Abstract
Protein restricted (PR) diets promote health and longevity in many species. While the precise components of a PR diet that mediate the beneficial effects to longevity have not been defined, we recently showed that many metabolic effects of PR can be attributed to reduced dietary levels of the branched-chain amino acids (BCAAs) leucine, isoleucine, and valine. Here, we demonstrate that restricting dietary BCAAs increases the survival of two different progeroid mouse models, delays frailty and promotes the metabolic health of wild-type C57BL/6J mice when started in midlife, and leads to a 30% increase in lifespan and a reduction in frailty in male, but not female, wild-type mice when fed lifelong. Our results demonstrate that restricting dietary BCAAs can increase healthspan and longevity in mice, and suggest that reducing dietary BCAAs may hold potential as a translatable intervention to promote healthy aging.
Keywords: branched-chain amino acids, lifespan, healthspan, protein restriction, mTOR, mTORC1, rapamycin, progeria
Introduction
Dietary interventions can robustly promote organismal health and longevity. The most well-known of these is calorie restriction (CR), which extends the lifespan and healthspan of diverse species including rodents 1, 2. While the role of reduced levels of specific dietary macronutrients in the benefits of a CR diet is still under investigation, dietary macronutrient balance has a strong influence on longevity and health 3, 4. A recent meta-analysis of protein restriction in rodents found that while the reduction in calories, not protein, is responsible for the effects of a CR diet on longevity, lifespan increases as dietary protein content decreases 5.
Many human diet plans call for high protein consumption, as protein promotes satiety and benefits of dietary protein have been noted in short-term studies 6, 7, 8. Decreased protein intake is also associated with frailty and sarcopenia 9, 10. However, a growing number of studies suggest that low protein diets may be healthier and associated with longer life. A retrospective clinical trial found that lower protein consumption is associated with decreased mortality and decreased incidence of diabetes 11; further, several prospective clinical trials have shown that high protein diets are associated with insulin resistance, diabetes, obesity, and mortality 12, 13, 14. While long-term randomized clinical trials (RCTs) of protein restriction (PR) in healthy humans have not yet been undertaken, a recent short-term RCT of PR observed improved metabolic health, including reduced adiposity and improved insulin sensitivity.
Positive metabolic effects of low protein diets are also observed in rodents, where PR promotes leanness, energy expenditure, and improved glucose homeostasis 15, 16, 17, 18, and PR promotes longevity in both Drosophila and rodents 5, 19, 20, 21, 22. The precise amino acids (AAs) altered in a PR diet that mediate the beneficial effects of PR have not been defined. In Drosophila, methionine restriction extends lifespan, though levels of other essential AAs are also influential 23, 24, 25. In rodents, adding back essential AAs to mice on a 40% CR diet blunts the effect of CR on longevity, demonstrating that essential AAs have a powerful effect on lifespan 26. Restriction of individual essential AAs has found that methionine restriction extends lifespan and improves health 27, 28, 29, and tryptophan restriction may increase maximum lifespan 30, 31. The role of other dietary AAs in mammalian lifespan has largely been unexplored.
Blood and dietary levels of the three branched-chain amino acids (BCAAs), leucine, isoleucine, and valine, have been linked to insulin resistance and obesity in both humans and rodents in numerous studies over the last decade 17, 18, 32, 33, 34, 35. Rodent studies have shown that specifically restricting dietary BCAAs improves metabolic health and recapitulates the metabolic benefits of PR 17, 18, 36. The effect of restricting dietary levels of the BCAAs on the healthspan and longevity of mice has not been extensively investigated. While one study found a small, male-specific increase in median lifespan in mice provided BCAA-enriched drinking water 37, dietary supplementation with BCAAs leads to obesity and a reduction in median and maximal lifespan 38.
The BCAAs are potent agonists of the AA-sensitive protein kinase mTORC1, a central regulator of metabolism and aging 39, 40. In rats, the negative effect of additional BCAAs on insulin sensitivity is accompanied by increased mTORC1 activity in skeletal muscle, and this BCAA-induced insulin resistance is reversible by rapamycin, an acute inhibitor of mTORC1 32. In combination with human data suggesting that plasma BCAAs are associated with an increased risk of age-associated diseases 34, and the fact that mTORC1 signaling is a strong negative regulator of lifespan in mice and many model organisms (41, 42, 43, 44, 45 and reviewed in 46), this suggested to us that dietary BCAAs may promote mortality and inhibit healthspan.
Here, we tested the hypothesis that reduced BCAA consumption would extend lifespan. We find that restricting dietary BCAAs extends the lifespan of two progeroid mouse models. Further, a reduced BCAA diet improves the metabolic health and decreases frailty of wild-type C57BL/6J mice when feeding is begun in midlife, and extends the lifespan of male, but not female wild-type mice when started early in life. Finally, a reduced BCAA diet has sex-specific effects on the skeletal muscle transcriptome, with males showing alterations in multiple longevity regulating pathways, including mTOR signaling. In conclusion, these results demonstrate that reducing dietary levels of BCAAs promotes metabolic health, reduces frailty, and increases longevity in mice, and should be investigated as a potential method for preventing and intervening in age-related disease.
Results
Branched-chain amino acid restriction extends the lifespan of progeroid mice
We first assessed the effect of BCAA restriction on the survival of Lamin A/C-deficient mice (Lmna−/−), originally described as a mouse model of Hutchinson-Gilford Progeria Syndrome (HGPS), but now considered a model of muscular dystrophy 47. We utilized an AA-defined Control diet based on a natural source 21% protein mouse diet, as well as a Low BCAA diet and Low AA diet which have BCAAs or all dietary AAs, respectively, restricted by two-thirds (67%). All three of these amino acid-defined diets are isocaloric with identical levels of dietary fat (Table S1).
We fed Lmna−/− mice Control, Low BCAA, or Low AA diets starting at weaning, and determined their survival (Fig. 1A-B, Table S2). As the shape of the survival curves for Low BCAA-fed and Low AA-fed mice suggested that the hazard ratio changed during the lifespan, we analyzed this data using both a log-rank test, which is most powerful in the case of proportional hazards, and the Wilcoxon test, which is more sensitive to early deaths. We observed that Low BCAA-fed Lmna−/− females lived longer (p=0.0794, log-rank test; p=0.0098, Wilcoxon test), with an 86% increase in median lifespan (Fig. 1A, Table S3). In contrast, Low BCAA-fed Lmna−/− males did not live longer (Fig. 1B, Table S3). Similarly, Low AA-fed Lmna−/− female mice lived longer (p=0.366, log-rank test, p=0.0355 Wilcoxon test), with a 59% increase in median lifespan, while Low AA-fed Lmna−/− male mice did not live longer despite a 38% increase in median lifespan (Figs. 1A-B, Table S3). The survival of the longest-lived quartile (hereafter, maximum lifespan), evaluated using the Wang-Allison test 48 as the proportion of mice still alive at the age of 75% mortality in the combined lifespan distribution, was not extended by either diet (Table S3).
Figure 1: Branched-chain amino acid restriction extends the lifespan of progeroid mice.
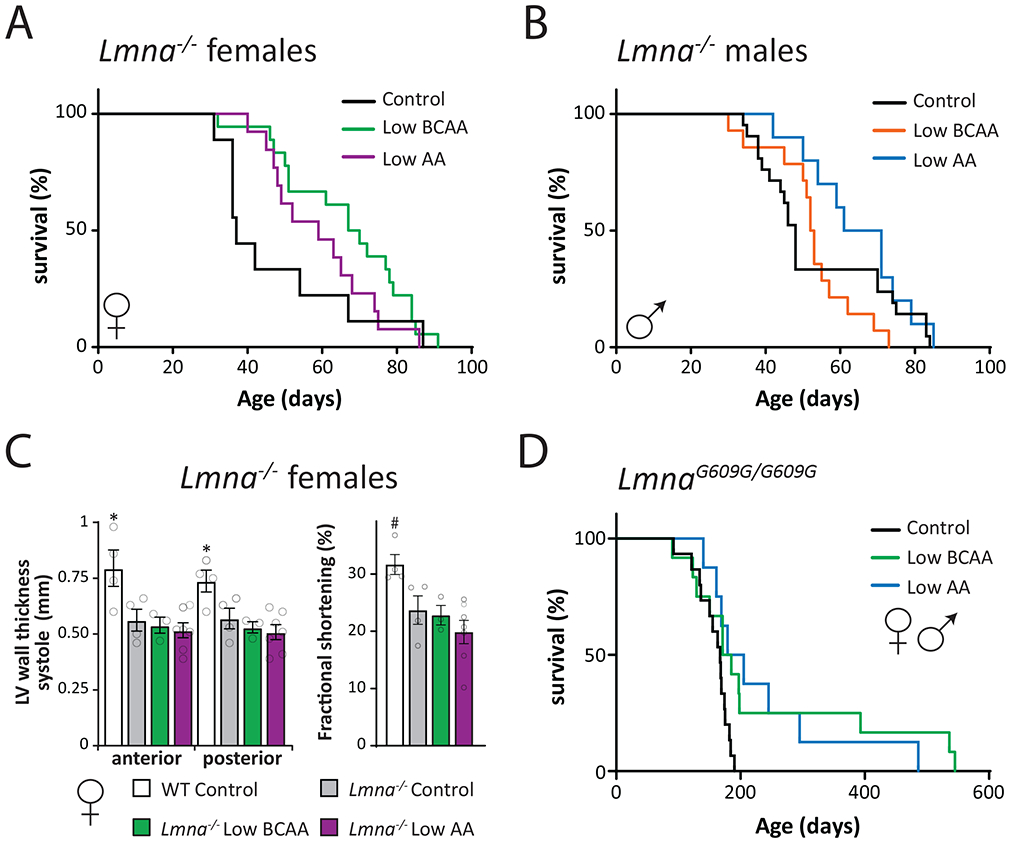
(A-B) Kaplan-Meier plots of the survival of Lmna−/− (A) females (N; Control = 9, Low BCAA = 18, Low AA = 13 biologically independent animals) and (B) males fed the indicated diets starting at weaning (N; Control = 20, Low BCAA = 14, Low AA = 10 biologically independent animals), see also Tables S2 and S3. (C) Echocardiography data of anterior and posterior left ventricular wall thickness (mm) in systole, and fractional shortening (%) in female wild-type and Lmna−/− mice fed the indicated diets from weaning to 40-55 days of age; full echocardiography data can be found in Table S4 (N; WT and Lmna−/− Control = 4, Low BCAA = 3, Low AA = 7 biologically independent animals; two-sided Dunnett’s test in comparison to Lmna−/− Control values post one-way ANOVA; LVAWs *p = 0.0224, LVPWs *p = 0.0406, FS #p = 0.0724). (D) Kaplan-Meier plot of the survival of LmnaG609G/G609G mice fed the indicated diets starting at weaning (N; Control = 15, Low BCAA = 12, Low AA = 8 biologically independent animals, Tables S3 and S5). Data are represented as mean ± SEM.
We performed echocardiography on female Lmna−/− mice to detect diet-induced differences in heart structure and function. While Lmna−/− mice had clear cardiac deficits, no diet treatment corrected the thinning ventricular walls or decreased fractional shortening characteristic of dilated cardiomyopathy (Fig. 1C, Table S4).
We next utilized a mouse model of HGPS, LmnaG609G/G609G, in which the mouse Lmna gene has the same mutation found in most human cases of HGPS 49, 50. For this and all subsequent experiments, we utilized the same AA-defined isocaloric Control and Low AA diets as above, and an advanced Low BCAA diet with the same percentage of calories derived from AAs as the Control diet; this was achieved by proportionally increasing the non-essential AAs (Table S1). We examined the survival of LmnaG609G/G609G mice fed these diets starting at weaning. We observed an increase in the median lifespan of Low BCAA-fed and Low AA-fed mice (7% and 15%, respectively; p=0.0274 and 0.0144, log-rank test) (Fig. 1D, Tables S3 and S5), and a substantial increase in maximum lifespan (125%, p < 0.01 (Low BCAA); 69%, p < 0.05 (Low AA)) (Table S3).
A diet low in branched-chain amino acids improves the metabolic health of aged mice
These results suggested to us that BCAA restriction might be geroprotective in wild-type mice. To test this starting in midlife, we randomized 16-month old male and female C57BL/6J.Nia mice from the NIA Aged Mouse Colony to the Control or Low BCAA diet. We followed these animals longitudinally, with periodic assessments of metabolic health, frailty, and physical performance, and determined their survival (Fig. 2A).
