Abstract
Isoprene was efficiently converted to 1,6-dimethyl-1,5-cyclooctadiene (DMCOD) by selective [4+4]-cycloaddition with a catalyst formed by in situ reduction of [(MePI)FeCl(μ-Cl)]2 (MePI = [2-(2,6-(CH3)2-C6H3–N=C(CH3))–C4H5N]). DMCOD was isolated in 92% yield, at the preparative scale, with a catalyst loading of 0.025 mol%, and a TON of 3680. Catalytic hydrogenation of DMCOD yielded 1,4-dimethylcyclooctane (DMCO). The cyclic structure and ring strain of DMCO afforded gravimetric and volumetric net heats of combustion 2.4 and 9.2% higher, respectively, than conventional jet fuel. In addition, the presence of methyl branches at two sites resulted in a –20 °C kinematic viscosity of 4.17 mm2 s−1, 48 % lower than the maximum allowed value for conventional jet fuel. The ability to derive isoprene and related alcohols readily from abundant biomass sources, coupled with the highly efficient [Fe]-catalyzed [4+4]-cycloaddition described herein, suggests that this process holds great promise for the economical production of high-performance, bio-based jet fuel blendstocks.
Graphical Abstract
Bio-based isoprene is converted to a high performance jet fuel blendstock by Fe-catalyzed [4+4] cycloaddition and hydrogenation.
Introduction
The development of advanced bio-based fuels provides a unique opportunity to reduce greenhouse gas emissions and the environmental impact of jet aircraft, while delivering performance properties that in many cases exceed those of conventional jet fuel.1−22 Nature provides a dizzying array of substrates that can be efficiently converted into reduced hydrocarbon species suitable for incorporation into jet fuel blends. Isoprene is a particularly intriguing substrate due to the ability to readily generate it, or precursor molecules, from biomass sources through fermentation with metabolically engineered microorganisms. For example, Keasling and Lee have demonstrated the production of 3-methyl-3-buten-1-ol with metabolically engineered E. coli at moderate titer (2.2 g/L).23 Isoprene can then be readily generated from the alcohol by dehydration.24−26 Isoprene can also be generated from biomass sources by hybrid biological/chemical routes. For example, biomass sugars can be efficiently converted to mevalonolactone, followed by catalytic conversion to isoprene at temperatures above 300 °C in the presence of a heterogenous acid catalyst.27 Alternatively, biomass sugars can be fermented to generate mesaconic acid which can then be hydrogenated and dehydrated to form isoprene.28,29 The direct production of isoprene, and subsequent removal from the fermentation broth via low temperature distillation, is a more straightforward approach that obviates the need for a chemical upgrading step. This route has been extensively explored,30a with reported isoprene titers of >60 g/L.30b Regardless of the pathway, additional improvements in conversion efficiency and lignocellulosic biomass utilization will be required to allow for bio-based isoprene to become competitive with petroleum feedstocks.
Isoprene is an ideal starting material for the synthesis of high-performance jet fuel by virtue of its high reactivity (arising from the conjugated pi system) and methyl substitution. The latter feature allows for the introduction of branching motifs useful for decreasing the viscosity and lowering the freezing point of derivative fuels. To maximize the environmental benefits afforded by a bio-based fuel, it is important to utilize high-throughput catalytic methods that are selective for formation of molecules with desirable structures and fuel properties.2,6,13,31 From a design standpoint, conversion of isoprene to a cyclic paraffin should maximize the density of the fuel while providing good combustion properties and low viscosity. Selective dimerization of isoprene to a cyclic product, followed by hydrogenation, would yield a C10 hydrocarbon, which falls within the range of conventional jet fuel (typically C9−C14).
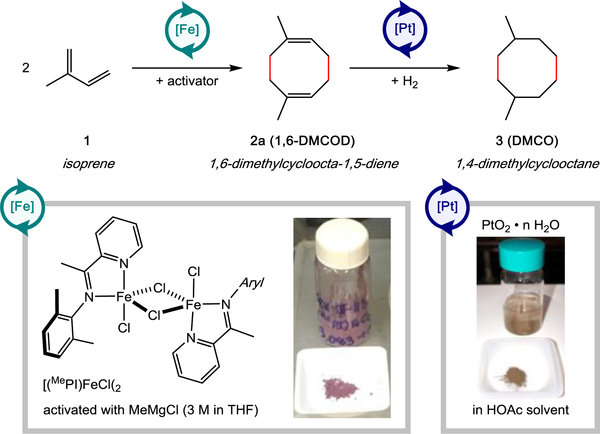
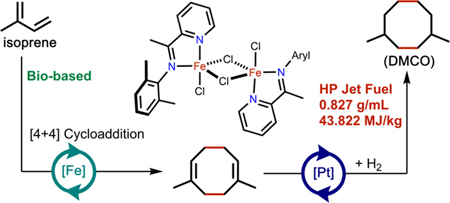
Recent work by the Chirik group32 has shown that isoprene can be selectively dimerized to 1,6-dimethyl-1,5-cyclooctadiene (1,6-DMCOD, 2a) through [4+4]-cycloaddition catalyzed by well-defined, reduced iron iminopyridine complexes. The proposed catalytic cycle proceeds through coordination of two equivalents of isoprene followed by oxidative cyclization, rearrangement, and reductive elimination.32 In situ activation protocols were reported that allowed the dimerization to be conducted using low loadings of bench-stable iron (II) halide precatalysts. Hydrogenation of 2a would yield 1,4-dimethylcyclooctane (DMCO, 3), a C10H20 hydrocarbon that would be expected to have favorable fuel properties due to its cyclic structure, and chain branching (Scheme 1). To explore the potential of DMCO as an alternative jet fuel blendstock, this paper reports the preparative-scale synthesis of DMCO and describes key fuel properties of DMCO and blends with a common synthetic paraffinic kerosene.
Scheme 1.
Synthesis of 1,4-DMCO from isoprene using iron-catalyzed cycloaddition followed by hydrogenation,. Aryl = 2,6-dimethylphenyl.
EXPERIMENTAL SECTION
Materials and Methods.
