Abstract
Objective
To compare the risk of cardiovascular events between sodium glucose cotransporter 2 (SGLT2) inhibitors and dipeptidyl peptidase-4 (DPP-4) inhibitors among people with type 2 diabetes in a real world context of clinical practice.
Design
Multi-database retrospective cohort study using a prevalent new user design with subsequent meta-analysis.
Setting
Canadian Network for Observational Drug Effect Studies (CNODES), with administrative healthcare databases from seven Canadian provinces and the United Kingdom, 2013-18.
Population
209 867 new users of a SGLT2 inhibitor matched to 209 867 users of a DPP-4 inhibitor on time conditional propensity score and followed for a mean of 0.9 years.
Main outcome measures
The primary outcome was major adverse cardiovascular events (MACE, a composite of myocardial infarction, ischaemic stroke, or cardiovascular death). Secondary outcomes were the individual components of MACE, heart failure, and all cause mortality. Cox proportional hazards models were used to estimate site specific adjusted hazards ratios and 95% confidence intervals, comparing use of SGLT2 inhibitors with use of DPP-4 inhibitors in an as treated approach. Site specific results were pooled using random effects meta-analysis.
Results
Compared with DPP-4 inhibitors, SGLT2 inhibitors were associated with decreased risks of MACE (incidence rate per 1000 person years: 11.4 v 16.5; hazard ratio 0.76, 95% confidence interval 0.69 to 0.84), myocardial infarction (5.1 v 6.4; 0.82, 0.70 to 0.96), cardiovascular death (3.9 v 7.7; 0.60, 0.54 to 0.67), heart failure (3.1 v 7.7; 0.43, 0.37 to 0.51), and all cause mortality (8.7 v 17.3; 0.60, 0.54 to 0.67). SGLT2 inhibitors had more modest benefits for ischaemic stroke (2.6 v 3.5; 0.85, 0.72 to 1.01). Similar benefits for MACE were observed with canagliflozin (0.79, 0.66 to 0.94), dapagliflozin (0.73, 0.63 to 0.85), and empagliflozin (0.77, 0.68 to 0.87).
Conclusions
In this large observational study conducted in a real world clinical practice context, the short term use of SGLT2 inhibitors was associated with a decreased risk of cardiovascular events compared with the use of DPP-4 inhibitors.
Trial registration
ClinicalTrials.gov NCT03939624.
Introduction
Randomised controlled trials have shown that sodium glucose cotransporter 2 (SGLT2) inhibitors reduce the incidence of major adverse cardiovascular events (MACE) among people with type 2 diabetes and previous cardiovascular disease.1 2 In the EMPAgliflozin Removal of Excess of Glucose OUTCOME trial, participants randomised to empagliflozin had decreased rates of MACE (a composite endpoint of death from cardiovascular causes, non-fatal myocardial infarction, or non-fatal stroke) (hazard ratio 0.86, 95% confidence interval 0.74 to 0.99) and of hospital admission for heart failure (0.65, 0.50 to 0.85) compared with those randomised to placebo.3 Similar benefits were found in the CANagliflozin cardioVascular Assessment Study of canagliflozin.4 In contrast, the Dapagliflozin Effect on Cardiovascular Events-Thrombolysis in Myocardial Infarction 58 trial5 found that dapagliflozin was non-inferior to placebo for MACE (0.93, 0.84 to 1.03) and superior for hospital admission due to heart failure (0.73, 0.61 to 0.88).6 Although these randomised controlled trials found that SGLT2 inhibitors are efficacious compared with placebo, the cardiovascular effects of SGLT2 inhibitors compared with other second line to third line antidiabetic treatments remain unknown. Furthermore, the generalisability of data from these randomised controlled trials to a real world setting is uncertain.7
To date, several observational studies have examined the association between SGLT2 inhibitors and cardiovascular outcomes, with most of these studies showing a reduced risk in comparisons with other antidiabetic drugs.8 9 10 11 12 13 14 15 A few of these studies, however, had important limitations that make it difficult to interpret the results. These limitations included the presence of immortal time bias16 17 18 in three studies.8 9 13 In addition, all these studies used new user designs and thus excluded individuals with recent use of the comparator drugs. Given the highly dynamic treatment of type 2 diabetes and the frequent use of other second line or third line treatments before the initiation of SGLT2 inhibitors, such exclusions can greatly affect the generalisability of study results and might even introduce selection bias.19 Furthermore, limited data are available on the cardiovascular effects of individual SGLT2 inhibitors. We compared the risks of MACE, its components, all cause mortality, and heart failure associated with SGLT2 inhibitors versus dipeptidyl peptidase-4 (DPP-4) inhibitors (a class of oral antidiabetic drugs usually prescribed as a second line or third line treatment of type 2 diabetes) among people with type 2 diabetes by applying a prevalent new user design to population based data from eight jurisdictions. This study was conducted by the Canadian Network for Observational Drug Effect Studies (CNODES).20
Methods
Data sources
We implemented a prevalent new user design in a retrospective multi-database cohort study using administrative healthcare databases from the Canadian provinces of Alberta, British Columbia, Manitoba, Nova Scotia, Ontario, Quebec, and Saskatchewan, and the United Kingdom Clinical Practice Research Datalink (CPRD). The Canadian databases include population wide data on doctor claims, hospital admission records, and prescription drug claims. Prescription drug data are restricted to those aged 18 years or more in Alberta, those aged 65 years or more in Ontario, and those aged 65 years or more, receiving social assistance, or without access to a private insurance plan in Quebec. Prescription drug data are available for all ages in the other jurisdictions. The CPRD is a primary care database that contains the records of more than 15 million people registered with more than 700 general practices in the UK.21 Importantly, it includes clinical data not typically found in administrative databases. CPRD data were linked to the Hospital Episode Statistics database, which contains information on hospital admissions; linkage is only available for general practices in England that have consented to the linkage scheme (currently representing 75% of all practices in England).
The study protocol was registered at clinicalTrials.gov (https://clinicaltrials.gov/ct2/show/NCT03939624).
Study population
In each participating site, we identified a source population of all individuals who received an antidiabetic drug (metformin, sulfonylureas, thiazolidinediones, DPP-4 inhibitors, SGLT2 inhibitors, glucagon-like peptide-1 receptor agonists, α-glucosidase inhibitors, meglitinides, insulin, or combinations of these drugs) between 1 January 2006 and 30 June 2018 (or the latest date of data availability at each site). Supplementary table 1 provides the dates of data availability at each site. Owing to availability of prescription drug data, the source population in Nova Scotia was restricted to those with an antidiabetic drug dispensed between 1 November 2017 and 30 June 2018. Entry into the source population was defined by the date the antidiabetic drug was first dispensed (or prescribed for CPRD) during this period. We selected 2006 as the beginning of observation for the source population because 2006 to 2018 corresponds to the period during which DPP-4 inhibitors were approved.
The study cohort included all individuals from the source population who received a SGLT2 inhibitor or DPP-4 inhibitor between the date a SGLT2 inhibitor was first dispensed in each site and 30 June 2018 (or the latest date of data availability at each site). Supplementary table 1 lists the dates that the SGLT2 inhibitors were first dispensed at each site. Using a prevalent new user cohort design,22 we matched each SGLT2 inhibitor user to a DPP-4 inhibitor user from their exposure set. The study cohort entry date was defined by the date the SGLT2 inhibitor was dispensed or the corresponding date the DPP-4 inhibitor was dispensed in the matched exposure set.
We excluded individuals younger than 18 years (<19 years in Alberta and <66 years in Ontario) and those with fewer than 365 consecutive days of healthcare (including prescription drug) coverage before the date of cohort entry. Among incident users of a SGLT2 inhibitor, we excluded those who also initiated a DPP-4 inhibitor on the same date. In addition, we excluded users of a DPP-4 inhibitor who were dispensed a SGLT2 inhibitor before the date of cohort entry. These individuals were eligible for inclusion in the SGLT2 inhibitor group if they met all the inclusion criteria and no exclusion criteria at the time of their first prescription for a SGLT2 inhibitor. Patients were followed until the occurrence of an event or censoring as a result of discontinuation of the study drug, death, end of healthcare coverage, or end of the study period, whichever occurred first. We determined separate follow-up times for each outcome. Patients were eligible to enter the cohorts a maximum of two times, first with a DPP-4 inhibitor and second with a SGLT2 inhibitor (but not vice versa given our use of the prevalent new user design).
