Abstract
Background: The novel severe acute respiratory syndrome related corona virus-2 (SARS-CoV-2) belongs to the “Coronaviridae” family and order “Nidovirales”, which has caused the pandemic coronavirus disease 2019 (COVID-19). SARS-CoV-2 has been spread in more than a 100 countries, and more than a million have lost their lives. Vaccination and immunization could be an effective strategy to combat fatal COVID-19.
Methods: For identification of effective vaccine candidate against COVID-19, various immunoinformatics online tools and softwares were used to predict epitopes. Cytotoxic T cell epitopes, helper T cell epitopes, and B cell epitopes from three structural polyproteins (Spike, Membrane, and Nucleocapsid (SMN) based on the binding affinity towards MHC, antigenicity, non-allergenicity, and non-toxicity) were identified for vaccine development. The multiepitope based vaccine was constructed linking two additional adjuvants human beta-defensin-3 and human beta-defensin-2 at N and C terminal, respectively.
Results: The constructed vaccine sequence was found to be a good antigen and non-allergen for the human body. The constructed vaccine was docked with the TLR-3 receptor. The docked complex was further taken for molecular dynamics simulations and RMSD was calculated, which showed stable binding of the complex. The codon adaptation index (CAI) of 0.92 and GC content of 55.5% for E. coli (K12 strain) suggested efficient expression of the predicted vaccine.
Conclusion: The current study can be helpful in the reduction of time and cost for further experimental validations and could give a valuable contribution against this pandemic.
Keywords: SARS-CoV-2, COVID-19, Immunoinformatics, multiepitope, docking, simulations.
Introduction
Coronaviruses (CoVs) belong to the family of coronaviridae in the order Nidovirales, and have single-strand positive-sense RNA 1. The size of the RNA of coronavirus is the largest among the viruses (~30 kb) 2. They have glycoprotein projections on the envelope, which gives the corona appearance. CoVs are pathogens mainly involved in respiratory and gastrointestinal diseases in a wide range of animals and humans 1 , 2. CoVs are divided into four sub-categories, namely alpha, beta, gamma, and delta, out of which alpha and beta coronavirus are known to infect humans 1 , 3. From alpha and beta, four strains are responsible for the common cold, and two strains were found to be responsible for severe acute respiratory syndrome (SARS-CoV) and Middle East respiratory syndrome CoV (MERS-CoV) 4 , 5. Recently in December 2019, a novel coronavirus SARS-CoV-2 was detected from patients of novel coronavirus disease 2019 (COVID-19) in the Wuhan, China 6- 8. The symptoms of COVID-19 infection include headache, fever, pneumonia, and asthenia 9 , 10. A severe and fatal outbreak of this virus has taken many lives and has created enormous economic loss worldwide. The treatment and prevention from this infection is the need of the hour.
COVID-19 has given a serious and tough challenge to biomedical research scientists and researchers all around the world. Much research is looking at repurposing antiviral drugs, and developing new drugs and vaccines against the SARS-CoV-2 11- 15. Here, an attempt has been given to construct an in-silico vaccine, which can be further validated through experimental assays and could play a major role in the management of this pandemic. In this study, three structural proteins form SARS-CoV-2, based on the antigenicity has been selected for the construction of the vaccine. The first structural protein is Spike (S) glycoprotein, which has been reported to be a crucial surface protein of SARS-CoV-2, which facilitates the entry of the virus inside the host cell. It has been reported that for the entry of the SARS-CoV-2, S protein first binds with Angiotensin-Converting Enzyme-2 (ACE-2) receptor, and then is primed by the host serine protease (TMPRSS2) 16 , 17. This priming of the S protein lets it to fuse into the host cell membrane and entry inside the cell. The second crucial structural protein, which induces a strong immune response, is Membrane (M) glycoprotein. It plays a crucial role in virus morphogenesis and assembly by interacting with several other viral proteins 18. The third structural protein chosen for vaccine construct is Nucleocapsid (N) phosphoprotein; it links the viral genome to the envelope. It consists of two domains, N terminal and C terminal, and both can bind to RNA. It has been reported that the C terminal domain facilitates the physical interaction of the RNA genome and envelope 19 , 20. All these three structural proteins are predicted to be good antigens and could induce the immune response.