All air- and moisture-sensitive manipulations were performed using standard Schlenk techniques on a dual-manifold vacuum line under ultra-high purity argon, or in an M. Braun glove box with an inert atmosphere of purified nitrogen. Isoprene, MeMgCl solution (3M in THF), platinum (IV) oxide hydrate, and glacial acetic acid were purchased from Millipore Sigma, while a sample of Jet-A/F-24 was obtained from the fuel depot at the China Lake Naval Air Warfare Center. A sample of HEFA-Jet was obtained from the Air Force Research Laboratory, Dayton, OH. Prior to use, isoprene was stirred over calcium hydride for >48 hours, degassed by 3 freeze-pump-thaw cycles, and distilled under vacuum. Without exposure to air, the dried isoprene was transferred to a glovebox, stored over activated 4 Å molecular sieves at –35 °C, and (as needed) filtered through a plug of activated Al2O3 prior to use. Deuterated chloroform (CDCl3) for NMR spectroscopy was purchased from Cambridge Isotopes and stored over anhydrous potassium carbonate. All other reagents were used as received. The iminopyridine iron dihalide precatalyst used herein [(MePI)FeCl(μ-Cl)]2 was prepared as previously described.32,33
Proton nuclear magnetic resonance (1H NMR) spectra were recorded at 25 °C on a Bruker Avance III 500 spectrometer operating at 500.46 MHz. Proton-decoupled 13C{1H} NMR, 13C{1H} APT NMR, and quantitative q13C{1H} NMR nuclear magnetic resonance spectra were recorded at 25 °C on a Bruker Avance III 500 instrument operating at 125.86 MHz. All experiments were performed at the Princeton University Nuclear Magnetic Resonance Facility or the China Lake Naval Air Warfare Center. Chemical shifts are reported in parts per million downfield from tetramethylsilane (SiMe4) and are referenced relative to the NMR solvent according to literature values: δ(1H) = 7.26, δ(13C) = 77.0 for CDCl3. NMR spectra were processed using the MestReNova or TopSpin software suites.
Analytical gas chromatography was performed using a Shimadzu GC-2010 instrument equipped with a Shimadzu AOC-20s autosampler. For samples analyzed on a Shimadzu SHRXI-5MS achiral stationary phase capillary column (15 m × 250 μm), the instrument was set to an injection volume of 1.0 μL, an inlet split ratio of 20:1, and inlet and detector temperatures of 250 °C and 275 °C, respectively. UHP-grade S3 helium was used as carrier gas with a flow rate of 1.96 mL/min. The column temperature was varied as follows: 0–2 minutes, isotherm, 30 °C; 2–12 minutes, temperature ramp, +3 °C/min; 12–15 minutes, isotherm, 60 °C; 15–19 minutes, temperature ramp, +10 °C/min; 19–20 minutes, isotherm, 100 °C. Products were further characterized via GC-MS (electron-impact ionization). The GC-MS system was equipped with an RTX-5MS 30-meter column and the analysis was conducted under the following conditions: inlet temperature, 250 °C; initial column temperature, 40 °C; temperature ramp, 4 °C/min to 100 °C; 2nd temperature ramp, 20 °C/min to 300 °C.
Net heat of combustion (NHOC) measurements were conducted by the Southwest Research Institute (SwRI) using ASTM D240N. The derived cetane number (DCN) of DMCO was measured via ignition quality testing (IQT) by SwRI using ASTM D6890. The kinematic viscosity and density of DMCO were measured with a Stabinger Viscometer, SVM 3001 connected to a TC-502 circulation cooler. A 5 mL disposable syringe was used to inject the sample into the viscometer. The flash point of DMCO was measured with a Grabner Instruments/Ametek Miniflash FLP Touch according to ASTM D7094. For each measurement, 2 mL of fuel were transferred via auto pipette to a 7 mL stainless steel sample cup. The initial temperature of each run was set to 28 °C and the final temperature was set to 64 °C.
1,6-dimethyl-1,5-cyclooctadiene (DMCOD)
[Caution: The [4+4]-cycloaddition of 1,3-dienes is highly exothermic. When performing the reaction on preparative scale, special care should be taken to ensure adequate heat transfer. Maintaining a controlled temperature throughout the course of the reaction is necessary to prevent dangerous pressure build-up.]
Procedure A (200 mmol scale):
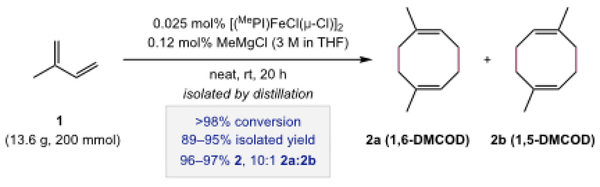
The dimerization of isoprene was conducted on a 200 mmol scale as depicted in Scheme 2. In a glovebox, an oven-dried 50-mL round bottom flask was charged with [(MePI)FeCl(μ-Cl)]2 (0.035 g, 0.05 mmol dimer, 0.1 mmol [Fe], 0.05 mol% [Fe]), isoprene (1.0 g, 15 mmol), and an oven-dried PTFE-coated magnetic stir bar. The reaction was initiated by the addition of MeMgCl (3 M in THF, 0.080 mL, 0.24 mmol, 0.12 mol%). The reaction flask was covered with a vacuum-dried virgin rubber septum or an oven-dried ground-glass stopper. The reaction mixture was stirred vigorously in the glovebox for 10 min or until a blue/green color persisted. Additional isoprene (12.6 g, 185 mmol; to a total of 200 mmol, 1.0 equiv) was then added in the glovebox. The reaction flask was sealed, and the reaction mixture was maintained with vigorous stirring at ambient temperature (~23 °C). Aliquots (50 uL) of the reaction mixture were removed regularly by syringe to monitor the reaction progress. For each aliquot, the sample was removed from the glovebox, diluted with CDCl3 (0.7 mL), filtered through a plug of Celite, and analyzed by 1H NMR spectroscopy (500 MHz, CDCl3, 25 °C). After 20 h, or upon complete consumption of isoprene, the reaction flask was removed from the glovebox and opened to air in a fume-hood. The flask was fitted with an oven-dried short-path distillation head, which was connected to a high vacuum manifold. DMCOD (89–95% isolated yield, 96–97% 2, 10:1 2a:2b) was isolated by vacuum distillation into a tared, 50- or 100-mL receiving flask cooled by dry ice/acetone. The combined yield of product isomers was determined from the mass of the isolated material. The composition of [4+4]-cycloadducts 1,6-DMCOD (2a) and 1,5-DMCOD (2b) relative to other dimeric species was assessed by gas-chromatography and corroborated by 1H NMR and 13C NMR spectroscopy. The regioisomer ratio (1,6-DMCOD:1,5-DMCOD; 2a:2b) of the [4+4]-cycloadducts was determined from the relative integration of diagnostic resonances in the quantitative 13C NMR spectrum. Spectral data were consistent with values reported previously.32,34 1H NMR (500 MHz, CDCl3): δ 5.44–5.15 (m, 2H, (=CH-)), 2.34 (s, 4H, CH2), 2.32–2.24 (m, 4H, CH2), 1.70 (s, 6H, 1,6-DMCOD, CH3), 1.68 (s, 6H, 1,5-DMCOD, CH3). 13C{1H} NMR (126 MHz, CDCl3): δ 136.0 (=C(CH3)-), 122.5 (=CH-), 32.4 (CH2), 27.6 (CH2), 26.5 (CH3) for 1,6-DMCOD; 135.9 (=C(CH3)-), 122.7 (=CH-), 33.5 (CH2), 26.5 (CH2), 26.4 (CH3) for 1,5-DMCOD. GC (30TO60TO100_20MIN): Rt = 14.0 min for both 1,6- and 1,5-DMCOD
Scheme 2.