Matching
For each new user of SGLT2 inhibitors, we defined exposure sets based on level of antidiabetic treatment, previous use of glucagon-like peptide-1 receptor agonists, duration of DPP-4 inhibitor treatment for prevalent new users, and calendar time (DPP-4 inhibitor prescription within 120 days of SGLT2 inhibitor initiation). Level of antidiabetic treatment was determined as 1) one insulin prescription or more in the past 365 days; 2) two or more classes of antidiabetic drugs (excluding insulin) in the past 365 days; or 3) other (including those without any antidiabetic drug treatment in the past 365 days). Previous use of glucagon-like peptide-1 receptor agonists was not used to define exposure sets in Ontario because these drugs were not reimbursed through provincial drug insurance, and thus data on use were not available. We matched incident SGLT2 inhibitor users to incident DPP-4 inhibitor users who initiated treatment in the same period, whereas we matched patients switching from a DPP-4 inhibitor to a SGLT2 inhibitor or adding a SGLT2 inhibitor to a DPP-4 inhibitor (prevalent users) to patients who had been using DPP-4 inhibitors for the same duration in their exposure sets. DPP-4 inhibitor use was considered incident if the patients had no DPP-4 inhibitor dispensed in the past 12 months.
We constructed time conditional propensity scores using conditional logistic regression stratified by exposure set to predict the probability (or propensity) of receiving a SGLT2 inhibitor compared with a DPP-4 inhibitor using covariates defined a priori (see supplementary table 2). Specifically, we assessed comorbidities using the eighth (Ontario outpatient billing only), ninth, and 10th revisions of the International Statistical Classification of Diseases and Related Health Problems with Canadian enhancement (ICD-9-CM and ICD-10-CA) diagnostic codes present in physician billing and hospital records in the three years before cohort entry. We assessed prescription drug use and healthcare use in the year before cohort entry. Comorbidities in the CPRD were assessed using ICD-10 codes and Read codes. In the CPRD, several covariates were also included in the propensity score model: body mass index, smoking status, race, blood pressure, estimated glomerular filtration rate, and glycated haemoglobin (HbA1c). Age and duration of diabetes were modelled continuously using restricted cubic splines with four knots.
We matched patients using SGLT2 inhibitors 1:1 without replacement to patients using DPP-4 inhibitors in their exposure set on nearest time conditional propensity score and in chronological order. However, five sites experienced a substantial loss of exposure sets when matching without replacement. In sites with more than 10% of exposure sets with no suitable match available after trimming the distribution of time conditional propensity scores, we performed matching with replacement. Supplementary table 3 summarises the matching approach adopted at each site.
Drug exposure definition
Patients were classified into one of the two mutually exclusive categories at entry into the study cohort: current use of SGLT2 inhibitors (canagliflozin, dapagliflozin, empagliflozin) alone or in combination with other antidiabetic drugs, or current use of DPP-4 inhibitors (alogliptin, linagliptin, saxagliptin, sitagliptin, vildagliptin) alone or in combination with other non-SGLT2 inhibitor antidiabetic drugs. Vildagliptin was only available in the UK. Exposure was defined using an as treated approach; specifically, we considered exposure time fixed and defined by the cohort entry drug, and patients were followed until treatment discontinuation, defined as either a gap of 30 days or more between successive prescriptions or the initiation of a SGLT2 inhibitor within the DPP-4 inhibitor cohort.
DPP-4 inhibitors were used as the reference category as both DPP-4 inhibitors and SGLT2 inhibitors are oral agents usually prescribed as second line or third line treatment for type 2 diabetes. Given that these drugs are used at a similar point in the management of type 2 diabetes, DPP-4 inhibitors as the reference group avoided time lag bias, a severe form of confounding by disease severity.23 Furthermore, DPP-4 inhibitors have no known association with the cardiovascular outcomes of interest.24 25 26 27
Outcomes
The primary outcome was MACE, defined as a composite of myocardial infarction, ischaemic stroke, or cardiovascular death. Secondary outcomes included the individual components of MACE, all cause mortality, and hospital admission for heart failure (see supplementary table 4 for ICD-10-CA codes). Myocardial infarction, ischaemic stroke, and heart failure were defined using hospital admission data, with a diagnosis recorded in the primary (ie, most responsible) position and the event date defined by the admission date. For MACE, the event date was determined by the first occurrence of any component of the composite endpoint. It was not feasible to use vital statistics data to define cardiovascular death because of the recent entry of SGLT2 inhibitors into the market and the lag in the availability of vital statistics data at several sites. Therefore, we defined cardiovascular death using an algorithm: in-hospital death with a cardiovascular diagnosis, or out-of-hospital death without documentation of cancer in the previous year or trauma in the preceding month. The date of death defined the event date for both cardiovascular death and all cause mortality.
Statistical analysis
Descriptive statistics were used to summarise patient characteristics, with frequencies and percentages for categorical variables and means (standard deviations) for continuous variables. Potential imbalances in covariates were assessed using the absolute value of the standardised difference, with a value of 0.1 or more considered important.
In our primary analysis, we used Cox proportional hazards models to estimate site specific adjusted hazard ratios and corresponding 95% confidence intervals for MACE among users of a SGLT2 inhibitor compared with users of a DPP-4 inhibitor. Models were adjusted for age (in years; continuous), sex, diabetes duration (in years; continuous), and 10ths of time conditional propensity score; sites that implemented matching with replacement used a robust sandwich estimator for the covariance matrix.
We conducted 13 prespecified secondary analyses. Firstly, we repeated our primary analysis for the individual components of MACE (myocardial infarction, ischaemic stroke, and cardiovascular death), all cause mortality, and hospital admission for heart failure. Then we conducted stratified analyses for MACE and heart failure by age (≥70 and <70 years), sex, previous insulin use (in the past year), and SGLT2 inhibitor molecule. In addition, we conducted stratified analyses for MACE by history of cardiovascular disease, defined by a diagnosis of coronary artery disease, peripheral arterial disease, or cerebrovascular disease in the previous three years. Stratified analyses for heart failure were conducted by history of heart failure, defined by two outpatient codes or one inpatient code for heart failure in the previous three years.
In post hoc subgroup analyses, we conducted stratified analyses for the individual components of MACE and all cause mortality by history of cardiovascular disease. In addition, we repeated our MACE analyses stratified by follow-up time (≤1 year and >1 year) to examine the impact of follow-up duration on our results.
In prespecified sensitivity analyses, we analysed MACE and heart failure as follows: firstly, using an intention-to-treat approach in which exposure was defined at cohort entry and patients were followed until the occurrence of an event or censored on death, end of healthcare coverage, end of the study period, entry into SGLT2 inhibitor cohort for patients who used DPP-4 inhibitors, or a maximum of one year follow-up, whichever occurred first; secondly, varying the grace period to define continuous drug use to 0 and 60 days; thirdly, stratifying by incident and prevalent new user status; and, finally, stratifying prevalent users by the addition of a SGLT2 inhibitor to a DPP-4 inhibitor versus switching to a SGLT2 inhibitor from a DPP-4 inhibitor. Furthermore, we conducted an analysis for MACE restricted to the subset of patients for whom vital statistics were available to define cardiovascular death. In addition, CPRD analyses were repeated with data restricted to variables found in the Canadian databases to examine the amount of residual confounding removed by the inclusion of these variables in the time conditional propensity scores. In post hoc sensitivity analyses, we repeated these analyses with data restricted to variables found in the Canadian databases and to estimated glomerular filtration rate. In a single site (CPRD) analysis, we repeated our primary analysis using a robust sandwich estimator to examine the impact on precision of estimates of some patients contributing to both groups. Finally, to explore residual confounding as a potential explanation for our observed results, we used the rule-out approach described elsewhere.28
Meta-analysis
Site specific adjusted hazard ratios were pooled using DerSimonian and Laird random effects meta-analytical models with inverse variance weighting. Heterogeneity between sites was estimated using the I2 statistic. All site specific analyses were conducted using SAS (versions varied across sites), and meta-analyses were conducted using Review Manager version 5.3.
Patient and public involvement
This study was a secondary data analysis and was done without patient involvement. Patients were not invited to comment on the study design and were not consulted to develop patient relevant outcomes or interpret the results. Patients were not invited to contribute to the writing or editing of this document for readability or accuracy, and there are no plans to involve patients in the dissemination of study results.