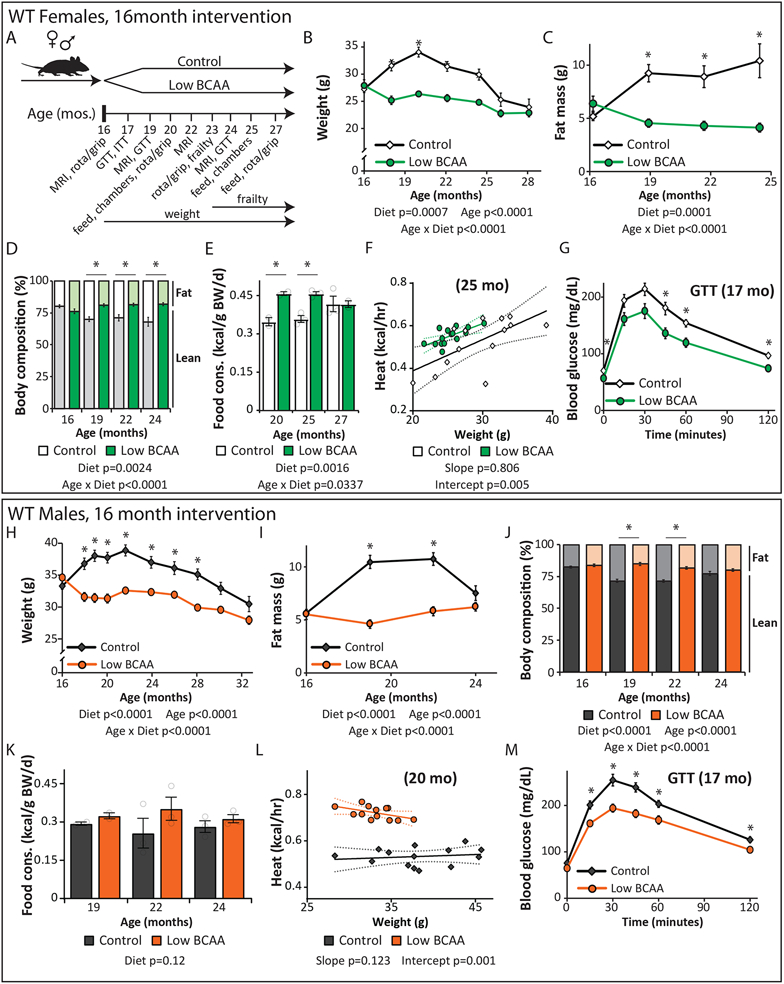
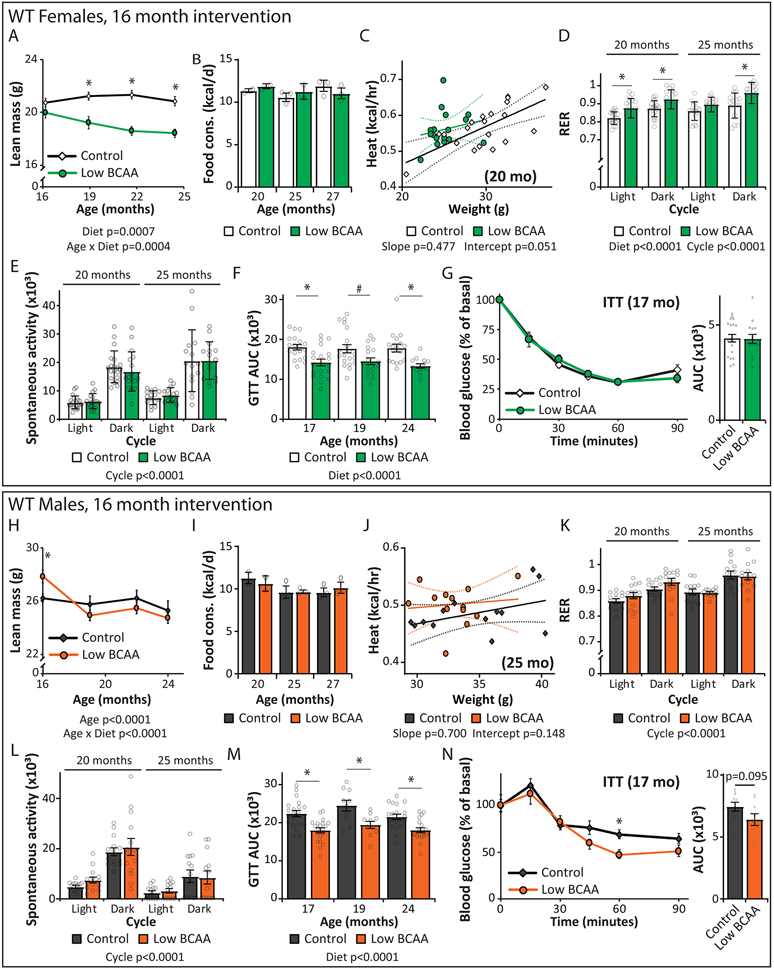
Figure 2: A Low BCAA diet promotes the metabolic health of aged mice.
(A) Female and male C57BL/6J.Nia mice were fed Control or Low BCAA diets beginning at 16 months of age, schematic relevant to Figures 2-3 and Supplementary Tables 1 and 2. (B-G) Female C57BL/6J.Nia mice were fed the indicated diets beginning at 16 months of age. (B) The weight of the mice was tracked starting at 16 months of age (n varies by month; maximum N; Control = 20, Low BCAA = 19 biologically independent animals; * p < 0.05 (p-values by month of age: 18 mo. < 0.0001, 20 mo. < 0.0001, 22 mo. = 0.0016, 24.5 mo. = 0.0222). (C-D) The (C) fat mass and (D) body composition of a subset of mice was tracked (n varies by month; maximum N = 10 biologically independent animals for both groups; * p < 0.05 (p-values for (C) by month of age: 19 mo. = 0.0007, 21.5 mo. = 0.0052, 24.5 mo. = 0.016; p-values for (D) by month of age: 19 mo. = 0.0005, 21.5 mo. = 0.0134, 24.5 mo. = 0.0193). (E) Food consumption over time (maximum N = 3 independent cages for both groups; * p < 0.05 (p-values by month of age: 20 mo. = 0.0141, 25 mo. = 0.0082). (F) Energy expenditure (Heat) was assessed using metabolic chambers at 25 months of age (N; Control = 15, Low BCAA = 15 biologically independent animals). (G) Glucose tolerance test after three weeks of diet feeding (N; Control = 19, Low BCAA = 20 biologically independent animals; * p < 0.05; p-values by time: 0m = 0.0369, 45m = 0.0118, 60m = 0.0066, 120m = 0.0005). (H-M) Male C57BL/6J.Nia mice were fed the indicated diets beginning at 16 months of age. (H) The weight of the mice was tracked starting at 16 months of age (n varies by month; maximum N = 20 biologically independent animals for both groups; * p < 0.05, p-values by month of age: 18 mo. = 0.0005, 19-21.5 mo. <0.0001, 24 mo. = 0.0023, 26 mo. = 0.0073, 28 mo. = 0.0251). (I-J) The (I) fat mass and (J) body composition of a subset of mice was tracked (n varies by month; maximum N = 20 biologically independent animals for both groups; * p < 0.05 (p-values for (I) by month of age: 19-22 mo. < 0.0001; p-values for (J) by month of age: 19-22 mo. < 0.0001). (K) Food consumption of mice over time (maximum N = 3 independent cages for both groups). (L) Energy expenditure (Heat) was assessed using metabolic chambers at 20 months of age (N; Control = 14, Low BCAA = 13 biologically independent animals). (M) Glucose tolerance test after three weeks of diet feeding (N = 20 biologically independent animals for both groups; * p < 0.05, p-values by time: 15m = 0.0034, 30m = 0.0019, 45m = 0.0006, 60m = 0.0279, 120m = 0.0469). (B-E, H-K) Statistics for the overall effects of diet, age, and the interaction represent the p value from a mixed-effects model (restricted maximum likelihood [REML]) or two-way repeated measures ANOVA, multiple comparisons by two-sided Sidak’s post-test. (F, L) Energy expenditure data was analyzed by linear regression of energy expenditure by body weight (ANCOVA). (G, M) two-way repeated measures ANOVA, multiple comparisons by two-sided Sidak’s post-test. Data are represented as mean ± SEM.
Low BCAA-fed female mice weighed less than Control-fed animals throughout their life (Fig. 2B), due principally to weight gain by Control-fed mice. In contrast, Low BCAA-fed female mice did not gain fat mass with age, and therefore remained leaner than age-matched Control-fed females despite a small reduction in lean mass (Figs. 2C-D, Extended Data 1A). This decreased adiposity was not due to caloric restriction, as Low BCAA-fed females consumed more, not less, food than aged-matched Control-fed females relative to their body weight (Fig. 2E, Extended Data 1B).
This seeming paradox in energy balance could result from altered energy expenditure; we found that Low BCAA-fed females had increased energy expenditure at 20 and 25 months of age (Fig. 2F, Extended Data 1C). Although the Control and Low BCAA diets have an identical percentage of calories derived from AAs, carbohydrates, and fats, the respiratory exchange ratio (RER) was higher in Low BCAA-fed females (Extended Data 1D), suggesting a higher reliance on carbohydrates. These animals showed no difference in activity (Extended Data 1E).
Low BCAA-fed midlife females were more glucose tolerant than Control-fed mice as early as three weeks after starting the diet, and with increasing age remained more glucose tolerant (Fig. 2G, Extended Data 1F). However, there was no difference in insulin tolerance between Control-fed and Low BCAA-fed females (Extended Data 1G). Overall, Low BCAA-fed female mice had improved metabolic health relative to Control-fed mice.
The effects of the Low BCAA diet was similar in males; Low-BCAA fed males weighed less, principally due to weight and fat mass gain by Control-fed mice (Fig. 2H-I), although Low BCAA-fed males initially lost a small amount of lean mass (Extended Data 1H). Low BCAA-fed males mice were overall leaner than Control-fed males, though the body composition of the two groups was more similar when last measured at 24 months of age, as Control-fed males began to lose adipose mass (Fig. 2J). As with females, Low BCAA-fed males were not calorically restricted, with an increase in food consumption relative to body weight that did not reach statistical significance (p=0.12) (Fig. 2K, Extended Data 1I). The increased caloric intake of Low BCAA-fed males was balanced by increased energy expenditure, which was significantly higher at 20 months of age and higher, but not significantly (p=0.148) at 25 months of age without increased activity (Fig. 2L, Extended Data 1J).; but in contrast to females, RER was not significantly increased; there was also no difference in activity levels (Extended Data 1K-L).
Low BCAA-fed midlife males rapidly became more glucose tolerant than Control-fed animals, and remained more glucose tolerant as they aged (Fig. 2M, Extended Data 1M). In contrast to Low BCAA-fed females, Low BCAA-fed males had improved insulin sensitivity relative to Control-fed males, but this did not reach statistical significance (p=0.095) (Extended Data 1N).
A Low BCAA diet fed to middle-aged mice reduces frailty but does not extend lifespan
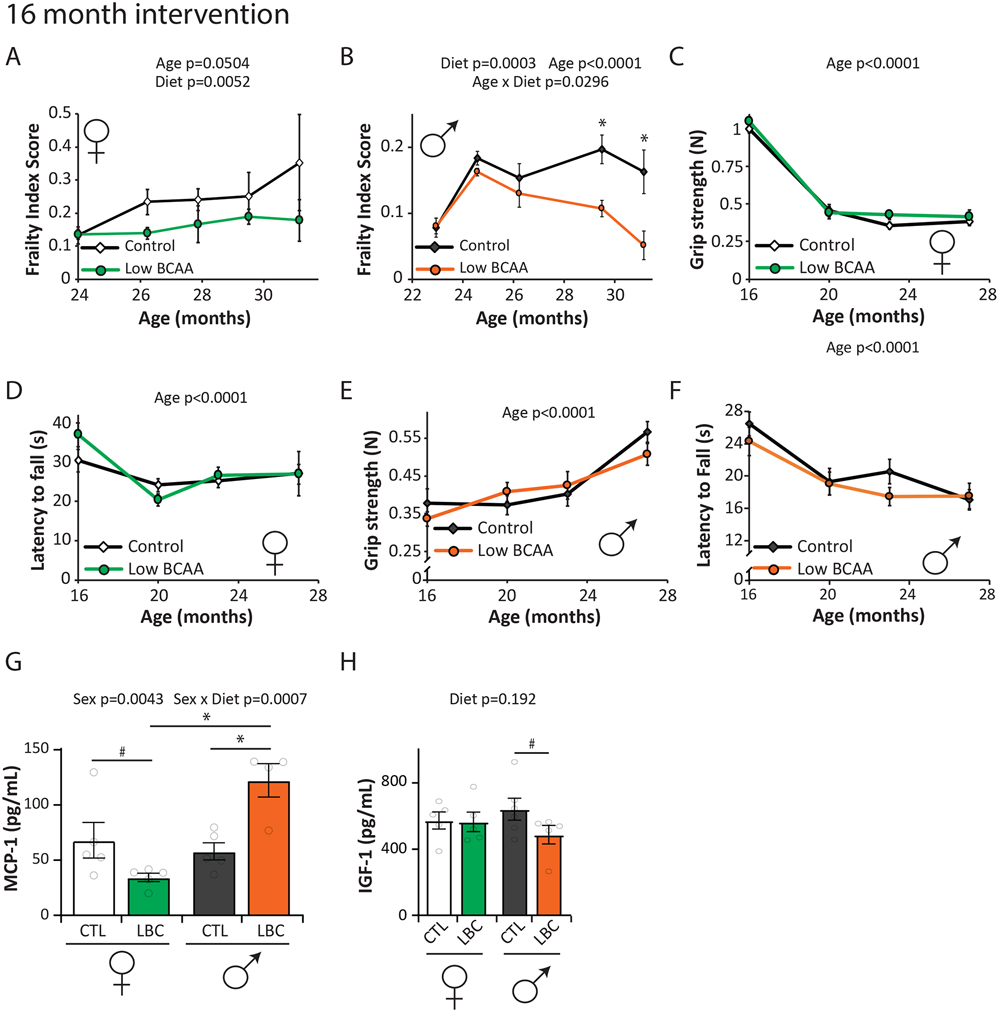
Mice and humans become increasingly frail with age, and starting at approximately 23 months of age, we utilized a recently developed mouse frailty index to assess the impact of a Low BCAA diet on frailty 51, 52. As expected, we observed increased frailty with age in Control-fed mice of both sexes (Figs. 3A-B). Overall, frailty scores were significantly lower in the low BCAA diet groups, in both males and females (Figs. 3A-B). The low BCAA diet did not negatively or positively impact grip strength or rotarod performance in either sex (Figs. 3C-F). Detailed frailty data can be found in Tables S6-7.
Figure 3: A Low BCAA diet promotes healthy aging.
C57BL/6J.Nia mice were fed the indicated diets beginning at 16 months of age. (A-B) Frailty was assessed longitudinally in (A) female (n varies by month; maximum N; Control = 7, Low BCAA = 8 biologically independent animals) and (B) male mice starting at 23-24 months of age (n varies by month; maximum N = 10 biologically independent animals for both groups; * p < 0.05 (adjusted p-values by month of age: 29.5 mo. = 0.0075, 31 mo. = 0.003); average frailty by index measured can be found in Tables S6-7, respectively. (C-D) Grip strength (C) and rotarod (D) performance in females was assessed longitudinally. (E-F) Grip strength (E) and rotarod performance (F) in males was assessed longitudinally. (C-F) N varies by month; maximum N = 20 biologically independent animals for all groups. (A-F) Statistics for the overall effects of diet, age, and the interaction represent the p value from a mixed-effects model (restricted maximum likelihood [REML]) or two-way ANOVA; * p-values reported in (B) represent a two-sided Sidak’s post-test. (G-H) Levels of MCP-1 (G) and IGF-1 (H) in serum was determined by ELISA (22 months old female, 25 months male; N: females and Control males = 5, Low BCAA males = 4 biologically independent animals; statistics for the overall effects of diet, age/sex, and the interaction represent the p value from a two-way ANOVA, multiple comparisons by two-sided Sidak’s post-test; *p < 0.05, # p < 0.16, adjusted p-values in (G): CTL female vs. LBC female = 0.1016, CTL male vs. LBC male = 0.0034, LBC female vs. LBC male = 0.0002; # adjusted p-value in (H) CTL male vs. LBC male = 0.1525). CTL=Control, LBC=Low BCAA. Data are represented as mean ± SEM.
Circulating levels of monocyte chemoattractant protein 1 (MCP-1) increase with age and frailty in both rodents and humans 53. We found a significant interaction between sex and diet on the levels of MCP1 in aged mice, with a significant increase in MCP-1 in Low BCAA-fed male mice despite the decrease in frailty (Fig. 3G). Levels of insulin-like growth factor-I (IGF-1) are associated with aging and mortality, and are decreased by geroprotective interventions, including PR, CR, methionine restriction, and rapamycin 54, 55, 56, 57. We did not detect a significant change in the level of IGF-1 in either male or female mice fed a Low BCAA diet (Fig. 3H).
