Abstract
The SARS-CoV-2 replication and transcription complex (RTC) comprising nonstructural protein (nsp) 2–16 plays crucial roles in viral replication, reducing the efficacy of broad-spectrum nucleoside analog drugs such as remdesivir and evading innate immune responses. Most studies target a specific viral component of the RTC such as the main protease or the RNA-dependent RNA polymerase. In contrast, our strategy is to target multiple conserved domains of the RTC to prevent SARS-CoV-2 genome replication and to create a high barrier to viral resistance and/or evasion of antiviral drugs. We show that the clinically safe Zn-ejector drugs disulfiram and ebselen can target conserved Zn2+ sites in SARS-CoV-2 nsp13 and nsp14 and inhibit nsp13 ATPase and nsp14 exoribonuclease activities. As the SARS-CoV-2 nsp14 domain targeted by disulfiram/ebselen is involved in RNA fidelity control, our strategy allows coupling of the Zn-ejector drug with a broad-spectrum nucleoside analog that would otherwise be excised by the nsp14 proofreading domain. As proof-of-concept, we show that disulfiram/ebselen, when combined with remdesivir, can synergistically inhibit SARS-CoV-2 replication in Vero E6 cells. We present a mechanism of action and the advantages of our multitargeting strategy, which can be applied to any type of coronavirus with conserved Zn2+ sites.
Keywords: COVID-19, structural Zn sites, nsp13, nsp14, replication and transcription complex
The COVID-19 pandemic, caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), is affecting billions of people around the world. The recent approval of several vaccines against SARS-CoV-21 would help to reduce transmission. However, it is not known how long immunity stays and whether the vaccinated person, though protected from COVID-19, may still carry the SARS-CoV-2 for some period and possibly infect others. To treat hospitalized COVID-19 patients, antivirals such as small-molecule drugs and neutralizing antibodies targeting SARS-CoV-2 are needed. Neutralizing antibodies derived from convalescent plasma obtained from a COVID-19 patient have shown only moderate success in clinical studies.2De novo antiviral development is lengthy and costly. A shortcut approach is to repurpose widely available, inexpensive drugs that can be easily administered against SARS-CoV-2. Most studies target a single SARS-CoV-2 protein3 such as the spike protein that mediates cell entry,4 the main protease (Mpro)5,6 or the papain-like protease (PLpro)7 that cleaves the viral polyprotein into its constituent proteins, or the RNA-dependent RNA polymerase (RdRp) that catalyzes viral RNA synthesis.8,9 However, the virus may evade antiviral drugs that target a single viral protein by mutations10 or deletions,11 and the spike receptor-binding domain is the most variable.12 One strategy is to create a high barrier to viral resistance/evasion of antiviral drugs by targeting sites with slow mutation rates in the multiprotein SARS-CoV-2 replication and transcription complex (RTC) that enables viral replication and transcription (see below). Here, we show that FDA-approved drugs (disulfiram/ebselen and remdesivir) can synergistically inhibit SARS-CoV-2 replication by targeting conserved sites in multiple nonstructural proteins (nsps) constituting the core of the RTC.
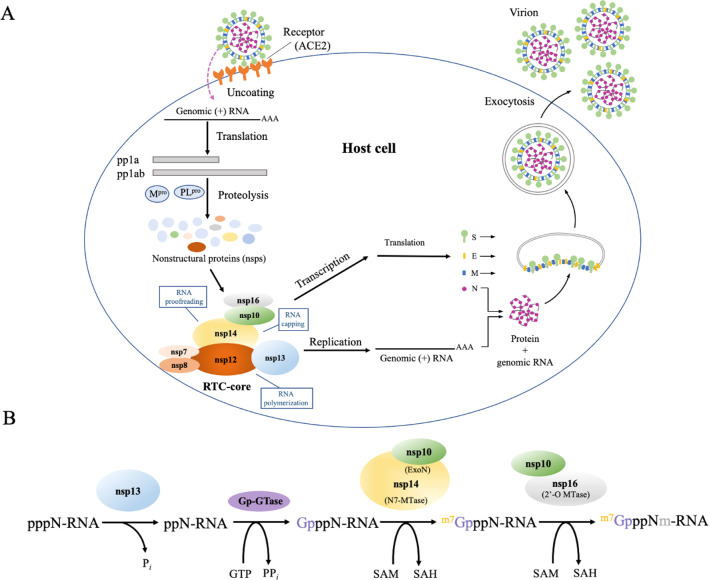
SARS-CoV-2 is an enveloped β-coronavirus containing a positive-sense single-stranded RNA (+ssRNA) genome that encodes 16 nonstructural, 4 structural, and 9 accessory proteins.13 It infiltrates cells by binding to human cell surface receptors such as angiotensin-converting enzyme 2 (ACE2) and possibly other cell receptors such as CD14714 via its spike glycoprotein, which upon cleavage by human proteases enables virus–cell membrane fusion (Figure 1A).3,15,16 Once inside, the viral RNA genome is released into the cytoplasm. Its 5′ end is translated by the host protein synthesis machinery to polyproteins (pp1a and pp1ab) that are cleaved by virus-encoded proteases (Mpro and PLpro) to 16 constituent nsps.17 The viral RTC is formed from nsp2–1618 and plays two crucial functions: (i) transcription of subsets of the viral genome to produce mRNAs that are translated into accessory and structural proteins and (ii) replication of the full-length +ssRNA genome. The viral proteins are then assembled into a virion consisting of the spike (S), envelope (E), membrane (M), and nucleocapsid (N) proteins, with the latter encapsidating the +ssRNA.18 Multiple virions leave from a single infected cell.
Figure 1.
Importance of RTC in the SARS-CoV-2 life cycle. (A) SARS-CoV-2 enters cells by the binding of its surface spike (S) protein to human ACE2 and possibly other cell receptors. Once inside the cell, the 5′ end of its genomic RNA is translated by host ribosomes to yield viral nsps. Nsp7–8, nsp10, nsp12–14, and nsp16 form the core of the viral RTC, which drives the synthesis of full-length and subsets of the genomic ssRNA. (B) Capping of newly synthesized viral mRNA is initiated by nsp13 catalyzing the hydrolysis of the 5′-γ-phosphate of the nascent pppN-RNA to ppN-RNA. A yet to be identified GTase transfers a GMP (Gp) to ppN-RNA, yielding GpppN-RNA. Subsequently, the nsp14 N7-MTase methylates the guanosine N7, forming m7GpppN-RNA via the demethylating coenzyme S-adenosyl methionine (SAM) to S-adenosyl homocysteine (SAH). Finally, the nsp16 2′-O-MTase demethylates SAM to SAH and adds the methyl group to the ribose 2′-O, yielding m7GpppNm-RNA. nsp10 is needed to activate the enzymatic activities of nsp14 N7-MTase and nsp16 2′-O-MTase.
