Abstract
Purpose:
To assess the associations between inter-site texture heterogeneity parameters derived from computed tomography (CT), survival, and BRCA mutation status in women with high-grade serous ovarian cancer (HGSOC).
Material and methods:
retrospective study of 88 HGSOC patients undergoing CT and BRCA mutation status testing prior to primary cytoreductive surgery. Associations between texture metrics – namely inter-site cluster variance (SCV), inter-site cluster prominence (SCP), inter-site cluster entropy (SE) – and overall survival (OS), progression-free survival (PFS) as well as BRCA mutation status were assessed.
Results:
Higher inter-site cluster variance (SCV) was associated with lower PFS (p=0.006) and OS (p=0.003). Higher inter-site cluster prominence (SCP) was associated with lower PFS (p=0.02) and higher inter-site cluster entropy (SE) correlated with lower OS (p=0.01). Higher values of all three metrics were significantly associated with lower complete surgical resection status in BRCA negative patients (SE p=0.039, SCV p=0.006, SCP p=0.02), but not in BRCA positive patients (SE p=0.7, SCV p=0.91, SCP p=0.67). None of the metrics were able to distinguish between BRCA mutation carrier versus non-mutation carrier.
Conclusion:
The assessment of tumoral heterogeneity in the era of personalized medicine is important, as increased heterogeneity has been associated with distinct genomic abnormalities and worse patient outcomes. A radiomics approach using standard-of-care CT scans might have a clinical impact by offering a noninvasive tool to predict outcome and therefore improving treatment effectiveness. However, it was not able to assess BRCA mutation status in women with HGSOC.
Keywords: High grade serous ovarian cancer, tumor heterogeneity, texture analysis, BRCA mutation status, radiomics
INTRODUCTION
Ovarian cancer is the fifth most common cause of cancer deaths among women in the United States, accounting for almost half of all gynecologic cancer deaths. High-grade serous ovarian cancer (HGSOC) is the most common histological subtype and has the least favorable prognosis. The fact that five-year survival rates remain poor and inferior to most other tumor entities emphasizes the importance of developing new stratification approaches to optimize therapeutic strategies and finally outcomes [1,2].
Late stage detection, as is common in ovarian cancer due to the vague, non-specific symptoms, is associated with delay in treatment but also fails to explain the variable clinical outcome. There is increasing evidence hinting that the underlying genetic alteration is relevant for determining the course of disease, e.g. response to treatment, and therefore prognosis. Bolton et al. showed that women with positive BRCA1 or BRCA2 mutation status had a better 5-year overall survival (OS) compared with non-carriers, and BRCA2 was associated with the most favorable prognosis [3]. These findings are in line with another study demonstrating an OS benefit in HGSOC patients who had BRCA2 mutation status compared with patients who had BRCA1 or BRCA negative mutation status [4].
In contrast, another multicenter study did not find a difference in progression-free survival (PFS) between HGSOC patients with germline BRCA mutation and those with BRCA wild-type, despite the fact that the BRCA mutation carriers initially presented more frequently with peritoneal spread, bulky lymph nodes and a high tumor load [5]. Furthermore, this study found that in the BRCA wild-type group, women who received primary debulking surgery had PFS that was 8 months longer than women who received neoadjuvant chemotherapy, but in the BRCA mutation carrier group, there was no significant difference between the treatments [5]. Although these results are interesting especially in the light of higher chemosensitivity in BRCA mutation carriers, they also underpin the importance of BRCA mutation status testing for a more personalized treatment stratification. The presence of tumor heterogeneity has been demonstrated in many different malignancies [6–8] including ovarian cancer and pancreatic cancer [9,10]. Gerlinger et al. was able to reveal branched evolutionary tumor development in metastatic renal cell carcinoma manifesting inter-site tumor heterogeneity, with the clinical implication that single site biopsy underrepresents genetic alterations and is not able to display the complete mutational burden [11]. Therefore, heterogeneity on a genetic level may be responsible for variable treatment responses and overall outcomes, suggesting that standard treatment regiments should be phased out in favor of personalized patient-tailored therapeutic approach based on the individual genetic profiling.
