Abstract
Wastewater-based epidemiology is an emerging tool to monitor COVID-19 infection levels by measuring the concentration of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) RNA in wastewater. There remains a need to improve wastewater RNA extraction methods’ sensitivity, speed, and reduce reliance on often expensive commercial reagents to make wastewater-based epidemiology more accessible. We present a kit-free wastewater RNA extraction method, titled “Sewage, Salt, Silica and SARS-CoV-2” (4S), that employs the abundant and affordable reagents sodium chloride (NaCl), ethanol, and silica RNA capture matrices to recover sixfold more SARS-CoV-2 RNA from wastewater than an existing ultrafiltration-based method. The 4S method concurrently recovered pepper mild mottle virus (PMMoV) and human 18S ribosomal subunit rRNA, which have been proposed as fecal concentration controls. The SARS-CoV-2 RNA concentrations measured in three sewersheds corresponded to the relative prevalence of COVID-19 infection determined via clinical testing. Lastly, controlled experiments indicate that the 4S method prevented RNA degradation during storage of wastewater samples, was compatible with heat pasteurization, and in our experience, 20 samples can be processed by one lab technician in approximately 2 h. Overall, the 4S method is promising for effective, economical, and accessible wastewater-based epidemiology for SARS-CoV-2, providing another tool to fight the global pandemic.
Introduction
Wastewater-based epidemiology (WBE) enables the indirect assessment of viral infection prevalence in populations.1−3 The quantity of viral nucleic acids shed into wastewater by infected individuals, whether symptomatic or not, serves as a proxy for the relative prevalence of infection.1 WBE can provide population-level infection information for up to many thousands of individuals in a community to complement individual-level testing and aid public health decision making.4
WBE is now being applied to monitor and even predict population-level coronavirus disease 2019 (COVID-19) outbreaks.1,5 Local COVID-19 prevalence is difficult to assess due to insufficient individual testing capacity, rendering effective response more challenging.6 Wastewater can provide insights into COVID-19 prevalence, as COVID-19 patients shed SARS-CoV-2 RNA in their stool and thus into wastewater.7,8 Emerging studies report wastewater SARS-CoV-2 concentrations that correspond to reported clinical prevalence of COVID-19, with potential for early detection of COVID-19 outbreaks and identification of newly emerging SARS-CoV-2 variants.9−12 To extract and quantify the concentration of SARS-CoV-2 RNA shed into wastewater, researchers are using size- and charge-based concentration methods that concentrate intact SARS-CoV-2 virus prior to RNA extraction.13−15 These methods employ a primary concentration step via sieving by particle size, enmeshment of viral particles in precipitates that can be separated by mass, or adsorption via electrostatic interactions, prior to RNA extraction.15 These methods can be relatively time-consuming and become inaccessible if they are dependent on reliable supply of commercial reagents, a paucity of which has already hampered clinical SARS-CoV-2 testing efforts.13−17 Furthermore, the use of primary concentration assumes the recovery of intact virus and is therefore not geared toward cocapturing RNA from SARS-CoV-2 viruses that have already lysed or capture of nonviral RNAs suitable as fecal concentration controls. Lastly, current CDC safety guidelines recommend a biosafety level 2 facility with unidirectional airflow and BSL-3 precautions when employing environmental sampling procedures that concentrate viruses presumed to be intact.18 To mitigate concerns of concentrating potentially infectious virus, heat-based wastewater sample pasteurization and subsequent extraction could allow for easier and safer wastewater processing after collection.
We aimed to develop an economical, kit-free method for the direct capture (extraction) of SARS-CoV-2 RNA from wastewater. The resulting method, described herein, employs lysis of biological particles via sodium chloride (NaCl), heat-based pasteurization, coarse filtration, ethanol precipitation, and RNA capture via silica-based columns (4S-column) or silicon dioxide slurry (4S-Milk-of-Silica). This approach was developed with the aim to provide recovery of wastewater RNA without mass, size, or charge bias, the ability to cocapture RNA from intact and lysed SARS-CoV-2 virus, as well as RNA from other biological particles in wastewater that are suitable as fecal concentration controls, such as pepper mild mottle virus (present in dietary peppers and shed in feces) and human 18S ribosomal RNA.19 As compared to other wastewater RNA extraction procedures, the 4S method is to our knowledge the only procedure to simultaneously concentrate and extract RNA from a large volume (40–400 mL) of wastewater, enabling the processing of 20 wastewater samples by one lab technician within approximately 2 h.
The 4S method was evaluated in terms of its sensitivity to detect wastewater SARS-CoV-2 and ability to remove RT-qPCR inhibitors. Furthermore, we assessed whether the lysis salts added to wastewater during the 4S method could also protect wastewater RNA from degradation. We found that 4S method’s omission of a primary concentration step and extraction from a relatively large wastewater volume (40 mL) allowed for highly sensitive, same-day measurement of wastewater SARS-CoV-2 abundance and removal of RT-qPCR inhibitors. Furthermore, the sodium chloride and ethylenediaminetetraacetic acid (EDTA) lysis additives served to protect wastewater RNA from degradation. Lastly, we found that the 4S method was compatible with heat pasteurization, which makes wastewater samples safer to process.
Materials and Methods
Sample Collection
For this study, we obtained composite 24 h wastewater influent samples from East Bay Municipal Utility District’s wastewater treatment plant. These samples represent three discrete sampling areas: North and West Berkeley, El Cerrito, Kensington, and Albany (subsewershed “N”), Oakland/Piedmont (subsewershed “S”), and Berkeley/Oakland Hills (subsewershed “A”) (interceptor coverage detailed in Figure S2A). Samples processed via the 4S-column method and ultrafiltration were kept at 4 °C on ice during transport and processed within 24 h. Samples processed via the “Milk-of-Silica” procedure were kept at −80 °C and processed within two weeks. Biological replicates were defined as aliquots of the same wastewater sample, processed independently through the entire method. For example, three aliquots from the same wastewater were processed via the 4S-column method.
