Abstract
Objective
To describe clinical features, diagnostic findings, treatments, and outcomes in patients with new-onset postural orthostatic tachycardia syndrome (POTS) and other autonomic disorders following SARS-CoV-2 infection (COVID-19).
Methods
We retrospectively reviewed medical records for patients who presented with persistent neurologic and cardiovascular complaints between April and December 2020 following COVID-19 infection.
Results
Twenty patients (70% female) were included in this study.Fifteen had POTS, 3 had neurocardiogenic syncope, and 2 had orthostatic hypotension. Six patients had abnormalities on cardiac or pulmonary testing, and 4 had elevated autoimmune or inflammatory markers. All patients were treated with non-pharmacologic therapies, and most required pharmacologic therapies. Six to 8 months after COVID-19, 17 (85%) patients had residual autonomic symptoms, with 12 (60%) unable to return to work.
Conclusions
POTS can follow COVID-19 in previously healthy patients. Appropriate diagnostic investigations and therapies are necessary to identify and treat autonomic dysfunction after COVID-19.
Keywords: COVID-19, Neurologic complications, Postural orthostatic tachycardia syndrome, Neurocardiogenic syncope, Orthostatic hypotension
Introduction
Coronavirus disease 2019 (COVID-19), caused by SARS-CoV-2 virus, is associated with various neurologic, including autonomic, manifestations in both hospitalized and non-hospitalized patients [1, 2]. Isolated case reports and a case series of 6 patients presenting with autonomic nervous system dysfunction after COVID-19 have been reported [2–5].
Postural orthostatic tachycardia syndrome (POTS), one of the most common autonomic disorders, has a wide range of clinical manifestations, such as postural tachycardia, dizziness, orthostatic intolerance, presyncope, and exercise intolerance. POTS commonly occurs after viral or bacterial infections, such as Epstein-Barr virus, influenza, and Borrelia burgdorferi infection [6, 7]. Due to increasing reports of post-COVID-19 POTS, we aimed to investigate patients with new-onset autonomic disorders following COVID-19 infection. In this case series, we report the clinical features, diagnostic findings, treatment, and outcomes of 20 patients with new-onset autonomic dysfunction after COVID-19 infection.
Methods
We performed a retrospective chart review of patients who presented to Dysautonomia Clinic, an outpatient referral clinic, with persistent neurologic and cardiovascular complaints after acute COVID-19 infection, and who had evidence of orthostatic intolerance (OI) on a tilt table test (TTT) or a 10-min stand test between April 2020 and December 2020 following either presumed or laboratory-confirmed COVID-19 infection. Patients were either self-referred or referred to Dysautonomia Clinic by their healthcare provider for an evaluation and treatment of a suspected autonomic disorder following COVID-19. Patients were diagnosed with POTS if they had a heart rate increase of 30 beats per minute (bpm) or more, or over 120 bpm within 10 min of standing, in the absence of orthostatic hypotension (OH) [8]; orthostatic hypotension (OH) if they had a decrease in systolic blood pressure of 20 mm Hg or a decrease in diastolic blood pressure of 10 mm Hg within 3 min of standing or a TTT [9]; or neurocardiogenic syncope (NCS) if they experienced loss of consciousness with abrupt blood pressure and heart rate drop during standing or tilt table test [9]. Out of 28 charts that were reviewed for this study, 3 patients with persistent complaints after COVID-19 were excluded due to having no evidence of OI, and 5 patients were excluded due to a personal history of autonomic disorders, such POTS, NCS, or OH prior to developing COVID-19, which yielded 20 patients who were included in this study. All patients were evaluated and followed by one author (SB).
Standard protocol approvals, registrations, and patient consents
The study was approved by the Institutional Review Board at State University of New York at Buffalo Jacobs School of Medicine and Biomedical Sciences.
Results
A total of 20 patients, (70% female), median age 40 (age range 25–65) years, were included in this study. Six patients had pre-existing minor autonomic symptoms, such as occasional dizziness, syncope, or palpitations, and 4 had a remote history of concussion. Prior to COVID-19, none had chronic OI, and all patients were fully functional and employed.