Although a Low BCAA diet improved metabolic health and promoted robustness in both sexes, there was no significant effect on the overall survival of either sex when started at midlife (Figs. 4A-B, Tables S3 and S8). However, we there was an excess of early mortality among female mice switched to the Low BCAA diet, with 26% dying prior to 717 days (the lifespan of the last mouse in the Low BCAA group prior to the curves crossing), vs only 8% of the female mice switched to a Control diet. Similar initial increases in mortality have been observed in methionine-restricted mice 27 and tryptophan restricted rats 30, as well as calorie restricted mice 58.
Figure 4: A Low BCAA diet does not extend the lifespan of middle-aged mice.
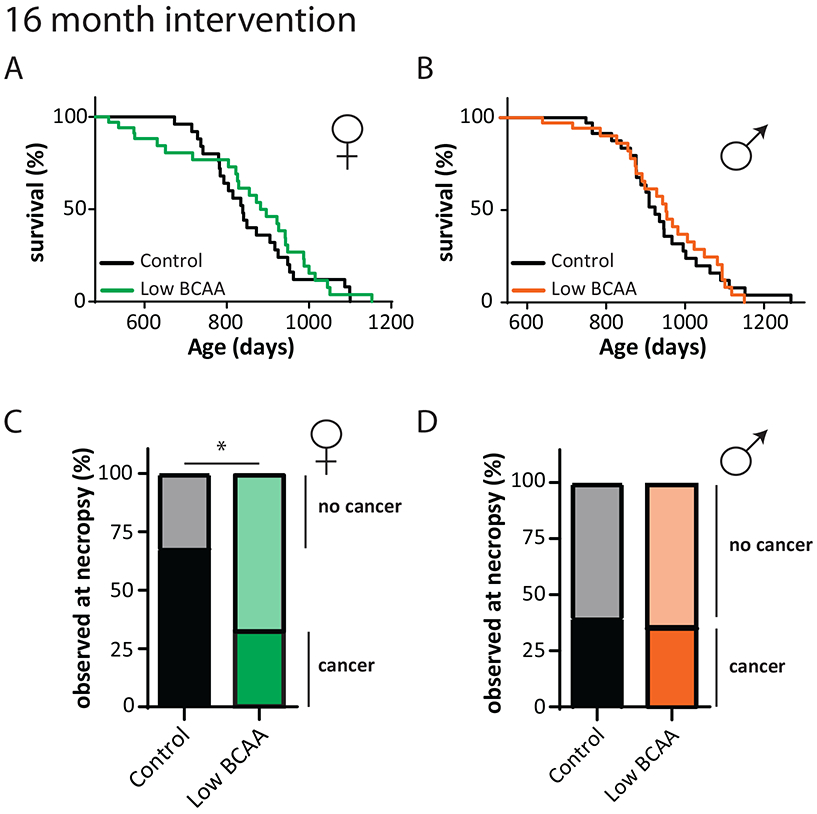
(A-B) Kaplan-Meier plots showing the survival of (A) female (N = 34 biologically independent animals for both groups) and (B) male C57BL/6J.Nia mice fed the indicated diets starting at 16 months of age (N = 35 biologically independent animals for both groups) see also Table S8. (C) Percent of female mice with and without cancer observed at necropsy from lifespan in (A) (N; Control cancer = 17, Low BCAA cancer = 8, Control non-cancer = 8, Low BCAA non-cancer = 17 biologically independent animals). (D) Percent of males with and without cancer observed at necropsy from lifespan in (B) (N; Control cancer = 10, Low BCAA cancer = 9, Control non-cancer = 15, Low BCAA non-cancer = 16 biologically independent animals). (C-D) two-sided Fisher’s exact test, * p = 0.0254.
While cause of death analysis is difficult in mice, we noted the presence or absence of observable cancers by gross necropsy. We observed cancer in 68% of the Control-fed females; in contrast, we observed cancer in only 33% of Low BCAA-fed females (Fig. 4C). There was no observed decrease in the cancer rate of Low BCAA-fed males (Fig. 4D).
Low BCAA diet feeding throughout life extends male lifespan
Dietary interventions, including CR, have a more pronounced effect on longevity when begun early in life 59. As the survival of progeroid mice was increased when Low BCAA and Low AA diets were initiated at weaning, we examined how lifelong feeding of these diets impacts the healthspan and longevity of wild-type mice (Extended Data 2A).
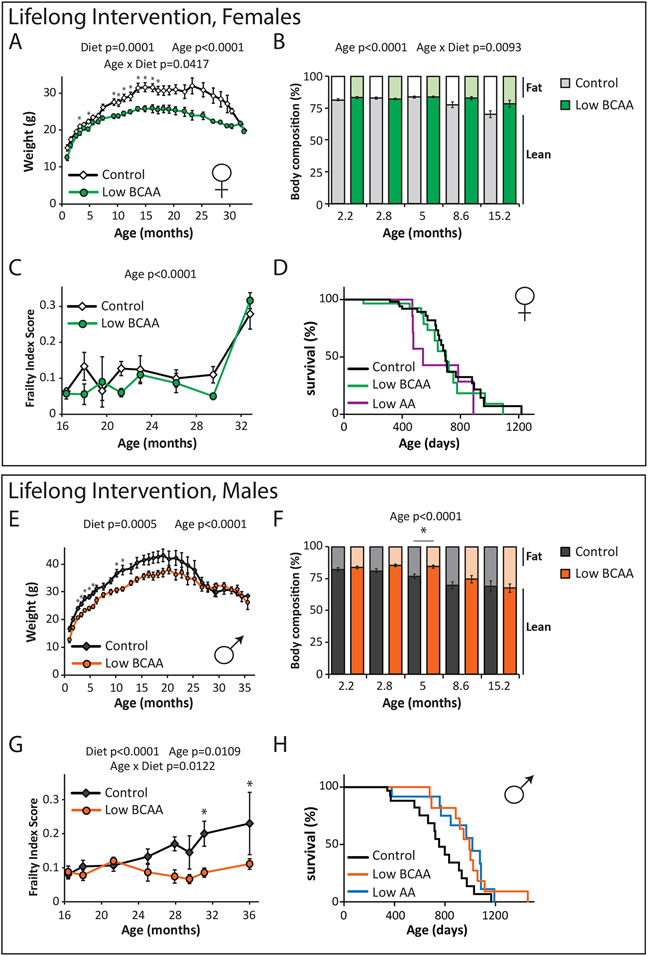
Low BCAA-fed females weighed significantly less than Control-fed mice throughout the majority of their life (Fig. 5A). While the body composition of these groups were initially similar, as the mice aged Low BCAA-fed females became leaner than their Control-fed counterparts due to reduced fat mass gain (Figs. 5B, Extended Data 2B-C). In contrast to females fed a Low BCAA diet starting in midlife, the food consumption, RER, activity, and energy expenditure of Low BCAA-fed females after four months of diet feeding was similar to that of Control-fed mice (Extended Data 2D-H). We observed that a Low BCAA diet improved glucose tolerance in females at multiple ages (Extended Data 2I), with no change in systemic insulin sensitivity as assessed by insulin tolerance test (Extended Data 2J).
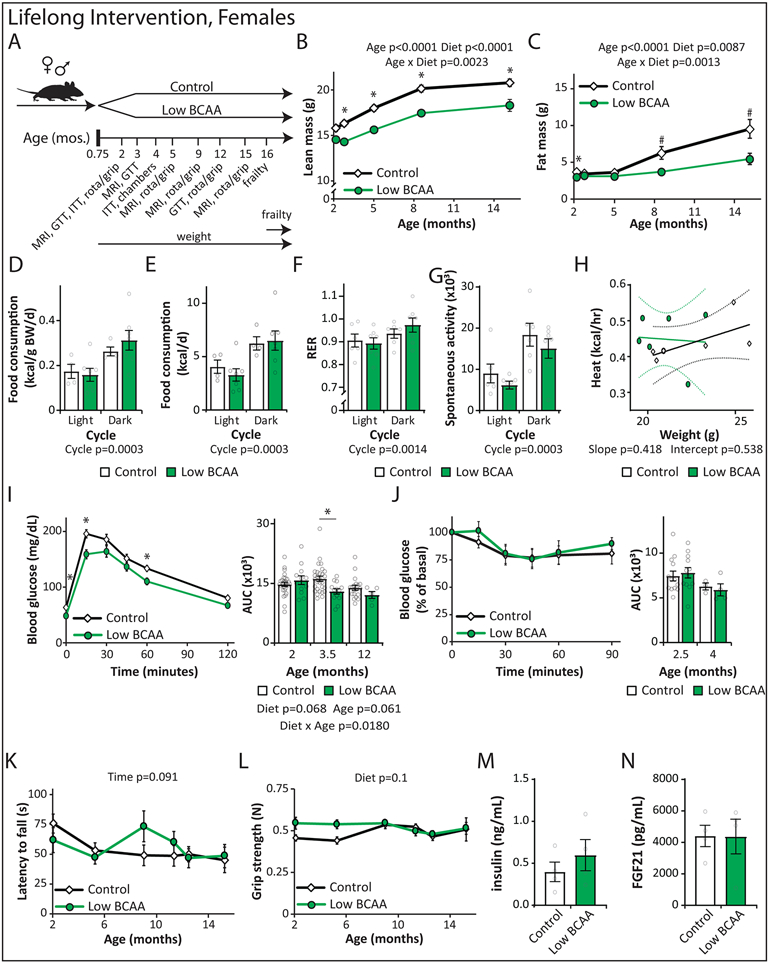
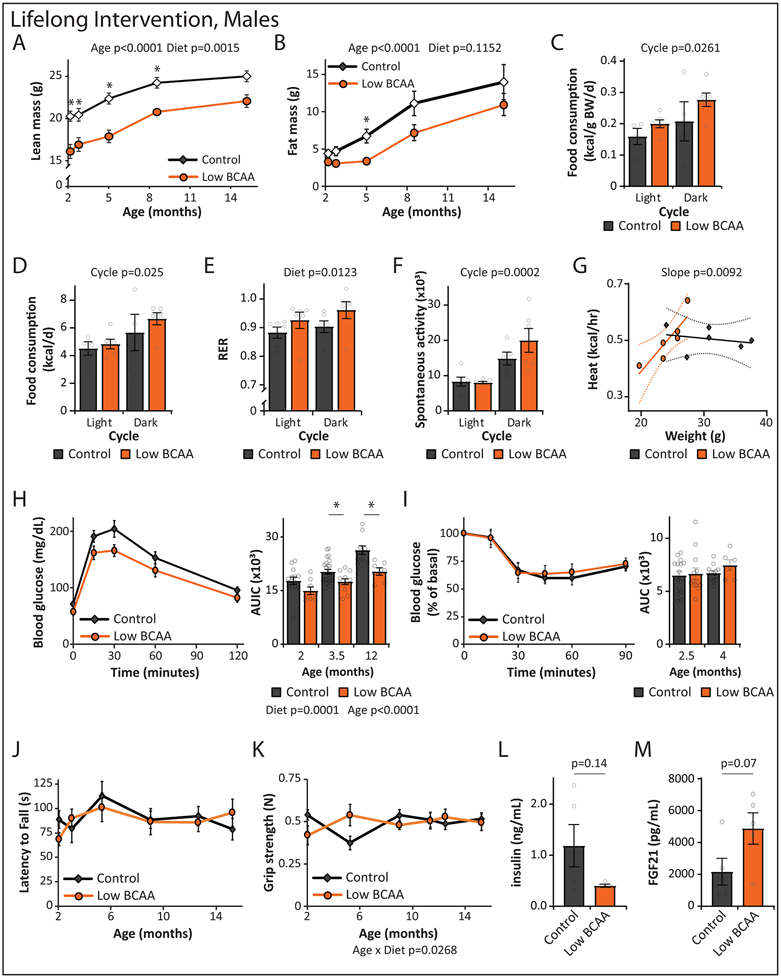
Figure 5: Lifelong consumption of a Low BCAA diet promotes healthspan and extends male lifespan.
Wild-type female (A-E) and male (F-J) mice were placed on either Control or Low BCAA diets at weaning. (A) The weight of the female mice was tracked longitudinally (n varies by month, maximum N; Control = 35, Low BCAA = 22 biologically independent animals; * p < 0.05, adjusted p-values by month of age: 3.25 mo. = 0.0015, 4.75 mo. = 0.0136, 9.5 mo. = 0.0102, 10 mo. = 0.0341, 11 mo. = 0.0065, 12.25 mo. = 0.0393, 13.5 mo. = 0.0047, 15 mo. = 0.0057, 16.25 mo. = 0.0150, 17.25 mo. = 0.0323). (B) Female body composition (n varies by month, maximum N; Control = 15, Low BCAA = 12 biologically independent animals). (C) Female frailty was assessed longitudinally starting at 16 months of age (n varies by month, maximum N; Control = 25, Low BCAA = 18 biologically independent animals); average frailty by index measured can be found in Table S9. (D) Kaplan-Meier plot showing the survival of females fed the indicated diets from weaning (N; Control = 60, Low BCAA = 29, Low AA = 9 biologically independent animals, Table S10). (E) The weight of the male mice was tracked longitudinally (n varies by month, maximum N; Control = 22, Low BCAA = 23 biologically independent animals; * p < 0.05, adjusted p-values by month of age: 2.5 mo. = 0.0014, 3.25 mo. = 0.0190, 4 mo. = 0.0014, 4.75 mo. = 0.0028, 5.5 mo. = 0.0005, 10 mo. = 0.025, 11.25 mo. = 0.0283). (F) Male body composition, (n varies by month, maximum N; Control = 19, Low BCAA = 18 biologically independent animals; *p = 0.0154). (G) Male frailty was assessed longitudinally starting at 16 months of age (n varies by month, maximum N = 13 biologically independent animals for both groups; * p < 0.05, adjusted p-values by month of age: 31 mo. = 0.0409, 36 mo. = 0.0199); average frailty by index measured can be found in Table S11. (H) Kaplan Meier plot showing the survival of males fed the indicated diets from weaning (N; Control = 54, Low BCAA = 30, Low AA = 21 biologically independent animals, Table S10). (A-C, E-G) Statistics for the overall effects of diet, age, and the interaction represent the p value from a mixed-effects model (restricted maximum likelihood [REML]) or two-way repeated measures ANOVA, multiple comparisons by two-sided Sidak’s post-test. Data are represented as mean ± SEM.
During the first year, we performed assays to determine the effect of a Low BCAA diet on physiological development, muscle strength, and neuromuscular coordination. We found no change in either rotarod performance or grip strength in female mice on a Low BCAA diet (Extended Data 2K-L). We collected blood at 16 months of age, and quantified insulin and fibroblast growth factor 21 (FGF21); we observed no effect of diet on the levels of these hormones (Extended Data 2M-N). Starting at 16 months of age, we switched to non-invasive frailty assessment; we observed no statistically significant differences between Control-fed and Low BCAA-fed females (Fig. 5C, Table S9). The overall lifespan of Low BCAA-fed female mice was indistinguishable from that of Control female mice (Fig. 5D, Tables S3 and S10). A small cohort of female mice fed a Low AA diet in parallel showed decreased survival, with a 22% reduction in median lifespan, but the effect failed to reach statistical significance (Fig. 5D and Table S3).