SARS-CoV-2 replication involves RNA synthesis, proofreading, and modification/capping. Viral RNA synthesis is catalyzed by the C-terminal RdRp domain of nsp12 with the help of nsp7 and nsp8 cofactors.8,19,20 RNA proofreading, the maintenance of the SARS-CoV-2 genome integrity, is carried out by the N-terminal 3′–5′ exoribonuclease (ExoN) domain of nsp14, which recognizes erroneous nucleotides and catalyzes their excision.21 The nsp14 ExoN is stabilized by the nsp10 zinc-finger protein, which enhances its nucleolytic activity.22 Viral RNA capping, the addition of a cap structure to the newly synthesized viral mRNAs, ensures their efficient translation by host cell ribosomes and evasion of the host immune response. Without the cap structure, the viral RNA molecules are degraded and may be detected as “non-self” by the host, triggering innate immune responses.17 SARS-CoV-2 mRNA cap synthesis involves nsp10 and four enzymes, viz., (i) nsp13 helicasetriphosphatase, (ii) an unknown guanylyltransferase (GTase), (iii) nsp14 C-terminal (N7 guanine)-methyltransferase (N7-MTase), and (iv) nsp16 2′-O-methyltransferase (2′-O-MTase). nsp10 serves as an allosteric activator of the two methyltransferase enzymes and stabilizes the conserved domains involved in fidelity control (nsp14) and mRNA capping (nsp13–16).17,21Figure 1B summarizes the reactions involved in capping newly synthesized viral mRNA (pppN-RNA).
The viral proteins responsible for RNA synthesis, proofreading, and capping (nsp7, -8, -10, -12–14, and -16) form the RTC core. The following mechanism to produce stable viral RNA has been proposed:20,21 First, a RNA template is unwound by nsp13 helicase and subsequently translocated to the nsp12 active site where a nascent ssRNA is synthesized.20 The neighboring nsp14 excises any erroneous nucleotides from the nascent pppN-RNA, which is dephosphorylated by nsp13. An unknown GTase, nsp14, and nsp16 cap the viral ppN-RNA yielding m7GpppNm-RNA. nsp10, an allosteric activator of nsp14 and nsp 16, acts as a molecular connector between proofreading and capping activities.21 Because the RTC core is indispensable for SARS-CoV-2 replication, using clinically safe drugs to target its constituent proteins would reduce viral load.
Interestingly, the nsp3 PLpro domain, nsp10 zinc-finger, nsp12, nsp13, and nsp14 possess several Zn2+ sites that are conserved among β-coronaviruses.23 The Zn2+ sites in these nsps serve an essential structural role19,23−26 as well as a vital catalytic role for nsp13 helicase activity26 and nsp14 ExoN exoribonuclease activity.24 Among the multiple Zn2+ sites in SARS-CoV-2, the structural Zn2+ sites in nsp3 PLpro and nsp10 have been found to be labile,23 i.e., Zn2+ ions that contribute to structural stability are released upon reaction of the Zn2+-bound Cys thiolates with Zn2+-ejecting agents.27−30 However, it is not known whether the Zn2+ sites in nsp13 and nsp14, which constitute the RTC, are also labile and can be targeted by clinically safe Zn2+-ejector drugs such as disulfiram, an approved antialcoholic drug, and ebselen, in phase III clinical trials for hearing loss. To address this possibility, we have overexpressed SARS-CoV-2 nsp13 and nsp14 proteins to see if their Zn2+ ions could be ejected by Zn2+-ejecting agents and if clinically safe disulfiram and ebselen can inhibit their enzymatic activities. On the basis of the finding herein that the Zn2+-ejector drugs could indeed inhibit the enzymatic activities of SARS-CoV-2 nsp13 and nsp14, we further assessed if disulfiram/ebselen combined with remdesivir, which stops RNA synthesis, could synergistically inhibit SARS-CoV-2 replication.
Results
Zn2+ Ions Are Released from SARS-CoV-2 nsp13 and nsp14 by Zn2+-Ejecting Agents
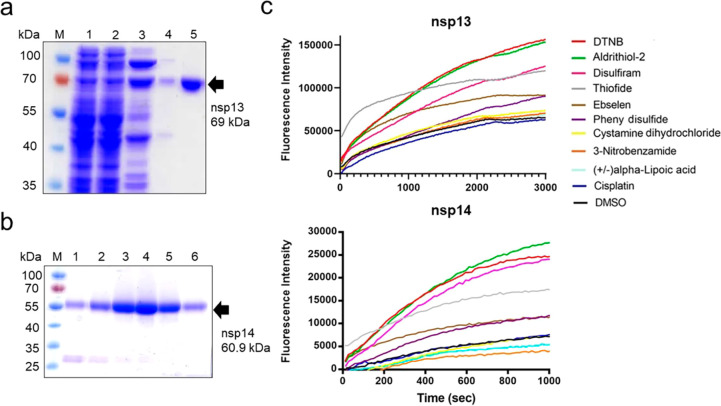
His-tagged full-length SARS-CoV-2 nsp13 and nsp14 were expressed in Escherichia coli, and the recombinant proteins were purified by chromatographic methods to a high homogeneity (Figure 2a,b). After incubating SARS-CoV-2 nsp13 and nsp14 with the Zn2+-specific fluorophore FluoZin-3 (1 μM), a Zn2+-ejecting compound (5 μM) was added. Ten Zn2+-ejecting compounds were tested: 2,2′-dithiobisbenzothiazole (also known as thiofide), cystamine dihydrochloride, 5,5′-dithiobis2-nitrobenzoic acid (DTNB), phenyl disulfide, 3-nitrobenzamide, tetraethylthiuram disulfide (disulfiram), 2,2′-dithiodipyridine (aldrithiol-2), (±)alpha-lipoic acid, ebselen, and cisplatin. Release of Zn2+ ions was monitored by the increase of the fluorescence signal from FluoZin-3 (Figure 2c). Five of the Zn2+-ejecting compounds (DTNB, aldrithiol-2, disulfiram, thiofide, and ebselen) effectively ejected Zn2+ ions from the SARS-CoV-2 nsp13 and nsp14 proteins.
Figure 2.
Zn2+ ions are released from SARS-CoV-2 nsp13 and nsp14 by Zn2+-ejecting compounds. (a) His-tagged nsp13 was purified to high homogeneity, as shown by SDS-PAGE. The final purified nsp13 (in lane 5) was used for Zn2+-ejecting and enzymatic assays. The protein marker (M), cell extract (lane 1), the flow through from the HisTrap FF column at different imidazole concentrations (lanes 2 and 3), and the flow through from the Hitrap SP HP column (in lanes 4) are shown, respectively. (b) Purified His-tagged nsp14 (lanes 3–6) had high homogeneity, as shown by SDS-PAGE. (c) nsp13 (5 μM) and nsp14 (5 μM) were each incubated with FluoZin-3 and one of the Zn2+-ejecting compounds (5 μM). The released Zn2+ ions were detected by the increase in the fluorescence signal from FluoZin-3 with excitation and emission wavelengths of 494 and 516 nm, respectively.