Patients' clinical characteristics are presented in Table 1, and a summary of important findings is outlined in Table 2.
Table 1.
Clinical features, diagnostic findings, treatment, and outcomes of patients with POTS and other autonomic disorders after COVID-19
| Patient | Age | Sex | SARS-CoV-2 test (PCR/IgG) | Acute COVID-19 presentation | Post-COVID-19 most disabling symptoms | Autonomic disorder/test | Treatment | Outcomes | Additional information |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 25 | F | − | None | Tachycardia, fatigue, SOB, hypersomnolence | POTS/TTT | NPH, propranolol | Symptomatic 6 months later, unable to work from home | Air trapping on pulmonary function test |
| 2 | 27 | M | + (IgG) | Respiratory syndrome | Episodic tachycardia, panic attacks, exercise intolerance, anosmia, ageusia | POTS/ST | NPH, citalopram, propranolol | Symptomatic 8 months later, unable to work | History of concussion with LOC |
| 3 | 30 | M | + (PCR) | Respiratory syndrome | Postural tachycardia, fatigue, anosmia, ageusia | POTS/ST | NPH | Resolved after 2 months, returned to full-time work | Sister with POTS |
| 4 | 30 | F | − | GI symptoms | Tachycardia, fatigue, headache, anosmia, ageusia | NCS/TTT | NPH, steroids, diphenhydramine | Resolved after 8 months, returned to full-time work | Abnormal EMG with minor neuropathic changes |
| 5 | 36 | F | + (PCR) | Fever, headache | Postural tachycardia, fatigue, exercise intolerance, anosmia, ageusia | POTS/ST | NPH, midodrine | Symptoms improved somewhat after 4 months, unable to work | |
| 6 | 36 | M | + (PCR) | Respiratory, syndrome, GI symptoms | Tachycardia, fatigue, SOB, chest pain | POTS/TTT as part of AFT | NPH, intravenous saline, iron infusions | 50% recovered 8 months later, returned to work part-time from home | + GAD antibody, + SARS CoV-2-positive staining in gastric, duodenal and ileal biopsy, mild atrial and ventricular enlargement on cardiac MRI |
| 7 | 37 | F | NC | GI symptoms, headache, neuropathic pain | Tachycardia, SOB, neuropathic pain | NCS/TTT | NPH, anticoagulants | 65% recovered after 2 months, unable to work | + cardiolipin and + beta 2 glycoprotein antibodies |
| 8 | 37 | M | − | Respiratory syndrome, GI symptoms, pneumonia | Tachycardia, fatigue, SOB, high blood pressure, anosmia, ageusia | POTS/ST | NPH, atenolol, steroids | Symptomatic after 4 months, works part-time from home | High ESR 79, history of post-concussion syndrome |
| 9 | 38 | M | − | Respiratory syndrome | Postural tachycardia, SOB, chest tightness, anosmia, ageusia | POTS/ST | NPH, fludrocortisone | 50% recovered after 8 months, unable to work | |
| 10 | 38 | F | − | Respiratory syndrome | Postural tachycardia, headache, orthostatic intolerance | POTS/ST | NPH, fludrocortisone, ivabradine | Symptoms improved, able to work full-time from home only with accommodations | History of + ANA, post-viral syndrome as a teen, mild orthostatic dizziness |
| 11 | 43 | F | − | Fever, arthralgia | Postural tachycardia, fatigue, SOB, recurrent fevers, anosmia, ageusia | POTS/ST | NPH, steroids | Symptomatic 8 months later, unable to work | History of Hashimoto’s, ADHD, migraine |
| 12 | 44 | F | NC | Respiratory syndrome | Dizziness, presyncope, low blood pressure | NCS/TTT | NPH, fludrocortisone | Symptoms improved 50% after 8 months, unable to work | History of presyncope |
| 13 | 50 | F | − | Respiratory syndrome | Tachycardia, SOB, exercise intolerance | POTS/ST | NPH, antihistamines | Residual symptoms, works from home full-time | Previously very healthy and athletic, but post-COVID-19 with low VO2 max at 74 on exercise stress test |
