Abstract
In healing wounds, the regression of blood vessels during the resolution phase creates a significant number of apoptotic endothelial cells (ApoECs). Surprisingly few studies have investigated the fate of apoECs in wounds, or the consequence of their removal. The current study employed both in vitro and in vivo models to investigate if macrophages ingest apoECs and to determine if such phagocytosis alters macrophage phenotype. To examine the capability of macrophages to ingest apoECs in in vivo wounds, pHrodo green labeled apoECs were injected into skin wounds 6 days after injury. The results demonstrated that 2.2% of macrophages in the wounds had engulfed apoECs 24 hours after injection. Macrophages that had engulfed apoECs expressed the markers CD80 (100%), CD86 (93.8%), and CD163 (22.8%), while no expression of CD206 marker was observed. In in vitro studies, 76.1% and 81.1% of PMA differentiated THP-1 macrophages engulfed apoECs at 6 and 24 hours, respectively. mRNA expression levels of IL-1β, iNOS, and TGF-β1 decreased in THP-1 macrophages after exposure to apoECs, while the expression of IL-6 increased. THP-1 macrophages that were incubated with apoECs for 6hours expressed CD80 (30.2%), CD163 (62.9%), and CD206 (45.3%), while expression levels in untreated group were 0.5%, 45.0%, and 2.4%, respectively. Taken together, our studies showed that macrophages phagocytize dermal apoECs both in vitro and in vivo. The engulfment of apoECs leads to a unique macrophage phenotype, which has characteristics of both M1 and M2 macrophage phenotypes. These findings provide a new mechanism by which macrophage phenotypes can be modified during wound resolution.
Keywords: apoptosis, endothelial cell, macrophage, phagocytosis, skin, wound healing
Graphical Abstract
Introduction
Wound healing is a complex pathophysiological process that begins immediately after an injury and can continue for weeks, months, or even years. The process involves interactions among resident and infiltrating cells, autocrine and paracrine cytokines, chemokines, growth factors, and extracellular matrix molecules [1-4]. The macrophage is one of the major types of infiltrating leukocytes after injury [1-4]. Numerous studies over the past few decades have described macrophages as key, indispensable regulatory inflammatory cells in normal acute wound healing and tissue regeneration [5-8]. A pioneering study published by Leibovich and Ross in 1975 first showed that macrophage depletion caused severely impaired skin wound healing including failed clearance of fibrin and damaged tissues, delayed re-epithelialization, decreased fibroblast accumulation, and down-regulated collagen synthesis [6]. Mills and colleagues first classified macrophages into two phenotypes, M1, Th1 cytokine (IFN-γ) activated macrophages and M2, Th2 cytokine (IL-4) activated macrophages [9]. M1 and M2 macrophages are also called classically activated macrophages and alternatively activated macrophages, respectively. Macrophages in the wound micro-environment play several critical roles during the healing process. M1 macrophages engulf necrotic/apoptotic neutrophils or damaged cells and produce proinflammatory cytokines such as IL-1β, IL-6, and TNF-α, which amplify the inflammatory response in the early stages of wound healing. In the later stages of healing, M2 macrophages produce anti-inflammatory cytokines and growth factors such as IL-10, TGF-β1, PDGF, VEGF-A, FGF2, and TGF-α. In this latter function, macrophages play pivotal roles in down-regulating inflammation and stimulating the proliferation of keratinocytes, fibroblasts, and endothelial cells (ECs), thereby promoting angiogenesis and the synthesis of extracellular matrix (ECM) molecules to restore damaged tissue [1, 2, 4, 10-12].
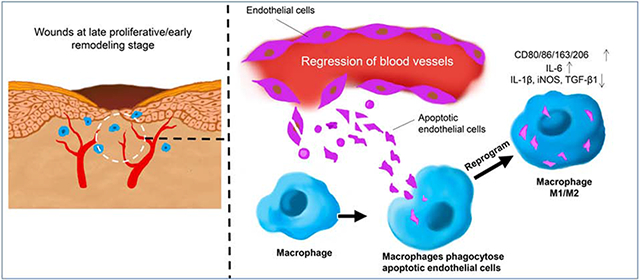
Macrophages are also known to influence angiogenesis, a hallmark feature of healing wounds that begins with the exuberant growth of new capillaries. Due to the need for oxygenation of healing tissue, a robust angiogenic response was long considered necessary for wound repair. However, the angiogenic response in wounds ultimately creates a vascular bed that is several times denser than normal tissue, but is dilated, tortuous, and poorly perfused [13]. As the wound resolves, most of the excess vessels regress [14], and a large number of apoECs are generated during the degeneration of the vascular endothelium [15]. A similar pattern of robust angiogenesis and vascular pruning occurs in many regenerative materials as well [16]. The fate of apoECs in healing wounds and regenerative materials is poorly understood. During normal development, most apoptotic bodies are phagocytosed by macrophages and are cleared locally [17]. We hypothesized that macrophages in the wound would engulf apoECs in the wounds by the process of efferocytosis, and that engulfment of apoECs would change the phenotype of the macrophages. Such an interaction might tie the pruning of the vasculature to other aspects of wound resolution, including the remodeling of the ECM and maturation of the tissue. Our current study demonstrates that both human and mouse macrophages ingest apoECs and that macrophage efferocytosis of apoECs leads to a unique macrophage phenotype that exhibits features of altered the expression of both M1 and M2 markers. The data provided a distinct link between vascular overgrowth, vascular regression, endothelial cell apoptotic load, and the function of wound macrophages.
Materials and Methods
Cell culture
Primary GFP-human dermal microvascular ECs (GFP-hECs, Angio-Proteomie, Boston, MA), primary human dermal microvascular ECs (hECs, ATCC, Manassas, VA), C57BL/6 mouse primary dermal microvascular ECs (mECs, Cell biologics, Chicago, IL), and the human monocyte cell line THP-1 (ATCC) were used in the study. GFP-hECs and hECs were cultured in Vascular Cell Basal Medium (ATCC) containing Microvascular Endothelial Cell Growth Kit-VEGF (ATCC). mECs were cultured in Complete Mouse Endothelial Dell Medium (Cell Biologics). THP-1 cells were cultured in RPMI 1640 (Corning, Corning, NY) with 10% FBS (Gemini Bio-Products, West Sacramento, CA) and 0.05 mM 2-mercaptoethanol (Sigma-Aldrich, St. Louis, MO). To differentiate THP-1 cells into macrophages, cells were treated with 50 ng/ml phorbol 12-myristate 13-acetate (PMA, Sigma-Aldrich) for 72 hours. All cells were cultured at 37°C in a 5% CO2 atmosphere. To induce apoptosis of GFP-hECs or hECs, 80-90% confluent cells were cultured in serum free Vascular Cell Basal Medium for 72 hours. To induce apoptosis of mECs, cells were cultured in PBS for 48 hours. Under these conditions, 77.2 of hECs, 88.1% of GFP-hECs, and 88.6% of mECs were apoptotic (Annexin V+) as shown in Appendix A. For functional assays, apoptotic EC were harvested from apoptosis induced cultures as floating cells.
