Abstract
The Swiss Alpine environments are poorly described from a microbiological perspective. Near the Greina plateau in the Camadra valley in Ticino (southern Swiss Alps), a green-turquoise-coloured water spring streams off the mountain cliffs. Geochemical profiling revealed naturally elevated concentrations of heavy metals such as copper, lithium, zinc and cadmium, which are highly unusual for the geomorphology of the region. Of particular interest, was the presence of a thick biofilm, that was revealed by microscopic analysis to be mainly composed of Cyanobacteria. A metagenome was further assembled to detail the genes found in this environment. A multitude of genes for resistance/tolerance to high heavy metal concentrations were indeed found, such as, various transport systems, and genes involved in the synthesis of extracellular polymeric substances (EPS). EPS have been evoked as a central component in photosynthetic environments rich in heavy metals, for their ability to drive the sequestration of toxic, positively-charged metal ions under high regimes of cyanobacteria-driven photosynthesis. The results of this study provide a geochemical and microbiological description of this unusual environment in the southern Swiss Alps, the role of cyanobacterial photosynthesis in metal resistance, and the potential role of such microbial community in bioremediation of metal-contaminated environments.
Introduction
Microbial mats are composed of different horizontally stratified biofilms of microorganisms building a connected network with the ability to endure extreme environments such as hypersaline basins, sulphuretums, aquifers and sulfur springs, under prohibitive conditions for the growth of eukaryotic organisms [1, 2].
Sedimentary rock finds indicate a worldwide presence of microbial mats throughout the history of the Earth, as representative of first ecosystems together with stromatolites, and their role as modifiers of early atmosphere [3].
Modern biomats typically host a high biological diversity that includes bacterial, but also archaeal and eukaryotic communities [4]. This is partly due to dynamic physicochemical conditions that accommodate the needs of the different communities into interacting ecological niches [5–7], allowing them to carry out biological processes such as methanogenesis, denitrification, metal and sulfate reduction [8–10], as well as photosynthesis and nitrogen fixation in which Cyanobacteria play an important role [11]. Cyanobacteria are frequently found in diverse ecological niches including those harbouring high heavy metal concentrations [12]. Cyanobacteria’s tolerance to such conditions is supposed to derive from their ability to synthesize extracellular polymeric substances (EPS). However, it is unclear how the oxidative stress induced by the combination of heavy metal ions present in polluted environments affects cyanobacterial physiology [13, 14]. Secreted EPS, membrane-bound or soluble, surround the cells in microbial communities through the formation of protective layers against oxidative and other sources of stress. In particular, their negative charge has been shown to play an important role in the protection against heavy metals-mediated oxidative stress, by depleting positively charged metal ions [15–17].
Therefore, microbial mats are of particular interest for studying microbial communities’ diversity, structure and evolution, which contributes to their adaptation to extreme environments [18–20], and for their potential applications in bioremediation [4].
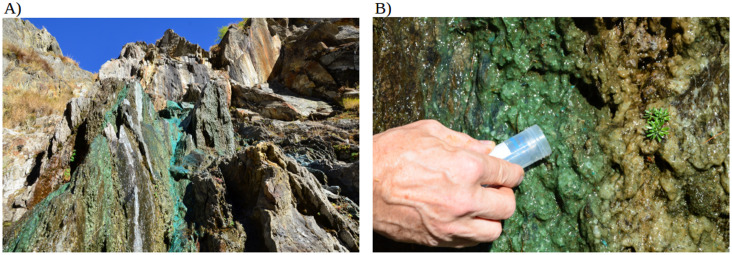
While biomats in different environments (for example hypersaline, acid, thermophillic, psychrophilic, oligotrophic, coastal mats) have been described previously [4, 7, 21–23], biomats in Alpine environments have been poorly studied so far. Here we investigate the microbial and chemical compositions of this biomat adapted to the Swiss Alpine environment (Fig 1). The peculiar chemical compositions of the biomat spring described in this study, principally composed of copper, cadmium and zinc, is unusual for the Swiss southern Alpine environment from a geological perspective [24, 25]. It is therefore of interest to investigate the formation of this biomat as a microbial adaptation to this unusual environment.
Fig 1. Biomat spring in the Greina region, Camadra di Fuori / Sassina location in the Camadra valley.
This spring is well known in the region because of the green-turquoise colour of the biomat on the rocks [26]. The geographical position of the biomat spring is: CH1903+ / LV95 2’715’235, 1’160’895, at an elevation of 1’726 m asl. A) Overview of the biomat spring (summer 2015). B) Details of the sampling.
Materials and methods
Chemical profiling of the spring water
Water samples were taken using 500 ml disposable polypropylene bottles (Carl Roth, Arlesheim, Germany), they were kept at 4°C and transported to the laboratory within 2 h.
The water chemical composition was measured using inductively coupled plasma mass spectrometry (ICP-MS), after the water samples were acidified using 0.3% HCl and 0.3% NHO3. The conductivity was determined using a 5-ring conductivity measuring cell with cell constant c = 0.7 cm−1 with integrated Pt1000 temperature sensor (Metrohm, art. 6.0915.100). The quantity of the anions (sulfate, fluoride, nitrate, chloride, nitrite and bromide) and cations (potassium and sodium) was measured using ion chromatography (IC, Metrohm, 850 Professional IC). Phosphate concentration was determined using UV-Vis colorimetric analysis (method SOP MSDA 628.1 [27]). Calcium and magnesium concentrations were measured using a calcium-selective electrode with polymer membrane (Metrohm, art. 6.0508.110). Strontium and zinc concentrations were measured with an inductively coupled plasma optical emission spectrometer (ICP-OES VISTA MPX Axial, Varian). Before analysis the samples were acidified with 1% NHO3. All the other metal concentrations were measured using an inductively coupled plasma mass spectrometry (ICP-MS, iCAP-Q, ThermoScientific). Before analysis the samples were acidified with 0.3% HCl and 0.3% NHO3.
Microscopy
Fluorescence microscopy
The biofilm probes were examined by microscopy with a Zeiss Axiolab microscope in bright field and epifluorescence, using the F41 filter sets (AHF Analysentechnik HQ535/50, Q565LP and HQ610/75) for detection of phycoerythrin-containing autofluorescent cells [28].
X-ray & scanning electron microscopy
The rock samples were mounted on aluminum supports. They were covered with an ultra-thin coating of gold (10 nm) by low vacuum sputter, prior to imaging with a scanning electron microscope JEOL JSM 70001 FA (department of Earth Sciences, University of Geneva, Switzerland). Scanning electron microscope energy dispersive X-ray spectroscopy analyses (EDXS) were lead with a JEOL EX-94300S4L1Q detector. These analyses were acquired with an accelerating voltage of 15 kV, a beam current of 3.5 nA (acquisition times of 30 s). Gold (Au) is not taken into account in the semiquantitative quantification of the elements, as it is not part of the sample. Although not labelled, the characteristic energy peak of Au is visible on the spectrum at 2.12 KeV (S1 File).
Sample collection and DNA extraction
The green-turquoise mucilage was collected during summer 2015 from the stream located in the Greina region, Camadra di Fuori / Sassina location in the Camadra valley (CH1903+ / LV95 2’715’235, 1’160’895, 1’726 m asl) using a falcon tube and stored at -20°C. DNA was extracted from 10 g of mucilage using the DNeasy PowerMax Soil Kit (Qiagen) following the manufacturer protocol. After extraction, DNA was precipitated with ethanol and NaCl following the procedure suggested by the manufacturer. Finally, DNA was eluted in 50 μl of molecular grade H2O. Quality and quantity of DNA was assessed spectrophotometrically using Nanodrop as well as the Quant-iT™ PicoGreen™ dsDNA Assay Kit (Invitrogen) combined with a TD700 Fluorometer (Turner Design) and the Qbit4 instrument (Thermofisher). In addition, the integrity of the DNA was checked by agarose gel electrophoresis.
