Abstract
Amyloidosis is a disorder characterized by misfolded precursor proteins that form depositions of fibrillar aggregates with an abnormal cross-beta-sheet conformation, known as amyloid, in the extracellular space of several tissues. Although there are more than 30 known amyloidogenic proteins, both hereditary and non-hereditary, cardiac amyloidosis (CA) typically arises from either misfolded transthyretin (ATTR amyloidosis) or immunoglobulin light-chain aggregation (AL amyloidosis). Its prevalence is more common than previously thought, especially among patients with heart failure and preserved ejection fraction (HFpEF) and aortic stenosis. If there is a clinical suspicion of CA, focused echocardiography, laboratory screening for the presence of a monoclonal protein (serum and urinary electrophoresis with immunofixation and serum free light-chain ratio), and cardiac scintigraphy with 99mtechnetium-labeled bone-tracers are sensitive and specific initial diagnostic tests. In some cases, more advanced/invasive techniques are necessary and, in the last several years, treatment options for both AL CA and ATTR CA have rapidly expanded. It is important to note that the aims of therapy are different. Systemic AL amyloidosis requires treatment targeted against the abnormal plasma cell clone, whereas therapy for ATTR CA must be targeted to the production and stabilization of the TTR molecule. It is likely that a multistep treatment approach will be optimal for both AL CA and ATTR CA. Additionally, treatment of CA includes the management of restrictive cardiomyopathy with preserved or reduced ejection fraction in addition to treating the amyloid deposition. Future studies are necessary to define optimal management strategies for AL CA and ATTR CA and confirm cardiac response to therapy.
Keywords: cardiac amyloidosis, light chain amyloidosis, transthyretin amyloidosis
Introduction
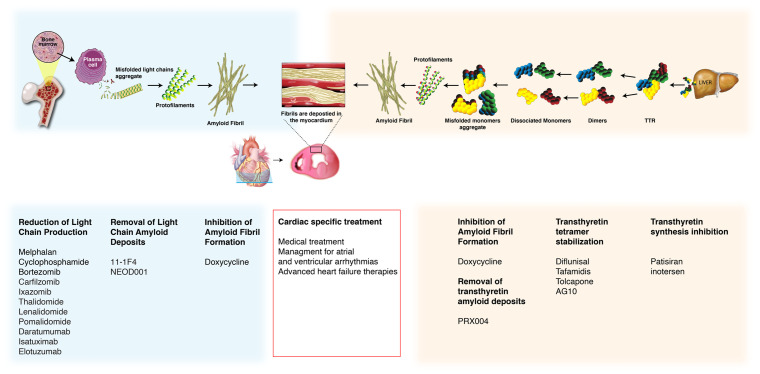
Amyloidosis is characterized by organ dysfunction as a result of deposition of misfolded precursor proteins that form cross-beta-sheet amyloid fibrils in the extracellular spaces. Originally identified via pathology samples, amyloid fibrils’ affinity for Congo Red staining produces a pathognomonic “apple-green” birefringence when visualized under polarized light microscopy. Amyloid fibrils consist of proteins such as serum amyloid P (SAP), glycosaminoglycans, and calcium. There are more than 30 known amyloidogenic proteins (hereditary and non-hereditary), but cardiac amyloidosis (CA) arises from just two major causes: 1) immunoglobulin light-chain aggregation or 2) misfolded transthyretin (TTR)1 (Figure 1). Other amyloid types, including amyloid A, apolipoprotein AI, heavy chain, and atrial natriuretic peptide, can also involve the heart but they are extremely rare. Amyloid light-chain (AL) amyloidosis results from misfolding of overproduced immunoglobulin light-chains (kappa or lambda) secreted by abnormal plasma cells such as those in multiple myeloma. It is a rare disease, with about 3,000 new cases per year in the United States, but the heart is involved in 50–75% of these cases2. The other major cause of CA is the misfolding of TTR (formerly named pre-albumin), which is produced by the liver and serves as a transport protein for thyroxine and other proteins. The TTR protein contains four identical subunits rich in beta-sheets that assemble into a tetramer as two associated dimers. Formation of TTR amyloid fibril requires the dissociation of the tetramer into alternatively folded monomers, which then self-assemble to form insoluble amyloid fibrils3. TTR amyloidosis can be due to wild-type (ATTRwt, no mutation, previously called “senile cardiac amyloidosis”) or a hereditary genetic variant of TTR (ATTRm), of which there are currently more than 100 described. It is not well understood why ATTRwt proteins become kinetically unstable and aggregate, but it appears to involve the aging process4. In fact, up to 25% of patients 85 years and older show ATTRwt amyloid deposits on autopsy studies5. In addition, ATTRwt is much more common than previously thought and is likely underdiagnosed, particularly among patients with HFpEF and calcific aortic stenosis6,7. It is therefore conceivable (but speculative) that pressure loading may promote myocardial TTR deposition. Another possible etiologic mechanism is that the inflammatory processes and oxidative stress associated with calcified aortic stenosis may promote amyloid deposition8,9.
Figure 1. Cardiac amyloidosis: pathogenesis and treatment targets.
The two main types of amyloidosis that affect the heart are immunoglobulin light-chain (AL) amyloidosis (blue), which results from aberrant plasma cell production of monoclonal light-chains, and transthyretin (TTR) amyloidosis (ATTR; orange), resulting from TTR produced by the liver. Misfolded proteins aggregate into amyloid fibrils that deposit extracellularly in the interstitial space of the myocardium. Different treatments and their targets in the pathogenesis of cardiac amyloidosis are presented.
Natural history and prognosis of cardiac amyloidosis
In general, ATTR CA is characterized by years of relative clinical stability despite advanced disease shown by imaging, hemodynamics, and reduced functional capacity. This is commonly followed by a slow but steady decline that transitions to severe and refractory HF. Accordingly, patients with ATTR CA often “look much better” clinically than cardiac imaging and invasive hemodynamics would suggest4. This is in contrast to AL amyloidosis, where the imaging findings may be subtle despite rapidly progressive HF10. The discrepancy has been attributed to the direct cardiotoxicity of circulating free light-chains (FLCs) and pre-fibrillar aggregates in CA caused by AL amyloidosis2,4.
The presence and extent of cardiac involvement is a major determinant of outcome in both AL amyloidosis and ATTR amyloidosis. Despite AL amyloidosis being a disease that fundamentally arises as a result of a hematologic malignancy, the prognosis is primarily driven by the extent of cardiac involvement. The median untreated survival is less than 6 months in patients who present with AL CA and HF11. A validated staging system can be used to predict 5-year survival of CA caused by AL amyloidosis based on the presence of elevated levels of biomarkers such as cardiac troponin, natriuretic peptides, and FLC ratio (Table 1). Prognosis has markedly improved in AL amyloidosis over the last two decades. With current treatment regimens, patients in all but the highest risk group have a median survival of >4 years and some groups have a median survival of >10 years12. However, median survival in stage 4 patients remains poor (around 6 months)13. In contrast, the median survival of untreated patients diagnosed with ATTRm is 2–3 years but depends on the underlying genetic mutation and is around 4 years for ATTRwt4,14 (Table 1).
Table 1. Amyloidosis staging systems and risk stratification.
| Mayo AL Amyloidosis Staging23 | Mayo ATTRwt CA Staging24 | NAC ATTR CA Staging14,25 |
|---|---|---|
|
Prognostic variables:
• cTnT ≥0.025 ng/ml = 1 point • NT-proBNP ≥1,800 pg/ml = 1 point • FLC-diff ≥18 mg/dL = 1 point |
Prognostic variables:
• cTnT ≥0.05 ng/ml = 1 point • NT-proBNP ≥3,000 pg/ml = 1 point |
Prognostic variables:
• NT-proBNP ≥3,000 pg/ml = 1 point • eGFR <45 ml/min/1.73 m2 = 1 point |
|
5-year survival estimate
Stage I (0 points): 59% Stage II (1 point): 42% Stage III (2 points): 20% Stage IV (3 points): 14% |
4-year survival estimate
Stage I (0 points): 57% Stage II (1 point): 42% Stage III (2 points): 18% |
5-year survival estimate
Stage I (0 points): 56–62% Stage II (1 point): 22–38% Stage III (2 points): 12–23% |
Abbreviations: ATTR, transthyretin amyloidosis; ATTRwt, wild-type transthyretin amyloidosis; CA, cardiac amyloidosis; cTnT, cardiac troponin T; eGFR, estimated glomerular filtration rate; FLC-diff, free light-chain difference; NAC, National Amyloidosis Center; NT-proBNP, N-terminal of the prohormone of B-type natriuretic peptide
Advances in the diagnosis of cardiac amyloidosis
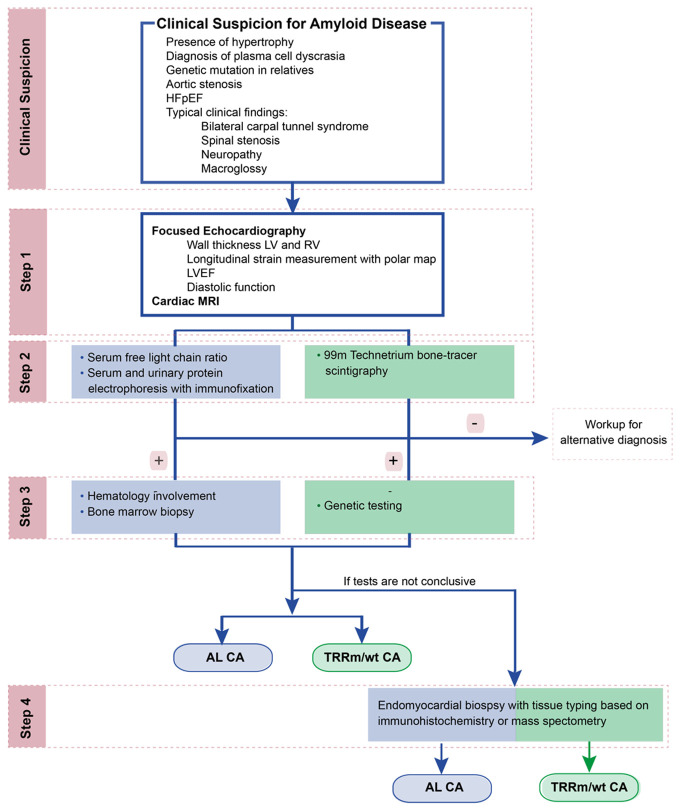
Early diagnosis of CA is crucial, since mortality is high without prompt recognition and treatment. Figure 2 gives a general overview of the diagnostic algorithm for CA used in our center, which is comparable to previously published algorithms15. Often a workup for CA starts after a clinical suspicion of CA, which may include the presence of unexplained ventricular hypertrophy or stiffness on echocardiography, the presence of a non-specific cardiac magnetic resonance imaging (MRI) pattern of late gadolinium enhancement (LGE) suspicious of CA, the diagnosis of a plasma cell dyscrasia, the diagnosis of a genetic variant in a family member, or an antecedent medical history suggestive of an infiltrative disease. For example, bilateral carpal tunnel syndrome is predominantly present in patients with ATTR amyloidosis and can precede heart symptoms by 5–15 years14,16. Spinal stenosis is common in patients with ATTRwt variant and is due to amyloid infiltration in the ligamentum flavum17. Peripheral and autonomic neuropathy can occur in both AL and ATTRm amyloidosis but are uncommon in ATTRwt amyloidosis18 (Table 2).
