, Labonte B, Wen XL, Turecki G, Meaney MJ (2013): Epigenetic mechanisms for the early environmental regulation of hippocampal glucocorticoid receptor gene expression in rodents and humans. Neuropsychopharmacology 38:111–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anisman H, Zaharia MD, Meaney MJ, Merali Z (1998): Do early-life events permanently alter behavioral and hormonal responses to stressors? Int J Dev Neurosci 16:149–164. [DOI] [PubMed] [Google Scholar]
- 42.Francis DD, Meaney MJ (1999): Maternal care and the development of stress responses. Curr Opin Neurobiol 9:128–134. [DOI] [PubMed] [Google Scholar]
- 43.Hellstrom IC, Dhir SK, Diorio JC, Meaney MJ (2012): Maternal licking regulates hippocampal glucocorticoid receptor transcription through a thyroid hormone-serotonin-NGFI-A signalling cascade. Philos Trans R Soc Lond B Biol Sci 367:2495–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bredy TW, Humpartzoomian RA, Cain DP, Meaney MJ (2003): Partial reversal of the effect of maternal care on cognitive function through environmental enrichment. Neuroscience 118:571–576. [DOI] [PubMed] [Google Scholar]
- 45.Muller Smith K, Fagel DM, Stevens HE, Rabenstein RL, Maragnoli ME, Ohkubo Y, et al. (2008): Deficiency in inhibitory cortical interneurons associates with hyperactivity in fibroblast growth factor receptor 1 mutant mice. Biol Psychiatry 63:953–962. [DOI] [PubMed] [Google Scholar]
- 46.Smith KM, Williamson TL, Schwartz ML, Vaccarino FM (2012): Impaired motor coordination and disrupted cerebellar architecture in Fgfr1 and Fgfr2 double knockout mice. Brain Res 1460: 12–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stevens HE, Jiang GY, Schwartz ML, Vaccarino FM (2012): Learning and memory depend on fibroblast growth factor receptor 2 functioning in hippocampus. Biol Psychiatry 71:1090–1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aurbach EL, Inui EG, Turner CA, Hagenauer MH, Prater KE, Li JZ, et al. (2015): Fibroblast growth factor 9 is a novel modulator of negative affect. Proc Natl Acad Sci U S A 112:11953–11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE (2005): Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:617–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