In this pandemic situation, an immunoinformatics approach could be a fast, scientifically sound, and reliable option for quicker vaccine development. These three proteins chosen for the present study were predicted to be good antigens, which gives the opportunity to predict B and T cell epitopes. When naive B cells interact with the antigenic B cell epitopes via its transmembrane bound antibody, they differentiate into two types of cells plasma and memory cells 21 , 22. Plasma cells lack receptors, but they produce a large number of antibodies against the antigen. Memory cells express membrane-bound antibody molecules, but they are functionally inactive unless they encounter the same antigen again 23- 25. Furthermore, T cell epitopes are recognized by Major Histocompatibility Class (MHC), a glycoprotein present on the variety of the cells, which display the antigen to T cells 26 , 27. Antigen-presenting MHCs are divided into major two classes, MHC class-I are expressed on nucleated cells while MHC class-II are only expressed by antigen-presenting cells. Class II MHC interacts with T helper cells and activates B cells via cytokines, while Class-I MHC interacts with cytotoxic T cells, which kills virus-infected host cells 28 , 29. Further to know the ability of a constructed vaccine for inducing innate as well as antigen-specific acquired immunity, the constructed vaccine must dock with Toll-Like Receptor-3 (TLR-3). TLRs are mainly expressed on various leukocytes such as dendritic cells, natural killer cells, and cells of adaptive immunity such as T cells and B cells 30 , 31. Hence, in this study, an attempt has been made to construct the multiepitope vaccine consists of Helper T cells (HTLs), Cytotoxic T Cells (HTLs), and B cell epitopes, which could interact with TLR-3 and generate the immune response. This constructed multiepitope vaccine may induce both humoral as well as cell-mediated immune responses.
Methods
Retrieval of structural polyproteins of the SARS-CoV-2
The complete sequence of all three structural polyproteins from SARS-CoV-2 reference sequence ( NC045512.2) were retrieved from NCBI on the basis of their antigenicity. The spike (S) glycoprotein ( YP_009724390.1), Membrane (M) glycoprotein ( YP_009724393.1) and Nucleocapsid (N) phosphoprotein ( YP_009724397.2) were retrieved in FASTA format. These three proteins together are referred to as SMN (Spike, Membrane, and Nucleocapsid) polyprotein in this study.
Cytotoxic T cell epitopes (CTL) prediction
First, the CTL epitopes for SMN polyproteins were predicted using Netctl 1.2 server 32. Prediction of the epitopes depends on three major attributes: (1) binding affinity of MHC-1 class; (2) ability of the proteasome cleavage; and (3) TAP transport efficiency. The first two are predicted with the artificial neural network algorithm while third one using weight matrix. For the prediction of the epitopes threshold for epitopes, identification was chosen to be 0.75, weight on C terminal cleavage was set on 0.15, while the weight on TAP transport efficiency was set on 0.05. The predicted epitopes were ranked according to the combined score.
Helper T cell (HTL) epitope prediction
For the prediction of HTL epitopes, the IEDB MHC II server was used 33. The species/locus was selected as Human/HLA-DR, and a 7-allele HLA reference set was selected for the prediction. Further, 15 mer length of the epitopes were retrieved and ranked according to the percentile. The percentile rank is given after comparing the peptides score with five million 15 mers from the SWISSPROT database. The higher percentile value means a lower binding affinity of MHC-II. For further refinements of the HTL epitopes, these selected HTL epitopes were subjected to investigate whether they can induce IFN gamma immune response using the IFN epitope server 34. For IFN gamma inducing epitopes selection, the Motif/SVM hybrid approach was chosen, and the model was set to be IFN gamma versus non-IFN gamma. Finally, the epitopes whose results were positive for the IFN gamma response were chosen for the in-silico vaccine development.
B cell epitope prediction
B cell epitopes were predicted using the ABCpred server 35. This server predicts B cell epitopes using recurrent neural network algorithm. For the identification of the epitopes, the threshold was set on 0.51, while the window length for the prediction was chosen to be 16, keeping overlapping filter on. Top predicted epitopes having scored more than 0.9 was only chosen for the development of the candidate vaccine. Further, after the construction of the vaccine, linear as well as discontinuous conformational B cell epitopes were identified in the vaccine construct using ElliPro, an online server 36. Elipro predicts the antibody epitopes taking protein antigen tertiary structure as input.
Antigenicity, allergenicity, and toxicity prediction
The important attributes such as the antigenicity, allergenicity and toxicity were predicted for all the predicted epitopes individually as well as after construction of the vaccine. First of all, the antigenicity was investigated using the VaxiJen 2.0 server 37, and only probable antigen epitopes were chosen for the construction of the vaccine. Further, the allergenicity was predicted using the AlgPred server 38 and only non-allergenic epitopes were selected. Finally, all the epitopes were investigated for toxicity using the ToxinPred server 39 and non-toxic epitopes were selected. All the predicted epitopes had to cross all these barriers. The overall construct of the vaccine was also tested for these attributes.
Construction of multiepitope vaccine sequence
The vaccine sequence was constructed using the best identified CTL, HTL, and B cell epitopes. For the construction of the sequence at the N terminal and C terminal, an adjuvant was added using EAAAK linkers. While HTL epitopes were linked using GPGPG, linkers and CTL epitopes were linked using AAY linkers. In the C terminal, HHHHHH was added for the easy purification of the vaccine.