Wastewater RNA Extraction
Wastewater RNA extraction via the 4S-column and 4S-Milk-of-Silica methods is detailed in depth at https://www.protocols.io/view/v-4-direct-wastewater-rna-capture-and-purification-bpdfmi3n and dx.doi.org/10.17504/protocols.io.biwfkfbn.20,21 For 4S RNA extraction using a silica column (4S-column), samples were lysed via the addition of sodium chloride (NaCl) to a final concentration of 4 M and EDTA to a final concentration of 1 mM and buffered via the addition of tris(hydroxymethyl)aminomethane pH 7.2 to a final concentration of 10 mM. Bovine coronavirus vaccine stock (Bovilis Coronavirus Calf Vaccine, Merck Animal) was resuspended in 2 mL of PBS, diluted in PBS at a 1:10 ratio, and 50 μL of diluted BCoV vaccine was added to each sample as a process control. Samples were heat-inactivated in a water bath (unless indicated otherwise) at 70 °C for 45 min and filtered using a 5 μM DuraPore PVDF filter membrane (Millipore Sigma) and syringe filter. Ethanol was added to the sample filtrate to a final concentration of 35%. Samples were passed through Zymo-IIIP silica columns (Zymo Research) using a vacuum manifold. For all experiments other than the wash buffer tests (Figure 4, Supporting Information Figure S4), samples were washed with 25 mL of high NaCl (1.5 M) and ethanol (20%) containing wash buffer #1 (4S-WB1) and 50 mL of low NaCl (100 mM) and ethanol (80%) containing wash buffer #2 (4S-WB2). Columns were detached from the vacuum manifold and centrifuged at 10,000 g for 2 min to remove any residual 4S-WB2 present in the column. Washed RNA was eluted from silica columns using 200 uL of ZymoPURE elution buffer (Zymo Research) preheated to 50 °C and eluted from the final step of the “Milk-of-Silica” procedure using pH 8 Tris-EDTA buffer preheated to 50 °C. Eluted RNA was stored at 4 °C for same-day use or frozen at −80 °C for later use and storage.
Figure 4.
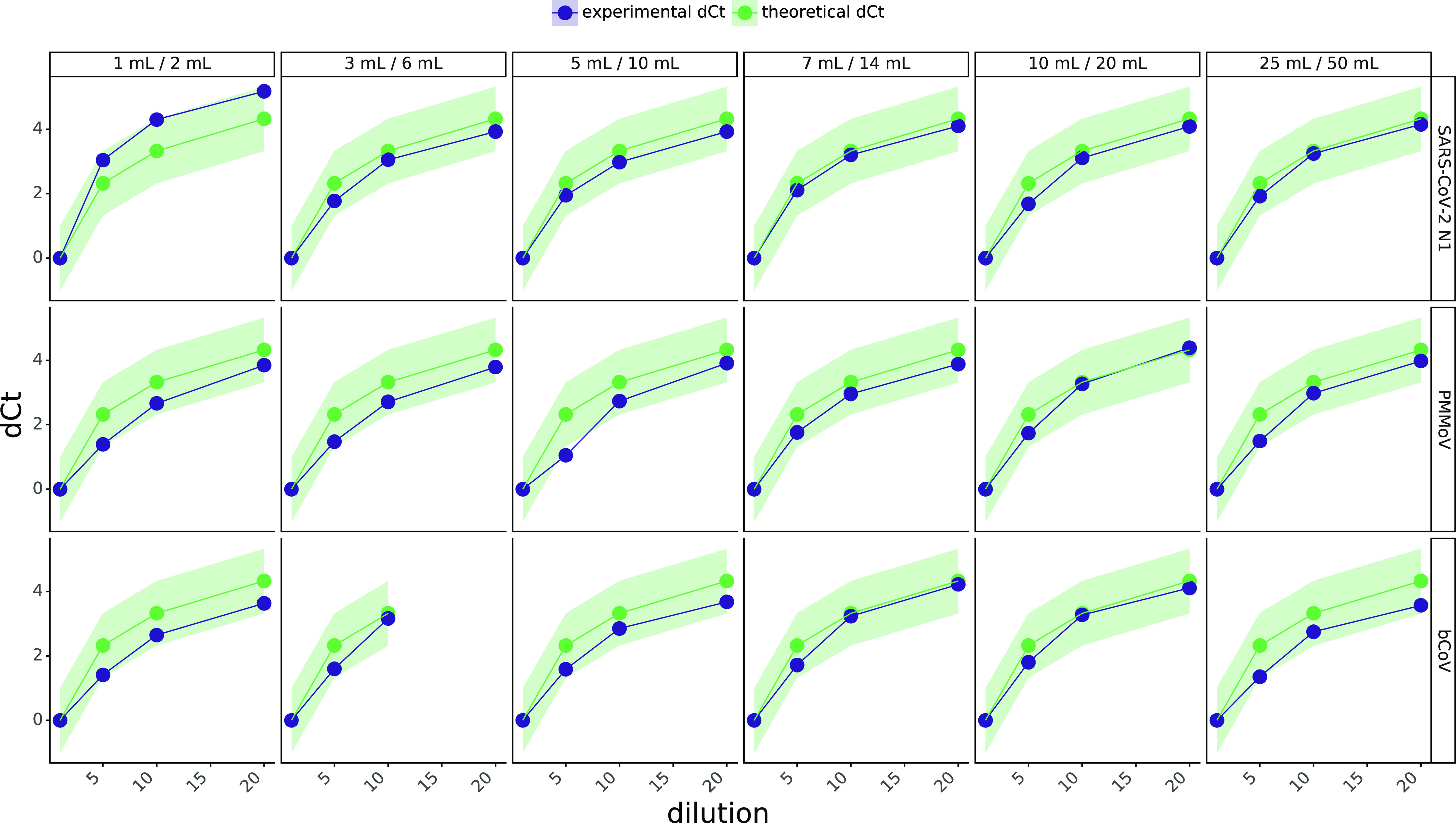
Assessment of RT-qPCR assay inhibition of the SARS-CoV-2 N1, PMMoV and BCoV assays via the “spike and dilute” method for different volumes of 4S-WB1 and 4S-WB2 (volumes reported at the top of each panel). Sample dilutions shown are 1 ×, 5 ×, 10 ×, and 20 ×. The green line with circular points represents theoretically expected increase in Ct due to sample dilution, and the blue line with triangular points indicates actual increase in Ct with sample dilution. The green band indicates +/– 1 Ct tolerance range around the expected Ct values, due to variability. An increase in the measured Ct that is lower than the expected increase was interpreted as inhibition. The RNA sample dilution factor is indicated on the x-axis.
For 4S-Milk-of-Silica extraction, samples were lysed, heat inactivated, and filtered as in the 4S-column extraction. Next, a 1 g/mL silicon dioxide slurry in water was added to the filtered lysate and incubated at room temperature for 10 min. The lysate and silica slurry were centrifuged at 4000 × g for 5 min, pelleting wastewater RNA bound to silica particulates. The lysate supernatant was decanted, and the silica pellet was washed with 40 mL 4S-WB1 and 40 mL of 4S-WB2 via centrifugation and wash buffer decanting. The washed silica pellet was resuspended in 20 mL of pure water preheated to 37 °C to elute bound RNA. Next, the silicon dioxide particulate was pelleted via centrifugation and the eluted RNA was separated and concentrated via isopropanol precipitation, as previously described.21,22 Here, 20 mL of 100% volume isopropanol and 4 mL of 3 M pH 5.2 sodium acetate were added to the eluted RNA. The mixture was centrifuged for 4000 × g in a swinging bucket rotor for 1 h, forming a semitranslucent RNA pellet. The excess supernatant was decanted from the RNA pellet, and the pellet was washed with 40 mL of 75% volume ethanol by vortexing the pellet until fully suspended. The pellet was reprecipitated via centrifugation at 4000 × g for 30 min and excess ethanol was decanted from the RNA pellet. The washed pellet was resuspended in 1 mL 75% ethanol, transferred to a 1.5 mL tube, and precipitated via centrifugation at 5000 × g for 5 min. Excess ethanol was carefully aspirated from the pellet and the pellet was dried via incubation at 37 °C for 10 min. The dried pellet was resuspended in 200 μL of pH 8 TE buffer and stored at 4 °C for same-day use or frozen at −80 °C for later use and storage. The 4S-column and 4S-Milk-of-Silica reagent costs are listed in Supporting Information Table S6.