Phagocytosis assay
Apoptotic GFP-hECs or hECs were harvested after 72 hours serum starvation. 8×105 apoECs were added to wells containing 8×105 PMA induced THP-1 macrophages in a 6-well plate or 2-well chamber slide. After various time periods of incubation, THP-1 macrophages were washed with PBS three times to remove the remaining apoptotic cells. The cells were then subjected to flow cytometry (6 and 24 hours), immunofluorescence staining (24 hours) or real time PCR (2, 6, and 24 hours) analyses as described below.
Preparation of pHrodo-green labeled apoptotic mouse ECs
Apoptotic mECs prepared as described above were collected and treated with 0.15 mg/ml DNAse I (Sigma-Aldrich) for 15 min in room temperature with gentle shaking. The cells were then incubated with 10μM pHrodo green (Invitrogen, Carlsbad, CA) in 2ml PBS for 30 min with gentle shaking at room temperature. The labeled cells were washed in PBS, centrifuged at 2000 rpm for 5 min, and then resuspended in PBS before being injected into mouse wounds as described below.
Animals, wound model, and PEDF treatment
Nine week old female C57BL/6j mice (Jackson Laboratory, Bar Harbor, ME) were used in this study. The mice were housed in groups at 24°C on a 12-hour/12-hour light/dark cycle. Food and water were provided ad libitum. Mice were anesthetized by intraperitoneal injection of 100mg/kg ketamine and 5mg/kg xylazine. The hair on dorsal skin was shaved and skin was cleansed with 70% isopropyl alcohol. Four excisional full thickness dermal wounds were made on each mouse, with 2 on either side of the midline using a 6 mm diameter punch biopsy instrument (Acu Punch, Acuderm, Ft. Lauderdale, FL). Recombinant pigment epithelium-derived factor (rPEDF) was prepared and used as described in our previous publications [18, 19]. In brief, 2μg rPEDF in 10μl 25% pluronic gel (Sigma-Aldrich) was applied directly to the surface of the wound for the first 3 days. From day 4 to day 9, the same dose of rPEDF in 20ul PBS was injected intradermally into the wounds. Control wounds were treated with PBS. N=4 mice in each group (rPEDF and PBS) at each time point (day 7 and day 10). On day 7 and 10 post wounding, 16 wounds from 4 mice (4 wounds from each mouse) in each group were harvested and pooled for flow cytometry analysis as described below. For the in vivo phagocytosis study, 5×105 pHrodo green (Invitrogen) labeled apoptotic mECs in 50μl PBS or 50μl of cell-free PBS as control was injected intradermally into each healing wound on day 6. After 24 hours, 8 wounds from 4 mice (2 wounds from each mouse) in each group were harvested and pooled for flow cytometry analysis, and the rest of the wounds were used for immunofluorescent histochemistry and real time PCR analyses. Four mice in each group (apoptotic mECs and PBS only) were included in this study. All animal procedures were approved by the University of Illinois at Chicago Institutional Animal Care and Use Committee.
Flow cytometry
An Annexin V Apoptosis Detection Kit APC (Catalog No. 88-8007-72, ThermoFisher, Waltham, MA) was used to detect apoptotic cells by following the manufacturer’s directions. The cells were then subjected to flow cytometry analysis using LSR Fortessa (BD bioscience, San Jose, CA) at the Flow Cytometry Core, Research Resources Services, University of Illinois at Chicago RRC. To analyze M1 and M2 macrophage cell surface M1 and M2 markers on macrophages in skin wounds, single cell suspensions were prepared by following a published protocol [20]. Briefly, the harvested wounds were pooled and cut into small pieces in RPMI 1640 with 5% FBS and an enzyme cocktail containing 1.2mg/ml DNAse I, 1mg/ml hyaluronidase, 1mg/ml collagenase type 1A, 1mg/ml magnesium chloride hexahydrate, and 200μg/ml gentamicin sulfate (all purchased from Sigma-Aldrich) for 2 hours at 37°C with gentle shaking. The treated minced tissues were passed through a 40μm cell strainer (BD Biosciences, San Jose, CA) and centrifuged. The resulting cells were resuspended with 3 ml RBC Lysis buffer (eBioscicence) for 5 min, washed with PBS, and then resuspended in PBS with 1% bovine serum albumin (Sigma-Aldrich). Cells were blocked with an anti-CD16/CD32 FC blocker (eBioscience, dilution 1:100) for 15 min followed by incubation with the following anti-mouse antibodies: anti-CD80-PE (Miltenyi Biotec, Bergisch Gladbach, Germany, dilution 1:20), anti-CD86 PE-Cyanine7 (eBioscience, dilution 1:80), anti-CD163 PerCP-eFluor 710 (eBioscience, dilution 1:80), anti-CD206-APC (eBiosciences, dilution 1:80) and anti-F4/80-BV421 (BD Bioscience, dilution 1:40) for 20 minutes on ice. After washing with PBS, cells were resuspended in flow cytometry buffer. 7-amino-actinomycin D (BD Biosciences) was added to the cells before they were subjected to flow cytometry. 7-amino-actinomycin D was used to exclude the dead cells during analysis.
To detect cell surface M1 and M2 macrophage markers on THP-1 macrophages, THP-1 macrophages were washed 3 times with PBS to remove any floating cells and were dissociated by 0.25% EDTA Typsin treatment. THP-1 macrophages were then treated with the FC blocker as described above. The cells were further incubated with the following anti-human antibodies: anti-CD80-PE (Miltenyi Biotec, dilution 1:11), anti-CD163 PE-Cyanine7 (eBioscience, dilution 1:20), anti-CD206-eFluor 450 (eBioscience, dilution1:20), and anti-CD44-APC (eBioscience, dilution 1:50). The CD44 marker was selected because nearly 100% of THP-1 cells express CD44 after PMA stimulation [21] which was also confirmed in our study (Appendix B). Cells were washed with PBS and fixed in 1% paraformaldehyde for 10 min. After PBS washing, the cells were kept in flow cytometry buffer at 4°C and subjected to flow cytometry analysis. For all flow cytometry, results were analyzed using Summit 4.3 software (Dako Cytomation, Glostrup, Denmark) or Kaluza 2.1 (Beckman Coulter, Brea, CA). The sources for all antibodies used this study are listed in Table 1.
Table 1.
Antibodies used in flow cytometry and immunofluorescent histochemistry analyses
| Manufacturer | Catalog No. | Clone name | Dilution | |
|---|---|---|---|---|
| Anti-human | ||||
| Anti-CD80-PE | Miltenyi Biotec | 130-110-371 | REA661 | 1:11 |
| Anti-CD163 PE-Cyanine7 | eBioscience | 25-1639-41 | eBioGHI/61(GHI/61) | 1:20 |
| Anti-CD206 eFlour 450 | eBioscience | 48-2069-41 | 19.2 | 1:20 |
| Anti-CD44-APC | eBioscience | 17-0441-81 | IM7 | 1:50 |
| Anti-mouse | ||||
| Anti-CD16/32 (FC Blocker) | eBioscience | 14-0161-85 | 93 | 1:100 |
| Anti-CD80-PE | Miltenyi Biotec | 130-102-883 | 16-10A1 | 1:20 |
| Anti-CD86 PE-Cyanine7 | eBioscience | 25-0862-80 | GL1 | 1:80 |
| Anti-CD163 PerCP-eFlour710 | eBioscience | 46-1631-80 | TNKUPJ | 1:80 |
| Anti-CD206-APC | eBioscience | 17-2061-80 | MR6F3 | 1:80 |
| Anti-F4/80-BV421 | BD Biosciences | 565411 | T45-2342 | 1:40 |
| Anti-CD68 | Bio-Rad Lab | MCA1957 | FA-11 | 1:1000 |
| Goat anti-rat Alexa fluor 594 | Invitrogen | A-11007 | N/A | 1:1000 |
Real time PCR
Total RNA from mouse skin wound tissues stored in RNALater (Invitrogen) or PMA induced THP-1 macrophages treated with GFP-hECs were extracted using TRIzol Reagent (Invitrogen). One μg of total RNA from each sample was treated with DNase I (Invitrogen) to remove genomic DNA contamination and subjected to reverse transcription using a High-Capacity cDNA Reverse Transcription Kit (ThermoFisher). mRNA expression levels of TNF-α, IL-1β, IL-6, iNOS, IL-10, TGF-β1, IGF-1, and VEGF-A were examined by real time PCR using SYBR Green PCR mix and gene specific primers (Table 2). GAPDH was used as a house-keeping gene for calibration. Relative expression was calculated using the 2−ΔΔCT method.
