Abstract
Objective.
The purpose of this study is to compare the growth factor and cytokine content found within the amnion and chorion layers and to determine the effects of dehydration on them.
Materials and Methods.
Placentas were collected from 5 to 6 consented donors following elective cesarean section, and 1-cm2 sections of either amnion or chorion were immediately stored at −80°C or dehydrated prior to −80°C storage until proteomic analysis. Signaling molecules from tissue samples were evaluated using quantitative multiplex proteomics microarrays, and data were analyzed based on a per cm2 basis and also on pg/mg of extracted protein for potency.
Results.
Fresh chorion contained more of some signaling molecules per cm2 compared with amnion. Specifically, the chorion contained significantly higher levels of adiponectin, APN, ANG-2, bFGF, EG-VEGF, HGF, IGF-1, PDGF-AA, PDGF-BB, TIMP-2, and TIMP-4. When samples were dehydrated, a significant drop in total growth factor and cytokine content was observed in both amnion and chorion samples with a loss of 51.1% ± 20.2% and 55.5% ± 37.3%, respectively. When evaluating the potency of fresh amnion and fresh chorion, there were similar levels of signaling molecules found with some exceptions. Amnion had significantly higher GAL-7, TGF-β1, and IL-1F5, and chorion had significantly more EG-VEGF, PDGF-BB, and TIMP-2.
Conclusion.
The processing of placental membranes can have a dramatic effect on the total growth factor and cytokine load found within these tissues.
Keywords: amnion, chorion, dehydration, proteomic analysis, regenerative, wound healing
Amniotic membranes have a long history of use for the treatment of wounds, dating back to 1910.1 In their fresh state, amniotic membranes contain various multipotential cells, growth factors, and extracellular matrix proteins that support healing. These membranes are especially suited for chronic wound healing, because they are known to naturally address many of the contributing factors of chronic wound development, including suppressing dysregulated/uncontrolled inflammatory responses, increasing levels of matrix metalloproteinase inhibitors in the wound environment, stimulating proliferation and migration of important cell types, and promoting angiogenesis.2–4 Most commercially available membrane products are processed by dehydration or lyophilization, which alter those characteristics to varying degrees, and the layers included in these grafts vary with most containing a double-layer amnion, amnion and chorion, or the amnion layer alone.
The purpose of this study is to compare the growth factor and cytokine content found within the amnion and chorion layers and to determine the effects of dehydration on these signaling molecules.
Materials and Methods
Placentas were collected following elective cesarean sections from 6 donors who gave written informed consent. The placental disk was removed and amnion and chorion layers were separated via blunt dissection, with a total of 6 donors used for amnion analysis and 5 donors for chorion. Membranes were washed in sterile saline and cut into 1-cm2 sections. To compare the structural differences between the fresh and dehydrated samples, tissue was paraffin embedded, sectioned, and stained with H&E. For proteomic assays, 1-cm2 sections were either immediately stored at −80°C or dehydrated using standard techniques prior to storage at −80°C until analysis. Of note, all sections (fresh and dehydrated) were deep frozen for a short period of time to equally preserve protein content until analysis of all donors and groups. Growth factor and cytokine content were assessed using a quantitative multiplex enzyme-linked immunosorbent assay (ELISA) proteomics microarray (RayBiotech, Inc, Norcross, GA). Signaling molecules evaluated in this study are thought to be relevant to wound healing and have previously been identified within placental-derived tissues.2,4,5 Tissue samples were first homogenized using a Retsch CryoMill (Verder Scientific Inc, Newtown, PA). After cryomilling, the tissue was incubated overnight in a total protein extraction buffer with a protease inhibitor cocktail (EMD Millipore, Billerica, MA) at 4°C with agitation. Following incubation, the supernatant was removed and loaded into the microarray chambers and the assay carried out per the manufacturer’s instructions. The slides were imaged using a GenePix 4000B Microarray Scanner (Molecular Devices, Sunnyvale, CA), and scanned images were imported and analyzed using GenePix Pro 7 Software (Molecular Devices, Sunnyvale, CA). Total growth factor and cytokine content were then represented as pg/cm2. To compare the potency of the signaling molecules within each membrane, the extracted protein was quantified using a Pierce BCA Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA), and the growth factor and cytokine loads were normalized to the total extracted protein from either amnion or chorion. For this study, growth factors and cytokines have been categorized into general functional areas (Table). A Student’s t-test was used to determine significance between the groups, and an asterisk was used to indicate P < .05.