For sample RNA concentration via ultrafiltration, Amicon 100-kDa ultrafilters (Millipore Sigma) were pretreated to block virus adsorption using 2 mL bovine serum albumin 1% (w/v) in 1 × PBS and then washed with PBS. Wastewater samples were divided into 40 mL aliquots and solids were removed via slow centrifugation with a swinging bucket rotor at 4700 × g for 30 min. The supernatant was decanted and passed through a 0.2 μm flat membrane filter (Steriflip, EMD Millipore). The filtrate was loaded onto the ultrafilter in increments of up to 15 mL and ultrafilters were spun for 10 min at 4700 × g for each increment. Flow-through was discarded and samples were concentrated until they were reduced to a final volume of ∼250 μL. RNA was extracted from the ultrafiltration concentrate using an AllPrep DNA/RNA Mini kit (QIAGEN) following manufacturer instructions.
RNA Detection and Quantification Via RT-qPCR
This study employed four primer/probe sets: the SARS-CoV-2 N1 assay, pepper mild mottle virus (PMMoV) coat protein gene assay, bovine coronavirus transmembrane protein gene assay, and a newly developed human 18S ribosomal rRNA assay. (Supporting Information, Table S3) RT-qPCR reaction conditions are detailed in Table S1, assay thermocycling conditions are detailed in Table S2, and primer sequence information is in Table S3. RT-qPCR assay performance is detailed in Table S4 (validation) and Table S5 (limit of detection). Three technical replicate RT-qPCR wells were analyzed for each sample and standard. After outlier detection and removal, the arithmetic mean of Cq values for the technical replicates was determined; this value was used to determine the concentration for each biological replicate. RT-qPCR minimum information for publication of quantitative real-time PCR experiment (MIQE) documentation is detailed in Table S7. RT-qPCR analysis is detailed in the Supporting Information. Full data set and associated code are available in the Supporting Information as well as through Zenodo at DOI 10.5281/zenodo.4570691.
Results and Discussion
Many current methods of wastewater viral RNA extraction assume that most viral particles within wastewater are intact and that the concentration of these intact viruses prior to extraction is necessary to achieve sensitive detection of SARS-CoV-2 in wastewater. Given this assumption, these methods typically employ precipitation-, charge-, or size-based viral concentration and subsequent RNA extraction of unpasteurized wastewater to preserve viruses in an intact state.14,15 Despite concentration, some methods were shown to recover as little as 0–1% of SARS-CoV-1 from wastewater during the SARS-CoV-1 epidemic.23 We hypothesized that direct extraction could avoid loss of virus during the primary concentration step, and we therefore designed the 4S (sewage, salt, silica, and SARS-CoV-2) method to lyse viruses and microorganisms present in wastewater using sodium chloride and subsequently capture the free RNA using a silica RNA-binding matrix.
Direct Wastewater RNA Extraction Via the 4S Method
To benchmark the performance of the 4S method, we analyzed a 24 h composite wastewater sample treated with and without heat pasteurization and compared the recovery of endogenous SARS-CoV-2 to that of an ultrafiltration-based method. In addition, we compared the recovery of endogenous PMMoV RNA, which may be useful to control for variable fecal concentrations in wastewater, and a spiked-in bovine coronavirus vaccine (BCoV), used as an RNA extraction process control (Figure 1). We observed that the 4S-column method recovered sixfold more SARS-CoV-2 RNA than ultrafiltration (Figure 1).
Figure 1.
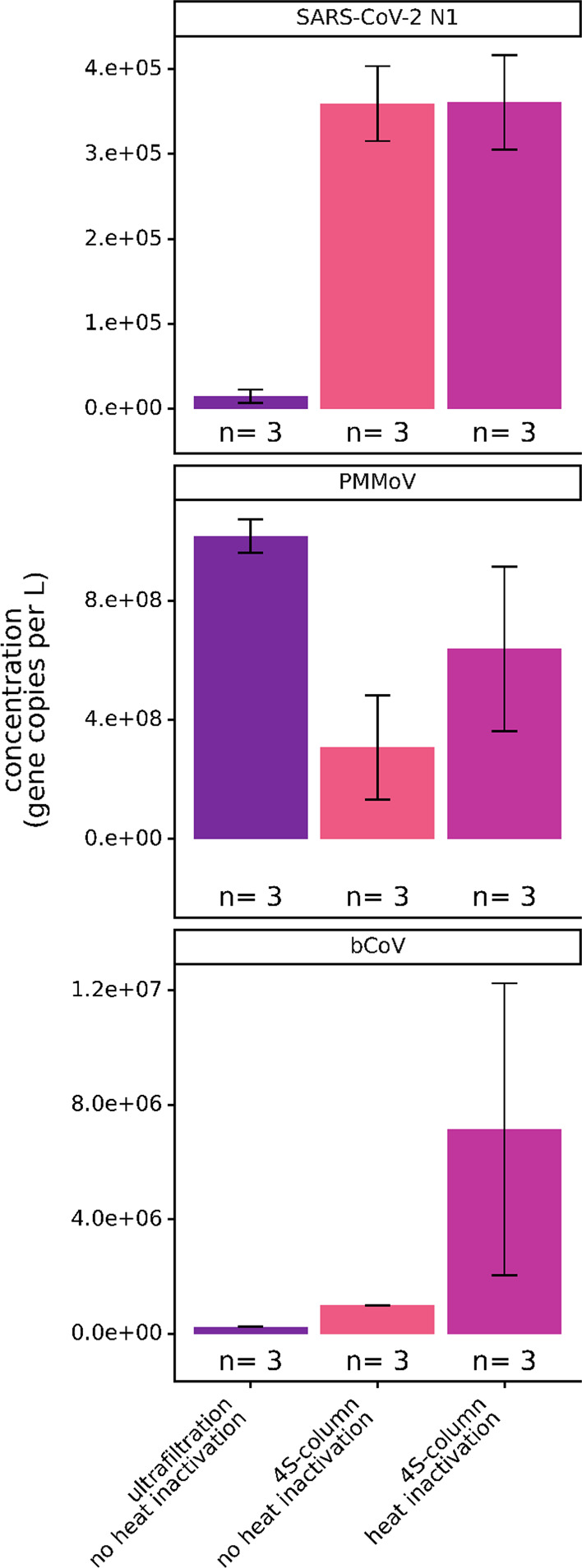
Comparison of SARS-CoV-2, PMMoV, and BCoV spike-in assay signals in gene copies per liter between the 4S-column method with and without heat inactivation and ultrafiltration. “n” represents the number of wastewater biological replicates per condition. Bars are plotted at the arithmetic mean of biological replicates and error bars represent the variation associated with biological triplicates as quantified by the arithmetic standard deviation of the biological triplicates.
