Abstract
Mechanistic details of the modulation by cAMP of Trypanosoma cruzi host cell invasion remain ill-defined. Here we report that activation of host’s Epac1 stimulated invasion, whereas specific pharmacological inhibition or maneuvers that alter Epac1 subcellular localization significantly reduced invasion. Furthermore, while specific activation of host cell PKA showed no effect, its inhibition resulted in an increased invasion, revealing a crosstalk between the PKA and Epac signaling pathways during the process of invasion. Therefore, our data suggests that subcellular localization of Epac might be playing an important role during invasion and that specific activation of the host cell cAMP/Epac1 pathway is required for cAMP-mediated invasion.
Keywords: Trypanosoma cruzi, invasion, Invasion, cAMP, Epac, PKA
Trypanosoma cruzi, a protozoan parasite transmitted by blood-sucking triatomine insects from the Reduviidae family, is the etiological agent of Chagas disease, a serious health threat among people living in poor rural populations of Central and South America. Estimations indicate 6 to 8 million infected people and 65 million individuals at risk of contracting the disease, an annual incidence of 28.000 cases and 12.000 deaths (http://www.paho.org). Additionally, human migration from endemic areas of Latin America to Europe, North America, and the Western Pacific, significantly increased the prevalence of T. cruzi infection outside of Latin America, resulting in a global economic burden of $7.19 billion [1].
T. cruzi, has a complex life cycle including a bug-vector and a vertebrate host. In the mammalian host, T. cruzi is an obligate intracellular parasite that posses the ability of infecting different kind of tissues. To ensure successful cell invasion the parasite has developed multiple mechanisms of internalization, including the recruitment and fusion of lysosomes to the entry site [2]. In this process, attachment of trypomastigotes to the cellular host is accompanied by an elevation of intracellular cAMP levels. It has been shown that cAMP is able to potentiate the Ca2+-dependent exocytosis of lysosomes and lysosome-mediated cell invasion by T. cruzi [3]. Accordingly, pharmacologic intervention of the cAMP pathway is able to modulate parasite invasion [3]. However, cAMP effectors involved in T. cruzi invasion remain unknown. In mammalian cells, both cAMP effector pathways, i.e. Protein Kinase A (PKA) and Exchange protein activated directly by cAMP (Epac), are involved in Ca2+-triggered exocytic events (i.e. secretion) [4]. Moreover, members of the cAMP signaling pathway were localized to late endosomes/lysosomes [5], and Epac-mediated Rap activation is involved in regulated exocytosis in human sperm [6], insulin secretion [7] and pancreatic amylase release [8]. Therefore, we hypothesized that the cAMP/Epac pathway might play a role during T. cruzi invasion of the host cell. In this work, using a set of pharmacological tools, we demonstrated that Epac1-mediated signaling represents the main mechanism for cAMP-mediated host cell invasion.
