Abstract
With the demand for rapid COVID-19 vaccine development and evaluation, this paper aimed to describe the prevalence and correlates of willingness to participate in COVID-19 vaccine trials among university students in China. A cross-sectional survey with 1912 Chinese university students was conducted during March and April 2020. Bivariate and multivariate analyses were performed to identify variables associated with willingness to participate. The majority of participants (64.01%) indicated willingness to participate in COVID-19 vaccine trials. Hesitancy over signing informed consent documents, concerns over time necessary for participating in a medical study, and perceived COVID-19 societal stigma were identified as deterrents, whereas lower socioeconomic status, female gender, perception of likely COVID-19 infection during the pandemic, and COVID-19 prosocial behaviors were facilitative factors. Further, public health mistrust and hesitancy over signing informed consent documents had a significant interactive effect on vaccine trial willingness. High standards of ethical and scientific practice are needed in COVID-19 vaccine research, including providing potential participants full and accurate information and ensuring participation free of coercion, socioeconomic inequality, and stigma. Attending to the needs of marginalized groups and addressing psychosocial factors including stigma and public health mistrust may also be important to COVID-19 vaccine development and future uptake.
Keywords: COVID-19, Vaccine trials willingness, China, Young adult, Vaccine hesitancy
1. Introduction
The novel coronavirus disease 2019 (COVID-19) pandemic has created an unprecedented global health challenge. Besides prevention, diagnosis, and treatment research, successful development and implementation of COVID-19 vaccines will be crucial to end the pandemic. Willingness to participate in COVID-19 vaccine trials among healthy populations will be key to evaluate COVID-19 vaccines, select promising candidates, and reduce the burden of COVID-19-related mortality and morbidity. Given that vaccine trials rely on volunteers, understanding reasons for hesitancy and predictors of willingness is important to inform ethical and scientific decisions in COVID-19 vaccine trials. Such information might also be relevant to anticipating demographic and psychosocial factors associated with future vaccine uptake (Fadda et al., 2020).
The current study investigated willingness to participate in COVID-19 vaccine trials and reasons for hesitancy among young adult students in China, a population considered to be at low risk for COVID-19 mortality and with high health literacy. Demographic and psychosocial variables were explored as willingness correlates, including region of residence, gender, socioeconomic status, reasons for participation hesitancy (e.g., potential harm and procedural issues), and four social-cognitive variables (public health mistrust, perceived COVID-19 societal stigma, perceived COVID-19 infection likelihood, and COVID-19 prosocial behaviors). As there is no research on vaccine trials willingness regarding COVID-19, predictors were selected based on established knowledge regarding vaccine willingness and hesitancy regarding other infectious diseases (e.g., HIV, Human Papillomavirus, influenza) (Strauss et al., 2001, Jenkins et al., 2000, Karafillakis et al., 2019, Yang et al., 2020).
Mistrust of health authorities has shown to affect willingness for vaccination and vaccine trials of HPV and HIV, in Europe and United States (Strauss et al., 2001, Karafillakis et al., 2019). Mistrust could be relevant to COVID-19 vaccine trials enrollment in China due to the lack of transparency during the initial outbreak, as well as vaccine scandals in recent years (Kavanagh, 2020, Yuan, 2018). Previous HIV vaccine research identified stigma as a barrier (Nyblade et al., 2011), yet its role has not been examined in the context of COVID-19. Consistent with previous vaccine willingness research (Jenkins et al., 2000); specific reasons for hesitancy, such as potential harms (physical, social) and procedural and logistical issues (e.g., consent form, time for participation), were also explored as potential deterrents. As to potential facilitators, individuals in hotspot regions (i.e., Hubei province, China’s hotspot) and from lower socioeconomic background may be particularly motivated due to their communities being heavily impacted by the pandemic (Ahmed et al., 2020). COVID-19 prosocial behaviors was explored, given that altruism appears to motivate participants in other trials (e.g., HIV) (Strauss et al., 2001, Harro et al., 2004). Perceived COVID-19 infection likelihood may be a motivating factor due to potential indirect medical benefit in participation.