Surprisingly, SARS-CoV-2 recovery by the 4S method was not impacted by heat pasteurization, suggesting that further SARS-CoV-2 virus lysis did not occur. This result may imply that a large fraction of SARS-CoV-2 RNA was not bound to virus particles; this unbound RNA was captured by the 4S method but was not efficiently concentrated by ultrafiltration.
The 4S-column method without heat pasteurization also recovered sixfold more BCoV than ultrafiltration and 28-fold more BCoV with heat pasteurization. In this case, heat pasteurization may promote additional lysis of encapsidated BCoV, releasing its RNA for subsequent capture. However, we have observed that the BCoV vaccine used as a process control is subject to potential degradation during storage and handling and potential incomplete lysis during heat inactivation, possibly explaining the large variance in BCoV recovery with the 4S method. Recovery of PMMoV by 4S was also higher with heat pasteurization (twofold increase in recovery), but ultrafiltration was more effective in enriching PMMoV (1.6-fold higher than using the 4S-column method with heat pasteurization). Here, ultrafiltration may be effective in concentrating intact virus that is able to persist in wastewater, which is consistent with previous reports on PMMoV.24,25 Although we did not directly assess the state of SARS-CoV-2 in wastewater, these results may suggest that a fraction of SARS-CoV-2 RNA in the analyzed wastewater was not bound to viral particles but was present as free or ribonucleoprotein-bound RNA. This possibility is consistent with reports indicating reduced viability of SARS-CoV-2 and related coronaviruses spiked into wastewater, as well as preliminary reports demonstrating that wastewater SARS-CoV-2 genomes are predominantly decapsidated.26,27 Given that the 4S method is designed to lyse and extract wastewater RNAs without requiring the enrichment of viral particles, we also investigated whether the 4S method could recover human RNAs present in wastewater. Using the 4S method, we were able to recover and detect human ribosomal subunit RNA (18S rRNA) in wastewater influent (Supporting Information Figure S1A). 18S rRNA recovery was enhanced 2.5-fold by heat pasteurization, suggesting the lysis of human cells or 18S rRNA bound to ribonucleoprotein complexes present in wastewater (Supporting Information Figure S1A). Therefore, the 4S method enabled the recovery and detection of human RNA, another potential indicator of wastewater fecal concentration, which could allow direct normalization of SARS-CoV-2 RNA quantity to human RNA content of wastewater. As heat pasteurization did not affect 4S recovery of SARS-CoV-2 and improved the recovery of PMMoV, BCoV, and 18S rRNA, we recommend integrating this pathogen-inactivation step to increase the safety of processing wastewater samples.
We sought to adapt the 4S strategy to employ silica powder for RNA capture rather than silica columns to circumvent reliance on commercially manufactured silica columns. In this approach, we added a slurry of silicon dioxide particles to lysed wastewater and used centrifugation to separate particle-bound RNA from the wastewater matrix, an approach we named “4S-Milk-of-Silica”. We observed that the 4S-Milk-of-Silica method recovered equivalent SARS-CoV-2 and PMMoV signals to the 4S method using a silica column (Supporting Information Figure S1B). Thus, the 4S-Milk-of-Silica method presents an even more cost-effective (∼$8 per sample vs ∼$13 per sample, using the 4S-column extraction method, Supporting Information Table S6) and accessible method to extract wastewater RNA without reliance on commercially manufactured silica columns and a vacuum manifold. However, the “Milk-of-Silica” version of the 4S protocol requires an isopropanol precipitation RNA concentration step, lengthening the protocol time. Therefore, we recommend using the 4S-column method to enable faster sample processing, while “4S-Milk-of-Silica” presents an alternate protocol for use in resource-limited settings.
Wastewater-Based Epidemiology Via the 4S Method
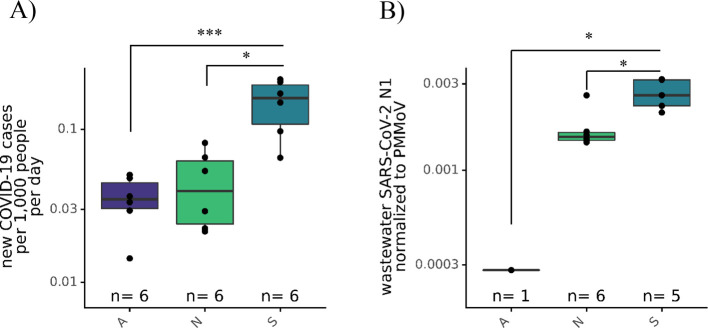
WBE can provide an assessment of different areas’ relative COVID-19 infection prevalence, so we assessed whether the 4S-column extraction method could detect differential SARS-CoV-2 RNA levels in wastewaters derived from different subsections of a collection system. We surveyed three wastewater influent interceptors serving North and West Berkeley and El Cerrito (N), East Berkeley/Berkeley Hills (A), and Oakland (S) (interceptor area coverage shown in Supporting Information Figure S2A). These interceptors served areas exhibiting differential incidence of clinically confirmed COVID-19 cases, ranging from three (A interceptor) to 68 (S interceptor) reported cases per day within the week of our sampling (Figure S2A). To compare clinical COVID-19 clinical case data and wastewater SARS-CoV-2 concentration, we normalized the clinical case data by population, and we normalized the SARS-CoV-2 quantity by PMMoV abundance, to control for fecal concentration in the wastewater. Detected wastewater SARS-CoV-2 concentrations are likely dependent on wastewater fecal concentration, which in turn varies based on the number of individuals contributing to the wastewater, wastewater flow rates, and water utilization. Future work into relating wastewater flow, solid content, and fecal concentration controls such as PMMoV may clarify true wastewater SARS-CoV-2 concentrations. Normalized wastewater SARS-CoV-2 signals followed per capita clinical cases per day in the three subsewersheds (Figure 2B). Raw SARS-CoV-2 and PMMoV abundance is available in Supporting Information Figure S2B. The normalized SARS-CoV-2 RNA concentration was highest in wastewater representing the S interceptor area, where the highest daily per capita new cases also occurred. Normalized SARS-CoV-2 RNA concentrations in wastewaters representing the N interceptor area were only 2.3-fold lower than those of S interceptor wastewaters, despite 11.6-fold fewer per capita daily cases being reported in the A interceptor area during the week of our sample collection. One possible reason for this difference could be the presence of undiagnosed infections in the N interceptor service area in which case wastewater SARS-CoV-2 RNA concentrations may provide a more accurate view of the relative COVID-19 infection prevalence in the week prior to sampling. Alternatively, the variability associated with wastewater measurements may be too large to detect differences of this magnitude.9,13 Ongoing research seeks to better quantify the measurement variability in wastewater samples over temporal and spatial scales. We emphasize that SARS-CoV-2 RNA levels were quantifiable in the A subsewershed despite only 18 cases being reported in an estimated population of 90,000 during the weeklong period of our sampling. This result implies that the 4S method is highly sensitive and can be used to monitor areas with low COVID-19 prevalence.