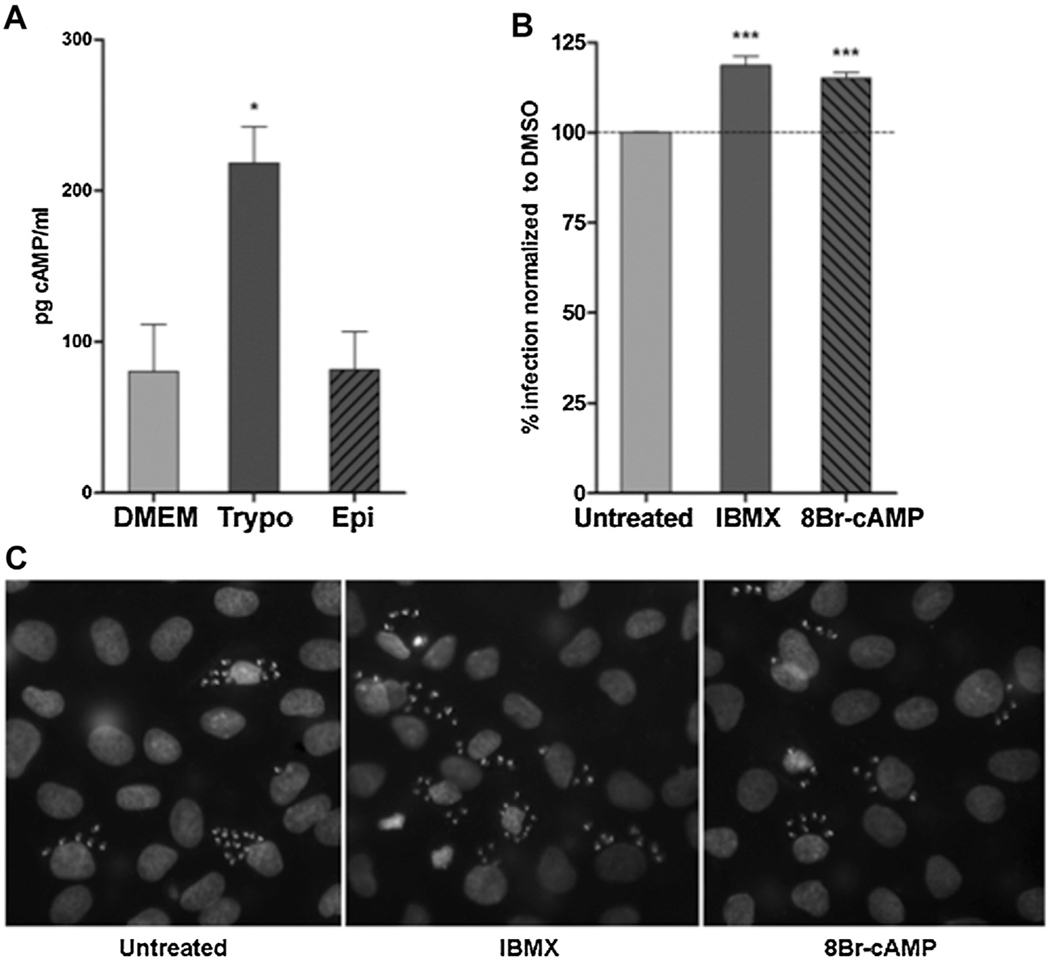
In order to evaluate our hypothesis, we first demonstrated the ability of tissue-cultured trypomastigotes of the CL Brener strain to induce an increase in the intracellular levels of cAMP in NRK cells (Normal Rat Kidney cell-line) (Fig. 1A). In contrast, epimastigotes from the same strain failed to promote the production of cAMP by the host cell (Fig. 1A). To confirm the positive modulation of cAMP on T. cruzi invasion of NRK cells, elevation of the intracellular cyclic nucleotide was attained by pretreatment of host cells with the Phosphodiesterase (PDE) inhibitor IBMX (3-Isobutyl-1-methylxanthine) (Sigma) or the non-hydrolysable cAMP analog 8-Br-cAMP (8-Bromoadenosine-3′,5′-cyclic monophosphate) (Biolog). In both cases, treatment led to an increased in the total number of infected cells, as reflected in an increased percentage of invasion (Fig. 1B and C).
Fig. 1.
A) T. cruzi trypomastigotes trigger elevation in host cell cAMP intracellular levels. NRK cells were incubated with trypomastigotes or epimastigotes of the CL Brener strain (100:1 parasite to cell ratio) or vehicle. After 30 min incubation cells were lysed and cAMP intracellular concentration measured with the cAMP Screen System Kit, following instructions by supplier (Applied Biosystem). Data represent the mean ± SD of three independent experiments. * p < 0.05, with unpaired Student’s t-test. B) T. cruzi modulates host’s cAMP-mediated signaling to promote invasion. Pretreated NRK cells (30 min at 300 μM IBMX or 300 μM 8-Br-cAMP) were infected with trypomastigotes of the T. cruzi CL Brener strain. 48 hs post-infection cells were fixed, stained with DAPI and percentage of invasion ((#Infected cells/3000 cells)*100) was calculated by fluorescence microscopy. Invasion of untreated cells was considered as basal invasion. Data represent the mean ± SD of three independent experiments. * p < 0.05 and *** p < 0.001, ANOVA with posttest comparisons. C) Representative images of DAPI staining of infected cells pretreated with the indicated inhibitor/activator.