Results
Patient characteristics
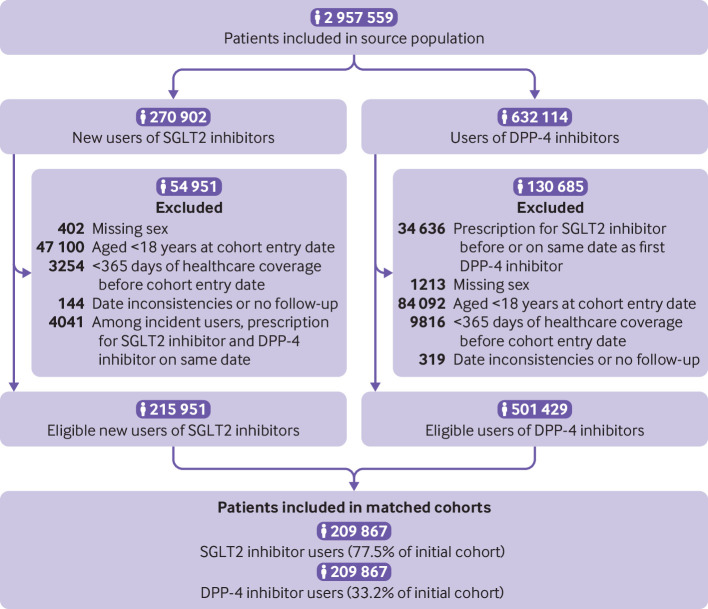
Among 270 902 eligible new users of SGLT2 inhibitors and 632 114 users of DPP-4 inhibitors (fig 1), 209 867 matched pairs were included in the study cohort. The study population included 103 797 pairs of incident new users and 106 070 pairs of prevalent new users. Table 1 describes the baseline characteristics of new users of SGLT2 inhibitors and their matched DPP-4 inhibitor users (also see supplementary table 5). Baseline covariates were well balanced between the two cohorts after matching on time conditional propensity score. Among 209 867 users of SGLT2 inhibitors, 88 862 (42.3%) initiated canagliflozin, 64 291 (30.7%) dapagliflozin, and 56 714 (27.0%) empagliflozin at cohort entry. Supplementary table 6 presents the distribution of the additional characteristics of the users of SGLT2 inhibitors and DPP-4 inhibitors in CPRD. Some imbalance was present in estimated glomerular filtration rate, with SGLT2 inhibitor users having a lower prevalence of rates less than 60 mL/min/1.73m2; groups were balanced on other covariates. The median (interquartile range) duration between measurement of estimated glomerular filtration rate and cohort entry was 30 days (13-100 days) among SGLT2 inhibitor users and 62 (19 to 161) days among DPP-4 inhibitor users.
Fig 1.
Selection of study cohort. Numbers might not add up because site specific cells with a value of less than six were suppressed owing to privacy restrictions. Patients aged less than 19 years in Alberta and less than 66 years in Ontario were excluded. Patients were eligible to enter the study cohort a maximum of two times, first with a prescription for a dipeptidyl peptidase-4 (DPP-4) inhibitor and second time with a prescription for a sodium glucose cotransporter 2 (SGLT2) inhibitor
Table 1.
Baseline characteristics of users of sodium glucose cotransporter 2 (SGLT2) inhibitors and matched users of dipeptidyl peptidase-4 (DPP-4) inhibitors. Values are numbers (percentages) unless stated otherwise
| Characteristics | Pre-matching* | Post-matching† | |||||
|---|---|---|---|---|---|---|---|
| SGLT2 inhibitors (n=215 762) | DPP-4 inhibitors (n=215 762) | aSD | SGLT2 inhibitors (n=209 867) | DPP-4 inhibitors (n=209 867) | aSD | ||
| Mean (SD) age (years): | 63.6 (9.5) | 67.9 (11.0) | 0.414 | 63.8 (9.5) | 64.0 (9.6) | 0.028 | |
| Age group (years): | |||||||
| 18-35 | 3744 (1.7) | 2806 (1.3) | 0.036 | 3536 (1.7) | 3636 (1.7) | 0.004 | |
| 36-45 | 12 984 (6.0) | 9724 (4.5) | 0.068 | 12 456 (5.9) | 11 990 (5.7) | 0.009 | |
| 46-55 | 32 528 (15.1) | 24 062 (11.2) | 0.116 | 31 302 (14.9) | 30 472 (14.5) | 0.011 | |
| 56-65 | 49 997 (23.2) | 40 588 (18.8) | 0.107 | 48 290 (23.0) | 48 486 (23.1) | 0.002 | |
| 66-75 | 92 107 (42.7) | 78 374 (36.3) | 0.130 | 90 031 (42.9) | 88 813 (42.3) | 0.012 | |
| 76-85 | 22 371 (10.4) | 46 363 (21.5) | 0.307 | 22 226 (10.6) | 24 251 (11.6) | 0.031 | |
| >85 | 2031 (0.9) | 13 845 (6.4) | 0.294 | 2026 (1.0) | 2219 (1.1) | 0.009 | |
| Site: | |||||||
| Alberta | 26 459 (12.3) | 26 459 (12.3) | - | 26 186 (12.5) | 26 186 (12.5) | - | |
| British Columbia | 44 629 (20.7) | 44 629 (20.7) | - | 44 043 (21.0) | 44 043 (21.0) | - | |
| Manitoba | 12 539 (5.8) | 12 539 (5.8) | - | 12 204 (5.8) | 12 204 (5.8) | - | |
| Nova Scotia | 1268 (0.6) | 1268 (0.6) | - | 1119 (0.5) | 1119 (0.5) | - | |
| Ontario | 66 549 (30.8) | 66 549 (30.8) | - | 65 556 (31.2) | 65 556 (31.2) | - | |
| Quebec | 46 751 (21.7) | 46 751 (21.7) | - | 44 504 (21.2) | 44 504 (21.2) | - | |
| Saskatchewan | 11 363 (5.3) | 11 363 (5.3) | - | 10 832 (5.2) | 10 832 (5.2) | - | |
| CPRD | 6204 (2.9) | 6204 (2.9) | - | 5423 (2.6) | 5423 (2.6) | - | |
| Women | 89 361 (41.4) | 97 954 (45.4) | 0.080 | 87 076 (41.5) | 87 650 (41.8) | 0.006 | |
| Calendar year at cohort entry: | |||||||
| 2013 | 333 (0.2) | 351 (0.2) | 0.002 | 323 (0.2) | 325 (0.2) | 0.000 | |
| 2014 | 7447 (3.5) | 8497 (3.9) | 0.026 | 7131 (3.4) | 8082 (3.9) | 0.024 | |
| 2015 | 53 249 (24.7) | 52 426 (24.3) | 0.009 | 52 091 (24.8) | 51 361 (24.5) | 0.008 | |
| 2016 | 68 440 (31.7) | 68 822 (31.9) | 0.004 | 66 816 (31.8) | 66 569 (31.7) | 0.003 | |
| 2017 | 63 715 (29.5) | 63 014 (29.2) | 0.007 | 61 792 (29.4) | 61 504 (29.3) | 0.003 | |
| 2018 | 22 578 (10.5) | 22 652 (10.5) | 0.001 | 21 714 (10.3) | 22 026 (10.5) | 0.005 | |
| Mean (SD) diabetes duration (years) | 12.5 (6.5) | 13.1 (6.7) | 0.093 | 12.5 (6.5) | 12.5 (6.5) | 0.001 | |
| Diabetes duration (years): | |||||||
| <1 | 7379 (3.4) | 7193 (3.3) | 0.005 | 7194 (3.4) | 7412 (3.5) | 0.006 | |
| 1-4.9 | 25 833 (12.0) | 24 043 (11.1) | 0.026 | 25 401 (12.1) | 25 570 (12.2) | 0.002 | |
| 5-10 | 53 982 (25.0) | 49 729 (23.0) | 0.046 | 52 681 (25.1) | 52 685 (25.1) | 0.000 | |
| >10 | 128 568 (59.6) | 134 797 (62.5) | 0.059 | 124 591 (59.4) | 124 200 (59.2) | 0.004 | |
| Comorbidities‡: | |||||||
| Alcohol related disorders | 3060 (1.4) | 4632 (2.1) | 0.055 | 2975 (1.4) | 2992 (1.4) | 0.001 | |