Preparation of DMCOD by Procedure A.
Procedure B (2 mol scale):
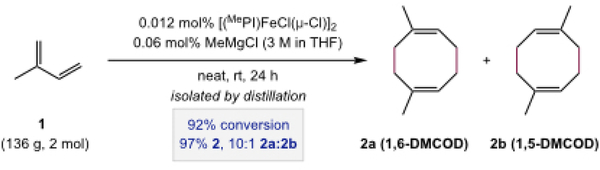
The dimerization of isoprene was conducted on the 2 mol scale as depicted in Scheme 3. In a glovebox, an oven-dried 500-mL round-bottom Schlenk flask was charged with [(MePI)FeCl(μ-Cl)]2 (0.175 g, 0.25 mmol dimer, 0.50 mmol [Fe], 0.025 mol% [Fe]), an oven-dried football-shaped stir bar, and isoprene (10 g, 0.15 mol). A separate, oven-dried 250-mL round-bottom flask was charged with additional isoprene (126 g, 1.85 mol; to a total of 2.0 mol, 1.0 equiv). Both flasks were sealed with virgin rubber septa and removed from the glovebox. The Schlenk flask side-arm was fit with a gas-inlet hose, and the hose was evacuated and back-filled with argon three times before the stopcock was opened, placing the flask under a positive pressure of argon. The Schlenk flask was then lowered into a cold-water bath (10–15 °C; maintained with ice chips), and stirring was initiated. While stirring vigorously under argon, the catalyst was activated with the addition of MeMgCl solution (3 M in THF, 0.4 mL, 1.2 mmol, 0.6 mol%) injected through the septum. The reaction was maintained for 10 minutes as the mixture changed colors from purple to green to blue-green. After 10 minutes, the flask containing the remaining isoprene was placed under a positive pressure of argon. An oven-dried stainless-steel cannula was connected between the two-flasks, and the Schlenk flask was fit with an N2-flushed, but unpressurized balloon. The stopcock on the Schlenk flask side-arm was closed, and the isoprene was added over the span of 20 minutes by an argon-driven cannula transfer, releasing pressure into the balloon. After the addition was complete, the stopcock was reopened, and the cannula and needle were removed. The puncture marks in the septum were covered with grease and PTFE-tape. The reaction was maintained with stirring under gentle argon pressure for 24 hours, and the water bath was allowed to warm gradually to ambient temperature (~23 °C). After 24 hours, the reaction vessel was then opened to air, and DMCOD (92% conversion; 97% 2; 10:1 2a:2b) was isolated by vacuum distillation directly from the reaction vessel. Characterization data were consistent with those above.
Scheme 3.
Preparation of DMCOD by Procedure B.
Dimethylcyclooctane (DMCO).
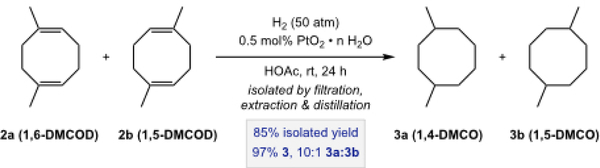
The hydrogenation of DMCOD was conducted on the 1.6 mol scale as depicted in Scheme 4. A 2.5 L reinforced glass Parr reaction vessel was charged with 2 (220 g, 1.62 mol), platinum(IV) oxide hydrate (2.05 g, ~80 wt% [Pt], 0.008 mol [Pt], 0.5 mol% [Pt] ), and acetic acid (50 mL). The flask was sealed with a rubber septum and the headspace was filled with nitrogen. The vessel was evacuated and then placed within the hydrogenation apparatus. The flask was charged with hydrogen, evacuated, and recharged with hydrogen four times. The vessel was finally charged with hydrogen (0.34 MPa) and mechanically shook. Rapid consumption of hydrogen was immediately observed, and the vessel was recharged multiple times with 50 psi of hydrogen until the pressure stabilized. At this point the flask was recharged with hydrogen again and shook overnight. The vessel was then removed from the hydrogenation apparatus and quickly put under nitrogen. The contents were filtered through Celite and the filter cake was washed with pentane. The filtrate was transferred to a 500 mL separatory funnel and washed with DI water (300 mL), saturated sodium bicarbonate solution (300 mL), and lastly additional DI water (300 mL). The organic layer was collected and dried with magnesium sulfate, filtered, and the volatiles were carefully removed on a rotary evaporator to give a pale yellow solution. The solution was then fractionally distilled under reduced pressure. Residual pentane was removed with the first fraction at ambient temperature under a static vacuum induced by opening the distillation apparatus to a standard high vacuum line for several seconds. A second distillate fraction was collected under dynamic vacuum (0.01 Torr) between 25 °C and 40 °C to yield DMCO (equimolar mixture of cis and trans isomers) as a clear, colorless liquid (192.06 g, 85% yield, 97% 3, 10:1 3a:3b). Anal. Calcd for C10H20: C, 85.63; H, 14.37. Found: C, 85.48; H, 14.54. 1H NMR (500 MHz, CDCl3): δ 1.760 – 1.210 (alkyl-H), 0.939 – 0.906 (alkyl-H). 13C{1H} NMR (126 MHz, CDCl3): δ 34.46 (s), 33.87 (s), 33.85 (s), 33.74 (s), 33.05 (s), 32.64 (s), 26.14 (s), 26.12 (s), 24.79 (s), 23.91 (s). IR (neat, cm−1): 2950, 2914, 2868, 1457, 1379. GC-MS (DCM): 8.58–8.64 min (140 m/z).