Figure 2.
(A) New COVID-19 clinical cases per day per 100,000 population in three areas served by the distinct A, N, and S wastewater interceptors over 6 days from 7/15 to 7/21. “n” represents the number of days during which clinical case data were collected. (B) Comparison of SARS-CoV-2 N1 assay represented as SARS-CoV-2 gene copies per liter normalized to PMMoV gene copies per liter between interceptors serving the A, N, and S East Bay areas. “n” represents the number of biological replicates (the wastewater was collected on a single day in the 6-day window). The Kruskal-Wallis test followed by Dunn’s test was performed to determine significance, where * = p < 0.05 and *** = p < 0.001.
Wastewater RNA Preservation and Purification Via the 4S Method
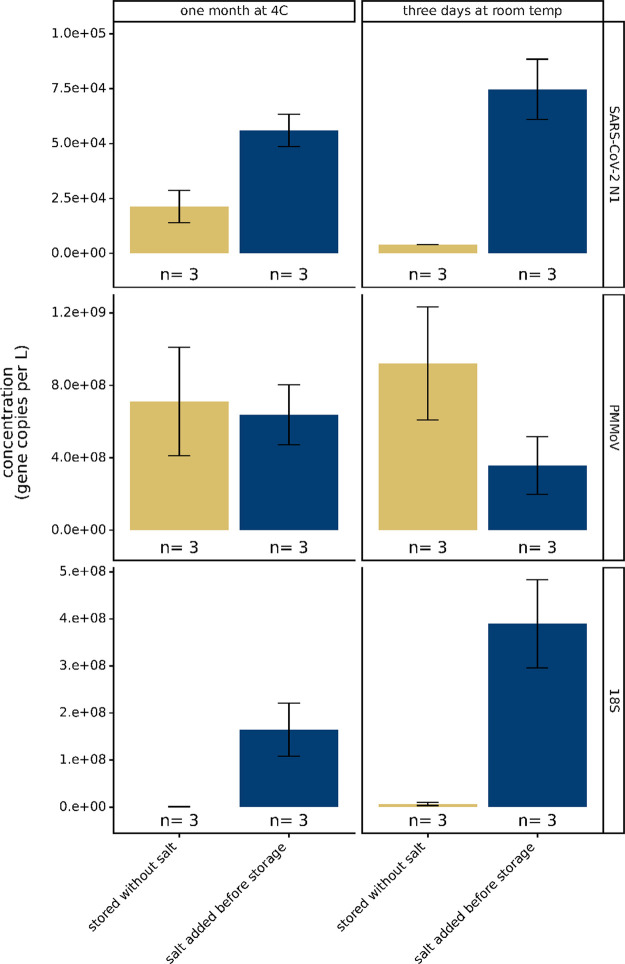
Wastewater contains many contaminants with the potential to degrade nucleic acids, and it has been previously observed that SARS-CoV-2 RNA in wastewater is degraded during storage.26,28,29 Viral detection relying on wastewater RNA extraction methods that concentrate intact viruses may be strongly affected by variable amounts of virus and viral RNA degradation in wastewater. Therefore, we sought to assess whether EDTA and sodium chloride, added to wastewater in the 4S method to promote lysis, could dually act to preserve RNA in wastewater. Upon receipt of each wastewater sample, we added NaCl to a final concentration of 4 M, added EDTA to a final concentration of 1 mM, and stored the samples either at 4°C for a month or three days at room temperature (20°C). We observed that salt and EDTA addition prior to storage improved the SARS-CoV-2 N1 assay signal after storage at both 4°C for one month (2.6-fold higher signal when stored with salt and EDTA) or at 20°C for three days (22-fold higher signal when stored with salt and EDTA) (Figure 3). Interestingly, the PMMoV assay signal remained similar throughout storage with or without salt, implying that PMMoV remains resistant to RNAses in the wastewater matrix. This observation corroborates previous reports indicating the persistence of PMMoV in sea and river water.24,25 As with the SARS-CoV-2 N1 signal, we observed that salt and EDTA addition preserved the human 18S rRNA signal at 4 °C for one month (126-fold higher) or at 20 °C for three days (56-fold higher) (Figure 3). These results may suggest that a portion of SARS-CoV-2 in wastewater has been lysed, rendering its RNA more susceptible to degradation, while a greater portion of PMMoV may remain encapsidated, protecting its RNA from degradation. The nonenveloped enteric Coxsackievirus B5 was also recently reported to be more stable in wastewater than SARS-CoV-2.27 Overall, the lysis salts added to wastewater as part of the normal 4S method workflow conveniently preserved wastewater RNAs and may mitigate degradation-mediated variation in SARS-CoV-2 and fecal concentration controls caused by RNA degradation during shipping and storage. However, we recommend extracting RNA from wastewater as soon as possible after sampling to ensure maximal RNA integrity.
Figure 3.
Effect of lysis salt addition prior to wastewater storage on SARS-CoV-2 N1, PMMoV, and 18S rRNA assay signals. “n” represents the number of storage and extraction biological replicates per condition. Bars are plotted at the arithmetic mean of biological triplicates and error bars represent the variation associated with biological triplicates as quantified by the arithmetic standard deviation of the biological triplicates.
Given the impact of RNA degradation on the SARS-CoV-2 N1 assay signal during wastewater storage, we investigated whether the bulk RNA yield could approximate the fecal load. However, the bulk RNA yield per mL of wastewater input correlated poorly with SARS-CoV-2 and PMMoV detection (Supporting Information Figure S3A), suggesting that the bulk of wastewater RNA is not contributed by fecal inputs. We also observed that extracting nucleic acids from increasing volumes of wastewater (up to 400 mL) did not strongly increase the total RNA yield per extraction past 100 mL of wastewater sample input, implying potential saturation of the RNA capture matrix (Supporting Information Figure S3B). From these experiments, we conclude that the RT-qPCR detection of human fecal concentration indicators such as PMMoV and human 18S rRNA, the latter of which is preserved during storage similarly to SARS-CoV-2, are suitable estimators of wastewater fecal concentration. Lastly, we observed that the 4S method enriched up to 8 μg of DNA per 100 mL of wastewater, suggesting that the 4S method could be employed for future wastewater surveillance of DNA-based pathogens and DNA-sequencing-based wastewater surveys (Supporting Information Figure S3C).