| Aortic aneurysm | 1534 (0.7) | 2125 (1.0) | 0.030 | 1503 (0.7) | 1568 (0.7) | 0.004 | |
| Atherosclerosis | 4356 (2.0) | 6649 (3.1) | 0.067 | 4221 (2.0) | 4226 (2.0) | 0.000 | |
| Atrial fibrillation | 7553 (3.5) | 14 351 (6.7) | 0.144 | 7336 (3.5) | 7516 (3.6) | 0.005 | |
| Cancer | 22 019 (10.2) | 28 067 (13.0) | 0.088 | 21 575 (10.3) | 21 882 (10.4) | 0.005 | |
| Cerebrovascular disease | 10 217 (4.7) | 16 896 (7.8) | 0.128 | 10 024 (4.8) | 10 218 (4.9) | 0.004 | |
| Cirrhosis | 3694 (1.7) | 3973 (1.8) | 0.010 | 3586 (1.7) | 3497 (1.7) | 0.003 | |
| COPD | 21 450 (9.9) | 26 604 (12.3) | 0.076 | 20 824 (9.9) | 20 885 (10.0) | 0.001 | |
| Coronary artery disease | 47 048 (21.8) | 53 160 (24.6) | 0.067 | 45 532 (21.7) | 44 871 (21.4) | 0.008 | |
| Dementia | 2234 (1.0) | 9643 (4.5) | 0.211 | 2203 (1.0) | 2359 (1.1) | 0.007 | |
| Diabetic nephropathy | 7838 (3.6) | 17 126 (7.9) | 0.185 | 7610 (3.6) | 7796 (3.7) | 0.005 | |
| Diabetic neuropathy | 4343 (2.0) | 5750 (2.7) | 0.043 | 4033 (1.9) | 3944 (1.9) | 0.003 | |
| Diabetic retinopathy | 5755 (2.7) | 7158 (3.3) | 0.038 | 5371 (2.6) | 5618 (2.7) | 0.007 | |
| Dialysis | 293 (0.1) | 1970 (0.9) | 0.108 | 284 (0.1) | 316 (0.2) | 0.004 | |
| Dyslipidaemia | 175 880 (81.5) | 174 482 (80.9) | 0.017 | 170 806 (81.4) | 170 146 (81.1) | 0.008 | |
| Heart failure | 11 933 (5.5) | 21 850 (10.1) | 0.172 | 11 625 (5.5) | 11 762 (5.6) | 0.003 | |
| Hypertension | 110 915 (51.4) | 123 768 (57.4) | 0.120 | 108 231 (51.6) | 108 768 (51.8) | 0.005 | |
| Hypoglycaemia | 1096 (0.5) | 2018 (0.9) | 0.051 | 1051 (0.5) | 1086 (0.5) | 0.002 | |
| Ischaemic stroke | 2543 (1.2) | 4623 (2.1) | 0.075 | 2499 (1.2) | 2664 (1.3) | 0.007 | |
| Myocardial infarction | 5836 (2.7) | 7910 (3.7) | 0.055 | 5585 (2.7) | 5415 (2.6) | 0.005 | |
| Other kidney disease | 10 262 (4.8) | 31 457 (14.6) | 0.337 | 10 011 (4.8) | 10 939 (5.2) | 0.020 | |
| Peripheral arterial disease | 5035 (2.3) | 6898 (3.2) | 0.053 | 4862 (2.3) | 4852 (2.3) | 0.000 | |
| Use of antidiabetic drugs‡: | |||||||
| α-glucosidase inhibitors | 3310 (1.5) | 2750 (1.3) | 0.022 | 3107 (1.5) | 3130 (1.5) | 0.001 | |
| GLP-1 receptor agonists | 13 047 (6.0) | 12 943 (6.0) | 0.002 | 9180 (4.4) | 9180 (4.4) | 0.000 | |
| Insulin | 61 166 (28.3) | 61 167 (28.3) | 0.000 | 58 330 (27.8) | 58 330 (27.8) | 0.000 | |
| Meglitinides | 4879 (2.3) | 6146 (2.8) | 0.037 | 4736 (2.3) | 4773 (2.3) | 0.001 | |
| Metformin | 190 198 (88.2) | 186 916 (86.6) | 0.046 | 185 681 (88.5) | 185 426 (88.4) | 0.004 | |
| Sulfonylureas | 112 044 (51.9) | 107 293 (49.7) | 0.044 | 109 139 (52.0) | 109 132 (52.0) | 0.000 | |
| Thiazolidinediones | 5629 (2.6) | 4367 (2.0) | 0.039 | 5315 (2.5) | 5114 (2.4) | 0.006 | |
aSD=absolute value of standardised difference; COPD=chronic obstructive pulmonary disease; CPRD=Clinical Practice Research Datalink; GLP-1=glucagon-like peptide-1.
Pre-matching cohort represents new users of SGLT2 inhibitors and a randomly selected member (DPP-4 inhibitor user) of their exposure sets, selected before matching on time conditional propensity score.
Matched from exposure set (defined on level of antidiabetic treatment, past use of GLP-1 receptor agonists, time receiving DPP-4 inhibitors for prevalent new users, and calendar time) on time conditional propensity score. Site specific cells that contained a value <6 were suppressed owing to privacy restrictions and were assumed to have a value of 3.
Assessed in the three years before entry to the study cohort. Drug use was assessed in the year before cohort entry.
Cardiovascular outcomes
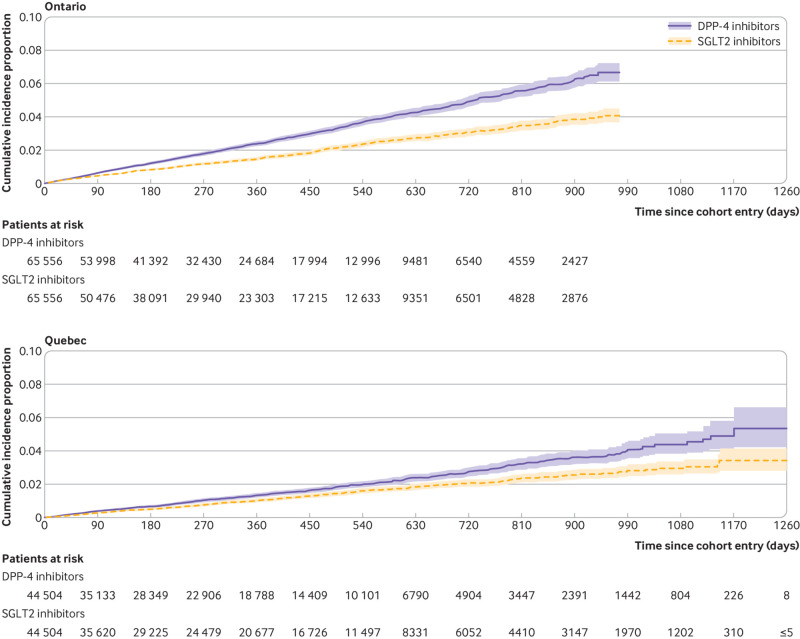
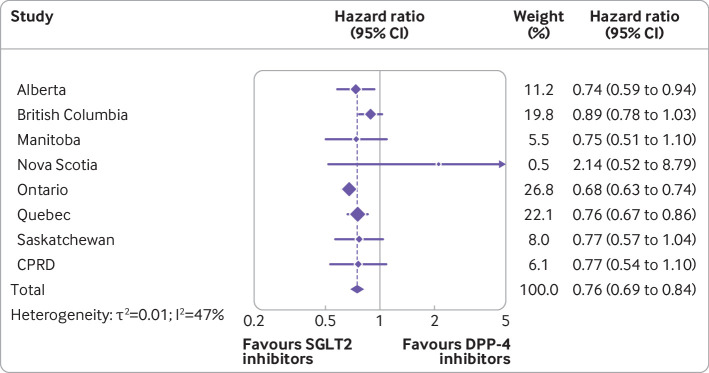
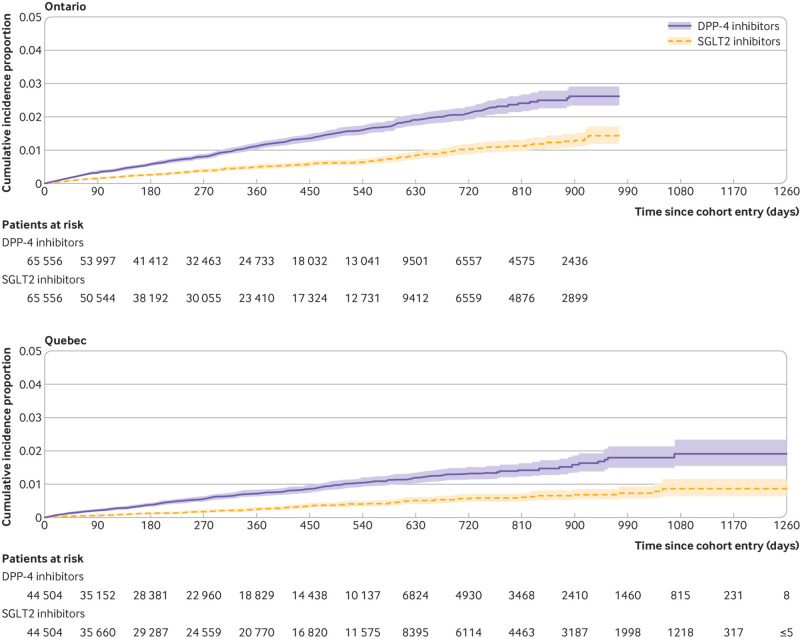
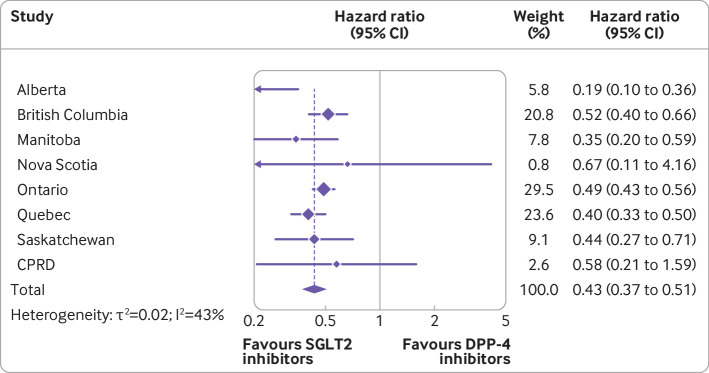
Overall, the mean duration of follow-up in the matched cohort for the primary outcome of MACE was 0.9 (SD 0.76) years, generating a total of 370 515 person years of observation time. During follow-up, MACE occurred in 2146 users of SGLT2 inhibitors (incidence rate 11.4 per 1000 person years) and 3001 users of DPP-4 inhibitors (16.5 per 1000 person years) (see fig 2 for timing of events at the two largest sites). Table 2 shows the crude incidence rates and crude and adjusted hazard ratios for all outcomes. Numbers of events for MACE by site are reported in supplementary table 7. Compared with DPP-4 inhibitors, SGLT2 inhibitors were associated with a decreased risk of MACE (hazard ratio 0.76, 95% confidence interval 0.69 to 0.84; I2=47%; fig 3). These drugs were also associated with decreased risks of myocardial infarction (0.82, 0.70 to 0.96; I2=53%; also see supplementary fig 1) and cardiovascular death (0.60, 0.54 to 0.67; I2=14%; also see supplementary fig 2), with a more modest effect for ischaemic stroke (0.85, 0.72 to 1.01; I2=28%; also see supplementary fig 3). In addition, SGLT2 inhibitors were associated with decreased risks of all cause mortality (0.60, 0.54 to 0.67; I2=42%; also see supplementary fig 4) and hospital admission for heart failure (0.43, 0.37 to 0.51; I2=43%; figs 4 and 5).