Scheme 4.
Hydrogenation of DMCOD to afford DMCO (3).
RESULTS AND DISCUSSION
Isoprene was readily converted to DMCO on a 100+ gram scale by a straightforward, high-throughput, two-step method. Prior to conducting the cycloaddition reaction, commercial isoprene was carefully purified to remove traces of water, oxygen, oligomers, and inhibitor. With precatalyst loadings as low as 0.025 mol% [Fe], the dimerization reaction proceeded with 92% conversion and 97% selectivity to [4+4] products (91% 1,6-dimethyl-2,5-cyclooctadiene; Figure S6) over 24 hours, with complete conversion of substrate achieved over the same time frame with modestly higher catalyst loadings (0.05 mol% [Fe]). At the lower catalyst loading, a relatively high turnover number (TON) of 3680 was achieved. This TON is more than 42-times higher than that obtained for the pyridine(diimine) iron-catalyzed [2+2]-cycloaddition of unactivated α-olefins,2,31 and on the same order as TONs achieved with the Cp2ZrCl2/MAO-catalyzed dimerization of 1-hexene.6 Hydrogenation was conducted with PtO2 under mild conditions (ambient temperature, 50 psi H2) using acetic acid as a heterogeneous co-solvent. Complete reduction of 2 yielded DMCO (3) as a 50:50 mixture of the cis- and trans-isomer. The structure and purity of 3 was confirmed by 1H and 13C{1H} NMR spectroscopy, as well as GC-MS/FID (Figures S8–S11). Traces of other isomers (~3%), likely formed by [4+2] cycloaddition of isoprene prior to hydrogenation, were observed in the gas chromatogram of DMCO, but were not distinguishable in the NMR spectra.
With significant quantities of DMCO available for further study, key fuel properties including density, NHOC, kinematic viscosity, flashpoint, and derived cetane number were measured (Table 1). DMCO exhibited a density of 0.827 g/mL at 15 °C. This value is 6.7% higher than the lower limit for conventional jet fuel (>0.775 g/mL) and 12.8% higher than the acyclic hydrogenated isoprene dimer 2,6-dimethyloctane (0.73 g/mL). As expected, the higher density of DMCO resulted in a volumetric net heat of combustion (NHOC) of 36.222 MJ/L, 9.2% higher than the lower limit of conventional jet fuel (33.17 MJ/L). Similarly, due to the lack of aromatic compounds, the gravimetric NHOC of DMCO was 43.822 MJ/kg, 2.4% higher than that of conventional jet fuel.
Table 1.
Key Fuel Properties of DMCO and Conventional Jet Fuel.
| Property | DMCO | Jet-A/F-24* |
|---|---|---|
| Gravimetric Net Heat of Combustion (NHOC) | 43.822 MJ/kg | >42.8 MJ/kg |
| Volumetric NHOC | 36.222 MJ/L | >33.17 MJ/L |
| Density (15 °C) | 0.827 g/mL | >0.775 g/mL |
| Kinematic Viscosity (−20 °C) | 4.17 mm2s−1 | <8.0 mm2s−1 |
| Freezing Point | <−78 °C | <−40 °C |
| Flash Point | 50 °C | >38 °C |
The values listed here are based on the specification for Jet-A/F-24 (ASTM D1655) and should not be confused with those obtained for the authentic sample used as a benchmark below.
DMCO also compares favorably to recently reported alkyl cyclobutane fuels that can be prepared by [2+2]-cycloaddition of unactivated alkenes.2,31 For example, 1,2-dipropylcyclobutane (DPC), a C10 cyclic hydrocarbon prepared from 1-pentene, has a similar gravimetric heat of combustion (43.74 MJ/kg) to DMCO. One would expect to observe similar values considering both molecules have the same hydrogen content and molecular weight. The cyclobutane ring in DPC imparts greater strain energy (~26 kcal for cyclobutane vs. ~10 kcal for cyclooctane), 35,36 which should increase the gravimetric NHOC of DPC by ~1.1% compared to DMCO. However, other factors, including differences in heat of vaporization, and the presence of acyclic isomers in the DPC samples, result in comparable gravimetric NHOCs. In contrast, the volumetric NHOC of DMCO is nearly 8% higher than DPC (36.222 MJ/L compared to 33.55 MJ/L) due to the higher density of DMCO (0.827 g/mL compared to 0.767 g/mL). This higher density is a result of having more carbon atoms constrained within the ring system.