Figure 2. Diagnostic algorithm for the diagnosis of CA.
AL, amyloid light-chain; ATTRm, hereditary (or mutant) transthyretin amyloidosis; ATTRwt, wild-type transthyretin amyloidosis; CA, cardiac amyloidosis; HFpEF, heart failure with preserved ejection fraction; LV, left ventricle; LVEF, left ventricular ejection fraction; MRI, magnetic resonance imaging; RV, right ventricle.
Table 2. Typical characteristics for AL and ATTR CA.
| AL CA | ATTR CA | |
|---|---|---|
| Age of onset | Median age 63 years | ≥60 years |
| Gender | Male | |
| Race | White (T60A mutation, Irish descent) African American (V122I mutation) |
|
|
Common clinical symptoms |
Atrial fibrillation/flutter | Atrial fibrillation/flutter |
| Carpal tunnel | (Bilateral) carpal tunnel | |
| Spinal stenosis | ||
| Peripheral neuropathy | Peripheral neuropathy | |
| Heart block and/or bundle branch block | Heart block and/or bundle branch block | |
| Low flow, low grade aortic stenosis | Low flow, low grade aortic stenosis | |
| Hypertrophic CMP Low voltage ECG (overall degree of voltage relative to the degree of LV thickening) |
Hypertrophic CMP in older age Low voltage ECG (overall degree of voltage relative to the degree of LV thickening) |
|
| Renal (nephrotic syndrome), GI symptoms | Distal biceps tendon rupture | |
| Macroglossia | ||
| Periorbital purpura | ||
| Median survival | 1–3 years | 2–6 years |
| Therapy | Combination chemotherapy (alkylating agents, proteasome inhibitors, steroids) Monoclonal antibodies Immunomodulatory therapy Autologous stem cell transplant Heart transplant |
siRNA TTR stabilizer Amyloid fibril disruptor Heart transplant Combined heart and liver transplant |
Abbreviations: AL, amyloid light-chain; ATTR, transthyretin amyloidosis; CA, cardiac amyloidosis; CMP, cardiomyopathy; ECG, electrocardiogram; GI, gastrointestinal; LV, left ventricle; siRNA, small interfering RNA; TTR, transthyretin
Echocardiography
The appropriate next step in diagnosing CA is a focused echocardiogram if not already performed or, in case of insufficient image quality, a cardiac MRI (Figure 3). Typical findings are left ventricular (LV) wall thickness >12 mm, the presence of right ventricular (RV) hypertrophy, and abnormalities of diastolic function or the presence of restrictive physiology. Longitudinal strain imaging is used to measure the actual deformation of myocardium in specific LV segments, and quantification is displayed as a polar map. Several distinguishing characteristics of CA can be seen on echocardiogram. For example, apical sparing, in which the apical LV segments have normal or near-normal strain compared with the mid and basal segments, is commonly seen in CA. The easily recognizable bull’s-eye (“cherry-on-top”) pattern on the polar map can help differentiate CA from other forms of LV hypertrophy with good sensitivity and specificity19. Historically, the characteristic myocardial “sparkling” pattern has low sensitivity and specificity and should not be relied on18. Although LV ejection fraction is usually preserved in CA, cardiac output is low because of decreased ventricular volume20. Another important echocardiographic clue that can differentiate CA from other diseases is the presence of biventricular and concomitant valvular thickening, which is not often seen in hypertensive heart disease of hypertrophic cardiomyopathy21. A final classic hallmark of CA to look for is the combination of low voltage on electrocardiogram (ECG) and increased LV wall thickness on echocardiogram22.
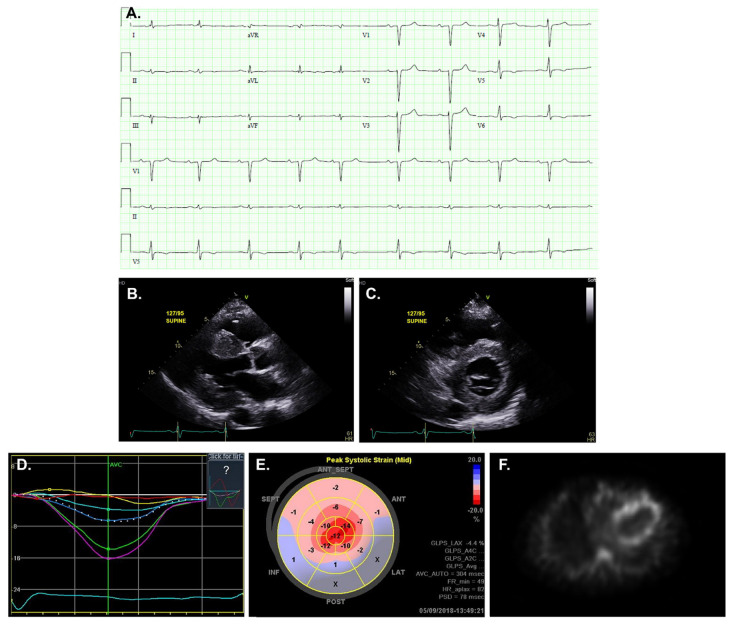
Figure 3. Diagnostic tests for cardiac amyloidosis.
(A) Electrocardiogram showing characteristic low voltages relative to left ventricle wall thickness, with a pseudo-infarct pattern with Q waves in the early precordial leads mimicking a prior anteroseptal myocardial infarction. (B and C) Transthoracic echocardiogram, parasternal long-axis and short-axis view, showing increased ventricular wall thickness and thickening of the mitral valve. (D and E) Longitudinal strain imaging using two-dimensional speckle tracking echocardiography reveals the characteristic bull’s-eye pattern of apical sparing. (F) 99mTechnetium pyrophosphate scan shows grade 3 myocardial radiotracer uptake characteristic of transthyretin cardiac amyloidosis.
In the future, the diagnostic accuracy of echocardiography will likely increase by combining several parameters. A recent study showed that amyloidosis can be confirmed or excluded on the basis of echocardiogram scores in about 50% of cases26. These scores include wall thickness, E/e’ ratio, longitudinal strain, and tricuspid annular plane excursion. In another study, Zhang and coauthors investigated the potential of automated cardiac image interpretation for the detection of CA and other cardiac diseases and demonstrated that deep machine learning models may help to automatically detect these diseases in large healthcare imaging systems27. At this point, however, despite increasing interest, future studies are necessary to understand how machine learning can be used to improve the diagnostics of CA.
Cardiac magnetic resonance imaging
Cardiac MRI is useful for the diagnosis of CA. In addition to the typical structural abnormalities in CA seen with echocardiography, global subendocardial enhancement and transmural LGE or focal patchy LGE are typical MRI features of CA28. Traditional LGE imaging techniques require an operator-determined null point, which is defined as the inversion recovery time at which the normal myocardium appears black. This can be challenging in CA, and difficulty nulling is strongly suggestive of CA with sensitivity of 100%29. Additionally, cardiac MRI can reveal the characteristic LGE pattern that is diffuse and subendocardial without following a coronary artery distribution. The LGE pattern can also be seen in the right ventricle, and even the atrial walls, and can be transmural and patchy in ATTRwt CA. This LGE pattern is highly sensitive (93%) and specific (70%) for CA with an overall negative predictive accuracy of 84%29. Of note, this high negative predictive value of cardiac MRI makes this test very interesting to screen asymptomatic family members of patients with genetic forms of amyloidosis. In addition, CA patients with transmural LGE have a fivefold increase in mortality when compared to with those without LGE29. In addition to LGE, native (pre-gadolinium contrast) T1-myocardial mapping techniques, which provide a combined signal from myocyte and extracellular space, are also useful in CA diagnosis. Significantly increased native T1 times are seen in CA and have been associated with outcomes in patients with systemic AL amyloidosis30. Native T1 mapping offers a promising alternative for LGE, especially in patients with severe renal dysfunction in whom gadolinium contrast is contraindicated. However, because of the lack of reproducibility for different scanners, a normal T1 value should be established in each institution28,30,31.
Laboratory testing of circulating proteins
If amyloidosis is suspected, then testing for paraproteins should be performed as part of the workup for diagnosis/exclusion of AL amyloidosis. The traditional approach is to perform serum and urine protein electrophoresis (SPEP and UPEP), with the finding of an M-spike suggestive of a possible monoclonal protein. However, these tests have low sensitivities for AL amyloidosis and should be complemented by immunofixation of the serum and urine and the calculation of the serum FLC (sFLC) ratio (ratio of free kappa over lambda light-chain levels). Mass spectrometry of blood and urine may replace serum and urine immunofixation to further improve sensitivity32. In AL amyloidosis, the sFLC assay will show an abnormal kappa–lambda ratio that can additionally be used to track responses to therapy. Notably, an abnormal sFLC ratio must be examined in the context of each patient because a significant subset of individuals may have low-grade, non-specific circulating light-chains, and it is not uncommon (20 to 40% of cases) for patients with ATTR CA to also have an unrelated monoclonal gammopathy of undetermined significance (defined as an overgrowth of clonal plasma cells <10% along with a serum monoclonal protein <3 g/dl but without end-organ damage) without overproduction of light-chains33. Therefore, hematology input can be very helpful in understanding the exact cause of CA in these cases. In general, an abnormally low kappa–lambda ratio of <0.26 is suggestive of a monoclonal lambda light-chain process, while an abnormally high kappa–lambda ratio of >1.65 is suggestive of a monoclonal kappa light-chain process. Because light-chains are excreted by the kidney, the absolute serum levels of both kappa and lambda will be elevated in renal dysfunction but the ratio should remain normal21. It is important to recognize that while circulating TTR is detectable, tetramer stability and overall “burden” of abnormal TTR deposition cannot be quantified by routine measurements of circulating pre-albumin.
Recently, a proof-of-concept study showed that an expert-independent machine learning prediction model for CA relying on routinely determined laboratory parameters was able to generate a CA-related HF profile that may help increase disease awareness among physicians and improve timely diagnosis34. With the continuous improvement in machine learning techniques and the integration of imaging and laboratory parameters, this approach holds promise for the future.