Lifelong Low BCAA diet-fed males also weighed less than Control-fed males for the majority of their life (Fig. 5E). In contrast to females, Low BCAA-fed males rapidly became leaner due to reduced accretion of fat mass, but lean mass gain was also substantially impaired, and by 15 months of age the body composition of Low BCAA-fed males was similar to that of Control-fed males (Figs. 5F, Extended Data 3A-B). Low BCAA-fed males did not consume more food, alter activity level, or increase energy expenditure, but RER was increased (Extended Data 3C-G). Glucose tolerance was robustly and consistently improved, with no change in systemic insulin sensitivity as assessed by insulin tolerance test (Extended Data 3H-I).
As in females, a Low BCAA diet did not impair rotarod performance or grip strength during the first year of life (Extended Data 3J-K). At 16 months of age, Low BCAA-fed males showed decreased fasting insulin (Extended Data 3L) and increased levels of FGF21 (Extended Data 3M) but these effects were not statistically significant. We observed that Low BCAA-fed males had reduced frailty relative to Control-fed males after 25 months of age (Fig. 5G, Table S11). In agreement with this significant difference in frailty, we observed that lifelong consumption of a Low BCAA diet significantly increased lifespan, with a 31.8% increase in median lifespan relative to Control-fed males, and a 12.3% increase in maximum lifespan (p = 0.0251) (Fig. 5H, Tables S3 and S10). A parallel cohort of Low AA-fed males also lived significantly longer, with a 34.9% increase in median lifespan and an 18.2% increase in maximum lifespan (p = 0.0066), and were indistinguishable from Low BCAA-fed males (Fig. 5H and Table S3). The longest-lived Low BCAA-fed male lived to 1456 days, just shy of 4 years (Fig. 5H and Table S10), exceeding the lifespan of the longest-lived survivor in several previous studies of ad libitum fed C57BL/6J mice 60, 61 by approximately 25%.
Lack of detrimental or beneficial effects of prolonged BCAA restriction on cardiovascular function
Blood levels of BCAAs are associated with risk for cardiovascular diseases 62, cardiac dysfunction and remodeling following myocardial infarction, and an increased incidence of subsequent adverse events 63, 64, 65. To determine if a Low BCAA diet might prevent age-associated cardiac dysfunction, or alternatively if lifelong Low BCAA-feeding might lead to impairment in cardiovascular structure or function, we performed echocardiograms on a subset of lifelong Control-fed and Low BCAA-fed mice of both sexes at 16 months of age (Table S12). We observed no significant differences in cardiovascular parameters, including fractional shortening and ejection fraction, in Low BCAA-fed mice (Table S12). We also found a significant sex x diet interaction in parameters related to aortic stiffening with age (Table S12). Thus, lifelong Low BCAA diet feeding did not clearly improve or impair cardiac function.
Transcriptional profiling of skeletal muscle identifies sex-specific changes in longevity-associated signaling pathways
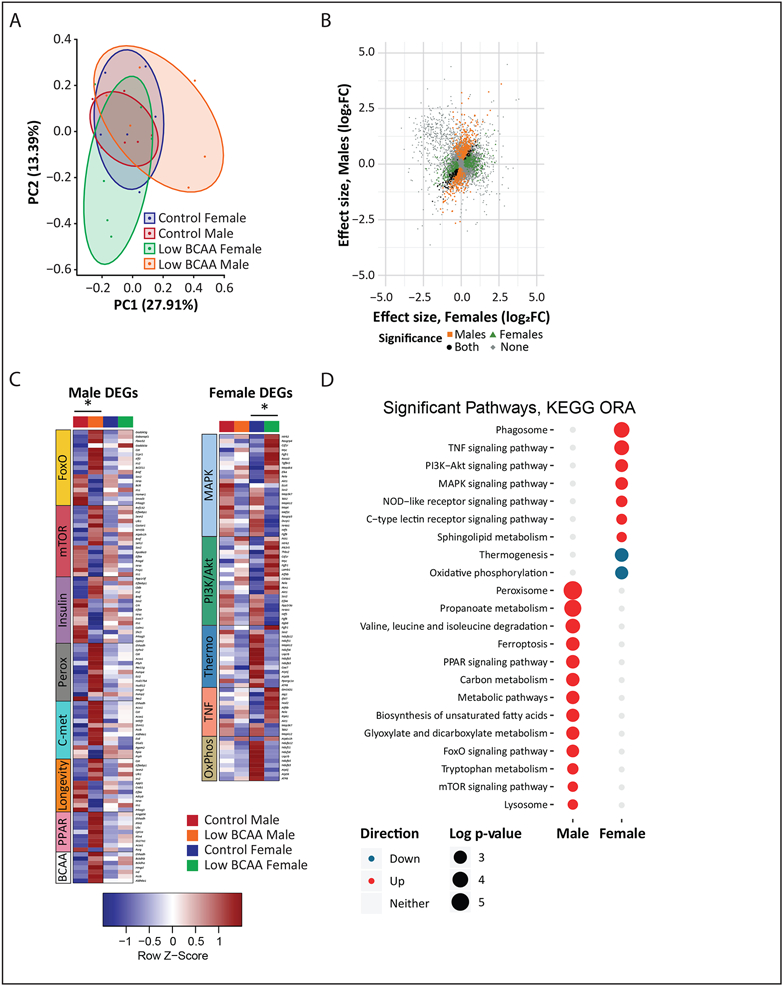
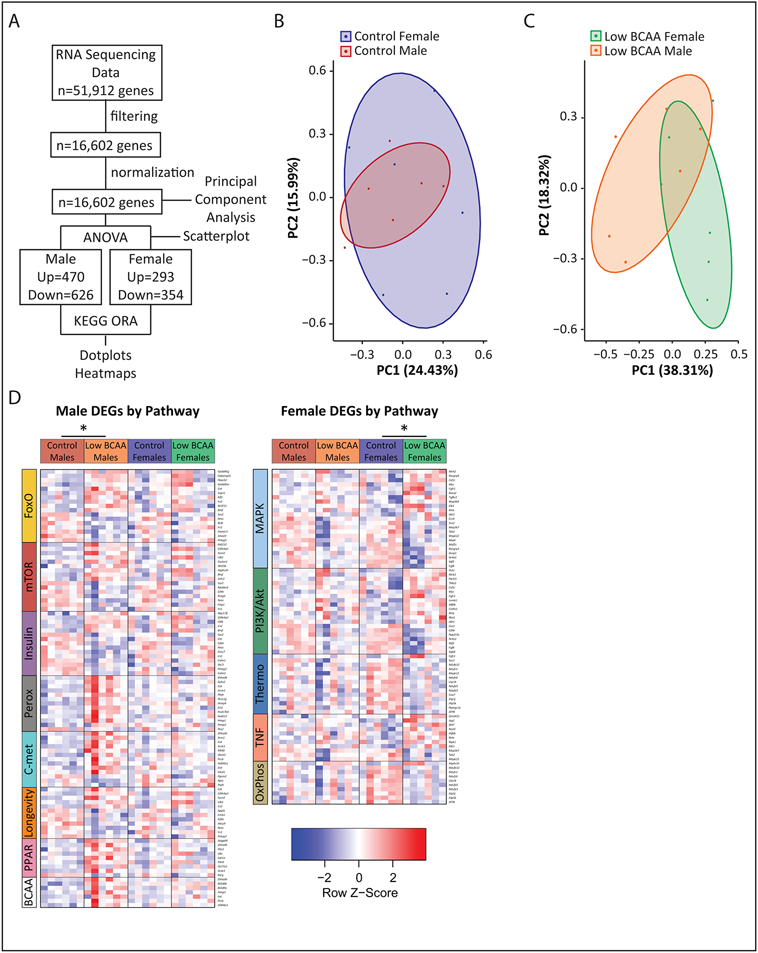
To obtain insight into the sex-specific effects of a Low BCAA diet, we performed transcriptional profiling of the quadriceps muscle of Control and Low BCAA lifelong fed mice at 16 months of age (Extended Data 4A, Tables S13 and S14). Principal component analysis showed that while the muscle transcriptome of male and female Control-fed mice substantially overlapped, it diverged upon Low BCAA diet feeding (Figs. 6A, Extended Data 4B-C). Approximately 80% more genes were significantly altered by a Low BCAA diet in males than females, with relatively little overlap between the sexes, as visualized by a scatterplot of the effect size of Low BCAA-feeding (Fig. 6B, Tables S13A and S14A).
Figure 6: Transcriptional profiling of skeletal muscle identifies male-specific changes in longevity-associated signaling pathways.
Transcriptional profiling was performed on mRNA from the skeletal muscle of male and female mice that consumed either Control or Low BCAA diets from weaning until 16 months of age (N = 6 biologically independent animals for all groups; Tables S13-14). (A) Principal component analysis. (B) Scatterplot showing effect size of Low BCAA diet feeding in males and females, colored according to significance in one, both, or neither group. (C) Heatmaps of differentially expressed genes from significant KEGG over-representation analysis (ORA) pathways of interest identified in Tables S13B, S14B. DEGs were identified using an empirical Bayes moderated linear model, * two-sided p-values adjusted with the Benjamini-Hochberg procedure. (D) Dotplot of significant pathways and direction changed from KEGG ORA in Table S13C, S14C. CTL=Control, LBC=Low BCAA. All overrepresentation analysis was performed using designated by an adjusted p value <0.3 for female and <0.2 for male contrasts in a one-sided hypergeometric test, and p-values were adjusted using the Benjamini-Hochberg procedure.
To identify pathways altered by a Low BCAA diet in each sex, we performed KEGG over-representation analysis (ORA) on the differentially expressed genes, regardless of directionality. In males, we found that a Low BCAA diet resulted in significantly altered multiple KEGG pathways related to metabolism and longevity, including the FoxO, insulin, and mTOR signaling pathways (Table S13B). A Low BCAA diet altered a distinct and non-overlapping set of pathways in females (Table S14B). Visualizing the individual transcriptional changes in pathways of interest, we noted that several KEGG pathways identified as significantly altered only in a single sex appeared to be altered to a lesser degree in the opposite sex (Fig. 6C, Extended Data 4D).
We also performed KEGG ORA with gene directionality (Fig. 6D, Tables S13C and S14C). Males subject to Low BCAA diet feeding significantly upregulated many pathways centered around metabolism and longevity, and again, a distinct set of non-overlapping metabolic pathways were altered in females. Notably, although the mTOR signaling pathway was listed as upregulated, several of the upregulated genes, including Sesn2 and Castor1, encode negative regulators of mTORC1 66, 67.
As inhibition of mTORC1 robustly extends longevity in mice and this pathway was significantly altered only in males, we examined mTORC1 signaling in more detail. We fed an additional cohort of young and aged mice Control and Low BCAA diets from 16 months of age until an age at which we expected 10% mortality, 22 months of age (females) and 25 months of age (males). Young mice were fed in parallel for an equivalent length of time beginning at 6.5 months of age. Mice were then euthanized and tissues collected for analysis of mTOR signaling.
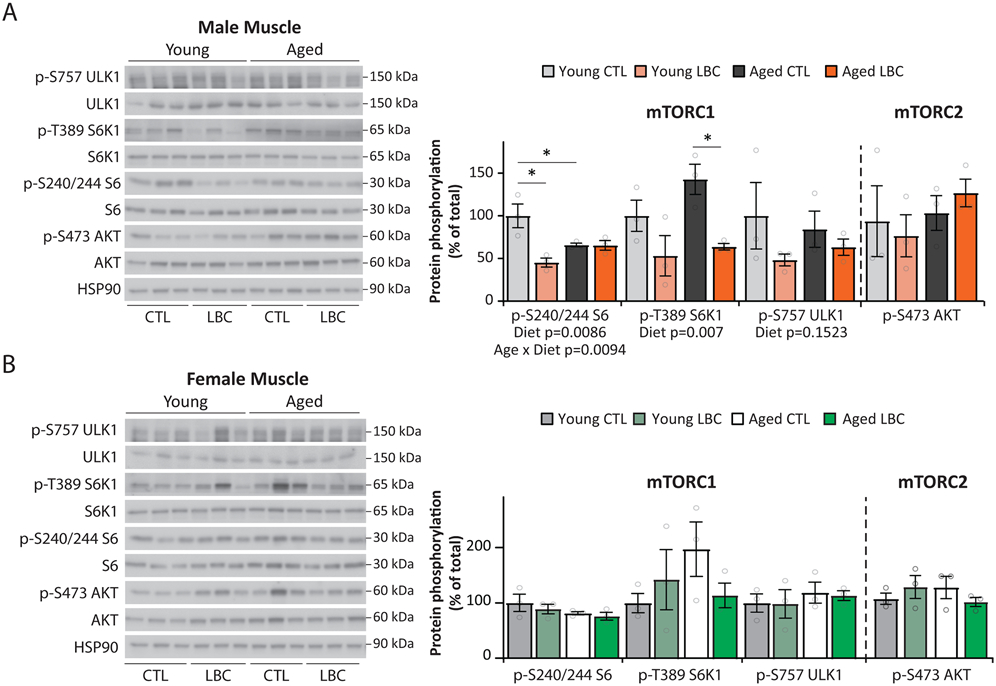
We found that males fed a Low BCAA diet had significantly decreased phosphorylation of the mTORC1 substrate S6K1 T389 in skeletal muscle, with a 47% decrease in young males and a 78% decrease in aged males (Extended Data 5A). Phosphorylation of S6 S240/S244, a downstream readout of mTORC1 activity, was likewise reduced by 55% in young males. There was a statistically significant overall effect of diet on the phosphorylation of both S6 and S6K1 (Extended Data 5A). We did not observe any changes in the phosphorylation of the mTORC2 substrate AKT S473 (Extended Data 5A), demonstrating that these effects were specific to mTORC1. No changes in the phosphorylation of S6K1 T389, S6 S240/S244, ULK1 S757, or AKT S473 were observed in Low-BCAA-fed females (Extended Data 5B).