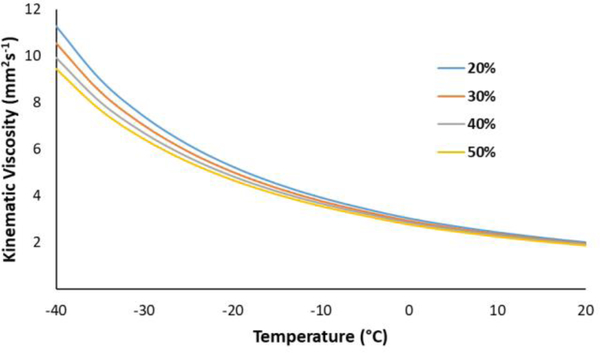
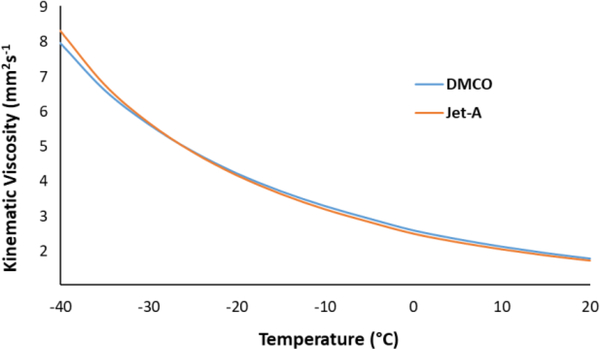
To further evaluate DMCO, the kinematic viscosity was measured from −40 to 20 °C (Figure 2). Maintaining low viscosity at low temperature is critical for jet fuels, both for safety, as well as to ensure complete combustion. A viscosity of less than 12 mm2s−1 at −40 °C is required to ensure that a jet engine can be relit at altitude if the engine has been extinguished.37 Low viscosity is also important for the rapid atomization of fuels. Higher viscosity fuels can lead to reduced combustion efficiency and the production of soot which can reduce engine life. DMCO exhibited a −20 °C kinematic viscosity of 4.17 mm2s−1, which is about half of the upper viscosity limit for Jet-A (8.0 mm2s−1). The −40 °C kinematic viscosity of DMCO was 7.95 mm2s−1, 34% lower than the specification limit. To provide a comparison, the kinematic viscosity of a sample of Jet-A/F-24 was measured over a comparable temperature range (Figure 1). The viscosity curves of DMCO and the Jet-A sample were virtually identical, with DMCO exhibiting a slightly lower viscosity at −40 °C.
Figure 2.
Kinematic viscosity of DMCO/HEFA-Jet blends (20–50% DMCO) from 20 to −40 °C.
Figure 1.
Kinematic viscosity of DMCO and Jet-A from 20 to −40 °C.
The freezing point of DMCO was first studied by differential scanning calorimetry (DSC). The DSC trace did not reveal an observable freezing point down to −80 °C. Similarly, no crystallization or gel formation was observed after placing a sample of DMCO in a dry ice/acetone bath (−78 °C) for up to one hour. Thus, the freezing point of DMCO is at least 40 °C lower than the requirement for Jet-A/F-24 (< −40 °C). This extremely low freezing point may be due, in part, to the presence of two diastereomers in the fuel mixture. In addition to an upper limit on freezing point, the specification for Jet-A/F-24 has a strict flashpoint requirement (>38 °C). This requirement is an important safety consideration, with more volatile fuels increasing the likelihood of a catastrophic fire. DMCO has a flashpoint of 50 °C, allowing for its use as a drop-in blendstock.
Although the focus of the current work was the development of a high-performance, bio-based jet fuel, it was also of interest to explore the potential of DMCO as a diesel fuel. Ignition Quality Testing (IQT) was conducted and a derived cetane number (DCN) of 18 was measured for DMCO. DCN directly relates to the ignition delay of the fuel, with higher values representing rapid combustion under compression ignition conditions, and lower values representing slow combustion.4,38 The lower cetane number limit for conventional diesel fuel (Diesel #2) is 40, with typical ranges of about 40–54 found throughout the U.S.39,40 The low DCN of DMCO, along with its low viscosity would preclude its use as a standalone diesel fuel. In addition, although there is no DCN requirement in jet fuel specifications, turbine fuels with DCNs below 30 can increase the likelihood of lean blowout.41–43 High density/high cetane fuel blends were recently prepared by blending sesquiterpanes with bio-derived synthetic paraffinic kerosenes.4 In a conceptually similar fashion, blending DMCO with other high cetane fuels is anticipated to remedy this shortcoming.
The high density of DMCO coupled with its high gravimetric NHOC suggest that it is an ideal candidate to replace aromatic compounds in high performance, bio-based fuel formulations. Most alternative jet fuels are composed of synthetic paraffinic kerosenes (SPKs) containing acyclic linear and branched alkanes that have moderate densities (~0.74–0.76 g/mL)44 and do not meet the minimum density requirement for Jet-A/F-24 (>0.775 g/mL). Blending DMCO with these SPKs can increase the density and help these fuels meet the specification. DMCO also has a much higher gravimetric NHOC (43.82 MJ/kg) compared to a typical aromatic compound like toluene (40.59 MJ/kg). This 8% increase in NHOC can increase the range or payload capability of both civilian and military aircraft. The low viscosity of DMCO suggests that it can be blended in almost any proportion with conventional SPKs. Alternatively, it could be applied to reduce the viscosity of higher molecular weight blendstocks.
Minimizing the amount of aromatics in jet fuel can improve the combustion efficiency of the fuel, while simultaneously reducing the production of soot, and the emission of unburned hydrocarbons, including carcinogenic polyaromatic hydrocarbons.45,46 Aromatics have traditionally been an important component of jet fuel, prized for their ability to effectively swell nitrile rubber elastomers, ensuring engine integrity. However, with the advent of modern elastomeric materials, the need for aromatic compounds in jet fuel has greatly diminished. Moreover, a recent study by Boeing has demonstrated that 30% blends of cyclic alkanes are capable of swelling nitrile o-rings to the same extent as existing low (8%) aromatic fuel blends, suggesting the viability of aromatic-free alternative jet fuels in modern aircraft.47,48 To evaluate the suitability of DMCO as a replacement for aromatic compounds in a full-performance, bio-based jet fuel, 20–50% (v/v) DMCO blends were prepared with HEFA-Jet (Table 2; Figure 2), which is a complex mixture of acyclic paraffins derived from a mixture of fatty acids and methyl esters by hydrotreatment and isomerization.49–52 Selected properties of these blends are listed in Table 2.