MinION sequencing
The metagenome of the green-turquoise mat was sequenced using a 1D ligation sequencing kit (SQK-LSK108). Sequencing was performed using an Oxford Nanopore Technologies (ONT) MinION flow cell R9.4 containing an initial number of 1’553 active nanopores for a duration run of 48 hours, using the MinKNOW software (v18.01.6). The ONT Guppy basecaller (v2.3.7) was further used to assign base names on the resulting chromatogram.
Metagenome assembly and annotation
The fastq reads obtained by MinION sequencing were used to perform taxonomic classification using MetaMaps (v0.1) [29] against its “miniSeq+H” database (updated March 12th 2020) with BLAST NCBI taxonomy from Krona Tools (v2.7.1) [30]. The reads were also used to run Canu (v1.9) for assembling a metagenome with the following parameters: genomeSize = 5m, corOutCoverage = 10000, corMhapSensitivity = high, corMinCoverage = 0, correctedErrorRate = 0.105, redMemory = 32, oeaMemory = 32, batMemory = 200, maxMemory = 230G, nanopore-raw. Prokka (v13.1) was subsequently used to annotate the 3’421 contigs resulting from Canu assembly in conjunction with the NCBI BLAST’s nt database (v2.10.0+). In addition, metagenomic binning was applied using the fastq reads and the assembled contigs longer than 10’000 nucleotides, using CONCOCT (v1.1.0) [31] with the “composition_file” option. Prokka was then used to annotate the contigs of the bins separately and CheckM (v1.1.2) [32] used to assess the completeness of the corresponding metagenome-assembled genomes (MAGs).
Results and discussion
Hydrochemical and geochemical analyses
The biomat spring is located at the contact between the migmatitic mica-alkali feldspar-plagioclase gneiss to the north and the biotite-muscovite-alkali feldspar gneiss to the south. These two units are the basement of Mels-, Röti- and Quarten-Formations (autochthonous cover of the Gotthard Massif deposited during the Triassic Period) composed by dolomitic/calcitic marble and cellular dolostone [24]. Just above the spring, a deposit of industrial minerals is inventoried in the Georesources information system of Switzerland [33]. This deposit belongs to the Triassic dolomitic marbles and contains, as elements, barium, fluorine, zinc, iron and copper, and, as minerals, barite, sphalerite, pyrite, chalcopyrite and fluorite, of hydrothermal origin [34].
Despite streaming through an area characterized by gneissic rocks with different mineralogical compositions, the hydrochemistry of the spring showed a calcium-sulfate type water strongly influenced by the presence of gypsum and carbonates in the Triassic rocks surrounding the area [24, 35]. In addition, the chemical profiling of the spring water indicated high concentrations of heavy metals such as aluminium (∼1.25-fold higher than expected from granitic gneiss), barium (∼2.6-fold higher than expected from dolomitic and calcitic marble and cellular dolostone) as well as lithium, manganese and strontium as expected from rocks of dolomitic origin, but also, surprisingly, it showed unusually high concentrations of copper (>60-fold), cadmium (>100-fold) and zinc (>650-fold) compared to the typical concentration range found in Alps (Table 1). The enrichment in the latter metals might be due to the presence of the hydrothermal veins previously mentioned, which contain minerals such as chalcopyrite and sphalerite [33, 35], and represents a significant difference with respect to concentrations found in typical Alpine environments, however being ∼100-fold lower than extreme acidophiles found in heavy-metal laden acid mine drainage waters [36, 37]. The presence of a metarhyolite vein to the north of the study site could potentially explain the observed enrichment primarily of copper and zinc, but also of barium, cadmium, manganese and nickel.
Table 1. Chemical analysis of the spring water.
| Chemical parameter | Measured value | Chemical parameter | Measured value |
|---|---|---|---|
| Conductivity at 20°C (μS/cm) | 539 | Phosphate (mg/L) | <0.01 |
| pH | 7.73 | Nitrite (mg/L) | <0.01 |
| Alcalinity at pH 4.3 (mmol/L) | 0.98 | Bromide (mg/L) | <0.01 |
| Sulfate (mg/L) | 254.5 | Molybdenum (μg/L) | 9.92 |
| Calcium (mg/L) | 116.4 | Uranium (μg/L) | 4.77 |
| Magnesium (mg/L) | 8 | Boron (μg/L) | 3.09 |
| Fluoride (mg/L) | 3.65 | Lead (μg/L) | 1.4 |
| Zinc (mg/L) | 3.39 | Selenium (μg/L) | 1.38 |
| Potassium (mg/L) | 2.8 | Antimony (μg/L) | 0.53 |
| Sodium (mg/L) | 1.2 | Cesium (μg/L) | 0.46 |
| Nitrate (mg/L) | 0.7 | Arsenic (μg/L) | 0.46 |
| Strontium (μg/L) | 574 | Cobalt (μg/L) | 0.34 |
| Copper (μg/L) | 305.14 | Tin (μg/L) | <0.20 |
| Chloride (mg/L) | 0.1 | Mercury (μg/L) | <0.20 |
| Ammonium (mg/L) | <0.1 | Bismuth (μg/L) | <0.20 |
| Aluminum (μg/L) | 40.19 | Vanadium (μg/L) | <0.1 |
| Manganese (μg/L) | 35.62 | Thallium (μg/L) | <0.1 |
| Cadmium (μg/L) | 30.86 | Silver (μg/L) | <0.1 |
| Barium (μg/L) | 25.32 | Iron (μg/L) | <0.1 |
| Nickel (μg/L) | 14.29 | Chromium (μg/L) | <0.1 |
| Lithium (μg/L) | 12.87 | Beryllium (μg/L) | <0.1 |
The geochemical composition of the green-turquoise deposit collected at the spring was also investigated with the scanning electron microscopy analysis, and similar results were obtained (see S1 File). The analysis showed in fact typical elements contained in the crystal lattice of silicates of the gneissic rocks and elements as copper, zinc, iron and sulfur forming the minerals of hydrothermal origin.
MinION metagenomics sequencing analysis
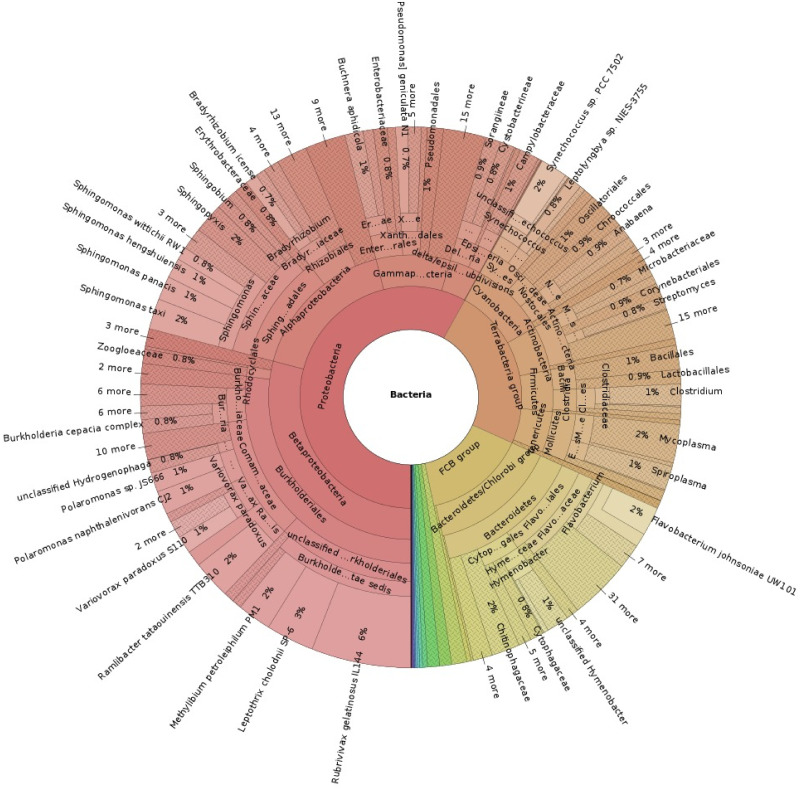
The sequencing generated a total of 5’874’348 reads with a mean length of 1’010 bp and a mean read quality of 9.7, which were further processed with the ONT Guppy basecaller resulting in 5’085’754 (86.6%) reads of quality score Q > 7. Taxonomic classification against the “miniSeq+H” index database (updated March 12th 2020) was used for evaluating biological diversity (Fig 2). The metagenome assembled contigs were annotated with Prokka before (S2 File) and after metagenomic binning (S3 File) in order to build different MAGs, further assessed for completeness, contamination and strain heterogeneity using CheckM (S4 File). These constituted the metagenomic data used to search for relevant genes and components involved in the biological functions described below.