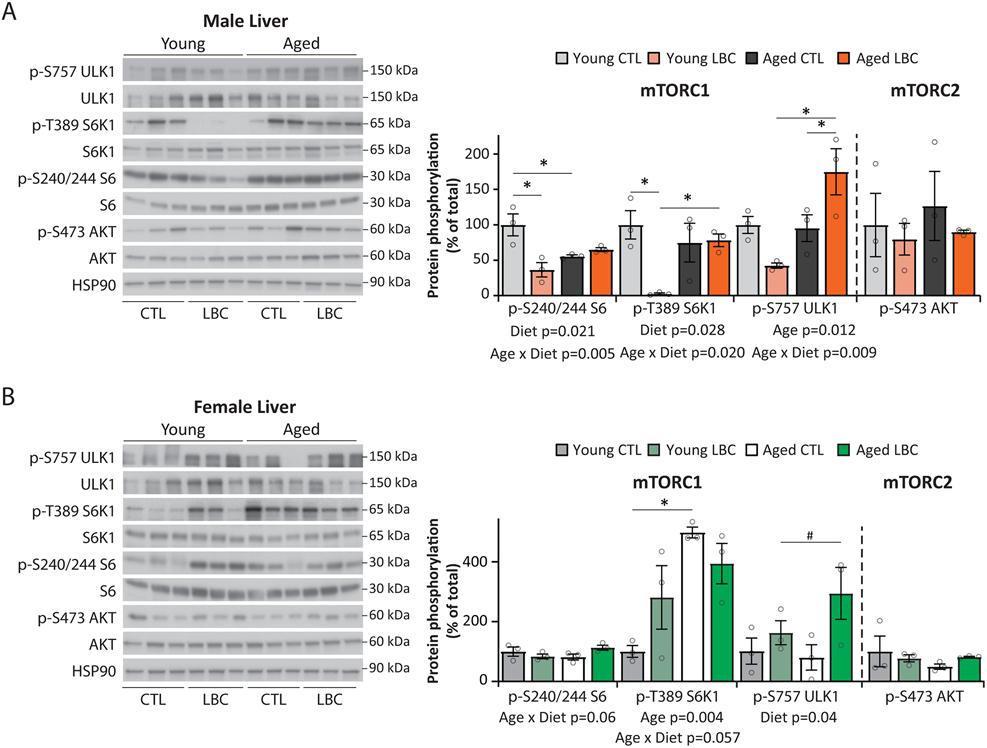
We also examined the phosphorylation of these mTOR substrates in the liver. We observed that phosphorylation of S6K1 T389 was decreased by 97% in young Low BCAA-fed males, and phosphorylation of S6 S240/244 was similarly decreased by 64% (Extended Data 6A). As in skeletal muscle, there was a statistically significant overall effect of diet on the phosphorylation of both S6 and S6K1 in male liver, as well as a statistically significant Age x Diet interaction on the phosphorylation of ULK1. We observed no effect of a Low BCAA diet on mTOR signaling in female liver (Extended Data 6B).
Discussion
Reduced consumption of dietary protein is associated with increased metabolic health and longevity in organisms including flies, mice, rats, and humans 11, 12, 13, 15, 17, 18, 19, 20, 21, 22. The beneficial effects of PR may be mediated by reduced consumption of specific AAs. Based on emerging evidence that reducing dietary levels of the three BCAAs recapitulates the metabolic benefits of a PR diet, and that elevated levels of BCAAs promote insulin resistance, obesity, and mortality 17, 18, 32, 36, 38, we tested the hypothesis that restricting dietary BCAAs would promote healthspan and longevity.
Here, we determined the effect of restricting dietary BCAAs on the longevity of both progeroid and wild-type C57BL/6J mice as well as multiple measures of healthspan. We show that a reduced BCAA diet promotes longevity in two distinct progeroid mouse models. A reduced BCAA diet begun in middle-aged C57BL/6J mice promotes metabolic health, keeping mice lean and glucose tolerant; decreases frailty; and also reduces cancer in female mice. Lifelong consumption of a reduced BCAA diet improves the metabolic health of wild-type mice, reduces frailty in males, and significantly extends male but not female lifespan. Finally, we show that a reduced BCAA diet has sex-specific effects on the skeletal muscle transcriptome, reducing mTORC1 signaling specifically in males.
We first utilized short-lived progeroid mice in order to rapidly analyze the effect of these diets; several other geroprotective interventions also promote the health of progeroid mice 47, 68, 69. We utilized two distinct mouse models: first, Lmna−/−, a model of Emery-Dreifuss muscular dystrophy 70; and second, a recently developed model of HGPS, LmnaG609G/G096G, which has the same mutation as most humans with HGPS 50. The Low BCAA and Low AA diets increased survival in both models, increasing the overall survival and maximum lifespan of LmnaG609G/G096G mice.
The effect on the survival of Lmna−/− mice was limited to females, and was significant only by the Wilcoxon test, which gives greater weight to early deaths than the log-rank test and does not assume proportional hazards; median but not maximum lifespan was increased in Low BCAA-fed Lmna−/− female mice. Although we did not conduct a detailed study of the physiological or molecular impacts of a Low BCAA diet on Lmna−/− mice, the shape of the survival curves suggest that the hazard ratio varies during the lifespan. The benefit of a Low BCAA diet to the survival of Lmna−/− female mice may therefore result from improved health during midlife rather than a reduction in the rate of aging. Alternatively, the inability of a Low BCAA diet to extend maximum lifespan may be related to the fact that Lmna−/− mice suffer from muscular dystrophy. While further research will be required in order to determine how Low BCAA and Low AA diets extend the survival of these progeroid strains, and to assess if these diets may provide insight into the treatment of muscular dystrophy or HGPS, these findings in progeroid mice support our overall conclusion that a Low BCAA diet promotes healthy aging.
We next tested the effects of a Low BCAA diet using wild-type C57BL/6J mice, beginning the diet at 16 months of age. The diet promotes metabolic health, keeping mice lean and glucose tolerant. A limitation of our study is that Control-fed mice gained weight and fat for several months, achieving a peak weight similar to those of other aged chow-fed mice in our animal facility 71, 72, 73. The weight gain we observed in Control-fed mice may be due to transition of these animals, previously housed at the NIA Aging Mouse Colony, from a NIH31 diet; further research will be required to clarify the effect of a Low BCAA diet on the weight and adiposity of aged mice.
Low protein intake is associated with increased frailty in adults 9, 11, and the role of dietary protein and BCAA intake on sarcopenia, frailty, and longevity in the aged has been debated extensively 74. Despite the importance of dietary protein in building and maintaining muscle, there were no negative effects on a Low BCAA diet on grip strength or rotarod performance in either sex, and a Low BCAA diet led to a reduction in age-associated frailty. Unexpectedly, the chemokine MCP-1, which has been suggested as a circulating biomarker of age and frailty 53, was increased in Low BCAA-fed males. Understanding the relationship between dietary BCAAs, MCP-1 and frailty during aging will require additional research; examining MCP-1 and frailty in Low AA-fed animals would also be informative.
Our results support the idea that reducing dietary BCAAs promotes multiple aspects of healthspan in mice. Beginning a reduced BCAA diet at midlife had no effect on male lifespan. While there was also no effect on overall lifespan in females, we observed significant excess mortality in the Low BCAA-fed females following the diet switch. A similar effect has previously observed in several other studies that have examined the lifespan of AA-restricted mice 27, 31, as well as some studies of CR animals 58, 73. Future studies, perhaps using a gradual “stepdown” of BCAAs rather than an abrupt 67% restriction in order to avoid any stress related to the diet switch, will be needed to unambiguously establish the effects of midlife BCAA restriction on female lifespan.
BCAAs are linked to the development and progression of cancer 75, 76, and we observed a 50% reduction in tumors in Low BCAA fed female mice. As cancer is the predominant cause of death in C57BL/6J mice, a reduction in the rate or progression of neoplasia would be expected to correlate with increased longevity. We did not identify cancer types or perform in-depth pathology, and more in-depth necropsies should be performed in these future studies to better understand the effect of a Low BCAA diet on cause of death, cancer incidence and progression. Future studies would also benefit from the inclusion of a chow-fed control group, which would enable any effects of AA-defined diets on lifespan or healthspan to be detected.
Dietary interventions often have a stronger effect on longevity when begun early in life 59. We observed that lifelong consumption of a Low BCAA diet improves metabolic health, and does not impair rotarod performance, grip strength, or cardiac function. A Low BCAA diet blunts the age-associated increase in frailty in males. Finally, we found that lifelong consumption of a Low BCAA diet robustly extends the median lifespan of male mice by 32%, and the survival of the longest-lived quartile was increased by 12%, recapitulating the effect of restricting all dietary AAs on lifespan.
Lifelong consumption of a Low BCAA or Low AA diet did not extend female lifespan. Although we did not anticipate that PR or BCAA restriction would have sex-specific effects, female and male mice benefit maximally from different levels of CR 58, and in Drosophila males and females have optimal reproduction on low and high protein diets, respectively 19, 77. As many PR have focused on males, a sexually dimorphic response to a PR diet may have been overlooked; a recent study suggests that female mice have a blunted metabolic response to PR 78. One early study found PR extends the lifespan of female rats when PR was started at 120 days of age 79. It is possible that the precise level of PR and BCAA restriction used here, or the age of initiation, was not optimal for female longevity.
Another limitation of the research presented here is our use of mice on a single genetic background, C57BL/6J. It has become clear that the effects of a CR diet vary not only by sex, but also by strain 58, 80. Strain-dependent effects have been observed with respect to the metabolic effects of a series of different healthy diets 81, as well as the phenotypes relating to genetic modifications 82. Genetic background will likely play a role in determining the effect of BCAA restriction on healthspan and longevity, and examining BCAA restriction in different strains or in a heterogeneous genetic background such as HET3 mice will be crucial in understanding if these effects are strain-independent and the relevance to the genetically heterogeneous human population.
Our results broadly agree with a recent study showing that excess dietary BCAAs shorten lifespan 38. However, Dr. Solon-Biet and colleagues did not observe increased longevity or a statistically significant improvement in glucose tolerance in mice fed a reduced BCAA diet. Key differences from our study include the age at which restriction was initiated, diet design and the degree of restriction. Dr. Solon-Biet and colleagues examined 50% and 80% restriction starting at 12 weeks of age, and the approach used for diet construction increased levels of other essential AAs, including methionine and tryptophan, that impact longevity 27, 28, 30. Our study specifically restricted BCAAs by 67%, with dietary levels of all other essential AAs kept constant; the level of restriction was selected based as our previous work which found that restricting dietary AAs to a greater degree led to a progressive loss of lean mass 18. Additional research will be required to define the optimal level of dietary BCAAs for healthspan and longevity in each sex. Finally, while we see an overall benefit to the survival of the longest-lived quartile, we do not have enough information to draw conclusions about survival of the longest-lived decile, which could be addressed in future, larger studies powered to detect these differences.
Since a Low BCAA diet reduces frailty, we performed transcriptional profiling of the skeletal muscle of both male and female mice fed either a Low BCAA or Control diet. In agreement with the male-specific effects of a Low BCAA diet on frailty and lifespan, we found that substantially greater changes were induced by a Low BCAA diet in males, and there was no overlap in the KEGG pathways significantly enriched in either sex.
One KEGG pathways enriched in Low BCAA-fed males is the mTOR signaling pathway, and we found that Low BCAA-fed males, but not females, had reduced phosphorylation of mTORC1 substrates. In particular, there were significant reductions in the phosphorylation of S6K1 and its substrate S6; reduced S6K1 signaling extends the lifespan of mice as well as other model organisms 42, 45. A trend towards increased mTORC1 signaling in aged male muscle, which we and others have previously observed 83, 84, was not observed in Low BCAA-fed males. Low BCAA-fed males, but not females, upregulate genes associated with BCAA degradation; as BCAA degradation is negatively associated with mTORC1 activity 85, 86, the male-specific decrease in mTORC1 activity we observe could result in part from male-specific alterations in BCAA catabolism. Additional work will be required to examine how BCAA requirements, utilization, and catabolism differ between the sexes.
Reduced mTORC1 signaling in skeletal muscle delays sarcopenia and improves markers of muscle health 84, and skeletal muscle mTORC1 signaling regulates lifespan 87. There are some similarities between the effects of a Low BCAA diet and the effects of rapamycin, including a decrease in cancer and a trend towards improvements in cardiovascular parameters, including ejection fraction and fractional shortening 88, 89. While the effects of a Low BCAA diet and rapamycin are not equivalent, it is likely that the beneficial effects of a Low BCAA diet are mediated in part by inhibition of mTORC1.
FGF21 is elevated in male Low BCAA-fed mice. Transgenic expression of FGF21 extends the lifespan of mice 90, and as this hormone is a key mediator of many metabolic effects of PR 15, it may also contribute to both the lifespan and metabolic phenotypes of Low BCAA-fed males. Additional research will be required to elucidate the role of reduced mTORC1 signaling and FGF21 in the beneficial effects of a Low BCAA diet. Further, it will be important to determine if, like rapamycin, the beneficial effects of reduced dietary BCAAs persist after resumption of a normal diet.
In summary, we have shown that dietary BCAAs regulate both healthspan and lifespan in progeroid as well as wild-type mice. Restricting dietary BCAAs broadly improves metabolic health and decreases frailty without overt negative consequences on muscle performance or cardiac function. Additional research will be required to determine if there are potentially negative effects of BCAA restriction, and to examine how optimal levels of BCAAs for lifespan and healthspan vary with age. We find that lifelong BCAA restriction reduces mTORC1 signaling, reduces frailty, and extends the lifespan of wild-type males, while BCAA restriction begun in midlife promotes healthspan of both sexes and reduces the incidence of cancer in females. Our results demonstrate that dietary levels of BCAAs are critically important in healthy aging, and provide new evidence that protein quality – the specific amino acid composition of dietary protein – is as important as the amount of dietary protein consumed. Finally, while additional research and clinical trials will be required to determine how our findings apply to humans, our results support an emerging model that suggests that limiting dietary levels of BCAAs may be a key to a long and healthy life.
Materials and Methods
Animals use and care
All procedures were performed in conformance with institutional guidelines and were approved by the Institutional Animal Care and Use Committee of the William S. Middleton Memorial Veterans Hospital (Madison, WI, USA). C57BL/6J.Nia were mice obtained from the National Institute on Aging Aged Rodent Colony. Lmna−/− mice were cryorecovered by The Jackson Laboratory (stock 009125) 70, 91, and heterozygous parents were bred to produce Lmna−/− progeny. Homozygous male LmnaG609G/G609G mice 50 were obtained from Dr. Brian Kennedy at The Buck Institute for Research on Aging by the kind permission of Dr. Carlos López-Otín, and their sperm used for in vitro fertilization of female C57BL/6J mice to obtain Lmna G609G/+ heterozygous mice suitable for breeding. Lmna G609G/+ heterozygous mice were then crossed to obtain experimental LmnaG609G/G609G mice and wild-type (Lmna+/+) mice. Mice were housed in a specific pathogen free (SPF) mouse facility with a 12:12 hour light/dark cycle maintained at 22°C, with free access to food and water. SPF status was monitored by the use of sentinal animals and serology testing every four months, as well as a parasitology and Helicobacter screening twice yearly, and these tests remained negative for the duration of these experiments. Animals were group housed in static microisolator cages, except when temporarily housed in a Columbus Instruments Oxymax/CLAMS metabolic chamber system.
Diets
All amino acid defined diets were obtained from Envigo (Madison, WI): Control (TD.140711), Low AA (TD.140712), and Low BCAA (TD.140714 in Figs. 1A, 1B, TD.150662 in all other figures) diet compositions are provided in Supplemental Table 1. Mice not consuming experimental diets were fed standard chow diet (LabDiet 5001), and breeding mice were fed breeder diet (LabDiet 5015).