Nuclear imaging
Myocardial bone-avid radiotracer (such as 99mtechnetium pyrophosphate [99mTc-PYP], 3.3-diphosphone-1,2-propanodicarboxylic acid [99mTc-DPD], or hydroxymethylene diphosphonate [99mTc-HMDP]) uptake is highly specific for ATTR CA. The exact mechanism underlying the myocardial retention of these tracers is unknown but may be due to the presence of cardiac tissue microcalcifications that are more common in ATTR amyloidosis than in AL amyloidosis4. Multiple guidelines support a non-biopsy diagnosis of ATTR CA using 99mTc-PYP/DPH/HMDP scintigraphy but emphasize both its use in the appropriate clinical context (e.g. patients with high pre-test likelihood) and the crucial need to first rule out AL amyloid cardiomyopathy with the combination of SPEP and UPEP with immunofixation and sFLC ratio33. In our center, laboratory testing and a 99mTc-bone scintigraphy are often planned together in the work up for CA because of logistic reasons and physician preference (Figure 2, Step 2). Technetium-labeled cardiac scintigraphy scans with bone-seeking tracers are evaluated using either a qualitative visual grading score (grade 0 means no myocardial uptake and normal rib uptake while grade 3 means myocardial uptake greater than rib uptake) or a semi-quantitative method using the heart to contralateral lung (HCL) uptake ratio or heart to whole body ratio. Several studies have shown that there is a significant myocardial uptake in ATTR CA (grade 2 or 3) but no to mild uptake in AL CA (grade <2)21,35,36. A large multicenter study assessed the diagnostic accuracy of all three previously mentioned tracers in 1,217 patients with suspected CA and found that grade 2 or 3 myocardial radiotracer uptake and the absence of a monoclonal protein in serum or urine (based on serum and urine immunofixation and sFLC assay) had a specificity and positive predictive value for ATTR CA of 100%, albeit with a sensitivity of 70%37. If there is any inconsistency between different tests, further investigation (e.g. with endomyocardial biopsy) is necessary.
Lately, there is increasing interest in using thioflavin-analogue tracers, such as 18F-florbetapir, 18F-florbetapen, 18F-flutemetamol, and 11C-labeled Pittsburg compound-B. These radiotracers are promising for the quantification and assessment of response to therapy in the future, but further data are needed to define their accuracy and additive value to the care of patients with suspected CA.
Genetic testing
Differentiating the ATTRwt variant and the ATTRm variant is done by testing the TTR gene for a mutation38. The ATTRm variant can manifest as a polyneuropathy, cardiomyopathy, or a mixed phenotype. The most common mutation in the United States, present in 3.4% of African Americans, is V122I, in which there is an isoleucine substitution for valine at the 122nd amino acid position4. The second most common mutation in the United States, T60A, is seen in patients of Irish descent39. Outside the US, the V30M is the most frequent pathogenic mutation and is especially present in endemic areas in Portugal, Sweden, Japan, and South America40.
Beyond diagnostics, genetics may also help to improve therapy-related outcomes in AL amyloidosis. Fluorescence in situ hybridization genetics show a t(11;14) variation in approximately 50% of patients. Patients with this translocation appear to have lower response rates to bortezomib-containing regimens but may see a greater benefit from an alkylating agent than those without the translocation41,42.
Endomyocardial or fat pad biopsy
When amyloid fibrils infiltrate the heart, deposition is most commonly diffuse with amyloid depositions surrounding each myocyte. Thus, endomyocardial biopsy is 100% sensitive for the diagnosis of CA43. However, it should be noted that the myocardial deposits may be heterogeneous, so multiple biopsies are recommended, and each biopsy carries a small risk of right ventricular perforation (1%)44. Although birefringence under polarized light microscopy is considered histopathologically diagnostic for amyloidosis, Congo Red staining of the biopsy tissue alone is sometimes not sufficient and other methods need to be used45. In particular, Congo Red staining requires a skilled and experienced pathologist who may not be available in all institutions to accurately visualize the apple-green birefringence under polarized light microscopy. Some pathology laboratories rely on fluorochrome dyes such as thioflavin S staining, which can be a more sensitive technique even though it is not a specific stain for amyloid fluorescence. Moreover, subtyping the type of amyloidosis by the pathologist is absolutely crucial. Subtyping can be performed by immunohistochemistry and/or mass spectrometry for accurate identification of the precursor protein type18,46.
While commonly performed, fat pad biopsy has diagnostic limitations, and a negative fat pad biopsy does not rule out amyloidosis. The sensitivity is 60–80% sensitive in AL amyloidosis but only 14% in ATTRwt. Nevertheless, if amyloid is present in a fat pad biopsy and clinical features of CA are evident in imaging studies, endomyocardial biopsies are no longer necessary for diagnosis.
Right heart catherization
Hemodynamic analysis is important for managing HF in CA but is less useful in the diagnosis of amyloidosis. Right heart catheterization is nonspecific but often shows restrictive filling patterns that can inform disease severity and prognosis. Cardiac output can be preserved but is more commonly low in patients with progressive HF symptoms.
Advances in the treatment of cardiac amyloidosis
Until recently, treatment options for AL CA and ATTR CA were limited. However, several therapeutics have been recently approved to target the amyloid deposits directly, and multiple novel therapeutics are in development, holding the promise to revolutionize the clinical management of these conditions. Figure 1 and Table 3 present different targets of therapy as well as practical aspects of each treatment. The aims of therapy for ATTR and AL CA are different. Systemic AL amyloidosis requires treatment targeted against the abnormal plasma cell clone, whereas therapies for ATTR CA target the production and destabilization of the TTR molecule. The majority of these treatments interrupt particular steps during amyloidogenesis3, while some use monoclonal antibodies to target the removal of existing deposits. A multistep approach will likely be the most optimal way to manage both AL CA and ATTR CA, and meticulously conducted clinical trials are necessary to decide which approach is best3. Additionally, treatment of CA must include the management of restrictive cardiomyopathy with preserved or reduced ejection fraction in addition to treating the amyloid disease.
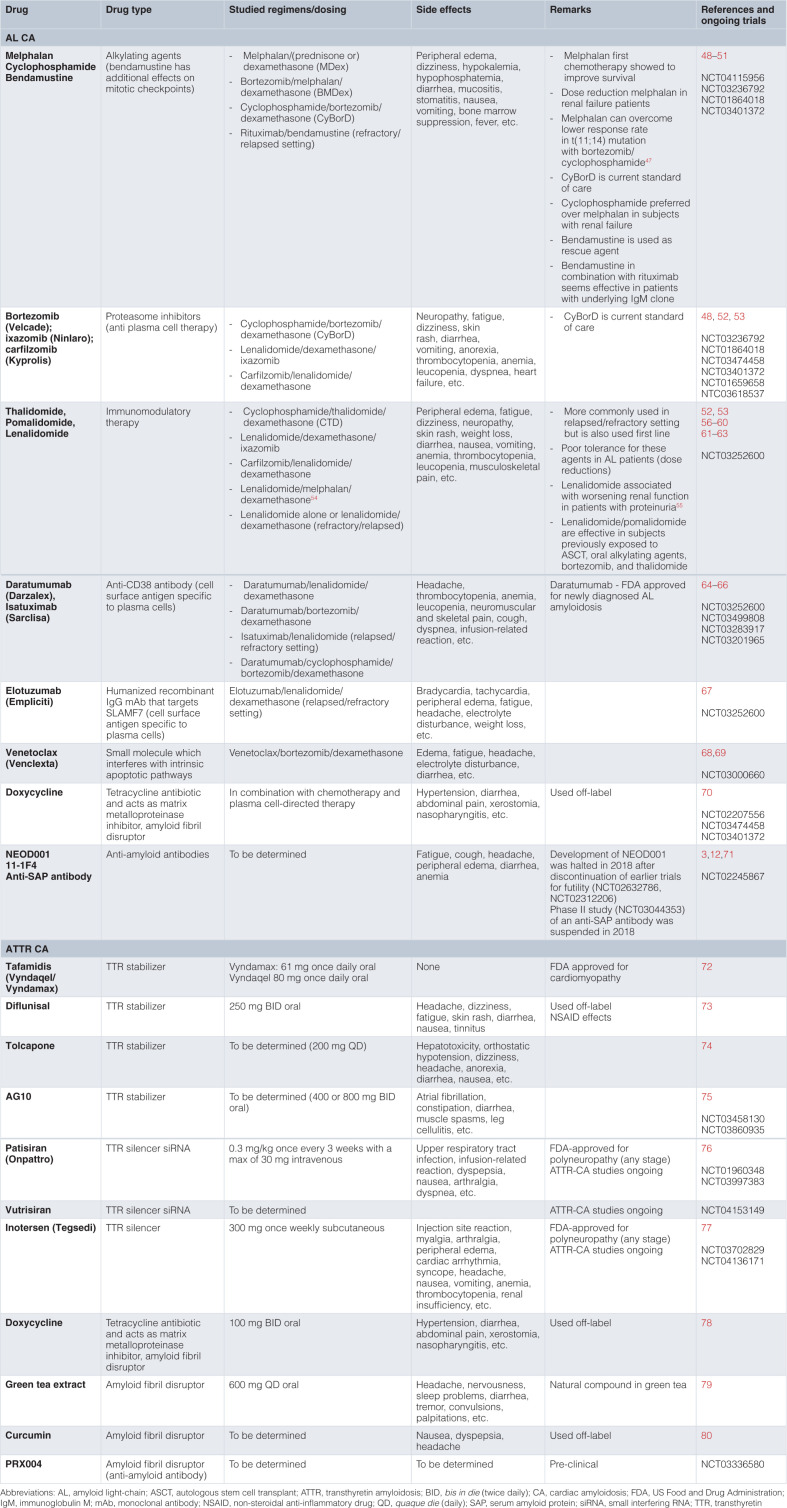
Table 3. Therapies for current therapies for AL and ATTR CA.
Light-chain amyloidosis
Unusual serum light-chains compel disease progression in AL CA, and thus the main focus during treatment is maximum reduction of pathogenic light-chain levels. The majority of studies show that overall survival and the degree of hematologic response are firmly connected, highlighting the necessity to change the treatment approach if light-chain levels are not suitably reduced by the existing regimen12.