Fig 2.
Cumulative incidence of major adverse cardiovascular events among users of sodium glucose cotransporter 2 (SGLT2) inhibitors and matched users of dipeptidyl peptidase-4 (DPP-4) inhibitors in Ontario and Quebec, the two largest study sites
Table 2.
Crude and adjusted hazard ratios for association between sodium glucose cotransporter 2 (SGLT2) inhibitors versus dipeptidyl peptidase-4 (DPP-4) inhibitors and risk of cardiovascular outcomes
| Cardiovascular outcomes by drug | No of events | Person years | Crude incidence rate per 1000 person years | Crude hazard ratio (95% CI)* | Adjusted models*† | |
|---|---|---|---|---|---|---|
| Hazard ratio (95% CI) | I2 (%) | |||||
| MACE: | ||||||
| SGLT2 inhibitors | 2146 | 188 782 | 11.4 | 0.72 (0.65 to 0.80) | 0.76 (0.69 to 0.84) | 47 |
| DPP-4 inhibitors | 3001 | 181 733 | 16.5 | 1.00 (Reference) | 1.00 (Reference) | |
| Myocardial infarction: | ||||||
| SGLT2 inhibitors | 995 | 196 503 | 5.1 | 0.81 (0.72 to 0.92) | 0.82 (0.70 to 0.96) | 53 |
| DPP-4 inhibitors | 1169 | 182 398 | 6.4 | 1.00 (Reference) | 1.00 (Reference) | |
| Ischaemic stroke: | ||||||
| SGLT2 inhibitors | 501 | 190 047 | 2.6 | 0.78 (0.68 to 0.89) | 0.85 (0.72 to 1.01) | 28 |
| DPP-4 inhibitors | 636 | 182 731 | 3.5 | 1.00 (Reference) | 1.00 (Reference) | |
| Cardiovascular death: | ||||||
| SGLT2 inhibitors | 738 | 189 276 | 3.9 | 0.55 (0.47 to 0.65) | 0.60 (0.54 to 0.67) | 14 |
| DPP-4 inhibitors | 1399 | 182 746 | 7.7 | 1.00 (Reference) | 1.00 (Reference) | |
| All cause mortality: | ||||||
| SGLT2 inhibitors | 1651 | 189 278 | 8.7 | 0.54 (0.48 to 0.60) | 0.60 (0.54 to 0.67) | 42 |
| DPP-4 inhibitors | 3156 | 183 075 | 17.3 | 1.00 (Reference) | 1.00 (Reference) | |
| Heart failure: | ||||||
| SGLT2 inhibitors | 587 | 189 058 | 3.1 | 0.40 (0.35 to 0.46) | 0.43 (0.37 to 0.51) | 43 |
| DPP-4 inhibitors | 1401 | 181 956 | 7.7 | 1.00 (Reference) | 1.00 (Reference) | |
MACE=major adverse cardiovascular events.
Users of SGLT2 inhibitors were matched to users of DPP-4 inhibitors from their exposure set (defined on level of antidiabetic therapy, time on DPP-4 inhibitors for prevalent new users only, prior use of glucagon-like peptide-1 receptor agonists, and within 120 days of the SGLT2 prescription) on time-conditional propensity score.
Adjusted for age (continuous), sex, diabetes duration (continuous), and 10ths of time conditional propensity score.
Fig 3.
Adjusted hazard ratios (95% confidence intervals) of major adverse cardiovascular events associated with use of sodium glucose cotransporter 2 (SGLT2) inhibitors compared with dipeptidyl peptidase-4 (DPP-4) inhibitors. Outcome models were adjusted for age (continuous), sex, diabetes duration (continuous), and 10ths of time conditional propensity score. CPRD=Clinical Practice Research Datalink
Fig 4.
Cumulative incidence of hospital admission for heart failure among users of sodium glucose cotransporter 2 (SGLT2) inhibitors and matched users of dipeptidyl peptidase-4 (DPP-4) inhibitors in Ontario and Quebec, the two largest study sites
Fig 5.
Adjusted hazard ratios (95% confidence intervals) of hospital admission for heart failure associated with use of sodium glucose cotransporter 2 (SGLT2) inhibitors compared with dipeptidyl peptidase-4 (DPP-4) inhibitors. Outcome models were adjusted for age (continuous), sex, diabetes duration (continuous), and 10ths of time conditional propensity score. CPRD=Clinical Practice Research Datalink
Stratified and sensitivity analyses
Table 3 shows the stratified analyses for MACE and heart failure. These analyses showed no evidence of effect modification by age, sex, previous insulin use, or SGLT2 inhibitor molecule. Additionally, no difference was found in the estimated associations for MACE when analyses were stratified by a history of cardiovascular disease or for heart failure when analyses were stratified by a history of heart failure. Additional analyses stratified by history of cardiovascular disease suggest greater benefits for cardiovascular death and all cause mortality among those with a history of cardiovascular disease, although benefits were present for those with and without a history of cardiovascular disease (supplementary table 8). Overall, sensitivity analyses produced results that were consistent with those of our primary analyses for both MACE and heart failure (table 4), although the analysis stratified by incident user versus prevalent user cannot exclude potentially stronger benefits with SGLT2 inhibitors among prevalent users. In addition, similar estimates were obtained with and without the use of clinical covariates available in the CPRD only for all outcomes except ischaemic stroke, when a higher point estimate was obtained in analyses that did not include these variables, although 95% confidence intervals largely overlapped (supplementary table 9). A sensitivity analysis restricted to the subset of patients with available information on vital statistics produced results that were consistent with the primary analysis but with a wider confidence interval (hazard ratio 0.78, 95% confidence interval 0.63 to 0.97). Similar estimates were also obtained in subgroup analyses stratified by follow-up of less than one year versus more than one year (supplementary fig 5). The sensitivity analysis using the robust sandwich estimator in the CPRD produced results that were identical to those of our primary CPRD analysis (adjusted hazard ratio 0.77, 95% confidence interval 0.54 to 1.10). Finally, the rule-out method suggested that it was unlikely that residual confounding from an unmeasured confounder would explain the observed results (supplementary fig 6).
Table 3.
Summary results for stratified analyses of pooled adjusted hazard ratios (95% confidence intervals) for major adverse cardiovascular events (MACE) and heart failure associated with use of sodium glucose cotransporter 2 (SGLT2) inhibitors versus dipeptidyl peptidase-4 (DPP-4) inhibitors
| Subgroup | Adjusted hazard ratio (95% CI)* | I2 (%) |
|---|---|---|
| MACE | ||
| Main analysis | 0.76 (0.69 to 0.84) | 47 |
| Age (years): | ||
| ≥70 | 0.75 (0.67 to 0.85) | 19 |
| <70 | 0.77 (0.69 to 0.87) | 33 |
| Sex: | ||
| Women | 0.65 (0.58 to 0.72) | 2 |
| Men | 0.81 (0.72 to 0.91) | 39 |
| History of cardiovascular disease†: | ||
| Yes | 0.71 (0.59 to 0.86) | 67 |
| No | 0.78 (0.69 to 0.88) | 40 |
| Previous insulin use‡: | ||
| Yes | 0.75 (0.66 to 0.86) | 32 |
| No | 0.76 (0.68 to 0.86) | 38 |
| SGLT2 inhibitor molecule: | ||
| Canagliflozin | 0.79 (0.66 to 0.94) | 67 |
| Dapagliflozin | 0.73 (0.63 to 0.85) | 32 |
| Empagliflozin | 0.77 (0.68 to 0.87) | 1 |
| Heart failure | ||
| Main analysis | 0.43 (0.37 to 0.51) | 43 |
| Age (years): | ||
| ≥70 | 0.46 (0.36 to 0.61) | 53 |
| <70 | 0.39 (0.30 to 0.50) | 49 |
| Sex: | ||
| Women | 0.42 (0.35 to 0.49) | 0 |
| Men | 0.50 (0.39 to 0.65) | 62 |
| History of heart failure§: | ||
| Yes | 0.44 (0.35 to 0.55) | 33 |
| No | 0.47 (0.41 to 0.53) | 0 |
| Past insulin use‡: | ||
| Yes | 0.45 (0.39 to 0.52) | 1 |
| No | 0.47 (0.40 to 0.55) | 9 |
| SGLT2 inhibitor molecule: | ||
| Canagliflozin | 0.41 (0.32 to 0.52) | 42 |
| Dapagliflozin | 0.44 (0.36 to 0.54) | 0 |
| Empagliflozin | 0.52 (0.43 to 0.65) | 4 |
Nova Scotia had zero events in one of the treatment groups and thus was not included in the cardiovascular disease (yes) analysis for MACE or in the age (≥70 years), sex, history of heart failure, and SGLT2 inhibitor molecule analyses for heart failure.