| 14 | 50 | F |
+ (PCR) |
Respiratory syndrome | Fatigue, dizziness, headache | POTS/ST | NPH, cyproheptadine | Symptomatic after 6 months, unable to work | History of SVT and mild concussion, taking atenolol for many years |
| 15 | 51 | F | − | Respiratory syndrome | Presyncope, weight loss, low blood pressure, anosmia, ageusia | OH/ST | NPH, antihistamines, gabapentin | 85% recovered after 3 months, unable to work | History of NCS since teenage years, concussion without LOC |
| 16 | 54 | M | + (PCR) | Respiratory, syndrome, pneumonia | Postural tachycardia, fatigue, SOB, diarrhea, weight loss | POTS/ST | NPH, atenolol, antihistamines | Symptomatic 3 months later, unable to work | High d-dimer |
| 17 | 55 | F | NC | Respiratory syndrome | Postural tachycardia, fatigue, SOB, anosmia, ageusia | POTS/ST | NPH, midodrine | 50% recovered after 8 months, unable to work | Small pericardial effusion-resolved, negative cardiac MRI, remote history of seizures and migraine |
| 18 | 58 | F | − | Respiratory syndrome | Tachycardia, fatigue, presyncope | POTS/ST | NPH, antihistamines | 65% recovered after 6 months, returned to work part-time from home | History of allergies |
| 19 | 59 | F | − | Respiratory syndrome, GI symptoms | Tachycardia, bradycardia, dizziness, oxygen desaturation | POTS/ST | NPH | Resolved after 3 months, returned to full-time work | Night time oxygen desaturation episodes to 80 s |
| 20 | 65 | F | − | Respiratory syndrome | Fatigue, SOB, dizziness, chest pain, anosmia, ageusia | OH/ST | NPH, aspirin | Symptomatic 8 months later, unable to work |
Stroke-like episodes, labile blood pressure, small pericardial effusion-resolved |
ADHD, attention-deficit hyperactivity disorder; AFT, autonomic function tests; ANA, antinuclear antibody; EMG, electromyography; ESR, erythrocyte sedimentation rate; GAD, glutamic acid decarboxylase; GI, gastrointestinal; IgG, immunoglobulin G; LOC, loss of consciousness; MRI, magnetic resonance imaging; NC, not completed; NCS, neurocardiogenic syncope; NPH, non-pharmacologic treatment (increased fluids and salt intake, compression stockings, exercise); PCR, polymerase chain reaction; POTS, postural orthostatic tachycardia syndrome; SARS, severe acute respiratory syndrome; SOB, shortness of breath; ST, 10-minute stand test; SVT, supraventricular tachycardia
Table 2.
Summary of important findings
| Number of patients (%) | |
|---|---|
| Sex | |
| Female | 14 (70) |
| Male | 6 (30) |
| SARS-CoV-2 PCR or IgG test | |
| Positive | 6 (30) |
| Negative or not completed | 14 (70) |
| Acute COVID-19 presentation | |
| Respiratory syndrome | 15 (75) |
| GI symptoms | 5 (25) |
| Anosmia and ageusia | 10 (50) |
| Pneumonia | 2 (10) |
| Hospitalizations | 0 (0) |
| Post-COVID-19 autonomic disorder | |
| POTS | 15 (75) |
| NCS | 3 (15) |
| OH | 2 (10) |
| Outcomes | |
| Recovered (100%) | 3 (15) |
| Residual symptoms | 17 (85) |
| Able to work part-time to full-time | 8 (40) |
| Unable to work | 12 (60) |
| Clinical features | |
| Abnormal cardiac or pulmonary tests | 6 (30) |
| Elevated markers of autoimmunity/inflammation | 4 (20) |
| History of minor autonomic symptoms before COVID-19 | 6 (30) |
| Remote history of concussion | 4 (20) |
GI, gastrointestinal; IgG, immunoglobulin G; NCS, neurocardiogenic syncope; OH, orthostatic hypotension; PCR, polymerase chain reaction; POTS, postural orthostatic tachycardia syndrome
Acute COVID-19 presentation