Table 2.
Fuel properties of HEFA-Jet/DMCO blends.
| Fuel | Density (at 15 °C) | η (−40 °C, mm2s−1) | η (−20 °C, mm2s−1) | NHOC (MJ/kg) |
| HEFA | 0.762 | 12.77 | 5.65 | 43.73 |
| 20% DMCO | 0.773 | 11.26 | 5.25 | 43.75 |
| 30% DMCO | 0.780 | 10.54 | 5.03 | 43.76 |
| 40% DMCO | 0.788 | 9.93 | 4.84 | 43.77 |
| 50% DMCO | 0.793 | 9.43 | 4.68 | 43.78 |
η denotes kinematic viscosity. The NHOC values are calculated based on measured values of pure HEFA and DMCO.
Addition of only 20% DMCO yields a fuel blend with a density close to that required for the Jet-A/F-24 specifications, while the 30–50% DMCO blends meet the density requirement. The kinematic viscosity of pure HEFA-Jet at −40 °C is higher than the preferred value of 12 mm2s−1, while all of the DMCO blends have values below this limit. All of the fuels, including pure HEFA, have kinematic viscosities at −20 °C well within the specification for Jet-A/F-24. The impact on the gravimetric NHOC of HEFA is modest due to the similarities in NHOC between the two blendstocks.
With the benefits of DMCO as a jet fuel blendstock firmly established, some perspective on the further development of this technology is warranted. A recent study by the US Department of Energy suggests that up to 30% of domestic transportation fuels could be generated from sustainable biomass sources.53 Although manufacturers of automobiles and light trucks are transitioning away from liquid fuels, and toward the use of electric motors, there is currently no alternative to hydrocarbons for propulsion of large aircraft, over long distances, and at high speed. In 2018, jet fuel accounted for 12% of transportation fuels used in the US,54 thus it is conceivable that petroleum jet fuel could be replaced with high performance, zero-aromatic bio-based fuels as these fuels transition to industrial scale production. Despite the promise of this approach, there are many barriers to the implementation of alternative jet fuels, with price representing the largest hurdle. A recent study by Scown estimates that biosynthetic limonane (a cyclic, C10H20 hydrogenated isoprene dimer) can potentially be sold for as low as $0.73/L, or $2.77/gal, assuming significant improvements in biomass deconstruction, sugar conversion, and host optimization.55 This value is similar to the 10-year average for petroleum-derived jet fuel ($0.66/L or $2.50/gal). Using $0.73/L as the lower limit for the cost of DMCO, it is plausible that DMCO could eventually be a cost-competitive blendstock for bio-based and conventional jet fuels, particularly as governments around the world, and the commercial aviation industry, strive to reduce greenhouse gas emissions.
CONCLUSION
Iron-catalyzed [4+4]-cycloaddition of isoprene, followed by hydrogenation, allowed for a selective, high-throughput process for the synthesis of 1,4-dimethylcyclooctane (DMCO). DMCO has a higher gravimetric and volumetric NHOC compared to conventional jet fuel, along with higher density and lower viscosity. The results suggest that DMCO can be blended with a wide variety of jet fuel blendstocks, including bio-based SPKs, to create high-performance jet fuel blends with enhanced properties. Improved catalyst stability and a transition to robust heterogeneous systems for the [4+4]-cycloaddition step are expected to further enhance the already impressive TONs achieved by the first-generation homogeneous system described here. These tractable challenges can likely be addressed by ligand design and immobilization of the catalysts on solid supports to enable development of a viable commercial process. Studies along these lines are currently being pursued by our groups.
Supplementary Material
ACKNOWLEDGMENT
B.G.H. and K.E.R. thank the Naval Air Warfare Center, Weapons Division PL-219 program for financial support of this work. We also thank Dr. Josanne Woodroffe for conducting viscosity studies of DMCO/HEFA blends, Ms. Alicia Hughes for conducting flash point studies, Dr. Patrick Fedick for GC-MS analysis of DMCOD and DMCO, and Dr. Tim Edwards (AFRL) for supplying HEFA-Jet. C.R.K. thanks the NIH for a Ruth L. Kirschstein National Research Service Award (F32 GM126640).
Footnotes
ASSOCIATED CONTENT
Supporting Information
The Supporting Information is available free of charge via the internet at http://pubs.acs.org.. Characterization data for DMCOD and DMCO including 1H and 13C{1H}NMR spectra and GC traces (PDF)
A patent application has been filed for the methods and materials described in this manuscript.