Fig 2. Bacterial taxonomy based on MinION metagenomics sequencing, only reads representing more than 1% occurrence are represented (an interactive diagram is available in S5 File).
Overall bacterial diversity
Proteobacteria were the most diverse group of bacteria present in the environment with a proportion of 58% (Fig 2). Among them, Polaromonas spp. of the Betaproteobacteria is known for being tolerant to elevated metal concentrations thanks to the metal-resistance genes for mercury, arsenate, chromate, and other heavy metals [38], and its role in pollutant degradation [39], as well as Rhizobiales (Alphaproteobacteria) such as Rhodopseudomonas palustris also involved in the removal of environmental pollutants by degrading chlorinated compounds [40].
Cytophaga, Bacteroidia, and Flavobacteriia are classes of the Bacteroidetes phylum that represented about 15% of the bacterial species and have been previously found in hypersaline mats [41] having a role in scavenging of Cyanobacteria biomass [42].
Terrabacteria represented 24% of the bacterial diversity, including Actinobacteria, Firmicutes, Tenericutes and Deinococci, known to be part of aquatic microbial biomat communities at low temperatures [43], aside of the most diverse group, i.e., Cyanobacteria.
Cyanobacteria diversity
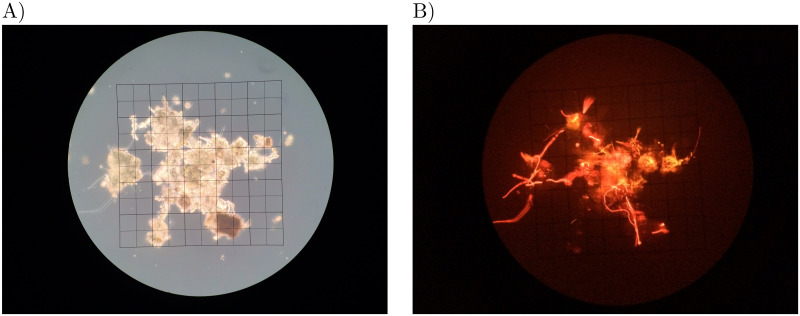
Fluorescence microscopy of the biofilm indicated the presence of Cyanobacteria (Fig 3) highlighted by the fluorescence emission range of the Cyanobacteria characteristic pigments.
Fig 3. Microscopy of biomat samples.
A) Biomat sample under 200x light microscopy magnification (grid side length = 635μm). B) Fluorescence microscopy at 552 nm of biomat’s Cyanobacteria.
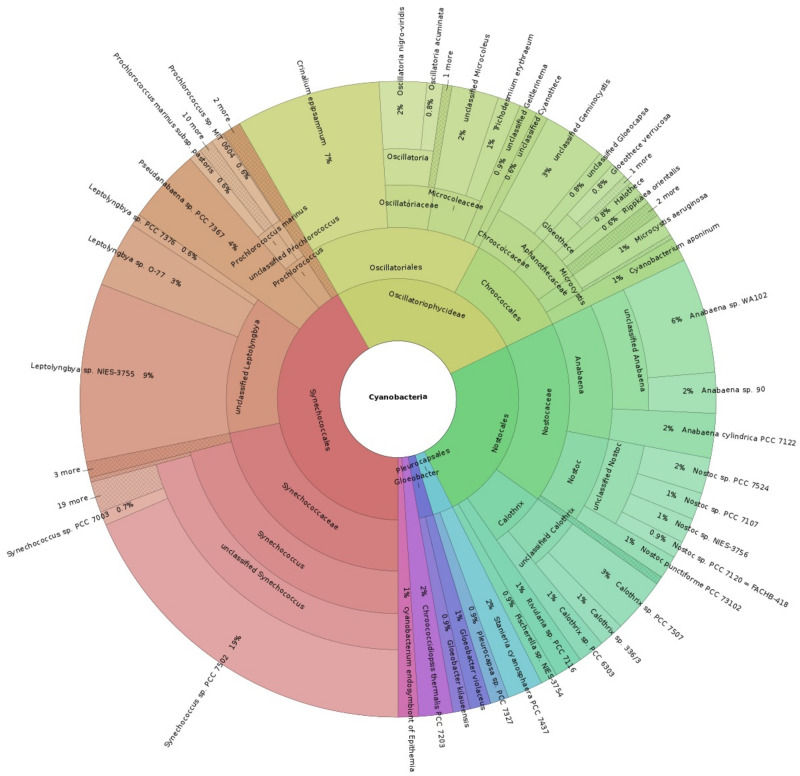
Cyanobacteria diversity could be assessed based on MetaMaps analysis (Fig 4). Synechococcales were the most represented (42%), known to be involved in metal cycling in oceans’ photic zone [44], followed by Oscillatoriophycideae (26%), such as Gloeocapsa and Gloeothece spp., and Nostocales (25%), that are both considered as primary producers of phototrophic mats [4].
Fig 4. Cyanobacterial taxonomy based on MinION metagenomics sequencing, only reads representing more than 1% occurrence are represented (an interactive diagram is available in S5 File).
In addition, functional prediction of the genes based on the assembled metagenome indicated the presence of several genes involved in cyanobacterial metabolic activities. Cyanobacteria activity was suggested by cyanophycinase and cyanophycin synthetase genes, involved in the degradation and polymerization of Cyanobacteria-specific cyanophycin, respectively [45]. Diverse phycocyanobilin lyase subunits (CpcE,F,T,S) as well as phycocyanobilin:ferredoxin oxidoreductase (PcyA) and a putative phycocyanobilin lyase (CpcS) were also predicted by the metagenome annotation. The latter are involved in the light harvesting complexes [46], together with other photosynthetic antenna proteins (ApcA-E; CpcA,B,D,E-I,S,T; PetA-H,J,M; PsaA-F,I-M; PsbA-E,F,H-J,K,M,N,O,U,V,X,Y,Z), proteins involved in cyanobacterial oxidative phosphorylation (CyoE; NdhA-E; NdhH-N; Ppa; Ppk; SdhA,B,E) [47].
Genes for nitrogen fixation
Genes involved in nitrogen fixation were also found, such as the Nif-specific regulatory protein (NifA and NifS) and the nitrogen fixation protein (VnfA) found in Cyanobacteria (Anabaena genus) [48], as well as in bacteria, such as Azotobacter (Pseudomonadales) [49, 50], Rhizobiales [51, 52] and Azospirillum (Alphaproteobacteria) [53, 54], together with associated regulator proteins, such as a nitrogen regulatory protein P-II (GlnB) and a global nitrogen regulator (NtcA) commonly found in (cyano)bacteria, archaea and plants [55].
Genes for microbial metal resistance
Several genes involved in conferring tolerance/resistance to high metal concentrations were found in the annotated metagenome (S2 and S3 Files).
In particular, resistance against arsenic was represented by Acr3 and ArsA,C,H,M resistance effectors, which detect and stimulate the cellular response to arsenic [56, 57]. The genes CzcA and B, CnrA and R, and CopA and B, were found in the annotated metagenome representing resistance mechanisms against elevated concentrations of cobalt, zinc, cadmium, nickel and copper [58–62], all of which were present in the sampled environment according to the chemical analysis (Table 1).
Further, genes coding for permeases related to the transport of iron were found (FeoB; FecA,E) described previously with the role of regulators of intracellular iron concentration [63–65], as well as PhnE,D and PstA,C for the uptake of phosphate at low extracellular concentrations [66] and for sulfate (CysT,W) [67], and sodium-translocating NADH-quinone reductase (subunits A,B,F).