Physiochemical properties of the vaccine sequence
The physicochemical properties such as molecular weight, PI, half-life, aliphatic index, and hydropathicity were predicted using online tool ProtParam 40.
Secondary structure of the vaccine sequence
Protein secondary structure prediction gives further opportunity to predict the tertiary structure as well as gives information about the activity and function of the protein. The secondary structure of the final multiepitope vaccine sequence was predicted by the free online web tool CFSSP 41.
Tertiary structure prediction
The tertiary structure of the constructed vaccine was predicted using the Rosetta web tool 42. Rosetta tool applies a deep neural network algorithm to predict the inter-residue distances as well as orientations. Then these orientations are converted to smooth inter-residue constraints followed by gradient descent energy minimization. Further, the coarse-grained models are generated, and full atom refinement is done. The validation of the model has been done through Ramachandran plot analysis using VADAR web tool 43. Further, the modelled structure was validated through the ProSA web tool 44, which gives the quality Z score of the modelled protein based on the already known similar size of the proteins crystal structures.
Minimization and equilibration of the predicted structure
To achieve a more stable structure, the predicted structure was further taken for molecular dynamics (MD) simulations using Gromacs software 45. The structure was minimized using the steepest descent algorithm with 50000 steps, followed by NVT and NPT equilibration for 100 picoseconds, followed by MD simulations of 500 picoseconds. The last frame of the MD trajectory was taken for further analysis.
Molecular docking of constructed vaccine with TLR-3 receptor
For molecular docking, the last frame from the MD simulations of the constructed vaccine was taken, and the TLR-3 structure was retrieved from Protein Data Bank (PDB; ID 1ZIW). The downloaded structure was prepared and processed for docking using dock prep tool UCSF Chimera software. For the docking, the vaccine construct and TLR-3 was uploaded to patchDock server 46. Further, for refinement of the rigid body molecular docking solutions, FireDock server was used 47. It gave the best 10 docked confirmation based on global energy and Van der Waal’s interactions.
Reverse translation and codon optimization
Finally, for expressing the constructed multiepitope, the vaccine needs to be expressed in the suitable vector inside the prokaryotic system. Hence, reverse translation and codon optimization were analysed using the Java codon adaptation web tool ( Jcat) 48. The codon optimization was performed for E. coli strain K12 as a host. Jcat gives the codon adaption index (CAI) and percentage GC content as output. The CAI gives the information of codon usage, generally score between 1 and 0.8, while GC contents should be between 40 % to 70%, values lying outside the given margin is suggested to be inefficient 49.
Results
Retrieval of the polyproteins and antigenicity
The amino acid sequence of all three (SMN) structural proteins were retrieved from the NCBI database in fasta format. The proteins were investigated for antigenicity by Vaxijen web tool, and it was found that all the three chosen proteins could be good antigens. The default threshold of 0.4 was chosen as the criteria for the antigenicity in the Vaxijen tool. The spike protein showed a score of 0.46; membrane glycoprotein showed a score of 0.51; while nucleocapsid protein showed a score of 0.50. Hence, all three proteins were chosen for further predictions of B cell and T cell epitopes and the construction of the vaccine.
Prediction of CTL epitopes
CTL epitopes were predicted using Netctl 1.2 server for all the three selected proteins. A total of 38 epitopes was predicted from spike glycoprotein; 10 epitopes were predicted from membrane glycoprotein; while 9 were predicted from nucleocapsid protein. Out of all these predicted CTL epitopes, only 8 were selected for the construction of the vaccine, based on a high binding affinity towards MHC-I, antigenicity, non-allergenicity, and non-toxicity predictions, as shown in Table 1 .
Table 1. List of the final selected CTL epitopes which have fulfilled all the criteria for antigenicity, non-allergenicity, non-toxicity and could bind efficiently to MHC-I.
| Peptide sequence | MHC binding affinity | Rescale binding affinity | C -terminal cleavage affinity | Transport efficiency | Prediction score | Polyprotein |
|---|---|---|---|---|---|---|
| WTAGAAAYY | 0.6735 | 2.8596 | 0.7339 | 2.8630 | 3.1128 | S protein |
| WMESEFRVY | 0.3902 | 1.6569 | 0.7993 | 2.9290 | 1.9232 | S protein |
| AGDSGFAAY | 0.1341 | 0.5695 | 0.9652 | 2.6730 | 0.8480 | M protein |
| LVGLMWLSY | 0.2694 | 1.1440 | 0.7240 | 2.8970 | 1.3974 | M protein |
| VATSRTLSY | 0.2752 | 1.1684 | 0.9679 | 3.0130 | 1.4642 | M protein |
| LSPRWYFYY | 0.4837 | 2.0538 | 0.9746 | 2.8150 | 2.3408 | N protein |
| DLSPRWYFY | 0.2866 | 1.2167 | 0.9760 | 2.7250 | 1.4994 | N protein |
| NTASWFTAL | 0.1772 | 0.7523 | 0.9557 | 1.1280 | 0.9521 | N protein |
Prediction of HTL epitopes
HTL epitopes were predicted using the IEDB MHC II server for all the three SMN structural proteins. Finally, 4 HTL epitopes were selected on the basis of binding affinity, antigenicity, non-allergenicity, and non-toxicity, as shown in Table 2 . Four human alleles and position of predicted epitopes are HLA-DRB1*07:01 (166-180), HLA-DRB4*01:01 (298-312), HLA-DRB5*01:01 (232-246), HLA-DRB5*01:01 (345-359).