Wastewater samples contain many contaminants that have previously been reported to inhibit RT-qPCR reactions.30 Therefore, we sought to assess whether the 4S method could generate purified RNA free of RT-qPCR contaminants by employing the “spike and dilute” method to assess PCR inhibition.31 Here, we spiked purified wastewater RNA with a synthetic RNA standard and sequentially diluted the sample and observed whether SARS-CoV-2 N1, PMMoV, and BCoV detection followed corresponding sample dilutions, indicating an absence of inhibition. We assessed the impact of a range (1–50 mL) of wash buffer volumes during RNA extraction on PCR inhibition and SARS-CoV-2 N1, PMMoV, and BCoV assay signals to identify the optimal wash buffer volume for RNA purity and recovery. There was no evidence of inhibition for the SARS-CoV-2 N1 assay using the 4S procedure with any wash buffer volume, and slight inhibition of the PMMoV assay when using 5 mL of 4S-Wash buffer #1 (4S-WB1) and 10 mL of 4S-Wash buffer #2 (4S-WB2) (Figure 4). To limit ethanol waste generation, we therefore recommend using at least 7 mL of 4S-WB1 and 14 mL of 4S-WB2 to yield inhibitor-free RNA.
Next, we assessed potential assay signal loss due to excess washing of the silica columns. Here, we observed highest SARS-CoV-2, PMMoV, and BCoV assay signals using 3 mL of 4S-WB1 and 6 mL of Wash 4S-WB2, with no trend toward loss in SARS-CoV-2 and PMMoV signals up until 25 mL of 4S-WB1 and 50 mL of 4S-WB2, whereas we noticed a trend toward BCoV signal loss after 3 mL of 4S-WB1 and 6 mL of 4S-WB2 (Supporting Information Figure S4). Using too little wash buffer may not sufficiently wash away lysis salts and contaminants from the silica matrix, reducing RNA recovery and increasing inhibition, whereas too much wash buffer may partially elute bound RNA, decreasing the RNA yield. Therefore, we recommend using 7–10 mL of 4S-WB1 and 14–20 mL of 4S-WB2 to extract PCR inhibitor-free RNA while maximizing target RNA recovery.
The results presented here are representative of only three wastewater sources which may differ in composition from wastewater collected at other times and from other locations. Different wastewaters may contain different types and quantities of PCR inhibitors, so we recommend assessing PCR inhibition in all sample types, and if necessary, adjusting the wash buffer volumes to effectively remove inhibitors from the purified RNA. Different wastewater samples may also contain varying biological and chemical species influencing RNA stability, potentially impacting the RNA preservation documented here by the 4S method. Furthermore, the 4S method may be less effective in capturing the nucleic acids from wastewater viruses or other microorganisms resistant to the sodium chloride and heat-based lysis evaluated here.
Overall, we demonstrate that the 4S method enabled efficient extraction of SARS-CoV-2, PMMoV, BCoV, and human 18S rRNA. Combined with RT-qPCR, the 4S method allowed monitoring of relative COVID-19 infection prevalence with high sensitivity. These results are consistent with those of a recent interlaboratory comparison of 36 different wastewater SARS-CoV-2 RNA detection methods. In this comparison, the concentration of SARS-CoV-2 measured with the 4S method, identified as “1S.2H”, was one of the highest reported (direct measurement, without correcting for recovery efficiency) and the recovery efficiency of a spiked-in OC43 virus efficiency control was the highest reported, among all methods.32 The 4S method also preserved RNA in wastewater, was compatible with heat pasteurization, and yielded purified RNA free of PCR inhibitors. Given the high efficiency, low cost, and same-day assessment of wastewater SARS-CoV-2 and fecal concentration controls, the 4S method presents an affordable and accessible method for implementing wastewater-based epidemiology for SARS-CoV-2. This method also appears promising for the application of WBE for other RNA- and DNA-based pathogens and facilitating research on the wastewater microbial community more broadly.
Acknowledgments
We thank all members of the Nelson laboratory and UC Berkeley COVID-WEB pop-up lab for their contributions, reading, insights and helpful discussions of this paper. We also thank all members of the Tjian and Darzacq lab for their support designing and carrying out molecular assays and experiments. We thank the East Bay Municipal Utility District for sample collection.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acs.est.0c08129.
-
The supporting information details supplementary figures, methods, and data analysis.
Impact of heat inactivation on 4S-column recovery of 18 s rRNA, “Milk-of-Silica” silicon dioxide RNA extraction strategy performance; wastewater interceptor coverage, individual A, N, and S interceptor SARS-CoV-2 and PMMoV concentration measurements associated with Figure 2; relation of extracted RNA concentration to SARS-CoV-2 N1 and PMMoV assay signals, relationship between the wastewater input and RNA yield using the 4S-column, Milk-of-Silica, ultrafiltration and ultrafiltration and solids extraction methods, and relationship between the wastewater input volume and DNA yield; impact of wash buffer volume use on SARS-CoV-2 N1, PMMoV, and BCoV recovery; RT-qPCR reaction conditions (Table S1); RT-qPCR thermocycling parameters (Table S2); qPCR assay information for the SARS-CoV-2 nucleocapsid N gene (N1), the bovine coronavirus transmembrane protein gene (bCoV), the pepper mild mottle virus coat protein gene (PMMoV), and human 18S ribosomal rRNA (18S) (Table S3); RT-qPCR validation information (Table S4); evidence for LoD/LoQ (Table S5); reagent cost of the 4S method using a silica column or silicon dioxide particulate (Table S6) MIQE guideline essential information checklist (Table S7); and nucleic acid quantification, target RNA detection via RT-qPCR, and data analysis methods (PDF)
O.N.W. is supported by the NIH training program grant T32GM007232. We gratefully acknowledge funding from the Howard Hughes Medical Institute (grant CC34430 to R.T.) and from rapid response grants from the Center for Information Technology Research in the Interest of Society (CITRIS), the Catena Foundation and the Innovative Genomics Institute (IGI) at UC Berkeley to K.L.N.
The authors declare no competing financial interest.