Disulfiram and Ebselen Inhibit SARS-CoV-2 nsp13 ATPase Activity
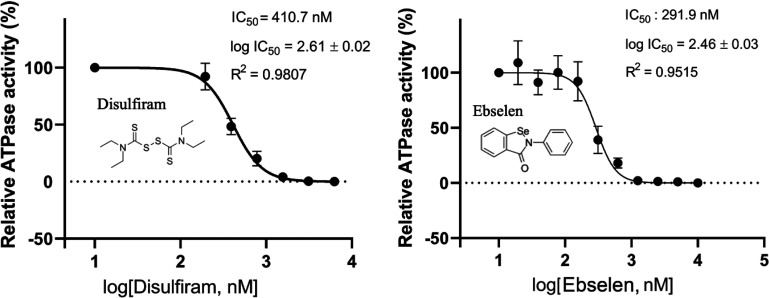
SARS-CoV-2 nsp13 consists of an N-terminal Zn2+-binding domain that is connected to an inserted domain (1B) by a stalk region and two RecA ATPase domains. These domains work together to complete the helicase unwinding function.26,31 The Zn2+-binding domain is not directly involved in unwinding double-stranded DNA/RNA, but nevertheless, it is critical for nsp13 helicase activity.32 Can the release of Zn2+ ions from SARS-CoV-2 nsp13 affect its helicase activity? To address this, we exploited the following: SARS-CoV-2 nsp13 is an NTP-dependent helicase, and its unwinding activity/helicase activity depends on ATPase activity.26 Hence, we measured the ATPase activity of SARS-CoV-2 nsp13 by the molybdenum blue method33 in the presence and absence of disulfiram/ebselen to examine if these Zn2+-ejecting compounds can inhibit nsp13 enzymatic activity. The relative ATPase activities of nsp13 were estimated from the concentration of phosphate ions produced during ATP hydrolysis in the presence of single-stranded DNA and disulfiram (0.2–12.5 μM) or ebselen (0.2–10.0 μM) (Figure 3). The Zn2+-ejecting assays in Figure 2c suggest that Zn2+ ions can be ejected comparably by disulfiram and ebselen after 10 min incubation with nsp13. We thus incubated nsp13 with disulfiram/ebselen for 10 min and then measured nsp13 ATPase activity. On the basis of the measurements, disulfiram and ebselen inhibited the ATPase activity of nsp13 with comparable log IC50 values of 2.6 and 2.5 nM, respectively. The corresponding IC50 values indicate that ebselen (IC50 = 291 nM) is slightly more potent than disulfiram (IC50 = 410.7 nM), consistent with its slightly more efficient Zn2+ release from nsp 13 in the reaction time of 10 min (600 s, Figure 2c).
Figure 3.
Inhibition of the ATPase activity of SARS-CoV-2 nsp13 by disufiram and ebselen. The ATPase activity of SARS-CoV-2 nsp13 (0.25 μM) was estimated by measurement of the phosphate ion concentrations using the molybdenum blue method in the presence of 0.25 μM ssDNA and different concentrations of disulfiram (0.2–12.5 μM) or ebselen (0.02–10.0 μM). The half-maximal inhibitory activity (IC50) of disufiram and ebselen in inhibiting the ATPase activity of SARS-CoV-2 nsp13 is shown. Error bars shown in the two panels represent the standard errors from three replicates of the experiment.
Disulfiram and Ebselen Inhibit SARS-CoV-2 nsp14 Exoribonuclease Activity
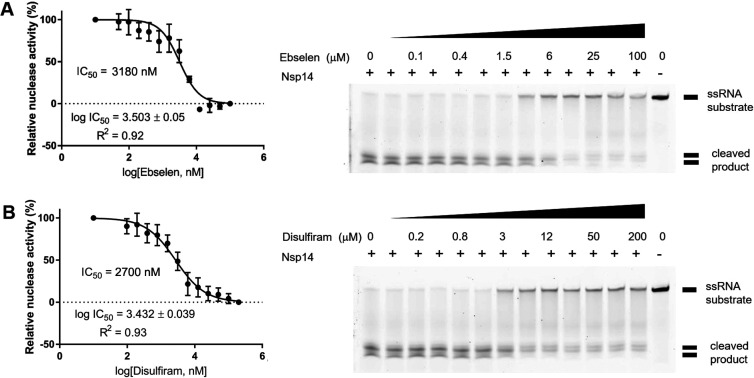
SARS-CoV nsp14 has two zinc-finger motifs (Zf1 and Zf2) in the ExoN domain responsible for proofreading and one zinc-finger motif (Zf3) in the N7-MTase domain involved in mRNA capping.24 Truncating the C-terminal region of Zf3 disrupted local hydrophobic interactions, causing a decrease in the N7-MTase activity.24 To see if release of Zn2+ cations from SARS-CoV-2 nsp14 could inhibit its exoribonuclease function, we measured the 3′–5′ exoribonuclease activity of nsp14 in cleaving a 5′-end-fluorophore-labeled ssRNA (5′-FAM-C7U30-3′) in the presence and absence of the Zn2+-ejecting drugs. The 37-nucleotide ssRNA was degraded by nsp14 into small fragments. However, upon addition of ebselen (from 0 to 100 μM) or disulfiram (from 0 to 200 μM), the amount of cleaved RNA products was gradually reduced (Figure 4). The relative (%) nuclease activity with respect to different concentrations of a given Zn2+ ejector was calculated based on the band intensity of the cleaved products. This gave an estimated IC50 of 3.18 μM for ebselen and 2.70 μM for disulfiram in inhibiting SARS-CoV-2 nsp14 exoribonuclease activity.
Figure 4.
Inhibition of the exoribonuclease activity of SARS-CoV-2 nsp14 by ebselen and disulfiram. (A) SARS-CoV-2 nsp14 degraded the FAM-labeled 37-nucleotide ssRNA (5′-FAM-C7U30-3′) into small fragments as revealed in the TBE gel (right panel). Increased concentrations of ebselen (0–100 μM) gradually inhibited nsp14 exoribonuclease activity with an estimated IC50 of 3.18 μM (left panel). (B) Disulfiram inhibited the exoribonuclease activity of nsp14 with an estimated IC50 of 2.70 μM. In the two gels, the relative nuclease activity (%) was estimated based on the band intensity of the cleaved products, normalized to that in the first lane (no drug, 100% activity) and that in the second to last lane corresponding to 0% activity with 100 μM ebselen or 200 μM disulfiram. Error bars shown in the two left panels represent the standard errors from three replicates of the experiment.