Several components of other transport systems related to metals or chemical species measured in the environmental chemical analysis were found in the metagenomic data. Transporting ATPases for copper (ActP) [68], silver (SilP) [69, 70], cadmium/zinc/cobalt (CadA) [71, 72], zinc (ZiaA) [73, 74], calcium (PacL, YloB) [75, 76], magnesium (MgtA and MgtB) [77] and potassium (KdpA-C) [78], as well as other transporters were found for iron (FieF) [63, 79], ammonium (NrgA) [80, 81], magnesium (MgtE) [82], manganese (MntB,H,R) [83], nitrate/nitrite (NrtA and NrtP) [84], sodium (SdcS) [85], Zinc (ZitB) [86], cobalt/magnesium (CorA) [87, 88]. Import systems were found for phosphate (PstB, PhnD) [66, 89] and an antiporter for cadmium/cobalt/zinc vs. proton/potassium (CzcD) [62, 90, 91], as well as for sodium (NhaA,C,D, GerN, NhaS3) [92], molybdenum (ModA,B), nickel (LarO) or potassium (NhaP2) [93]vs. protons. Finally, genes coding for transcriptional regulators involved in sensing and uptake of phosphate (PhoB and PhoR) [94, 95] and zinc (Zur) [86] were identified.
Genes involved in EPS synthesis
The metagenome analysis revealed several genes predicted to be involved in EPS synthesis, which have been studied in various contexts from biosynthesis to biotechnological applications in bioremediation, for their capacity of heavy metal sorption [15, 16, 96–98]. Putative glycosyl-/acetyltransferases (EpsL, EpsM) and components of the Type II secretion system protein (EpsE, EpsF) were found in the annotated metagenome [99]. Also genes coding for glucans were found, such as 1,4-alpha-glucan branching enzyme (GlgB) [100], the 1,4-beta-D-glucan glucohydrolase (GghA) [101], 4-alpha-glucanotransferase (MalQ) [102], alpha-1,4-glucan:maltose-1-phosphate maltosyltransferase (GlgE) [103, 104], beta-glucanase (BglA) [105, 106], glucan synthase (NdvB) [107] and the Endoglucanase (Egl) [108]. Osmoregulated periplasmic glucans are part of EPS and have been found to play a role in bacteria that respond to harsh conditions such as osmotic [109] and heavy-metal stresses [110, 111]. The role of EPS in photosynthetic environments rich in metal has been demonstrated [17, 112–114] as the negative charge assumed in high pH, i.e., during high photosynthetic regimes, might sequestrate toxic, positively charged metal ions.
Potential and limitations
The metagenomics analysis approach benefits of modern sequencing tools, that encompass the sequencing itself made easier by user-friendly hardware such as the MinION sequencer, together with the accompanying nucleic acid extraction kits, as well as software for data analysis such as MetaMaps that take advantage of long reads for optimizing information retrieval from public databases. A limitation of metagenomic studies is that they rely on the genomic DNA and therefore only allow to infer the genetic composition of the organisms living in a studied environment, without providing evidence for actual activity of the genes that are identified, for which further analysis would be required for the assessment of gene expression, such as metatranscriptomics. However, the validity of our metagenome-based approach is supported by our findings that revealed a multitude of genes conferring tolerance to the chemicals we detected by chemical profiling the environment, as they were likely retained through natural selection.
Conclusions
In this study we described an environment in the southern Swiss Alps which is atypical for the region from its peculiar chemical composition. We characterized this environment from a geochemical and microbiological perspective.
Geochemical analysis revealed a complex rock composition due to the merging of different geological units during rock formation of the surrounding area. This determines the unusual chemical composition of the outflowing water, characterized by high concentrations of heavy metals. Interestingly, microbiological analyses based on microscopy and metagenomics revealed an ecosystem adapted to these conditions. The ecosystems appeared as a green-turquoise biomat mainly composed of Proteobacteria, Bacteroidetes, and Cyanobacteria, along with other taxonomic groups. In particular, the substantial presence of Cyanobacteria in the biomat underlines its photosynthetic activity, where Cyanobacteria play a crucial role of primary producers and nitrogen fixators, thereby providing the underlying building blocks for the complex nutrient flow network involving the other microorganisms composing the biomat. The photosynthetic activity in such environments was suggested to improve tolerance to high heavy metal concentrations, by an increased pH that drives metal cations sequestration by EPS. Indeed, genes for synthesis of EPS were found in the assembled biomat metagenome, along with several other genes involved in diverse mechanisms of metal resistance. Altogether, this study allowed the first characterization of this unusual Swiss Alpine ecosystem from a geochemical and microbiological perspective. Several open questions remain, for example regarding the ecosystem’s seasonal dynamics, as well as potential applications to adapt such microbial community for bioremediation of anthropogenically contaminated environments rich in heavy metals.
Supporting information
(TXT)
(TXT)
(TXT)
(TXT)
(TXT)
(TXT)
Data Availability
All Supporting information files are available from the Figshare database (accession number(s) DOI:10.6084/m9.figshare.12482660, 10.6084/m9.figshare.11967684, 10.6084/m9.figshare.13547438, 10.6084/m9.figshare.13523960, 10.6084/m9.figshare.11871444, PRJNA689378).
Funding Statement
This study was funded through the canton of Ticino.
References
- 1.Joseph Seckbach and Aharon Oren. Microbial Mats: Modern and Ancient Microorganisms in Stratified Systems. 01 2010.
- 2.Dudeja Suman, Bhattacherjee Aranya, and J. Chela-Flores. Cellular origin, life in extreme habitats and astrobiology. 09 2012.
- 3. Hoehler Tori M., Bebout Brad M., and Des Marais David J. The role of microbial mats in the production of reduced gases on the early Earth. Nature, 412(6844):324–327, July 2001. 10.1038/35085554 [DOI] [PubMed] [Google Scholar]
- 4. Prieto-Barajas Cristina M., Eduardo Valencia-Cantero, and Santoyo Gustavo. Microbial mat ecosystems: Structure types, functional diversity, and biotechnological application. Electronic Journal of Biotechnology, 31:48—56, 2018. 10.1016/j.ejbt.2017.11.001 [DOI] [Google Scholar]
- 5. van Gemerden Hans. Microbial mats: A joint venture. Marine Geology, 113(1):3—25, 1993. Marine Sediments, Burial, Pore Water Chemistry, Microbiology and Diagenesis. 10.1016/0025-3227(93)90146-M [DOI] [Google Scholar]
- 6. Ley Ruth E., J. Kirk Harris, Wilcox Joshua, Spear John R., Miller Scott R., Bebout Brad M., et al. Unexpected diversity and complexity of the Guerrero Negro hypersaline microbial mat. Applied and Environmental Microbiology, 72(5):3685–3695, 2006. 10.1128/AEM.72.5.3685-3695.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bolhuis Henk, Cretoiu Mariana Silvia, and Stal Lucas J. Molecular ecology of microbial mats. FEMS Microbiology Ecology, 90(2):335–350, 11 2014. [DOI] [PubMed] [Google Scholar]