Six patients had COVID-19 confirmed by positive SARS-CoV-2 polymerase chain reaction (PCR) or antibody (IgG) test (Table 1). A majority of patients had either a negative test or could not be tested in a timely manner due to the limited testing capabilities in March–April of 2020, but those with a negative test were presumed to have COVID-19 by their primary care physician based on clinical features, timing of onset, and prevalence of COVID-19 in their area. Additionally, due to the limited availability and access to testing, a number of patients who tested negative with SARS-CoV-2 PCR had a significant delay in the timing of their test in relationship to the onset of COVID-19 symptoms, which resulted in falsely negative test result. During COVID-19 infection, 15 patients experienced a typical acute respiratory syndrome, 10 patients experienced anosmia and ageusia, 2 patients were diagnosed with pneumonia, but none was hospitalized for COVID-19. The youngest patient in the series, a previously healthy 25-year-old woman, had no acute viral illness, but developed sudden onset of shortness of breath, exercise intolerance, postural tachycardia, hypersomnolence, and severe fatigue in March of 2020 that, in conjunction with abnormal pulmonary function tests, were presumed to follow an asymptomatic COVID-19 infection, given a high prevalence of COVID-19 in her area and her living in an apartment building where other infected individuals resided.
Post-COVID-19 presentation
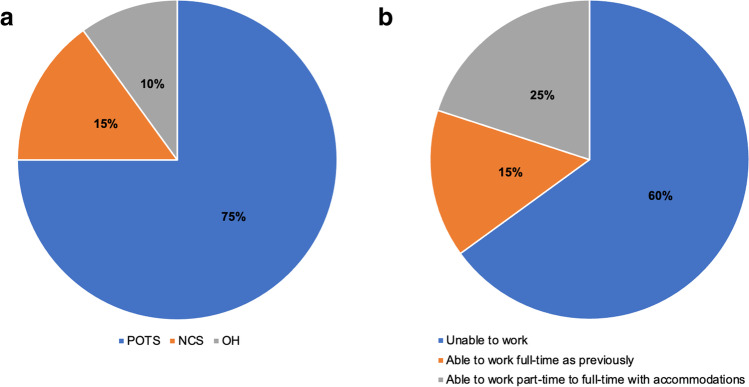
After resolution of COVID-19 infection, most patients experienced fatigue, postural tachycardia, OI, dizziness, and exercise intolerance that were chronic and disabling. Six had mild abnormalities on cardiac or pulmonary testing as described in Table 1, and 4 had elevated markers of autoimmunity and/or inflammation; however, not all patients were tested with thorough diagnostic studies based on autonomic testing protocols due to limited access to clinical facilities during lockdowns. Based on a 10-min stand tests or TTTs where available, 15 patients were diagnosed with POTS, 3 with NCS, and 2 with OH (Fig. 1a). All patients were advised to utilize non-pharmacologic therapy for autonomic dysfunction, which consisted of increased sodium chloride and fluids intake, waist-high compression stockings and abdominal binders, and sitting or supine exercise. Sixteen (80%) patients required pharmacotherapy for the autonomic dysfunction, which included beta blockers, fludrocortisone, midodrine, ivabradine, and other medications used for treatment of comorbid conditions, such as headache, neuropathic pain, or allergic symptoms associated with mast cell activation disorder.
Fig. 1.
a Autonomic disorders and b Patient outcomes 6–8 months after COVID-19. POTS, postural orthostatic tachycardia syndrome; NCS, neurocardiogenic syncope; OH, orthostatic hypotension
Patient outcomes
Most patients (85%) self-reported residual symptoms 6–8 months after COVID-19, although many felt that they had improved with treatment. Only 3 patients returned to work full time with near or complete resolution of symptoms, and an additional 5 patients were able to work full time from home with some accommodations within 8 months after COVID-19 (Fig. 1b).
Discussion
In this largest case series to date, we found that POTS and other common autonomic disorders can follow COVID-19 in previously healthy non-hospitalized patients who experience significant disability 6–8 months after an acute infection, and these patients require appropriate diagnostic and therapeutic interventions to improve their symptoms and functional status.