Disulfiram/Ebselen and Remdesivir Exhibit Synergistic Antiviral Activity
Remdesivir, an adenosine analog RdRp inhibitor, has gained emergency FDA approval to treat acute COVID-19 patients.9,34 It outcompetes the natural ATP substrate for binding to nsp12 RdRp, and upon RNA chain elongation, its cyano (CN) group has steric clashes with RdRp, inducing delayed chain termination.34−36 Although remdesivir is better than other nucleotide analogs in avoiding removal by the proofreading nsp14 ExoN, it may still be removed by nsp14, as it is more effective in a model β-coronavirus (murine hepatis virus) lacking the proofreading ExoN activity compared to that of the wild-type virus, as well as in viral mutants with reduced exonuclease activity:37,38 Indeed, remdesivir has shown only moderate success in clinical studies: The ACTT-1 trial39 reported that remdesivir moderately reduced time to recovery, but the WHO SOLIDARITY trial did not.40 Mortality results from four trials of remdesivir suggested some benefit in only low-risk patients.40
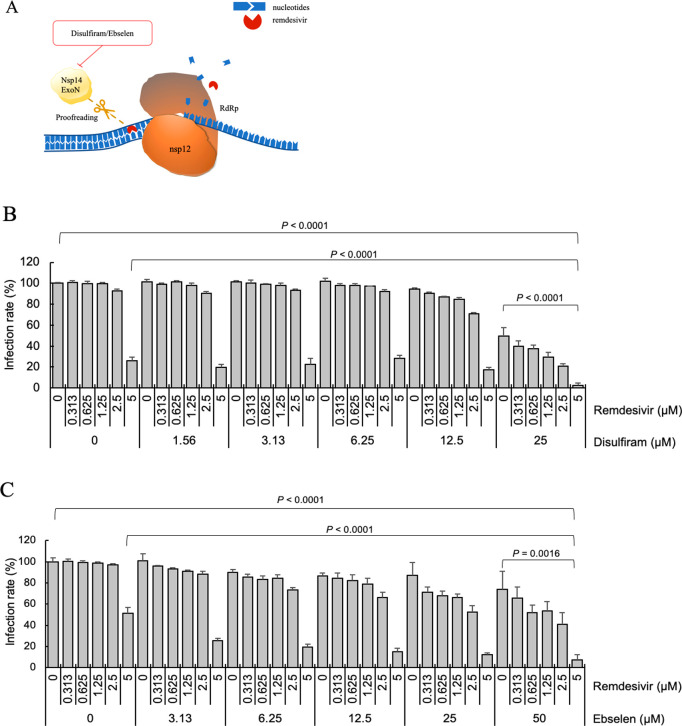
We hypothesize that inhibition of SARS-CoV-2 proofreading activity by disulfiram/ebselen may allow remdesivir to escape nsp14 ExoN-mediated removal; thus, combining remdesivir with disulfiram/ebselen may help to improve inhibition of viral RNA synthesis (Figure 5A).41 To see if remdesivir combined with disulfiram/ebselen could exhibit synergistic inhibition of SARS-CoV-2 replication, Vero E6 cells were treated with a given concentration of remdesivir and/or disulfiram/ebselen (Figures S1A and S2A). Figure 5B,C shows the SARS-CoV-2 infection rate as the mean and corresponding standard deviation of four replicates. Disulfiram/ebselen combined with remdesivir exhibited enhanced antiviral effect compared to each drug alone with p values < 0.05.
Figure 5.
Antiviral potential of disulfiram/ebselen and remdesivir. (A) The active form of remdesivir inhibits nsp12 RdRp by competing with the natural ATP substrate. Disulfiram/ebselen can inhibit nsp14 ExoN proofreading activity, as shown above. The SARS-CoV-2 infection rates in Vero E6 cells treated with remdesivir and (B) disulfiram or (C) ebselen were determined and are shown as means and standard deviations (n = 4). The infection rate of no-compound treatment was set as 100%. The p values were calculated by t-test.
To quantify the synergistic antiviral effect, the synergy score between two drugs was calculated using the SynergyFinder.42 As there are different reference models for quantifying degrees of synergy and their synergy scores may sometimes disagree, we used several well-known models that are based on different assumptions (dose–response-independent scores, HSA and Bliss, and dose–response-dependent scores, ZIP).43 All models gave consistent results; hence, we report the ZIP score below. To make our prediction robust, we consider interaction between two drugs to be synergetic if the average ZIP score is >10, i.e., >10% of response beyond expectation, antagonistic if it is < −10, and additive if it is between −10 and 10. The ZIP scores between disulfiram and remdesivir are ≥0 for all concentrations with a mean of 11.4 for the most synergistic area and a maximum of 23.7, indicating synergistic interaction (Figure S1B). The ZIP scores between ebselen and remdesivir are also ≥0 for all concentrations; its average value for the most synergistic area is 24.6 with a maximum of 32.7, indicating synergistic interaction (Figure S2B). The results verify that remdesivir combined with disulfiram/ebselen show synergistic SARS-CoV-2 inhibition, and the synergy is greater when doses of the drug pair are increased.
Discussion
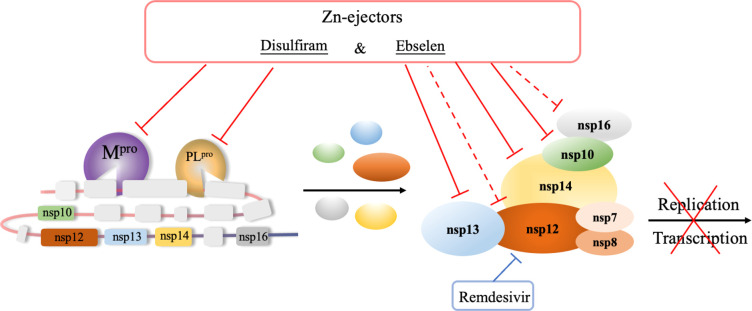
The RTC core, which plays critical roles in SARS-CoV-2 replication as well as evasion of nucleoside analog drugs and innate immune responses, is evidently an important drug target. To date, drugs have been developed/repurposed to target a specific component of this complex such as nsp12 RdRp.3,44 In sharp contrast, we employ clinically safe Zn2+-ejector drugs to target multiple conserved sites in the RTC core. We show that disulfiram/ebselen can target conserved Zn2+ sites in nsp13 and nsp14, and their combination with remdesivir can synergistically inhibit SARS-CoV-2 replication in Vero E6 cells. We propose the following mechanism for the observed effects: By ejecting Zn2+ from the nsp13 Zn2+-binding domain, which is crucial for helicase and triphosphatase activities and interactions with nsp12,26,31 disulfiram/ebselen not only inhibits nsp13 enzymatic activities but also may disrupt nsp12—nsp13 interactions and affect nsp12 RdRp-catalyzed synthesis of viral RNA. It may also affect virus–host interactions since many human proteins involved in organization of the centrosome and Golgi, transcriptional regulation, protein kinase A and immune signaling, and vesicle trafficking have been identified as interacting with nsp13 using affinity purification of 26 SARS-CoV-2 proteins followed by mass spectrometry.45 Notably, nsp13 targets innate immune signaling proteins along the interferon and nuclear factor-κB (NF-κB) pathways.45,46 By ejecting “structural” Zn2+ ions from the nsp14 ExoN domain, disulfiram/ebselen can inhibit SARS-CoV-2 nsp14 exoribonuclease activity. This would mitigate the efficacy of nsp14 to excise erroneous nucleotides and allow nucleotide/nucleoside analog drugs such as remdesivir to inhibit viral RNA synthesis. In addition to nsp13 and nsp14, disulfiram/ebselen can also eject “structural” Zn2+ from the conserved nsp10 zinc finger domain,23 which would destabilize the nsp10–nsp14–nsp16 complex that is essential for mRNA capping (see Figure 1).17,47,48 This would allow the host’s antiviral sensors to detect viral uncapped RNA, which can be degraded, and to prevent the virus from evading host immune responses.
Altogether, this work and previous studies show that disulfiram/ebselen can target multiple conserved sites in the viral RTC that are crucial for SARS-CoV-2 RNA genome replication as well as translation of the viral proteins (Figure 1). It can destabilize nsp10 cofactor,23 a crucial mediator of protein–protein interactions, and inhibit the enzyme activities of nsp3 PLpro,23 nsp5 Mpro,6 nsp13, and nsp14, and probably nsp12 and nsp16 through their interactions with nsp13 and nsp10–nsp14, respectively. Disulfiram/ebselen can target not only the Zn2+-bound cysteines but also catalytic cysteines23 and thereby inhibit SARS-CoV-2 Mpro,6 which does not possess a Zn2+-site. By inhibiting Mpro and PLpro viral proteolysis, disulfiram/ebselen can prevent efficient cleavage of the replicase polyproteins into component nsps. In case the virus produces resistance against these proteases, disulfiram/ebselen can also inhibit the RTC core that is crucial for viral RNA synthesis, proofreading, and capping, thus restoring remdesivir’s ability to function as a delayed chain terminator. As SARS-CoV-2 nsp3, nsp5, nsp13, and nsp14 enzymes target the interferon and/or NF-κB pathway,45,46 their inhibition by disulfiram/ebselen may help to restore innate immune responses.