Lifespans and necropsy
Lmna−/− progeny from parent heterozygous crosses were identified at weaning (confirmed with genotyping) and co-housed with a wild type or heterozygous littermate of the same sex. LmnaG609G/G609G mice were obtained from parent heterozygous crosses and identified by genotyping before removing excess littermates and co-housing with a wild-type littermate of the same sex. C57BL/6J.Nia mice were obtained from the NIA Aged Mouse Colony at 15 months of age and allowed to acclimate to the animal facility for one month. Mice were fed Control, Low BCAA, or Low AA diets. As Lmna−/− mice are particularly short-lived, rigorous daily inspection of welfare, hydration and body temperature were assessed. Mice were euthanized for humane reasons if moribund, if the mice developed other specified problems (e.g. excessive tumor burden), or upon the recommendation of the facility veterinarian. No mice in these studies contracted dermatitis requiring treatment or removal. Mice found dead were noted at each daily inspection and saved in a refrigerator for gross necropsy, during which the abdominal and thoracic cavities were examined for the presence of solid tumors, metastases, splenomegaly, and infection; on the basis of this inspection the presence or absence of observable cancer was recorded. A subset of mice were removed from the study for cross-sectional analysis (e.g. echocardiography) or to control colony size; these mice were selected on the basis of age and cage grouping without visual inspection of the animals or reference to their health status or frailty. Mice were censored as of the date of death if removed for cross-sectional analysis, to control colony size, or if death was due to experimenter error. The lifespans of Lmna−/−, LmnaG609G/G609G, and wild-type mice are provided in Supplemental Tables 2, 5, 8, and 10.
Genotyping
Genotyping primers were obtained from Integrated DNA Technologies (Coralville, IA). Genotyping of Lmna−/− mice utilized primers 8965: (5’-CAAGTCCCCATCACTTGGTT-3’), 8966: (5’-CTGTGACACTGGAGGCAGAA-3’), and oIMR7415: (5’-GCCAGAGGCCACTTGTGTAG-3’) as recommended by The Jackson Laboratory, with the following PCR program: 1 x 4m 94°C, 35 x (30 s 94°C, 30s 52°C, 1m 72°C), 1 x 10m 72°C, hold 4°C, using DreamTaq Green PCR Master Mix (Thermo Fisher Scientific, Waltham, MA, USA). Genotyping of LmnaG609G/G609G mice utilized primers as described by Osorio et al. 50: 5’-AAG GGGCTGGGAGGACAGAG-3’, 5’-AGTAGAAGGTGGCGCGAAGG-3’, and 5’-AGCATGCAATAGGGTGGAAGGA-3’, with the following PCR program as advised by the Kennedy laboratory: 1 x 3m 98°C, 30 x (30 s 98°C, 1m 64°C, 30s 62°C), 2m x 72°C, hold 4°C using Phusion Taq DNA Polymerase (Thermo Fisher Scientific, Waltham, MA, USA).
Body composition
Body composition was measured by magnetic resonance imaging using an EchoMRI 700 Body Composition Analyzer (EchoMRI LLC, Houston, TX, USA).
Glucose and insulin tolerance tests
Glucose tolerance tests were performed by fasting the mice overnight for 16 hours and then injecting glucose (1g/kg) intraperitoneally 18, 92. Insulin tolerance tests were performed by fasting mice for 4 hours starting at lights on, and then injecting insulin (0.75U/kg) intraperitoneally. Glucose measurements were taken using a Bayer Contour blood glucose meter and test strips.
Metabolic chambers
To assess metabolic physiology (O2, CO2, respiratory exchange ratio (RER), food consumption) and spontaneous activity, mice were acclimated to housing in a Columbus Instruments Oxymax/CLAMS metabolic chamber system (Columbus, OH, USA) for approximately 24 hours, and data from a continuous 24 hour period was then recorded and analyzed.
Frailty assessment
Frailty was assessed longitudinally in a subset of mice using a 26-item frailty index 51. This frailty index reflects an accumulation of deficits associated with aging, akin to Rockwood’s frailty index in humans 93, and is conceptually distinct from Fried’s frailty syndrome 94. The items scored included alopecia, loss of fur color, dermatitis, loss of whiskers, coat condition, tumors, distended abdomen, kyphosis, tail stiffening, gait disorders, tremor, body condition score, vestibular disturbance, cataracts, corneal opacity, eye discharge/swelling, microphthalmia, vision loss, menace reflex, nasal discharge, malocclusions, rectal prolapse, vaginal/uterine/penile prolapse, diarrhea, breathing rate/depth, mouse grimace score , and piloerection. Average scores per group over time of these specific items can be found in Tables S6-7 for the midlife intervention and Tables S9 and 11 for the lifelong intervention.
Rotarod and grip strength
Forelimb grip strength was measured by the average of three tests using a grip strength meter (1027SM). Performance on a rotating rod was measured by the average of two runs after two acclimating runs the day prior, using a Rota Rod Rotamex 5 (0890M). The rotarod was intially set at 3 rpm, with speed increasing by 1 rpm every 3 seconds. Both instruments were purchased from Columbus Instruments (Columbus, OH, USA).
Echocardiography
Mice used for echocardiography were selected on the basis of age without reference to frailty status, and following the procedure were euthanized, with animals censored from the lifespan study on the date of euthanasia. Transthoracic echocardiography was performed using a Visual Sonics Vevo 770 ultrasonograph with a 30-MHz transducer as detailed previously 95. For acquisition of two-dimensional guided M-mode images at the tips of papillary muscles and Doppler studies, mice were sedated by facemask administration of 1% isoflurane, hair removed, and maintained on a heated platform. Blood velocities across the mitral, aortic and pulmonary valves were measured using Doppler pulse wave imaging angling the probe to obtain a nearly parallel orientation to the blood flow.
End diastolic and systolic left ventricular (LV) diameter as well as anterior and posterior wall (AW and PW respectively) thickness were measured on line from M-mode images obtained in a parasternal long axis view using the leading edge-to-leading edge convention. All parameters were measured over at least three consecutive cardiac cycles and averaged. Left ventricular fractional shortening was calculated as [(LV diameterdiastole −LV diametersystole )/LV diameterdiastole] x 100; ejection fraction [(7.0/(2.4 + LV diameterdiastole)(LV diameterdiastole)3 - (7.0/(2.4 + LV diametersystole)( LV diametersystole)3/(7.0/(2.4 + LV diameterdiastole)( LV diameterdiastole)3 x 100 and LV mass was calculated by using the formula [1.05 x ((Posterior Wall diastole+Anterior Walldiastole+LV diameterdiastole)3 − (LV diameterdiastole)3)]. Heart rate was determined from at least three consecutive intervals from the pulse wave Doppler tracings of the LV outflow tract. Ejection time was measured from the same outflow track tracings from the onset of flow to the end of flow. Isovolumic relaxation time was measured as the time from the closing of the aortic valve to the opening of the mitral valve from pulse wave Doppler tracings of the LV outflow tract and mitral inflow region. The same person obtained all images and measures.
Transcriptional profiling
Animals used for transcriptional profiling were sacrificed after an overnight fast of 16 hours. RNA was extracted from quadriceps muscle as previously described 17. The concentration and purity of RNA was determined using a NanoDrop 2000c spectrophotometer (Thermo Fisher Scientific, Waltham, MA) and RNA was diluted to 100-400 ng/μl for sequencing. The RNA was then submitted to the University of Wisconsin-Madison Biotechnology Center Gene Expression Center & DNA Sequencing Facility, and RNA quality was assayed using an Agilent RNA NanoChip. RNA libraries were prepared using the TruSeq Stranded Total RNA Sample Preparation protocol (Illumina, San Diego, CA) with 250ng of mRNA, and cleanup was done using RNA Clean beads (lot #17225200). Reads were aligned to the mouse (Mus musculus) with genome-build GRCm38.p5 of accession NCBI:GCA_000001635.7 and expected counts were generated with ensembl gene IDs (Zerbino et al., 2018).
Analysis of significantly differentially expressed genes (DEGs) was completed in R version 3.6.1 96 using edgeR 97 and limma 98. Gene names were converted to gene symbol and Entrez ID formats using the mygene package. Initially, 51,912 transcripts were identified across all Genes with zero counts per million (cpm) in four or more individuals were removed, leaving 16,602 transcripts. To reduce the impact of external factors not of biological interest that may affect expression, data was normalized to ensure the expression distributions of each sample are within a similar range. We normalized using the trimmed mean of M-values (TMM), which scales to library size. Heteroscedascity was accounted for using the voom function, DEGs were identified using an empirical Bayes moderated linear model, and log coefficients and Benjamini-Hochberg adjusted p-values were generated for each comparison of interest 99. DEGs (designated based on an adjusted p value of 0.3 for female and 0.2 for male contrasts) were used to identify enriched pathways, and KEGG enriched pathways were determined for each contrast.
Plasma hormone measurements
Plasma MCP-1 and FGF21 were quantified using a mouse MCP-1 ELISA kit (MJE00B) , and mouse/rat FGF21 ELISA kit (MF2100) from R&D Systems (Minneapolis, MN, USA). Plasma insulin (90080) and IGF-1 (80574) were quantified using ELISA kits from Crystal Chem (Elk Grove Village, IL, USA).
Immunoblotting
Animals used for Western blotting were sacrificed following a four hour refeeding period after an overnight fast. Tissue samples from muscle were lysed in cold RIPA buffer supplemented with phosphatase inhibitor and protease inhibitor cocktail tablets (Thermo Fisher Scientific, Waltham, MA, USA) as previously described 83 using a FastPrep 24 (M.P. Biomedicals, Santa Ana, CA, USA) with bead-beating tubes (16466–042) from (VWR, Radnor, PA, USA) and zirconium ceramic oxide bulk beads (15340159) from (Thermo Fisher Scientific, Waltham, MA, USA). Protein lysates were then centrifuged at 13,300 rpm for 10 min and the supernatant was collected. Protein concentration was determined by Bradford (Pierce Biotechnology, Waltham, MA, USA). 20 μg protein was separated by SDS–PAGE (sodium dodecyl sulfate–polyacrylamide gel electrophoresis) on 8%, 10%, or 16% resolving gels (Thermo Fisher Scientific, Waltham, MA, USA) and transferred to PVDF membrane (EMD Millipore, Burlington, MA, USA). pT389-S6K1 (9234), S6K1 (9202), pS240/244-S6 (2215), S6 (2217), pS757-ULK1 (6888), ULK1 (8054), pS473-AKT (9271), AKT (9272), and HSP90 (4877) were purchased from Cell Signaling Technologies (CST, Danvers, MA, USA) and used at a dilution of 1:1000. Imaging was performed using a GE ImageQuant LAS 4000 imaging station (GE Healthcare, Chicago, IL, USA). Quantification was performed by densitometry using NIH ImageJ software.
Blinding
Investigators were blinded to diet groups during data collection whenever feasible, but this was not usually possible or feasible as as cages were clearly marked to indicate the diet provided, diets were color-coded to prevent feeding mistakes, and the size and body composition of the mice was altered by genotype and diet. However, blinding is not relevant to the majority of the studies conducted here, as the data is collected in numeric form which is not readily subject to bias due to the need for subjective interpretation. Investigators were not blinded during necropsies.
Statistics
Statistical analyses were conducted using Prism 8 (GraphPad Software, La Jolla, CA, USA). Statistical analyses were performed by one-way ANOVA, or in the case of factor analysis, by two-way ANOVA. Dunnett’s post-test comparisons were used in one-way ANOVA analyses, Sidak’s post-test comparisons were used in two-way ANOVA analyses. Where measurements were taken longitudinally, a mixed-effects model (restricted maximum likelihood [REML]) or two-way repeated measures ANOVA were used. Energy expenditure data was analyzed by linear regression of energy expenditure by body weight (ANCOVA). Statistical significance for cancer incidence at death was calculated using a two-sided Fisher’s exact test. Lifespan comparisons were calculated by log-rank test or Gehan Breslow Wilcoxon test, as specified in figure legends. A log-rank test was performed unless the assumption of proportional hazards was not satisfied, in these cases a Gehan Breslow Wilcoxon test which does not rely on this assumption was used as a fallback. Transcriptomics data were analyzed using R (version 3.6.1). Additional comparisons, if any, were corrected for multiple comparisons using the Benjamini-Hochberg method. Maximum lifespan calculations were made by generating a cutoff of the top 25% longest lived animals in each cohort, coupled with Boschloo’s test for significance testing between groups 48. All statistical analyses were performed as specified in the figure legends. Sample sizes for longevity studies were determined in consultation with previously published power tables 100. Sample sizes for metabolic studies was determined based on our previous experimental results with the effects of Low BCAA diet feeding, as published 17, 18, with the goal of having > 90% power to detect a change in area under the curve during a glucose tolerance test, p < 0.05. Data distribution was assumed to be normal but this was not formally tested.
Randomization
Middle-aged animals obtained from the NIA were randomized into groups of equivalent weight prior to the beginning of the in vivo studies; all other animals, which were bred in-house, were enrolled by birth order. Mice removed from the lifespan studies for cross-sectional studies were selected from a database without information about the health status of the mice as they reached the age previously chosen for phenotyping and there was availability at the cardiac phenotyping core. Young animals removed from the lifespan study were removed at the cage level without assessing health status of individual mice.
Data availability
RNA-seq data have been deposited with the Gene Expression Omnibus and are accessible through accession number GSE155064. The data that support the plots within this article and other findings of this study are available from the corresponding author upon reasonable request. Full scans of western blot images are provided as Source Data files.
Reporting Summary
Detailed information on experimental design and reagents can be found in the accompanying Life Sciences Reporting Summary.
Extended Data
Extended Data Fig. 1. A Low BCAA diet promotes the metabolic health of aged wild-type mice.
