Abstract
Aminolevulinic acid based photodynamic therapy (ALA-PDT) is a popular and efficacious treatment for actinic keratosis (AK). However, standard PDT can elicit stinging pain during illumination, and hence is not always favored by patients. In a new regimen called metronomic PDT (mPDT), similar to daylight PDT but using blue light, the illumination is delivered concurrently with ALA application rather than after a 1-hour pre-incubation (conventional regimen, cPDT). In the clinic, mPDT is not only painless but also nearly as effective as cPDT for AK lesion clearance. In this investigation, a murine AK model (generated by repeated UVB exposure) was treated with either mPDT or cPDT. Lesion clearance was followed by area measurement, and samples were harvested for mechanistic analyses. Compared to pretreatment (100%), the average lesion area was reduced to 47% and 32% in cPDT, and to 57% and 40% in mPDT at 1- and 2-weeks post PDT, respectively. Relative to untreated controls, enhanced cell death (histomorphology by H&E staining and apoptosis by TUNEL assay), and generation of Reactive Oxygen Species (ROS; CM-H2DCFDA staining) were observed in both cPDT and mPDT samples. Activation of cleaved Caspase-3 was specifically observed only in cPDT samples. Immunomodulation by inflammatory cells was observed by enhanced infiltration/retention of neutrophils and macrophages in metronomic PDT samples. Our results suggest that metronomic PDT can be just as effective as conventional PDT for treatment of AK, but the mechanisms may be quite different.
Keywords: Photodynamic therapy (PDT), aminolevulinic acid (ALA), protoporphyrin IX (PpIX), metronomic PDT (mPDT), actinic keratosis, murine model, cell death, reactive oxygen species (ROS), In Vivo Imaging System (IVIS)
1. INTRODUCTION
Nonmelanoma skin cancers (NMSC), comprising squamous cell carcinoma (SCC) and basal call carcinoma (BCC), account for the majority of skin malignancies with an increasing incidence in Caucasian populations worldwide [1]. Chronic sun-exposure in the lifespan of an individual plays the main etiological role in the incidence of NMSCs [2]. SCCs develop from sun-exposed epidermal keratinocytes, which undergo neoplastic transformation from precursor lesions called actinic keratosis (AK) [3]. Sun-induced TP53 mutations in epidermal keratinocytes play causative role in the neoplastic progression from normal skin to actinic keratosis, and eventually to SCCs that can invade and metastasize, if left untreated [4, 5]. In humans, AK lesions appear as rough, red, scaly patches mainly on sun-exposed areas like the face, scalp, arms and forearms. Cryosurgery, i.e. freezing the lesion with liquid nitrogen, is the most popular therapy for AKs. One of the major limitations for cryosurgery is lesion size, because lesions must be large enough to be visualized by the treating physician. Neoplastic changes are often missed because they are microscopic, and spread over wide areas of sun-damaged skin (a phenomenon called ‘field cancerization’) [6]. Photodynamic therapy (PDT), a treatment modality specifically utilized for treatment of AK lesions in broad areas of field cancerization, is therefore becoming popular in both Europe and America [7–10]. In dermatologic oncology clinics, PDT represents a non-scarring and repeatable alternative to cryotherapy for treatment of cutaneous carcinomas and precancers, and is currently being widely utilized [11–13].
1.1. Photodynamic therapy (PDT):
Photodynamic therapy (PDT) for treatment of cancer employs a photosensitizer (PS) and visible light in the presence of oxygen to kill tumor cells [14–17]. PDT offers dual selectivity for the treatment of cancer; first, the cancer cells accumulate higher levels of PS than normal cells; and second, the light source that triggers the cell death is directly focused onto the tumor eliminating undesired off-target tissue damage [18]. The PS once activated inside the cancer cell by visible light, triggers cell death by releasing free radicals called reactive oxygen species (ROS) [14, 17, 19]. The PS for PDT in its early days was hematoporphyrin derivative (HPD) or a benzylated porphyrin derivative (BPD), systemically-delivered effectively but often associated with the risk of increased phototoxicity (sunburn) [17]. To reduce phototoxicity and improve tumor selectivity, a newer mechanism of PDT that uses a prodrug, aminolevulinic acid (ALA) instead of the pre-formed PS, was developed [20]. ALA, a precursor for PpIX, given orally, systemically or topically, is taken up and enzymatically converted into protoporphyrin IX (PpIX) through heme biosynthetic pathway within mitochondria. PpIX is then activated by strong visible blue or red light to generate free radicals or reactive oxygen species (ROS) that kill the cancer cells through a cascade of cell death events [14–17]. Cutaneous cancers can be easily illuminated using a blue (405 nm) or red light (633 nm) for ALA-PDT. However, red light can penetrate up to 1 cm into skin tissue, making it a suitable light source for thicker or deeper lesions. ALA/methyl ALA (mALA)-based PDT is now being successfully used in Europe for the treatment of superficial basal cell carcinoma (BCC) and cutaneous squamous cell carcinoma (SCC). Unlike the conventional treatment option of surgical excision, ALA-PDT represents a treatment modality with several advantages (scar-less healing; repeatability) over other routine therapies for management of precancer and cancer of skin.