Supplementary Material
References
- Bivins A.; North D.; Ahmad A.; Ahmed W.; Alm E.; Been F.; Bhattacharya P.; Bijlsma L.; Boehm A. B.; Brown J.; Buttiglieri G.; Calabro V.; Carducci A.; Castiglioni S.; Cetecioglu Gurol Z.; Chakraborty S.; Costa F.; Curcio S.; de los Reyes F. L.; Delgado Vela J.; Farkas K.; Fernandez-Casi X.; Gerba C.; Gerrity D.; Girones R.; Gonzalez R.; Haramoto E.; Harris A.; Holden P. A.; Islam M. T.; Jones D. L.; Kasprzyk-Hordern B.; Kitajima M.; Kotlarz N.; Kumar M.; Kuroda K.; La Rosa G.; Malpei F.; Mautus M.; McLellan S. L.; Medema G.; Meschke J. S.; Mueller J.; Newton R. J.; Nilsson D.; Noble R. T.; van Nuijs A.; Peccia J.; Perkins T. A.; Pickering A. J.; Rose J.; Sanchez G.; Smith A.; Stadler L.; Stauber C.; Thomas K.; van der Voorn T.; Wigginton K.; Zhu K.; Bibby K. Wastewater-Based Epidemiology: Global Collaborative to Maximize Contributions in the Fight Against COVID-19. Environ. Sci. Technol. 2020, 54, 7754–7757. 10.1021/acs.est.0c02388. [DOI] [PubMed] [Google Scholar]
- Zhou N. A.; Fagnant-Sperati C. S.; Komen E.; Mwangi B.; Mukubi J.; Nyangao J.; Hassan J.; Chepkurui A.; Maina C.; van Zyl W. B.; Matsapola P. N.; Wolfaardt M.; Ngwana F. B.; Jeffries-Miles S.; Coulliette-Salmond A.; Peñaranda S.; Shirai J. H.; Kossik A. L.; Beck N. K.; Wilmouth R.; Boyle D. S.; Burns C. C.; Taylor M. B.; Borus P.; Meschke J. S. Feasibility of the Bag-Mediated Filtration System for Environmental Surveillance of Poliovirus in Kenya. Food Environ. Virol. 2020, 12, 35–47. 10.1007/s12560-019-09412-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazama S.; Miura T.; Masago Y.; Konta Y.; Tohma K.; Manaka T.; Liu X.; Nakayama D.; Tanno T.; Saito M.; Oshitani H.; Omura T. Environmental Surveillance of Norovirus Genogroups I and II for Sensitive Detection of Epidemic Variants. Appl. Environ. Microbiol. 2017, 83, e03406–e03416. 10.1128/AEM.03406-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaliner E.; Kopel E.; Anis E.; Mendelson E.; Moran-Gilad J.; Shulman L. M.; Singer S. R.; Manor Y.; Somekh E.; Rishpon S.; Leventhal A.; Rubin L.; Tasher D.; Honovich M.; Moerman L.; Shohat T.; Bassal R.; Sofer D.; Gdalevich M.; Lev B.; Gamzu R.; Grotto I. The Israeli Public Health Response to Wild Poliovirus Importation. Lancet Infect. Dis. 2015, 15, 1236–1242. 10.1016/S1473-3099(15)00064-X. [DOI] [PubMed] [Google Scholar]
- Hata A.; Honda R. Potential Sensitivity of Wastewater Monitoring for SARS-CoV-2: Comparison with Norovirus Cases. Environ. Sci. Technol. 2020, 54, 6451–6452. 10.1021/acs.est.0c02271. [DOI] [PubMed] [Google Scholar]
- Li R.; Pei S.; Chen B.; Song Y.; Zhang T.; Yang W.; Shaman J. Substantial Undocumented Infection Facilitates the Rapid Dissemination of Novel Coronavirus (SARS-CoV-2). Science 2020, 368, 489–493. 10.1126/science.abb3221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y.; Guo C.; Tang L.; Hong Z.; Zhou J.; Dong X.; Yin H.; Xiao Q.; Tang Y.; Qu X.; Kuang L.; Fang X.; Mishra N.; Lu J.; Shan H.; Jiang G.; Huang X. Prolonged Presence of SARS-CoV-2 Viral RNA in Faecal Samples. Lancet Gastroenterol. Hepatol. 2020, 5, 434–435. 10.1016/S2468-1253(20)30083-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holshue M. L.; DeBolt C.; Lindquist S.; Lofy K. H.; Wiesman J.; Bruce H.; Spitters C.; Ericson K.; Wilkerson S.; Tural A.; Diaz G.; Cohn A.; Fox L.; Patel A.; Gerber S. I.; Kim L.; Tong S.; Lu X.; Lindstrom S.; Pallansch M. A.; Weldon W. C.; Biggs H. M.; Uyeki T. M.; Pillai S. K. First Case of 2019 Novel Coronavirus in the United States. N. Engl. J. Med. 2020, 382, 929–936. 10.1056/NEJMoa2001191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peccia J.; Zulli A.; Brackney D. E.; Grubaugh N. D.; Kaplan E. H.; Casanovas-Massana A.; Ko A. I.; Malik A. A.; Wang D.; Wang M.; Warren J. L.; Weinberger D. M.; Arnold W.; Omer S. B. Measurement of SARS-CoV-2 RNA in Wastewater Tracks Community Infection Dynamics. Nat. Biotechnol. 2020, 38, 1164–1167. 10.1038/s41587-020-0684-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W.; Angel N.; Edson J.; Bibby K.; Bivins A.; O’Brien J. W.; Choi P. M.; Kitajima M.; Simpson S. L.; Li J.; Tscharke B.; Verhagen R.; Smith W. J. M.; Zaugg J.; Dierens L.; Hugenholtz P.; Thomas K. V.; Mueller J. F. First Confirmed Detection of SARS-CoV-2 in Untreated Wastewater in Australia: A Proof of Concept for the Wastewater Surveillance of COVID-19 in the Community. Sci. Total Environ. 2020, 728, 138764. 10.1016/j.scitotenv.2020.138764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medema G.; Heijnen L.; Elsinga G.; Italiaander R.; Brouwer A. Presence of SARS-Coronavirus-2 RNA in Sewage and Correlation with Reported COVID-19 Prevalence in the Early Stage of the Epidemic in The Netherlands. Environ. Sci. Technol. Lett. 2020, 7, 511–516. 10.1021/acs.estlett.0c00357. [DOI] [PubMed] [Google Scholar]
- Crits-Christoph A.; Kantor R. S.; Olm M. R.; Whitney O. N.; Al-Shayeb B.; Lou Y. C.; Flamholz A.; Kennedy L. C.; Greenwald H.; Hinkle A.; Hetzel J.; Spitzer S.; Koble J.; Tan A.; Hyde F.; Schroth G.; Kuersten S.; Banfield J. F.; Nelson K. L.. Genome Sequencing of Sewage Detects Regionally Prevalent SARS-CoV-2 Variants. medRxiv; 2020, 10.1101/2020.09.13.20193805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitajima M.; Ahmed W.; Bibby K.; Carducci A.; Gerba C. P.; Hamilton K. A.; Haramoto E.; Rose J. B. SARS-CoV-2 in Wastewater: State of the Knowledge and Research Needs. Sci. Total Environ. 2020, 739, 139076 10.1016/j.scitotenv.2020.139076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Rosa G.; Bonadonna L.; Lucentini L.; Kenmoe S.; Suffredini E. Coronavirus in Water Environments: Occurrence, Persistence and Concentration Methods - A Scoping Review. Water Res. 2020, 179, 115899 10.1016/j.watres.2020.115899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D.; Huang Z.; Luo J.; Zhang X.; Sha S. Primary Concentration – The Critical Step in Implementing the Wastewater Based Epidemiology for the COVID-19 Pandemic: A Mini-Review. Sci. Total Environ. 2020, 747, 141245 10.1016/j.scitotenv.2020.141245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satyanarayana M. Shortage of RNA extraction kits hampers efforts to ramp up COVID-19 coronavirus testing https://cen.acs.org/analytical-chemistry/diagnostics/Shortage-RNA-extraction-kits-hampers/98/web/2020/03 (accessed Nov 9, 2020).