Adjusted for age (continuous), sex, diabetes duration (continuous), and 10ths of time conditional propensity score.
Coronary artery disease, peripheral arterial disease, or cerebrovascular disease in the past three years.
Prescription for insulin in past year.
Two outpatient codes or one inpatient code in the past three years.
Table 4.
Summary results for sensitivity analyses of pooled adjusted hazard ratios (95% confidence intervals) for major adverse cardiovascular events (MACE) and heart failure associated with use of sodium glucose cotransporter 2 (SGLT2) inhibitors versus dipeptidyl peptidase-4 (DPP-4) inhibitors
| Sensitivity analyses | Adjusted hazard ratio (95% CI)* | I2 (%) |
|---|---|---|
| MACE | ||
| Main analysis | 0.76 (0.69 to 0.84) | 47 |
| Intention-to-treat approach | 0.80 (0.73 to 0.88) | 45 |
| Grace period (days): | ||
| 0 | 0.75 (0.67 to 0.85) | 0 |
| 60 | 0.75 (0.69 to 0.81) | 34 |
| New user status: | ||
| Incident users | 0.81 (0.71 to 0.93) | 44 |
| Prevalent users | 0.71 (0.65 to 0.76) | 0 |
| Prevalent users: | ||
| Adding a SGLT2 inhibitor | 0.72 (0.63 to 0.82) | 0 |
| Switching to a SGLT2 inhibitor | 0.70 (0.64 to 0.77) | 0 |
| Heart failure | ||
| Main analysis | 0.43 (0.37 to 0.51) | 43 |
| Intention-to-treat approach | 0.52 (0.45 to 0.61) | 43 |
| Grace period (days): | ||
| 0 | 0.47 (0.32 to 0.69) | 48 |
| 60 | 0.43 (0.35 to 0.53) | 68 |
| New user status: | ||
| Incident users | 0.46 (0.38 to 0.56) | 26 |
| Prevalent users | 0.41 (0.30 to 0.55) | 55 |
| Prevalent users: | ||
| Adding a SGLT2 inhibitor | 0.40 (0.31 to 0.51) | 0 |
| Switching to a SGLT2 inhibitor | 0.39 (0.27 to 0.56) | 62 |
Nova Scotia had zero events in one of the treatment groups and thus was not included in the following analyses for MACE: grace period (0 days), new user status (prevalent users), and prevalent users. Nova Scotia, Alberta, Saskatchewan, and the Clinical Practice Research Datalink had zero events in one of the treatment groups in the prevalent user analysis involving the addition of a SGLT2 inhibitor and were thus excluded from this analysis.
Adjusted for age (continuous), sex, diabetes duration (continuous), and 10ths of time conditional propensity score.
Discussion
In this large multi-database retrospective cohort study, we found that the use of SGLT2 inhibitors was associated with a decreased risk of MACE compared with use of DPP-4 inhibitors among individuals with type 2 diabetes (hazard ratio 0.76, 95% confidence interval 0.69 to 0.84). Beneficial effects were observed for the individual endpoints of MACE (myocardial infarction, ischaemic stroke, and cardiovascular death). The strong association with MACE was mainly driven by cardiovascular death (0.60, 0.54 to 0.67). We also observed decreased risks of all cause mortality (0.60, 0.54 to 0.67) and heart failure (0.43, 0.37 to 0.51) in individuals using SGLT2 inhibitors compared with those using DPP-4 inhibitors. Similar results were observed for canagliflozin, dapagliflozin, and empagliflozin and across patient subgroups defined by age, sex, past insulin use, and history of cardiovascular disease or history of heart failure. Although some heterogeneity was present in subgroup analyses by cardiovascular disease history, consistent benefits were present for all outcomes except ischaemic stroke, where results were inconclusive because of wide 95% confidence intervals.
Strengths and limitations of this study
Our study has several strengths. An active comparator used at a similar stage of diabetes treatment and rigorous matching minimised potential confounding bias. Our large sample size permitted the calculation of precise estimates for the primary and secondary outcomes. This sample size also allowed for the examination of molecule specific associations, representing a key addition to the literature. The consistency of results across several sensitivity analyses further supports the robustness of our results. Finally, the registration of our study protocol enhanced the transparency of reporting.
Our study also has potential limitations. This study is observational, and residual or unmeasured confounding bias remains possible. Confounding is perhaps most likely among prevalent new users, when individuals using a DPP-4 inhibitor who switched to or added on a SGLT2 inhibitor were compared with those who continued DPP-4 inhibitor treatment. We used different approaches to minimise potential confounding, including the use of an active comparator, the prevalent new user design, propensity score matching, and extensive sensitivity analyses. We adjusted for covariates measured at cohort entry, therefore we cannot rule out confounding from changes in health status during follow-up. In addition, although imperfectly measured and recorded renal function impairment in the Canadian claims data represents a potentially important source of residual confounding, we assessed the possible effect of residual confounding using the CPRD, which includes clinical measures (including estimated glomerular filtration rate) not typically found in administrative data. Sensitivity analyses conducted in the CPRD suggest that these variables are unlikely to explain the observed association.
Misclassification of exposure is possible as data for prescriptions represent dispensed drugs (or prescribed drugs in the CPRD) and not actual consumption. Outcome misclassification is possible for cardiovascular death defined using our algorithm. A sensitivity analysis restricted to the subset of patients for whom vital statistics were available produced results that were consistent with the primary analysis but with wider confidence intervals. Although we had a large sample size, the number of events were limited in some stratified analyses. In addition, ertugliflozin was not available during the study period and was thus excluded from our assessment. Furthermore, despite our use of a common protocol, some heterogeneity was present across sites. This heterogeneity might be due to differences in populations, data capture, and formulary restrictions. For example, British Columbia, Manitoba, and Nova Scotia capture all dispensed drugs (regardless of payer), whereas Quebec and Ontario only capture those reimbursed by the provincial government (with Ontario data restricted to those aged ≥65 years in this study), Alberta only captures reimbursed outpatient prescriptions, and Saskatchewan captures dispensed drugs covered by provincial and federal governments. Therefore, we used random effects models to account for variance both within sites and between sites. Finally, the mean duration of follow-up was only 0.9 years, so it is possible that the observed findings are related to short term haemodynamic effects of SGLT2 inhibitors rather than disease modifying benefits in the long term. However, we conducted subgroup analyses by duration of follow-up and found similar results for the first year of follow-up and for subsequent years. Nonetheless, a need remains to assess the long term comparative effectiveness and safety of these drugs as additional real world evidence becomes available.
Comparison with other studies
Placebo controlled randomised controlled trials of SGLT2 inhibitors have reported a decreased risk of MACE in participants randomised to canagliflozin or empagliflozin, with dapagliflozin reaching non-inferiority but not superiority for MACE.3 4 5 6 A decrease in hospital admissions for heart failure was observed in randomised controlled trials for all three molecules. Although these placebo controlled trials provided important information about the cardiovascular effects of SGLT2 inhibitors, they also had important limitations. All three were conducted in participants with either established cardiovascular disease or who were at high risk of cardiovascular disease, further limiting the generalisability of the results to a real world setting. Furthermore, although the use of placebo offers greater assay sensitivity (ie, the ability to determine if a treatment is or is not effective) compared with the use of an active comparator,29 the differential use of rescue drugs among those with poorly controlled blood glucose hampers this sensitivity, particularly given the known cardiotoxic effects of some antidiabetic drugs (eg, thiazolidinedione and heart failure,30 sulfonylureas and cardiovascular death31). An active comparator used at the same point in the management of type 2 diabetes overcomes these limitations and provides more clinically and policy relevant comparisons.