2. Methods
2.1. Participants and recruitment
Study was approved by [masked for review]. Data was collected via an anonymous online survey between March 20th 2020 and April 10th 2020, two months following the COVID-19 outbreak announcement in China and during a period of state-enforced quarantine. We recruited the sample via advertisements on WeChat-linked platforms (websites and groups for students in 19 Chinese universities located in geographical diverse regions). Eligibility included (1) ≥18 years, (2) enrolled in a Chinese university, and (3) fluent in Chinese. Participants were asked to read through and indicate their eligibility and consent before starting the survey. No compensation was provided.
Participants were 1912 university students. Average age was 20.38 (SD = 2.10, Range = [18, 49]). The majority were female (69.77%). Recruited participants resided in 30 provinces out of the 34 provinces of China. Study areas included medicine (36.40%), science (16.21%), engineering (13.34%), economics (12.24%), industrial organization (8.53%), literature (6.80%), art (2.25%), education (1.41%), law (1.31%), history (1.10%), and agriculture (0.41%).
2.2. Instruments
Demographic information included age, gender, region, and socioeconomic status, assessed by a single-item: “Your socioeconomic status compared to school classmates” (1 = lower than average; 2 = average; 3 = higher than average). One item assessed perceived COVID-19 infection likelihood: “I believe I will NOT be infected by COVID-19” (1 = completely disagree; 4 = completely agree). Responses were then categorized to perceiving infection as unlikely and likely. As measures below have not been validated in China, a small group of university students (n = 40) completed pre-testing and provided feedback for improvement prior to launching the survey.
Willingness to Participate in COVID-19 Vaccine Trials. Both willingness and hesitancy items were adapted from previous HIV vaccine research (Jenkins et al., 2000). Willingness was assessed by a single-item: “Research on COVID-19 vaccines has started; would you be willing to participate in future human COVID-19 vaccine trials when they become available?” (1 = absolutely unwilling; 2 = probably unwilling; 3 = probably willing; 4 = absolutely willing). Consistent with previous research (Jenkins et al., 2000), willingness was dichotomized (1 = willing; 0 = not willing), such that those indicted “absolutely” and “probably” willing were designated “willing” and compared against the reminder, termed as “not willing”.
Reasons for Hesitancy in participation consisted10 items, including a physical harm index (five items), a social harm index (two items), and three items of other concerns (full list described in Results). Participants indicated if they have each of the concerns (Yes/No/Not sure). “Yes” and “not sure” responses were treated as endorsements, and “no” was treated as absence of concerns (Jenkins et al., 2000). Cronbach’s α was 0.92.
Public Health Mistrust Scale (Eisenman et al., 2012) consisted of four Likert-scale items (1 = strongly agree; 4 = strongly disagree) that assessed mistrust toward the public health system in responding to an emergency (e.g., “The public health system will provide honest information to the public”). Cronbach’s α was 0.91.
Perceived COVID-19 Societal Stigma was adapted from the Perceived External Stigma of the Ebola-related Stigma Questionnaire (Overholt et al., 2018). Six Likert scale items (1 = strongly disagree; 4 = strongly agree) assessed perceived societal stigma against COVID-19 (e.g., “Most people who have had COVID-19 are rejected when others find out”). Cronbach’s α was 0.90.
COVID-19-related Prosocial Behaviors was adapted using items from two scales: the Empathic Responding to SARS scale (Lee-Baggley et al., 2004) and Prosocialness Scale (Caprara et al., 2005). Nine statements assessed prosocial behaviors specific to COVID-19 (1 = strongly disagree; 5 = strongly agree), such as donating resources, providing help to those affected by COVID-19. Cronbach’s α was 0.93.