Unlike most studies that target a specific viral protein such as Mpro5,6 or the spike protein1,2 by developing potent (nanomolar) inhibitors or antibodies, our strategy simultaneously targets multiple conserved viral proteins responsible for viral polypeptide proteolysis as well as viral genome replication and viral protein translation using clinically safe Zn2+-ejector drugs. This has several advantages: Whereas a single viral drug target such as Mpro or the spike protein can undergo mutations to produce resistance against specific drugs, it would be less likely for the multiple conserved viral proteins that are jointly responsible for viral replication and transcription to simultaneously mutate and achieve drug resistance. Mutations of the conserved Zn2+-binding cysteines in nsp3, nsp10, and nsp12–14 that are targeted by disulfiram/ebselen would likely disrupt binding to Zn2+ cations that serve essential structural and/or catalytic roles in several SARS-CoV-2 proteins. Furthermore, disulfiram/ebselen targets the “upstream” part of the SARS-CoV-2 life cycle (Figure 1) before multiple replicated viruses leave the infected cell. Importantly, disulfiram is inexpensive, widely available, and easily administered, and may allow other broad-spectrum antivirals to synergistically inhibit a new virus, as shown herein. A third advantage is that our strategy is not specific to a particular SARS-CoV-2 variant or any type of coronavirus. This is because the proteins essential for RNA replication such as the RTC core are the most conserved among coronaviruses. Indeed, analysis of the SARS-CoV-2 gene sequences show the least divergence in the gene encoding these conserved domains (https://nextstrain.org/ncov/global). Thus, our approach may prove useful not only for the current COVID-19 pandemic but also in future coronavirus outbreaks where it may be deployed as a first line of defense in the absence of monoclonal antibodies and vaccines.
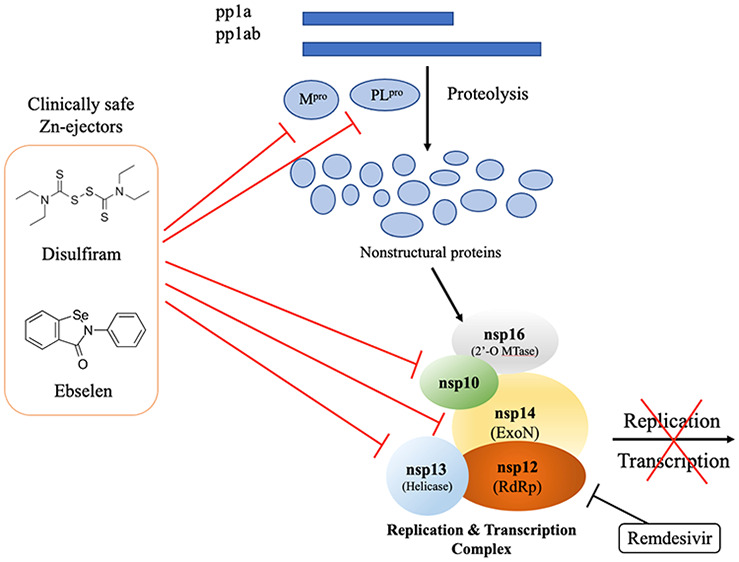
In summary, we show that clinically safe Zn2+-ejector drugs (disulfiram/ebselen) can target conserved functionally important Zn2+ sites in the multiprotein RTC and, when combined with remdesivir, that these can synergistically inhibit SARS-CoV-2 replication in Vero E6 cells (Figure 6).
Figure 6.
Disulfiram and ebselen can target multiple SARS-CoV-2 nsps to block the viral polyprotein cleavage and RNA replication/transcription. These Zn2+-ejector drugs target PLpro of nsp3 and Mpro of nsp5 to inhibit viral polypeptide proteolysis. They also directly target nsp10, nsp13, and nsp14 as well as indirectly target nsp12 and nsp16 to inhibit viral RNA genome replication and translation of the structural and accessory viral proteins. They can act in concert with remdesivir to synergistically inhibit SARS-CoV-2 replication.
Experimental Section
Protein Expression and Purification
The cDNA of SARS-CoV-2 nsp13 (residues 5302–5902 of pp1a/pp1ab) and nsp14 (residues 5903–6429 pp1a/pp1ab) were synthesized from Protech, Inc. (Taiwan) and cloned into pET28a and pRSFDuet-1 vectors, respectively, for expression of the N-terminal His-tagged nsp13 and the C-terminal His-tagged nsp14. Both plasmids were transformed into E. coli BL21 (DE3) pLysS strain cultured in LB medium supplemented with 50 μg/mL kanamycin. Cells were grown to an optical density of 0.6 measured at a wavelength of 600 nm and induced by 0.2 mM IPTG at 18 °C for 14–16 h to express His-tagged SARS-CoV-2 nsp13 and nsp14.
The harvested cells expressing nsp13 were disrupted by a microfluidizer (Microfluidics M-110P) in lysis buffer containing 50 mM Tris-HCl (pH 8.0), 500 mM NaCl, protease inhibitor, 5% glycerol, and 10 mM β-mercaptoethanol. The supernatant was run through a HisTrap FF column (5 mL, GE HealthCare) and washed with a washing buffer containing 50 mM Tris-HCl (pH 8.0), 200 mM NaCl, 5% glycerol, and 30 mM imidazole. The eluted nsp13 was further purified by a HiTrap SP HP column (5 mL, GE HealthCare) to produce a homogeneous nsp13 protein sample.
For the purification of nsp14, the harvested cells were resuspended in lysis buffer (20 mM HEPES (pH 7), 150 mM NaCl, 4 mM MgCl2, 5% glycerol, and 10 mM β-mercaptoethanol). After homogenization, the supernatant was loaded into a HisTrap FF column (5 mL, GE HealthCare) and washed with a washing buffer containing 20 mM HEPES (pH 7.0), 150 mM NaCl, 4 mM MgCl2, 5% glycerol, 10 mM β-mercaptoethanol, and 40 mM imidazole. The protein sample was collected and further loaded into a gel filtration column (HiLoad 16/60 Superdex 200, GE HealthCare), then eluted by a buffer of 50 mM HEPES (pH 7.4), 50 mM NaCl, 5 mM MgCl2, and 5% glycerol.
Zn2+-Ejection Assays
Zn2+-ejecting agents were purchased from Sigma-Aldrich (USA), including 2,2′-dithiobis(benzothiazole), cystamine dihydrochloride, 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), phenyl disulfide, 3-nitrobenzamide, tetraethylthiuram disulfide (disulfiram), 2,2′-dithiodipyridine, (±)alpha-lipoic acid, ebselen, and cisplatin. The Zn2+-specific fluorophore FluoZinTM-3 (Invitrogen/Life Technologies) was used to monitor the release of Zn2+ ions from SARS-CoV-2 nsp13 and nsp14. The Zn2+-ejecting agents were dissolved in DMSO to a stock solution of 100 μM. SARS-CoV-2 His-tagged nsp13 (5 μM) and nsp14 (5 μM) were respectively mixed with each Zn2+-ejecting agent (5 μM) and FluoZin-3 (1 μM) in a total reaction volume of 200 μL at room temperature. Fluorescence emission was then measured by EnSpire Multilabel Plate Reader (PerkinElmer, USA) at an excitation wavelength of 494 nm and emission wavelength of 516 nm.