Table 2. List of the final selected HTL epitopes which fulfilled all the criteria for antigenicity, non-allergenicity, non-toxicity and could also induce the IFN gamma immune response.
| Allele | Start | End | Peptide sequence | Percentile score | Polyprotein |
|---|---|---|---|---|---|
| HLA-DRB5*01:01 | 232 | 246 | GINITRFQTLLALHR | 0.52 | S protein |
| HLA-DRB5*01:01 | 345 | 359 | TRFASVYAWNRKRIS | 0.52 | S protein |
| HLA-DRB1*07:01 | 166 | 180 | KEITVATSRTLSYYK | 2.1 | M protein |
| HLA-DRB4*01:01 | 298 | 312 | YKHWPQIAQFAPSAS | 11 | N protein |
B cell epitope prediction
For the prediction of B cell epitopes, ABCpred server was used. Based on the binding score (>0.9), non-allergenicity and non-toxicity, a total of four B cell epitopes were finally selected, as shown in Table 3 .
Table 3. Predicted linear B cell epitopes, binding score better than 0.9, are only selected for the final vaccine construct.
| Peptide sequence | Predicted score | Polyprotein |
|---|---|---|
| AGTITSGWTFGAGAAL | 0.97 | S protein |
| GVSVITPGTNTSNQVA | 0.95 | S protein |
| TRRIRGGDGKMKDLSP | 0.94 | N protein |
| KSAAEASKKPRQKRTA | 0.93 | N protein |
Construction of multiepitope based vaccine
The four B cell epitopes, four HTL epitopes and 8 CTL epitopes were selected for vaccine construction, which fulfilled all the criteria of binding affinity, antigenicity, non-toxicity and non-allergenicity. Besides these epitopes, two adjuvants were also added at the N terminal (human beta defensin-3) and at C terminal (human beta defensin-2) of the vaccine to increase the antigenicity. Adjuvant were linked via EAAAK linkers to the epitopes, HTL epitopes were linked via GPGPG linkers, while CTL epitopes were linked with AAY linkers, as shown in Figure 1 . The constructed vaccine sequence was again checked for antigenicity, non-allergenicity, non-toxicity and it fulfilled all the criteria.
Figure 1. Structure of the final multiepitope based vaccine.
At the C terminal, an adjuvant human B defesin3 has been added, and then it is linked with B cell epitopes using EAAAK linkers. B cell epitopes are linked with HTL with GPGPG linkers, and HTL are linked with CTL with AYY linkers. At N terminal, another adjuvant human B defensin-2 has been linked with six histidine sequences.
Prediction of physicochemical parameters of the constructed vaccine sequence
The physiochemical parameters of the vaccine sequence were predicted by the ProtParam server. The molecular weight of the construct was predicted to be 38.8 KDa, and the theoretical PI value was 9.92. The predicted half-life in E. coli was more than 10 hours, and the instability index in the test tube was found to be stable. The aliphatic value of the vaccine sequence was 58.7 and the grand average of hydropathicity (GRAVY) was -0.348.
Secondary structure prediction of the vaccine sequence
Secondary structure prediction was made using the CFSSP web tool. The result showed the presence of helix at 44.5%, sheet at 35.6%, and turns at 14 %.
Tertiary structure prediction of the vaccine sequence
The 3D structure of the multiepitope predicted vaccine was predicted using the Rosetta web tool. It uses de-novo structure prediction using deep neural network algorithm to predict the inter-residue distances as well as orientations. Then these orientations are converted to smooth inter-residue constraints followed by gradient descent energy minimization. Further, coarse-grained models are generated, and full atom refinement is done. It gave five best-predicted models, and based on the TM score, one model was selected for further investigation, as shown in Figure 2A . Further to validate the predicted model, Ramachandran plot analysis was done, and results showed that 96.3% residues were in the favourable region, 2.5% were in the allowed region while ~ 1% were in the outlier region ( Figure 3 ). Additionally, the PROSA web tool was used to predict the quality of the modelled vaccine, which predicted a Z score of -6.34. Ramachandran plot and Z score have suggested that the predicted model of protein was valid and could be taken for further analysis.