2.3. Statistical analysis
Preliminary analysis was performed to detect univariate outliers and non-normal distributions. No outlier was detected. Bivariate analyses (Chi-square statistics, t-tests) were conducted to explore potential correlates of COVID-19 vaccine trials participation willingness. Gender (male or female), region (Hubei or non-Hubei), and socioeconomic status (low SES or not) were dummy coded. Variables that were significant at the bivariate level were entered into a logistic regression simultaneously, with willingness as the outcome variable. Consistent with prior research using the vaccine willingness scale (Jenkins et al., 2000); items within the same index were collapsed into a composite score to improve the efficiency of logistic model.
3. Results
3.1. Willingness to participate in COVID-19 vaccine trials and reasons for hesitancy
Among 1912 young adults, most (64.01%) indicated willingness to participate in COVID-19 vaccine trials (13.70% “absolutely willing”, 50.31% “probably willing”, 29.29% “probably unwilling”, and 6.70% “absolutely unwilling”). Concerns for participation were prevalent: 88.91% endorsed concerns about vaccine side effects, followed by “family may not want me to take part” (86.72%), “handicap or death from vaccines” (84.36%), “becoming sick sooner if I ever contract COVID-19” (80.60%), “contracting COVID-19 through vaccines” (79.86%), “time necessary to be in a medical study” (74.01%), “vaccines might contain the COVID-19 virus” (73.69%), “having to sign informed consent documents” (70.82%), “others may refuse contact with me” (65.48%), and “taking part may be seen as having COVID-19” (63.23%).
3.2. Predictors of COVID-19 vaccine trials participation willingness
Following bivariate analysis (Table 1), variables that remained significant in predicting willingness in logistic regression were two demographic variables (lower SES, aOR = 1.49, 95% CI = [1.21, 1.83], and female, aOR = 1.27[1.03, 1.57]), two motivating factors including COVID-19 prosocial behaviors, aOR = 1.19[1.07, 1.33], and perceived COVID-19 infection likelihood, aOR = 1.48[1.15, 1.91], and three barriers including perceived COVID-19 societal stigma aOR = 0.86[0.78, 0.95], informed consent hesitancy, aOR = 0.55[0.40, 0.75], and time for a medical study, aOR = 0.60[0.43, 0.83]. Variance inflation factor (VIF) analysis did not suggest multicollinearity (VIF values <3).
Table 1.
Bivariate and multivariate analysis of willingness to participate in COVID-19 vaccine trials (N = 1912).
| Not willing |
Willing |
Logistic Regression |
|||||
|---|---|---|---|---|---|---|---|
| Continuous Variables | M (SD) | M (SD) | t | p | aOR | 95%CI | p |
| Age | 20.43 (2.31) | 20.35 (1.97) | 0.78 | 0.434 | |||
| COVID-19 prosocial behaviors | 26.12 (5.71) | 27.03 (5.53) | −3.40 | <0.001*** | 1.19 | [1.07, 1.33] | 0.002** |
| Perceived COVID-19 societal stigma | 12.53 (4.16) | 11.66 (3.84) | 4.01 | <0.001*** | 0.86 | [0.78, 0.95] | 0.002** |
| Public health mistrust | 6.72 (2.43) | 6.30 (2.43) | 3.55 | <0.001*** | 0.95 | [0.85, 1.06] | 0.331 |
| Categorical Variables | n (%) | n (%) | χ2 | p | |||
| Socioeconomic status | 10.43 | 0.001** | |||||
| Average or higher than average | 478 (38.64) | 759 (61.36) | ref | ||||
