Abstract
The stability of plasma-sprayed hydroxyapatite (HA) coatings on metallic implants in vivo remains a significant challenge for load-bearing orthopedic implants despite their excellent mechanical and osteoconductive properties. This study focuses on oxide layer formation on the surface of Ti6Al4V samples through furnace heating at 600, 700, and 800 °C for 10 min for optimization of the most effective oxide layer to increase plasma coating crystallinity and improve plasma coating bond strength with the metal surface. The 800 °C heat treatment shows an effective oxide layer which increases coating crystallinity from 64 to 75% and coating adhesive bond strength from 25.9 ± 2.3 to 30.7 ± 1.1 MPa, while simultaneously reducing the dissolution rate of HA coatings. The addition of biologically relevant dopants, MgO and SiO2, show negligible effects on crystallinity and adhesive bond strength on plasma-sprayed HA coatings and additionally show an enhancement effect on osteoblast proliferation and differentiation. Moreover, the inclusion of these additivess shows an increase in osteogenesis in a rat distal femur model after 6 and 10 weeks of implantation. Overall, this study provides a direct solution to improve the crystallinity, adhesive bond strength, and osteogenic properties of plasma-sprayed HA coatings on orthopedic implants that is more manufacturable and translational from research to an industrial scale.
Keywords: plasma-sprayed hydroxyapatite coating, thermal oxidation, coating crystallinity, adhesive bond strength, osteogenic properties
Graphical Abstract

1. INTRODUCTION
Nearly 4 million total joint arthroplasties are expected to be performed by 2030 in the United States.1 Of these surgeries, aseptic loosening is still one of the most common failure modes.2 Metal implants, such as Ti6Al4V, are widely used for arthroplasties by virtue of their excellent biocompatibility in vivo.3,4 Although Ti6Al4V is somewhat osteoconductive, a property that facilitates early-stage bone ingrowth and superior implant integration, cementless implants for joint replacement depend on stringent initial mechanical stability for bone ingrowth/apposition to occur.5,6 Initial implant integration and avoidance of late aseptic loosening are even more challenging in the revision (redo) scenario, in which the bone bed is often sclerotic and dysvascular. To address this limitation, hydroxyapatite (HA), a bioceramic coating material for load-bearing implants, has been used to enhance osteoconductivity. In addition, because of its compositional similarity to bone mineral, HA coatings can promote bonding between host tissues and implant surface, therefore increasing osseointegration and reducing implant fixation time.7,8
Plasma-spray is considered the most advantageous coating technique in the industry because of its high deposition rates and cost efficiency. However, coatings deposited by this method usually combine both impurity and amorphous phases due to the high-temperature processing and rapid cooling rate. These amorphous phase can increase the dissolution rate of the coating, resulting in reduced stability in vivo.9 To mitigate the phase changes of the bioceramic implant surface, thermal oxidation can be employed. The addition of an oxide layer creates a thermal barrier between the substrate and plasma coating, which can reduce rapid cooling during the high-temperature plasma coating process. Thermal oxidation has also been used to decrease the wear rate, increase corrosion resistance, and improve the bioactivity of titanium implants.10–12 To the best of the authors’ knowledge, this is the first study reporting the oxidation of Ti6Al4V to improve the crystallinity of plasma-sprayed HA coatings.
In addition, biologically relevant metal oxide additives incorporated within the HA coating have also been shown to strengthen implant osseointegration. In this study, MgO and SiO2 additives, which have been previously studied, were utilized to enhance the biological properties of the plasma-sprayed HA coatings. In vitro studies have shown the presence of MgO in calcium phosphate materials improved the osteoblast attachment and proliferation, as well as alkaline phosphatase (ALP) expression.13,14 MgO was also reported to improve the osteogenesis of HA in vivo using a rabbit femoral defect model.15 In vivo studies demonstrated that the presence of SiO2 enhanced the osteogenesis of calcium phosphate materials by showing improved bone growth.16–19 Furthermore, SiO2 can enhance the vascular network formation and reduce osteoclastogenesis in vitro.20–22
The objective of this study is to understand the effects of thermal oxidation of Ti6Al4V toward improving the crystallinity of plasma-sprayed HA coatings. We hypothesize that the presence of TiO2 would increase the crystallinity of plasma-sprayed HA coating and, therefore, decrease the dissolution in vitro by providing a thermal barrier during the high-temperature plasma coating process. In this study, a layer of TiO2 is formed on Ti6Al4V via thermal oxidation at 600, 700, and 800 °C. Then, an induction radio-frequency (RF) plasma system is used to prepare HA and MgO/SiO2-HA coatings on thermally oxidized Ti6Al4V. X-ray diffraction (XRD), adhesive bond strength tests, and field emission scanning electron microscopy (FESEM) results are presented for characterization of the oxide layer and plasma-sprayed HA coatings. A dissolution study, performed in a simulated body fluid (SBF), is used to evaluate the effects of the oxide layer and MgO/SiO2 on the dissolution kinetics of plasma-sprayed HA coatings. An osteoblast cell line along with 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) colorimetric and alkaline phosphatase (ALP) assays are used to evaluate the effects of MgO/SiO2 on the osteogenic properties of plasma-sprayed HA coatings in vitro. Finally, results from an in vivo rat distal femur model is presented to showcase the osteogenic potential of MgO/SiO2 additives within plasma HA coatings.