Common light-chain suppressive therapies, used alone or in combination, are alkylators, proteasome inhibitors, steroids, immunomodulatory drugs (thalidomide-related drugs), antibodies directed to cell surface antigens on plasma cells, and autologous stem cell transplantation (ASCT). However, there are very few controlled studies investigating the most appropriate treatment regimen for different settings, and the design of individual treatment strategies relies on expert opinions mostly based on retrospective trials47. The first regimen to improve outcomes in AL amyloidosis was a combination of melphalan and a steroid. Owing to the poor outcomes with standard chemotherapy (median survival of 6 to 18 months), high-dose melphalan followed by ASCT was investigated and showed a significant improvement in both short- and long-term survival81–84. However, there is conflicting evidence regarding the role of ASCT for the treatment of amyloidosis. Trials that suggest superiority of ASCT over standard chemotherapy are mostly retrospective case(-control) studies, and these studies are criticized for the fact that patients who underwent ASCT were overall healthier than those who did not. The only randomized trial to date showed no superiority of ASCT over standard chemotherapy, but this trial was published more than 10 years ago and the chemotherapy arm is not representative of current “standard” chemotherapy/immunotherapy regimens85. Currently, most patients with a new diagnosis of AL amyloidosis are started on a regimen combining the alkylator cyclophosphamide, the proteasome inhibitor bortezomib, and the steroid dexamethasone (CyBorD). This regimen yielded an overall hematologic response rate of 60% in a European study of 230 patients and even higher hematologic response rates up to 94% in smaller studies86–88. Other proteasome inhibitors, which cause cell cycle arrest and cellular apoptosis, especially in proliferating malignant cells, such as carfilzomib and the oral agent ixazomib have shown promise but because of their treatment toxicities are not typically chosen as first-line therapy12,89,90. Immunomodulatory agents (e.g. thalidomide, pomalidomide, and lenalidomide), which inhibit pro-inflammatory cytokine production and angiogenesis and induce apoptosis, are also commonly used in the treatment of AL amyloidosis, though rarely as first-line therapies3. For example, pomalidomide was shown to be effective in relapsed and refractory AL amyloidosis patients91. Of note, these therapies are associated with an increased thrombosis risk.
One of the most revolutionary advancements in AL amyloidosis management in the last several years comes from the ANDROMEDA phase III trial (NCT03201965), which demonstrated promising results of up-front subcutaneous daratumumab added to CyBorD. Daratumumab is a monoclonal antibody directed against CD38, a cell surface-specific antigen on myeloma cells, that can lead to plasma cell death through antibody-dependent and complement-dependent cytotoxicity. In the ANDROMEDA trial, daratumumab in combination with CyBorD significantly improved the rate of hematologic complete response compared to CyBorD alone (53.3% versus 18.1%, odds ratio [OR] 5.1, 95% confidence interval [CI] 3.2 to 8.2, P <0.0001)92. Approximately 20% of AL amyloidosis patients have advanced cardiac involvement at diagnosis (stage IIIb), and treatment of these patients remains an unmet need. Ideally, these patients need a very rapidly acting, safe regimen93. The safety profile and rapidity of action of subcutaneous daratumumab make this agent very appealing in this setting, and a phase II trial is currently ongoing (NCT04131309). Other monoclonal antibodies of interest are isatuximab and elotuzumab (directed against SLAM7)3,94. Notable toxicities of monoclonal antibodies are infusion reaction and hypogammaglobulinemia12. Venetoclax is a small molecule that binds with high affinity to intrinsic pro-apoptotic proteins and induces apoptosis95,96 and is used as a second- or third-line therapy or used in conjunction with other standard therapies.
Recently, it was shown in a phase II trial that bendamustine, an alkylating agent with additional effects on mitotic checkpoints, in combination with dexamethasone can prolong survival in patients with relapsed or refractory AL amyloidosis and is relatively well tolerated, so this regimen can compete with other drug regimens97.
Besides light-chain suppressive therapies, pharmacologic strategies focused at the removal of amyloid deposits are also of interest in the treatment of AL amyloidosis. Doxycycline, a tetracycline antibiotic, may mitigate the cardiotoxic effects of light-chain amyloid fibril deposition in the extracellular cardiac environment. In a retrospective cohort study of 103 patients with AL CA, 24-month survival improved from 40% to 82% by administering doxycycline along with chemotherapy, while cardiac response to therapy improved threefold70. Although this early study is promising, rigorous clinical trial data are currently lacking98.
Animal studies have suggested that exogenous administration of anti-amyloid antibodies (11-1F4, NEOD001) may expedite the clearance of amyloid deposits by phagocytes70,99,100. In a human study of patients with cardiac involvement, 11-1F4 led to a statistically significant improvement in global longitudinal strain after 12 weeks of follow up3. NEOD001 is an antibody with the potential to both bind to soluble aggregates of amyloid protein and help in the clearance of amyloid deposits in tissues. Despite promising results in a phase I/II study, a phase III study of NEOD001 treatment failed to meet its endpoint and a second phase III trial was stopped for futility71,100,101. Another treatment approach targets SAP, a glycoprotein that stabilizes and protects amyloid fibrils from degradation40. In a phase I trial that administered both an agent to deplete circulating SAP and a monoclonal antibody targeting SAP, there was modest evidence of decreased amyloid deposits102. However, a phase II study (NCT03044353) of an anti-SAP antibody specifically focusing on CA (both ATTR and AL CA) was suspended in 2018 owing to a “change in benefit/risk profile”12. Therefore, additional studies are needed to determine the efficacy and practicality of an anti-SAP treatment approach.
Transthyretin amyloidosis
Previously, ATTR CA could be treated only with organ transplantation, but now pharmaceutical therapies can slow or halt ATTR CA progression and favorably affect clinical outcome. Early recognition remains essential to afford the best treatment efficacy.
Orthotopic liver transplantation was first proposed in 1990 as a potentially curative treatment for ATTRm-related polyneuropathy. Liver transplantation removes the source of mutated TTR molecules, so is not a treatment option for patients with ATTRwt, and was shown to prolong survival in ATTRm patients. However, tissue accumulation of TTR fibrils can continue after liver transplantation, likely because existing TTR amyloid fibrils promote subsequent deposition of TTRwt40,103. Combined heart and liver transplant is feasible in selected patients with ATTRm cardiomyopathy, and small studies suggest that dual organ transplant is associated with a better prognosis than cardiac transplant alone, although this remains a point of controversy40,104,105. Based on current insights and newly available therapies to slow disease progression, heart transplant alone is likely sufficient to treat advanced forms of ATTR CA40.
TTR tetramer stabilizers (diflunisal, tafamidis, AG10, tolcapone) have emerged as a novel class of therapeutics for ATTR and stabilize the tetramers so that the formation of misfolded monomers is inhibited. The non-steroidal anti-inflammatory drug (NSAID) diflunisal was one of the first TTR tetramer stabilizers identified106. A phase I study showed that diflunisal can stabilize TTR tetramers and prevent amyloid fibril formation in vitro40. However, a small single-arm open-label study with an average follow up of <1 year could not show an effect on cardiac dysfunction107. Diflunisal appears to be safe, but the adverse effects from chronic NSAID treatment are of concern, especially in a HF population. The small molecular ligand tafamidis effectively and selectively binds TTR without NSAID influence. It dose-dependently inhibits wild-type TTR amyloidogenesis as well as provides stability to V30M and V122I with similar potency and efficacy108. In the ATTR-ACT trial, a phase III trial of 441 patients with wild-type and hereditary ATTR CA, tafamidis led to a reduction in all-cause mortality (hazard ratio [HR] 0.70, 95% CI 0.51 to 0.96), with a number needed to treat for the combined endpoint of all-cause mortality and cardiovascular-related hospitalization of 7.572. The Kaplan-Meier survival curves started diverging after 18 months of treatment, which is in agreement with the concept of tafamidis being a disease-modifying drug40. It is currently unknown if tafamidis is also effective in patients with advanced amyloid disease (NYHA class IV HF), since these patients were excluded from the study.
AG10 is a selective, oral TTR stabilizer that mimics a protective TTR mutation and binds to wild-type TTR with higher affinity than tafamidis or diflunisal do. Recently, the positive results of a phase II trial with AG10, demonstrating that the drug is well tolerated and can achieve near-complete stabilization of TTR, has led to the initiation of a phase III trial (NCT03860935)75. Tolcapone, an FDA-approved adjuvant treatment for Parkinson’s disease, binds to the thyroxine-binding pocket at the TTR dimer–dimer interface with higher affinity than tafamidis and is a stronger aggregation inhibitor than tafamidis109. However, despite its high potential for TTR stabilization, tolcapone should be used with caution because of a serious risk of acute fulminant liver failure21.
TTR synthesis inhibitors (patisiran and inotersen) work by targeting the TTR mRNA3. Patisiran, a small interfering RNA, targets the TTR gene76. It is delivered to the cytoplasm via endosomal endocytosis of the lipid nanoparticle, which is given as an intravenous infusion. Patisiran binds to the RNA-induced silencing complex, inducing the separation of the two RNA strands and enabling the antisense strand to bind to TTR mRNA. The substrate is cleaved and is subsequently degraded, resulting in TTR protein level reduction110. Patisiran was approved in the US and Europe for the treatment of ATTRm-related polyneuropathy after the positive findings regarding neurological status in the phase III APOLLO trial76. Based on a substudy of patients with evidence of CA in the APOLLO trial, it seems that patisiran may halt or reverse the progression of cardiac manifestations of ATTRm111. Indeed, a separate small study demonstrated that patisiran could reduce extracellular volume measured by cardiac magnetic resonance and thus provides evidence for ATTR cardiac amyloid regression112. The purpose of the ongoing APOLLO-B study (NCT03997383) is to specifically evaluate the efficacy of patisiran on functional capacity, hospitalizations, and mortality in patients with ATTR CA. Inotersen, a second-generation antisense oligonucleotide that binds to a region of TTR mRNA, elicits enzymatic degradation and reduced protein expression in both ATTRwt and ATTRm21,113. In the NEURO-TTR trial, which was a multicenter, randomized placebo-controlled phase III trial, inotersen demonstrated a significant improvement in neurologic functioning and quality of life compared to the placebo arm to the extent that inotersen has now received FDA approval for patients with ATTRm-related polyneuropathy77. However, this study was not powered to measure the effects on cardiac disease, leaving the effects of inotersen on CA unclear. An open-label study is currently investigating the effect of inotersen on cardiac structure changes in patients with both wild-type and hereditary ATTR CA (NCT03702829).
As discussed for AL amyloidosis, antibodies directed at SAP are of interest for treating ATTR CA, but the efficacy of this treatment approach in CA has yet to be demonstrated. After discontinuation of a phase II and a phase I trial (https://adisinsight.springer.com/drugs/800038353), there are no evaluations of anti-SAP antibody ongoing40. A monoclonal antibody designed to target TTR amyloid deposits (PRX004) did enter clinical evaluation with an ongoing phase I study (NCT03336580) in ATTRm patients.
Cardiac-specific treatment
Although management of the amyloid disease is the backbone of treatment for CA, treating cardiac dysfunction in parallel is of great importance. The natural history of CA includes progressive HF, complicated by arrhythmias and conduction system disease4.
Medical therapy. The mainstay of medical therapy is often volume management. Keeping patients in a euvolemic state can be very challenging, since both excessively low and high filling pressures are not well tolerated21. Patients with CA can be prone to the challenging combination of hypotension with concomitant volume overload due to diastolic dysfunction, coexisting renal or hepatic dysfunction leading to reduced serum albumin concentration, and concomitant autonomic dysfunction from amyloid deposition leading to postural hypotension. Salt restriction and appropriate diuretic doses are essential for most patients. If symptomatic hypotension limits the ability to achieve euvolemia, midodrine can be useful. Classic HF drugs are not as useful in the setting of CA. For example, beta-blockers and angiotensin-converting enzyme inhibitors are not well tolerated or not effective in CA, most likely because of dependence of the cardiac output on heart rate and the tendency for orthostatic hypotension114. Moreover, non-dihydropyridine calcium channel blockers bind to amyloid fibrils and are relatively contraindicated owing to the risk of profound hypotension and syncope21,115–117. Digoxin is usually avoided in CA because of concerns of increased risk of toxicity; however, it may be used with caution for rate control in atrial fibrillation given its lack of negative inotropy21.