| Lower than average | 210 (31.11) | 465 (68.89) | 1.49 | [1.21, 1.83] | <0.001*** | ||
| Gender | 4.98 | 0.026* | |||||
| Male | 230 (39.79) | 348 (60.21) | ref | ||||
| Female | 458 (34.33) | 876 (65.67) | 1.27 | [1.03, 1.57] | 0.021* | ||
| Residence | 2.99 | 0.084 | |||||
| Non-Hubei | 679 (36.29) | 1192 (63.71) | |||||
| Hubei | 9 (21.95) | 32 (78.05) | |||||
| Perceived COVID-19 infection likelihood during the pandemic | 10.32 | 0.001** | |||||
| Not likely | 577 (37.79) | 950 (62.21) | ref | ||||
| Likely | 111 (28.83) | 274 (71.17) | 1.48 | [1.15, 1.91] | 0.002** | ||
| Reasons for hesitancy | |||||||
| Physical harm concerns | 15.28 | 0.009** | |||||
| None | 40 (30.77) | 90 (69.23) | ref | ||||
| 1 endorsement (yes/not sure) | 19 (23.17) | 63 (76.83) | 1.91 | [0.97, 3.87] | 0.066 | ||
| 2 endorsements | 28 (27.45) | 74 (72.55) | 1.57 | [0.83, 2.99] | 0.167 | ||
| 3 endorsements | 61 (39.61) | 93 (60.39) | 0.97 | [0.54, 1.74] | 0.929 | ||
| 4 endorsements | 57 (32.02) | 121 (67.98) | 1.45 | [0.80, 2.61] | 0.217 | ||
| 5 endorsements | 483 (38.15) | 783 (61.85) | 1.31 | [0.75, 2.26] | 0.335 | ||
| Social harm concerns | 11.82 | 0.003** | |||||
| None | 184 (30.82) | 413 (69.18) | ref | ||||
| 1 endorsement (yes/not sure) | 57 (33.73) | 112 (66.27) | 1.04 | [0.70, 1.54] | 0.857 | ||
| 2 endorsements | 447 (39.01) | 699 (60.99) | 1.05 | [0.78, 1.42] | 0.750 | ||
| Other concerns: | |||||||
| My family may not want me to take part | 8.61 | 0.003** | |||||
| No | 70 (27.56) | 184 (72.44) | ref | ||||
| Yes/not sure | 618 (37.27) | 1040 (62.73) | 1.21 | [0.79, 1.83] | 0.382 | ||
| Having to sign informed consent documents | 48.27 | <0.001*** | |||||
| No | 134 (24.01) | 424 (75.99) | ref | ||||
| Yes/not sure | 554 (40.92) | 800 (59.08) | 0.55 | [0.40, 0.75] | <0.001*** | ||
| Time necessary to be in a medical study | 42.97 | <0.001*** | |||||
| No | 118 (23.74) | 379 (76.26) | ref | ||||
| Yes/not sure | 570 (40.28) | 845 (59.72) | 0.60 | [0.43, 0.83] | 0.002** | ||
Note. Socioeconomic status (0 = average or higher than average; 1 = lower than average), gender (0 = male; 1 = female), residence (0 = non-Hubei; 1 = Hubei), and perceived COVID-19 infection likelihood during the pandemic (0 = not likely; 1 = likely) were dummy coded.
3.3. Post-hoc analysis: the interaction of mistrust X informed consent hesitancy
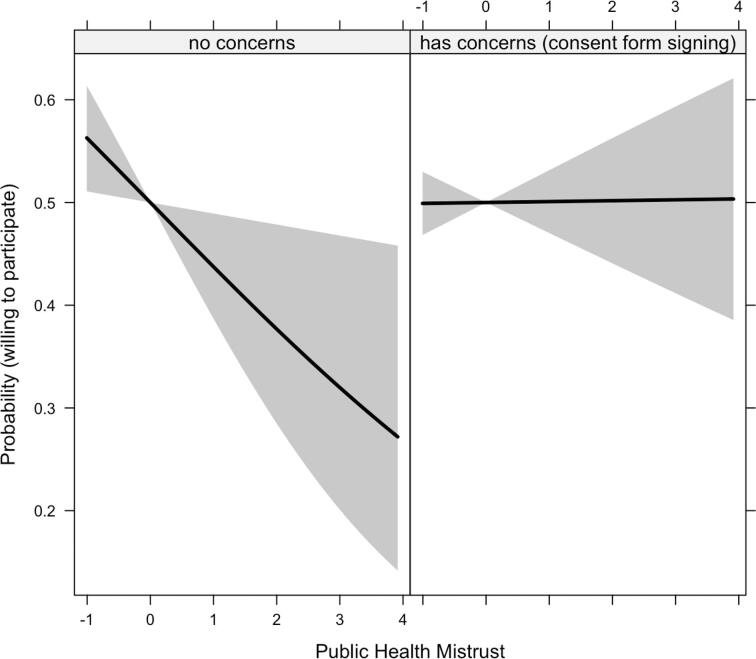
We explored the role of public health system mistrust as a psychological characteristic that may interact with informed consent hesitancy to predict willingness. Accounting for all variables in the logistic regression above, public health mistrust X informed consent hesitancy was significant, aOR = 1.29[1.03, 1.62]. Main effects of mistrust (aOR = 0.78[0.63, 0.96]) and informed consent hesitancy (aOR = 0.55[0.40,0.76]) were also significant. Interaction slopes indicate that mistrust had an additive and negative effect on willingness for individuals without concerns about signing consent documents; for those who were concerned about signing consent documents, there was no additional effect of public health mistrust (Fig. 1). Model comparison via analysis of variance revealed that the regression model including the interaction term accounted for more variance than the model without the interaction term, p = .028.