Previous observational studies also suggest a reduced risk of heart failure and all cause mortality.8 9 10 11 12 13 14 15 32 However, observational studies have provided more heterogeneous results for MACE, with some studies finding a protective effect8 9 13 14 and others finding no benefit.10 12 15 Notably, the definition of MACE varied across studies.8 9 10 12 14 Some of this heterogeneity might also be explained by the use of different comparators, with some studies comparing SGLT2 inhibitors to a reference group consisting of “other antidiabetic drugs.”8 9 10 13 With a heterogeneous reference group, the results can be difficult to interpret and, depending on the distribution of antidiabetic drugs in the reference group, time lag bias (confounding by disease severity23) can occur. In addition, some studies8 9 13 could have been affected by immortal time bias, which tends to exaggerate effectiveness.16 17
Our use of a prevalent new user design allowed us to include patients with a recent history of DPP-4 inhibitor use, thus better reflecting real world practice. Indeed, the use of an active comparator new user approach33 would have resulted in the exclusion of about 50% of our study cohort. Thus, this methodological approach, combined with the use of data from seven Canadian provinces and the UK and broad inclusion criteria, has greatly increased the generalisability of results relative to previous studies in this area. Our use of time conditional propensity scores produced treatment groups that were well balanced for baseline characteristics. Indeed, the one characteristic for which an imbalance remained was renal insufficiency (defined by an estimated glomerular filtration rate <60 ml/min/1.73m2) in analyses restricted to the CPRD. This finding is not unexpected given that SGLT2 inhibitors are generally not recommended among patients with renal insufficiency.34 In addition, although the two largest sites included in this study were either restricted to older individuals (Ontario) or disproportionately included older individuals (Quebec), subgroup analyses suggested that the association did not vary with age. Ultimately, with data from eight jurisdictions across two countries, our study adds clarity to the heterogeneous treatment effects reported by previous observational studies, providing precise estimates of the beneficial cardiovascular effects of SGLT2 inhibitors in a real world setting.
Conclusions
In this large multi-database cohort study, the short term use of SGLT2 inhibitors was associated with a decreased risk of MACE compared with the use of DPP-4 inhibitors among people with type 2 diabetes. Benefits were observed for the individual endpoints of MACE, all cause mortality, and heart failure. Similar reductions in MACE were observed for canagliflozin, dapagliflozin, and empagliflozin and across patient subgroups defined by age, sex, previous use of insulin, and history of cardiovascular disease. These findings suggest that SGLT2 inhibitors offer cardioprotective benefits among people with type 2 diabetes in a real world setting, although additional studies are needed to determine if these benefits persist long term.
What is already known on the topic
Sodium glucose cotransporter 2 (SGLT2) inhibitors are increasingly being used to treat type 2 diabetes
Randomised controlled trials have shown that SGLT2 inhibitors reduce the risk of major adverse cardiovascular events (MACE) and heart failure compared with placebo
What this study adds
SGLT2 inhibitors are associated with a decreased risk of serious cardiovascular events compared with dipeptidyl peptidase-4 (DPP-4) inhibitors among people with type 2 diabetes in a real world setting
The consistent results across individual SGLT2 inhibitors suggest a class effect for the cardiovascular benefits of SGLT2 inhibitors
Acknowledgments
The CNODES Investigators are: Samy Suissa (principal investigator), Colin R Dormuth (British Columbia), Brenda R Hemmelgarn (Alberta), Jacqueline Quail (Saskatchewan), Dan Chateau (Manitoba), J Michael Paterson (Ontario), Jacques LeLorier (Quebec), Adrian R Levy (Atlantic: Nova Scotia, Newfoundland and Labrador, New Brunswick, Prince Edward Island), Pierre Ernst and Kristian B Filion (UK Clinical Practice Research Datalink), Lisa M Lix (database team), Robert W Platt (methods team), and Ingrid S Sketris (knowledge translation team).
This study was made possible through data sharing agreements between the CNODES member research centres and the respective provincial governments of Alberta, British Columbia (BC), Manitoba (Health Information Privacy Committee (HIPC) No 2018/2019-58), Nova Scotia, Ontario, Quebec, and Saskatchewan. This study was approved by the Independent Scientific Advisory Committee (protocol No 19_007A2) of the Clinical Practice Research Datalink; the approved protocol was made available to journal reviewers. The BC Ministry of Health approved access to and use of BC data for this study. Data sources were as follows (www.popdata.bc.ca/data): BC Ministry of Health (creator) (2018): Medical Services Plan (MSP) payment information file. BC Ministry of Health (publisher). Ministry of Health (2018); BC Ministry of Health (creator) (2018): consolidation file (MSP registration and premium billing). BC Ministry of Health (publisher). Ministry of Health (2018); BC Ministry of Health (creator) (2018): PharmaNet. BC Ministry of Health (publisher). Data Stewardship Committee (2018); and Canadian Institute for Health Information (creator) (2018): Discharge Abstract Database (Hospital Separations). BC Ministry of Health (publisher). Ministry of Health (2018). BC Ministry of Health (publisher). Ministry of Health (2018); BC Vital Statistics Agency (creator) (2018): Vital Statistics Deaths. Version 2. BC Ministry of Health (publisher). Parts of this material are based on data and information compiled and provided by the Ontario Ministry of Health and Long term Care. This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long Term Care. Parts of this material are based on data and/or information compiled and provided by the Canadian Institute for Health information (CIHI). The opinions, results, and conclusions reported in this paper are those of the authors. No endorsement by the provinces, data stewards, ICES, CIHI, or the Institut national d’excellence en santé et en services sociaux is intended or should be inferred.
We thank Corine Mizrahi at the CNODES Coordinating Centre for her important contributions to this work; the programming and analytical support of the analysts at each site: Greg Carney and Jason Kim (British Columbia), Zhihai Ma and Jianguo Zhang (Alberta), Matthew Dahl (Manitoba), Yan Wang and Steve Doucette (Nova Scotia), C Fangyun Wu (Ontario), Jean-Marc Daigle (Quebec), and Hui Yin and Christopher Filliter (Clinical Practice Research Datalink); and Michael Fralick, Hala Tamim, and Vanessa Brunetti for their contributions to this study. KBF is supported by a senior salary support award from the Fonds de recherche du Québec-santé (FRQS; Quebec Foundation for Research-Health) and a William Dawson Scholar award from McGill University. LML is supported by a tier I Canada Research Chair. OHY and AD are supported by salary support awards from the FRQS.
Web extra.
Extra material supplied by authors
Supplementary information: additional tables 1-9 and additional figures 1-6
Contributors: KBF drafted the manuscript. All authors contributed to the study design and implementation, interpretation of results, and critical review of the manuscript for important intellectual content. LL conducted the meta-analyses. All authors approved the final version of the manuscript. KBF is the guarantor. The corresponding author attests that all listed authors meet authorship criteria and that no others meeting the criteria have been omitted.
Funding: The Canadian Network for Observational Drug Effect Studies, a collaborating centre of the Drug Safety and Effectiveness Network, is funded by the Canadian Institutes of Health Research (grant No DSE-146021). The funders had no role in the design, analysis, interpretation of data, writing of the report, or decision to submit the article for publication.
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: SA-S received research grants from Pfizer and Merck for projects not involving SGLT2 inhibitors or DPP-4 inhibitors. SS has attended advisory meetings or received speaking fees from Atara, Boehringer-Ingelheim, Bristol-Myers-Squibb, Merck, and Pfizer; SS is on the scientific advisory board of the EMPRISE study of SGLT2 inhibitors conducted by Harvard University and funded by Boehringer-Ingelheim; no other relationships or activities that could appear to have influenced the submitted work.
Ethical approval: Ethical approval was obtained at each participating site except Ontario (where ethical approval was waived), including the research ethics board of the Jewish General Hospital in Montreal, Canada (REB No 2019-1636). This study used anonymised administrative data, and the requirement of informed consent was therefore waived.
Data sharing No additional data available.