Table.
Relevant growth factor and cytokine categories
| Angiogenic | |
|---|---|
| aFGF | PlGF |
| ANG | SDF-1 |
| ANG-2 | TSP-1 |
| bFGF | VEGF |
| EG-VEGF | VEGF-D |
| PDGF-AA | ANGPTL4 |
| PDGF-BB | |
| Regenerative | |
| APN | IGF-1 |
| EGF | IGF-2 |
| GAL-7 | TGF-α |
| HGF | TGF-β1 |
| IGFBP-1 | TGF-β3 |
| IGFBP-5 | |
| Immune Modulating | |
| IL-1F5 | IL-10 |
| il-1ra | TIMP-1 |
| IL-4 | TIMP-2 |
| IL-6 | TIMP-4 |
| IL-8 | TNF-α |
Results
Qualitative analysis of the H&E tissue samples indicated that dehydration of the membranes resulted in a thinner, more condensed structure, with a loss of visible porosity (Figure 1). In general, both unprocessed amnion and chorion had similar growth factor and cytokine compositions; however, there were some differences in distribution (Figure 2). Fresh chorion contained more growth factors and cytokines per cm2 compared with amnion, likely due to the overall increased thickness compared with amnion. Specifically, fresh chorion contained significantly higher levels of APN, ANG, ANG-2, bFGF, EG-VEGF, HGF, IGF-1, PDGF-AA, PDGF-BB, TIMP-2, and TIMP-4 (data not shown). When samples were dehydrated, a significant drop in total growth factor and cytokine content was observed in both amnion and chorion samples with a loss of 51.1% ± 20.2% and 55.5% ± 37.3%, respectively (Figure 3). When comparing the potency of amnion and chorion membranes (pg/mg extracted protein), the investigators found the membranes were similar in overall composition with some exceptions. Amniotic membranes had significantly higher levels of GAL-7, TGF-β1, and IL-1F5, and chorion membranes had significantly higher levels of EG-VEGF, PDGF-BB, and TIMP-2 (Figure 4).
Figure 1.

Histological assessment of effects of processing. (A) H&E of unprocessed human amnion/chorion; (B) H&E of dehydrated human amnion/chorion. Yellow and blue outlines indicate amnion and chorion, respectively. During dehydration, removal of moisture content results in a collapse of the extracellular matrix, as shown by the overall reduction of thickness (scale bar: 100μm) and removal of white space (or pores) throughout the matrix shown in unprocessed membranes (A).