Fig. 1.
The interactive effect of public health mistrust X hesitancy over signing informed consent documents on the probability of being willing to participate in COVID-19 vaccine trials Note. Public health mistrust was mean-centered (M = 0, SD = 1). Grey area represents 95% confidence intervals. Main effects and effects of co-variates were accounted in modeling the figure.
4. Discussion
To our knowledge, this is the first study investigating COVID-19 vaccine trials participation willingness and its demographic and social-cognitive correlates. The current study found overall high willingness among young adults in China while highlighting deterrents and facilitators important to incorporate into considerations for COVID-19 vaccine research.
Informed consent hesitancy was the strongest (and negative) predictor of willingness. Biomedical research has been growing in China, yet implementation of appropriate regulatory processes lags behind. Adverse events such as the “Golden Rice Event,” which involved deceptive language and incomplete consent content regarding risks in a health research with children, resulted in public outcries (Yu and Li, 2014). Substandard consent practice is prevalent, including poor readability, lack of description on alternatives, and failure to inform procedures and rights to withdrawal (Wen et al., 2016, Lynöe et al., 2004). Additionally; although designed to emphasize individual agency, some Chinese participants may view it as a transfer of responsibility for adverse consequences from researchers to participants and therefore disempowering. Thus, COVID-19 vaccine trials will need a thorough informed consent process with accessible language and adequate explanations on risks, alternatives, and rights to withdrawal to ensure rights of potential participants and research integrity. Adequate information on time required for participation will also be needed.
Public health mistrust decreased the likelihood of willingness more strongly for individuals without consent hesitancy (Fig. 1). Enhancing the public’s confidence may be crucial for successful vaccine development and uptake. Such efforts may involve increasing transparency about vaccine research and its potential risks, effective regulation of vaccine production, and crisis management in unexpected health emergencies.
Perceived COVID-19 societal stigma emerged as another deterrent. Given the high prevalence of COVID-19 stigma in China (He et al., 2020), vaccine trials should consider potential psychosocial harm, including risks of social isolation and stigmatization. Stigma mitigation efforts will be necessary to promote willingness for COVID-19 vaccine trials and future vaccination uptake.
Facilitative factors included lower income, being female, perception of likely COVID-19 infection, and COVID-19 prosocial behaviors. COVID-19 vaccine trials must be careful to minimize economic coercion that might occur through incentives that inequitably drive participation among economically marginalized people. Gender-specific communication about trials participation may be needed. As participants tend to view research as therapeutic interventions (Lidz et al., 2004), those anxious about infection may be inclined to participate, perhaps due to unrealistic expectations about trial success. Similarly, young adults motivated by altruism may view societal benefits of vaccine research surpassing any concerns over personal risks. COVID-19 vaccine trials will need to facilitate potential participants to gain an accurate understanding on their infection likelihood (without the vaccine trial) and provide adequate information on risks and benefits, including a realistic depiction on the magnitude of societal benefits and influencing factors (e.g., vaccine efficacy, scale of implementation, etc.).