2. MATERIALS AND METHODS
2.1. Thermal Oxidation of Ti6Al4V and Coating Preparation.
Ti6Al4V plates were attained from President Titanium (MA). Substrates with a diameter of 25.4 and 12.2 mm were prepared by cutting Ti6Al4V plates using a water jet cutter for adhesive bond strength tests and other characterizations, respectively. Samples were sandblasted and washed with deionized (DI) water and subsequently with acetone under ultrasonication. The thermal oxidation was conducted by heating substrates in a muffle furnace to 600, 700, and 800 °C with a heating rate of 10 °C/min and a dwelling time of 10 min. After heat treatment, samples were furnace cooled to room temperature prior to the plasma coating process. A commercial grade HA powder (150–212 μm, Monsanto) was used. MgO/SiO2-HA powder was achieved by mixing 0.5 wt % SiO2 and 1 wt % MgO with HA using 1:1 powder to milling medium ratio for 2 h at 70 rpm. An induction RF plasma-spray system (Tekna Plasma Systems, Sherbrooke, Canada) was used to spray HA coatings with a supersonic nozzle and an axial powder feeding system. Table 1 lists the detailed plasma running parameters used in this study. Plasma-sprayed HA coatings were prepared on Ti6Al4V (HA/Ti6Al4V), Ti6Al4V with oxidation at 600 °C (HA/600TiO2/Ti6Al4V), Ti6Al4V with oxidation at 700 °C (HA/700TiO2/Ti6Al4V), and Ti6Al4V with oxidation at 800 °C (HA/800TiO2/Ti6Al4V). Post oxide layer optimization, 800 °C processing was deemed the most effective to then study the presence of additives into the plasma coating. Plasma-sprayed MgO/SiO2-HA coating was prepared on Ti6Al4V with oxidation at 800 °C (MgO/SiO2-HA/800TiO2/Ti6Al4V) to see the effects of MgO and SiO2 on physical, mechanical, and biological properties compared to HA/800TiO2/Ti6Al4V. Plasma coatings were approximately 80–100 μm thick.
Table 1.
Plasma Running Parameters
| central gas flow rate (s.l.p.m.) | 25 Ar |
| sheath gas flow rate (s.l.p.m.) | 60 Ar + 6 H2 |
| carrier gas flow rate (s.l.p.m.) | 10 Ar |
| power (kW) | 25 |
| Working coordinate (mm) | 100 |
2.2. Phase Identification.
An X-ray diffractometer (Siemens D5000, Aubrey, TX) using Cu Kα radiation at 30 mA and 40 kV was used to identify the phase formation of the oxide layers, plasma-sprayed HA coatings, and plasma-sprayed MgO/SiO2-HA coatings. Samples were scanned by an X-ray with a step size of 0.05° and a dwelling of 0.5 s. The quantitative crystallinity of the HA was calculated based on the X-ray diffraction plots using the equation reported in previous studies.23–25 Briefly, the crystallinity of HA was estimated based on the intensity of crystalline HA divided by the intensity of the total HA phase including crystalline HA and amorphous HA between 25 and 37°. The intensity of crystalline HA was calculated through multiplying the area of 100% HA (211) peak by 3.23. The intensity of the total HA phase was calculated as the area under X-ray diffraction plots between 25 and 37°. The calculation was conducted by MDI JADE software.
2.3. Adhesive Bond Strength and Coating Microstructure.
The plasma-sprayed HA coating adhesive bond strength was tested for HA/Ti6Al4V, HA/700TiO2/Ti6Al4V, HA/800TiO2/Ti6Al4V, and MgO/SiO2-HA/800TiO2/Ti6Al4V. The coating thickness of all samples used specifically in the adhesive bond strength testing was around 300 μm. The testing procedures were performed based on ASTM C633. Samples were mounted to two stainless steel posts using Armstrong-12 epoxy resin adhesive. Tests were performed using an Instron tensile apparatus with a constant crosshead speed of 13 μm/s until failure. The failure force divided by the coating surface area was employed to calculate the adhesive bond strength. Four samples were tested for each composition to ensure reproducibility. Just epoxy was tested using identical procedures to ensure that strength exceeded 50 MPa, well over the HA coating bond strengths. Surface topography FESEM images were also captured for Ti6Al4V and 800TiO2/Ti6Al4V. For cross-sectional microstructural analysis, samples were first mounted with polymer resin, then cut by a low-speed diamond saw at 300 rpm to expose the interfaces. Cross-sections were polished and etched using a solution of nitric acid, hydrofluoric acid, and DI water in a ratio of 2:1:25 followed by the observation under FESEM.