- 8. Paerl H. W. and Pinckney J. L. A mini-review of microbial consortia: Their roles in aquatic production and biogeochemical cycling. Microbial Ecology, 31(3), 1996. 10.1007/BF00171569 [DOI] [PubMed] [Google Scholar]
- 9. H. Hübel. Yehuda cohen and eugene rosenberg (editors), microbial mats: Physiological ecology of benthic microbial communities. Journal of Basic Microbiology, 30(8):560–560, 1990. [Google Scholar]
- 10. Woebken Dagmar, Burow Luke C, Behnam Faris, Mayali Xavier, Schintlmeister Arno, Fleming Erich D, et al. Revisiting N2 fixation in Guerrero Negro intertidal microbial mats with a functional single-cell approach. The ISME Journal, 9(2):485–496, October 2014. 10.1038/ismej.2014.144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. L. I. Falcón, Cerritos R., Eguiarte L. E., and V. Souza. Nitrogen fixation in microbial mat and stromatolite communities from Cuatro Cienegas, mexico. Microbial Ecology, 54(2):363–373, April 2007. 10.1007/s00248-007-9240-3 [DOI] [PubMed] [Google Scholar]
- 12. Kiran Bala and Kaushik Anubha. Chromium binding capacity of Lyngbya putealis exopolysaccharides. Biochemical Engineering Journal, 38(1):47—54, 2008. 10.1016/j.bej.2007.06.007 [DOI] [Google Scholar]
- 13. Baptista Mafalda S. and M. Teresa Vasconcelos. Cyanobacteria metal interactions: Requirements, toxicity, and ecological implications. Critical Reviews in Microbiology, 32(3):127–137, 2006. 10.1080/10408410600822934 [DOI] [PubMed] [Google Scholar]
- 14. Burnat Mireia, Diestra Elia, Esteve Isabel, and Antonio Solé. In Situ determination of the effects of lead and copper on cyanobacterial populations in microcosms. PloS one, 4:e6204, February 2009. 10.1371/journal.pone.0006204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. De Philippis Roberto, Sili Claudio, Paperi Raffaella, and Vincenzini Massimo. Exopolysaccharide-producing cyanobacteria and their possible exploitation: A review. Journal of Applied Phycology, 13(4):293–299, Aug 2001. 10.1023/A:1017590425924 [DOI] [Google Scholar]
- 16. De Philippis Roberto and M. Vincenzini. Outermost polysaccharidic investments of cyanobacteria: nature, significance and possible applications. 7:13–22, 01 2003. [Google Scholar]
- 17. Pereira Sara, Zille Andrea, Micheletti Ernesto, Pedro Moradas-Ferreira, De Philippis Roberto, and Tamagnini Paula. Complexity of cyanobacterial exopolysaccharides: composition, structures, inducing factors and putative genes involved in their biosynthesis and assembly. FEMS Microbiology Reviews, 33(5):917–941, 09 2009. 10.1111/j.1574-6976.2009.00183.x [DOI] [PubMed] [Google Scholar]
- 18. Inskeep William, Jay Zackary, Tringe Susannah, Herrgard Markus, and Rusch Douglas. The ynp metagenome project: Environmental parameters responsible for microbial distribution in the yellowstone geothermal ecosystem. Frontiers in Microbiology, 4:67, 2013. 10.3389/fmicb.2013.00067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Villanueva Laura, Navarrete Antoni, Urmeneta Jordi, White David C., and Guerrero Ricardo. Analysis of diurnal and vertical microbial diversity of a hypersaline microbial mat. Archives of Microbiology, 188(2):137–146, March 2007. 10.1007/s00203-007-0229-6 [DOI] [PubMed] [Google Scholar]
- 20. Ward David M., Ferris Michael J., Nold Stephen C., and Bateson Mary M. A natural view of microbial biodiversity within hot spring cyanobacterial mat communities. Microbiology and Molecular Biology Reviews, 62(4):1353–1370, 1998. 10.1128/MMBR.62.4.1353-1370.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Ruvindy Rendy, White Richard A., Neilan Brett A, and Burns Brendan Paul. Unravelling core microbial metabolisms in the hypersaline microbial mats of Shark Bay using high-throughput metagenomics. The ISME Journal, 10:183–196, 2016. 10.1038/ismej.2015.87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Olalla López-López, Cerdán María Esperanza, and González-Siso María Isabel. Hot spring metagenomics. Life, 3(2):308–320, 2013. 10.3390/life3020308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kozubal M. A., Romine M., Jennings R. d., Jay Z. J., Tringe S. G., Rusch D. B., et al. Geoarchaeota: a new candidate phylum in the Archaea from high-temperature acidic iron mats in Yellowstone National Park. ISME J, 7(3):622–634, Mar 2013. 10.1038/ismej.2012.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baumer Adrian, Egli Willy, Johann Dietrich Frey, Jung Walter, Riemann Astrid, Uhr Albert, et al. Blatt 1233 Greina. Geol Atlas Schweiz 1:25 000. 2013.
- 25.Johann Dietrich Frey. Geologie des Greinagebietes: Val Camadra—Valle Cavalasca—Val di Larciolo—Passo della Greina. PhD thesis, 1967.
- 26.Angelo Valsecchi and Sandro Oldrati. L’oro blu del Ticino. Armando Dadò editore, 2017.
- 27.Bundesamt für Gesundheit (2003): Schweizerisches Lebensmittelbuch, Kapitel 27A Trinkwasser, Bern, http://www.slmb.ch.
- 28. Cátia Carreira, Staal M., Middelboe Mathias, and Brussaard Corina. Autofluorescence imaging system to discriminate and quantify the distribution of benthic cyanobacteria and diatoms. Limnology and Oceanography: Methods, 13, 04 2015. [Google Scholar]
- 29. Dilthey Alexander T., Jain Chirag, Koren Sergey, and Phillippy Adam M. Strain-level metagenomic assignment and compositional estimation for long reads with MetaMaps. Nature Communications, 10(1), July 2019. 10.1038/s41467-019-10934-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ondov Brian D, Bergman Nicholas H, and Phillippy Adam M. Interactive metagenomic visualization in a web browser. BMC Bioinformatics, 12(1), September 2011. 10.1186/1471-2105-12-385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Alneberg Johannes, Bjarnason Brynjar Smári, de Bruijn Ino, Schirmer Melanie, Quick Joshua, Ijaz Umer Z, et al. Binning metagenomic contigs by coverage and composition. Nat Methods, 11, 1144–1146, 2011. 10.1038/nmeth.3103 [DOI] [PubMed] [Google Scholar]
- 32. DH Parks, M Imelfort, CT Skennerton, P Hugenholtz, Tyson GW. CheckM: assessing the quality of microbial genomes recovered from isolates, single cells, and metagenomes. Genome Res., 11, 25(7):1043–1055, 2015. 10.1101/gr.186072.114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rohstoffinformationssystem Schweiz (RIS), https://map.georessourcen.ethz.ch/purl/obid/795 (last access 14th May 2020).
- 34. Schmidt S. Th. and Amstutz G. C. Mineralogical investigations of strata-bound Pb-Zn-Ba occurrences in the triassic of the Central Alps (Switzerland). In Schneider Hans-J., editor, Mineral Deposits of the Alps and of the Alpine Epoch in Europe, pages 117–127, Berlin, Heidelberg, 1983. Springer Berlin Heidelberg. [Google Scholar]
- 35.Marc-Henri Derron. Géochimie des eaux de sources et interaction eau-roche dans les Alpes. PhD thesis, 2004.