Inhibition of the nsp13 ATPase Activity by Zn2+ Ejector Drugs
ATPase activity of SARS-CoV-2 nsp13 was analyzed by the molybdenum blue method33 to measure the phosphate ion concentration in ATP hydrolysis reaction in the presence of the Zn2+ ejector drugs disulfram and ebselen. nsp13 (2.5 μM, 10 μL) was mixed with or without disulfiram (2–125 μM, 10 μL) or ebselen (0.2–100 μM, 10 μL) in 60 μL of the reaction buffer containing 33 mM Tris-HCl (pH 8.0) and 5 mM MgCl2 at room temperature for 10 min. ATP (50 mM, 10 μL) and ssDNA (2.5 μM, 10 μL) with a sequence of 5′-(dT)24-3′ were then added into the nsp13 solution and incubated for 10 min. The ATPase reaction was stopped by adding 100 μL of 10% SDS, and the blue complexes between inorganic phosphate and molybdate were developed by adding 100 μL of 1.25% ammonium molydbate in 6.5% sulfuric solution and 100 μL of freshly prepared 10% (w/v) ascorbic acid. A total of 400 μL of reaction solution was incubated at 25 °C for 10 min and then read at 660 nm by Enspire plate reader (PerkinElmer). Various concentrations of Na2HPO4 ranging from 0.05 to 0.5 mM were also prepared as a standard curve. The relative ATPase activity of SARS-CoV-2 nsp13 in the presence of each Zn2+ ejector was compared to that without adding Zn2+ ejector, and the half-maximal inhibitory activity (IC50) of each inhibitor was calculated by GraphPad Prism.
Inhibition of the nsp14 Exoribonuclease Activity by Zn2+ Ejector Drugs
nsp14 (50 μM, 100 μL) was mixed with 4 μL of 5′-end FAM-labeled ssRNA (1 μM, 5′-FAM-C7U30-3′) in 200 μL of the reaction buffer (100 mM HEPES (pH 7.4), 100 mM NaCl, 10 mM MgCl2 and 10% glycerol), and 100 μL of ddH2O. Disulfiram (1 μL, 0–200 μM) or ebselen (1 μL, 0–100 μM) in different concentrations was then added to the nsp14 and RNA mixture (19 μL), and incubated for 30 min at 37 °C. The RNA loading dye (20 μL, 89 mM Tris-HCl, pH 8, 89 mM boric acid, 2 mM EDTA, 0.01% bromophenol blue, 0.02% xylene cyanol FF, and 7 M urea) was then added into the mixture, and the sample was heated at 95 °C for 5 min to stop the reaction. The RNA in the mixtures were separated on TBE gel and visualized by Biomolecular Imager (Amersham Typhoon, GE HealthCare). The resolved RNA bands in the gel were analyzed by ImageJ, and the IC50 of each inhibitor was calculated by GraphPad Prism.
Cell-Based Assays
Remdesivir was produced following the previous protocol.49 Vero E6 cells were pretreated with the disulfiram/ebselen and/or remdesivir at various concentrations for 1 h at 37 °C and then adsorbed with SARS-CoV-2 TCDC#4 (hCoV-19/Taiwan/4/2020) at MOI 0.005 (100 PFU/well) for 1 h at 37 °C. After virus adsorption, the cells were replenished with fresh medium and compounds at the indicated concentrations for 1 day of incubation. The cells were fixed with 10% formaldehyde, permeabilized with 0.5% Triton X-100, and stained with anti-SARS-CoV-2 N protein antibody (provided by Dr. An-Suei Yang, Genomic Research Center, Academia Sinica, Taiwan) and anti-human IgG-488. The N protein expression was measured using a high-content image analysis system (Molecular Devices), and the average infection rate of no-drug treatment was set as 100%. The synergy score is calculated using the SynergyFinder to quantify the synergistic antiviral effect between disulfiram/ebselen and remdesivir.
Acknowledgments
The cell-based experiments were performed by Dr. Jian-Jong Liang (jjliang1234@yahoo.com.tw) and Dr. Chun-Che Liao (jfliao@ibms.sinica.edu.tw) in Dr. Yi-Ling Lin’s laboratory at the Institute of Biomedical Sciences (IBMS), Academia Sinica, Taipei, Taiwan. We thank Taiwan CDC for providing SARS-CoV-2 TCDC#4 (hCoV-19/Taiwan/4/2020) and funding support from Academia Sinica for IBMS P3 facility (AS-CFII-108-102) and the Ministry of Science and Technology, Taiwan for COVID-19 study (MOST 109-3114-Y-001-001). We thank Dr. Karine Mazmanian for help with the figures. We acknowledge the DNA Sequencing Core Facility (IBMS, AS-CFII-108-115) of the Scientific Instrument Center in Academia Sinica for DNA sequencing. This work was supported by the Ministry of Science & Technology (MOST-107-2113-M-001-018 to C.L.) and Academia Sinica (AS-IA-107-L03 to C. L. and AS-IA-110-L02 to H.S.Y.) Taiwan.
Supporting Information Available
The Supporting Information is available free of charge at https://pubs.acs.org/doi/10.1021/acsptsci.1c00022.
Synergistic antiviral potential of disulfiram with remdesivir, and synergistic antiviral potential of ebselen with remdesivir (PDF)
The authors declare no competing financial interest.
This article is made available via the ACS COVID-19 subset for unrestricted RESEARCH re-use and analyses in any form or by any means with acknowledgement of the original source. These permissions are granted for the duration of the World Health Organization (WHO) declaration of COVID-19 as a global pandemic.