Figure 2.
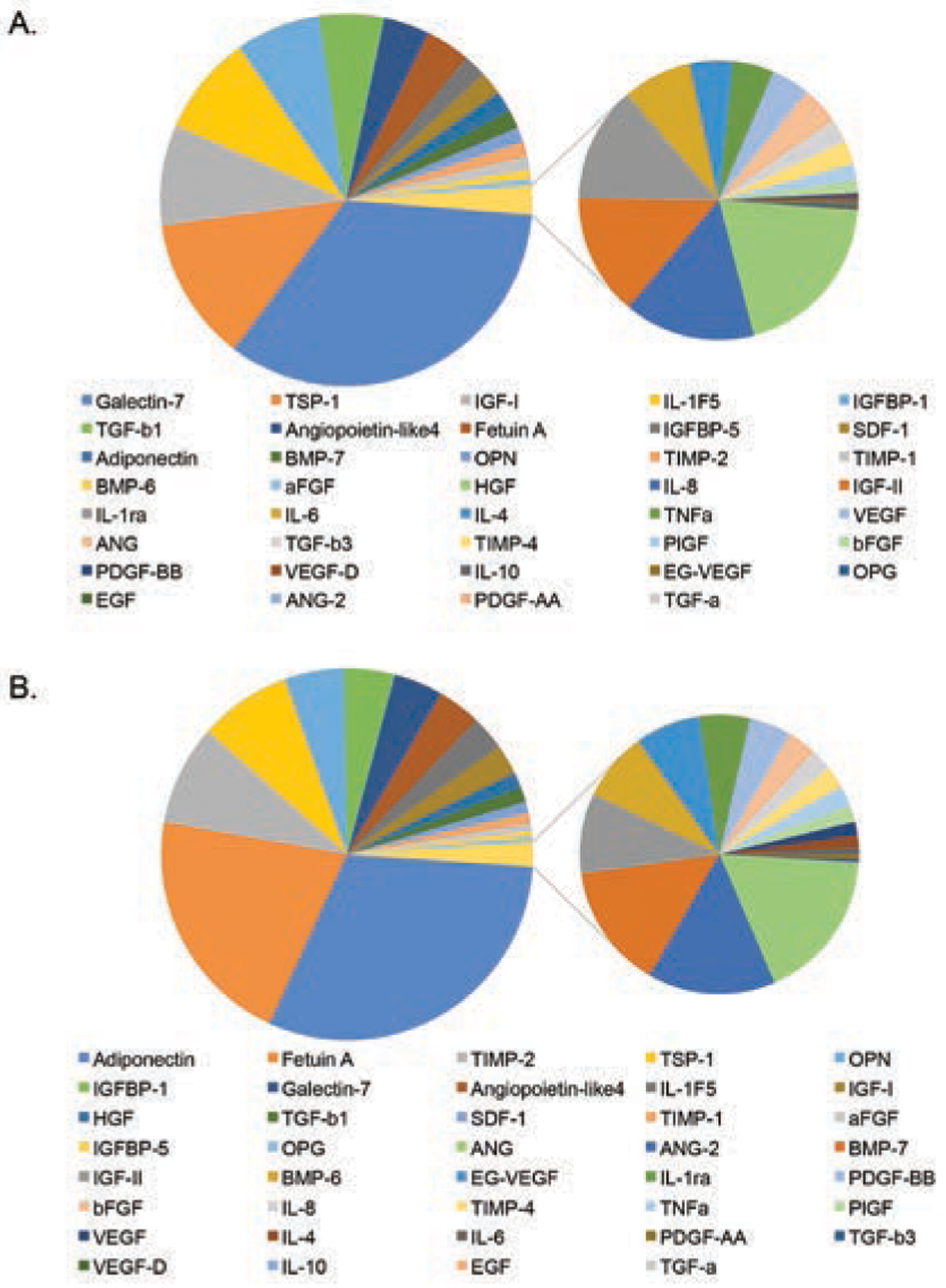
Relative levels for all growth factors and cytokines measured within unprocessed (A) human amnion and (B) chorion in pg/cm2.
Figure 3.
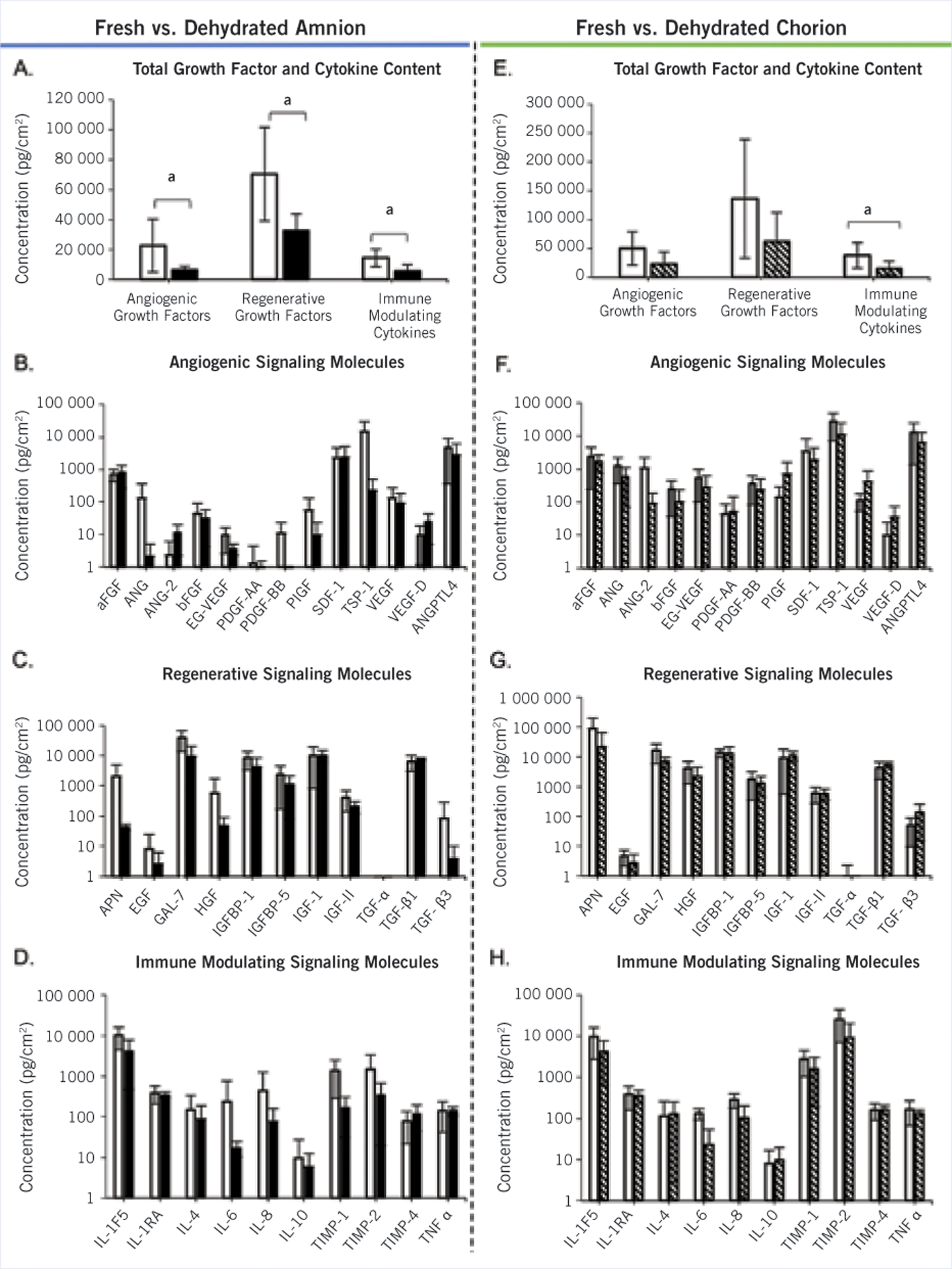
Effect of dehydration on amnion and chorion membranes. Growth factor and cytokine levels for amnion (n=6; A-D) and chorion (n=5; E-H) were measured with Multiplex ELISA proteomic microarray. (A-D) White bars represent fresh amnion and black bars represent dehydrated amnion. Data shown include (A) group totals, (B) angiogenic, (C) regenerative, and (D) immune modulating signaling molecules. (E-H) White bars represent fresh chorion and stripped bars represent dehydrated chorion. Data shown include (E) group totals, (F) angiogenic, (G) regenerative, and (H) immune modulating signaling molecules. Average results are shown. Error bar represents 1 standard deviation.
a significant difference: P<.05
Figure 4.