1.2. Painless or metronomic PDT (mPDT) for treatment of actinic keratosis:
Actinic keratoses (AK) are dysplastic lesions of the skin with the potential to develop into SCC, if left untreated. Therefore, diagnosis and treatment of AK at an early stage is very important to prevent these lesions from developing into skin cancer. Photodynamic therapy (PDT) with topical 5-aminolevulinic acid (ALA) is an FDA-approved treatment that has gained popularity due to its efficacy as a treatment for widespread field cancerization [6, 11, 21]. In the traditional protocol for ALA-PDT treatment of AK, topical ALA is applied to the skin and left on for 1–4 hours to allow the photosensitizing molecule within mitochondria (PpIX) to build up within the precancer cells. Upon excitation by visible light, PpIX becomes activated and induces cell death damage, primarily by generation of reactive oxygen species (ROS) [16, 17]. Although ALA-PDT is a well-accepted and effective therapy in dermatology clinics, it can elicit stinging pain during illumination which is often very troublesome for patients. In fact, pain may the primary reason for refusing the treatment altogether [22–24].
To address the pain associated with PDT treatment for AK, a new regimen called metronomic PDT (mPDT) has been recently advanced [25, 26]. The term “metronomic PDT” was originally used by Bisland et al. to refer to simultaneous delivery of low doses of photosensitizer and light over long periods of time, which proved effective in studies of PDT for malignant brain tumors in rats [27]. In the clinic, mPDT is not only painless but also nearly as effective as PDT for AK lesion clearance. The underlying hypothesis is that the PpIX photosensitizer may in fact only be needed in small amounts, and that it might be possible to photoactivate PpIX as soon as it is synthesized within mitochondria, rather than waiting for high levels of PpIX to build up. The latter high levels of PpIX might drive PpIX diffusion into nerves, cause pain when activated light, which is highly objectionable from the patient’s perspective. A real-life human example of metronomic PDT is a technique called ‘Daylight PDT’, first promoted in Europe, in which methyl-ALA is applied to the skin and the patient is immediately sent outside into the sun to receive ~2 hours of daylight exposure [25, 26]. Interestingly, this procedure elicits minimal pain, yet results in the inflammatory reaction and lesion clearance typically seen with a more traditional approach that uses a 1–3 hours preincubation of methyl-ALA prior to exposure to light [23, 24].
We recently completed a clinical trial in which we simulated Daylight PDT, but using artificial blue lights (which are the standard device used in U.S. dermatology clinic, which delivers a highly reproducible light dose). In this randomized, bilaterally-controlled clinical trial with a split-body design, the AK lesion clearance response was compared between metronomic PDT (on Side A) and conventional PDT (on Side B); the results showed that treatment efficacy with respect to lesion clearance was the same for both regimens. Just as importantly, the treatment procedure on Side A (metronomic PDT) was less painful than on Side B (conventional PDT) [41]. It is difficult to study detailed mechanisms in human patients; animal models are therefore desirable for this purpose. In the study reported here, we have employed a mouse model to investigate the mechanistic details that might explain the similar lesion clearance between metronomic and conventional PDT. Using a murine model in which AK are generated by repeated UVB exposures, we examined lesion clearance, cell death, and inflammatory cell infiltration following conventional PDT and mPDT. The results suggest that metronomic PDT can be just as effective as conventional PDT for AK treatment, but the mechanisms may be quite different.
2. MATERIALS AND METHODS
2.1. UV-induced AK mouse model:
To create an actinic keratosis (AK) model in mice, the dorsal side of SKH-1 hairless female mice at 8 weeks of age was UV-irradiated three times weekly, using a set of UV lamps that provide 80% UVB and 20% UVA. Due to this chronic UV exposure, lesions of early squamous cell carcinoma (SCC) that resemble actinic keratosis both morphologically and histologically began to appear on the dorsal skin of the mice by about week 15. All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of the Cleveland Clinic.
2.2. Analysis of protoporphyrin IX (PpIX) in normal skin and AK lesions:
A multispectral in vivo fluorescence imaging system (Maestro EX, Perkin-Elmer) was used to map the distribution of PpIX in AK lesions following 1h of ALA application. This system works on the principle of exciting fluorescent molecules at a fixed excitation wavelength, and recording the intensity of the emitted fluorescence over a range of wavelengths. Mice were placed in the light-tight camera box with continuous exposure to anesthesia (isoflurane, 3%) from nose cones on the imaging platform. Excitation was accomplished with blue light (435–480 nm, centered on the 455 nm peak) and fluorescence images were obtained using 490 nm long pass filter, to monitor the emission in 10 nm steps from 500 to 720 nm [28, 29]. Two images were obtained, the first before Levulan® application, and the second at 1 h post-application. The image obtained before applying ALA was used to subtract the autofluorescence present in AK lesions at 1 h post-Levulan® application, and thereby to obtain the PpIX-specific fluorescence (see Fig. 2). Spectral processing (i.e. unmixing) of PpIX fluorescence was done as per the manufacturer’s established protocols using Maestro EX 3.0 Image Processing software [29].