POTS is a disorder of the autonomic nervous system characterized by a rise in heart rate of at least 30 bpm from supine to standing position in the absence of OH, and in conjunction with symptoms of presyncope and OI; POTS is diagnosed by a TTT or a 10-min stand test [6, 8]. In this case series, a majority of patients were diagnosed via a 10-min stand test performed either at a doctor’s office or via self-administered stand test observed by the author (SB) as part of the tele-neurology exam. All patients were treated with non-pharmacologic therapies, and most required pharmacologic treatment for the autonomic dysfunction and comorbid conditions. Six to 8 months after COVID-19, 85% of patients had residual autonomic symptoms, with 60% unable to return to work.
POTS can be triggered by infection, surgery, pregnancy, or concussion, with the post-infectious being the most common mode of onset [6]. Isolated cases and one case series consisting of 6 patients with POTS and other autonomic disorders that followed COVID-19 infection have been reported [2–5]. Similar to our case series, treatment outcomes of these reported patients have been variable. Nevertheless, in our case series, many patients experienced improvement with treatment of POTS, which included beta blockers, fludrocortisone, midodrine, ivabradine, and other medications used for treatment of comorbid conditions, such as headache, neuropathic pain, or allergic symptoms associated with mast cell activation disorder.
In this case series, almost a third of the patients had a history of occasional autonomic symptoms, such as dizziness, syncope, or palpitations, and 20% had a remote history of concussion. While it is difficult to draw any conclusions from a case series, it is possible that a pre-existing history of minor autonomic symptoms or concussion, a known trigger of the autonomic dysfunction, might be risk factors for post-COVID-19 autonomic disorders.
Of interest is that nearly a third of the patients in this case series had confirmed mild abnormalities on cardiac or pulmonary testing, and 20% had abnormal markers of autoimmunity or inflammation, which suggests that patients with persistent cardiovascular and neurologic symptoms after COVID-19 may have an underlying autoimmune and/or inflammatory process that affects cardiopulmonary, neurologic, and immunologic systems. This is in agreement with the consideration that autoimmunity is one of the major mechanisms in the pathophysiology of POTS. Previously, we have demonstrated that patients with POTS had a higher prevalence of the autoimmune markers, such as anti-nuclear antibodies and anti-phospholipid antibodies, and comorbid autoimmune disorders, including Hashimoto's thyroiditis, rheumatoid arthritis, and celiac disease, than the general population [10]. More specifically to the autonomic nervous system, ganglionic N-type and P/Q type acetylcholine receptor antibodies, alpha 1, beta 1 and beta 2 adrenergic antibodies, muscarinic M2 and M4 antibodies, angiotensin II type 1 receptor antibodies, and opioid-like 1 receptor antibodies have been identified in patients with POTS [11–14]. Although the etiology of post-COVID-19 autonomic disorders is largely unknown, it is possible that the SARS-CoV-2-generated antibodies cross-react with components of the autonomic ganglia, autonomic nerve fibers, G-protein-coupled receptors, or other neuronal or cardiovascular receptors, which can lead to dysfunction of the autonomic nervous system. Further studies are needed to determine whether post-COVID-19 autonomic disorders are rooted in autoimmunity and what type of antibodies or cytokines may be mediating the autoimmune and/or inflammatory process.
Strengths and limitations
To the best of our knowledge, this is the largest case series to date of patients presenting with POTS and other autonomic disorders following COVID-19. Due to the pandemic, access to medical facilities was limited, and therefore a TTT, other autonomic and cardiopulmonary function tests, and serum autoimmune studies were not performed in all 20 patients. Similarly, there was limited access to SARS-CoV-2 PCR tests between March and April of 2020, which resulted in a substantial number of patients having no laboratory confirmation of the clinically diagnosed COVID-19. Due to the retrospective nature of our case series, standardized patient-reported outcome measures were not collected. Prospective studies with complete diagnostic investigation in a large cohort of patients are needed to delineate the pathophysiology, etiology, and the best treatment approaches in patients with post-COVID-19 autonomic disorders.