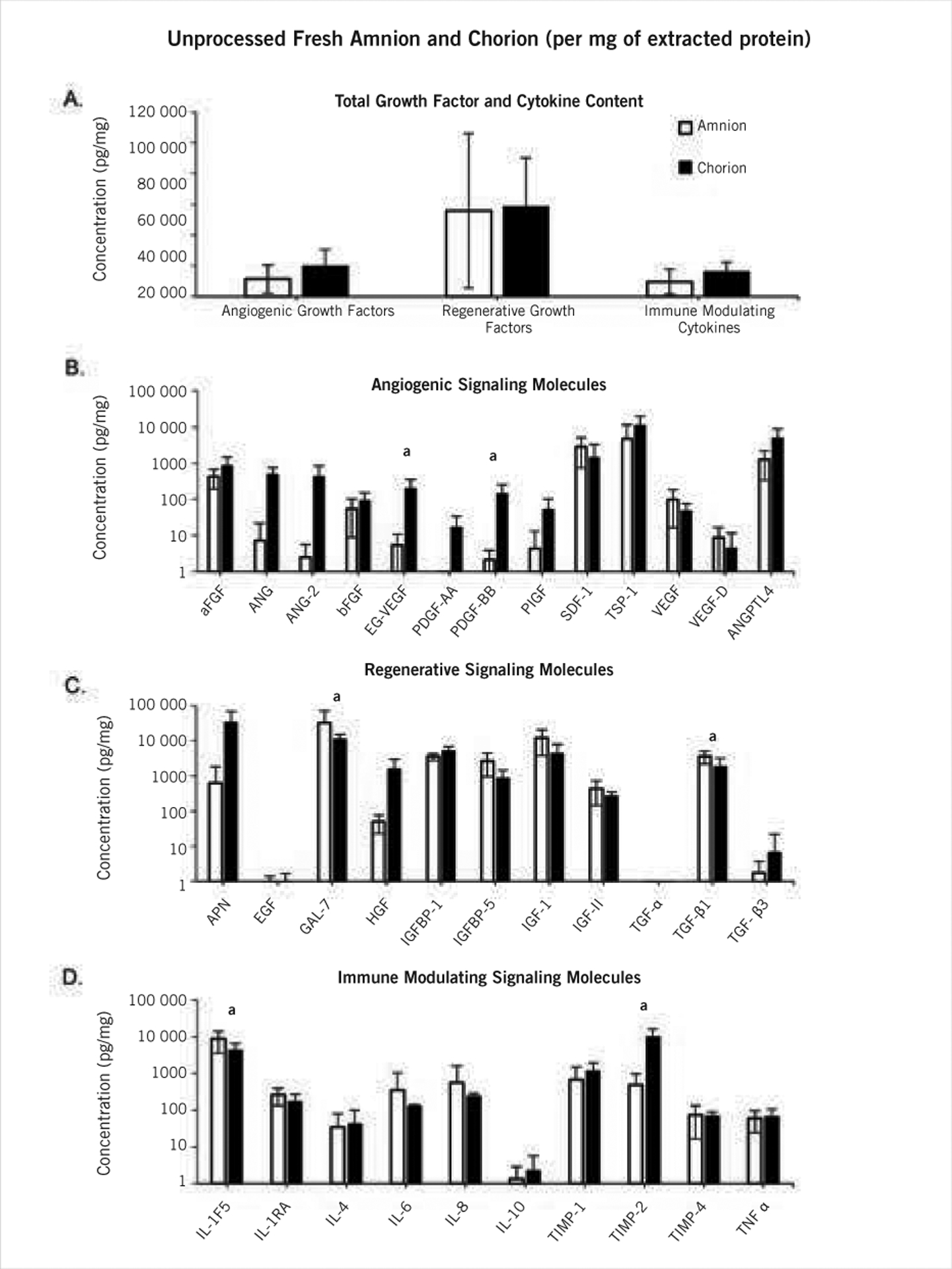
Potency comparison between unprocessed/fresh amnion (n=6) and chorion (n=5). White bars represent amnion values and black bars represent chorion for all graphs. (A) Group totals, (B) angiogenic, (C) regenerative, and (D) immune modulating signaling molecules. Bars represent the average ± standard deviation.
a significant difference: P<.05
Discussion
While previous studies have evaluated the cytokine content within the placental membrane for specific commercial products6–9 and have investigated the effects of processing on the amnion,10,11 there is a lack of literature comparing the cytokine content of the amnion and chorion and evaluating how these tissues are altered by dehydration. Likely related to the overall thicker nature of the chorion, the investigators found a greater growth factor and cytokine load within the fresh chorion compared with the fresh amnion (pg/cm2). The levels of growth factors within both membranes were significantly lower following dehydration (compared with fresh tissue). Previous studies that have compared dehydrated amnion with either fresh or cryopreserved amnion have found significant changes to the structure of the membrane, and with the exception of Paolin et al,11 other groups have also observed a decrease in cytokine content after dehydration.8,9,11 Researchers hypothesize the observed drop in growth factor content following dehydration may be due in part to perturbations of the protein structure known to occur during the dehydration process.12 When comparing the potency of the amnion and chorion by normalizing growth factor concentration to the quantity of extracted protein, the investigators found that overall both membranes had similar compositions in terms of potency; however, there were some significant differences between the composition of the membranes in regards to specific growth factors.