Figure 2.
(A) The crude 3D modelled structure of the vaccine (grey) has been superimposed with the simulated model (yellow). ( B) The top 3 conformational B cell epitopes predicted in the vaccine has been shown with yellow spheres.
Figure 3. Ramachandran plot of the 3D modelled vaccine construct.
Conformational B cell epitope analysis from modelled vaccine
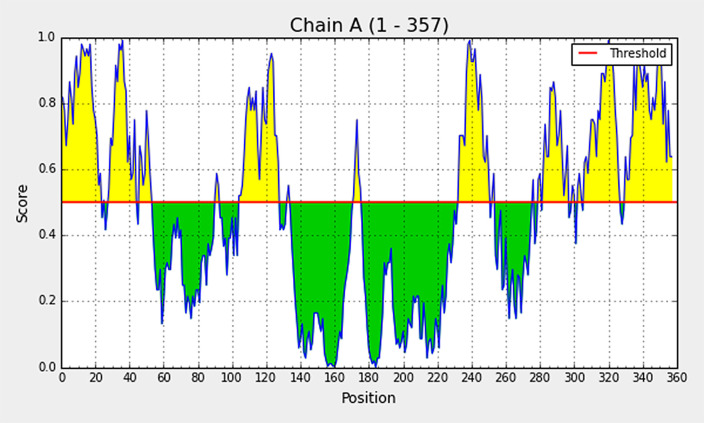
Elipro predicts the antibody epitopes taking protein 3D structure as input. Linear B, as well as discontinuous conformational epitopes, were identified in the vaccine construct using ElliPro, an online server. A total of 8 linear epitopes were predicted, and the sequence of the top 3 epitopes have been reported in Table 4 and has been shown structurally in Figure 2B . Various discontinuous epitope residues were predicted from vaccine sequence length 232-253 (21 epitope residues), between 299-357 (55 epitope residues), between 1-54 (52 epitope residues), between 69-128 (33 epitope residues) and between 168-176 (9 epitope residues) were predicted. The individual score of each of the discontinuous epitopes has been shown in Figure 4 .
Table 4. Predicted top three conformational B cell epitopes from the 3D modelled vaccine construct.
| Start | End | Peptide sequence | score |
|---|---|---|---|
| 329 | 357 | FCPRRYKQIGTCGLPGTKCCKKPHHHHHH | 0.78 |
| 232 | 253 | VYAAYAGDSGFAAYAAYLVGLM | 0.74 |
| 1 | 54 | GIINTLQKYYCRVRGGRCAVLSCLPKEEQIGKCSTRGRKCCRRKKEAAAKAGTI | 0.73 |
Figure 4. The individual score of discontinuous B cell epitopes predicted in the modelled vaccine.
Docking of vaccine with TLR-3 receptor
The modelled structure of the vaccine was taken through energy minimization, equilibration, and MD simulations before docking. The last frame from the simulated trajectory was taken further for docking. The simulated structure has been compared with the crude modelled structure, as shown in Figure 2A . The TLR-3 structure was retrieved from PDB (ID 1ZIW). The downloaded structure was prepared and processed for docking using the dock prep tool UCSF Chimera software 50. The simulation was done using the PatchDock server and further refined using FireDock. The best-docked complex had global energy of -14.91 Kcal/mol, and attractive Van der Waal’s energy was -18.1 Kcal/mol, which shows a decent binding affinity of the vaccine towards TLR-3. Further, the best binding pose was investigated for polar interactions using discovery studio visualizer 51 between TLR-3 and vaccine, and it was found that GLN352, SER428, ILE370 of TLR-3 was making the hydrogen bond with TYR260, ARG321, and LYS166 of vaccine respectively ( Figure 5 ).
Figure 5. The docked structure of TLR-3 (green)-modelled vaccine (orange) complex illustrating critical residues involved in the interactions.
Reverse translation and codon optimization
Jcat was used for the optimization of the codon for the proper expression of the protein. E. coli strain K12 was chosen as a host, with additional options such as avoid rho-independent transcription terminators, avoid prokaryotic ribosome binding sites, and avoid Cleavage Sites of Restriction Enzymes. The CAI gives the information of codon usage, generally score between 1 and 0.8, while GC contents should be between 40 % to 70%, values lie outside the given margin is suggested to be inefficient. CAI of the optimized nucleotide sequence of the vaccine was found to be 0.92, with a GC content of 55.6%, which indicates the effective expression of the protein in the E. coli.