Study limitations include (1) lack of assessment on other factors that may contribute to participation willingness including specifics on research protocols (e.g., phase of vaccine trial, monetary compensation, medical care provision, and vaccine administration mode); (2) cross-sectional research precluding causal inferences; (3) limited generalizability given the focus on young adults; (4) online recruitment and selective respondents, and (5) bias due to self-report (e.g., social desirability).
Findings have implications for COVID-19 vaccine research and uptake. Rapid vaccine development has been called for, yet the high stakes and public interests involved in COVID-19 vaccine also require high standards of scientific and ethical practice. This includes adequate ethical supervision, providing potential participants accurate, transparent, and accessible information about their rights, risks and benefits associated with participation, and efforts to ensure recruitment free of coercion, socioeconomic inequality, and stigma. Public health efforts to reduce COVID-19 stigma, enhance transparency and public trust including communication to improve accurate understanding and willingness for participation, and adequately protect marginalized communities may be critical to COVID-19 vaccine development and successful immunization implementation in the future.
Author statement
SS conceptualized the study, contributed to building the questionnaire, analyzed the data, and wrote the paper. DL initiated the study, allocated funding, collected data, and provided critical feedback to the paper. DO contributed to conceptualization and provided editing and critical feedback to the paper. All authors contributed to and have approved the final manuscript.
Funding
Work by Shufang Sun was supported by the Providence/Boston Center for AIDS Research (P30AI042853) and National Institute of Health (K23AT011173). This research project is supported by the Fighting COVID-19 Research Fund by Beijing Normal University, China, awarded to Danhua Lin.
Role of the funding source
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Work by Shufang Sun was supported by the National Institute of Health (K23AT011173) and the Providence/Boston Center for AIDS Research (P30AI042853). This research project is supported by the Fighting COVID-19 Research Fund by Beijing Normal University awarded to Danhua Lin. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. We would like to thank all participants and those who helped to distribute the survey.
References
- Ahmed, F., Ahmed, N., Pissarides, C., Stiglitz, J., 2020. Why inequality could spread COVID-19. Lancet Public Heal [Internet] 5(5):e240. Available from: http://dx.doi.org/10.1016/S2468-2667(20)30085-2 PMID: 32247329. [DOI] [PMC free article] [PubMed]
- Caprara G.V., Steca P., Zelli A., Capanna C. A new scale for measuring adults’ prosocialness. Eur. J. Psychol. Assess. 2005;21(2):77–89. [Google Scholar]
- Eisenman D.P., Williams M.V., Glik D., Long A., Plough A.L., Ong M. The public health disaster trust scale: Validation of a brief measure. J Public Heal Manag Pract. 2012;18(4):1–8. doi: 10.1097/PHH.0b013e31823991e8. [DOI] [PubMed] [Google Scholar]
- Fadda, M., Albanese, E., Suggs, L.S., 2020. When a COVID-19 vaccine is ready, will we all be ready for it? Int. J. Public Health [Internet]. Springer International Publishing; 2020;4(Latimer). Available from: 10.1007/s00038-020-01404-4. [DOI] [PMC free article] [PubMed]
- Harro C.D., Judson F.N., Gorse G.J., Mayer K.H., Kostman J.R., Brown S.J., Koblin B., Marmor M., Bartholow B.N., Popovic V. Recruitment and baseline epidemiologic profile of participants in the first phase 3 HIV vaccine efficacy trial. J. Acquir. Immune Defic. Syndr. 2004;37(3):1385–1392. doi: 10.1097/01.qai.0000122983.87519.b5. [DOI] [PubMed] [Google Scholar]