Modern radiomic analyses use computer-vision to extract image features from volumes of interest that are not assessible otherwise [12]. The purpose of this study was to evaluate intra- and inter-tumoral heterogeneity via an advanced computational radiomics method to reveal local heterogeneity within a single lesion and also inter-site heterogeneity between different metastatic lesions in patients with advanced stage HGSOC and to link this information to their genetic profile. We hypothesized that our quantitative assessment of standard-of-care computed tomography (CT) scans will reveal parameters that are able to determine BRCA mutation status and predict outcome.
MATERIALS AND METHODS
This was a retrospective study designed and conducted in compliance with the Health Insurance Portability and Accountability Act. The institutional review board approved the study protocol and waived the need for written informed consent for all patients.
Patients
In this study, we included patients meeting the following criteria: 1. pathologically confirmed HGSOC, 2. primary cytoreductive surgery performed at our institution (between 11/01/2005 and 02/20/2012), 3. genetic counseling and BRCA mutation testing, 4. preoperative CT with intravenous contrast administration, 5. no adjuvant chemotherapy and 6. no concurrent malignancy. One hundred and eight consecutive patients met all our inclusion criteria. All patients were included in a prior study that investigated the associations between qualitative CT imaging features and BRCA mutation status in patients with HGSOC [13]. In twenty patients of this initial population texture analysis was not feasible for the following reasons: (i) technical reasons, where the image data from outside patients could not be converted to the required format for the texture analysis program, (ii) patients with metal artifacts in the pelvic region from hip prostheses that would have influenced texture analysis and (iii) scans that did not cover the upper abdomen. In total, 88 patients were included in the final study population and texture analysis was performed.
Cytoreductive surgery was performed in compliance with our institutional guidelines, including total abdominal hysterectomy, bilateral salpingo-oophorectomy, omentectomy, and pelvic and para-aortic lymphadenectomy. Further reaching resection was performed at the physician’s discretion. The mean time between preoperative CT examination and cytoreductive surgery was 19 days (range: one to 137 days).
Histopathological diagnosis and genetic testing
All surgical specimens were reviewed by dedicated gynecologic pathologists. For the diagnosis of HGSOC, the Gilk’s modification of the World Health Organization criteria served as a reference standard [14].
For all patients, histopathologic characteristics such as tumor architecture, nuclear polymorphism or tumor-infiltrating lymphocytes were studied in multiple representative sections of tumors in the adnexa, while the pattern of metastatic disease was described by reviewing all available slides of metastatic deposits, as previously described [15,16].
Genetic counseling was received by all patients referred between 2005 and 2008. The referral for BRCA mutation status testing was based on the following criteria (at least one): 1. Family history of breast cancer under the age of 50 or history of ovarian cancer at any age in a first or second degree relative, 2. Eastern European (Ashkenazi) Jewish heritage, 3. Patient request, or 4. Physician’s request. All patients referred after 2008 were offered genetic counselling and BRCA testing notwithstanding a positive family history.
CT data acquisition
Abdomino-pelvic CT scans were performed on a CT scanner from General Electric (GE Medical Systems, Milwaukee, WI) with multiple detector rows (4 to 64). All images were acquired during breath hold. Patients scanned at our institution received 150 ml of intravenous contrast agent (iohexol 300, Omnipaque 300©; Amersham Health, GE Medical Systems) via an antecubital vein using a power injector and images were acquired in late portal venous phase. A full list of CT data acquisition parameters can be found in Online Resource 1. Patients with outside examinations met or exceeded the technical requirements. After data acquisition, images were sent to a picture archiving and communication system (PACS; Centricity, GE Healthcare).
Quantitative CT data analysis
To perform quantitative analysis in our study population, an oncologic imaging research fellow (XX) reviewed all exams and identified tumor sites involving primary ovarian mass(es) as well as omental, mesenteric and solid metastatic implants in abdominal organs. After that, segmentation and data extraction was performed using an open-source medical image processing software (ITK-SNAP) [17]. Each lesion was contoured individually on each single slice, and a region of interest (ROI) was generated and tagged with a color coded label according to its anatomical location (e.g., primary ovarian mass(es), omentum, peritoneal, left upper quadrant). A full list of labels used is displayed in Online Resource 2. By merging all ROIs of a connected lesion, a volume of interest (VOI) was generated and the whole volume of lesions was used for further computer-assisted imaging analysis of texture features.