REFERENCES
- 1.Leitner W; Klankermayer J; Pischinger S; Pitsch H; Kohse-Höinghaus K Advanced biofuels and beyond: chemistry solutions for propulsion and production. Angew. Chem. Int. Ed 2017, 56, 5412–5452 [DOI] [PubMed] [Google Scholar]
- 2.Morris DM; Quintana RL; Harvey BG High-performance jet fuels derived from bio-based alkenes by iron-catalyzed [2+2] cycloaddition. ChemSusChem, 2019, 12, 1646–1652 [DOI] [PubMed] [Google Scholar]
- 3.Harrison KW; Harvey BG Renewable high density fuels containing tricyclic sesquiterpanes and alkyl diamondoids. Sust. Energy Fuels, 2017, 1, 467–473 [Google Scholar]
- 4.Harvey BG; Merriman WW; Koontz TA High density renewable diesel and jet fuels prepared from multicyclic sesquiterpanes and a 1-hexene derived synthetic paraffinic kerosene. Energy Fuels, 2015, 29, 2431–2436 [Google Scholar]
- 5.Harvey BG; Meylemans HA; Gough RV; Quintana RL; Garrison MD; Bruno TJ High-density biosynthetic fuels: the intersection of heterogeneous catalysis and metabolic engineering. Phys. Chem. Chem. Phys 2014, 16, 9448–9457 [DOI] [PubMed] [Google Scholar]
- 6.Harvey BG; Meylemans HA 1-Hexene: a renewable C6 platform for full-performance jet and diesel fuels. Green Chem. 2014, 16, 770–776 [Google Scholar]
- 7.Meylemans HA; Baldwin LC; Harvey BG Low temperature properties of renewable high-density fuel blends. Energy Fuels 2013, 27, 883–888 [Google Scholar]
- 8.Meylemans HA; Quintana RL; Harvey BG Efficient conversion of pure and mixed terpene feedstocks to high density fuels. Fuel, 2012, 97, 560–568 [Google Scholar]
- 9.Meylemans HA; Quintana RL; Goldsmith BR; Harvey BG Solvent-free conversion of linalool to methylcyclopentadiene dimers: a route to renewable high-density fuels. ChemSusChem, 2011, 4, 465–469 [DOI] [PubMed] [Google Scholar]
- 10.Harvey BG; Meylemans HA The role of butanol in the development of sustainable fuel technologies J. Chem. Technol. Biotechnol 2011, 86, 2–9 [Google Scholar]
- 11.Harvey BG; Wright ME; Quintana RL High density renewable fuels based on the selective dimerization of pinenes. Energy Fuels, 2010, 24, 267–273 [Google Scholar]
- 12.Harvey BG; Quintana RL Synthesis of renewable jet and diesel fuels from 2-ethyl-1-hexene. Energy Environ. Sci 2010, 3, 352–357 [Google Scholar]
- 13.Wright ME; Harvey BG; Quintana RL Highly efficient zirconium-catalyzed batch conversion of 1-butene: a new route to jet fuels. Energy Fuels, 2008, 22, 3299–3302 [Google Scholar]
- 14.Zhang X; Pan L; Wang L; Zou J-J Review on synthesis and properties of high-energy-density liquid fuels: hydrocarbons, nanofluids and energetic ionic liquids. Chem. Engin. Sci 2018, 180, 95–125 [Google Scholar]
- 15.Nie G; Zhang X; Han P; Xie J; Pan L; Wang L; Zou J-J Lignin-derived multi-cyclic high density biofuel by alkylation and hydrogenated intramolecular cyclization. Chem. Engin. Sci 2017, 158, 64–69 [Google Scholar]
- 16.Yang J; Li S; Li N; Wang W; Wang A; Zhang T; Cong Y; Wang X; Huber GW Synthesis of jet-fuel range cycloalkanes from the mixtures of cyclopentanone and butanal. Ind. Eng. Chem. Res 2015, 54, 11825−11837 [Google Scholar]
- 17.Li G; Li N; Wang X; Sheng X; Li S: Wang A; Cong Y; Wang X; Zhang T Synthesis of diesel or jet fuel range cycloalkanes with 2-methylfuran and cyclopentanone from lignocellulose. Energy Fuels, 2014, 28, 5112–5118 [Google Scholar]
- 18.Li S; Li N; Wang W; Li L; Wang A; Wang X; Zhang T Synthesis of jet fuel range branched cycloalkanes with mesityl oxide and 2-methylfuran from lignocellulose. Sci. Rep 2016, 6, 32379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Z; Otsuki AL; Mascal M Production of cellulosic gasoline via levulinic ester self-condensation. Green Chem. 2018, 20, 3804 [Google Scholar]
- 20.Chen F; Li N; Li S; Li G; Wang A; Cong Y; Wang X; Zhang T Synthesis of jet fuel range cycloalkanes with diacetone alcohol from lignocellulose. Green Chem. 2016, 18, 5751–5755 [Google Scholar]
- 21.Zhang X; Lei H; Zhu L; Qian M; Chan JC; Zhu X; Liu Y; Yadavalli G; Yan D; Wang L; Bu Q; Wei Y; Wu J; Chen S Development of a catalytically green route from diverse lignocellulosic biomasses to high-density cycloalkanes for jet fuels. Catal. Sci. Technol 2016, 6, 4210–4220 [Google Scholar]
- 22.Sheng X; Li N; Li G; Wang W; Yang J; Cong Y; Wang A; Wang X; Zhang T Synthesis of high density aviation fuel with cyclopentanol derived from lignocellulose Sci. Rep. 2015, 5, 9565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.George KW; Thompson MG; Kang A; Baidoo E; Wang G; Chan LJG; Adams PD; Petzold CJ; Keasling JD; Lee TS Metabolic engineering for the high-yield production of isoprenoid-based C5 alcohols in E. coli. Sci. Rep 2015, 5, 11128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Riazi B; Karanjikar M; Spatari S Renewable rubber and jet fuel from biomass: evaluation of greenhouse gas emissions and land use trade-offs in energy and material markets. ACS Sust. Chem. Eng 2018, 6, 14414−14422 [Google Scholar]
- 25.Johnson MM; Kuper DG (Phillips Petroleum Co., Bartlesville, OK), US3809727, 1974 [Google Scholar]
- 26.Mueller H; Koernig W; Koehl H (BASF, Ludvigshafen), US3714285, 1975 [Google Scholar]
- 27.Dugar D; Nelter B (Visolis Inc. Berkely, CA), US10208008, 2019 [Google Scholar]
- 28.Schmidt LD; Dauenhauer PJ Hybrid routes to biofuels. Nature, 2007, 447, 914–915 [DOI] [PubMed] [Google Scholar]
- 29.Lundberg DJ; Lundberg DJ; Zhang K; Dauenhauer PJ Process design and economic analysis of renewable isoprene from biomass via mesaconic acid. ACS Sust. Chem. Eng 2019, 7, 5576–5586 [Google Scholar]