Table 2.
Primer sequences for real time PCR
| Targets | Forward (5’---3’) | Reverse (5’---3’) |
|---|---|---|
| Mouse | ||
| GAPDH | TCACCACCATGGAGAAGG | GCTAAGCAGTTGGTGGTGCA |
| IL-1β | CAACCAACAAGTGATATTCTCCAT | GATCCACACTCTCCAGCTGCA |
| IL-6 | GAGGATACCACTCCCAACAGACC | AAGTGCATCATCGTTGTTCATACA |
| IL-10 | GGTTGCCAAGCCTTATCGGA | ACCTGCTCCACTGCCTTGCT |
| IGF-1 | TCATGTCGTCTTCACACCTCTTCT | CCACACACGAACTGAAGAGCAT |
| iNOS | CAGCTGGGCTGTACAAACCTT | CATTGGAAGTGAAGCGTTTCG |
| TNF-α | CATCTTCTCAAAATTCGAGTGACAA | TGGGAGTAGACAAGGTACAACCC |
| TGF-β1 | TAAAGAGGTCACCCGCGTGCTAAT | ACTGCTTCCCGAATGTCTGACGTA |
| VEGF-A | TGCAGGCTGCTGTAACGA | GAACAAGGCTCACAGTGATTT TCT |
| Human | ||
| GAPDH | CAGGGCTGCTTTTAACTCTGG | TGGGTGGAATCATATTGGAACA |
| IL-1β | TACCTGAGCTCGCCAGTGAA | CAGAGGTCCAGGTCCTGGAA |
| IL-6 | TTTTGTACTCATCTGCACAGC | GGATTCAATGAGGAGACTTGC |
| IL-10 | AGGGAAGAAATCGATGACAGC | TCAAGGCGCATGTGAACTC |
| IGF-1 | AGGAAGTACATTTGAAGAACGCAAGT | CCTGCGGTGGCATGTCA |
| iNOS | TTCAGTATCACAACCTCAGCAAG | TGGACCTGCAAGTTAAAATCCC |
| TNF-α1 | TCTTCTCGAACCCCGAGTGA | CCTCTGATGGCACCACCAG |
| TGF-β1 | CAGACTCAGCCAAGACACTATT | GCTCACTTCCAGAGAGATGATT |
| VEGF-A | GCTCCCATGGCAGAAGG | GAGCAAGGCCCACAGGGATTT |
Immunofluorescent histochemistry
To identify macrophage phagocytosis of apoECs, THP1 cells that were exposed to apoptotic GFP-hECs for 24 h on a chamber slide were fixed in 4% paraformaldehyde for 10 minutes, and then permeabilized by incubation with 0.15% Triton X 100 for 10 minutes. The cells were then treated with anti-CD44-APC (1:50) for 1 hour. Fluorescent images were recorded after washing with PBS using a LSM 880 confocal microscope (ZEISS, Oberkochen, Germany). To identify macrophage phagocytosis of apoECs in skin wounds, wound tissues were embedded in frozen Tissue-Plus Optimal Cutting Temperature Compound (Fisher Scientific, Hampton, NH) and cut into 8μM sections for fluorescent microscopy or 12 μM sections for confocal imaging. Sections were blocked with 10% goat serum in PBS for 45 min and then incubated with rat anti-mouse CD68 (Bio-Rad Laboratories, Hercules, CA, dilution 1:1000) for 45 min. After washing with PBS, the sections were incubated with an Alexa fluor 594 goat anti-rat IgG (Invitrogen, Carlsbad, CA, dilution 1:1000). All procedures were performed at room temperature. Fluorescent images were acquired using a Zeiss fluorescence microscope, Axioskop 40, or LSM 880 confocal microscope (ZEISS) and analyzed using Zeiss Zen Blue software (ZEISS).
Statistical analysis
Results are expressed as mean + standard deviation (SD). t tests or two-way ANOVA followed by Bonferroni’s post hoc test were performed using GraphPad Prism 6.0 software (GraphPad Software, San Diego, CA). p values less than 0.05 were considered statistically significant.
Results
Phagocytosis of apoECs alters macrophage phenotypes in vitro.