Figure 2: Long-term UVB exposure results in generation of AK lesions in SKH-1 mice.

(A) Bright field images showing normal control (top) and AK bearing mouse (bottom). (B) Histological analysis of normal skin (top) and AK lesion (bottom) by hematoxylin and eosin (H&E) staining. (C) In vivo imaging of PpIX fluorescence in normal control mouse (top) and in AK bearing mouse (bottom) after application of ALA (Levulan® Kerastick) for 1 hour. Note the development of AK lesions in UVB-exposed mouse (red arrows in bottom image in A), H&E image showing hyperplastic epidermis in murine AK lesion (bottom image in B) and PpIX fluorescence originating from AK lesions (arrows in bottom image in C) after ALA application.
2.3. Photodynamic therapy:
Anesthetized mice with AK lesions, received either metronomic PDT (ALA + immediate illumination, 405 nm for 60 minutes, 36 Joules) or conventional PDT (1-hour incubation with ALA + illumination, 405 nm for 8.25 minutes, 5 Joules), using Blu-U light source. The light source was calibrated using a FieldMate laser power meter (Coherent). Mice were either sacrificed and lesions harvested at times shown in the figures or AK lesion clearance was measured in mice surviving one- or two-weeks post PDT treatment. Levulan Kerastick™ (Sun pharmaceuticals, Princeton, NJ) was used as the source of ALA [29].
2.4. Histological and Immunohistochemical staining of skin/AK samples:
Actinic keratosis lesions treated with either metronomic or conventional PDT were harvested at 1-, 3- or 5-days post PDT. Formalin-fixed, paraffin embedded sections were evaluated by Hematoxylin/Eosin (H&E) staining. For immunofluorescent staining of protein markers, skin was embedded in Histochoice (VWR) rather than formalin. For immunofluorescence analyses of Caspase-3 expression and cleavage, the primary antibody specific to cleaved Caspase 3 was purchased from BioVision Inc. and antibodies for macrophages (F4/80) and neutrophils (Ly6G) were from BioRad and Affymetrix, respectively. The secondary antibodies, Cy3-conjugated donkey anti-rabbit IgG and Cy3-conjugated donkey anti-rat IgG were from Jackson ImmunoResearch. Relative expression of marker proteins in sections stained by immunofluorescence was analyzed using fluorescence microscopy at the Lerner Research Institute Image Core [29, 30].
2.5. Analysis of Reactive Oxygen Species (ROS) in murine AK samples:
The Reactive Oxygen Species (ROS) generation was analyzed in frozen sections of murine AK lesions using diclorodihydrofluorescein di-acetate (CM-H2DCFDA). The CM-H2DCFDA is a non-fluorescent dye but it is converted into a fluorescent dichlorofluorescein (DCF) upon oxygenation with PDT-induced ROS production in AK lesions. Freshly cut OCT skin cryosections (10 μm) were incubated with 10 μM CM-H2DCFDA probe dissolved in ACAS buffer (127 mM NaCl, 0.8 mM MgCl2, 3.8 mM KCl, 1.2 mM KH2PO4, 1.2 mM CaCl2, 5 mM glucose & 10 mM HEPES PH-7.4) for 1 h at room temperature in dark, followed by washing three times with PBS in dark and mounting in Vectashield containing DAPI. Relative levels of ROS generated during metronomic or conventional PDT was analyzed using fluorescence microscopy at the Lerner Research Institute Image Core [31].
2.6. Treatment responses at 1 and 2 weeks after PDT of murine AK lesions:
The treatment efficacy of metronomic and conventional PDT was analyzed by measuring lesion volume at 1- and 2-weeks post PDT and expressed as –fold pre-PDT counts. On an average, 12–15 lesions from 5 mice for each treatment group were analyzed using digital photographs. Bars shown in figure 2 represent −/+ SEM [10].
