Abstract
A bilobed tongue base was identified in an infant with multiple other head, neck and cardiac congenital anomalies. This anatomical variation of the posterior tongue is rare, with only two other cases identified in the literature. We report a case of a 5-month-old boy with a bilobed posterior tongue incidentally identified during workup for cardiac surgery.
Keywords: ear, nose and throat/otolaryngology, paediatrics, head and neck surgery
Background
Tongue malformations have been reported in the literature. Cleft or bifid anterior tongue is a rare but known entity, which can be isolated or related to other congenital anomalies or syndromes.1 However, congenital bilobed posterior tongue is an extremely rare presentation. Only two previous case reports were identified in the literature. One case was seen in a non-syndromic child during workup for obstructive sleep apnoea, while the second was reported in a patient with Goldenhar syndrome and laryngomalacia.2 3 Here, we present a case of bilobed tongue base in a 5-month-old boy incidentally identified during workup for cardiac surgery.
Case presentation
A 3-month-old boy was found to have a soft oropharyngeal mass during routine intubation for diagnostic cardiac catheterisation. Intubation and anaesthetic were performed without complication and the patient was successfully extubated before formal referral to Otolaryngology for assessment.
Investigations
Perinatal/feeding history
The patient was born on term (39+2) after spontaneous vaginal delivery. Postnatally, the patient was adapting well, but he required Optiflow on the second day of life secondary to his cardiac condition. He was initially kept nil by mouth and enteral feeds were introduced on day five of life via nasogastric tube, which was switched later to bottle feeds with nasogastric tube top ups. He was born on the 36th centile of the growth chart, but he gradually dropped to first or less since August 2020.
Head and neck
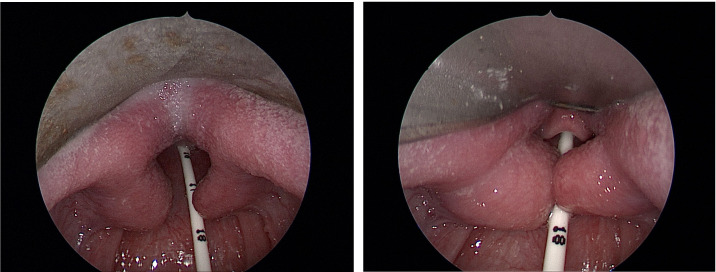
Prior to definitive cardiac surgery at 5 months of age, a complete airway examination was undertaken including microlaryngobronchoscopy under general anaesthesia. A midline sulcus of the tongue base tissue was seen with normal overlying mucosa (figure 1 and video 1). There was bilateral symmetrical outpouching of lateral posterior tongue tissue protruding into airway, giving a bilobed appearance of the tongue base. The epiglottis and vallecula were morphologically normal. The airway was otherwise unremarkable.
Figure 1.
Microlaryngoscopy images of bilobed tongue base.
Video 1.
The patient had multiple other head and neck abnormalities imaged using both CT and MRI under general anaesthesia. The abnormalities include bilateral microtia and a unilateral large pedunculated remnant ear lobule with insufficient blood supply which was removed shortly after birth left facial nerve palsy and a hypoplastic right vestibulocochlear nerve, bilateral atretic bony ear canals, abnormal ossicles/middle ear clefts (dysmorphic ossicular chain, oval window atresia) and abnormal vestibular complexes (dilated vestibules, lateral semicircular persistent anlage). The soft tissue of the tongue and oropharynx were not appreciated on imaging due to the presence of the endotracheal tube.
Heart
The patient’s cardiac history included antenatal diagnosis of tetralogy of Fallot with large doubly committed ventricular septal defect, severely hypoplastic pulmonary veins, pulmonary stenosis and Major Aorto-Pulmonary Collateral Arteries (MAPCA)-dependent pulmonary circulation confirmed by echocardiogram shortly after birth. This was repaired at 5 months of age via right ventricular outflow tract procedure (main pulmonary artery brought down to the right ventricle and roofed over with bovine pericardial patch).
Genetics
The Genetics service were consulted due to clinical suspicion of Treacher-Collins, Burn-McKeown or Goldenhar syndromes. However, bilateral Goldenhar screening and CHD7 and TXNL4A analysis were negative. To date, no causative genetic problem has been identified.
Outcome and follow-up
In the most recent review, at the age of 7 months, the patient has been tolerating nasogastric feeding well. He has no airway symptoms during the day, but at night there are possible obstructive episodes as described by the mother. Posterior bilobed tongue can be causing some obstructive element, so surgical reduction is planned after swallow assessment and sleep study have been performed.