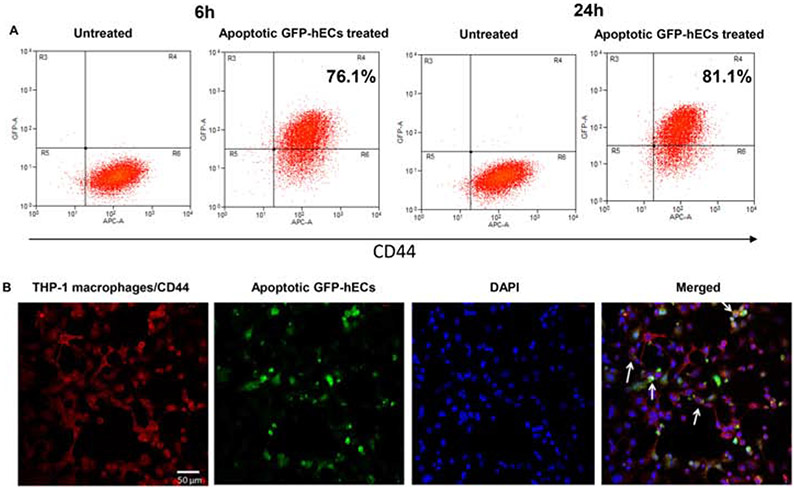
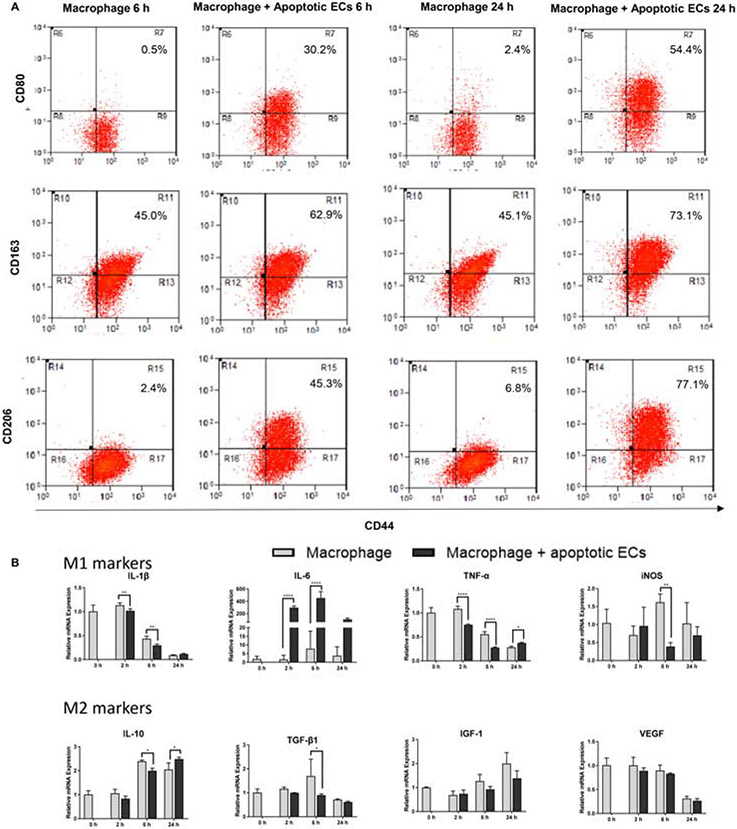
To determine if macrophages ingest apoECs, we first performed in vitro studies using THP-1 cells. THP1 cells, a monocytic cell line, were differentiated into macrophages by PMA stimulation. After incubation with apoptotic GFP-hECs, 76.1.0% and 81.1% of THP-1 cells were found to engulf GFP-hECs at 6 and 24 hours after incubation respectively (Fig. 1A&B), suggesting that the ability of macrophages to ingest apoECs is nearly universal. When the expression of macrophage markers was examined 6 hours after incubation with human apoptotic endothelial cells (hECs), 30.2%, 62.9%, and 45.3% of THP-1 macrophages expressed CD80, CD163, and CD206, respectively, compared to 0.5%, 45.0%, and 2.4% of macrophages in the untreated group. At 24 hours after incubation with apoptotic hECs, the expression of macrophage markers THP-1 cells increased to 54.4% of THP-1 macrophages were CD80+, 73.1% were CD163+, and 77.1% were CD206+ compared to 2.4%, 45.1%, and 6.8% in the untreated group, respectively (Fig. 2A). Analyses of the mRNA expression levels of M1 related cytokines in THP-1 cells showed that after incubation with apoECs, the M1 associated cytokines TNF-α, IL-1β, and iNOS decreased at some time points, while a tremendous upregulation of IL-6 was observed at all the time points tested (Fig. 2B). Expression levels of several M2 macrophage markers were also assessed. In THP-1 cells exposed to apoptotic hECs, the expression of IL-10 decreased significantly at 6 hours but increased at 24 hours, while TGF-β1 expression decreased significantly at 6 hours. There was no change in other M2 associated factors such as VEGF-A and IGF-1 (Fig. 2B). These results support the idea that exposure to and engulfment of apoECs by macrophages generates a unique phenotype with mixed M1 and M2 macrophage characteristics.
Figure 1: Macrophages ingest apoptotic human dermal ECs in vitro.
Serum starvation-induced apoptotic GFP-hECs were added to PMA-induced THP-1 macrophages for 6 and 24 hours. A) Flow cytometry scatter plots show percent of THP-1 macrophages engulfing apoptotic GFP-hECs. B) Confocal immunofluorescence images show macrophages (CD44+) ingesting apoptotic GFP-hECs indicated by white arrows. Representative results from two experiments are shown. Gating strategies are shown in Appendix B.
Figure 2: Exposure of macrophages to apoECs induces macrophage phenotype changes in vitro.
Serum starvation-induced apoptotic GFP-hECs or hECs were incubated with PMA-induced THP-1 macrophages for 6 and 24 hours. A) CD80, CD163 and CD206 expression on apoptotic GFP-hECs treated THP-1 macrophages by flow cytometry analysis. Cells were pooled from three wells of a 6 well plate at each time point. The number shown in the upper right on each scatter plot is the percentage of the target on F4/80 positive macrophages. Gating strategies are shown in Appendix B. B). mRNA expression of TNF-α, IL-1β, IL-6, IL-10, iNOS, TGF- β1, VEGF-A, and IGF-1 in apoptotic hECs treated THP-1 cells. The level of relative expression for each target at zero hour without treatment was used as the baseline control. N=3 triplicate wells at each time point. Representative results from two experiments are shown. Two-way ANOVA followed by Bonferroni’s post hoc test was used for statistical analysis. *p<0.05, **p<0.01
Phagocytosis of apoECs leads to altered macrophage phenotypes in vivo.
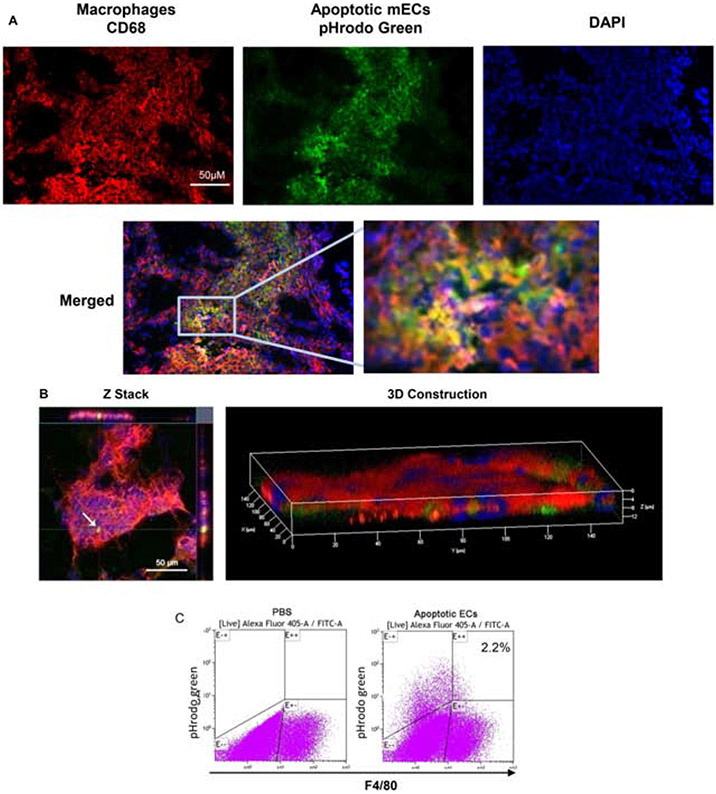
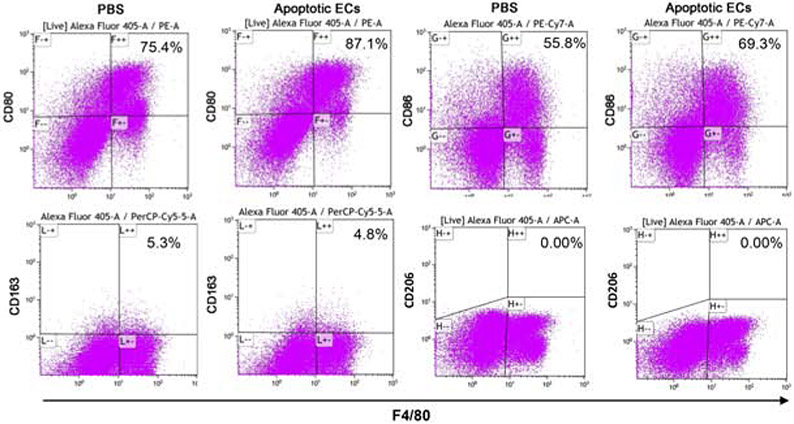
To determine if macrophages in wounds ingest apoECs, apoptotic mECs labeled with pHrodo green were injected intradermally into excisional mouse skin wounds at day 6 post-wounding. At 24 hours after apoECs injection, clusters of cells in the dermis of the injection site were found to be double positive (yellow) for CD68 (red) and apoECs (green) in regular fluorescence microscope observation, indicating wound site macrophages (CD68+) had engulfed apoECs (Fig. 3A). A similar finding was observed in 3D images by confocal microscopy (Fig. 3B) which further confirmed that macrophages in wounds ingest apoECs. Flow cytometric analysis showed that 2.2% of F4/80+ wound macrophages contained detectable pHrodo green labeled apoECs (located at E+/+quadrant in the right panel of Fig. 3C). We next investigated how injection of apoECs influenced overall wound macrophage phenotypes. In wounds injected with apoptotic mECs, the positive rate of both M1 (CD80, CD86) and M2 (CD163) macrophage surface