3. RESULTS and DISCUSSION
3.1. Chronic UVB-exposure results in generation of actinic keratosis lesions in SKH-1 mice:
Actinic keratosis (AK) also known as solar keratosis, are precancerous cutaneous lesions caused by prolonged sun-exposure. AK lesions are one of the most common reasons among Caucasians for a visit to the dermatology clinic [7, 21]. AK lesions must be treated at initial stages of their development to stop them from progressing to SCCs. Several groups including our own have shown that AK can be modeled in mice using a prolonged UVB-exposure regimen [28, 32]. Morphology and histology of AK lesions that develop in mice closely resemble those in humans. A recent study by Pillon et al. involving histological and phenotypic analyses with expression of p53, Ki67 and CD3 performed on human and mouse AK lesions showed that AK modeling in mice was clinically relevant [32]. Based on these observations, we generated AK lesions in SKH-1 mice following a 20-week UVB exposure regimen, as described [28, 29]. Rough, scaly and red AK lesions developed on the back of SKH-1 mice around week 15 of the exposure regimen (Fig. 1A; bottom vs. top image). Histological analysis of these lesions by hematoxylin and eosin (H&E) staining clearly showed an epidermal hyperplasia as compared to the normal epidermis (Fig. 1B; bottom vs. top image). The premalignant nature of the AK lesions was confirmed after application of ALA and in vivo visualization of PpIX fluorescence by Maestro imaging. Compared to the normal skin, PpIX fluorescence on the back of SKH-1 mice originating from AK lesions, validated their neoplastic nature (Fig. 1 C; bottom vs. top image). Following the confirmation of murine AK lesions by morphology, histology and PpIX fluorescence analysis, mice with AK lesions were used for comparative analyses of mechanistic details underlying the similar lesion clearance observed in the clinical trial involving metronomic or conventional PDT for treatment of AK in humans (see Introduction; 1.2).
Figure 1.
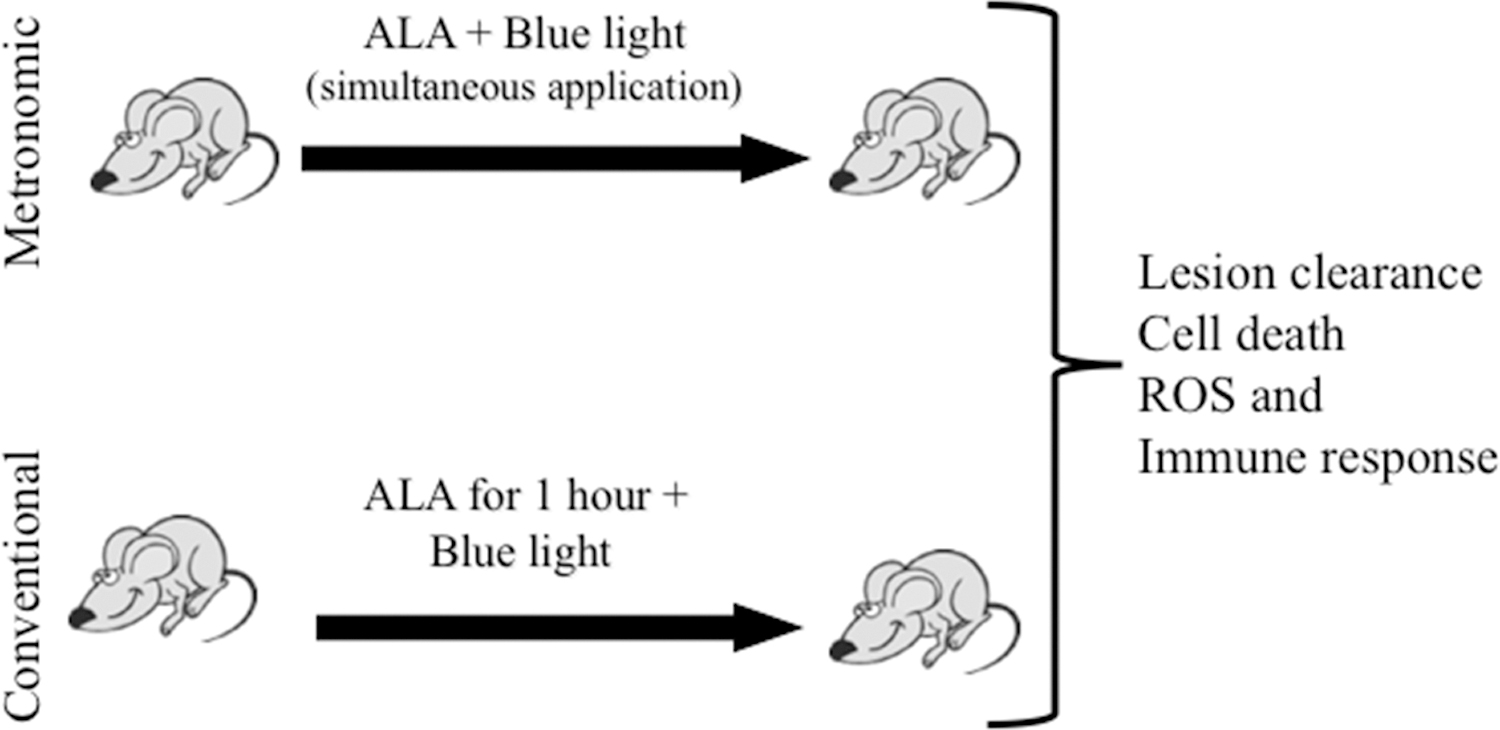
Schematic of conventional and metronomic PDT regimens for murine AK lesions
3.2. Metronomic and conventional PDT elicit similar treatment responses (lesion clearance) in murine actinic keratoses:
Mice with AK lesions were treated with either conventional PDT or metronomic PDT as described in Methods. We tested our hypothesis that small amounts of PpIX synthesized during metronomic regimen will be sufficient to induce photodamage, in contrast to high levels of PpIX produced during conventional regimen, which is the main source of pain associated with the procedure [22–24]. The lesion volume was measured before and 1- and 2-weeks post conventional or metronomic PDT of AK lesions. Compared to pretreatment (100%), the average lesion volume was reduced to 46% and 32% in conventional PDT, and to 57% and 40% in metronomic PDT at 1- and 2-weeks post PDT, respectively (Fig. 3). This observation was similar to the one observed during a clinical trial performed by Maytin et al. [unpublished data] which showed that clearance response between conventional an metronomic PDT regimens for human AK lesions were quite similar. Therefore, metronomic PDT for the treatment of AK could be as effective as conventional PDT.