Rhythm management. Atrial arrhythmias are common in patients with amyloidosis118. Maintenance of normal sinus rhythm is preferable owing to the importance of atrial contribution to cardiac output. Importantly, anticoagulation in patients with atrial fibrillation, and even in patients with normal sinus rhythm but poor atrial function, is important because of the high risk of thromboembolic complications in amyloid patients. It is therefore recommended to perform transesophageal echocardiography before elective cardioversion in all patients with amyloidosis, even if they have been on consistent therapeutic anticoagulation119. Pacemakers are indicated for heart block and symptomatic bradycardia, which is more prevalent in ATTR CA than in AL CA120,121. The role of intracardiac defibrillators, however, is controversial. Traditionally, implantable cardioverter defibrillator (ICD) placement was considered contraindicated in patients with CA owing to poor overall prognosis, a purported lack of successful resuscitation with ICD shocks in CA patients with ventricular arrhythmias, and many episodes of sudden cardiac death caused by bradyarrhythmias121. In general, though, cardiologists currently follow traditional ICD criteria for primary and secondary prevention, since the life expectancy of most CA patients now exceeds 1 year thanks to advancements in therapy122,123.
Advanced heart failure therapies. Because of the advancements in treatment of ATTR and AL amyloidosis, advanced HF therapies are increasingly considered in managing CA. Generally, CA patients are not considered suitable candidates for durable LV assist devices (LVAD) owing to a small LV cavity impeding outflow cannula placement or because of biventricular involvement. Recent case series, however, have shown that in carefully selected patients with CA, LVAD (and especially a total artificial heart) is feasible and may be a lifesaving therapy or bridge to transplantation124–126.
Early reports of cardiac transplantation for amyloidosis were overall discouraging, but there has been a significant improvement in outcomes in the last decade, with outcomes now matching those of non-CA restrictive cardiomyopathies. This likely reflects better light-chain suppressive therapies and a higher proportion of transplants for ATTR amyloidosis127. Patients with AL amyloidosis, more than ATTR amyloidosis, may have extra-cardiac involvement that would exclude heart transplantation or lead to worse post-transplant outcomes. Nevertheless, the relatively high mortality on the transplantation list has prompted their higher priority in the 2018 revision of the U.S. heart transplant policy (Status 4)128,129. SAP labeled with radioactive 123iodine has been used as a tracer to determine the extent and distribution of amyloid deposits by scintigraph and turnover studies130,131. Similarly, it has been shown that radioiodinated p5+14 can bind specifically to amyloid deposits in tissues. Currently, a single-center phase I study is evaluating if 124I-p5+14 positron emission tomography/computed tomography can be used as an imaging technique to identify and quantify whole body and/or organ amyloid deposition in systemic amyloidosis (NCT03678259). Recent data show that in AL CA patients who are young and healthy enough to receive chemotherapy, heart transplant followed or preceded by chemotherapy and eventual hematopoietic stem cell transplant is a reasonable treatment plan. Most heart transplant programs require that AL amyloidosis patients demonstrate a reasonable response to chemotherapy and are a candidate for bone marrow transplantation in order to be considered for heart transplantation. The most common cause of death after heart transplant/ASCT in AL amyloidosis is the recurrence of light-chain production with amyloid deposition in the heart and other organs129. In contrast, patients with ATTR CA are more likely to have isolated cardiac involvement, so require fewer systemic therapies, and have better survival following heart transplantation129. ATTR is predominantly a disease of the elderly, and progression is usually slow, so significant damage to the transplanted heart is unlikely. Dual heart-liver transplants were originally proposed for ATTR CA because of the hepatic production of TTR but are now seldomly performed.
Conclusion
CA is caused, in the vast majority of cases, by either TTR or AL amyloidosis. Its prevalence is more common than previously thought, especially among patients with HFpEF and aortic stenosis. If there is a clinical suspicion of CA, focused echocardiography, SPEP and UPEP with immunofixation and sFLC ratio, and/or 99mTc cardiac scintigraphy are the preferred initial diagnostic tests. In some cases, more advanced diagnostic techniques are necessary. Treatment depends on the underlying form of amyloidosis and targets the formation and deposition of amyloid fibrils. In case of AL CA, close collaboration with a hematologist is necessary. Besides treating the amyloid disease, these patients often need treatment of HF and other cardiac symptoms. In advanced stages, cardiac assist devices and transplantation can be pursued, especially in the case of ATTR CA. The fields of diagnosis and management of both AL CA and ATTR CA are rapidly evolving. Future research investigating optimal management strategies for AL CA and ATTR CA and techniques to follow cardiac response to therapy will further improve the management of CA and increase the quality of life of CA patients.
Abbreviations
99mTc-PYP, 99mTechnetrium pyrophosphate bone scintigraphy; 99mTc-DPD, 99mTechnetrium 3.3-diphosphone-1,2-propanodicarboxylic acid bone scintigraphy; 99mTc-HMDP, 99mTechnetrium hydroxymethylene diphosphonate bone scintigraphy; AL, amyloid light-chain; ASCT, autologous stem cell transplantation; ATTRwt, wild-type transthyretin amyloidosis; ATTRm, hereditary (or mutant) transthyretin amyloidosis; CA, cardiac amyloidosis; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; LGE, late gadolinium enhancement; LV, left ventricle; MRI, magnetic resonance imaging; NSAID, non-steroidal anti-inflammatory drug; NYHA, New York Heart Association Class; TTR, transthyretin; sFLC ratio, serum free light-chain ratio; SPEP, serum protein electrophoresis; UPEP, urinary protein electrophoresis
The peer reviewers who approve this article are:
Marina Ramirez-Alvarado, Department of Immunology, Mayo Clinic, Rochester, MN, USA
James C Fang, Division of Cardiovascular Medicine, University of Utah, Salt Lake City, UT, USA
Seung-Pyo Lee, Cardiovascular Center, Division of Cardiology, Department of Internal Medicine, Seoul National University Hospital, Seoul, South Korea
Funding Statement
P.N. is supported by the Frans Van de Werf Fund for Cardiovascular Research (KULeuven, Belgium). W.H.T. is supported by the National Institutes of Health (R01HL126827), the Collins Family Fund, and the Wortzman Family Fund.
The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wechalekar AD, Gillmore JD, Hawkins PN: Systemic amyloidosis. Lancet. 2016; 387(10038): 2641–54. 10.1016/S0140-6736(15)01274-X [DOI] [PubMed] [Google Scholar]
- 2. Falk RH, Alexander KM, Liao R, et al. : AL (Light-Chain) Cardiac Amyloidosis: A Review of Diagnosis and Therapy. J Am Coll Cardiol. 2016; 68(12): 1323–41. 10.1016/j.jacc.2016.06.053 [DOI] [PubMed] [Google Scholar]
- 3. Zhang KW, Stockerl-Goldstein KE, Lenihan DJ: Emerging Therapeutics for the Treatment of Light Chain and Transthyretin Amyloidosis. JACC Basic Transl Sci. 2019; 4(3): 438–448. 10.1016/j.jacbts.2019.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ruberg FL, Grogan M, Hanna M, et al. : Transthyretin Amyloid Cardiomyopathy: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019; 73(22): 2872–2891. 10.1016/j.jacc.2019.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 5. Tanskanen M, Peuralinna T, Polvikoski T, et al. : Senile systemic amyloidosis affects 25% of the very aged and associates with genetic variation in alpha2-macroglobulin and tau: A population-based autopsy study. Ann Med. 2008; 40(3): 232–9. 10.1080/07853890701842988 [DOI] [PubMed] [Google Scholar]
- 6. Mohammed SF, Mirzoyev SA, Edwards WD, et al. : Left Ventricular Amyloid Deposition in Patients With Heart Failure and Preserved Ejection Fraction. JACC Heart Fail. 2014; 2(2): 113–22. 10.1016/j.jchf.2013.11.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Scully PR, Treibel TA, Fontana M, et al. : Prevalence of Cardiac Amyloidosis in Patients Referred for Transcatheter Aortic Valve Replacement. J Am Coll Cardiol. 2018; 71(4): 463–464. 10.1016/j.jacc.2017.11.037 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 8. Gargiulo P, Perrone-Filardi P: Dangerous relationships: Aortic stenosis and transthyretin cardiac amyloidosis. Eur Heart J. 2017; 38(38): 2888–9. 10.1093/eurheartj/ehx513 [DOI] [PubMed] [Google Scholar]
- 9. Zhao L, Buxbaum JN, Reixach N: Age-Related Oxidative Modifications of Transthyretin Modulate Its Amyloidogenicity. Biochemistry. 2013; 52(11): 1913–26. 10.1021/bi301313b [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rapezzi C, Merlini G, Quarta CC, et al. : Systemic cardiac amyloidoses: disease profiles and clinical courses of the 3 main types. Circulation. 2009; 120(13): 1203–12. 10.1161/CIRCULATIONAHA.108.843334 [DOI] [PubMed] [Google Scholar]
- 11. Sperry BW, Ikram A, Hachamovitch R, et al. : Efficacy of Chemotherapy for Light-Chain Amyloidosis in Patients Presenting With Symptomatic Heart Failure. J Am Coll Cardiol. 2016; 67(25): 2941–8. 10.1016/j.jacc.2016.03.593 [DOI] [PubMed] [Google Scholar]
- 12. Witteles RM, Liedtke M: AL Amyloidosis for the Cardiologist and Oncologist: Epidemiology, Diagnosis, and Management. JACC: CardioOncology. 2019; 1(1): 117–30. 10.1016/j.jaccao.2019.08.002 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 13. Barrett CD, Dobos K, Liedtke M, et al. : A Changing Landscape of Mortality for Systemic Light Chain Amyloidosis. JACC Heart Fail. 2019; 7(11): 958–966. 10.1016/j.jchf.2019.07.007 [DOI] [PubMed] [Google Scholar]