- He J., He L., Zhou W., Nie X., He M. Discrimination and social exclusion in the outbreak of COVID-19. Int. J. Environ. Res. Public Health. 2020;17(8):2933. doi: 10.3390/ijerph17082933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins R.A., Torugsa K., Markowitz L.E., Mason C.J., Jamroentana V., Brown A.E., Nitayaphan S. Willingness to participate in HIV-1 vaccine trials among young Thai men. Sex. Transm. Dis. 2000;76(5):386–392. doi: 10.1136/sti.76.5.386. PMID: 11141858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karafillakis E., Simas C., Jarrett C., Verger P., Peretti-Watel P., Dib F., De Angelis S., Takacs J., Ali K.A., Pastore Celentano L., Larson H. HPV vaccination in a context of public mistrust and uncertainty: a systematic literature review of determinants of HPV vaccine hesitancy in Europe. Hum. Vaccines ImmunotherTaylor & Francis. 2019;15(7–8):1615–1627. doi: 10.1080/21645515.2018.1564436. PMID: 30633623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavanagh, M.M., 2020. Authoritarianism, outbreaks, and information politics. Lancet Public Heal. 5(3):e135–e136. PMID: 32061319. [DOI] [PMC free article] [PubMed]
- Lee-Baggley D., DeLongis A., Voorhoeave P., Greenglass E. Coping with the threat of severe acute respiratory syndrome: Role of threat appraisals and coping responses in health behaviors. Asian J. Soc. Psychol. 2004;7(1):9–23. doi: 10.1111/j.1467-839X.2004.00131.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidz C.W., Appelbaum P.S., Grisso T., Renaud M. Therapeutic misconception and the appreciation of risks in clinical trials. Soc. Sci. Med. 2004;58(9):1689–1697. doi: 10.1016/S0277-9536(03)00338-1. [DOI] [PubMed] [Google Scholar]
- Lynöe N., Sandlund M., Jacobsson L., Nordberg G., Jin T. Informed consent in China: Quality of information provided to participants in a research project. Scand J Public Health. 2004;32(6):472–475. doi: 10.1080/14034940410029432. [DOI] [PubMed] [Google Scholar]
- Nyblade L., Singh S., Ashburn K., Brady L., Olenja J. “ Once I begin to participate, people will run away from me”: Understanding stigma as a barrier to HIV vaccine research participation in Kenya. Vaccine Elsevier Ltd. 2011;29(48):8924–8928. doi: 10.1016/j.vaccine.2011.09.067. [DOI] [PubMed] [Google Scholar]
- Overholt L., Wohl D.A., Fischer W.A., Westreich D., Tozay S., Reeves E., Pewu K., Adjasso D., Hoover D., Merenbloom C., Johnson H., Williams G., Conneh T., Diggs J., Buller A., McMillian D., Hawks D., Dube K., Brown J., Sacks E. Stigma and Ebola survivorship in liberia: Results from a longitudinal cohort study. PLoS ONE. 2018;13(11):1–13. doi: 10.1371/journal.pone.0206595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R.P., Sengupta S., Kegeles S., McLellan E., Metzger D., Eyre S., Khanani F., Emrick C.B., MacQueen K.M. Willingness to volunteer in future preventive HIV vaccine trials: issues and perspectives from three U.S. communities. J. Acquir. Immune Defic. Syndr. 2001;26(1):63–71. doi: 10.1097/00126334-200101010-00010. [DOI] [PubMed] [Google Scholar]
- Wen G., Liu X., Huang L., Shu J., Xu N., Chen R., Huang Z., Yang G., Wang X., Xiang Y., Lu Y., Yuan H., Hills R.K. Readability and content assessment of informed consent forms for phase II-IV clinical trials in China. PLoS ONE. 2016;11(10):1–10. doi: 10.1371/journal.pone.0164251. PMID: 27701471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R., Penders B., Horstman K. Addressing vaccine hesitancy in china: A scoping review of Chinese scholarship. Vaccines. 2020;8(1):1–17. doi: 10.3390/vaccines8010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X., Li W. Informed consent and ethical review in Chinese human experimentation: Reflections on the “golden rice event”. Biotechnol. Law Rep. 2014;33(4):155–160. doi: 10.1089/blr.2014.9979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan X. Vaccine scandal and confidence crisis in China. Lancet. 2018;392(10145):371. doi: 10.1016/S0140-6736(18)31695-7. [DOI] [PubMed] [Google Scholar]