Gray-level spatial dependencies of the voxels within the VOI were analyzed to compute Haralick texture features [18] by first computing a gray-level co-occurrence matrix (GLCM). The GLCM represents the frequency of co-occurring pixel intensities as a two-dimensional histogram. Texture features were computed from the GLCM and consisted of energy (a measure of the amount of grey level variation within a given region), entropy (a measure of randomness or disorder in the distribution of signal intensities), homogeneity (a measure of uniformity in the distribution of co-occurrent intensity pairs) and constrast (a measure of variation in the distribution of co-occurrent intensity pairs). Haralick textures were computed using 5×5 neighborhood using an offset direction of [1–1] and 32 gray levels centered at each voxel using MATLAB (MathWorks Inc., Natick, Massachusetts, United States). The CT images were rescaled to 0–255 discrete intensity levels for meaningful texture extraction.
Next, the inter-site texture heterogeneity metrics were computed similar to the method outlined in [19], where the texture values from the tumor sites were grouped to produce sub-regions of consistent texture by using K-means clustering with (n=5) clusters. Next, an inter-site similarity matrix (ISM) was computed to capture the differences in the texture values between the clustered regions within the tumor sites. Finally, the inter-site texture heterogeneity metrics were computed from the ISM matrix. The inter-site texture heterogeneity metrics consisted of inter-site entropy (SE), inter-site cluster variance (SCV) and inter-site cluster prominence (SCP). We used only these three features as these were found to be relevant for distinguishing outcomes in patients with HGSOC in the previous study by (blinded for review) [19]. To calculate the above mentioned features, at least two metastatic sites are needed in order to reach sufficient validity. Otherwise, the inter-site similarity metrics cannot be calculated. However, this is not an actual restriction since most patients present with International Federation of Gynecology and Obstetrics (FIGO) stage III or stage IV and thus with multiple tumor sites. In our study population, all patients presented with two or more metastatic sites.
Additional data collection
Clinical and demographical information including the details of surgery and pathology results were collected for all patients from their electronic medical records. Data about surgery and pathology results were extracted likewise. For outcome evaluation, we assessed the date of progression based on follow-up CT examination and / or cancer antigen 125 (CA-125) level. On follow-up CT, progression was defined by applying Response Evaluation Criteria In Solid Tumors (RECIST, version 1.1) [20], namely the development of one or more new malignant lesions or progression of a preexisting lesion after completion of adjuvant chemotherapy. Similarly, a doubling of CA-125 level from the nadir or above normal value was considered as progression [21].
Statistical analysis
Complete gross surgical resection was defined as absence of residual disease following surgery as assessed by the operating surgeon as part of patient’s standard of care. OS was defined as the time between the preoperative CT exam and date of death or censure. PFS was defined as the time between the preoperative CT scan and progression (determined based on either follow-up CT examination and / or CA-125 level). All surviving patients were followed for up to 10.7 years (median 59 months). The survival curves were estimated by computing Kaplan-Meier curves with patients dichotomized at the median values of the texture heterogeneity metrics. Log-rank test was used to test for significant differences between the survival curves. Texture measures were summarized using median and interquartile range (IQR). Comparison between texture measures and BRCA mutation status and CSR was computed using Wilcoxon rank sum tests. P-values < 0.05 were considered to be significant. R software (R Foundation for Statistical Computing, Austria) was used for analysis.
RESULTS
Patient characteristics
In this study, we included 88 patients (median age 75 years, range 32–82 years). Twenty-eight patients (32%) were tested positive for BRCA mutation, of whom 18 (64%) had a BRCA1 mutation and 10 (36%) had a BRCA2 mutation. Sixty patients (68%) were tested negative for BRCA mutation.