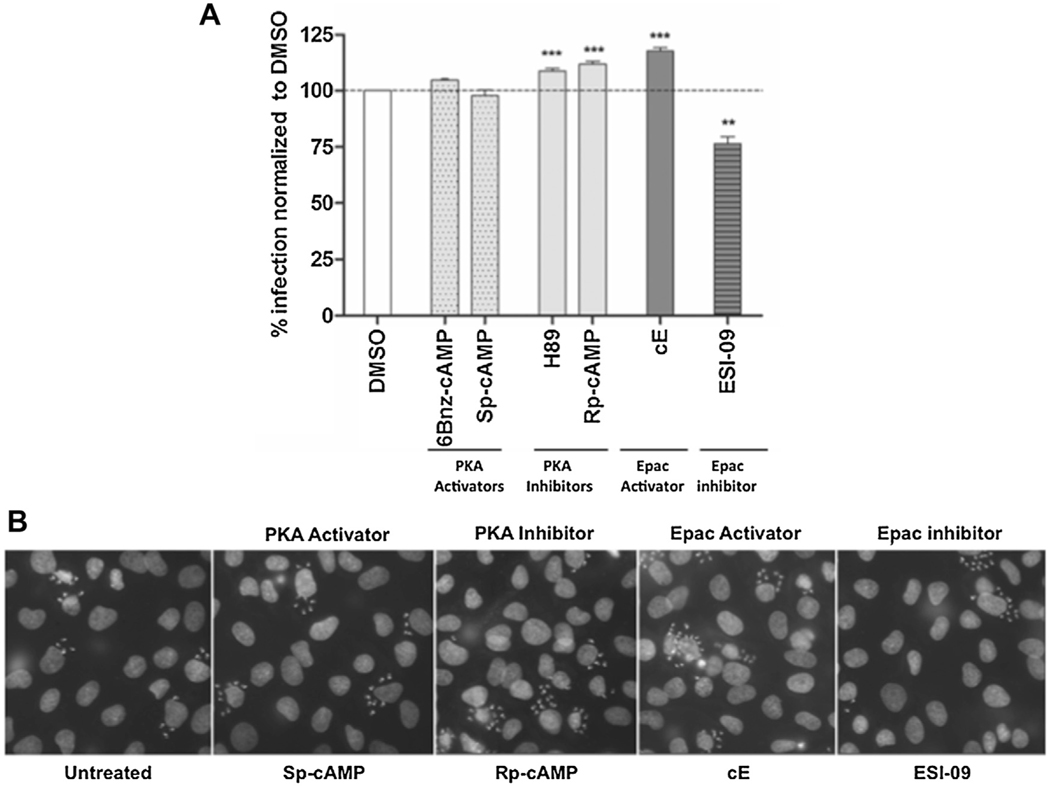
The specific roles of cAMP effectors, PKA and Epac, during host invasion by T. cruzi, were evaluated using a set of pharmacologic tools to selectively activate or inhibit these effectors. As shown in Fig. 2, pretreatment of host cells with cAMP analogs that triggered differential activation of PKA, such as 6-Bnz-cAMP (N6-Benzoyladenosine-3′,5′-cyclic monophosphate) (Biolog) or Sp-cAMP (Adenosine- 3′,5′-cyclic monophosphorothioate, Sp-isomer) (Biolog), had no significant effect on T. cruzi invasion. On the other hand, the percentage of invasion significantly increased when host cells were pretreated with a cAMP analog that exclusively activates Epac (8-(4- Methoxyphenylthio)-2′-O-methyladenosine-3′,5′ cyclic monophosphate) (Biolog) (Fig. 2). Consistent with this observation, differential inhibition of Epac by the recently discovered antagonist ESI-09 (Sigma) [9], showed a significant inhibition in invasion (Fig. 2). These results clearly showed the requirement of Epac activation for the positive modulation by cAMP of invasion. In addition, an increase in invasion was also observed when cells were pretreated with the PKA inhibitor H89 (Sigma) (Fig. 2). However, H89, a competitive inhibitor that blocks PKA activity through displacement of ATP from the catalytic site, could also inhibit at least 8 other kinases [10]. In order to avoid potential effects on other kinases, inhibition was achieved by using Rp-cAMP (Adenosine-3′,5′-cyclic monophosphorothioate, Rp-isomer) (Biolog), a permeable PDE-resistant cAMP analog shown to be an specific inhibitor of PKA. As for H89, pretreatment with Rp-cAMP had also a positive effect on T. cruzi invasion (Fig. 2). The increase in invasion observed as a result of PKA inhibition, could be translated in a PKA-dependent antagonistic