markers in the total macrophage population were changed. As shown in Fig 4, among macrophages from apoECs-injected wounds, 87.1% were CD80+ and 69.3% were CD86+, while 75.4% were CD80+ and 55.8% were CD86+ in control wounds injected with PBS only. No difference in expression of the M2 marker CD163 was observed between control wounds and apoECs-injected wounds. We then examined marker expression only in the small sub-population of macrophages (2.2%) that exhibited detectable engulfment of apoECs (demonstrated in the quadrant E++ of the right panel of Fig. 3C). Macrophages that had engulfed apoECs expressed the markers CD80 (100%), CD86 (93.8%), and CD163 (22.8%), while no expression of CD206 (C-type mannose receptor 1), a marker frequently observed in M2 macrophages, was observed (Appendix D). Taken together, the results therefore suggest that exposure to apoECs stimulates wound macrophages to differentiate into a unique phenotype that demonstrates both M1 and M2 characteristics in vivo.
Figure 3: Macrophages ingest apoptotic ECs in wounds in vivio.
pHrodo green labeled apoptotic mECs were injected into day 6 mouse skin wounds. The uptake of apoptotic mECs 24 hours after injection was examined. A) Images taken using a regular fluorescence microscope. B) Confocal microscopy images. The white arrow in Z stack images at the left panel indicates a macrophage with engulfed pHrodo green labeled apoptotic mEC. The 3D image at the right panel shows the pHrodo green labeled apoptotic mECs are embedded among red stained CD68 macrophages suggesting macrophages ingested apoptotic mECs. C) Cells were isolated from the wounds on day 7 and subjected to flow cytometry to identify F4/80 macrophages that had ingested apoptotic mECs. Cells were collected from 8 pooled wounds from 4 mice (two wounds from each mouse) in each group. The number shown in the right scatter plot is the percentage of F4/80 macrophages with engulfed pHrodo green labeled apoptotic mECs. Representative results from two experiments are shown. Gating strategies are shown in Appendix C
Figure 4: Exposure to apoptotic dermal ECs alters macrophages phenotypes in vivo.
pHrodo green labeled apoptotic mECs were injected into day 6 skin wounds. Eight wounds were harvested and pooled from 4 mice (two wounds from each mouse) in each group24 hours later. Single cell suspensions were prepared for CD80, CD86, CD163, and CD206 staining and then subjected to flow cytometry analysis. A) Expression of CD80, CD86, CD163, and CD206 on F4/80 macrophages. B) Expression of CD80, CD86, CD163, and CD206 on F4/80 macrophages with engulfed apoptotic mECs. The number showed in the upper right on each scatter plot is the percentage of the target marker on F4/80 positive macrophages. Representative results from two experiments are shown. Gating strategies are shown in Appendix C.
Having determined that exposure to apoECs could alter macrophage phenotypic markers, the expression levels of pro-inflammatory, anti-inflammatory cytokines and growth factors were compared in control whole wound tissue and wounds injected with apoptotic mECs. The expression levels of pro-inflammatory cytokines including IL-1β, IL-6, iNOS, and TNF-α were found to be elevated in the wounds of apoECs-injected group as compared to the control, although the difference was not statistically significant, perhaps due to high variability (Fig. 5A). However, among anti-inflammatory cytokines and growth factors (IL-10, TGF-β1 IGF-1, and VEGF-A), IL-10 expression was significantly up-regulated in apoECs-injected group as compared to the PBS treated group (Fig. 5B). These results suggest that apoECs may slightly modify pro-inflammatory factors, while significantly up regulating IL-10, thus promoting anti-inflammatory milieu.
Figure 5: mRNA expression of pro- and anti-inflammatory makers and growth factors in apoptotic mECs treated wounds.
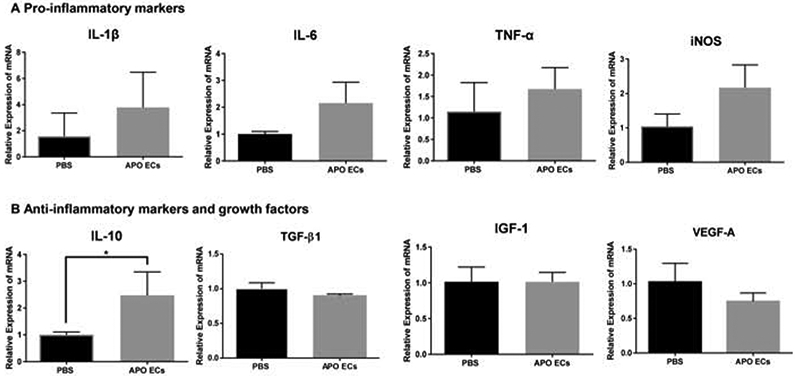
A) mRNA expression of pro-inflammatory/M1 markers (IL-1β, IL-6, iNOS, and TNF-α) in day 7 wound tissue 24 hours after apoptotic mECs injection. B) mRNA expression of anti-inflammatory markers and growth factors (IL-10, TGF- β1, IGF-1, and VEGF-A) in day 7 wound tissue 24 hours after apoptotic mECs injection. The level of relative expression of the individual target in the wounds treated with PBS was used as the baseline control. N=3 mice for each treatment group, t test was used for statistical analysis,*p<0.05.
Inhibition of angiogenesis by PEDF changes macrophages phenotype in wounds.