Figure 3: Treatment response after metronomic or conventional PDT of murine AK lesions.

Lesion clearance at 1 and 2 weeks after metronomic PDT (blue bars) or conventional PDT (grey bars). AK lesions were measured in three dimensions (Width x Length x Height) and expressed as –fold pretreatment for each individual lesion. Bars represent mean ± SEM, 12–15 lesions from 5 mice for each treatment group.
3.2. Cell death is induced to different levels by metronomic or conventional PDT:
A similar lesion clearance observed with conventional or metronomic PDT treatments were meaningful only if both regimens were able to induce similar cell death response post PDT. To test this hypothesis, AK lesions were harvested at 24 hours post PDT (Fig. 4A) and cell death was analyzed in H&E-stained sections of AK (Fig. 4B–D). During the 24 hours following PDT, both conventional and metronomic PDT treated lesions developed apoptotic cells characterized by shrunken, pyknotic nuclei, extravasated red cells, intracellular vacuoles and large areas of cell loss (empty spaces). However, the microscopic features of apoptotic cell death were more pronounced in lesions treated with conventional PDT regimen (Figs. 4D vs. 4C). A detail description of quantitation of cell death by H&E staining have been described previously [28, 29]. Cell death analysis by TUNEL staining of AK lesions showed a similar trend with robust staining in conventional PDT treated lesions as compared to lesions receiving metronomic PDT treatment (Fig. 5A; bottom vs. middle image). The therapeutic effect of PDT is mediated by generation of reactive oxygen species (ROS) which acts as an agent of tumor cell destruction and damage [14, 17, 33, 34]. To observe the relative levels of ROS generated by metronomic or conventional PDT regimens, ROS levels were analyzed in frozen sections from AK lesions harvested at 24 hours post activation of caspase-activation cascade which eventually results in activation and cleavage of caspase-3, a hallmark of cell death. We have previously reported the activation of the mitochondrial caspase-8 pathway resulting in caspase-3 activation and cleavage during PDT-induced cell death in a murine model of human SCC (A431) [30]. Caspase-3 activation in murine AK lesions was analyzed by immunofluorescence after conventional or metronomic PDT using an antibody specific to cleaved caspase-3. Interestingly, caspase-3 was activated and cleaved exclusively in AK lesions treated with conventional PDT regimen (Fig. 5C; bottom vs. middle image). This observation suggests that unlike metronomic, conventional PDT regimen involves classic caspase activation pathway for induction of apoptosis in murine AK lesions. A compromised cell death response observed in AK lesions treated with metronomic PDT suggests that PDT-induced apoptotic cell death may not be the primary mechanism causing a similar lesion clearance observed between metronomic or conventional PDT treatment regimens for the treatment of both murine and human AK lesions.
Figure 4: Histological analysis of PDT-induced cell death in AK lesions.

PDT-induced cell death was analyzed by comparing visual appearance and histomorphology of AK lesions at pre- and 24 hours post-PDT. (A) Visual appearance and PDT-induced lesion shrinkage (24 hours post-conventional PDT) as documented by 3D LifeViz Micro™ camera from Quantificare Inc. Note the reduction in lesion volume after PDT. (B–D) H&E images showing characteristic of AK lesion in untreated control (B) and cell death induced by metronomic (C) and conventional PDT (D). Note the presence of pyknotic nuclei and overall loss of staining in PDT treated samples. See results and discussion for details.
Figure 5: Histochemical analysis of PDT-induced cell death in AK lesions.