- 14. Gillmore JD, Damy T, Fontana M, et al. : A new staging system for cardiac transthyretin amyloidosis. Eur Heart J. 2018; 39(30): 2799–806. 10.1093/eurheartj/ehx589 [DOI] [PubMed] [Google Scholar]
- 15. Maurer MS, Bokhari S, Damy T, et al. : Expert Consensus Recommendations for the Suspicion and Diagnosis of Transthyretin Cardiac Amyloidosis. Circ Heart Fail. 2019; 12(9): e006075. 10.1161/CIRCHEARTFAILURE.119.006075 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 16. Eslam M, Newsome PN, Sarin SK, et al. : A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J Hepatol. 2020; 73(1): 202–9. 10.1016/j.jhep.2020.03.039 [DOI] [PubMed] [Google Scholar]
- 17. Yanagisawa A, Ueda M, Sueyoshi T, et al. : Amyloid deposits derived from transthyretin in the ligamentum flavum as related to lumbar spinal canal stenosis. Mod Pathol. 2015; 28(2): 201–7. 10.1038/modpathol.2014.102 [DOI] [PubMed] [Google Scholar]
- 18. Siddiqi OK, Ruberg FL: Cardiac amyloidosis: An update on pathophysiology, diagnosis, and treatment. Trends Cardiovasc Med. 2018; 28(1): 10–21. 10.1016/j.tcm.2017.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Phelan D, Collier P, Thavendiranathan P, et al. : Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography is both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart. 2012; 98(19): 1442–8. 10.1136/heartjnl-2012-302353 [DOI] [PubMed] [Google Scholar]
- 20. Mesquita ET, Jorge AJL, Junior CVS, et al. : Cardiac Amyloidosis and its New Clinical Phenotype: Heart Failure with Preserved Ejection Fraction. Arq Bras Cardiol. 2017; 109(1): 71–80. 10.5935/abc.20170079 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Donnelly JP, Hanna M: Cardiac amyloidosis: An update on diagnosis and treatment. Cleve Clin J Med. 2017; 84(12 Suppl 3): 12–26. 10.3949/ccjm.84.s3.02 [DOI] [PubMed] [Google Scholar]
- 22. Sperry BW, Vranian MN, Hachamovitch R, et al. : Are classic predictors of voltage valid in cardiac amyloidosis? A contemporary analysis of electrocardiographic findings. Int J Cardiol. 2016; 214: 477–81. 10.1016/j.ijcard.2016.04.030 [DOI] [PubMed] [Google Scholar]
- 23. Kumar S, Dispenzieri A, Lacy MQ, et al. : Revised prognostic staging system for light chain amyloidosis incorporating cardiac biomarkers and serum free light chain measurements. J Clin Oncol. 2012; 30(9): 989–95. 10.1200/JCO.2011.38.5724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Grogan M, Scott CG, Kyle RA, et al. : Natural History of Wild-Type Transthyretin Cardiac Amyloidosis and Risk Stratification Using a Novel Staging System. J Am Coll Cardiol. 2016; 68(10): 1014–20. 10.1016/j.jacc.2016.06.033 [DOI] [PubMed] [Google Scholar]
- 25. Law S, Petrie A, Chacko L, et al. : Disease progression in cardiac transthyretin amyloidosis is indicated by serial calculation of National Amyloidosis Centre transthyretin amyloidosis stage. ESC Heart Fail. 2020; 7(6): 3942–3949. 10.1002/ehf2.12989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Boldrini M, Cappelli F, Chacko L, et al. : Multiparametric Echocardiography Scores for the Diagnosis of Cardiac Amyloidosis. JACC Cardiovasc Imaging. 2020; 13(4): 909–920. 10.1016/j.jcmg.2019.10.011 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 27. Zhang J, Gajjala S, Agrawal P, et al. : Fully Automated Echocardiogram Interpretation in Clinical Practice. Circulation. 2018; 138(16): 1623–35. 10.1161/CIRCULATIONAHA.118.034338 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 28. Dorbala S, Cuddy S, Falk RH: How to Image Cardiac Amyloidosis: A Practical Approach. JACC Cardiovasc Imaging. 2020; 13(6): 1368–1383. 10.1016/j.jcmg.2019.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 29. White JA, Kim HW, Shah D, et al. : CMR Imaging With Rapid Visual T1 Assessment Predicts Mortality in Patients Suspected of Cardiac Amyloidosis. JACC Cardiovasc Imaging. 2014; 7(2): 143–56. 10.1016/j.jcmg.2013.09.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Banypersad SM, Fontana M, Maestrini V, et al. : T1 mapping and survival in systemic light-chain amyloidosis. Eur Heart J. 2015; 36(4): 244–51. 10.1093/eurheartj/ehu444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Fontana M, Banypersad SM, Treibel TA, et al. : Native T1 Mapping in Transthyretin Amyloidosis. JACC Cardiovasc Imaging. 2014; 7(2): 157–65. 10.1016/j.jcmg.2013.10.008 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 32. Kourelis T, Murray DL, Dasari S, et al. : MASS-FIX may allow identification of patients at risk for light chain amyloidosis before the onset of symptoms. Am J Hematol. 2018; 93(11): E368–E370. 10.1002/ajh.25244 [DOI] [PubMed] [Google Scholar]
- 33. Hanna M, Ruberg FL, Maurer MS, et al. : Cardiac Scintigraphy With Technetium-99m-Labeled Bone-Seeking Tracers for Suspected Amyloidosis: JACC Review Topic of the Week. J Am Coll Cardiol. 2020; 75(22): 2851–62. 10.1016/j.jacc.2020.04.022 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 34. Agibetov A, Seirer B, Dachs TM, et al. : Machine Learning Enables Prediction of Cardiac Amyloidosis by Routine Laboratory Parameters: A Proof-of-Concept Study. J Clin Med. 2020; 9(5): 1334. 10.3390/jcm9051334 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 35. Wizenberg TA, Muz J, Sohn YH, et al. : Value of positive myocardial technetium-99m-pyrophosphate scintigraphy in the noninvasive diagnosis of cardiac amyloidosis. Am Heart J. 1982; 103(4 Pt 1): 468–73. 10.1016/0002-8703(82)90331-3 [DOI] [PubMed] [Google Scholar]
- 36. Bokhari S, Castaño A, Pozniakoff T, et al. : (99m)Tc-Pyrophosphate Scintigraphy for Differentiating Light-Chain Cardiac Amyloidosis From the Transthyretin-Related Familial and Senile Cardiac Amyloidoses. Circ Cardiovasc Imaging. 2013; 6(2): 195–201. 10.1161/CIRCIMAGING.112.000132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gillmore JD, Maurer MS, Falk RH, et al. : Nonbiopsy Diagnosis of Cardiac Transthyretin Amyloidosis. Circulation. 2016; 133(24): 2404–12. 10.1161/CIRCULATIONAHA.116.021612 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 38. Merlini G, Bellotti V: Molecular Mechanisms of Amyloidosis. N Engl J Med. 2003; 349(6): 583–96. 10.1056/NEJMra023144 [DOI] [PubMed] [Google Scholar]
- 39. Hammarstrom P, Jiang X, Hurshman AR, et al. : Sequence-dependent denaturation energetics: A major determinant in amyloid disease diversity. Proc Natl Acad Sci U S A. 2002; 99 Suppl 4(Suppl 4): 16427–32. 10.1073/pnas.202495199 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 40. Emdin M, Aimo A, Rapezzi C, et al. : Treatment of cardiac transthyretin amyloidosis: An update. Eur Heart J. 2019; 40(45): 3699–3706. 10.1093/eurheartj/ehz298 [DOI] [PubMed] [Google Scholar]
- 41. Bochtler T, Hegenbart U, Kunz C, et al. : Translocation t(11;14) Is Associated With Adverse Outcome in Patients With Newly Diagnosed AL Amyloidosis When Treated With Bortezomib-Based Regimens. J Clin Oncol. 2015; 33(12): 1371–8. 10.1200/JCO.2014.57.4947 [DOI] [PubMed] [Google Scholar]
- 42. Bochtler T, Hegenbart U, Kunz C, et al. : Prognostic impact of cytogenetic aberrations in AL amyloidosis patients after high-dose melphalan: A long-term follow-up study. Blood. 2016; 128(4): 594–602. 10.1182/blood-2015-10-676361 [DOI] [PubMed] [Google Scholar]
- 43. Ardehali H, Qasim A, Cappola T, et al. : Endomyocardial biopsy plays a role in diagnosing patients with unexplained cardiomyopathy. Am Heart J. 2004; 147(5): 919–23. 10.1016/j.ahj.2003.09.020 [DOI] [PubMed] [Google Scholar]
- 44. Bennett MK, Gilotra NA, Harrington C, et al. : Evaluation of the Role of Endomyocardial Biopsy in 851 Patients With Unexplained Heart Failure From 2000-2009. Circ Heart Fail. 2013; 6(4): 676–84. 10.1161/CIRCHEARTFAILURE.112.000087 [DOI] [PubMed] [Google Scholar]
- 45. Yakupova EI, Bobyleva LG, Vikhlyantsev IM, et al. : Congo Red and amyloids: History and relationship. Biosci Rep. 2019; 39(1): BSR20181415. 10.1042/BSR20181415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Falk RH: Diagnosis and Management of the Cardiac Amyloidoses. Circulation. 2005; 112(13): 2047–60. 10.1161/CIRCULATIONAHA.104.489187 [DOI] [PubMed] [Google Scholar]
- 47. Milani P, Palladini G: Conventional Therapy for Amyloid Light-Chain Amyloidosis. Acta Haematol. 2020; 143(4): 365–372. 10.1159/000507072 [DOI] [PubMed] [Google Scholar]
- 48. Beel K, Vekemans MC, Bries G, et al. : Diagnosis and treatment of AL Amyloidosis in 2015: Consensus guidelines of the Belgian Hematological Society. Belg J Hematol. 2015; 6(5): 187–94. Reference Source [Google Scholar]
- 49. Palladini G, Milani P, Foli A, et al. : Melphalan and dexamethasone with or without bortezomib in newly diagnosed AL amyloidosis: A matched case-control study on 174 patients. Leukemia. 2014; 28(12): 2311–6. 10.1038/leu.2014.227 [DOI] [PubMed] [Google Scholar]
- 50. Manwani R, Sachchithanantham S, Mahmood S, et al. : Treatment of IgM-associated immunoglobulin light-chain amyloidosis with rituximab-bendamustine. Blood. 2018; 132(7): 761–4. 10.1182/blood-2018-04-846493 [DOI] [PubMed] [Google Scholar]
- 51. Milani P, Schönland S, Merlini G, et al. : Treatment of AL amyloidosis with bendamustine: A study of 122 patients. Blood. 2018; 132(18): 1988–1991. 10.1182/blood-2018-04-845396 [DOI] [PubMed] [Google Scholar]