- 36. Baker-Austin C, Dopson M, Wexler M, Sawers RG, Bond PL. Molecular insight into extreme copper resistance in the extremophilic archaeon’Ferroplasma acidarmanus’ Fer1. Microbiology (Reading), 151(Pt 8):2637–2646. August 2005 [DOI] [PubMed] [Google Scholar]
- 37. Bellenberg S, Buetti-Dinh A, Galli V, Ilie O, Herold M, Christel S, et al. Automated Microscopic Analysis of Metal Sulfide Colonization by Acidophilic Microorganisms. Appl Environ Microbiol.), 1;84(20):e01835–18. October 2018. 10.1128/AEM.01835-18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mattes Timothy E., Alexander Anne K., Richardson Paul M., Munk A. Christine, Han Cliff S., Stothard Paul, et al. The genome of Polaromonas sp. strain JS666: Insights into the evolution of a hydrocarbon- and xenobiotic-degrading bacterium, and features of relevance to biotechnology. Applied and Environmental Microbiology, 74(20):6405–6416, 2008. 10.1128/AEM.00197-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Coleman Nicholas V., Mattes Timothy E., Gossett James M., and Spain Jim C. Biodegradation of cis-dichloroethene as the sole carbon source by a β-proteobacterium. Applied and Environmental Microbiology, 68(6):2726–2730, 2002. 10.1128/AEM.68.6.2726-2730.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Larimer Frank, Chain Patrick, Hauser Loren, Lamerdin Jane, Malfatti Stephanie, Do Long, et al. Complete genome sequence of the metabolically versatile photosynthetic bacterium Rhodopseudomonas palustris. Nature biotechnology, 22:55–61, 02 2004. 10.1038/nbt923 [DOI] [PubMed] [Google Scholar]
- 41. Burganskaya Ekaterina, Bryantseva Irina, Krutkina Maria, Grouzdev Denis, and Gorlenko Vladimir. Bacterial communities of the microbial mats of Chokrak sulfide springs. Archives of Microbiology, 201, 03 2019. 10.1007/s00203-019-01648-6 [DOI] [PubMed] [Google Scholar]
- 42. Hania Wajdi Ben, Joseph Manon, Bunk Boyke, Cathrin Spröer, Klenk Hans-Peter, Fardeau Marie-Laure, et al. Characterization of the first cultured representative of a bacteroidetes clade specialized on the scavenging of cyanobacteria. Environmental Microbiology, 19(3):1134–1148, 2017. 10.1111/1462-2920.13639 [DOI] [PubMed] [Google Scholar]
- 43. Peeters Karolien, Verleyen Elie, Hodgson Dominic, Convey Peter, Ertz Damien, Vyverman Wim, et al. Heterotrophic bacterial diversity in aquatic microbial mat communities from Antarctica. Polar Biology, 35:543–554, 04 2011. 10.1007/s00300-011-1100-4 [DOI] [Google Scholar]
- 44. Liu Yuxia, Alessi Daniel, Owttrim George, Petrash Daniel, Mloszewska Aleksandra, Lalonde S., et al. Cell surface reactivity of Synechococcus sp. PCC 7002: Implications for metal sorption from seawater. Geochimica et Cosmochimica Acta, 169, 07 2015. 10.1016/j.gca.2015.07.033 [DOI] [Google Scholar]
- 45. Aboulmagd Elsayed, Oppermann-Sanio Fred Bernd, and Alexander Steinbüchel. Molecular characterization of the cyanophycin synthetase from Synechocystis sp. strain PCC6308. Archives of Microbiology, 174(5):297–306, November 2000. 10.1007/s002030000206 [DOI] [PubMed] [Google Scholar]
- 46.Malwade Chandrakant R., Maria C. Roda-Serrat, Christensen Knud V., Xavier Fretté, and Christensen Lars P. Kinetics of phycocyanobilin cleavage from c-phycocyanin by methanolysis. In Zdravko Kravanja and Miloš Bogataj, editors, 26th European Symposium on Computer Aided Process Engineering, volume 38 of Computer Aided Chemical Engineering, pages 61–66. Elsevier, 2016.
- 47. Mulkidjanian A. Y., Koonin E. V., Makarova K. S., Mekhedov S. L., Sorokin A., Wolf Y. I., et al. The cyanobacterial genome core and the origin of photosynthesis. Proceedings of the National Academy of Sciences, 103(35):13126–13131, August 2006. 10.1073/pnas.0605709103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Thiel Teresa and Pratte Brenda. Regulation of three nitrogenase gene clusters in the cyanobacterium Anabaena variabilis ATCC 29413. Life, 4(4):944–967, December 2014. 10.3390/life4040944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bennett L. T., Cannon F., and Dean D. R. Nucleotide sequence and mutagenesis of the nifA gene from Azotobacter vinelandii. Molecular Microbiology, 2(3):315–321, May 1988. 10.1111/j.1365-2958.1988.tb00034.x [DOI] [PubMed] [Google Scholar]
- 50. Woodley Paul, Buck Martin, and Kennedy Christina. Identification of sequences important for recognition of vnf genes by the VnfA transcriptional activator in Azotobacter vinelandii. FEMS Microbiology Letters, 135(2-3):213–221, January 1996. 10.1111/j.1574-6968.1996.tb07992.x [DOI] [PubMed] [Google Scholar]
- 51. Nienaber Andrea, Huber Alexander, Michael Göttfert, Hennecke Hauke, and Fischer Hans-Martin. Three new NifA-regulated genes in the Bradyrhizobium japonicum symbiotic gene region discovered by competitive DNA-RNA hybridization. Journal of Bacteriology, 182(6):1472–1480, March 2000. 10.1128/jb.182.6.1472-1480.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Prabha Ratna, Singh D. P., Gupta Shailendra, Gupta Vijai, Hesham El-Enshasy, and Verma Mukesh. Rhizosphere metagenomics of Paspalum scrobiculatum l. (kodo millet) reveals rhizobiome multifunctionalities. Microorganisms, 7:608, 11 2019. 10.3390/microorganisms7120608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Shigematsu Toru, Inoue Akio, Hidaka Makoto, Masaki Haruhiko, and Uozumi Takeshi. Oxygen sensitivity of NifA protein of Azospirillum lipoferum FS as suggested by gene cloning and expression in Escherichia coli. Bioscience, Biotechnology, and Biochemistry, 61(5):768–771, January 1997. 10.1271/bbb.61.768 [DOI] [PubMed] [Google Scholar]
- 54. Frise E, Green A, and Drummond M. Chimeric transcriptional activators generated in vivo from VnfA and AnfA of Azotobacter vinelandii: N-terminal domain of AnfA is responsible for dependence on nitrogenase Fe protein. Journal of Bacteriology, 176(21):6545–6549, 1994. 10.1128/jb.176.21.6545-6549.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huergo Luciano F., Chandra Govind, and Merrick Mike. PIIsignal transduction proteins: nitrogen regulation and beyond. FEMS Microbiology Reviews, 37(2):251–283, March 2013. 10.1111/j.1574-6976.2012.00351.x [DOI] [PubMed] [Google Scholar]
- 56. Fu Hseuh-Liang, Meng Yuling, Efrén Ordóñez, Villadangos Almudena F., Bhattacharjee Hiranmoy, José Gil A., et al. Properties of arsenite efflux permeases (Acr3) from Alkaliphilus metalliredigens and Corynebacterium glutamicum. Journal of Biological Chemistry, 284(30):19887–19895, June 2009. 10.1074/jbc.M109.011882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ibtissem Ben Fekih, Chengkang Zhang, Ping Li Yuan, Yi Zhao, Alwathnani Hend A., Quaiser Saquib, et al. Distribution of Arsenic Resistance Genes in Prokaryotes. Frontiers in Microbiology, 9:2473, 2018. 10.3389/fmicb.2018.02473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Cornelia Große, Grass Gregor, Anton Andreas, Franke Sylvia, Navarrete Alexander Santos, Lawley Blair, et al. Transcriptional organization of the czc heavy-metal homeostasis determinant from Alcaligenes eutrophus. Journal of Bacteriology, 181(8):2385–2393, 1999. 10.1128/JB.181.8.2385-2393.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Liu Pulin, Chen Xi, Huang Qiaoyun, and Chen Wenli. The role of CzcRS two-component systems in the heavy metal resistance of Pseudomonas putida X4. International Journal of Molecular Sciences, 16(8):17005–17017, July 2015. 10.3390/ijms160817005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Grass Gregor, Cornelia Große, and Nies Dietrich H. Regulation of the cnr cobalt and nickel resistance determinant from Ralstonia sp. strain CH34. Journal of Bacteriology, 182(5):1390–1398, 2000. 10.1128/jb.182.5.1390-1398.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nawapan S., Charoenlap N., Charoenwuttitam A., Saenkham P., Mongkolsuk S., and Vattanaviboon P. Functional and expression analyses of the cop operon, required for copper resistance in Agrobacterium tumefaciens. Journal of Bacteriology, 191(16):5159–5168, June 2009. 10.1128/JB.00384-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhang Yan, Rodionov Dmitry A, Gelfand Mikhail S, and Gladyshev Vadim N. Comparative genomic analyses of nickel, cobalt and vitamin B12 utilization. BMC Genomics, 10(1):78, 2009. 10.1186/1471-2164-10-78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Pi Hualiang and Helmann John D. Ferrous iron efflux systems in bacteria. Metallomics, 9(7):840–851, 2017. 10.1039/c7mt00112f [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Lau Cheryl K. Y., Krewulak Karla D., and Vogel Hans J. Bacterial ferrous iron transport: the Feo system. FEMS Microbiology Reviews, 40(2):273–298, 12 2015. 10.1093/femsre/fuv049 [DOI] [PubMed] [Google Scholar]