effect over cAMP-mediated invasion. If inhibition of PKA increased invasion, it could then be expected that activation of PKA would have the opposite effect. Therefore, under physiological conditions, PKA-mediated phosphorylation would be negatively regulating the Epac pathway. This inhibition could be achieved, at least, in two different ways: A) through direct phosphorylation of Epac or B) At the level of Rap1, a downstream effector of Epac, and potential target for PKA-mediated phosphorylation [11]. Supporting this idea, Low and Stork found peptides within the N-terminus of Epac1 that were capable of being phosphorylated in vitro by PKA [12].
Fig. 2.
Epac participates in T. cruzi invasion. A) Pretreated NRK cells (30min at 300uM of cAMP analogs or 37.5uM of ESI-09) were infected with trypomastigotes from T. cruzi CL Brener strain (20:1 parasite to cell ratio for 2 h). 48 hs post-infection cells were fixed, stained with DAPI and percentage of invasion determined by fluorescence microscopy. Infection of untreated cells was considered as basal infection. Results are expressed as mean ± SD (n ≥ 3). **p < 0.01, ***p < 0.001, ANOVA and Dunnett posttest. B) Representative images of DAPI staining of infected cells pretreated with the indicated inhibitor/activator.
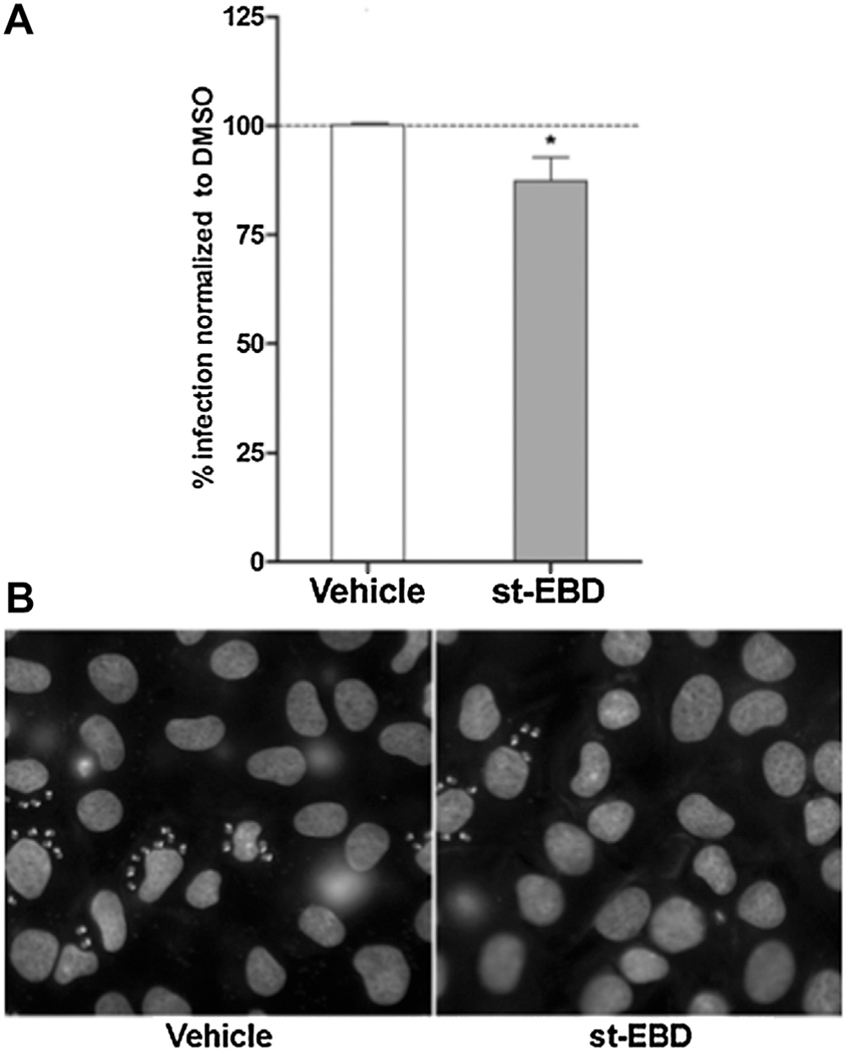
There is a growing body of evidence suggesting the formation microdomains at specific subcellular locations where the machinery for synthesis (adenylyl cyclase), degradation (PDEs) and effectors (PKA and Epac) of cAMP are located. This compartmentalization allows a confined cAMP signaling by regulating duration and strength of the signal. It is known that mammalian Epac proteins segregate into different intracellular compartments that determine its coupling and activation of specific downstream pathways [13]. Targeting Epac compartmentalization rather than its activity represents a logical approach towards achieving higher specificity. A construct containing the membrane anchoring domain but not the catalytic domain might displace Epac WT, blocking downstream events and disrupting the process of cAMP-mediated invasion. Such a tool, based on the expression of Epac regulatory domain [N-Epac (1–148)] devoid of its GEF catalytic activity was developed, and its dominant negative activity confirmed in a thyroid model [14]. Additionally, using a yeast two hybrid approach with the regulatory N-Epac (1–148) as bait, radixin, an ERM protein was identified as the target mediating the dominant negative effect [15]. Deletion mapping identified Epac (1–52), i.e. ERM-binding domain or EBD, and further mapping using peptide arrays identified a 15-mer sequence as the minimal ERM-binding domain sufficient for radixin interaction. A cell-permeant version of this 15-mer sequence (st-EBD), was able to inhibit specifically a cAMP-dependent (TSH-Insulin) but not a cAMP-independent (bFGF-Insulin) cell proliferation in thyroid cells, and provided the proof-of-principle for the compartmentalization targeting strategy (not shown). Evidence indicating that the Epac pathway is required for T. cruzi cAMP-mediated invasion (Fig. 2), prompted us to test the permeable peptide in the invasion assays. Pretreatment of NRK host cells with the st-EBD was able to partially block invasion (Fig. 3). This observation is consistent with the involvement in T. cruzi invasion of a pool of Epac1 compartmentalized with ERM-actin, as described for TSH stimulation in thyroid cells [15] and for integrin-mediated cell adhesion [16]. Taken together, these results suggest a critical role for the cAMP/Epac1 pathway during invasion by the parasite.
Fig. 3.