Discussion
The structural polyproteins from SARS-CoV-2 were selected for developing a multiepitope based vaccine. Initially, four structural proteins were chosen, namely spike, membrane, envelope, and nucleocapsid protein, based on their antigenicity prediction, role in facilitating the entry of the virus, and packaging inside the host cells. However, when CTL, HTL, and B cell epitopes were predicted, it was found that epitopes from envelope protein were not able to satisfy the criteria of non-allergenicity and antigenicity simultaneously, and hence envelope protein was not considered further in the study. While epitopes selected from SMN structural polyprotein were satisfying, all the criteria such as antigenicity, non-allergenicity, non-toxicity, and high binding affinity towards MHC and also HTL epitopes were able to induce IFN gamma immune response. The constructed multiepitope vaccine from the selected epitopes from all the three SMN polyproteins was again investigated for antigenicity, and it was found that vaccine construct predicted to be a potent antigen with score 0.60 (predicted by Vaxijen). Further, the vaccine construct was looked for its allergenicity, and it was found that the vaccine was a non-allergen with a score of -0.59 (threshold was set on -0.4, predicted by AlgPred tool). Further physicochemical parameters were analysed for vaccine sequence, and it was predicted to have a molecular weight of 38.8 KDa, PI of 9.92, and half-life inside the E. coli >10 hours, which shows that protein can easily express and isolated. The 3D model of the constructed vaccine sequence was predicted from the Rosetta web server. Eight conformational linear B cell epitopes were found in the modelled structure of the vaccine, as predicted by the Elipro web tool. It was evident from the prediction that the constructed vaccine model could easily produce adaptive immune response specific to the SARS-CoV-2 antigens. Further, to investigate the ability of the modelled vaccine to interact with TLR receptors on immune cells, the TLR-3 receptor was docked with the modelled vaccine. The results showed that the modelled vaccine had a good binding affinity towards TLR-3, and it was found that GLN352, SER428, ILE370 of TLR-3 was making the hydrogen bond with TYR260, ARG321, and LYS166 of vaccine, respectively. This interaction of vaccine with TLR-3 was predicting that vaccine have the potential to generate both innate as well as adaptive humoral and cell-mediated immune responses. For efficient expression of the protein inside the E. coli, codon optimization was done to improve the translation and transcription efficiency. The constructed vaccine sequence was reverse translated, and CAI and GC content were assessed, taking E. coli (K12) as a host organism. The CAI index of 0.92 and GC content of 55.6 % and half-life was already predicted to be more than 10 hours, suggests the efficient expression of recombinant protein inside the E. coli. This immunoinformatics study suggests that the predicted vaccine could generate specific adaptive immunity against SARS-CoV-2 and could provide a valuable contribution to the management of the COVID-19. This predicted vaccine candidate strongly warrant in-vitro and in-vivo study for the practical implications.
Conclusions
In this study, a multiepitope (CTL, HTL, and B cell) vaccine construct has been predicted and modelled through immunoinformatics techniques. The predictions suggest that the constructed vaccine could generate both humoral and cell-based adaptive immunity towards SARS-CoV-2. Further, it was also predicted that it may easily be expressed inside the E. coli strain (K12). This immunoinformatics study may reduce the expenditure and time for vaccine research and may give a significant value in the management of COVID-19. This in silico prediction warrants the in-vitro and in-vivo study to test the practical implications of the predicted vaccine.
Data availability
Underlying data
NCBI: Severe acute respiratory syndrome coronavirus 2 isolate Wuhan-Hu-1, complete genome, Accession number NC045512.2: https://www.ncbi.nlm.nih.gov/nuccore/NC_045512.2/
NCIB: surface glycoprotein [Severe acute respiratory syndrome coronavirus 2], Accession number YP_009724390.1: https://www.ncbi.nlm.nih.gov/protein/YP_009724390.1/
NCBI: membrane glycoprotein [Severe acute respiratory syndrome coronavirus 2], Accession number YP_009724393.1: https://www.ncbi.nlm.nih.gov/protein/YP_009724393.1
NCBI: nucleocapsid phosphoprotein [Severe acute respiratory syndrome coronavirus 2], Accession number YP_009724397.2: https://www.ncbi.nlm.nih.gov/protein/YP_009724397.2
Protein Data Bank: Human Toll-like Receptor 3 extracellular domain structure, Accession number 1ZIW: http://doi.org/10.2210/pdb1ZIW/pdb
Acknowledgements
A previous version of this article is available on Research Square: https://doi.org/10.21203/rs.3.rs-31779/v1
Funding Statement
The author(s) declared that no grants were involved in supporting this work.