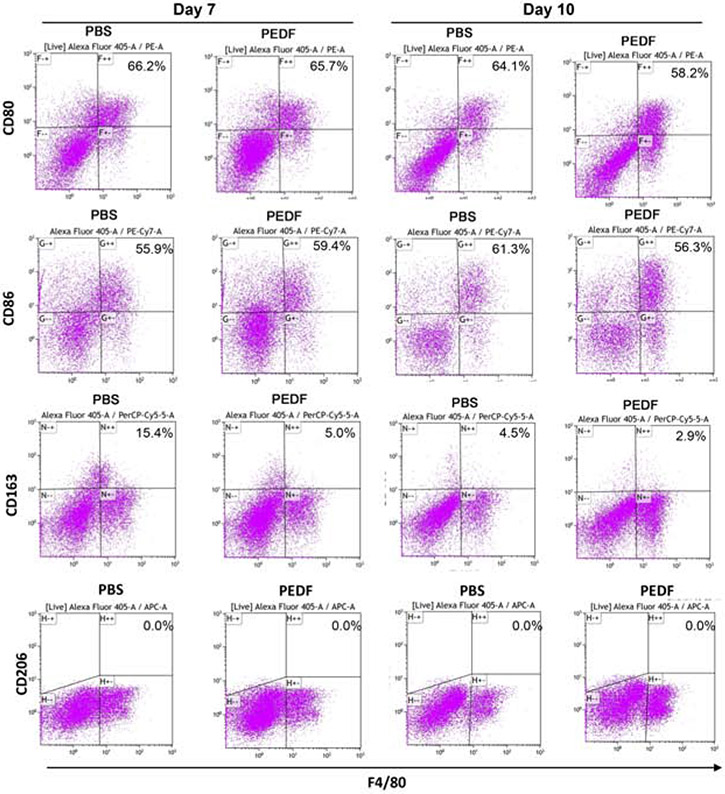
Having shown that macrophages can ingest apoECs in wounds, and this ingestion changes macrophage phenotype, we next investigated if a pharmacologic reduction in wound angiogenesis would lead to observable differences in macrophage phenotypes. To inhibit wound angiogenesis, wounds were treated with PEDF, a potent endogenous anti-angiogenic mediator, as our previous studies have demonstrated that PEDF treatment in mouse skin wounds results in a reproducible and significant reduction of angiogenesis [18, 19]. We next assessed if PEDF treatment could change macrophage phenotype in the wounds. Flow cytometric analysis demonstrated a decrease in the percentages of macrophages expressing the M1 markers CD80 and CD86 respectively in the PEDF versus control PBS treated groups on on day 7 (66.2% vs. 65.7% for CD80) and (55.9% vs. 59.4% for CD86, respectively) (Fig. 6). A decrease in CD80 and CD86 expression was also seen on day 10 post-injury after PEDF treatment(PBS group 64.1% vs. PEDF group 58.2% for CD80, PBS group 61.3% vs. PEDF group 56.3% for CD86), respectively (Fig. 6). The percentage of macrophages expressing the M2 marker CD163 markedly decreased (a 10.4% drop)in PEDF treated wounds (PBS group 15.4% vs. PEDF group 5.0% on day 7). However, there was only 1.6% drop in PEDF treated group at day 10 (PBS group 4.5% vs. PEDF group 2.9 % (Fig. 6). CD206 was not detected on macrophages in either PBS or PEDF group (Fig. 6). The results suggest that decreased angiogenesis in wounds (and the presumed resultant decrease in apoptotic EC load) may lead to differential macrophage phenotypes.
Figure 6: Inhibition of angiogenesis leads to macrophages phenotype changes in wounds.
rPEDF or PBS control was used to treat 6 mm skin wound to up to 9 days by combination of topical administration and intradermal injection. Wounds were harvested on day 7 and 10 after wounding and treatment. Single cell suspensions were prepared from the tissues as described in Materials and Methods. Cell surface expression of CD80, CD86 and CD163 on wound F4/80 macrophages was examined by flow cytometry. The cells were collected from 16 pooled wounds (4 mice per group at each time point). The number showed in the upper right on each scatter plot is the percentage of the target on F4/80 positive macrophages. Representative results from two experiments are shown. Gating strategies are shown in Appendix E.
Discussion
Macrophages are essential immune cells which play pivotal roles in wound healing and tissue regeneration. The number of macrophages in the wound gradually increases during the inflammatory phase, peaking shortly after the summit of neutrophil infiltration. Macrophages predominate during the proliferative phase of healing and are present during time periods of robust angiogenesis and granulation tissue formation. As wounds resolve and remodel, the number of macrophages declines [2, 22]. Ablation of macrophages at the early stage of wound healing leads to delayed wound closure, impaired angiogenesis, and decreased collagen deposition/granulation tissue formation [5-8], and it is now well accepted that macrophages are indispensable for wound healing. Gordon et al. proposed the concept that macrophages involved in the later stage of tissue repair had the alternatively activated or M2 phenotype [23], and this proposal was supported by others [24]. However, more extensive studies have shown that macrophages in wounds rarely have a single phenotype, but instead show characteristics of the classical M1 and M2 phenotypes. For example, macrophages isolated from wounds in both early and later stages of wound healing have been shown to express M2 markers such as CD206 (mannose receptor), arginase 1 and Ym1. However, day 1 wound macrophages do express more M1 markers, such as TNF-α and IL-6) and fewer M2 markers such as TGF-β1 than Day 7 wound macrophages[12]. Other studies confirm that macrophages predominately express pro-inflammatory cytokines in early wounds and anti-inflammatory cytokines or growth factors in the later stage of normal skin wound healing while there were sustained pro-inflammatory macrophages in diabetic wounds, which may contribute to delayed wound repair [25-27]. There is no evidence to suggest wound macrophages stratify cleanly into M1 and M2 phenotypes in either the early and late wound healing phases, respectively. Instead, multiple studies show that wound macrophages demonstrate complicated phenotypes which are not exclusively alternatively or classically activated macrophages, but a continuum with expression of multiple phenotypic markers which could be differentially regulated in the wound environment [12, 24]. This established knowledge is further supported by the current work, which demonstrates that the ingestion of apoECs by wound macrophages induces a unique macrophage phenotype that is neither M1 nor M2. This finding is in tandem with the emerging concept that the classification of macrophages into M1 and M2 subtypes is an oversimplification that does not adequately describe the in vivo macrophage population, especially in complex environments such as the healing wound.
The studies here describe a new function for macrophages during the remodeling phase of healing, and in particular, during the regression of the dense capillary bed that forms in healing wounds. The findings are likely to be applicable to any situation in which capillary growth and regression occurs, including many fibrotic diseases and many regenerative material strategies [28, 29].Although only a small percentage of wound macrophages clearly contained detectable apoECs, this percentage most likely represents only those cells that recently ingested the apoECs since once internalized, the apoptotic cells will quickly be digested in the phagolysosome. Thus the percentage of macrophages that ingest apoptotic EC in wounds is likely to be much greater than that measured by flow cytometry. We do not know the identities of the non-macrophage cells that engulfed apoECs (pHrodo green positive in E−/+ Fig. 1C which accounted for larger portion of engulfed apoECs. They could be other phagocytes such as neutrophils and/or dendritic cells, or non-professional phagocytes, an idea that can be further explored in future studies.
Following ingestion of apoECs, macrophages exhibit expression of both M1 and M2 markers, resulting in a mixed but unique phenotype both in vitro and in vivo. Similarly, mRNA expression of M1 pro-inflammatory factors and M2-associated factors was also mixed in macrophages that had ingested apoECs. This finding was recapitulated in in vitro studies with THP-1 macrophages, which also developed a heterogeneous phenotype with mixed M1 and M2 characteristics after after engulfment of apoECs. Similar data were obtained from the in vivo experiments.