(A-G) Female and (H-N) Male C57BL/6J.Nia mice were fed the indicated diets beginning at 16 months of age. (A) The lean mass of a subset of female mice was tracked (n varies by month; maximum N = 10 biologically independent animals for both groups; * p < 0.05 (p value by month of age: 19 mo. = 0.0091, 21.5 mo. <0.0001, 24.5 mo. = 0.0004). (B) Food consumption over time calculated as total kcal/d (maximum N = 3 independent cages for both groups). (C) Energy expenditure (Heat) was assessed using metabolic chambers at 20 months of age (N; Control = 20, Low BCAA = 17 biologically independent animals). (D) Respiratory exchange ratio and (E) ambulatory movement was assessed using metabolic chambers at 20 and 25 months of age (maximum N; Control = 20, Low BCAA = 17 biologically independent animals; * p < 0.05 (p value for (D) by month of age: 20 mo. light=0.0056, dark=0.0105, 25 mo. dark=0.0012). (F) Area under the curve (AUC) corresponding to the glucose tolerance test in Figure 2G as well as repeat tests performed at 19 and 24 months of age (maximum N = 20 biologically independent animals for both groups; * p < 0.05, # p < 0.1 (p value by month of age: 17 mo. = 0.0034, 19 mo. = 0.0714, 24 mo. = 0.0016). (G) Insulin tolerance test and corresponding area under the curve after four weeks of diet feeding (N; Control = 20, Low BCAA = 15 biologically independent animals). (H) The lean mass of a subset of male mice was tracked (n varies by month; maximum N = 20 biologically independent animals for both groups, * p = 0.0066). (I) Food consumption over time calculated as total kcal/d (maximum N = 3 independent cages for both groups). (J) Energy expenditure (Heat) was assessed using metabolic chambers at 25 months of age (N = 13 biologically independent animals for both groups). (K) Respiratory exchange ratio and (L) ambulatory movement was assessed using metabolic chambers at 20 and 25 months of age (maximum N = 14 biologically independent animals for both groups). (M) AUC corresponding to glucose tolerance test in Figure 2M as well as repeat tests performed at 19 and 24 months of age (maximum N = 20 biologically independent animals for both groups; * p < 0.05 (p-value by month of age: 17 mo. = 0.0006, 19 mo. =0.0240, 24 mo. = 0.0026). (N) Insulin tolerance test and corresponding area under the curve after four weeks of diet feeding (N; Control = 10, Low BCAA = 9 biologically independent animals; * p = 0.0192). (A-B, D-F, H-I, K-M) Statistics for the overall effects of diet, age, and the interaction represent the p value from a mixed-effects model (restricted maximum likelihood [REML]) or two-way repeated measures ANOVA, multiple comparisons by two-sided Sidak’s post-test. (C, J) Energy expenditure data was analyzed by linear regression of energy expenditure by body weight (ANCOVA). (G, N) AUC comparisons were made by two-sided t-test, * p < 0.05. Data are represented as mean ± SEM.
Extended Data Fig. 2. Effects of a lifelong Low BCAA diet on the healthspan of female mice.
(A) Schematic showing timeline of measurements taken from male and female mice fed Control or Low BCAA diets, relevant to Fig. 5 and Extended Data Figs. 2 and 3. (B-N) Wild-type female mice were placed on either Control or Low BCAA diets at weaning. (B-C) The lean mass (B) and fat mass (C) of a subset of mice was tracked (n varies by month, maximum N; Control = 15, Low BCAA = 12 biologically independent animals). (B) * p < 0.05 (p-values for by month of age: 2.75 mo. = 0.0004. 5 mo. < 0.0001, 8.5 mo. = 0.0003, 15 mo. = 0.0341). (C)* p < 0.05, # p < 0.1 (p-values by month of age: 2 mo. = 0.0102, 8.5 mo. = 0.0695, 15 mo. = 0.0652). (D-E) Food consumption was calculated per gram of body weight (D) and by animal (E) (N; Control = 4, Low BCAA = 7 biologically independent animals). (F-H) Respiratory exchange ratio (F), ambulatory movement (G), and energy expenditure (heat) (H) were assessed using metabolic chambers at 5 months of age (N; Control = 6, Low BCAA = 7 biologically independent animals). (I) Glucose tolerance test at 2 months of age (N; Control = 18, Low BCAA = 16 biologically independent animals; * p < 0.05 (p-value by time: 0m = 0.0005, 15m = 0.0120, 60m = 0.0267), and corresponding area under the curve (AUC), also from tests performed at 3.5 and 12 months of age (maximum N; Control = 24, Low BCAA = 18 biologically independent animals; * p = 0.0063). (J) Insulin tolerance test at 2.5 months of age (N; Control = 13, Low BCAA = 12 biologically independent animals), and corresponding area under the curve, also from a test at 4 months of age (N; Control = 3, Low BCAA = 4 biologically independent animals). (K) Rotarod performance (n varies by month, maximum N; Control = 12, Low BCAA = 8 biologically independent animals) and (L) grip strength (n varies by month, maximum N; Control = 15, Low BCAA = 12 biologically independent animals) was assessed longitudinally. (M-N) Levels of insulin (M) and fibroblast growth factor 21 (FGF21) (N) were measured in serum by ELISA (16 months of age; N = 4 biologically independent animals per group). (B-N) Statistics for the overall effects of diet, age, and the interaction represent the p value from a mixed-effects model (restricted maximum likelihood [REML]), two-way repeated measures ANOVA, or a two-tailed, unpaired t-test in (M-N); multiple comparisons by two sided Sidak’s post-test. Data are represented as mean ± SEM.
Extended Data Fig. 3. Effects of a lifelong Low BCAA diet on the healthspan of male mice.
(A-M), Wild-type male mice were placed on either Control or Low BCAA diets at weaning. The (A) lean mass and (B) fat mass of a subset of mice was tracked (n varies by month, maximum N; Control = 11, Low BCAA = 8 biologically independent animals; * p < 0.05 (p-values for (A) by month of age: 2 mo. = 0.0028, 2.75 mo. = 0.0194, 5 mo. = 0.0011, 8.5 mo. = 0.0246; for (B) * p = 0.0095). Food consumption (N; Control = 4, Low BCAA = 6 biologically independent animals), was calculated (C) per gram of body weight and (D) by animal. (E) Respiratory exchange ratio, (F) ambulatory movement, and (G) energy expenditure (heat) were assessed using metabolic chambers at 5 months of age (E-G; N = 6 biologically independent animals for both groups). (H) Glucose tolerance test at 2 months of age (N; Control = 14, Low BCAA = 8 biologically independent animals), and corresponding area under the curve (AUC), also from tests performed at 3.5 and 12 months of age (maximum N; Control = 23, Low BCAA = 12 biologically independent animals; * p < 0.05 (p-values by month of age: 3.5 mo. = 0.0379, 12 mo. = 0.0054). (I) Insulin tolerance test at 2.5 months of age (N; Control = 15, Low BCAA = 13 biologically independent animals), and corresponding area under the curve, also from a test at 4 months of age (maximum N; Control = 15, Low BCAA = 13 biologically independent animals). (J) Rotarod performance (n varies by month, maximum N; Control = 15, Low BCAA = 12 biologically independent animals) and (K) grip strength (n varies by month, maximum N; Control = 12, Low BCAA = 8 biologically independent animals) were assessed longitudinally. Levels of (L) insulin and (M) fibroblast growth factor 21 (FGF21) were measured in serum by ELISA (16 months of age; N; Control = 5, Low BCAA = 4 biologically independent animals per group). (A-M) Statistics for the overall effects of diet, age, and the interaction represent the p value from a mixed-effects model (restricted maximum likelihood [REML]), two-way repeated measures ANOVA, or a two-tailed, unpaired t-test in (L-M); multiple comparisons by two-sided Sidak’s post-test. Data are represented as mean ± SEM.
Extended Data Fig. 4. Transcriptional profiling of skeletal muscle.
Transcriptional profiling was performed on mRNA from the skeletal muscle of male and female mice that consumed either Control or Low BCAA diets from weaning until 16 months of age (N = 6 biologically independent animals for all groups; Tables S13-S14). (A) Workflow from raw RNA sequencing reads through data analysis and data representation. (B-C) Principal component analysis for (B) Control and (C) Low BCAA fed groups. (D) Heatmaps of differentially expressed genes by mouse from significant KEGG over-representation analysis (ORA) pathways of interest identified in Tables S13B, S14B. DEGs were identified using an empirical Bayes moderated linear model. *Two-sided P values adjusted with the Benjamini–Hochberg procedure ORA was performed on DEGs (designated by an adjusted P value of 0.3 for female and 0.2 for male contrasts) using a one-sided hypergeometric test, and P values were adjusted using the Benjamini–Hochberg procedure.
Extended Data Fig. 5. A Low BCAA diet reduces mTORC1 activity in male, but not female, muscle.
(A-B) mTORC1 activity determined by Western blotting and quantification of muscle tissue lysates from male and female mice. Young (12 months females; 15 months males) and aged (22 months females; 25 months males) mice were fed either a Control or Low BCAA diets from 6.5 months of age for young and 16 months of age for aged mice, then sacrificed following an overnight fast followed by 4 hours of refeeding. (A) Male and (B) Female muscle. Quantification was by ImageJ (N = 3 biologically independent animals for all groups). (A-B) * p < 0.05 (p-values for (B); pS6/S6, Young CTL vs. Young LBC=0.0025; Young CTL vs. Aged CTL=0.0327; pS6K1/S6K1, Aged CTL vs. Aged LBC=0.0254). Statistics for the overall effects of diet, age and the interaction represent the p value from a two-way repeated measures ANOVA, multiple comparisons by two-sided Sidak’s post-test. Full scans of the cropped western blots shown here are provided as Source Data files. CTL=Control, LBC=Low BCAA. Data are represented as mean ± SEM.
Extended Data Fig. 6. A Low BCAA diet reduces mTORC1 signaling in the liver of male mice.
(A-B) mTORC1 activity determined by Western blotting and quantification of liver tissue lysates from male and female mice. Young (12 months females; 15 months males) and aged (22 months females; 25 months males) mice were fed either a Control or Low BCAA diets from 6.5 months of age for young and 16 months of age for aged mice, then sacrificed following an overnight fast followed by 4 hours of refeeding. (A) Male and (B) female liver. Quantification was by ImageJ (N = 3 biologically independent animals for all groups). (A) * p < 0.05 (p-values for (A); pS6/S6, Young CTL vs. Young LBC=0.0028, Young CTL vs. Aged CTL=0.021; pS6K1/S6K1, Young CTL vs. Young LBC=0.0086, Young LBC vs. Aged LBC=0.0309; pULK1/ULK1, Aged CTL vs. Aged LBC = 0.0432, Young LBC vs. Aged LBC=0.003. (B) * p=0.0047, # = 0.056. Statistics for the overall effects of diet, age and the interaction represent the p value from a two-way repeated measures ANOVA, multiple comparisons by two-sided Sidak’s post-test. Full scans of the cropped western blots shown here are provided as Source Data files. CTL=Control, LBC=Low BCAA. Data are represented as mean ± SEM.
Supplementary Material
ACKNOWLEDGEMENTS
We would like to thank all members of the Lamming lab for their valuable insights and comments, and Drs. Caroline Alexander and Joseph Baur for critical reading of the manuscript. We thank Dr. Tina Herfel (Envigo) for assistance with the formulation of the amino acid defined diets. We thank Dr. Carlos López-Otin and Dr. Brian Kennedy for providing the Lmna(G609) mutant mice, and Drs. Yuehmei Hsu and Monique O’Leary for their assistance with initial genotyping of the Lmna(G609) mice. We thank Dr. Cara Green for assistance with analysis of RNA sequencing data, and generating panels in Figures 6 and Extended Data 4. Finally, we thank the reviewers of this manuscript and editor for their comments. We apologize for any papers not cited due to a lack of space. This work was supported in part by the NIH/National Institute on Aging (AG041765, AG050135, AG051974, AG056771, and AG062328 to D.W.L.), by a Glenn Foundation Award for Research in the Biological Mechanisms of Aging to D.W.L., and by funding from the University of Wisconsin-Madison School of Medicine and Public Health and Department of Medicine to D.W.L. This work was supported by a grant from the Progeria Research Foundation to D.W.L. This research was conducted in part while D.W.L. was an AFAR Research Grant recipient from the American Federation for Aging Research. N.E.C. was supported in part by a training grant from the UW Institute on Aging (NIA T32 AG000213). H.H.P. was supported in part by a NIA F31 predoctoral fellowship (NIA F31 AG066311). V.F. was supported in part by a Research Supplement to Promote Diversity in Health-Related Research (R01 AG056771-01A1S1). D.Y. was supported in part by a fellowship from the American Heart Association (17PRE33410983). The UW Carbone Cancer Center (UWCCC) Experimental Pathology Laboratory is supported by UWCCC Support Grant P30 CA014520 from the NIH National Cancer Institute. The Lamming laboratory is supported in part by the U.S. Department of Veterans Affairs (I01-BX004031), and this work was supported using facilities and resources from the William S. Middleton Memorial Veterans Hospital. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. This work does not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
ACCESSION CODES
CONFLICT OF INTEREST STATEMENT
D.W.L has received funding from, and is a scientific advisory board member of, Aeovian Pharmaceuticals, which seeks to develop novel, selective mTOR inhibitors for the treatment of various diseases. The University of Wisconsin-Madison has applied for a patent for the use of branched-chain amino acid restricted diets to promote metabolic health, for which NER and DWL are inventors.