2.4. Ca2+ Release.
An SBF solution, with pH 7.4, was used for the dissolution study of plasma-coated samples. The solution was prepared by mixing K2HPO4, KCl, Na2SO4·10H2O, NaHCO3, NaCl, CaCl2·2H2O, and MgCl2·6H2O into DI water and buffered at pH 7.4 with HCl and tris-hydroxymethyl aminomethane at room temperature. Coated samples were first rinsed with DI water to remove residual loose coatings. Then, Ti6Al4V control, HA/Ti6Al4V, MgO/SiO2-HA/Ti6Al4V, HA/800TiO2/Ti6Al4V, and MgO/SiO2-HA/800TiO2/Ti6Al4V were immersed in 5 mL of SBF at 37 °C under 150 rpm constant shaking for 12 weeks. For each sample collection time point, the SBF solution was first taken out and stored in a glass vial followed by refilling with 5 mL of fresh SBF. The collected solution was diluted 10 times with 2% HCl and 5% HNO3. Inductively coupled plasma-mass spectrometry (ICP-MS) (Agilent 7700, Agilent Technologies, Inc. CA) was used to measure the Ca2+ release at each time point. The temperature of the spray chamber was maintained at 2 °C. Argon gas flow conditions were 0.3 L/min make-up gas, 0.8 L/min nebulization gas, 1 L/min auxiliary gas, and 15 L/min plasma gas. Each measurement was deducted by Ti6Al4V control and Ca2+ in the SBF to get the accumulative Ca2+ release. The accumulative Ca2+ release was plotted against time with error bars (n = 3, p < 0.05). FESEM images were taken before and after the release.
2.5. Osteoblast Culture.
Ti6Al4V control, HA/800TiO2/Ti6Al4V, and MgO/SiO2-HA/800TiO2/Ti6Al4V samples were sterilized by an autoclave prior to the culture. Primary human osteoblasts (PromoCell, Heidelberg, Germany) were cultured for in vitro characterizations. Samples were of a 12 mm diameter and cells were seeded at a density of 1 × 104 cells/sample. The cell medium used in this study was prepared by mixing the osteoblast growth medium (PromoCell, Heidelberg, Germany) and osteoblast growth medium supplement mix (PromoCell, Heidelberg, Germany). Samples were cultured in an incubator with 37 °C and 5 vol % CO2, and cell medium was changed every other day until the end of the culture.
2.6. Cellular Proliferation.
A 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl tetrazolium bromide (MTT) assay (Sigma, MO) was used to evaluate the osteoblast proliferation. The MTT assay solution was prepared by adding 50 mg of MTT powder into 10 mL of phosphate-buffered saline (PBS) followed by sterilization using 0.45 μm filters. About 900 μL of cell media and 100 μL of MTT solution were added separately to each sample. Then samples were incubated at 37 °C for 2 h. After incubation, the MTT solution was removed and 600 μL of a solubilizing solution containing isopropanol, 0.1 N HCl, and 10% Triton X-100 was added on each sample. After pipet-mixing, the solution in each well was transferred into a 96-well plate and read by a Synergy 2 microplate reader (Biotek, VT) at 570 nm. Three duplicates were tested for each composition to ensure reproducibility.
2.7. Cellular Differentiation.
An ALP assay kit was purchased from SensoLyte (Fremont, CA) to characterize the osteoblast differentiation. First, cell media was aspirated, and samples were homogenized in the assay buffer provided by the manufacturer. Then, the reaction was initiated by adding 5 mM p-nitrophenyl phosphate solution followed by incubation in a dark environment at 25 °C for 60 min. Finally, the reaction was terminated by adding the stop solution provided by the manufacturer and ALP intensity was measured at 405 nm using a Synergy 2 microplate reader. Three samples were tested for each composition to ensure the reproducibility.