Discussion
Embryological development of the tongue
The embryological tongue forms in the fourth week of life. The body of the tongue arises from two lateral lingual swellings and one medial swelling, the tuberculum impar. The lateral lingual swellings from the first pharyngeal arch increase in size, fusing in the midline, overgrowing the tuberculum impar and forming the anterior two-thirds of the tongue. The posterior third of the tongue forms from the median swelling, the copula or hypobranchial eminence, originating from the second, third and fourth arches. Finally, the epiglottis is formed by another median swelling, arising from part of the fourth pharyngeal arch.4 To date, bifid anterior tongue has been reported and is thought to arise when the lateral swellings fail to merge completely. It presents as a deep grove on the dorsal surface of the anterior tongue at the midline and has been reported in both isolated cases and syndromic children.5 6
Differential diagnosis
Differential diagnosis of bilobed tongue base can include lingual abnormalities and posterior tongue lesions. Lingual abnormalities encompass accessory tongue and lingual hamartoma.7 Tongue base lesions can be categorised as congenital or acquired. Congenital lesions can be vascular (eg, haemangioma) or non-vascular (eg, ectopic thyroid gland).7 Acquired neoplasms can be benign (eg, papillomatosis) or malignant (eg, Ewing’s sarcoma).8 9
Other tongue abnormalities and genetic/syndromic associations
Common congenital lingual malformations include aglossia, microglossia, macroglossia, accessory tongue, long tongue, and cleft or bifid tongue. Aglossia and macroglossia usually present with other congenital anomalies and syndromes such as Pierre-Robin and Moebius syndrome. Macroglossia is found in children with a variety of congenital disorders including Beckwith-Weideman syndrome, trisomy 21, mucopolysaccharidoses, lymphovascular malformations and congenital hypothyroidism; however, it can also be an isolated finding. Long tongue had been associated with Ehlers-Danlos syndrome, while accessory tongue and ankyloglossia can be found in patients with Van der Wonde’s and cryptophthalmos syndrome.1 Cases of accessory tongue have been reported in non-syndromic patients as well.10 11
Lobulation of the anterior part of the tongue has been described in the literature as cleft or bifid tongue. It has been reported in association with different syndromes, more specifically Goldernhar’s syndrome and oro-facial-digital syndrome.1 12 Bifid tongue is usually associated with other congenital anomalies, including cleft palate, ankyloglossia and palatal hamartoma.12–16 This condition can rarely present as an isolated feature.5 12 Other unusual cases of lobulated tongue have been described in the literature, including bilobed tongue with distinct anterior and posterior segments in a patient with abnormal ears and a mutation of a rare gene associated with CHARGE syndrome, but inadequate anatomical features for a clinical diagnosis of CHARGE.17
Treatment of patients with congenital lingual malformation in syndromic cases usually required multidisciplinary involvement and focused on functional outcomes for patients in terms of eating, breathing and appearance. Isolated and non-syndromic tongue malformations are managed with surgical excision with or without local reconstruction.10–12
Branchial anomalies
Branchial arches are the embryological precursors of the structures in the head and neck and begin to develop in the fourth week of gestation. Branchial anomalies are composed of a heterogeneous group of congenital malformations and can present as fistulae, cysts, sinus tracts and cartilaginous remnants.18–20 They represent the second most common cause of congenital lesions of the head and neck in children.18 20
First branchial cleft anomalies are uncommon. They usually involve the externally auditory canal and submandibular area and, occasionally, the middle ear.19 20
Second branchial arch anomalies represent 95% of all branchial cleft malformations.18 20 The second arch contributes to the hyoid bone and adjacent area of the neck. Second branchial cleft malformation is often identified along the anterior border of the sternomastoid muscle, but can occur anywhere along the course of the second branchial arch tract which extends from the skin overlying the supraclavicular fossa, between the internal and external carotid arteries, to enter the supratonsillar fossa.18–20
Third and fourth branchial arch anomalies are extremely rare. They may appear similar to second branchial cleft anomalies as they are found along the anterior border of the sternomastoid muscle and present externally with a cutaneous opening in the supraclavicular area. However, internally, they enter the pharynx through the pyriform sinus below the hyoid bone.19 20
The patient described in this case has multiple abnormalities arising in structures originating across all of the branchial arches and pouches. They do not fit into any single entity previously described.
Conclusion
We describe a patient with a bilobed posterior tongue associated with multiple other head, neck and cardiac congenital anomalies. Bilobed tongue base is an extremely rare condition, with only two other cases reported in the literature. This child’s collection of congenital anomalies does not directly correlate with previously described syndromic associations or branchial cleft abnormalities.
Learning points.
Tongue congenital abnormalities are relatively rare and can present in isolation or in association with various syndromes.
Branchial anomalies are composed of a heterogeneous group of congenital malformations with different presentations.
Bilobed posterior tongue, in contrast with anterior tongue cleft, is an extremely rare entity.
Bilobed posterior tongue can be associated with multiple other head, neck and cardiac congenital anomalies.