The lead author (KBF) affirms that this manuscript is an honest, accurate, and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Dissemination to participants and related patient and public communities This study was commissioned by the Institut national d’excellence en santé et services sociaux (INESSS, Quebec’s provincial drug plan manger and health technology assessment agency) through the Drug Safety and Effectiveness Network (DSEN). A study report was provided to INESSS, which published a French language version of the report (with English abstract) as part of its optimal use of drugs guidelines. Available at www.inesss.qc.ca/en/publications/publications/publication/prevention-devenements-cardiovasculaires-et-innocuite-des-inhibiteurs-du-sglt2-comparativement-aux.html. A one-page summary will also be published by DSEN on its website (https://cihr-irsc.gc.ca/e/51381.html). Given the anonymised nature of the study data, results will not be disseminated directly to study participants.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
Collaborators: Canadian Network for Observational Drug Effect Studies (CNODES) Investigators, Samy Suissa, Colin R Dormuth, Brenda R Hemmelgarn, Jacqueline Quail, Dan Chateau, J Michael Paterson, Jacques LeLorier, Adrian R Levy, Pierre Ernst,, Kristian B Filion, Lisa M Lix, Robert W Platt,, and Ingrid S Sketris
References
- 1. Dalan R. Sodium-Glucose Cotransporter-2 Inhibition in Type 2 Diabetes Mellitus: A Review of Large-Scale Cardiovascular Outcome Studies and Possible Mechanisms of Benefit. Cardiol Rev 2018;26:312-20. 10.1097/CRD.0000000000000201. [DOI] [PubMed] [Google Scholar]
- 2. Secrest MH, Udell JA, Filion KB. The cardiovascular safety trials of DPP-4 inhibitors, GLP-1 agonists, and SGLT2 inhibitors. Trends Cardiovasc Med 2017;27:194-202. 10.1016/j.tcm.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 3. Zinman B, Wanner C, Lachin JM, et al. EMPA-REG OUTCOME Investigators . Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015;373:2117-28. 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 4. Neal B, Perkovic V, Mahaffey KW, et al. CANVAS Program Collaborative Group . Canagliflozin and cardiovascular and renal events in type 2 diabetes. N Engl J Med 2017;377:644-57. 10.1056/NEJMoa1611925 [DOI] [PubMed] [Google Scholar]
- 5. Raz I, Mosenzon O, Bonaca MP, et al. DECLARE-TIMI 58: Participants’ baseline characteristics. Diabetes Obes Metab 2018;20:1102-10. 10.1111/dom.13217. [DOI] [PubMed] [Google Scholar]
- 6. Wiviott SD, Raz I, Bonaca MP, et al. DECLARE–TIMI 58 Investigators . Dapagliflozin and Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2019;380:347-57. 10.1056/NEJMoa1812389. [DOI] [PubMed] [Google Scholar]
- 7. Dhruva SS, Redberg RF. Variations between clinical trial participants and Medicare beneficiaries in evidence used for Medicare national coverage decisions. Arch Intern Med 2008;168:136-40. 10.1001/archinternmed.2007.56. [DOI] [PubMed] [Google Scholar]
- 8. Kosiborod M, Cavender MA, Fu AZ, et al. CVD-REAL Investigators and Study Group* . Lower Risk of Heart Failure and Death in Patients Initiated on Sodium-Glucose Cotransporter-2 Inhibitors Versus Other Glucose-Lowering Drugs: The CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium-Glucose Cotransporter-2 Inhibitors). Circulation 2017;136:249-59. 10.1161/CIRCULATIONAHA.117.029190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cavender MA, Norhammar A, Birkeland KI, et al. CVD-REAL Investigators and Study Group . SGLT-2 Inhibitors and Cardiovascular Risk: An Analysis of CVD-REAL. J Am Coll Cardiol 2018;71:2497-506. 10.1016/j.jacc.2018.01.085. [DOI] [PubMed] [Google Scholar]
- 10. Patorno E, Goldfine AB, Schneeweiss S, et al. Cardiovascular outcomes associated with canagliflozin versus other non-gliflozin antidiabetic drugs: population based cohort study. BMJ 2018;360:k119. 10.1136/bmj.k119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Patorno E, Pawar A, Franklin JM, et al. Empagliflozin and the Risk of Heart Failure Hospitalization in Routine Clinical Care. Circulation 2019;139:2822-30. 10.1161/CIRCULATIONAHA.118.039177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shao SC, Chang KC, Hung MJ, et al. Comparative risk evaluation for cardiovascular events associated with dapagliflozin vs. empagliflozin in real-world type 2 diabetes patients: a multi-institutional cohort study. Cardiovasc Diabetol 2019;18:120. 10.1186/s12933-019-0919-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Udell JA, Yuan Z, Rush T, Sicignano NM, Galitz M, Rosenthal N. Cardiovascular Outcomes and Risks After Initiation of a Sodium Glucose Cotransporter 2 Inhibitor: Results From the EASEL Population-Based Cohort Study (Evidence for Cardiovascular Outcomes With Sodium Glucose Cotransporter 2 Inhibitors in the Real World). Circulation 2018;137:1450-9. 10.1161/CIRCULATIONAHA.117.031227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dawwas GK, Smith SM, Park H. Cardiovascular outcomes of sodium glucose cotransporter-2 inhibitors in patients with type 2 diabetes. Diabetes Obes Metab 2019;21:28-36. 10.1111/dom.13477. [DOI] [PubMed] [Google Scholar]
- 15. Pasternak B, Ueda P, Eliasson B, et al. Use of sodium glucose cotransporter 2 inhibitors and risk of major cardiovascular events and heart failure: Scandinavian register based cohort study. BMJ 2019;366:l4772. 10.1136/bmj.l4772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Suissa S. Lower Risk of Death With SGLT2 Inhibitors in Observational Studies: Real or Bias? Diabetes Care 2018;41:6-10. 10.2337/dc17-1223. [DOI] [PubMed] [Google Scholar]
- 17. Suissa S. Reduced Mortality With Sodium-Glucose Cotransporter-2 Inhibitors in Observational Studies: Avoiding Immortal Time Bias. Circulation 2018;137:1432-4. 10.1161/CIRCULATIONAHA.117.032799. [DOI] [PubMed] [Google Scholar]
- 18. Lévesque LE, Hanley JA, Kezouh A, Suissa S. Problem of immortal time bias in cohort studies: example using statins for preventing progression of diabetes. BMJ 2010;340:b5087. 10.1136/bmj.b5087. [DOI] [PubMed] [Google Scholar]
- 19. Filion KB, Suissa S. DPP-4 Inhibitors and Heart Failure: Some Reassurance, Some Uncertainty. Diabetes Care 2016;39:735-7. 10.2337/dci15-0036. [DOI] [PubMed] [Google Scholar]
- 20. Suissa S, Henry D, Caetano P, et al. Canadian Network for Observational Drug Effect Studies (CNODES) . CNODES: the Canadian Network for Observational Drug Effect Studies. Open Med 2012;6:e134-40. [PMC free article] [PubMed] [Google Scholar]
- 21. Herrett E, Gallagher AM, Bhaskaran K, et al. Data Resource Profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827-36. 10.1093/ije/dyv098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Suissa S, Moodie EE, Dell’Aniello S. Prevalent new-user cohort designs for comparative drug effect studies by time-conditional propensity scores. Pharmacoepidemiol Drug Saf 2017;26:459-68. 10.1002/pds.4107. [DOI] [PubMed] [Google Scholar]
- 23. Suissa S, Azoulay L. Metformin and the risk of cancer: time-related biases in observational studies. Diabetes Care 2012;35:2665-73. 10.2337/dc12-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Scirica BM, Bhatt DL, Braunwald E, et al. SAVOR-TIMI 53 Steering Committee and Investigators . Saxagliptin and cardiovascular outcomes in patients with type 2 diabetes mellitus. N Engl J Med 2013;369:1317-26. 10.1056/NEJMoa1307684. [DOI] [PubMed] [Google Scholar]
- 25. White WB, Cannon CP, Heller SR, et al. EXAMINE Investigators . Alogliptin after acute coronary syndrome in patients with type 2 diabetes. N Engl J Med 2013;369:1327-35. 10.1056/NEJMoa1305889. [DOI] [PubMed] [Google Scholar]
- 26. Green JB, Bethel MA, Armstrong PW, et al. TECOS Study Group . Effect of Sitagliptin on Cardiovascular Outcomes in Type 2 Diabetes. N Engl J Med 2015;373:232-42. 10.1056/NEJMoa1501352. [DOI] [PubMed] [Google Scholar]
- 27. Rosenstock J, Perkovic V, Johansen OE, et al. CARMELINA Investigators . Effect of Linagliptin vs Placebo on Major Cardiovascular Events in Adults With Type 2 Diabetes and High Cardiovascular and Renal Risk: The CARMELINA Randomized Clinical Trial. JAMA 2019;321:69-79. 10.1001/jama.2018.18269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schneeweiss S. Sensitivity analysis and external adjustment for unmeasured confounders in epidemiologic database studies of therapeutics. Pharmacoepidemiol Drug Saf 2006;15:291-303. 10.1002/pds.1200. [DOI] [PubMed] [Google Scholar]
- 29. Shapiro S, Fergusson D, Glass KC. Substituting placebo for established, effective therapy: why not? CMAJ 2010;182:1749-53. 10.1503/cmaj.090548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Nassif ME, Kosiborod M. A Review of Cardiovascular Outcomes Trials of Glucose-Lowering Therapies and Their Effects on Heart Failure Outcomes. Am J Cardiol 2019;124(Suppl 1):S12-9. 10.1016/j.amjcard.2019.10.025. [DOI] [PubMed] [Google Scholar]
- 31. Filion KB, Douros A, Azoulay L, Yin H, Yu OH, Suissa S. Sulfonylureas as initial treatment for type 2 diabetes and the risk of adverse cardiovascular events: A population-based cohort study. Br J Clin Pharmacol 2019;85:2378-89. 10.1111/bcp.14056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ueda P, Svanström H, Melbye M, et al. Sodium glucose cotransporter 2 inhibitors and risk of serious adverse events: nationwide register based cohort study. BMJ 2018;363:k4365. 10.1136/bmj.k4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep 2015;2:221-8. 10.1007/s40471-015-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.National Institute for Health and Care Excellence. Canagliflozin, dapagliflozin and empagliflozin as monotherapies for treating type 2 diabetes 2016 www.nice.org.uk/guidance/ta390/resources/canagliflozin-dapagliflozin-and-empagliflozin-as-monotherapies-for-treating-type-2-diabetes-pdf-82602903454405 (accessed 11 December 2019).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary information: additional tables 1-9 and additional figures 1-6