Placental membranes have been shown to be effective in treating difficult-to-heal chronic wounds13–15; however, the results presented herein indicate both processing and composition of amniotic grafts play an important role in their content. When evaluating amniotic products for clinical use, it is important to consider which tissue layers these grafts contain and how these grafts are processed (ie, dehydrated, cryopreserved, or fresh).
Limitations
Limitations for this study include the number of donors (N = 5–6), the cytokines evaluated, and the evaluation of only nonsterilized grafts (while most commercial grafts are terminally sterilized). All samples were snap frozen prior to cytokine and growth factor analysis; while this is an appropriate technique for protein preservation, there is the possibility of some sample degradation. In addition, this study evaluated signaling molecules by whether they were present and did not address protein denaturing and how that may affect bioactivity. Future studies will focus on the evaluation of other growth factors/cytokines of interest, the use of mechanistic studies on key cell types to evaluate the effect of processing and sterilization on the bioactivity of growth factors and cytokines within membranes, and the clinical relevance of these findings.
Conclusion
Fresh chorion contained more of some signaling molecules per cm2 compared with amnion, and both dehydrated samples saw a significant drop in total growth factor and cytokine content (51.1% ± 20.2% for amnion; 55.5% ± 37.3% for chorion). When evaluating the potency of fresh amnion and fresh chorion, there were similar levels of signaling molecules found with some exceptions. In conclusion, the processing of placental membranes can have a dramatic effect on the total growth factor and cytokine load found within these tissues.
Disclosure:
This study was supported and funded by NuTech, a divisionof Organogenesis, Inc (Birmingham, AL). All authors are employees of the company. This paper was presented as a poster at the Symposium on Advanced Wound Care Spring 2017.
Abbreviation Key
- aFGF
acidic fibroblast growth factor
- ANG
angiopoietin
- ANGPTL4
angiopoietin-like 4
- APN
adiponectin
- bFGF
basic fibroblast growth factor
- BMP
bone morphogenetic protein
- EG-VEGF
Endocrine gland-derived vascular endothelial growth factor
- EGF
endothelial growth factor
- ELISA
enzyme-linked immunosorbent assay
- GAL
galectin
- H&E
hematoxylin & eosin
- HGF
hepatocyte growth factor
- IGF
insulin-like growth factor
- IGFBP
insulin-like growth factor-binding protein
- IL
interleukin
- IL-1RA
interleukin-1receptor antagonist
- IL-1F5
interleukin 36 receptor antagonist/interleukin 1 family member
- OPG
osteoprotegerin
- OPN
osteopontin
- PDGF
platelet-derived growth factor
- PIGF
placental growth factor
- SDF
stromal cell-derived factor
- TGF
transforming growth factor
- TIMP
tissue inhibitor of metalloproteinase
- TNF
tumor necrosis factor
- TSP
thrombospondin
- VEGF
vascular endothelial growth factor
References
- 1.Davis JS. The use of skin grafts in the ambulatory treatment of ulcers. J Am Med Assoc. 1915;13;LXIV(7):558–560. [Google Scholar]
- 2.McQuilling JP, Vines JB, Mowry KC. In vitro assessment of a novel, hypothermically stored amniotic membrane for use in a chronic wound environment [published online ahead of print March 29, 2017]. Int Wound J. doi: 10.1111/iwj.12748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Litwiniuk M, Bikowska B, Niderla-Bielińska J, et al. Potential role of metalloproteinase inhibitors from radiation-sterilized amnion dressings in the healing of venous leg ulcers [published online ahead of print July 11, 2012]. Mol Med Rep. 2012;6(4):723–728. [DOI] [PubMed] [Google Scholar]