REFERENCES
- 1.Weindruch R, Walford RL, Fligiel S, Guthrie D. The retardation of aging in mice by dietary restriction: longevity, cancer, immunity and lifetime energy intake. The Journal of nutrition 116, 641–654 (1986). [DOI] [PubMed] [Google Scholar]
- 2.Cummings NE, Lamming DW. Regulation of metabolic health and aging by nutrient-sensitive signaling pathways. Molecular and cellular endocrinology 455, 13–22 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Solon-Biet SM, Mitchell SJ, de Cabo R, Raubenheimer D, Le Couteur DG, Simpson SJ. Macronutrients and caloric intake in health and longevity. J Endocrinol 226, R17–28 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Green CL, Lamming DW. Regulation of metabolic health by essential dietary amino acids. Mech Ageing Dev 177, 186–200 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Speakman JR, Mitchell SE, Mazidi M. Calories or protein? The effect of dietary restriction on lifespan in rodents is explained by calories alone. Exp Gerontol 86, 28–38 (2016). [DOI] [PubMed] [Google Scholar]
- 6.Gannon MC, Nuttall FQ, Saeed A, Jordan K, Hoover H. An increase in dietary protein improves the blood glucose response in persons with type 2 diabetes. Am J Clin Nutr 78, 734–741 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Dong JY, Zhang ZL, Wang PY, Qin LQ. Effects of high-protein diets on body weight, glycaemic control, blood lipids and blood pressure in type 2 diabetes: meta-analysis of randomised controlled trials. Br J Nutr 110, 781–789 (2013). [DOI] [PubMed] [Google Scholar]
- 8.Seino Y, Seino S, Ikeda M, Matsukura S, Imura H. Beneficial effects of high protein diet in treatment of mild diabetes. Hum Nutr Appl Nutr 37 A, 226–230 (1983). [PubMed] [Google Scholar]
- 9.Coelho-Junior HJ, Rodrigues B, Uchida M, Marzetti E. Low Protein Intake Is Associated with Frailty in Older Adults: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 10, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coelho-Júnior HJ, et al. Protein-Related Dietary Parameters and Frailty Status in Older Community-Dwellers across Different Frailty Instruments. Nutrients 12, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Levine ME, et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab 19, 407–417 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sluijs I, Beulens JW, van der AD, Spijkerman AM, Grobbee DE, van der Schouw YT. Dietary intake of total, animal, and vegetable protein and risk of type 2 diabetes in the European Prospective Investigation into Cancer and Nutrition (EPIC)-NL study. Diabetes Care 33, 43–48 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lagiou P, et al. Low carbohydrate-high protein diet and mortality in a cohort of Swedish women. J Intern Med 261, 366–374 (2007). [DOI] [PubMed] [Google Scholar]
- 14.Vergnaud AC, et al. Macronutrient composition of the diet and prospective weight change in participants of the EPIC-PANACEA study. PLoS One 8, e57300 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Laeger T, et al. FGF21 is an endocrine signal of protein restriction. J Clin Invest 124, 3913–3922 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maida A, et al. A liver stress-endocrine nexus promotes metabolic integrity during dietary protein dilution. J Clin Invest 126, 3263–3278 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummings NE, et al. Restoration of metabolic health by decreased consumption of branched-chain amino acids. The Journal of physiology 596, 623–645 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fontana L, et al. Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health. Cell reports 16, 520–530 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee KP, et al. Lifespan and reproduction in Drosophila: New insights from nutritional geometry. Proc Natl Acad Sci U S A 105, 2498–2503 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solon-Biet SM, et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab 19, 418–430 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mair W, Piper MD, Partridge L. Calories do not explain extension of life span by dietary restriction in Drosophila. PLoS Biol 3, e223 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross MH. Length of life and nutrition in the rat. The Journal of nutrition 75, 197–210 (1961). [DOI] [PubMed] [Google Scholar]
- 23.Lee BC, et al. Methionine restriction extends lifespan of Drosophila melanogaster under conditions of low amino-acid status. Nature communications 5, 3592 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grandison RC, Piper MD, Partridge L. Amino-acid imbalance explains extension of lifespan by dietary restriction in Drosophila. Nature 462, 1061–1064 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Juricic P, Gronke S, Partridge L. Branched-Chain Amino Acids Have Equivalent Effects to Other Essential Amino Acids on Lifespan and Aging-Related Traits in Drosophila. J Gerontol A Biol Sci Med Sci 75, 24–31 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshida S, et al. Role of dietary amino acid balance in diet restriction-mediated lifespan extension, renoprotection, and muscle weakness in aged mice. Aging Cell 17, e12796 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller RA, Buehner G, Chang Y, Harper JM, Sigler R, Smith-Wheelock M. Methionine-deficient diet extends mouse lifespan, slows immune and lens aging, alters glucose, T4, IGF-I and insulin levels, and increases hepatocyte MIF levels and stress resistance. Aging Cell 4, 119–125 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Orentreich N, Matias JR, DeFelice A, Zimmerman JA. Low methionine ingestion by rats extends life span. The Journal of nutrition 123, 269–274 (1993). [DOI] [PubMed] [Google Scholar]
- 29.Brown-Borg HM, et al. Growth hormone signaling is necessary for lifespan extension by dietary methionine. Aging Cell 13, 1019–1027 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ooka H, Segall PE, Timiras PS. Histology and survival in age-delayed low-tryptophan-fed rats. Mech Ageing Dev 43, 79–98 (1988). [DOI] [PubMed] [Google Scholar]
- 31.De Marte ML, Enesco HE. Influence of low tryptophan diet on survival and organ growth in mice. Mech Ageing Dev 36, 161–171 (1986). [DOI] [PubMed] [Google Scholar]
- 32.Newgard CB, et al. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9, 311–326 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Batch BC, et al. Branched chain amino acids are novel biomarkers for discrimination of metabolic wellness. Metabolism 62, 961–969 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Le Couteur DG, et al. Branched chain amino acids, cardiometabolic risk factors and outcomes in older men: the Concord Health and Ageing in Men Project. J Gerontol A Biol Sci Med Sci, (2019). [DOI] [PubMed] [Google Scholar]
- 35.Karusheva Y, et al. Short-term dietary reduction of branched-chain amino acids reduces meal-induced insulin secretion and modifies microbiome composition in type 2 diabetes: a randomized controlled crossover trial. Am J Clin Nutr 110, 1098–1107 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.White PJ, et al. Branched-chain amino acid restriction in Zucker-fatty rats improves muscle insulin sensitivity by enhancing efficiency of fatty acid oxidation and acyl-glycine export. Molecular metabolism 5, 538–551 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.D'Antona G, et al. Branched-chain amino acid supplementation promotes survival and supports cardiac and skeletal muscle mitochondrial biogenesis in middle-aged mice. Cell Metab 12, 362–372 (2010). [DOI] [PubMed] [Google Scholar]
- 38.Solon-Biet SM, et al. Branched chain amino acids impact health and lifespan indirectly via amino acid balance and appetite control. Nat Metab 1, 532–545 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wolfson RL, et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science 351, 43–48 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kennedy BK, Lamming DW. The Mechanistic Target of Rapamycin: The Grand ConducTOR of Metabolism and Aging. Cell Metab 23, 990–1003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wu JJ, et al. Increased mammalian lifespan and a segmental and tissue-specific slowing of aging after genetic reduction of mTOR expression. Cell reports 4, 913–920 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Selman C, et al. Ribosomal protein S6 kinase 1 signaling regulates mammalian life span. Science 326, 140–144 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrison DE, et al. Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vellai T, Takacs-Vellai K, Zhang Y, Kovacs AL, Orosz L, Muller F. Genetics: influence of TOR kinase on lifespan in C. elegans. Nature 426, 620 (2003). [DOI] [PubMed] [Google Scholar]
- 45.Kapahi P, Zid BM, Harper T, Koslover D, Sapin V, Benzer S. Regulation of lifespan in Drosophila by modulation of genes in the TOR signaling pathway. Curr Biol 14, 885–890 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arriola Apelo SI, Lamming DW. Rapamycin: An InhibiTOR of Aging Emerges From the Soil of Easter Island. J Gerontol A Biol Sci Med Sci 71, 841–849 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ramos FJ, et al. Rapamycin reverses elevated mTORC1 signaling in lamin A/C-deficient mice, rescues cardiac and skeletal muscle function, and extends survival. Sci Transl Med 4, 144ra103 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang C, Li Q, Redden DT, Weindruch R, Allison DB. Statistical methods for testing effects on “maximum lifespan”. Mech Ageing Dev 125, 629–632 (2004). [DOI] [PubMed] [Google Scholar]
- 49.Zaghini A, et al. Long term breeding of the Lmna G609G progeric mouse: Characterization of homozygous and heterozygous models. Exp Gerontol 130, 110784 (2020). [DOI] [PubMed] [Google Scholar]
- 50.Osorio FG, et al. Splicing-directed therapy in a new mouse model of human accelerated aging. Sci Transl Med 3, 106ra107 (2011). [DOI] [PubMed] [Google Scholar]
- 51.Whitehead JC, et al. A clinical frailty index in aging mice: comparisons with frailty index data in humans. J Gerontol A Biol Sci Med Sci 69, 621–632 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kane AE, et al. Impact of Longevity Interventions on a Validated Mouse Clinical Frailty Index. J Gerontol A Biol Sci Med Sci 71, 333–339 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yousefzadeh MJ, et al. Circulating levels of monocyte chemoattractant protein-1 as a potential measure of biological age in mice and frailty in humans. Aging Cell 17, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mitchell SE, et al. The effects of graded levels of calorie restriction: II. Impact of short term calorie and protein restriction on circulating hormone levels, glucose homeostasis and oxidative stress in male C57BL/6 mice. Oncotarget 6, 23213–23237 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chaumontet C, et al. Low-protein and methionine, high-starch diets increase energy intake and expenditure, increase FGF21, decrease IGF-1, and have little effect on adiposity in mice. Am J Physiol Regul Integr Comp Physiol 316, R486–R501 (2019). [DOI] [PubMed] [Google Scholar]
- 56.Miller RA, et al. Rapamycin-mediated lifespan increase in mice is dose and sex dependent and metabolically distinct from dietary restriction. Aging Cell 13, 468–477 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mao K, et al. Late-life targeting of the IGF-1 receptor improves healthspan and lifespan in female mice. Nature communications 9, 2394 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mitchell SJ, et al. Effects of Sex, Strain, and Energy Intake on Hallmarks of Aging in Mice. Cell Metab 23, 1093–1112 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hahn O, et al. A nutritional memory effect counteracts benefits of dietary restriction in old mice. Nat Metab 1, 1059–1073 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yuan R, et al. Genetic coregulation of age of female sexual maturation and lifespan through circulating IGF1 among inbred mouse strains. Proc Natl Acad Sci U S A 109, 8224–8229 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Turturro A, Witt WW, Lewis S, Hass BS, Lipman RD, Hart RW. Growth curves and survival characteristics of the animals used in the Biomarkers of Aging Program. J Gerontol A Biol Sci Med Sci 54, B492–501 (1999). [DOI] [PubMed] [Google Scholar]
- 62.Hu W, et al. Relationship between Branched-Chain Amino Acids, Metabolic Syndrome, and Cardiovascular Risk Profile in a Chinese Population: A Cross-Sectional Study. Int J Endocrinol 2016, 8173905 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Du X, et al. Relationships between circulating branched chain amino acid concentrations and risk of adverse cardiovascular events in patients with STEMI treated with PCI. Scientific reports 8, 15809 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wang W, et al. Defective branched chain amino acid catabolism contributes to cardiac dysfunction and remodeling following myocardial infarction. Am J Physiol Heart Circ Physiol 311, H1160–H1169 (2016). [DOI] [PubMed] [Google Scholar]
- 65.Sun H, et al. Catabolic Defect of Branched-Chain Amino Acids Promotes Heart Failure. Circulation 133, 2038–2049 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chantranupong L, et al. The CASTOR Proteins Are Arginine Sensors for the mTORC1 Pathway. Cell 165, 153–164 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Chantranupong L, et al. The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1. Cell reports 9, 1–8 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Barcena C, et al. Methionine Restriction Extends Lifespan in Progeroid Mice and Alters Lipid and Bile Acid Metabolism. Cell reports 24, 2392–2403 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vermeij WP, et al. Restricted diet delays accelerated ageing and genomic stress in DNA-repair-deficient mice. Nature 537, 427–431 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Frock RL, Kudlow BA, Evans AM, Jameson SA, Hauschka SD, Kennedy BK. Lamin A/C and emerin are critical for skeletal muscle satellite cell differentiation. Genes Dev 20, 486–500 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Arriola Apelo SI, et al. Ovariectomy uncouples lifespan from metabolic health and reveals a sex-hormone-dependent role of hepatic mTORC2 in aging. eLife 9, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chellappa K, et al. Hypothalamic mTORC2 is essential for metabolic health and longevity. Aging Cell 18, e13014 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu D, et al. Calorie-Restriction-Induced Insulin Sensitivity Is Mediated by Adipose mTORC2 and Not Required for Lifespan Extension. Cell reports 29, 236–248 e233 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sharples AP, Hughes DC, Deane CS, Saini A, Selman C, Stewart CE. Longevity and skeletal muscle mass: the role of IGF signalling, the sirtuins, dietary restriction and protein intake. Aging Cell 14, 511–523 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ericksen RE, et al. Loss of BCAA Catabolism during Carcinogenesis Enhances mTORC1 Activity and Promotes Tumor Development and Progression. Cell Metab 29, 1151–1165 e1156 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ananieva EA, Wilkinson AC. Branched-chain amino acid metabolism in cancer. Curr Opin Clin Nutr Metab Care 21, 64–70 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jensen K, McClure C, Priest NK, Hunt J. Sex-specific effects of protein and carbohydrate intake on reproduction but not lifespan in Drosophila melanogaster. Aging Cell 14, 605–615 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Larson KR, Russo KA, Fang Y, Mohajerani N, Goodson ML, Ryan KK. Sex Differences in the Hormonal and Metabolic Response to Dietary Protein Dilution. Endocrinology 158, 3477–3487 (2017). [DOI] [PubMed] [Google Scholar]
- 79.Miller DS, Payne PR. Longevity and protein intake. Experimental Gerontology 3, 231–234 (1968). [DOI] [PubMed] [Google Scholar]
- 80.Liao CY, Rikke BA, Johnson TE, Diaz V, Nelson JF. Genetic variation in the murine lifespan response to dietary restriction: from life extension to life shortening. Aging Cell 9, 92–95 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barrington WT, et al. Improving Metabolic Health Through Precision Dietetics in Mice. Genetics 208, 399–417 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sittig LJ, Carbonetto P, Engel KA, Krauss KS, Barrios-Camacho CM, Palmer AA. Genetic Background Limits Generalizability of Genotype-Phenotype Relationships. Neuron 91, 1253–1259 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Baar EL, Carbajal KA, Ong IM, Lamming DW. Sex- and tissue-specific changes in mTOR signaling with age in C57BL/6J mice. Aging Cell 15, 155–166 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Joseph GA, et al. Partial Inhibition of mTORC1 in Aged Rats Counteracts the Decline in Muscle Mass and Reverses Molecular Signaling Associated with Sarcopenia. Mol Cell Biol 39, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zhang YK, et al. Enoyl-CoA hydratase-1 regulates mTOR signaling and apoptosis by sensing nutrients. Nature communications 8, 464 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhou M, et al. Targeting BCAA Catabolism to Treat Obesity-Associated Insulin Resistance. Diabetes 68, 1730–1746 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Stephenson EJ, et al. Skeletal Muscle mTORC1 Activation Increases Energy Expenditure and Reduces Longevity in Mice. bioRxiv, 720540 (2019). [Google Scholar]
- 88.Dai DF, et al. Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell 13, 529–539 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Flynn JM, et al. Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell 12, 851–862 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Y, et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. eLife 1, e00065 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
METHODS-ONLY REFERENCES
- 91.Sullivan T, et al. Loss of A-type lamin expression compromises nuclear envelope integrity leading to muscular dystrophy. J Cell Biol 147, 913–920 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Arriola Apelo SI, et al. Alternative rapamycin treatment regimens mitigate the impact of rapamycin on glucose homeostasis and the immune system. Aging Cell 15, 28–38 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Mitnitski AB, Mogilner AJ, Rockwood K. Accumulation of deficits as a proxy measure of aging. ScientificWorldJournal 1, 323–336 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fried LP, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56, M146–156 (2001). [DOI] [PubMed] [Google Scholar]
- 95.Harris SP, et al. Hypertrophic cardiomyopathy in cardiac myosin binding protein-C knockout mice. Circ Res 90, 594–601 (2002). [DOI] [PubMed] [Google Scholar]
- 96.Team RC. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/. (ed^(eds) (2017). [Google Scholar]
- 97.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ritchie ME, et al. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res 43, e47–e47 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Benjamini Y, Hochberg Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. Journal of the Royal Statistical Society Series B (Methodological) 57, 289–300 (1995). [Google Scholar]
- 100.Liang H, Masoro EJ, Nelson JF, Strong R, McMahan CA, Richardson A. Genetic mouse models of extended lifespan. Exp Gerontol 38, 1353–1364 (2003). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq data have been deposited with the Gene Expression Omnibus and are accessible through accession number GSE155064. The data that support the plots within this article and other findings of this study are available from the corresponding author upon reasonable request. Full scans of western blot images are provided as Source Data files.