[version 1; peer review: 2 approved]
References
- 1. Denison MR, et al. : Coronaviruses: an RNA proofreading machine regulates replication fidelity and diversity. RNA Biol 2011;8(2):270–9. 10.4161/rna.8.2.15013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fehr AR, Perlman S: Coronaviruses: an overview of their replication and pathogenesis. Methods Mol Biol 2015;1282:1–23. 10.1007/978-1-4939-2438-7_1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ye ZW, et al. : Zoonotic origins of human coronaviruses. Int J Biol Sci 2020;16(10):1686–97. 10.7150/ijbs.45472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bhattacharya S, et al. : Recent apprise on coronavirus and its terrible insinuations. Virusdisease 2020;1–7. 10.1007/s13337-020-00582-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Vabret A, et al. : Human coronavirus NL63, France. Emerg Infect Dis 2005;11(8):1225–9. 10.3201/eid1108.050110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li Q, et al. : Early Transmission Dynamics in Wuhan, China, of Novel Coronavirus-Infected Pneumonia. N Engl J Med 2020. 10.1056/NEJMoa2001316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hui DS, et al. : The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int J Infect Dis 2020;91:264–6. 10.1016/j.ijid.2020.01.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Huang C, et al. : Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020. 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lechien JR, et al. : Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 2020;1–11. 10.1007/s00405-020-05965-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nicastri E, et al. : National institute for the infectious diseases "L. Spallanzani", IRCCS. Recommendations for COVID-19 clinical management. Infect Dis Rep 2020;12(1). 10.4081/idr.2020.8543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Sindhu T, et al. : Antiviral screening of Clerodol derivatives as COV 2 main protease inhibitor in Novel Corona Virus Disease: In silico approaches. Asian J Pharm Clin Res 2020;10(2):60–4. 10.5958/2231-5713.2020.00012.4 [DOI] [Google Scholar]
- 12. Yadav AR, Mohite SK: A Novel Approach for Treatment of COVID-19 with Convalescent Plasma. RJPDFT 2020;12(3):227–30. 10.5958/0975-4377.2020.00037.3 [DOI] [Google Scholar]
- 13. Shankhdhar PK, et al. : Turmeric: Plant Immunobooster against COVID-19. Res Rev J Pharmacogn Phytochem 2020;12(3):174–7. 10.5958/0975-4385.2020.00029.1 [DOI] [Google Scholar]
- 14. Goswami S, et al. : A Meticulous Interpretation on a Sanguinary Disease COVID-19. Res Rev J Pharmacogn Phytochem 2020;12(3):231–3. 10.5958/0975-4377.2020.00038.5 [DOI] [Google Scholar]
- 15. Naresh B: A Review of the 2019 Novel Coronavirus (COVID-19) Pandemic. Asian J Pharm Sci 2020;10(3):233–8. 10.5958/2231-5691.2020.00040.4 [DOI] [Google Scholar]
- 16. Hoffmann M, et al. : SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020. 10.1016/j.cell.2020.02.052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Iwata-Yoshikawa N, et al. : TMPRSS2 Contributes to Virus Spread and Immunopathology in the Airways of Murine Models after Coronavirus Infection. J Virol 2019;93(6). 10.1128/JVI.01815-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Prajapat M, et al. : Drug targets for corona virus: A systematic review. Indian J Pharmacol 2020;52(1):56–65. 10.4103/ijp.IJP_115_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Brunel J, et al. : Sequence of events in measles virus replication: role of phosphoprotein-nucleocapsid interactions. J Virol 2014;88(18):10851–63. 10.1128/JVI.00664-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Surjit M, Lal SK: The SARS-CoV nucleocapsid protein: a protein with multifarious activities. Infect Genet Evol 2008;8(4):397–405. 10.1016/j.meegid.2007.07.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nemazee D: Mechanisms of central tolerance for B cells. Nat Rev Immunol 2017;17(5):281. 10.1038/nri.2017.19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Oleinika K, et al. : B Cell Activation and B Cell Tolerance. The Autoimmune Diseases: Elsevier 2020;171–87. 10.1016/B978-0-12-812102-3.00009-9 [DOI] [Google Scholar]
- 23. Schmitt ME, et al. : The B cell antigen receptor of IgE-switched plasma cells regulates memory IgE responses. J Allergy Clin Immunol 2020. 10.1016/j.jaci.2020.02.015 [DOI] [PubMed] [Google Scholar]
- 24. Gold MR, Reth MG: Antigen receptor function in the context of the nanoscale organization of the B cell membrane. Annu Rev Immunol 2019;37:97–123. 10.1146/annurev-immunol-042718-041704 [DOI] [PubMed] [Google Scholar]
- 25. Brink R, Phan TG: Self-reactive B cells in the germinal center reaction. Annu Rev Immunol 2018;36:339–57. 10.1146/annurev-immunol-051116-052510 [DOI] [PubMed] [Google Scholar]