Epac1 subcellular localization is essential in T. cruzi invasion. A) NRK cells were incubated for 4 h with the st-EBD (10 uM of stearate-KPRACSYDLLLEHQRP-amide peptide), washed and infected with trypomastigotes from T. cruzi CL Brener strain (20:1 parasite to cell ratio for 2 h). 48 hs post-infection cells were fixed, stained with DAPI and percentage of invasion determined by fluorescence microscopy. Infection of non-treated cells was considered as basal infection. Results are expressed as mean ± SD (n ≥ 3). * p < 0.05, t-test. B) Representative images of DAPI staining of infected cells pretreated as indicated.
It has been shown that an intact, stiff and rapidly remodeling cytoskeleton would be required for the initial steps of parasite invasion and parasite retention [17]. Upon invasion, a time-dependent softening and disassembly of the cytoskeleton dictates the ability of internalized trypomastigotes to remain inside the cell, with changes occurring through PKA-mediated inhibition of the Rho/Rho kinase-signaling pathway [18]. The fact that Epac/Rap signaling could participate in reorganization of the cytoskeleton thru activation of RhoA [19], allowed us to raise the hypothesis that cAMP/Epac signaling pathway could be activated by T. cruzi at the initial steps of invasion, promoting Ca2+-dependent exocytosis of lysosomes and cytoskeletal reorganization, triggering lysosome-mediated cell invasion. Once the parasite gained access to the cell, PKA-mediated inhibition of the Epac pathway would abolish F-actin formation and lysosome-mediated invasion. In line with this hypothesis, our results showed that inhibition of PKA increased the percentage of invasion (Fig. 2), presumably by deregulation of Epac activity, confirming the requirement of a temporal loses of function of Epac for the establishment of the infection.
Although recognized as an important mechanism of invasion, knowledge about cAMP-mediated modulation of T. cruzi invasion has been obscure for long years. In this work, data obtained using a set of pharmacological tools provided us with convincing evidence to establish a critical role for Epac1 in host cell invasion by the parasite. Our results demonstrate that activation, and possibly compartmentalization, of Epac-mediated signaling would represent the central mechanism for cAMP-mediated host cell invasion. Dissecting the cAMP/Epac pathway would provide the detailed molecular mechanism by which Epac controls Ca2+ mobilization and modulates T. cruzi invasion.
Acknowledgments
Funding
Research reported in this publication was supported by the FIC-NIH award number R03TW009001 (to DLA/MME). MME and CAB are members of the Research Career of the Consejo Nacional de Investigaciones Científicasy Técnicas (CONICET). IMD is a CONICET Research Fellow. Research carried out in CAB laboratory is supported by the Agencia Nacional de Promoción Científica y Tecnológica (ANPCyT, Argentina).
References
- [1].Lee BY, Bacon KM, Bottazzi ME, Hotez PJ, Global economic burden of chagas disease: a computational simulation model, Lancet Infect. Dis 13 (4) (2013) 342–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Andrews NW, Lysosome recruitment during host cell invasion by Trypanosoma cruzi, Trends Cell Biol. 5 (3) (1995) 133–137. [DOI] [PubMed] [Google Scholar]
- [3].Rodriguez A, Martinez I, Chung A, Berlot CH, Andrews NW, cAMP regulates Ca2+-dependent exocytosis of lysosomes and lysosome-mediated cell invasion by trypanosomes, J. Biol. Chem 274 (24) (1999) 16754–16759. [DOI] [PubMed] [Google Scholar]
- [4].Seino S, Shibasaki T, PKA-dependent and PKA-independent pathways for cAMP-regulated exocytosis, Physiol. Rev 85 (4) (2005) 1303–1342. [DOI] [PubMed] [Google Scholar]