- 30. a).Ye L; Lv X; Yu H Engineering microbes for isoprene production. Metabol. Engin 2016, 38, 125–138. [DOI] [PubMed] [Google Scholar]; b) Whited GM; Feher FJ; Benko DA; Cervin MA; Chotani GK; McAuliffe JC; Laduca RJ; Ben-Shoshan EA; Sanford KJ Development of a gas-phase bioprocess for isoprene-monomer production using metabolic pathway engineering. Indust. Biotechnol 2010, 6, 152–163 [Google Scholar]
- 31.Hoyt JM; Schmidt VA; Tondreau AM; Chirik PJ Iron-catalyzed intermolecular [2+2] cycloadditions of unactivated alkenes. Science, 2015, 349, 960–963 [DOI] [PubMed] [Google Scholar]
- 32.Kennedy CR; Zhong H; Macaulay RL; Chirik PJ Regio- and diastereoselective iron-catalyzed [4+4]-cycloaddition of 1,3-dienes. J. Am. Chem. Soc 2019, 141, 8557–8573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lindley BM; Wolczanski P. t.; Cundari T. r.; Lobkovsky EB First-row transition metal and lithium pyridine-ene-amide complexes exhibiting N- and C-isomers and ligand-based activation of benzylic C−H bonds. Organometallics 2015, 34, 4656–4668 [Google Scholar]
- 34.van Leeuwen PWNM; Roobeek CF On the mechanism of the nickel-catalysed regioselective cyclodimerization of isoprene. Tetrahedron 1981, 37, 1973–1983 [Google Scholar]
- 35.Wiberg KB The concept of strain in organic chemistry. Angew. Chem. Int. Ed 1986, 25, 312–322 [Google Scholar]
- 36.Anslyn EV, Dougherty DA “Chapter 2: Strain and Stability.” In Modern Physical Organic Chemistry. University Science Books: Mill Valley, CA, 2006; pp 100–109. [Google Scholar]
- 37.Wilson GR III.; Edwards T; Corporan E; Feerks RL Certification of alternative aviation fuels and blend components. Energy Fuels, 2013, 27, 962–966 [Google Scholar]
- 38.Ghosh P; Jaffe SB Detailed composition-based model for predicting the cetane number of diesel fuels. Ind. Eng. Chem. Res 2006, 45, 346–351 [Google Scholar]
- 39.Infineum International, Ltd, Worldwide Winter Diesel Fuel Quality Survey 2014, Infineum International, Ltd., Abingdon, U.K., 2014, https://www.infineum.com/media/80722/wdfs-2014-fullscreen.pdf, accessed June 9, 2019. [Google Scholar]
- 40.Harrison KW; Harvey BG High cetane renewable diesel fuels prepared from bio-based methyl ketones and diols. Sust. Energy Fuels, 2018, 2, 367–371 [Google Scholar]
- 41.Esclapez L; Ma PC; Mayhew E; Xu R; Stouffer S; Lee T; Wang H; Ihme M Fuel effects on lean blow-out in a realistic gas turbine combustor. Comb. Flame, 2017, 181, 82–99 [Google Scholar]
- 42.Heyne JS; Peiffer E; Colket M; Moder J; Edwards JT; Roquemore WM; Shaw C; Li C; Rumizen M; Gupta M Year 3 of the national jet fuels combustion program: practical and scientific impacts. 2018 AIAA Aerospace Sciences Meeting 8−12 January 2018, Kissimmee, Florida: DOI: 10.2514/6.2018-1667 [DOI] [Google Scholar]
- 43.Hui X; Kumar K; Sung C-J; Edwards T; Gardner D Experimental studies on the combustion characteristics of alternative jet fuels. Fuel, 2012, 98, 176–182 [Google Scholar]
- 44.Corporan E; Edwards T; Shafer L; DeWitt MJ; Klingshirn C; Zabarnick S; West Z; Striebich R; Graham J; Klein J Chemical thermal stability, seal swell, and emissions studies of alternative jet fuels. Energy Fuels, 2011, 25, 955–966 [Google Scholar]
- 45.Brem BT; Durdina L; Siegerist F; Beyerle P; Bruderer K; Rindlisbacher T; Rocci-Denis S; Andac MG; Zelina J; Penanhoat O; Wang J. Effects of fuel aromatic content on nonvolatile particulate emissions of an in-production aircraft gas turbine. Environ. Sci. Technol 2015, 49, 13149−13157 [DOI] [PubMed] [Google Scholar]
- 46. http://www.caafi.org/resources/pdf/3.2_SAJF_Benefits.pdf.
- 47.Graham JL; Rahmes TF; Kay MC; Belières J-P; Kinder JD; Millett SA; Ray J; Vannice WL; Trela JA Impact of alternative jet fuel and fuel blends on non-metallic materials used in commercial aircraft fuel systems. Federal Aviation Administration Report DOT/FAA/AEE/2014–10 [Google Scholar]
- 48.Liu Y; Wilson CW Investigation into the impact of n-decane, decalin and isoparaffinic solvent on elastomeric sealing materials. Adv. Mech. Engin 2015. DOI: 10.1155/2012/127430 [DOI] [Google Scholar]
- 49.Vásquez MC; Silva EE; Castillo EF Hydrotreatment of vegetable oil: a review of the technologies and its developments for jet biofuel production. Biomass Bioenergy 2017, 105, 197–206 [Google Scholar]
- 50.Starck L; Pidol L; Jeuland N; Chapus T; Bogers P; Bauldreay J Production of hydroprocessed esters and fatty acids (HEFA)-optimization of process yield. Oil Gas Sci. Technol-Rev. IFP energies nouvelles 2016, 71, 10 DOI: 10.2516/ogst/2014007 [DOI] [Google Scholar]
- 51.Robota HJ; Alger JC; Shafer L Converting algal triglycerides to diesel and HEFA jet fuel fractions. Energy Fuels 2013, 27, 985–996 [Google Scholar]
- 52.Lestari S; Mäki-Arvela P; Beltramini J; Lu GQM; Murzin DY Transforming triglycerides and fatty acids into biofuels. ChemSusChem 2009, 2, 1109–1119 [DOI] [PubMed] [Google Scholar]
- 53.DOE, 2016 Billion-Ton Report, U.S. Department of Energy, Washington, DC, 2016 [Google Scholar]
- 54. [accessed 08/20/2019]. https://www.eia.gov/energyexplained/?page=us_energy_transportation.
- 55.Baral NR; Kavvada O; Mendez-Perez D; Mukhopadhyay A; Lee TS; Simmons BA; Scown CD Techno-economic analysis and life-cycle greenhouse gas mitigation cost of five routes to bio-jet fuel blendstocks. Energy Environ. Sci 2019, 12, 807–824 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.