Conclusion
New-onset POTS and other autonomic disorders can follow COVID-19 in previously healthy non-hospitalized patients who experience persistent neurologic and cardiovascular symptoms after resolution of acute infection. Physicians should be aware that POTS and other autonomic disorders may be a complication of COVID-19 and should consider appropriate diagnostic and therapeutic interventions in these patients.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Declarations
Conflict of interest
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Change history
4/13/2021
A Correction to this paper has been published: 10.1007/s12026-021-09191-7
References
- 1.Verstrepen K, Baisier L, De Cauwer H. Neurological manifestations of COVID-19 SARS and MERS. Acta Neurol Belg. 2020;120:1051–1060. doi: 10.1007/s13760-020-01412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dani M, Dirksen A, Taraborrelli P, et al. Autonomic dysfunction in ‘long covid’: rationale, physiology and management strategies. Clin Med (Lond) 2021;21:e63–e67. doi: 10.7861/clinmed.2020-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Novak P. Post COVID-19 syndrome associated with orthostatic cerebral hyperfusion syndrome, small fiber neuropathy and benefit of immunotherapy: a case report. eNeurologicalSci. 2020;21:100276. doi: 10.1016/j.ensci.2020.100276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miglis MG, Prieto T, Shaik R, Muppidi S, Sinn D, Jaradeh S. A case report of postural orthostatic tachycardia syndrome after COVID-19. Clin Auton Res. 2020;30:449–451. doi: 10.1007/s10286-020-00727-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kanjwal K, Jamal S, Kichloo A, Grubb BP. New-onset postural orthostatic tachycardia syndrome following coronavirus disease 2019 infection. J Innov Card Rhythm Manag. 2020;11:4302–4304. doi: 10.19102/icrm.2020.111102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thieben MJ, Sandroni P, Sletten DM, et al. Postural orthostatic tachycardia syndrome: the Mayo Clinic experience. Mayo Clin Proc. 2007;82:308–313. doi: 10.4065/82.3.308. [DOI] [PubMed] [Google Scholar]
- 7.Fedorowski A. Postural orthostatic tachycardia syndrome: clinical presentation, aetiology and management. J Intern Med. 2019;285:352–366. doi: 10.1111/joim.12852. [DOI] [PubMed] [Google Scholar]
- 8.Raj SR, Guzman JC, Harvey P, et al. Canadian Cardiovascular Society position statement on postural orthostatic tachycardia syndrome (POTS) and related disorders of chronic orthostatic intolerance. Can J Cardiol. 2020;36:357–372. doi: 10.1016/j.cjca.2019.12.024. [DOI] [PubMed] [Google Scholar]
- 9.Freeman R, Weiling W, Axelrod F, et al. Consensus statement on the definition of orthostatic hypotension, neurally mediated syncope and the postural tachycardia syndrome. Clin Auton Res. 2011;21:69–72. doi: 10.1007/s10286-011-0119-5. [DOI] [PubMed] [Google Scholar]
- 10.Blitshteyn S. Autoimmune markers and autoimmune disorders in patients with postural tachycardia syndrome (POTS) Lupus. 2015;24:1364–1369. doi: 10.1177/0961203315587566. [DOI] [PubMed] [Google Scholar]
- 11.Watari M, Nakane S, Mukaino A, et al. Autoimmune postural orthostatic tachycardia syndrome. Ann Clin Transl Neurol. 2018;5:486–492. doi: 10.1002/acn3.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li H, Yu X, Liles C, et al. Autoimmune basis for postural tachycardia syndrome. J Am Heart Assoc. 2014;3:e000755. doi: 10.1161/JAHA.113.000755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gunning WT, Kvale H, Kramer PM, Karabin BL, Grubb BP. Postural orthostatic tachycardia syndrome is associated with elevated G-protein coupled receptor antibodies. J Am Heart Assoc. 2019;8:e013602. doi: 10.1161/JAHA.119.013602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu X, Li H, Murphy TA, et al. Angiotensin II type 1 receptor autoantibodies in postural tachycardia syndrome. J Am Heart Assoc. 2018;7:e00835. doi: 10.1161/JAHA.117.008351. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.