- 52. Moreau P, Masszi T, Grzasko N, et al. : Oral Ixazomib, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016; 374(17): 1621–34. 10.1056/NEJMoa1516282 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 53. Stewart AK, Rajkumar SV, Dimopoulos MA, et al. : Carfilzomib, lenalidomide, and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015; 372(2): 142–52. 10.1056/NEJMoa1411321 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 54. Hegenbart U, Bochtler T, Benner A, et al. : Lenalidomide/melphalan/dexamethasone in newly diagnosed patients with immunoglobulin light chain amyloidosis: Results of a prospective phase 2 study with long-term follow up. Haematologica. 2017; 102(8): 1424–1431. 10.3324/haematol.2016.163246 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 55. Specter R, Sanchorawala V, Seldin DC, et al. : Kidney dysfunction during lenalidomide treatment for AL amyloidosis. Nephrol Dial Transplant. 2011; 26(3): 881–6. 10.1093/ndt/gfq482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Venner CP, Gillmore JD, Sachchithanantham S, et al. : A matched comparison of cyclophosphamide, bortezomib and dexamethasone (CVD) versus risk-adapted cyclophosphamide, thalidomide and dexamethasone (CTD) in AL amyloidosis. Leukemia. 2014; 28(12): 2304–10. 10.1038/leu.2014.218 [DOI] [PubMed] [Google Scholar]
- 57. Wechalekar AD, Goodman HJB, Lachmann HJ, et al. : Safety and efficacy of risk-adapted cyclophosphamide, thalidomide, and dexamethasone in systemic AL amyloidosis. Blood. 2007; 109(2): 457–64. 10.1182/blood-2006-07-035352 [DOI] [PubMed] [Google Scholar]
- 58. Sanchorawala V, Finn KT, Fennessey S, et al. : Durable hematologic complete responses can be achieved with lenalidomide in AL amyloidosis. Blood. 2010; 116(11): 1990–1. 10.1182/blood-2010-07-295485 [DOI] [PubMed] [Google Scholar]
- 59. Gay F, Mina R, Troia R, et al. : Pharmacokinetic evaluation of pomalidomide for the treatment of myeloma. Expert Opin Drug Metab Toxicol. 2013; 9(11): 1517–27. 10.1517/17425255.2013.827169 [DOI] [PubMed] [Google Scholar]
- 60. Dispenzieri A, Lacy MQ, Zeldenrust SR, et al. : The activity of lenalidomide with or without dexamethasone in patients with primary systemic amyloidosis. Blood. 2007; 109(2): 465–70. 10.1182/blood-2006-07-032987 [DOI] [PubMed] [Google Scholar]
- 61. Sanchorawala V, Wright DG, Rosenzweig M, et al. : Lenalidomide and dexamethasone in the treatment of AL amyloidosis: Results of a phase 2 trial. Blood. 2007; 109(2): 492–6. 10.1182/blood-2006-07-030544 [DOI] [PubMed] [Google Scholar]
- 62. Palladini G, Russo P, Foli A, et al. : Salvage therapy with lenalidomide and dexamethasone in patients with advanced AL amyloidosis refractory to melphalan, bortezomib, and thalidomide. Ann Hematol. 2012; 91(1): 89–92. 10.1007/s00277-011-1244-x [DOI] [PubMed] [Google Scholar]
- 63. Mahmood S, Venner CP, Sachchithanantham S, et al. : Lenalidomide and dexamethasone for systemic AL amyloidosis following prior treatment with thalidomide or bortezomib regimens. Br J Haematol. 2014; 166(6): 842–8. 10.1111/bjh.12973 [DOI] [PubMed] [Google Scholar]
- 64. Dimopoulos MA, Oriol A, Nahi H, et al. : Daratumumab, Lenalidomide, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016; 375(14): 1319–1331. 10.1056/NEJMoa1607751 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 65. Palumbo A, Chanan-Khan A, Weisel K, et al. : Daratumumab, Bortezomib, and Dexamethasone for Multiple Myeloma. N Engl J Med. 2016; 375(8): 754–66. 10.1056/NEJMoa1606038 [DOI] [PubMed] [Google Scholar]
- 66. Martin T, Baz R, Benson DM, et al. : A phase 1b study of isatuximab plus lenalidomide and dexamethasone for relapsed/refractory multiple myeloma. Blood. 2017; 129(25): 3294–3303. 10.1182/blood-2016-09-740787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lonial S, Dimopoulos M, Palumbo A, et al. : Elotuzumab Therapy for Relapsed or Refractory Multiple Myeloma. N Engl J Med. 2015; 373(7): 621–31. 10.1056/NEJMoa1505654 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 68. Kumar S, Kaufman JL, Gasparetto C, et al. : Efficacy of venetoclax as targeted therapy for relapsed/refractory t(11;14) multiple myeloma. Blood. 2017; 130(22): 2401–2409. 10.1182/blood-2017-06-788786 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 69. Moreau P, Chanan-Khan A, Roberts AW, et al. : Promising efficacy and acceptable safety of venetoclax plus bortezomib and dexamethasone in relapsed/refractory MM. Blood. 2017; 130(22): 2392–2400. 10.1182/blood-2017-06-788323 [DOI] [PubMed] [Google Scholar]
- 70. Wechalekar AD, Whelan C: Encouraging impact of doxycycline on early mortality in cardiac light chain (AL) amyloidosis. Blood Cancer J. 2017; 7(3): e546–e546. 10.1038/bcj.2017.26 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 71. Gertz MA, Landau H, Comenzo RL, et al. : First-in-Human Phase I/II Study of NEOD001 in Patients With Light Chain Amyloidosis and Persistent Organ Dysfunction. J Clin Oncol. 2016; 34(10): 1097–103. 10.1200/JCO.2015.63.6530 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 72. Maurer MS, Schwartz JH, Gundapaneni B, et al. : Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med. 2018; 379(11): 1007–16. 10.1056/NEJMoa1805689 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 73. Berk JL, Suhr OB, Obici L, et al. : Repurposing diflunisal for familial amyloid polyneuropathy: A randomized clinical trial. JAMA. 2013; 310(24): 2658–67. 10.1001/jama.2013.283815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Gamez J, Salvadó M, Reig N, et al. : Transthyretin stabilization activity of the catechol-O-methyltransferase inhibitor tolcapone (SOM0226) in hereditary ATTR amyloidosis patients and asymptomatic carriers: Proof-of-concept study. Amyloid. 2019; 26(2): 74–84. 10.1080/13506129.2019.1597702 [DOI] [PubMed] [Google Scholar]
- 75. Judge DP, Heitner SB, Falk RH, et al. : Transthyretin Stabilization by AG10 in Symptomatic Transthyretin Amyloid Cardiomyopathy. J Am Coll Cardiol. 2019; 74(3): 285–95. 10.1016/j.jacc.2019.03.012 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 76. Adams D, Gonzalez-Duarte A, O'Riordan WD, et al. : Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N Engl J Med. 2018; 379(1): 11–21. 10.1056/NEJMoa1716153 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 77. Benson MD, Waddington-Cruz M, Berk JL, et al. : Inotersen Treatment for Patients with Hereditary Transthyretin Amyloidosis. N Engl J Med. 2018; 379(1): 22–31. 10.1056/NEJMoa1716793 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 78. Obici L, Cortese A, Lozza A, et al. : Doxycycline plus tauroursodeoxycholic acid for transthyretin amyloidosis: A phase II study. Amyloid. 2012; 19 Suppl 1: 34–6. 10.3109/13506129.2012.678508 [DOI] [PubMed] [Google Scholar]
- 79. Kristen AV, Lehrke S, Buss S, et al. : Green tea halts progression of cardiac transthyretin amyloidosis: An observational report. Clin Res Cardiol. 2012; 101(10): 805–13. 10.1007/s00392-012-0463-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Alexander KM, Singh A, Falk RH: Novel pharmacotherapies for cardiac amyloidosis. Pharmacol Ther. 2017; 180: 129–38. 10.1016/j.pharmthera.2017.06.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Kyle RA, Gertz MA, Greipp PR, et al. : A Trial of Three Regimens for Primary Amyloidosis: Colchicine Alone, Melphalan and Prednisone, and Melphalan, Prednisone, and Colchicine. N Engl J Med. 1997; 336(17): 1202–7. 10.1056/NEJM199704243361702 [DOI] [PubMed] [Google Scholar]
- 82. Skinner M, Sanchorawala V, Seldin DC, et al. : High-Dose Melphalan and Autologous Stem-Cell Transplantation in Patients with AL Amyloidosis: An 8-Year Study. Ann Intern Med. 2004; 140(2): 85–93. 10.7326/0003-4819-140-2-200401200-00008 [DOI] [PubMed] [Google Scholar]
- 83. Sanchorawala V, Sun F, Quillen K, et al. : Long-term outcome of patients with AL amyloidosis treated with high-dose melphalan and stem cell transplantation: 20-year experience. Blood. 2015; 126(20): 2345–7. 10.1182/blood-2015-08-662726 [DOI] [PubMed] [Google Scholar]
- 84. Sidana S, Sidiqi MH, Dispenzieri A, et al. : Fifteen year overall survival rates after autologous stem cell transplantation for AL amyloidosis. Am J Hematol. 2019; 94(9): 1020–1026. 10.1002/ajh.25566 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 85. Jaccard A, Moreau P, Leblond V, et al. : High-Dose Melphalan versus Melphalan plus Dexamethasone for AL Amyloidosis. N Engl J Med. 2007; 357(11): 1083–93. 10.1056/NEJMoa070484 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 86. Palladini G, Sachchithanantham S, Milani P, et al. : A European collaborative study of cyclophosphamide, bortezomib, and dexamethasone in upfront treatment of systemic AL amyloidosis. Blood. 2015; 126(5): 612–5. 10.1182/blood-2015-01-620302 [DOI] [PubMed] [Google Scholar]
- 87. Venner CP, Lane T, Foard D, et al. : Cyclophosphamide, bortezomib, and dexamethasone therapy in AL amyloidosis is associated with high clonal response rates and prolonged progression-free survival. Blood. 2012; 119(19): 4387–90. 10.1182/blood-2011-10-388462 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 88. Mikhael JR, Schuster SR, Jimenez-Zepeda VH, et al. : Cyclophosphamide-bortezomib-dexamethasone (CyBorD) produces rapid and complete hematologic response in patients with AL amyloidosis. Blood. 2012; 119(19): 4391–4. 10.1182/blood-2011-11-390930 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 89. Sanchorawala V, Palladini G, Kukreti V, et al. : A phase 1/2 study of the oral proteasome inhibitor ixazomib in relapsed or refractory AL amyloidosis. Blood. 2017; 130(5): 597–605. 10.1182/blood-2017-03-771220 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 90. Cohen AD, Landau H, Scott EC, et al. : Safety and Efficacy of Carfilzomib (CFZ) in Previously-Treated Systemic Light-Chain (AL) Amyloidosis. Blood. 2016; 128(22): 645. 10.1182/blood.V128.22.645.645 [DOI] [Google Scholar]
- 91. Milani P, Sharpley F, Schönland SO, et al. : Pomalidomide and dexamethasone grant rapid haematologic responses in patients with relapsed and refractory AL amyloidosis: A European retrospective series of 153 patients. Amyloid. 2020; 27(4): 231–236. 10.1080/13506129.2020.1767566 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 92. https://ir.genmab.com/news-releases/news-release-details/genmab-announces-positive-topline-results-phase-iii-andromeda/ 2020 [Google Scholar]