Footnotes
Twitter: @ClaireFrau
Contributors: I can confirm that SK has contributed significantly in this manuscript, by collecting data, writing and editing the manuscript. She has approved the latest version of the manuscript. I can confirm that CF has contributed significantly in this manuscript, by being involved in patient’s care, collecting data, writing and editing the manuscript. She has approved the latest version of the manuscript. I can confirm that MW has contributed significantly in this manuscript, by being involved in patient’s care, writing and editing the manuscript. She has approved the latest version of the manuscript. I can confirm that CB has contributed significantly in this manuscript, by being involved in patient’s care, writing and editing the manuscript. He has approved the latest version of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Emmanouil-Nikoloussi EN, Kerameos-Foroglou C. Developmental malformations of human tongue and associated syndrome (review). Bull Gr Int Rech Sci Stomatol Odontol 1992;35:5–12. [PubMed] [Google Scholar]
- 2.Rajendran T, Ramalinggam G, Kamaru Ambu V. Rare presentation of bilobed posterior tongue in Goldenhar syndrome. BMJ Case Rep 2017;2017. 10.1136/bcr-2017-219726. [Epub ahead of print: 01 Aug 2017]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdel-Aziz M. Congenital bilobed posterior third of the tongue: a case report. Int J Pediatr Otorhinolaryngol Extra 2008;3:140–2. 10.1016/j.pedex.2008.02.001 [DOI] [Google Scholar]
- 4.Langman STW. Medical embryology. 12th editi: 273.p. [Google Scholar]
- 5.Surej KLK, Kurien NM, Sivan MP. Isolated congenital bifid tongue. Natl J Maxillofac Surg 2010;1:187. 10.4103/0975-5950.79228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jain P, Rathee M. Embryology, Tongue (StatPearls) [Internet]. August 11. 2020. [cited Nov 23, 2020]. Available: https://www.ncbi.nlm.nih.gov/books/NBK547697/
- 7.Chapman MC, Soares BP, Li Y, et al. Congenital oral masses: an anatomic approach to diagnosis. Radiographics 2019;39:1143–60. 10.1148/rg.2019180128 [DOI] [PubMed] [Google Scholar]
- 8.Gazia F, Galletti B, Freni F, et al. Use of intralesional cidofovir in the recurrent respiratory papillomatosis: a review of the literature. Eur Rev Med Pharmacol Sci 2020;24:956–62. 10.26355/eurrev_202001_20081 [DOI] [PubMed] [Google Scholar]
- 9.Canevari FR, Montevecchi F, Galla S, et al. Trans-oral robotic surgery for a Ewing’s sarcoma of tongue in a pediatric patient: a case report. Braz J Otorhinolaryngol 2020;86:26–9. 10.1016/j.bjorl.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naik SS, Surgery N. Congenital accessory tongue: a rare case of non syndromic tongue anomaly. Otolaryngol online J 2015;5:1–10. [Google Scholar]
- 11.Kumar S, Tiwary SK, Khanna AK. An accessory tongue. Singapore Med J 2009;50:1–2. [PubMed] [Google Scholar]
- 12.Siddiqua A, Abubaker P, Saraswati FK, et al. Bifid tongue: differential diagnosis and a case report. Journal of Oral and Maxillofacial Surgery, Medicine, and Pathology 2015;27:686–9. 10.1016/j.ajoms.2015.01.006 [DOI] [Google Scholar]
- 13.Daniel-Spiegel E, Ben-Ami M. Bifid tongue, a rare congenital malformation, is a prenatal clue for secondary cleft palate. J Ultrasound Med 2012;31:505–7. 10.7863/jum.2012.31.3.505 [DOI] [PubMed] [Google Scholar]
- 14.Widgerow AD. Klippel-Feil anomaly, cleft palate, and bifid tongue. Ann Plast Surg 1990;25:216–22. 10.1097/00000637-199009000-00014 [DOI] [PubMed] [Google Scholar]
- 15.Haghighi K, Milles M, Cleveland D, et al. Epignathus teratoma with bifid tongue and median glossal salivary mass: report of a case. J Oral Maxillofac Surg 2004;62:379–83. 10.1016/j.joms.2003.05.012 [DOI] [PubMed] [Google Scholar]
- 16.Chidzonga MM, Lopez Perez VM, Mzezewa S. Treatment of median cleft of the lower lip, mandible, and bifid tongue with ankyloglossia. A case report. Int J Oral Maxillofac Surg 1996;25:272–3. 10.1016/S0901-5027(06)80054-8 [DOI] [PubMed] [Google Scholar]
- 17.Schultz KP, Lambert EM, Buchanan EP. Presentation of a rare lobulated tongue anomaly. Cleft Palate Craniofac J 2018;55:1467–9. 10.1177/1055665618767304 [DOI] [PubMed] [Google Scholar]
- 18.Bajaj Y, Ifeacho S, Tweedie D, et al. Branchial anomalies in children. Int J Pediatr Otorhinolaryngol 2011;75:1020–3. 10.1016/j.ijporl.2011.05.008 [DOI] [PubMed] [Google Scholar]
- 19.Waldhausen JHT. Branchial cleft and arch anomalies in children. Semin Pediatr Surg 2006;15:64–9. 10.1053/j.sempedsurg.2006.02.002 [DOI] [PubMed] [Google Scholar]
- 20.Adams A, Mankad K, Offiah C, et al. Branchial cleft anomalies: a pictorial review of embryological development and spectrum of imaging findings. Insights Imaging 2016;7:69–76. 10.1007/s13244-015-0454-5 [DOI] [PMC free article] [PubMed] [Google Scholar]