- 4.Koob TJ, Rennert R, Zabek N, et al. Biological properties of dehydrated human amnion/chorion composite graft: implications for chronic wound healing [published online ahead of print August 1, 2013]. Int Wound J. 2013;10(5):493–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steed DL, Trumpower C, Duffy D, et al. Amnion-derived cellular cytokine solution: a physiological combination of cytokines for wound healing. Eplasty. 2008;8:e18. [PMC free article] [PubMed] [Google Scholar]
- 6.Koob TJ, Lim JJ, Zabek N, Massee M. Cytokines in single layer amnion allografts compared to multilayer amnion/chorion allografts for wound healing [published online ahead of print August 30, 2014]. J Biomed Mater Res B Appl Biomater. 2015;103(5):1133–1140. [DOI] [PubMed] [Google Scholar]
- 7.Koob TJ, Lim JJ, Massee M, Zabek N, Denozière G. Properties of dehydrated human amnion/chorion composite grafts: implications for wound repair and soft tissue regeneration [published online ahead of print March 25, 2014]. J Biomed Mater Res B Appl Biomater. 2014;102(6):1353–1362. [DOI] [PubMed] [Google Scholar]
- 8.Lim LS, Poh RW, Riau AK, Beuerman RW, Tan D, Mehta JS. Biological and ultrastructural properties of acelagraft, a freeze-dried γ-irradiated human amniotic membrane. Arch Ophthalmol. 2010;128(10):1303–1310. [DOI] [PubMed] [Google Scholar]
- 9.Russo A, Bonci P, Bonci P. The effects of different preservation processes on the total protein and growth factor content in a new biological product developed from human amniotic membrane [published online ahead of print June 18, 2011]. Cell Tissue Bank. 2012;13(2):353–361. [DOI] [PubMed] [Google Scholar]
- 10.Hopkinson A, McIntosh RS, Shanmuganathan V, Tighe PJ, Dua HS. Proteomic analysis of amniotic membrane prepared for human transplantation: characterization of proteins and clinical implications. J Proteome Res. 2006;5(9):2226–2235. [DOI] [PubMed] [Google Scholar]
- 11.Paolin A, Trojan D, Leonardi A, et al. Cytokine expression and ultrastructural alterations in fresh-frozen, freeze-dried and γ-irradiated human amniotic membranes [published online ahead of print April 12, 2016]. Cell Tissue Bank. 2016;17(3):399–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carpenter JF, Izutsu K, Randolph T. Freezing- and drying-induced perturbations of protein structure and mechanisms of protein protection by stabilizing additives. In: Rey L, May JC, eds. Freeze-Drying/Lyophilization Of Pharmaceutical & Biological Products. 3rd ed. London, UK: Informa Healthcare; 2010. [Google Scholar]
- 13.Serena TE, Carter MJ, Le LT, Sabo MJ, DiMarco DT; EpiFix VLU Study Group. A multicenter, randomized, controlled clinical trial evaluating the use of dehydrated human amnion/chorion membrane allografts and multilayer compression therapy vs. multilayer compression therapy alone in the treatment of venous leg ulcers. Wound Repair Regen. 2014;22(6):668–693. [DOI] [PubMed] [Google Scholar]
- 14.Frykberg RG, Gibbons GW, Walters JL, Wukich DK, Milstein FC. A prospective, multicentre, open-label, single-arm clinical trial for treatment of chronic complex diabetic foot wounds with exposed tendon and/or bone: positive clinical outcomes of viable cryopreserved human placental membrane [published online ahead of print August 3, 2016]. Int Wound J. 2017;14(3):569–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zelen CM, Serena TE, Snyder RJ. A prospective, randomised comparative study of weekly versus biweekly application of dehydrated human amnion/chorion membrane allograft in the management of diabetic foot ulcers [published online ahead of print February 21, 2014]. Int Wound J. 2014;11(2):122–128. [DOI] [PMC free article] [PubMed] [Google Scholar]