- 26. Karunakaran KP, et al. : Discordance in the Epithelial Cell-Dendritic Cell Major Histocompatibility Complex Class II Immunoproteome: Implications for Chlamydia Vaccine Development. J Infect Dis 2020;221(5):841–50. 10.1093/infdis/jiz522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Peters B, et al. : T Cell Epitope Predictions. Annu Rev Immunol 2020;38. 10.1146/annurev-immunol-082119-124838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gaudino SJ, Kumar P: Cross-talk between antigen presenting cells and T cells impacts intestinal homeostasis, bacterial infections, and tumorigenesis. Front Immunol 2019;10. 10.3389/fimmu.2019.00360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Miller CT: The Expression Of Connexin-43 By CD11c+ Dendritic Cells Is Required to Maintain CD4+ Foxp3+ Regulatory T Cell Population in Peripheral Lymphoid Organs. 2019. 10.25777/4efg-my55 [DOI]
- 30. Rich RR, Chaplin DD: The human immune response. Clinical Immunology: Elsevier 2019;e1:3–17. 10.1016/B978-0-7020-6896-6.00001-6 [DOI] [Google Scholar]
- 31. Toubi E, Vadasz Z: Innate immune-responses and their role in driving autoimmunity. Autoimmun Rev 2019;18(3):306–11. 10.1016/j.autrev.2018.10.005 [DOI] [PubMed] [Google Scholar]
- 32. Larsen MV, et al. : Large-scale validation of methods for cytotoxic T-lymphocyte epitope prediction. BMC Bioinformatics 2007;8:424. 10.1186/1471-2105-8-424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zhang Q, et al. : Immune epitope database analysis resource (IEDB-AR). Nucleic Acids Res 2008;36(Web Server issue):W513–8. 10.1093/nar/gkn254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dhanda SK, et al. : Designing of interferon-gamma inducing MHC class-II binders. Biol Direct 2013;8:30. 10.1186/1745-6150-8-30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saha S, Raghava GP: Prediction of continuous B-cell epitopes in an antigen using recurrent neural network. Proteins 2006;65(1):40–8. 10.1002/prot.21078 [DOI] [PubMed] [Google Scholar]
- 36. Ponomarenko J, et al. : ElliPro: a new structure-based tool for the prediction of antibody epitopes. BMC Bioinformatics 2008;9:514. 10.1186/1471-2105-9-514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Doytchinova IA, Flower DR: VaxiJen: a server for prediction of protective antigens, tumour antigens and subunit vaccines. BMC Bioinformatics. 2007;8:4. 10.1186/1471-2105-8-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Saha S, Raghava GP: AlgPred: prediction of allergenic proteins and mapping of IgE epitopes. Nucleic Acids Res 2006;34(Web Server):W202–9. 10.1093/nar/gkl343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gupta S, et al. : In silico approach for predicting toxicity of peptides and proteins. PLoS One 2013;8(9):e73957. 10.1371/journal.pone.0073957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Garg VK, et al. : MFPPI - Multi FASTA ProtParam Interface. Bioinformation 2016;12(2):74–7. 10.6026/97320630012074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kumar TA: CFSSP: Chou and Fasman secondary structure prediction server. Wide Spectrum 2013;1(9):15–9. [Google Scholar]
- 42. Yang J, et al. : Improved protein structure prediction using predicted interresidue orientations. Proc Natl Acad Sci U S A 2020;117(3):1496–503. 10.1073/pnas.1914677117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Willard L, et al. : VADAR: a web server for quantitative evaluation of protein structure quality. Nucleic Acids Res 2003;31(13):3316–9. 10.1093/nar/gkg565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wiederstein M, Sippl MJ: ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucleic Acids Res 2007;35(Web Server):W407–10. 10.1093/nar/gkm290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Hess B, et al. : GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. J Chem Theory Comput 2008;4(3):435–47. 10.1021/ct700301q [DOI] [PubMed] [Google Scholar]
- 46. Schneidman-Duhovny D, et al. : PatchDock and SymmDock: servers for rigid and symmetric docking. Nucleic Acids Res 2005;33(Web Server):W363–7. 10.1093/nar/gki481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mashiach E, et al. : FireDock: a web server for fast interaction refinement in molecular docking. Nucleic Acids Res 2008;36(Web Server):W229–32. 10.1093/nar/gkn186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Grote A, et al. : JCat: a novel tool to adapt codon usage of a target gene to its potential expression host. Nucleic Acids Res 2005;33(Web Server):W526–31. 10.1093/nar/gki376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Das S, et al. : Comparative Analysis of Predicted Gene Expression among Crenarchaeal Genomes. Genomics Inform 2017;15(1):38–47. 10.5808/GI.2017.15.1.38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Goddard TD, et al. : Software extensions to UCSF chimera for interactive visualization of large molecular assemblies. Structure 2005;13(3):473–82. 10.1016/j.str.2005.01.006 [DOI] [PubMed] [Google Scholar]
- 51. BIOVIA DS : Discovery Studio Modeling Environment. 2020.