When cytokine and growth factor expression levels were analyzed in wounds that were injected with apoECs, IL-10 was the only factor that was significantly elevated (Fig. 4), although the expression of IL-1β, IL-6, INOS, and TNF-α all were slightly increased. The IL-10 response is particularly interesting, as IL-10 has significant anti-inflammatory effects. IL-10 inhibits the expression of inflammatory cytokines and chemokines such as IL-1, IL-3, IL-6, IL-8, TNF-α, G-CSF and GM-CSF and CCL3 [30]. In the skin, it is produced by various cell types including T lymphocytes, macrophages, and keratinocytes [31]. Notably, high levels of IL-10 have been suggested to be responsible for the attenuated inflammatory response and scarless wound healing that is seen in fetal wounds [32]. Moreover, the overexpression of IL-10 has been shown to lead to reduced scar formation in adult tissue [33, 34]. The marked increase of IL-10 in the wounds after apoptotic EC injection may have a potential benefit in the inhibition of scar formation, a finding that warrants additional study.
Prior studies have shown that the phagocytosis of apoptotic cells by macrophages generally does not lead to pro-inflammatory cytokine production [35]. This outcome is in contrast to the inflammatory response seen in macrophages that ingest microbes[36], and is thought to be important for the maintenance of cell and tissue homeostasis in the presence of apoptotic bodies. Several studies have examined the effect of efferocytosis of apoptotic neutrophils on macrophages, finding that this activity prompts production of healing-associated factors such as TGF-β and inhibits expression of pro-inflammatory cytokine TNF-α and IL-8 in macrophages [35]. Macrophage engulfment of apoptotic peripheral blood lymphocytes has also been shown to enhances the production of anti-inflammatory cytokine IL-10 and inhibit production of TNF-α [37]. These studies and our current findings demonstrate that macrophage ingestion of apoptotic cells alters macrophage phenotype and that the response of the macrophages varies with the type of cell that is ingested. More specifically, unlike apoptotic neutrophils or lymphocytes, the ingestion of apoECs stimulates macrophages to adopt a distinctive mixed M1 and M2 phenotype.
In our previous studies, PEDF treated wounds showed significantly reduced angiogenesis [18, 19], indicating that there would be less apoECs generated during the remodeling phase of healing. Because wound macrophages were observed to engulf apoECs, we hypothesized that the phenotypes of macrophages would also be altered in PEDF treated wounds, which have fewer apoECs due to decreased angiogenesis. As shown in our results, the expression of M1 (CD80 and CD86) and M2 marker (CD163) on macrophages isolated from day 7 and day 10 wounds were decreased after PEDF treatment decreased, most especially M2 marker CD163, which was significantly reduced (Fig. 6). These data suggest that down regulation of angiogenesis by PEDF and the resultant decrease in apoEC load results in differential display of macrophage phenotypes. However, whether this macrophage phenotype shift results in other pathophysiological changes in wound healing in in PEDF treated mice such as repressed angiogenesis, improved vascular integrity/function, and increased collagen maturity [18, 19] warrants further investigations.
In this study we used serum starvation to induce EC apoptosis, and the resulting apototic EC were primarily both Annexin V and PI double positive. The finding of a significant level of positivity of both PI and Annevin V suggests that some of cells might be necrotic rather than apoptotic. While this is possible, recent studies show that apoptotic cells frequently exhibit positive PI staining due to uptake by RNA [38]. In addition, only small number of PI only positive cells were found, indicating that the samples do not likely contain large amounts of necrotic cells. Nevertheless, the possibility of the inclusion of necrotic cells represents a caveat to our findings, as small numbers of necrotic cells may have influenced the results.
Our study is the first to demonstrate that macrophages phagocytize apoECs. Our data show that the uptake of apoECs by macrophages reprograms macrophages to generate a phenotype that is not biased to either the M1 or M2 phenotype, but instead has characteristics of both M1 and M2 macrophage phenotypes. Importantly, the findings suggest that the regression of the vasculature may influence other aspects of the remodeling process by regulating the activity of resident and infiltrating macrophages. Our studies show that wounds that heal with refined angiogenesis and thus with decreased subsequent vascular regression do not experience the changes in resident macrophages that are observed in response to apoECs. A limitation of the current study is that we do not yet know how the observed change in macrophage phenotype during vascular regression influences ECM remodeling or other facets of wound resolution. Another limitation is that in vivo macrophage phenotype changes demonstrated in the study were observed after injection of apoECs into the wounds, which was not a physiological condition. Future studies are needed to completely understand the intersection of the phagocytosis of apoECs by macrophages and the related healing outcomes, such as scar formation and fibrosis.
Supplementary Material
Macrophages play a pivotal role in wound healing and tissue regeneration.
Regression of angiogenesis during remodeling phase of wound healing results in larger numbers of apoptotic endothelial cells.
Macrophages engulf apoptotic endothelial cells in healing wounds.
Engulfment of apoptotic endothelial cells creates a distinctive mixed M1 and M2 phenotype.
Acknowledgements
This work was supported by a National Institutes of Health Grant R01-GM-50875 (LAD), the First Affiliated Hospital, School of Medicine, Zhejiang University (MX), and a National Natural Science Foundation of China grant 81602383 (DM). The authors would like to thank May Barakat, Wendy Cerny, and Trevor Leonardo for their critical review of the manuscript. The content is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Abbreviations
- ApoECs
Apoptotic endothelial cells
- ECM
Extracellular matrix
- hECs
Human dermal microvascular endothelial cells
- mECs
Mouse dermal microvascular endothelial cells
- PEDF
Pigment epithelium-derived factor
Footnotes
Conflict of interests
No conflicts of interest are declared by the authors.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Eming SA; Krieg T; Davidson JM, Inflammation in wound repair: molecular and cellular mechanisms. J Invest Dermatol 2007, 127, (3), 514–25. [DOI] [PubMed] [Google Scholar]
- 2.Guo S; Dipietro LA, Factors affecting wound healing. J Dent Res 2010, 89, (3), 219–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin P, Wound healing--aiming for perfect skin regeneration. Science 1997, 276, (5309), 75–81. [DOI] [PubMed] [Google Scholar]
- 4.Werner S; Grose R, Regulation of wound healing by growth factors and cytokines. Physiol Rev 2003, 83, (3), 835–70. [DOI] [PubMed] [Google Scholar]