PDT-induced cell death in murine AK lesions was analyzed by histochemistry (TUNEL and ROS) and immunohistochemistry (cleaved Caspase −3). (A) TUNEL images show lack of cell death in untreated control sample (top) and dying TUNEL-labeled nuclei in PDT-treated AK samples (A; middle and bottom). Note the lower amounts of TUNEL labeling observed in metronomic PDT than in conventional PDT samples, reflecting the relatively higher levels of cell death in the latter. (B) Similarly, the ROS assay shows lack of ROS production in untreated control sample (B; top), and low levels of ROS in metronomic PDT samples (B; middle), and the highest ROS production in conventional PDT samples (B; bottom). (C) Immunofluorescence staining for cleaved Caspase-3 show lack of Caspase-3 cleavage in non-treated control sample (C; top) and in the metronomic PDT treated AK lesion (C; middle), but robust cleavage of Caspase-3 in the basal layer of an AK lesion treated with conventional PDT (C; bottom). Epidermal thinning and loss of viable keratinocytes (blue nuclei) is moderate in metronomic treated samples (C; middle image), but severe in conventional PDT treated samples (C; bottom image). These results show that cell death mechanisms are different between metronomic and conventional PDT. Dashed line, Dermal-epidermal junction.
3.4. Infiltration of macrophages and neutrophils in actinic keratosis lesions treated with conventional or metronomic PDT:
The tumor regression caused by photodynamic therapy is a cumulative response mediated through several mechanisms including enhanced cell death, damage of tumor vasculature and more importantly PDT-induced anti-tumor immunity [16]. PDT, in addition to causing direct tumor damage by cell death response, also exerts anti-tumor immunity by activating immune responses involving rapid infiltration of tumors by neutrophils and macrophages and accompanied by release of inflammatory cytokines [16, 35–37]. To investigate the role of inflammatory response following metronomic or conventional PDT that could result in similar lesion clearance observed between two regimens, we analyzed tissue sections from AK lesions harvested at different times after PDT, by immunofluorescence using antibodies against markers for macrophages and neutrophils. Increased infiltration/recruitment of macrophages as visualized by staining with F4/80 antibody (a marker for mature macrophages) was observed at 1-day post PDT in AK lesions treated with conventional PDT (Fig. 6A; top row, PDT vs. mPDT images). However, neutrophil recruitment/infiltration showed an opposite response, with higher presence in metronomic PDT treated samples at 1-day post PDT (Fig. 6A; bottom row, mPDT vs. PDT images). Interestingly, relative to conventional regimen, macrophage recruitment was much higher in metronomic PDT treated samples at 5-days post PDT (Fig. 6B; mPDT vs. PDT images). Krosl et al. have reported relative levels of major immune cell populations in SCCVII tumors following PDT showing a rapid infiltration by neutrophils (within minutes post PDT) and gradual enrichment of macrophages around 8 hours post PDT [38]. PDT has been shown to induce local acute inflammatory responses, resulting in massive infiltration of tumors with myeloid cells characterized by sequential arrival of neutrophils, mast cells and macrophages that contribute to tumor regression via their tumoricidal properties [35, 38–40]. Therefore, a delayed recruitment or prolonged retention of neutrophils (Fig. 6A; day 1) and macrophages (Fig. 6B; day 5) in metronomic PDT treated AK lesions could be a mechanism responsible for similar lesion clearance observation between the two regimens. Additionally, a compromised cell death response observed during metronomic PDT regimen would favor preservation of tumor vasculature which is required for systemic immune response resulting in anti-tumor immunity by metronomic PDT [39]. However, a more detailed analyses of the response of these and other immune cell types during the treatment of AK lesions by metronomic or conventional PDT regimens is required to better understand the mechanism of immunomodulation by PDT.
Figure 6: Immune response in murine AK lesions following conventional and metronomic PDT.

(A) Immunofluorescence staining for macrophages (F4/80) and neutrophils (Ly6G) shown in top and bottom panels, respectively. Note the increase in macrophage infiltration in conventional PDT (A; middle vs. right images in top row) and neutrophil infiltration in metronomic PDT (A; right vs. middle images in bottom row) treated lesions at 1-day post PDT, respectively. (B) Immunofluorescence staining for macrophages (F4/80) showing marked increase in macrophage recruitment in metronomic PDT treated lesions at 5-days post PDT B; right vs. middle images). No presence of neutrophils was seen in either of the treatment groups on day 5.
CONCLUSIONS
Based on findings from a recent clinical trial by the Maytin group [41] showing a similar lesion clearance observed following conventional and metronomic (pain less) PDT treatment regimens, we investigated underlying mechanisms using a murine model of actinic keratosis. The results presented in this paper show the following major findings:
The murine model of AK clearly simulated the morphological, histological and lesion clearance response observed after PDT of human AK lesions.
Relative to untreated controls, we observed that enhanced cell death, generation of reactive oxygen species (ROS) and activation of cleaved Caspase-3 (a hallmark of apoptosis) occurred at higher levels in conventional PDT relative to metronomic PDT treated AK lesions.
Immunomodulation by inflammatory cells (macrophages and neutrophils) showed a delayed response to recruitment or prolonged retention in mPDT treated AK lesions.
Based on the results presented here, metronomic PDT can be just as effective as conventional PDT for treatment of AK, but may involve entirely different mechanisms.