- [5].Pizon V, Desjardins M, Bucci C, Parton RG, Zerial M, Association of Rap1a and Rap1b proteins with late endocytic/phagocytic compartments and Rap2a with the golgi complex, J. Cell Sci 107 (Pt 6) (1994) 1661–1670. [DOI] [PubMed] [Google Scholar]
- [6].Branham MT, Bustos MA, De Blas GA, Rehmann H, Zarelli VE, Trevino CL, et al. , Epac activates the small G proteins Rap1 and Rab3A to achieve exocytosis, J. Biol. Chem 284 (37) (2009) 24825–24839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Seino S, Takahashi H, Fujimoto W, Shibasaki T, Roles of cAMP signalling in insulin granule exocytosis, Diabetes Obes. Metab 11 (Suppl. 4) (2009) 180–188. [DOI] [PubMed] [Google Scholar]
- [8].Sabbatini ME, Chen X, Ernst SA, Williams JA, Rap1 activation plays a regulatory role in pancreatic amylase secretion, J. Biol. Chem 283 (35) (2008) 23884–23894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Almahariq M, Tsalkova T, Mei FC, Chen H, Zhou J, Sastry SK, et al. , A novel EPAC-specific inhibitor suppresses pancreatic cancer cell migration and invasion, Mol. Pharmacol 83 (1) (2013) 122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lochner A, Moolman JA, The many faces of H89: a review, Cardiovasc. Drug Rev 24 (3–4) (2006) 261–274. [DOI] [PubMed] [Google Scholar]
- [11].Edreira MM, Li S, Hochbaum D, Wong S, Gorfe AA, Ribeiro-Neto F, et al. , Phosphorylation-induced conformational changes in Rap1b: allosteric effects on switch domains and effector loop, J. Biol. Chem 284 (40) (2009) 27480–27486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wang Z, Dillon TJ, Pokala V, Mishra S, Labudda K, Hunter B, et al. , Rap1-mediated activation of extracellular signal-regulated kinases by cyclic AMP is dependent on the mode of Rap1 activation, Mol. Cell. Biol 26 (6) (2006) 2130–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Pereira L, Rehmann H, Lao DH, Erickson JR, Bossuyt J, Chen J, et al. , Novel Epac fluorescent ligand reveals distinct Epac1 vs: epac2 distribution and function in cardiomyocytes, Proc. Natl. Acad. Sci. U. S. A 112 (13) (2015) 3991–3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Hochbaum D, Hong K, Barila G, Ribeiro-Neto F, Altschuler DL Epac, in synergy with cAMP-dependent protein kinase (PKA), is required for cAMP-mediated mitogenesis, J. Biol. Chem 283 (8) (2008) 4464–4468. [DOI] [PubMed] [Google Scholar]
- [15].Hochbaum D, Barila G, Ribeiro-Neto F, Altschuler DL, Radixin assembles cAMP effectors Epac and PKA into a functional cAMP compartment: role in cAMP-dependent cell proliferation, J. Biol. Chem 286 (1) (2011) 859–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gloerich M, Ponsioen B, Vliem MJ, Zhang Z, Zhao J, Kooistra MR, et al. , Spatial regulation of cyclic AMP-Epac1 signaling in cell adhesion by ERM proteins, Mol. Cell. Biol 30 (22) (2010) 5421–5431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Woolsey AM, Burleigh BA, Host cell actin polymerization is required for cellular retention of Trypanosoma cruzi and early association with endosomal/lysosomal compartments, Cell. Microbiol 6 (9) (2004) 829–838. [DOI] [PubMed] [Google Scholar]
- [18].Mott A, Lenormand G, Costales J, Fredberg JJ, Burleigh BA, Modulation of host cell mechanics by Trypanosoma cruzi, J. Cell. Physiol 218 (2) (2009) 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Jeyaraj SC, Unger NT, Eid AH, Mitra S, Paul El-Dahdah N, Quilliam LA, et al. , Cyclic AMP-Rap1A signaling activates RhoA to induce alpha(2c)-adrenoceptor translocation to the cell surface of microvascular smooth muscle cells, Am. J. Physiol. Cell Physiol 303 (5) (2012) C499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]