- 93. Palladini G, Milani P, Merlini G: Management of AL amyloidosis in 2020. Hematology Am Soc Hematol Educ Program. 2020; 2020(1): 363–371. 10.1182/hematology.2020006913 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. de Weers M, Tai YT, van der Veer MS, et al. : Daratumumab, a Novel Therapeutic Human CD38 Monoclonal Antibody, Induces Killing of Multiple Myeloma and Other Hematological Tumors. J Immunol. 2011; 186(3): 1840–8. 10.4049/jimmunol.1003032 [DOI] [PubMed] [Google Scholar]
- 95. Oltersdorf T, Elmore SW, Shoemaker AR, et al. : An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005; 435(7042): 677–81. 10.1038/nature03579 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 96. Bodet L, Gomez-Bougie P, Touzeau C, et al. : ABT-737 is highly effective against molecular subgroups of multiple myeloma. Blood. 2011; 118(14): 3901–10. 10.1182/blood-2010-11-317438 [DOI] [PubMed] [Google Scholar]
- 97. Lentzsch S, Lagos GG, Comenzo RL, et al. : Bendamustine With Dexamethasone in Relapsed/Refractory Systemic Light-Chain Amyloidosis: Results of a Phase II Study. J Clin Oncol. 2020; 38(13): 1455–62. 10.1200/JCO.19.01721 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 98. Witteles RM: Doxycycline and Ursodiol for ATTR Amyloidosis: Not Ready for Prime Time. J Card Fail. 2019; 25(3): 154–155. 10.1016/j.cardfail.2019.01.014 [DOI] [PubMed] [Google Scholar]
- 99. Renz M, Torres R, Dolan PJ, et al. : 2A4 binds soluble and insoluble light chain aggregates from AL amyloidosis patients and promotes clearance of amyloid deposits by phagocytosis. Amyloid. 2016; 23(3): 168–177. 10.1080/13506129.2016.1205974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Wall JS, Kennel SJ, Williams A, et al. : AL Amyloid Imaging and Therapy with a Monoclonal Antibody to a Cryptic Epitope on Amyloid Fibrils. PLoS One. 2012; 7(12): e52686. 10.1371/journal.pone.0052686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Varga C, Lentzsch S, Comenzo RL: Beyond NEOD001 for systemic light-chain amyloidosis. Blood. 2018; 132(18): 1992–3. 10.1182/blood-2018-07-865857 [DOI] [PubMed] [Google Scholar]
- 102. Richards DB, Cookson LM, Berges AC, et al. : Therapeutic Clearance of Amyloid by Antibodies to Serum Amyloid P Component. N Engl J Med. 2015; 373(12): 1106–14. 10.1056/NEJMoa1504942 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 103. Ericzon B-G, Wilczek HE, Larsson M, et al. : Liver Transplantation for Hereditary Transthyretin Amyloidosis: After 20 Years Still the Best Therapeutic Alternative? Transplantation. 2015; 99(9): 1847–54. 10.1097/TP.0000000000000574 [DOI] [PubMed] [Google Scholar]
- 104. Sack F-U, Kristen A, Goldschmidt H, et al. : Treatment options for severe cardiac amyloidosis: Heart transplantation combined with chemotherapy and stem cell transplantation for patients with AL-amyloidosis and heart and liver transplantation for patients with ATTR-amyloidosis. Eur J Cardiothorac Surg. 2008; 33(2): 257–62. 10.1016/j.ejcts.2007.10.025 [DOI] [PubMed] [Google Scholar]
- 105. Maurer MS, Hanna M, Grogan M, et al. : Genotype and Phenotype of Transthyretin Cardiac Amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J Am Coll Cardiol. 2016; 68(2): 161–72. 10.1016/j.jacc.2016.03.596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Peterson SA, Klabunde T, Lashuel HA, et al. : Inhibiting transthyretin conformational changes that lead to amyloid fibril formation. Proc Natl Acad Sci U S A. 1998; 95(22): 12956–60. 10.1073/pnas.95.22.12956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Castaño A, Helmke S, Alvarez J, et al. : Diflunisal for ATTR cardiac amyloidosis. Congest Heart Fail. 2012; 18(6): 315–9. 10.1111/j.1751-7133.2012.00303.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Bulawa CE, Connelly S, Devit M, et al. : Tafamidis, a potent and selective transthyretin kinetic stabilizer that inhibits the amyloid cascade. Proc Natl Acad Sci U S A. 2012; 109(24): 9629–34. 10.1073/pnas.1121005109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Sant'Anna R, Gallego P, Robinson LZ, et al. : Repositioning tolcapone as a potent inhibitor of transthyretin amyloidogenesis and associated cellular toxicity. Nat Commun. 2016; 7: 10787. 10.1038/ncomms10787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Bumcrot D, Manoharan M, Koteliansky V, et al. : RNAi therapeutics: A potential new class of pharmaceutical drugs. Nat Chem Biol. 2006; 2(12): 711–9. 10.1038/nchembio839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Solomon SD, Adams D, Kristen A, et al. : Effects of Patisiran, an RNA Interference Therapeutic, on Cardiac Parameters in Patients With Hereditary Transthyretin-Mediated Amyloidosis. Circulation. 2019; 139(4): 431–43. 10.1161/CIRCULATIONAHA.118.035831 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 112. Fontana M, Martinez-Naharro A, Chacko L, et al. : Reduction in CMR Derived Extracellular Volume With Patisiran Indicates Cardiac Amyloid Regression. JACC Cardiovasc Imaging. 2021; 14: 189–99. 10.1016/j.jcmg.2020.07.043 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 113. Rinaldi C, Wood MJA: Antisense oligonucleotides: The next frontier for treatment of neurological disorders. Nat Rev Neurol. 2018; 14(1): 9–21. 10.1038/nrneurol.2017.148 [DOI] [PubMed] [Google Scholar]
- 114. Malha L, Mann SJ: Loop Diuretics in the Treatment of Hypertension. Curr Hypertens Rep. 2016; 18(4): 27. 10.1007/s11906-016-0636-7 [DOI] [PubMed] [Google Scholar]
- 115. Giancaterino S, Urey MA, Darden D, et al. : Management of Arrhythmias in Cardiac Amyloidosis. JACC Clin Electrophysiol. 2020; 6(4): 351–61. 10.1016/j.jacep.2020.01.004 [DOI] [PubMed] [Google Scholar]
- 116. Gertz MA, Skinner M, Connors LH, et al. : Selective binding of nifedipine to amylold fibrils. Am J Cardiol. 1985; 55(13 Pt 1): 1646. 10.1016/0002-9149(85)90996-8 [DOI] [PubMed] [Google Scholar]
- 117. Gertz MA, Falk RH, Skinner M, et al. : Worsening of congestive heart failure in amyloid heart disease treated by calcium channel-blocking agents. Am J Cardiol. 1985; 55(13 Pt 1): 1645. 10.1016/0002-9149(85)90995-6 [DOI] [PubMed] [Google Scholar]
- 118. Goldsmith YB, Liu J, Chou J, Hoffman J, et al. : Frequencies and types of arrhythmias in patients with systemic light-chain amyloidosis with cardiac involvement undergoing stem cell transplantation on telemetry monitoring. Am J Cardiol. 2009; 104(7): 990–4. 10.1016/j.amjcard.2009.05.040 [DOI] [PubMed] [Google Scholar]
- 119. El-Am EA, Dispenzieri A, Melduni RM, et al. : Direct Current Cardioversion of Atrial Arrhythmias in Adults With Cardiac Amyloidosis. J Am Coll Cardiol. 2019; 73(5): 589–97. 10.1016/j.jacc.2018.10.079 [DOI] [PMC free article] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 120. Castaño A, Drachman BM, Judge D, et al. : Natural history and therapy of TTR-cardiac amyloidosis: Emerging disease-modifying therapies from organ transplantation to stabilizer and silencer drugs. Heart Fail Rev. 2015; 20(2): 163–78. 10.1007/s10741-014-9462-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Sayed RH, Rogers D, Khan F, et al. : A study of implanted cardiac rhythm recorders in advanced cardiac AL amyloidosis. Eur Heart J. 2015; 36(18): 1098–105. 10.1093/eurheartj/ehu506 [DOI] [PubMed] [Google Scholar]
- 122. Hamon D, Algalarrondo V, Gandjbakhch E, et al. : Outcome and incidence of appropriate implantable cardioverter-defibrillator therapy in patients with cardiac amyloidosis. Int J Cardiol. 2016; 222: 562–568. 10.1016/j.ijcard.2016.07.254 [DOI] [PubMed] [Google Scholar]
- 123. Patel KS, Hawkins PN, Whelan CJ, et al. : Life-saving implantable cardioverter defibrillator therapy in cardiac AL amyloidosis. BMJ Case Rep. 2014; 2014: bcr2014206600. 10.1136/bcr-2014-206600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Kittleson MM, Cole RM, Patel J, et al. : Mechanical circulatory support for cardiac amyloidosis. Clin Transplant. 2019; 33(10): e13663. 10.1111/ctr.13663 [DOI] [PubMed] [Google Scholar]; Faculty Opinions Recommendation
- 125. Swiecicki PL, Edwards BS, Kushwaha SS, et al. : Left ventricular device implantation for advanced cardiac amyloidosis. J Heart Lung Transplant. 2013; 32(5): 563–8. 10.1016/j.healun.2013.01.987 [DOI] [PubMed] [Google Scholar]
- 126. Grupper A, Park SJ, Pereira NL, et al. : Role of ventricular assist therapy for patients with heart failure and restrictive physiology: Improving outcomes for a lethal disease. J Heart Lung Transplant. 2015; 34(8): 1042–9. 10.1016/j.healun.2015.03.012 [DOI] [PubMed] [Google Scholar]
- 127. Davis MK, Lee PHU, Witteles RM: Changing outcomes after heart transplantation in patients with amyloid cardiomyopathy. J Heart Lung Transplant. 2015; 34(5): 658–66. 10.1016/j.healun.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 128. Gilstrap LG, Niehaus E, Malhotra R, et al. : Predictors of survival to orthotopic heart transplant in patients with light chain amyloidosis. J Heart Lung Transplant. 2014; 33(2): 149–56. 10.1016/j.healun.2013.09.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Sousa M, Monohan G, Rajagopalan N, et al. : Heart transplantation in cardiac amyloidosis. Heart Fail Rev. 2017; 22(3): 317–327. 10.1007/s10741-017-9601-z [DOI] [PubMed] [Google Scholar]
- 130. Hawkins PN, Lavender JP, Pepys MB: Evaluation of systemic amyloidosis by scintigraphy with 123I-labeled serum amyloid P component. N Engl J Med. 1990; 323(8): 508–13. 10.1056/NEJM199008233230803 [DOI] [PubMed] [Google Scholar]
- 131. Hawkins PN: Serum amyloid P component scintigraphy for diagnosis and monitoring amyloidosis. Curr Opin Nephrol Hypertens. 2002; 11(6): 649–55. 10.1097/00041552-200211000-00013 [DOI] [PubMed] [Google Scholar]