- 5.Goren I; Allmann N; Yogev N; Schurmann C; Linke A; Holdener M; Waisman A; Pfeilschifter J; Frank S, A transgenic mouse model of inducible macrophage depletion: effects of diphtheria toxin-driven lysozyme M-specific cell lineage ablation on wound inflammatory, angiogenic, and contractive processes. Am J Pathol 2009, 175, (1), 132–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leibovich SJ; Ross R, The role of the macrophage in wound repair. A study with hydrocortisone and antimacrophage serum. Am J Pathol 1975, 78, (1), 71–100. [PMC free article] [PubMed] [Google Scholar]
- 7.Lucas T; Waisman A; Ranjan R; Roes J; Krieg T; Muller W; Roers A; Eming SA, Differential roles of macrophages in diverse phases of skin repair. J Immunol 2010, 184, (7), 3964–77. [DOI] [PubMed] [Google Scholar]
- 8.Mirza R; DiPietro LA; Koh TJ, Selective and specific macrophage ablation is detrimental to wound healing in mice. Am J Pathol 2009, 175, (6), 2454–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mills CD; Kincaid K; Alt JM; Heilman MJ; Hill AM, M-1/M-2 macrophages and the Th1/Th2 paradigm. J Immunol 2000, 164, (12), 6166–73. [DOI] [PubMed] [Google Scholar]
- 10.Wetzler C; Kampfer H; Stallmeyer B; Pfeilschifter J; Frank S, Large and sustained induction of chemokines during impaired wound healing in the genetically diabetic mouse: prolonged persistence of neutrophils and macrophages during the late phase of repair. J Invest Dermatol 2000, 115, (2), 245–53. [DOI] [PubMed] [Google Scholar]
- 11.Novak ML; Koh TJ, Macrophage phenotypes during tissue repair. J Leukoc Biol 2013, 93, (6), 875–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Daley JM; Brancato SK; Thomay AA; Reichner JS; Albina JE, The phenotype of murine wound macrophages. J Leukoc Biol 2010, 87, (1), 59–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bluff JE; O'Ceallaigh S; O'Kane S; Ferguson MW; Ireland G, The microcirculation in acute murine cutaneous incisional wounds shows a spatial and temporal variation in the functionality of vessels. Wound Repair Regen 2006, 14, (4), 434–42. [DOI] [PubMed] [Google Scholar]
- 14.DiPietro LA, Angiogenesis and scar formation in healing wounds. Curr Opin Rheumatol 2013, 25, (1), 87–91. [DOI] [PubMed] [Google Scholar]
- 15.Meeson AP; Argilla M; Ko K; Witte L; Lang RA, VEGF deprivation-induced apoptosis is a component of programmed capillary regression. Development 1999, 126, (7), 1407–15. [DOI] [PubMed] [Google Scholar]
- 16.Park YK; Tu TY; Lim SH; Clement IJ; Yang SY; Kamm RD, In Vitro Microvessel Growth and Remodeling within a Three-dimensional Microfluidic Environment. Cell Mol Bioeng 2014, 7, (1), 15–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akers JC; Gonda D; Kim R; Carter BS; Chen CC, Biogenesis of extracellular vesicles (EV): exosomes, microvesicles, retrovirus-like vesicles, and apoptotic bodies. J Neurooncol 2013, 113, (1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Michalczyk ER; Chen L; Fine D; Zhao Y; Mascarinas E; Grippo PJ; DiPietro LA, Pigment Epithelium-Derived Factor (PEDF) as a Regulator of Wound Angiogenesis. Sci Rep 2018, 8, (1), 11142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wietecha MS; Krol MJ; Michalczyk ER; Chen L; Gettins PG; DiPietro LA, Pigment epithelium-derived factor as a multifunctional regulator of wound healing. Am J Physiol Heart Circ Physiol 2015, 309, (5), H812–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brubaker AL; Schneider DF; Palmer JL; Faunce DE; Kovacs EJ, An improved cell isolation method for flow cytometric and functional analyses of cutaneous wound leukocytes. J Immunol Methods 2011, 373, (1-2), 161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang G; Zhang H; Liu Y; He Y; Wang W; Du Y; Yang C; Gao F, CD44 clustering is involved in monocyte differentiation. Acta Biochim Biophys Sin (Shanghai) 2014, 46, (7), 540–7. [DOI] [PubMed] [Google Scholar]
- 22.Brancato SK; Albina JE, Wound macrophages as key regulators of repair: origin, phenotype, and function. Am J Pathol 2011, 178, (1), 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gordon S, Alternative activation of macrophages. Nat Rev Immunol 2003, 3, (1), 23–35. [DOI] [PubMed] [Google Scholar]
- 24.Mosser DM; Edwards JP, Exploring the full spectrum of macrophage activation. Nat Rev Immunol 2008, 8, (12), 958–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mirza R; Koh TJ, Dysregulation of monocyte/macrophage phenotype in wounds of diabetic mice. Cytokine 2011, 56, (2), 256–64. [DOI] [PubMed] [Google Scholar]
- 26.Mirza RE; Fang MM; Ennis WJ; Koh TJ, Blocking interleukin-1β induces a healing-associated wound macrophage phenotype and improves healing in type 2 diabetes. Diabetes 2013, 62, (7), 2579–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirza RE; Fang MM; Weinheimer-Haus EM; Ennis WJ; Koh TJ, Sustained inflammasome activity in macrophages impairs wound healing in type 2 diabetic humans and mice. Diabetes 2014, 63, (3), 1103–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martino MM; Brkic S; Bovo E; Burger M; Schaefer DJ; Wolff T; Gurke L; Briquez PS; Larsson HM; Gianni-Barrera R; Hubbell JA; Banfi A, Extracellular matrix and growth factor engineering for controlled angiogenesis in regenerative medicine. Front Bioeng Biotechnol 2015, 3, 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Johnson A; DiPietro LA, Apoptosis and angiogenesis: an evolving mechanism for fibrosis. FASEB J 2013, 27, (10), 3893–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pretolani M, Interleukin-10: an anti-inflammatory cytokine with therapeutic potential. Clin Exp Allergy 1999, 29, (9), 1164–71. [DOI] [PubMed] [Google Scholar]
- 31.King A; Balaji S; Le LD; Crombleholme TM; Keswani SG, Regenerative Wound Healing: The Role of Interleukin-10. Adv Wound Care (New Rochelle) 2014, 3, (4), 315–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liechty KW; Kim HB; Adzick NS; Crombleholme TM, Fetal wound repair results in scar formation in interleukin-10-deficient mice in a syngeneic murine model of scarless fetal wound repair. J Pediatr Surg 2000, 35, (6), 866–72; discussion 872–3. [DOI] [PubMed] [Google Scholar]
- 33.Gordon A; Kozin ED; Keswani SG; Vaikunth SS; Katz AB; Zoltick PW; Favata M; Radu AP; Soslowsky LJ; Herlyn M; Crombleholme TM, Permissive environment in postnatal wounds induced by adenoviral-mediated overexpression of the anti-inflammatory cytokine interleukin-10 prevents scar formation. Wound Repair Regen 2008, 16, (1), 70–9. [DOI] [PubMed] [Google Scholar]
- 34.Peranteau WH; Zhang L; Muvarak N; Badillo AT; Radu A; Zoltick PW; Liechty KW, IL-10 overexpression decreases inflammatory mediators and promotes regenerative healing in an adult model of scar formation. J Invest Dermatol 2008, 128, (7), 1852–60. [DOI] [PubMed] [Google Scholar]
- 35.Fadok VA; Bratton DL; Konowal A; Freed PW; Westcott JY; Henson PM, Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest 1998, 101, (4), 890–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weiss G; Schaible UE, Macrophage defense mechanisms against intracellular bacteria. Immunol Rev 2015, 264, (1), 182–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Voll RE; Herrmann M; Roth EA; Stach C; Kalden JR; Girkontaite I, Immunosuppressive effects of apoptotic cells. Nature 1997, 390, (6658), 350–1. [DOI] [PubMed] [Google Scholar]
- 38.Rieger AM; Hall BE; Luong le T; Schang LM; Barreda DR, Conventional apoptosis assays using propidium iodide generate a significant number of false positives that prevent accurate assessment of cell death. J Immunol Methods 2010, 358, (1-2), 81–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.