Association between texture measures and survival
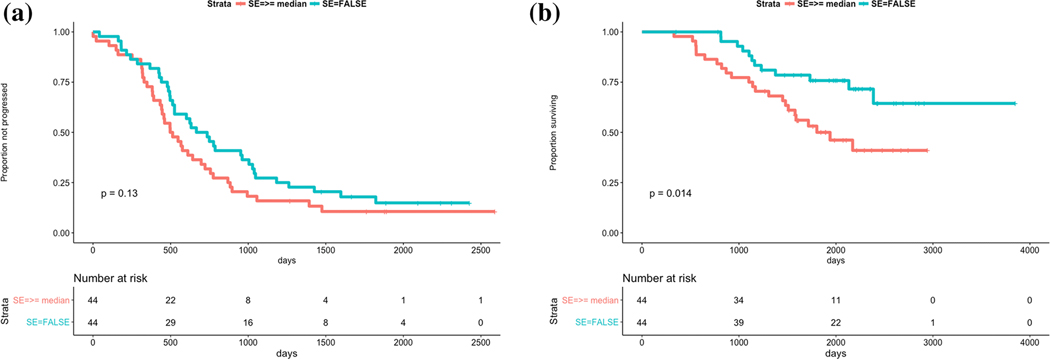
Lower values of SCV (p=0.006) and SCP (p=0.021) were significantly associated with longer PFS. Similarly, lower values of SCV (p=0.003) and smaller values of SE (p=0.014) were significantly associated with longer OS. SE (p=0.13) and SCP (p=0.16) were not significantly associated with PFS and OS, respectively (Fig. 1-3).
Fig. 1.
a Survival prediction using SE with regard to PSF
b Survival prediction using SE with regard to OS
Fig. 3.
a Survival prediction using SCP with regard to PSF
b Survival prediction using SCP with regard to OS
Association between cytoreductive outcome and texture metrics
Higher levels of all three inter-site textural heterogeneity metrics were significantly associated with incomplete surgical resection in BRCA negative patients (p<0.05 all) but not in BRCA positive patients (p>0.05 all), as displayed in Table 1. The results for the comparison of texture heterogeneity metrics and surgical resection status for all patients are listed in Online Resource 3.
Table 1:
Comparison of texture heterogeneity measures for patients with BRCA positive and BRCA negative mutation status and surgical resection status.
| BRCA mutation | Feature | Complete | Incomplete | p |
|---|---|---|---|---|
| BRCA positive | SE | 3.42(2.87–3.97) | 3.69(3.19–4.08) | 0.7 |
| SCV | 1.43(0.7–2.78) | 2.27(1.81–3.78) | 0.91 | |
| SCP | 3208(1581–5443) | 4195(2241–7269) | 0.67 | |
| BRCA negative | SE | 3.39(2.86–3.94) | 3.81(3.28–4.23) | 0.039 |
| SCV | 1.43(0.68–2.35) | 2.48(1.67–4.42) | 0.006 | |
| SCP | 2860(1462–5539) | 4907(2555–8474) | 0.02 |
Association between BRCA mutation status and texture metrics
None of the evaluated texture metrics were able to distinguish between BRCA mutation carriers and non-mutation carriers (Table 2). Also, the metrics did not reach a statistical significance for differentiating between BRCA1 and BRCA2 mutation (p>0.05 all) (Fig. 4, Table 2).
Table 2:
Comparison between texture heterogeneity measures and patients according to their BRCA mutation status.
| Feature | BRCA positive | BRCA negative | p | BRCA1 | BRCA2 | p |
|---|---|---|---|---|---|---|
| SE | 3.53(3.02–4.00) | 3.55(3.03–4.00) | 0.93 | 3.24(2.99–3.99) | 3.67(3.48–3.98) | 0.31 |
| SCV | 1.86(0.94–3.01) | 1.835(0.88–3.85) | 0.9 | 1.87(0.96–3.02) | 1.67(0.89–2.89) | 0.98 |
| SCP | 3425(1564–4913) | 3494(1811–6946) | 0.49 | 2662(1354–4741) | 3686(2688–5159) | 0.33 |
Fig. 4.
Boxplots displaying the similarity between BRCA positive mutation status versus BRCA negative mutation status for SE (a), SCV (b) and SCP (c).