- 65. Angerer Annemarie and Braun V. Iron regulates transcription of the Escherichia coli ferric citrate transport genes directly and through the transcription initiation proteins. Archives of Microbiology, 169(6):483–490, May 1998. 10.1007/s002030050600 [DOI] [PubMed] [Google Scholar]
- 66. Gebhard S., Tran S. L., and Cook G. M. The Phn system of Mycobacterium smegmatis: a second high-affinity ABC-transporter for phosphate. Microbiology, 152(11):3453–3465, November 2006. 10.1099/mic.0.29201-0 [DOI] [PubMed] [Google Scholar]
- 67. Sirko A, Zatyka M, Sadowy E, and Hulanicka D. Sulfate and thiosulfate transport in Escherichia coli K-12: evidence for a functional overlapping of sulfate- and thiosulfate-binding proteins. Journal of bacteriology, 177(14):4134–4136, 1995. 10.1128/jb.177.14.4134-4136.1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Reeve Wayne G., Tiwari Ravi P., Kale Neetin B., Dilworth Michael J., and Glenn Andrew R. ActP controls copper homeostasis in Rhizobium leguminosarum bv. viciae and Sinorhizobium meliloti preventing low pH-induced copper toxicity. Molecular Microbiology, 43(4):981–991, February 2002. 10.1046/j.1365-2958.2002.02791.x [DOI] [PubMed] [Google Scholar]
- 69. Gupta Amit, Matsui Kazuaki, Lo Jeng-Fan, and Silver Simon. Molecular basis for resistance to silver cations in Salmonella. Nature Medicine, 5(2):183–188, February 1999. 10.1038/5545 [DOI] [PubMed] [Google Scholar]
- 70. Sütterlin S., Dahlö M., Tellgren-Roth C., Schaal W., and Å. Melhus. High frequency of silver resistance genes in invasive isolates of Enterobacter and Klebsiella species. Journal of Hospital Infection, 96(3):256–261, July 2017. 10.1016/j.jhin.2017.04.017 [DOI] [PubMed] [Google Scholar]
- 71. Cécile Oger, Mahillon Jacques, and Petit Fabienne. Distribution and diversity of a cadmium resistance (cada) determinant and occurrence of IS257 insertion sequences in Staphylococcal bacteria isolated from a contaminated estuary (Seine, France). FEMS microbiology ecology, 43:173–83, 04 2003. 10.1111/j.1574-6941.2003.tb01056.x [DOI] [PubMed] [Google Scholar]
- 72. Tsai K J, P K Yoon, and Lynn A R. ATP-dependent cadmium transport by the cadA cadmium resistance determinant in everted membrane vesicles of Bacillus subtilis. Journal of Bacteriology, 174(1):116–121, 1992. 10.1128/jb.174.1.116-121.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Thelwell C., Robinson N. J., and Turner-Cavet J. S. An SmtB-like repressor from Synechocystis PCC 6803 regulates a zinc exporter. Proceedings of the National Academy of Sciences, 95(18):10728–10733, September 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Banci Lucia, Bertini Ivano, Simone Ciofi-Baffoni, Poggi Luisa, Vanarotti Murugendra, Tottey Stephen, et al. NMR structural analysis of the soluble domain of ZiaA-ATPase and the basis of selective interactions with copper metallochaperone Atx1. JBIC Journal of Biological Inorganic Chemistry, 15(1):87–98, July 2009. 10.1007/s00775-009-0568-7 [DOI] [PubMed] [Google Scholar]
- 75. Berkelman T, Garret-Engele P, and Hoffman N E. The PacL gene of Synechococcus sp. strain PCC 7942 encodes a Ca2+-transporting ATPase. Journal of Bacteriology, 176(14):4430–4436, 1994. 10.1128/jb.176.14.4430-4436.1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Raeymaekers L, Wuytack E.Y, Willems I, Michiels C.W, and Wuytack F. Expression of a P-type Ca2+-transport ATPase in Bacillus subtilis during sporulation. Cell Calcium, 32(2):93–103, August 2002. 10.1016/S0143-4160(02)00125-2 [DOI] [PubMed] [Google Scholar]
- 77. Smith Ronald L. and Maguire Michael E. Microbial magnesium transport: unusual transporters searching for identity. Molecular Microbiology, 28(2):217–226, April 1998. 10.1046/j.1365-2958.1998.00810.x [DOI] [PubMed] [Google Scholar]
- 78. Michael Gaßel, Siebers Annette, Epstein Wolfgang, and Altendorf Karlheinz. Assembly of the Kdp complex, the multi-subunit K+-transport ATPase of Escherichia coli. Biochimica et Biophysica Acta (BBA)—Biomembranes, 1415(1):77–84, December 1998. 10.1016/S0005-2736(98)00179-5 [DOI] [PubMed] [Google Scholar]
- 79. Grass Gregor, Otto Markus, Fricke Beate, Haney Christopher J., Rensing Christopher, Nies Dietrich H., et al. FieF (YiiP) from Escherichia coli mediates decreased cellular accumulation of iron and relieves iron stress. Arch Microbiol., 183(1):9–18, January 2005. 10.1007/s00203-004-0739-4 [DOI] [PubMed] [Google Scholar]
- 80. Ardin Arifah Chieko, Fujita Kazuyo, Nagayama Kayoko, Takashima Yukiko, Nomura Ryota, Nakano Kazuhiko, et al. Identification and functional analysis of an ammonium transporter in Streptococcus mutans. PLoS ONE, 9(9):e107569, September 2014. 10.1371/journal.pone.0107569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Detsch Christian and Jörg Stülke. Ammonium utilization in Bacillus subtilis: transport and regulatory functions of NrgA and NrgB. Microbiology, 149(11):3289–3297, November 2003. 10.1099/mic.0.26512-0 [DOI] [PubMed] [Google Scholar]
- 82. Chakravarty Shubham, Melton Cameron N., Bailin Adam, Yahr Timothy L., and Anderson Gregory G. Pseudomonas aeruginosa magnesium transporter MgtE inhibits type III secretion system gene expression by stimulating rsmYZ transcription. Journal of Bacteriology, 199(23), August 2017. 10.1128/JB.00268-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Bartsevich Victor V. and Pakrasi Himadri B. Membrane topology of MntB, the transmembrane protein component of an ABC transporter system for manganese in the cyanobacterium Synechocystis sp. strain pcc 6803. Journal of Bacteriology, 181(11):3591–3593, 1999. 10.1128/JB.181.11.3591-3593.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Frías J E, Flores E, and Herrero A. Nitrate assimilation gene cluster from the heterocyst-forming cyanobacterium Anabaena sp. strain PCC 7120. Journal of bacteriology, 179(2):477–486, 1997. 10.1128/jb.179.2.477-486.1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Hall J. A. and Pajor A. M. Functional characterization of a Na+-coupled dicarboxylate carrier protein from Staphylococcus aureus. Journal of Bacteriology, 187(15):5189–5194, July 2005. 10.1128/JB.187.15.5189-5194.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Choi Seung-Hwan, Lee Kang-Lok, Shin Jung-Ho, Cho Yoo-Bok, Cha Sun-Shin, and Roe Jung-Hye. Zinc-dependent regulation of zinc import and export genes by Zur. Nature Communications, 8(1), June 2017. 10.1038/ncomms15812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Kehres D. G., Lawyer C. H., and Maguire M. E. The CorA magnesium transporter gene family. Microbial & Comparative Genomics, 3(3):151–169, January 1998. 10.1089/omi.1.1998.3.151 [DOI] [PubMed] [Google Scholar]