ACKNOWLEDGEMENTS
We thank Dr. Tayyaba Hasan (Massachusetts General Hospital) and Dr. Brian Pogue (Dartmouth Medical Center) for their longstanding collaborations with us through an NIH Program Project that they co-direct. This work was financially supported by P01CA084203 (Tayyaba Hasan and Edward Maytin) from the National Cancer Institute, National Institutes of Health, U.S.A.
REFERENCES
- [1].Miller DL, and Weinstock MA, “Nonmelanoma skin cancer in the United States: incidence,” J Am Acad Dermatol, 30(5 Pt 1), 774–8 (1994). [DOI] [PubMed] [Google Scholar]
- [2].Kallini JR, Hamed N, and Khachemoune A, “Squamous cell carcinoma of the skin: epidemiology, classification, management, and novel trends,” Int J Dermatol, 54(2), 130–40 (2015). [DOI] [PubMed] [Google Scholar]
- [3].Ratushny V, Gober MD, Hick R et al. , “From keratinocyte to cancer: the pathogenesis and modeling of cutaneous squamous cell carcinoma,” J Clin Invest, 122(2), 464–72 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Brash DE, Rudolph JA, Simon JA et al. , “A role for sunlight in skin cancer: UV-induced p53 mutations in squamous cell carcinoma,” Proc Natl Acad Sci U S A, 88(22), 10124–8 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ziegler A, Jonason AS, Leffell DJ et al. , “Sunburn and p53 in the onset of skin cancer,” Nature, 372(6508), 773–6 (1994). [DOI] [PubMed] [Google Scholar]
- [6].Apalla Z, Sotiriou E, Chovarda E et al. , “Skin cancer: preventive photodynamic therapy in patients with face and scalp cancerization. A randomized placebo-controlled study,” Br J Dermatol, 162(1), 171–5 (2010). [DOI] [PubMed] [Google Scholar]
- [7].Zeitouni NC, Oseroff AR, and Shieh S, “Photodynamic therapy for nonmelanoma skin cancers. Current review and update,” Mol Immunol, 39(17–18), 1133–6 (2003). [DOI] [PubMed] [Google Scholar]
- [8].Ortel B, and Calzavara-Pinton P, “Advances in photodynamic therapy. A review,” G Ital Dermatol Venereol, 145(4), 461–75 (2010). [PubMed] [Google Scholar]
- [9].Szeimies RM, Hauschild A, Ortland C et al. , “Photodynamic therapy simplified: nonprepared, moderate-grade actinic keratosis lesions respond equally well to 5-aminolaevulinic acid patch photodynamic therapy as do mild lesions,” Br J Dermatol, 173(5), 1277–9 (2015). [DOI] [PubMed] [Google Scholar]
- [10].Maytin EV, Anand S, Riha M et al. , “5-Fluorouracil Enhances Protoporphyrin IX Accumulation and Lesion Clearance during Photodynamic Therapy of Actinic Keratoses: A Mechanism-Based Clinical Trial,” Clin Cancer Res, 24(13), 3026–3035 (2018). [DOI] [PubMed] [Google Scholar]
- [11].Zhao B, and He YY, “Recent advances in the prevention and treatment of skin cancer using photodynamic therapy,” Expert Rev Anticancer Ther, 10(11), 1797–809 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Wulf HC, “Increasing the acceptability of photodynamic therapy,” Photodermatol Photoimmunol Photomed, 31(1), 3–4 (2015). [DOI] [PubMed] [Google Scholar]
- [13].Cohen DK, and Lee PK, “Photodynamic Therapy for Non-Melanoma Skin Cancers,” Cancers (Basel), 8(10), (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ortel B, Shea CR, and Calzavara-Pinton P, “Molecular mechanisms of photodynamic therapy,” Front Biosci (Landmark Ed), 14, 4157–72 (2009). [DOI] [PubMed] [Google Scholar]
- [15].Celli JP, Spring BQ, Rizvi I et al. , “Imaging and photodynamic therapy: mechanisms, monitoring, and optimization,” Chem Rev, 110(5), 2795–838 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Agostinis P, Berg K, Cengel KA et al. , “Photodynamic therapy of cancer: an update,” CA Cancer J Clin, 61(4), 250–81 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Anand S, Ortel BJ, Pereira SP et al. , “Biomodulatory approaches to photodynamic therapy for solid tumors,” Cancer Letters, 326(1), 8–16 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Hasan T, Ortel B, Solban N et al. , [Photodynamic therapy of cancer] BC Decker, Inc., Hamilton, Ontario: (2006). [Google Scholar]
- [19].Kessel D, “Apoptosis, Paraptosis and Autophagy: Death and Survival Pathways Associated with Photodynamic Therapy,” Photochem Photobiol, 95(1), 119–125 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Kennedy JC, Pottier RH, and Pross DC, “Photodynamic therapy with endogenous protoporphyrin IX: basic principles and present clinical experience,” J Photochem Photobiol B, 6(1–2), 143–8 (1990). [DOI] [PubMed] [Google Scholar]