DISCUSSION
In this study, we validate previous findings regarding the associations of inter-site heterogeneity texture metrics and survival in women with HGSOC [19]. However, we did not find significant associations between those same texture metrics and BRCA mutation status. A recent study that evaluated the same patients in our analysis found a significant difference between some qualitative CT features of patients who were BRCA mutation carriers and those who were non-carriers [13]. Therefore, we hypothesized that an advanced computational analysis would be able to quantitatively analyze phenotypic traits of standard of care CT scans not apparent to the naked eye. We found that patients with higher SE, SCV and SCP were more highly associated with incomplete cytoreductive surgery in BRCA negative women but not in BRCA positive patients. In the BRCA negative subpopulation of the previous study, patients with peritoneal disease in the left upper quadrant and in the lesser sac, mesenteric involvement, supradiaphragmal lymphadenopathy and lymphatic spread in the suprarenal paraaortic regions were also associated with a higher likelihood of incomplete cytoreductive surgery.
Tumoral heterogeneity is a feature of solid tumors including ovarian cancer. The quantification of spatial heterogeneity on imaging may offer an opportunity to non-invasively predict outcome and to optimally triage and granting the best treatment option for every single patient. In our study population, higher metrics were associated with inferior outcome. These results are in line with previous results, in which low SCP values were associated with a better outcome [19]. These results underpin the importance of quantifying tumoral heterogeneity of single primary tumor sites and moreover different disease sites in the same patient.
Positive BRCA mutation status has been associated with a better prognosis and a longer median survival [22,23], making it an important factor in the individual treatment setting. We hypothesized that this genotypic distinction would also be reflected in distinguishable phenotypic traits. However, in our study the texture metrics we used were not able to determine BRCA mutation status. This may be due to the relatively small study population and in particular to the small BRCA1/2 subpopulations. Larger study populations are therefore warranted to eventually validate our assumption.
Limitations of this study are as follows: First, it is retrospective in nature. Second, not all patients with HGSOC underwent genetic testing perhaps resulting in a selection bias. Third, as mentioned above, the sample size was relatively small, potentially hindering higher significance. For that reason, larger study populations are warranted to confirm our findings. Lastly, the manual segmentation is a time consuming process hindering translation into clinical daily practice. However, advances in artificial intelligence supported algorithm may facilitate automatic segmentation and thus making texture features available faster and without extra effort.
In conclusion, the assessment of tumoral heterogeneity in the era of personalized medicine is important, as increased heterogeneity has been associated with distinct genomic abnormalities and worse patient outcomes. Our radiomics approach in these standard-of-care CT scans may have a clinical impact by offering a noninvasive tool to predict outcome and therefore improve treatment selection and effectiveness. However, it was not able to assess BRCA mutation status in women with HGSOC.
Supplementary Material
Fig. 2.
a Survival prediction using SCV with regard to PSF
b Survival prediction using SCV with regard to OS
Acknowledgments
Funding information:
All authors received funding from the National Cancer Institute (P30 CA008748) Andreas Meier received funding from the Professor Dr Max Cloëtta Foundation
Footnotes
ETHICAL APPROVAL
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Conflict of Interest:
The authors declare that they have no conflict of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Jemal A: Cancer statistics, 2017. CA: A Cancer Journal for Clinicians 2017. January 5;67:7–30. [DOI] [PubMed] [Google Scholar]
- 2.Prat J, FIGO Committee on Gynecologic Oncology: Staging classification for cancer of the ovary, fallopian tube, and peritoneum. Int J Gynaecol Obstet 2014. January;124:1–5. [DOI] [PubMed] [Google Scholar]