- 88. Lunin Vladimir V., Dobrovetsky Elena, Khutoreskaya Galina, Zhang Rongguang, Joachimiak Andrzej, Doyle Declan A., et al. Crystal structure of the CorA Mg2+ transporter. Nature, 440(7085):833–837, April 2006. 10.1038/nature04642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Hudek L., Premachandra D. W., Webster A. J., and Bräu L. Role of phosphate transport system component PstB1 in phosphate internalization by Nostoc punctiforme. Applied and Environmental Microbiology, 82(21):6344–6356, 2016. 10.1128/AEM.01336-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Anton A., Grosse C., Reissmann J., Pribyl T., and Nies D. H. CzcD is a heavy metal ion transporter involved in regulation of heavy metal resistance in Ralstonia sp. strain CH34. J. Bacteriol., 181(22):6876–6881, November 1999. 10.1128/JB.181.22.6876-6881.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Nies D. H. CzcR and CzcD, gene products affecting regulation of resistance to cobalt, zinc, and cadmium (czc system) in Alcaligenes eutrophus. J. Bacteriol., 174(24):8102–8110, Dec 1992. 10.1128/jb.174.24.8102-8110.1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Tsunekawa K., Shijuku T., Hayashimoto M., Kojima Y., Onai K., Morishita M., et al. Identification and characterization of the Na+/H+ antiporter NhaS3 from the thylakoid membrane of Synechocystis sp. PCC 6803. J. Biol. Chem., 284(24):16513–16521, June 2009. 10.1074/jbc.M109.001875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Resch C. T., Winogrodzki J. L., Patterson C. T., Lind E. J., Quinn M. J., Dibrov P., et al. The putative Na+/H+ antiporter of Vibrio cholerae, Vc-NhaP2, mediates the specific K+/H+ exchange in vivo. Biochemistry, 49(11):2520–2528, March 2010. 10.1021/bi902173y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Tiwari Balkrishna, Singh Savita, Kaushik Manish, and Mishra Arun. Regulation of organophosphate metabolism in cyanobacteria—a review. Microbiology, 84:291–302, 04 2015. 10.1134/S002626171503020026263689 [DOI] [Google Scholar]
- 95. Yamada M., Makino K., Amemura M., Shinagawa H., and Nakata A. Regulation of the phosphate regulon of Escherichia coli: analysis of mutant phoB and phoR genes causing different phenotypes. J. Bacteriol., 171(10):5601–5606, October 1989. 10.1128/jb.171.10.5601-5606.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Sheng Guo-Ping, Yu Han-Qing, and Li Xiao-Yan. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: A review. Biotechnology Advances, 28(6):882—894, 2010. 10.1016/j.biotechadv.2010.08.001 [DOI] [PubMed] [Google Scholar]
- 97. De Philippis Roberto, Colica Giovanni, and Micheletti Ernesto. Exopolysaccharide-producing cyanobacteria in heavy metal removal from water: molecular basis and practical applicability of the biosorption process. Applied Microbiology and Biotechnology, 92(4):697–708, October 2011. 10.1007/s00253-011-3601-z [DOI] [PubMed] [Google Scholar]
- 98. Gupta Pratima and Diwan Batul. Bacterial exopolysaccharide mediated heavy metal removal: A review on biosynthesis, mechanism and remediation strategies. Biotechnology Reports, 13:58—71, 2017. 10.1016/j.btre.2016.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Marvasi Massimiliano, Visscher Pieter T., and Martinez Lilliam Casillas. Exopolymeric substances (EPS) from Bacillus subtilis: polymers and genes encoding their synthesis. FEMS Microbiology Letters, 313(1):1–9, November 2010. 10.1111/j.1574-6968.2010.02085.x [DOI] [PubMed] [Google Scholar]
- 100. Garg S. K., Alam M. S., Kishan K. V., and Agrawal P. Expression and characterization of alpha-(1,4)-glucan branching enzyme Rv1326c of Mycobacterium tuberculosis H37Rv. Protein Expr. Purif., 51(2):198–208, February 2007. 10.1016/j.pep.2006.08.005 [DOI] [PubMed] [Google Scholar]
- 101. Yernool D. A., McCarthy J. K., Eveleigh D. E., and Bok J. D. Cloning and characterization of the glucooligosaccharide catabolic pathway beta-glucan glucohydrolase and cellobiose phosphorylase in the marine hyperthermophile Thermotoga neapolitana. J. Bacteriol., 182(18):5172–5179, September 2000. 10.1128/jb.182.18.5172-5179.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Pugsley A. P. and Dubreuil C. Molecular characterization of malQ, the structural gene for the Escherichia coli enzyme amylomaltase. Mol. Microbiol., 2(4):473–479, July 1988. 10.1111/j.1365-2958.1988.tb00053.x [DOI] [PubMed] [Google Scholar]
- 103. Leiba J., Syson K., Baronian G., Zanella-Cléon I., Kalscheuer R., Kremer L., et al. Mycobacterium tuberculosis maltosyltransferase GlgE, a genetically validated antituberculosis target, is negatively regulated by Ser/Thr phosphorylation. J. Biol. Chem., 288(23):16546–16556, June 2013. 10.1074/jbc.M112.398503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Syson K., Stevenson C. E., Rejzek M., Fairhurst S. A., Nair A., Bruton C. J., et al. Structure of Streptomyces maltosyltransferase GlgE, a homologue of a genetically validated anti-tuberculosis target. J. Biol. Chem., 286(44):38298–38310, November 2011. 10.1074/jbc.M111.279315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105. Iwashita Kazuhiro, Nagahara Tatsuya, Kimura Hitoshi, Takano Makoto, Shimoi Hitoshi, and Ito Kiyoshi. The BglA gene of Aspergillus kawachii encodes both extracellular and cell wall-bound β-glucosidases. Applied and Environmental Microbiology, 65(12):5546–5553, 1999. 10.1128/AEM.65.12.5546-5553.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Kosugi A., Arai T., and Doi R. H. Degradation of cellulosome-produced cello-oligosaccharides by an extracellular non-cellulosomal beta-glucan glucohydrolase, BglA, from Clostridium cellulovorans. Biochem. Biophys. Res. Commun., 349(1):20–23, October 2006. 10.1016/j.bbrc.2006.07.038 [DOI] [PubMed] [Google Scholar]
- 107. Hall C. W., Hinz A. J., Gagnon L. B., Zhang L., Nadeau J. P., Copeland S., et al. Pseudomonas aeruginosa Biofilm Antibiotic Resistance Gene ndvB Expression Requires the RpoS Stationary-Phase Sigma Factor. Appl. Environ. Microbiol., 84(7), 04 2018. 10.1128/AEM.02762-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Huang J. Z., Sukordhaman M., and Schell M. A. Excretion of the egl gene product of Pseudomonas solanacearum. J. Bacteriol., 171(7):3767–3774, July 1989. 10.1128/jb.171.7.3767-3774.1989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Bohin Jean-Pierre. Osmoregulated periplasmic glucans in Proteobacteria. FEMS Microbiology Letters, 186(1):11–19, 05 2000. 10.1111/j.1574-6968.2000.tb09075.x [DOI] [PubMed] [Google Scholar]
- 110. Toner Brandy, Manceau Alain, Marcus Matthew A., Millet Dylan B., and Sposito Garrison. Zinc sorption by a bacterial biofilm. Environmental Science and Technology, 39(21):8288–8294, 11 2005. 10.1021/es050528+ [DOI] [PubMed] [Google Scholar]
- 111. Guiné V., Spadini L., Sarret G., Muris M., Delolme C., Gaudet J.-P., et al. Zinc sorption to three gram-negative bacteria: combined titration, modeling, and EXAFS study. Environmental Science & Technology, 40(6):1806–1813, 2006. 10.1021/es050981l [DOI] [PubMed] [Google Scholar]
- 112. De Philippis Roberto, Margheri Maria Cristina, Materassi Riccardo, and Vincenzini Massimo. Potential of unicellular cyanobacteria from saline environments as exopolysaccharide producers. Applied and Environmental Microbiology, 64(3):1130–1132, 1998. 10.1128/AEM.64.3.1130-1132.1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Li Pengfu, Harding Stephen E., and Liu Zhili. Cyanobacterial exopolysaccharides: Their nature and potential biotechnological applications. Biotechnology and Genetic Engineering Reviews, 18(1):375–404, 2001. 10.1080/02648725.2001.10648020 [DOI] [PubMed] [Google Scholar]
- 114. Mota Rita, Pereira Sara B., Meazzini Marianna, Fernandes Rui, Santos Arlete, Evans Caroline A., et al. Effects of heavy metals on Cyanothece sp. CCY 0110 growth, extracellular polymeric substances (EPS) production, ultrastructure and protein profiles. Journal of Proteomics, 120:75—94, 2015. 10.1016/j.jprot.2015.03.004 [DOI] [PubMed] [Google Scholar]