Supplementary Material
References
- Kim P. S.; Read S. W.; Fauci A. S. (2020) Therapy for early COVID-19: A critical need. JAMA 324, 2149–2150. 10.1001/jama.2020.22813. [DOI] [PubMed] [Google Scholar]
- Chen P.; Nirula A.; Heller B.; Gottlieb R. L.; Boscia J.; Morris J.; Huhn G.; Cardona J.; Mocherla B.; Stosor V.; et al. (2021) SARS-CoV-2 neutralizing antibody LY-CoV555 in outpatients with Covid-19. N. Engl. J. Med. 384, 229–237. 10.1056/NEJMoa2029849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q. A.; Kato-Weinstein J.; Li Y.; Deng Y.; Granet R.; Garner L.; Liu C.; Polshakov D.; Gessner C.; Watkins S. (2020) Potential therapeutic agents and associated bioassay data for COVID-19 and related human coronavirus infections. ACS Pharmacol. Transl. Sci. 3, 813–834. 10.1021/acsptsci.0c00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wrapp D.; Wang N.; Corbett K. S.; Goldsmith J. A.; Hsieh C.-L.; Abiona O.; Graham B. S.; McLellan J. S. (2020) Cryo-EM structure of the 2019-nCoV spike in the prefusion conformation. Science 367, 1260–1263. 10.1126/science.abb2507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Lin D.; Sun X.; Curth U.; Drosten C.; Sauerhering L.; Becker S.; Rox K.; Hilgenfeld R. (2020) Crystal structure of SARS-CoV-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 368, 409–412. 10.1126/science.abb3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Z.; Du X.; Xu Y.; Deng Y.; Liu M.; Zhao Y.; Zhang B.; Li X.; Zhang L.; Peng C.; Duan Y.; Yu J.; Wang L.; Yang K.; Liu F.; Jiang R.; Yang X.; You T.; Liu X.; Yang X.; Bai F.; Liu H.; Liu X.; Guddat L. W.; Xu W.; Xiao G.; Qin C.; Shi Z.; Jiang H.; Rao Z.; Yang H. (2020) Structure of Mpro from COVID-19 virus and discovery of its inhibitors. Nature 582, 289–293. 10.1038/s41586-020-2223-y. [DOI] [PubMed] [Google Scholar]
- Maiti B. K. (2020) Can papain-like protease inhibitors halt SARS-CoV-2 replication?. ACS Pharmacol. Transl. Sci. 3, 1017–1019. 10.1021/acsptsci.0c00093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y.; Yan L.; Huang Y.; Liu F.; Zhao Y.; Cao L.; Wang T.; Sun Q.; Ming Z.; Zhang L.; Ge J.; Zheng L.; Zhang Y.; Wang H.; Zhu Y.; Zhu C.; Hu T.; Hua T.; Zhang B.; Yang X.; Li J.; Yang H.; Liu Z.; Xu W.; Guddat L. W.; Wang Q.; Lou Z.; Rao Z. (2020) Structure of the RNA-dependent RNA polymerase from COVID-19 virus. Science 368, 779–782. 10.1126/science.abb7498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin W.; Mao C.; Luan X.; Shen D.-D.; Shen Q.; Su H.; Wang X.; Zhou F.; Zhao W.; Gao M.; et al. (2020) Structural basis for inhibition of the RNA-dependent RNA polymerase from SARS-CoV-2 by remdesivir. Science 368, 1499–1504. 10.1126/science.abc1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starr T. N.; Greaney A. J.; Addetia A.; Hannon W. W.; Choudhary M. C.; Dingens A. S.; Li J. Z.; Bloom J. D. (2021) Prospective mapping of viral mutations that escape antibodies used to treat COVID-19. Science 371, 850–854. 10.1126/science.abf9302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy K. R.; Rennick L. J.; Nambulli S.; Robinson-McCarthy L. R.; Bain W. G.; Haidar G.; Duprex W. P. (2021) Recurrent deletions in the SARS-CoV-2 spike glycoprotein drive antibody escape. Science 371, 1139. 10.1126/science.abf6950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson N. J.; Lehner P. J. (2020) How does SARS-CoV-2 cause COVID-19?. Science 369, 510–511. 10.1126/science.abc6156. [DOI] [PubMed] [Google Scholar]
- (2020) The species Severe acute respiratory syndrome-related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 5, 536–544. 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K.; Chen W.; Zhang Z.; Deng Y.; Lian J.-Q.; Du P.; Wei D.; Zhang Y.; Sun X.-X.; Gong L.; et al. (2020) CD147-spike protein is a novel route for SARS-CoV-2 infection to host cells. Signal Transduct. Target. Ther. 5, 283. 10.1038/s41392-020-00426-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letko M.; Marzi A.; Munster V. (2020) Functional assessment of cell entry and receptor usage for SARS-CoV-2 and other lineage B betacoronaviruses. Nat. Microbiol. 5, 562–569. 10.1038/s41564-020-0688-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffmann M.; Kleine-Weber H.; Schroeder S.; Krüger N.; Herrler T.; Erichsen S.; Schiergens T. S.; Herrler G.; Wu N.-H.; Nitsche A.; et al. (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181, 271–280. 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder E. J.; Decroly E.; Ziebuhr J. (2016) The nonstructural proteins directing coronavirus RNA synthesis and processing. Adv. Virus Res. 96, 59–126. 10.1016/bs.aivir.2016.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- V’kovski P.; Kratzel A.; Steiner S.; Stalder H.; Thiel V. (2021) Coronavirus biology and replication: implications for SARS-CoV-2. Nat. Rev. Microbiol. 19, 155–170. 10.1038/s41579-020-00468-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirchdoerfer R. N.; Ward A. B. (2019) Structure of the SARS-CoV nsp12 polymerase bound to nsp7 and nsp8 co-factors. Nat. Commun. 10, 2342. 10.1038/s41467-019-10280-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L.; Zhang Y.; Ge J.; Zheng L.; Gao Y.; Wang T.; Jia Z.; Wang H.; Huang Y.; Li M.; et al. (2020) Architecture of a SARS-CoV-2 mini replication and transcription complex. Nat. Commun. 11, 5874. 10.1038/s41467-020-19770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano M.; Ruggiero A.; Squeglia F.; Maga G.; Berisio R. (2020) A structural view of SARS-CoV-2 RNA replication machinery: RNA synthesis, proofreading and final capping. Cells 9, 1267. 10.3390/cells9051267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouvet M.; Lugari A.; Posthuma C. C.; Zevenhoven J. C.; Bernard S.; Betzi S.; Imbert I.; Canard B.; Guillemot J. C.; Lécine P.; Pfefferle S.; Drosten C.; Snijder E. J.; Decroly E.; Morelli X. (2014) Coronavirus Nsp10, a critical co-factor for activation of multiple replicative enzymes. J. Biol. Chem. 289, 25783–25796. 10.1074/jbc.M114.577353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargsyan K.; Lin C. C.; Chen T.; Grauffel C.; Chen Y. P.; Yang W. Z.; Yuan H. S.; Lim C. (2020) Multi-targeting of functional cysteines in multiple conserved SARS-CoV-2 domains by clinically safe Zn-ejectors. Chem. Sci. 11, 9904–9909. 10.1039/D0SC02646H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y.; Wu L.; Shaw N.; Gao Y.; Wang J.; Sun Y.; Lou Z.; Yan L.; Zhang R.; Rao Z. (2015) Structural basis and functional analysis of the SARS coronavirus nsp14–nsp10 complex. Proc. Natl. Acad. Sci. U. S. A. 112, 9436–9441. 10.1073/pnas.1508686112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao W.; Wojdyla J. A.; Zhao R.; Han R.; Das R.; Zlatev I.; Manoharan M.; Wang M.; Cui S. (2017) Crystal structure of Middle East respiratory syndrome coronavirus helicase. PLoS Pathog. 13, e1006474 10.1371/journal.ppat.1006474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Z.; Yan L.; Ren Z.; Wu L.; Wang J.; Guo J.; Zheng L.; Ming Z.; Zhang L.; Lou Z.; Rao Z. (2019) Delicate structural coordination of the Severe Acute Respiratory Syndrome coronavirus Nsp13 upon ATP hydrolysis. Nucleic Acids Res. 47, 6538–6550. 10.1093/nar/gkz409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang M.; Maynard A.; Turpin J. A.; Graham L.; Janini G. M.; Covell D. G.; Rice W. G. (1998) Anti-HIV agents that selectively target retroviral nucleocapsid protein zinc fingers without affecting cellular zinc finger proteins. J. Med. Chem. 41, 1371–1381. 10.1021/jm9708543. [DOI] [PubMed] [Google Scholar]