- 3.Bolton KL, Chenevix-Trench G, Goh C, Sadetzki S, Ramus SJ, Karlan BY, et al. : Association between BRCA1 and BRCA2 mutations and survival in women with invasive epithelial ovarian cancer. JAMA 2012. January 25;307:382–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyman DM, Zhou Q, Iasonos A, Grisham RN, Arnold AG, Phillips MF, et al. : Improved survival for BRCA2-associated serous ovarian cancer compared with both BRCA-negative and BRCA1-associated serous ovarian cancer. Cancer 2012. August 1;118:3703–3709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Petrillo M, Marchetti C, De Leo R, Musella A, Capoluongo E, Paris I, et al. : BRCA mutational status, initial disease presentation, and clinical outcome in high-grade serous advanced ovarian cancer: a multicenter study. Am J Obstet Gynecol 2017. September;217:334.e1–334.e9. [DOI] [PubMed] [Google Scholar]
- 6.Parsons DW, Jones S, Zhang X, Lin JC-H, Leary RJ, Angenendt P, et al. : An integrated genomic analysis of human glioblastoma multiforme. Science 2008. September 26;321:1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Varela I, Tarpey P, Raine K, Huang D, Ong CK, Stephens P, et al. : Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature 2011. January 27;469:539–542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bailey P, Chang DK, Nones K, Johns AL, Patch A-M, Gingras M-C, et al. : Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 2016. March 3;531:47–52. [DOI] [PubMed] [Google Scholar]
- 9.Network TCGAR: Integrated genomic analyses of ovarian carcinoma. Nature 2011. June 1;474:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campbell PJ, Yachida S, Mudie LJ, Stephens PJ, Pleasance ED, Stebbings LA, et al. : The patterns and dynamics of genomic instability in metastatic pancreatic cancer. Nature 2010. October 28;467:1109–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, et al. : Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 2012. March 8;366:883–892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gillies RJ, Kinahan PE, Hricak H: Radiomics: Images Are More than Pictures, They Are Data. Radiology 2016. February;278:563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nougaret S, Lakhman Y, Gonen M, Goldman DA, Micco M, D’Anastasi M, et al. : High-Grade Serous Ovarian Cancer: Associations between BRCA Mutation Status, CT Imaging Phenotypes, and Clinical Outcomes. Radiology 2017. November;285:472–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gilks CB, Ionescu DN, Kalloger SE, Köbel M, Irving J, Clarke B, et al. : Tumor cell type can be reproducibly diagnosed and is of independent prognostic significance in patients with maximally debulked ovarian carcinoma. Human Pathology 2008. August;39:1239–1251. [DOI] [PubMed] [Google Scholar]
- 15.Soslow RA: Histologic Subtypes of Ovarian Carcinoma. International Journal of Gynecological Pathology 2008. February;PAP:1–14. [DOI] [PubMed] [Google Scholar]
- 16.Reyes MC, Arnold AG, Kauff ND, Levine DA, Soslow RA: Invasion patterns of metastatic high-grade serous carcinoma of ovary or fallopian tube associated with BRCA deficiency. Modern Pathology 2014. October;27:1405–1411. [DOI] [PubMed] [Google Scholar]
- 17.Yushkevich PA, Piven J, Hazlett HC, Smith RG, Ho S, Gee JC, et al. : User-guided 3D active contour segmentation of anatomical structures: significantly improved efficiency and reliability. Neuroimage 2006. July 1;31:1116–1128. [DOI] [PubMed] [Google Scholar]
- 18.Haralick RM, Shanmugam K: Textural features for image classification. Systems 1973; [Google Scholar]
- 19.Vargas HA, Veeraraghavan H, Micco M, Nougaret S, Lakhman Y, Meier AA, et al. : A novel representation of inter-site tumour heterogeneity from pre-treatment computed tomography textures classifies ovarian cancers by clinical outcome. Eur Radiol 2017;38:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. : New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). European Journal of Cancer 2009. January;45:228–247. [DOI] [PubMed] [Google Scholar]
- 21.Rustin GJ, Marples M, Nelstrop AE, Mahmoudi M, Meyer T: Use of CA-125 to define progression of ovarian cancer in patients with persistently elevated levels. J Clin Oncol 2001. October 15;19:4054–4057. [DOI] [PubMed] [Google Scholar]
- 22.Safra T, Lai WC, Borgato L, Nicoletto MO, Berman T, Reich E, et al. : BRCA mutations and outcome in epithelial ovarian cancer (EOC): experience in ethnically diverse groups. Ann Oncol 2013. November 1;24:viii63–viii68. [DOI] [PubMed] [Google Scholar]
- 23.Sun C, Li N, Ding D, Weng D, Meng L, Chen G, et al. : The role of BRCA status on the prognosis of patients with epithelial ovarian cancer: a systematic review of the literature with a meta-analysis. PLoS ONE 2014;9:e95285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.