- [21].Szeimies RM, Torezan L, Niwa A et al. , “Clinical, histopathological and immunohistochemical assessment of human skin field cancerization before and after photodynamic therapy,” Br J Dermatol, 167(1), 150–9 (2012). [DOI] [PubMed] [Google Scholar]
- [22].Warren CB, Karai LJ, Vidimos A et al. , “Pain associated with aminolevulinic acid-photodynamic therapy of skin disease,” J Am Acad Dermatol, 61(6), 1033–43 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ang JM, Riaz IB, Kamal MU et al. , “Photodynamic therapy and pain: A systematic review,” Photodiagnosis Photodyn Ther, 19, 308–344 (2017). [DOI] [PubMed] [Google Scholar]
- [24].Paragh G, and Zeitouni NC, “Two-Step Irradiance Treatment Can Achieve Excellent Pain Control During Red Light 5-Aminolevulinic Acid Photodynamic Therapy for Actinic Keratoses,” Photomed Laser Surg, 36(3), 174–176 (2018). [DOI] [PubMed] [Google Scholar]
- [25].Wiegell SR, Fabricius S, Gniadecka M et al. , “Daylight-mediated photodynamic therapy of moderate to thick actinic keratoses of the face and scalp: a randomized multicentre study,” Br J Dermatol, 166(6), 1327–32 (2012). [DOI] [PubMed] [Google Scholar]
- [26].Wulf HC, “Photodynamic Therapy in Daylight for Actinic Keratoses,” JAMA Dermatol, 152(6), 631–2 (2016). [DOI] [PubMed] [Google Scholar]
- [27].Bisland SK, Lilge L, Lin A et al. , “Metronomic photodynamic therapy as a new paradigm for photodynamic therapy: rationale and preclinical evaluation of technical feasibility for treating malignant brain tumors,” Photochem Photobiol, 80, 22–30 (2004). [DOI] [PubMed] [Google Scholar]
- [28].Rollakanti KR, Anand S, Davis SC et al. , “Noninvasive Optical Imaging of UV-Induced Squamous Cell Carcinoma in Murine Skin: Studies of Early Tumor Development and Vitamin D Enhancement of Protoporphyrin IX Production,” Photochem Photobiol, 91(6), 1469–78 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Anand S, Rollakanti KR, Brankov N et al. , “Fluorouracil Enhances Photodynamic Therapy of Squamous Cell Carcinoma via a p53-Independent Mechanism that Increases Protoporphyrin IX levels and Tumor Cell Death,” Mol Cancer Ther, 16(6), 1092–1101 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Anand S, Wilson C, Hasan T et al. , “Vitamin D3 enhances the apoptotic response of epithelial tumors to aminolevulinate-based photodynamic therapy,” Cancer Res, 71(18), 6040–50 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Srivastava RK, Li C, Weng Z et al. , “Defining cutaneous molecular pathobiology of arsenicals using phenylarsine oxide as a prototype,” Sci Rep, 6, 34865 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Pillon A, Gomes B, Vandenberghe I et al. , “Actinic keratosis modelling in mice: A translational study,” PLoS One, 12(6), e0179991 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Kessel D, Vicente MG, and Reiners JJ Jr., “Initiation of apoptosis and autophagy by photodynamic therapy,” Lasers Surg Med, 38(5), 482–8 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kessel D, and Reiners JJ Jr., “Apoptosis and autophagy after mitochondrial or endoplasmic reticulum photodamage,” Photochem Photobiol, 83(5), 1024–8 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Korbelik M, “Induction of tumor immunity by photodynamic therapy,” J Clin Laser Med Surg, 14(5), 329–34 (1996). [DOI] [PubMed] [Google Scholar]
- [36].Brackett CM, and Gollnick SO, “Photodynamic therapy enhancement of anti-tumor immunity,” Photochem Photobiol Sci, 10(5), 649–52 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Firczuk M, Nowis D, and Golab J, “PDT-induced inflammatory and host responses,” Photochem Photobiol Sci, 10(5), 653–63 (2011). [DOI] [PubMed] [Google Scholar]
- [38].Krosl G, Korbelik M, and Dougherty GJ, “Induction of immune cell infiltration into murine SCCVII tumour by photofrin-based photodynamic therapy,” Br J Cancer, 71(3), 549–55 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Garg AD, Nowis D, Golab J et al. , “Photodynamic therapy: illuminating the road from cell death towards anti-tumour immunity,” Apoptosis, 15(9), 1050–71 (2010). [DOI] [PubMed] [Google Scholar]
- [40].Gollnick SO, “Photodynamic therapy and antitumor immunity,” J Natl Compr Canc Netw, 10 Suppl 2, S40–3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kaw U, Ilyas M, Bullock T, et al. , A regimen to minimize pain during blue light photodynamic therapy of actinic keratoses: bilaterally controlled, randomized trial of simultaneous versus conventional illumination. J Am Acad Dermatol, 82(4), 862–868 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]