- LaTurner Z. W.; Zong D. M.; Kalvapalle P.; Gamas K. R.; Terwilliger A.; Crosby T.; Ali P.; Avadhanula V.; Santos H. H.; Weesner K.; Hopkins L.; Piedra P. A.; Maresso A. W.; Stadler L. B. Evaluating Recovery, Cost, and Throughput of Different Concentration Methods for SARS-CoV-2 Wastewater-Based Epidemiology. medRxiv; 2020, 10.1101/2020.11.27.20238980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.cdc.gov/coronavirus/2019-ncov/cases-updates/wastewater-surveillance/testing-methods.html
- Kitajima M.; Sassi H. P.; Torrey J. R. Pepper Mild Mottle Virus as a Water Quality Indicator. Npj Clean Water 2018, 1, 1–9. 10.1038/s41545-018-0019-5. [DOI] [Google Scholar]
- Whitney O.Direct Wastewater RNA Capture and Purification via the "Sewage, Salt, Silica and SARS-CoV-2 (4S)". Method. 2020. 10.17504/protocols.io.biwekfbe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney O.Direct Wastewater RNA Extraction via the "Milk of Silica (MoS)" Method - A Companion Method to "Sewage, Salt, Silica and SARS-CoV-2 (4S)" 2020. 10.17504/protocols.io.biwfkfbn. [DOI] [Google Scholar]
- Graham T. G. W.; Dugast-Darzacq C.; Dailey G. M.; Nguyenla X. H.; Van Dis E.; Esbin M. N.; Abidi A.; Stanley S. A.; Darzacq X.; Tjian R.. ,Inexpensive, Versatile and Open-Source Methods for SARS-CoV-2 Detection. preprint. Infectious Diseases (except HIV/AIDS). 2020. 10.1101/2020.09.16.20193466. [DOI] [Google Scholar]
- Wang X.-W.; Li J.-S.; Guo T.-K.; Zhen B.; Kong Q.-X.; Yi B.; Li Z.; Song N.; Jin M.; Xiao W.-J.; Zhu X.-M.; Gu C.-Q.; Yin J.; Wei W.; Yao W.; Liu C.; Li J.-F.; Ou G.-R.; Wang M.-N.; Fang T.-Y.; Wang G.-J.; Qiu Y.-H.; Wu H.-H.; Chao F.-H.; Li J.-W. Concentration and Detection of SARS Coronavirus in Sewage from Xiao Tang Shan Hospital and the 309th Hospital. J. Virol. Methods 2005, 128, 156–161. 10.1016/j.jviromet.2005.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamza I. A.; Jurzik L.; Überla K.; Wilhelm M. Evaluation of Pepper Mild Mottle Virus, Human Picobirnavirus and Torque Teno Virus as Indicators of Fecal Contamination in River Water. Water Res. 2011, 45, 1358–1368. 10.1016/j.watres.2010.10.021. [DOI] [PubMed] [Google Scholar]
- Symonds E. M.; Rosario K.; Breitbart M. Pepper Mild Mottle Virus: Agricultural Menace Turned Effective Tool for Microbial Water Quality Monitoring and Assessing (Waste)Water Treatment Technologies. PLoS Pathog. 2019, 15, e1007639 10.1371/journal.ppat.1007639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bivins A.; Greaves J.; Fischer R.; Yinda K. C.; Ahmed W.; Kitajima M.; Munster V. J.; Bibby K. Persistence of SARS-CoV-2 in Water and Wastewater. Environ. Sci. Technol. Lett. 2020, 7, 937–942. 10.1021/acs.estlett.0c00730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtzer S.; Waldman P.; Ferrier-Rembert A.; Frenois-Veyrat G.; Mouchel J.; Boni M.; Maday Y.; Consortium O.; Marechal V.; Moulin L.. Several Forms of SARS-CoV-2 RNA Can Be Detected in Wastewaters : Implication for Wastewater-Based Epidemiology and Risk Assessment. medRxiv; 2020, 10.1101/2020.12.19.20248508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed W.; Bertsch P. M.; Bibby K.; Haramoto E.; Hewitt J.; Huygens F.; Gyawali P.; Korajkic A.; Riddell S.; Sherchan S. P.; Simpson S. L.; Sirikanchana K.; Symonds E. M.; Verhagen R.; Vasan S. S.; Kitajima M.; Bivins A. Decay of SARS-CoV-2 and Surrogate Murine Hepatitis Virus RNA in Untreated Wastewater to Inform Application in Wastewater-Based Epidemiology. Environ. Res. 2020, 191, 110092 10.1016/j.envres.2020.110092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael-Kordatou I.; Karaolia P.; Fatta-Kassinos D. Sewage Analysis as a Tool for the COVID-19 Pandemic Response and Management: The Urgent Need for Optimised Protocols for SARS-CoV-2 Detection and Quantification. J. Environ. Chem. Eng. 2020, 8, 104306 10.1016/j.jece.2020.104306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader C.; Schielke A.; Ellerbroek L.; Johne R. PCR Inhibitors – Occurrence, Properties and Removal. J. Appl. Microbiol. 2012, 113, 1014–1026. 10.1111/j.1365-2672.2012.05384.x. [DOI] [PubMed] [Google Scholar]
- Cao Y.; Griffith J. F.; Dorevitch S.; Weisberg S. B. Effectiveness of QPCR Permutations, Internal Controls and Dilution as Means for Minimizing the Impact of Inhibition While Measuring Enterococcus in Environmental Waters. J. Appl. Microbiol. 2012, 113, 66–75. 10.1111/j.1365-2672.2012.05305.x. [DOI] [PubMed] [Google Scholar]
- Pecson B. M.; Darby E.; Haas C. N.; Amha Y.; Bartolo M.; Danielson R.; Dearborn Y.; Giovanni G. D.; Ferguson C.; Fevig S.; Gaddis E.; Gray D.; Lukasik G.; Mull B.; Olivas L.; Olivieri A.; Qu Y.; Consortium, S.-C.-2 I . Reproducibility and Sensitivity of 36 Methods to Quantify the SARS-CoV-2 Genetic Signal in Raw Wastewater: Findings from an Interlaboratory Methods Evaluation in the U.S. medRxiv 2020, 10.1101/2020.11.02.20221622. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.