- Briknarova K.; Thomas C. J.; York J.; Nunberg J. H. (2011) Structure of a zinc-binding domain in the Junin virus envelope glycoprotein. J. Biol. Chem. 286, 1528–1536. 10.1074/jbc.M110.166025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. M.; Lin Y. F.; Lim C. (2014) Factors controlling the role of Zn and reactivity of Zn-bound cysteines in proteins: Application to drug target discovery. J. Chin. Chem. Soc. 61, 142–150. 10.1002/jccs.201300392. [DOI] [Google Scholar]
- Lee Y. M.; Duh Y.; Wang S. T.; Lai M. M. C.; Yuan H. S.; Lim C. (2016) Using an old drug to target a new drug site: Application of disulfiram to target the Zn-site in HCV NS5A protein. J. Am. Chem. Soc. 138, 3856–3862. 10.1021/jacs.6b00299. [DOI] [PubMed] [Google Scholar]
- Chen J.; Malone B.; Llewellyn E.; Grasso M.; Shelton P. M.; Olinares P. D. B.; Maruthi K.; Eng E. T.; Vatandaslar H.; Chait B. T.; et al. (2020) Structural basis for helicase-polymerase coupling in the SARS-CoV-2 replication-transcription complex. Cell 182, 1560–1573. 10.1016/j.cell.2020.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seybert A.; Posthuma C. C.; Van Dinten L. C.; Snijder E. J.; Gorbalenya A. E.; Ziebuhr J. (2005) A complex zinc finger controls the enzymatic activities of nidovirus helicases. J. Virol. 79, 696–704. 10.1128/JVI.79.2.696-704.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z.; Honeycutt C. W. (2005) A modified molybdenum blue method for orthophosphate determination suitable for investigating enzymatic hydrolysis of organic phosphates. Commun. Soil Sci. Plant Anal. 36, 1373–1383. 10.1081/CSS-200056954. [DOI] [Google Scholar]
- Eastman R. T.; Roth J. S.; Brimacombe K. R.; Simeonov A.; Shen M.; Patnaik S.; Hall M. D. (2020) Remdesivir: A review of its discovery and development leading to emergency use authorization for treatment of COVID-19. ACS Cent. Sci. 6, 672–683. 10.1021/acscentsci.0c00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Badgujar K. C.; Ram A. H.; Zanznay R.; Kadam H.; Badgujar V. C. (2020) Remdesivir for COVID-19: A review of pharmacology, mechanism of action, in-vitro activity and clinical use based on available case studies. J. Drug Delivery Ther. 10, 264–270. 10.22270/jddt.v10i4-s.4313. [DOI] [Google Scholar]
- Gordon C. J.; Tchesnokov E. P.; Woolner E.; Perry J. K.; Feng J. Y.; Porter D. P.; Gotte M. (2020) Remdesivir is a direct-acting antiviral that inhibits RNA-dependent RNA polymerase from severe acute respiratory syndrome coronavirus 2 with high potency. J. Biol. Chem. 295, 6785–6797. 10.1074/jbc.RA120.013679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agostini M. L.; Andres E. L.; Sims A. C.; Graham R. L.; Sheahan T. P.; Lu X.; Smith E. C.; Case J. B.; Feng J. Y.; Jordan R.; et al. (2018) Coronavirus susceptibility to the antiviral remdesivir (GS-5734) is mediated by the viral polymerase and the proofreading exoribonuclease. mBio 9, e00221-18 10.1128/mBio.00221-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo J.; Dangerfield T. L.; Taylor D. W.; Johnson K. A. (2021) Remdesivir is a Delayed Translocation Inhibitor of SARS CoV-2 Replication. Mol. Cell 35. 10.1016/j.molcel.2021.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beigel J. H.; Tomashek K. M.; Dodd L. E.; Mehta A. K.; Zingman B. S.; Kalil A. C.; Hohmann E.; Chu H. Y.; Luetkemeyer A.; Kline S.; et al. (2020) Remdesivir for the treatment of Covid-19—final report. N. Engl. J. Med. 383, 1813–1826. 10.1056/NEJMoa2007764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2020) Repurposed antiviral drugs for COVID-19—interim WHO SOLIDARITY trial results. N. Engl. J. Med. 384, 497–511. 10.1056/NEJMoa2023184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon A.; Le N. T. T.; Selisko B.; Eydoux C.; Alvarez K.; Guillemot J.-C.; Decroly E.; Peersen O.; Ferron F.; Canard B. (2020) Remdesivir and SARS-CoV-2: Structural requirements at both nsp12 RdRp and nsp14 Exonuclease active sites. Antiviral Res. 178, 104793. 10.1016/j.antiviral.2020.104793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ianevski A.; Giri A. K.; Aittokallio T. (2020) SynergyFinder 2.0: visual analytics of multi-drug combination synergies. Nucleic Acids Res. 48, W488–W493. 10.1093/nar/gkaa216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlot A. H.; Aniceto N.; Menden M. P.; Ulrich-Merzenich G.; Bender A. (2019) Applying synergy metrics to combination screening data: agreements, disagreements and pitfalls. Drug Discovery Today 24, 2286–2298. 10.1016/j.drudis.2019.09.002. [DOI] [PubMed] [Google Scholar]
- Li G.; De Clercq E. (2020) Therapeutic options for the 2019 novel coronavirus (2019-nCoV). Nat. Rev. Drug Discovery 19, 149–150. 10.1038/d41573-020-00016-0. [DOI] [PubMed] [Google Scholar]
- Gordon D. E.; Jang G. M.; Bouhaddou M.; Xu J.; Obernier K.; White K. M.; O'Meara M. J.; Rezelj V. V.; Guo J. Z.; Swaney D. L. (2020) A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 583, 459–468. 10.1038/s41586-020-2286-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X.; Dong X.; Ma R.; Wang W.; Xiao X.; Tian Z.; Wang C.; Wang Y.; Li L.; Ren L.; et al. (2020) Activation and evasion of type I interferon responses by SARS-CoV-2. Nat. Commun. 11, 3810. 10.1038/s41467-020-17665-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decroly E.; Debarnot C.; Ferron F.; Bouvet M.; Coutard B.; Imbert I.; Gluais L.; Papageorgiou N.; Sharff A.; Bricogne G.; Ortiz-Lombardia M.; Lescar J.; Canard B. (2011) Crystal structure and functional analysis of the SARS-coronavirus RNA cap 2’-O-methyltransferase nsp10/nsp16 complex. PLoS Pathog. 7, e1002059 10.1371/journal.ppat.1002059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruijssers A. J.; Denison M. R. (2019) Nucleoside analogues for the treatment of coronavirus infections. Curr. Opin. Virol. 35, 57–62. 10.1016/j.coviro.2019.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siegel D.; Hui H. C.; Doerffler E.; Clarke M. O.; Chun K.; Zhang L.; Neville S.; Carra E.; Lew W.; Ross B.; Wang Q.; Wolfe L.; Jordan R.; Soloveva V.; Knox J.; Perry J.; Perron M.; Stray K. M.; Barauskas O.; Feng J. Y.; Xu Y.; Lee G.; Rheingold A. L.; Ray A. S.; Bannister R.; Strickley R.; Swaminathan S.; Lee W. A.; Bavari S.; Cihlar T.; Lo M. K.; Warren T. K.; Mackman R. L. (2017) Discovery and synthesis of a phosphoramidate prodrug of a pyrrolo[2,1-f][triazin-4-amino] adenine C-nucleoside (GS-5734) for the treatment of Ebola and emerging viruses. J. Med. Chem. 60, 1648–1661. 10.1021/acs.jmedchem.6b01594. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.