2.8. Rat Distal Femur Model.
An in vivo rat distal femur model was studied using implants of Ti6Al4V control, HA/Ti6Al4V, and MgO/SiO2-HA/Ti6Al4V to assess the osteogenic potential of adding the MgO/SiO2 additives. Surgical procedures were followed in accordance to the Institutional Animal Care and Use Committee (IACUC) at the Washington State University. In brief, six male Sprague Dawley rats (Simonsen Laboratories, Gilroy, CA) were implanted producing duplicates of each composition, with half euthanized at 6 weeks and the other half euthanized at 10 weeks. The average body mass was 300 g. Buprenorphine was employed for pain management followed by isoflurane (Abbott Laboratories, North Chicago, IL) anesthesia coupled with oxygen before surgery. Surgery sites were shaved, cleaned, and disinfected using chlorohexidine and 70% ethanol. Following incision, critically sized circular bone defects were formed using a drill with gradually increasing diameter bit sizes up to 3 mm diameter. Implants were tightly placed through both cortices with lengths of 5 mm. Absorbable synthetic sutures were used to close surgical sites followed by a 5% povidone iodine cleaning. Postoperative procedures included meloxicam administration via subcutaneous injection for 3 days and physical monitoring. Euthanasia was induced with an overdose of CO2 followed by bilateral pneumothorax.
2.9. Histological Analysis.
Implants were harvested, fixed in 10% buffered formalin for 3 days, followed by a series of dehydration, and finally embedded in Spurr’s resin. Sections were cut using a low-speed diamond saw, mounted onto glass slides, and polished prior to staining. A modified Masson–Goldner trichrome staining was utilized to assess osteoid (red/orange) and mineralized bone formation (blue/green). Histology images were obtained using a light microscope.
2.10. Statistical Analysis.
A one-way ANOVA statistical analysis was used and p < 0.05 was considered statistically significant.
3. RESULTS AND DISCUSSION
Plasma-spray HA coatings on metal implants to facilitate osseointegration can be used for younger patients with active lifestyles because of the higher osteoconductive capabilities that HA provides on top of the superior mechanical properties of a metal. These composite implants could also be advantageous in revision surgeries, in which the implant is placed in a biologically compromised bone bed. Although HA coatings are beneficial for osseointegration by promoting a reduction in healing time for patients, poor stability of HA coatings within physiological environments is still a concern, which needs to be addressed. Coatings that degrade too quickly are susceptible to limited or poor osseointegration with the surrounding tissue. This study aims to address this challenge by utilizing a titanium oxide layer in between the HA coating and metal implant surface. The authors acknowledge that an inherent oxide layer may be generated during plasma-spray processing due to the sample preheating prior to coating. However, the results of this work show that the extent of the oxide layer formation, which can especially be affected by temperature, has a positive impact on the crystallinity of the HA coating. The reason for the enhancement of crystallinity could be from lower cooling rates during plasma-spray processing due to the thermal barrier the oxide layer provides. The control of temperature, and subsequently the degree of oxidation, within the chamber prior to plasma coating is limited or impossible; therefore, heat treatment of the samples prior to plasma spray is vital when using this system. This enables controlled fabrication of the oxide layer with uniformity and predictability in the process, allowing for a more feasible translation from research into the industry.
3.1. Phase Analysis and Characterization of Samples.
Phase purity and crystallinity are two major challenges in plasma-sprayed HA coatings that can affect dissolution kinetics. The oxide layer utilized in this study aids in both these challenges. The oxide layer has a thickness of less than 10 μm, measured by a gauge micrometer caliper with a tolerance of 2.5 μm. The dwell reaction time to form the oxide layer at temperature is short at only 10 min. Oxidation kinetics is highly dependent on the surrounding environment including pressure, temperature, and time. Other studies have noted that longer reaction times will increase the oxide scale.26,27 Variation in color due to differing oxidation temperatures is observed, as shown in Figure 1. The intensity of the brown color increases as the temperature of oxidation increases. The X-ray diffraction plots of Ti6Al4V and oxide layers are also shown in Figure 1. Ti6Al4V substrates contain a large amount of α-Ti (JCPDS file #44–1294) and a small amount of β-Ti (JCPDS file #44–1288), as expected. After oxidation at 600 °C, only a small amount of the rutile phase is formed. On increasing the temperature to 700 °C, the rutile phase increases and becomes the second major phase. On further increasing the temperature to 800 °C, the rutile phase starts to dominate.
Figure 1.

X-ray diffraction plots of Ti6Al4V and oxide layers at 600, 700, and 800 °C, with each respective sample image, show an increase in the amount of rutile phase when the temperature of oxidation increases. Variation in color at different temperatures is also observed.
HA can decompose into other calcium phosphate materials, such as tricalcium phosphate and tetracalcium phosphate, under high temperatures. All forms of calcium phosphate materials aside from HA have comparatively higher dissolution rates and can reduce the lifespan of HA-coated orthopedic implants.9 The decomposition of HA is common in the plasma-sprayed coating process since the temperature near the plasma arc can be as high as 10 000 °C, while the decomposition temperature of HA is only 1670 °C.28 In this study, the HA powder is distributed by a supersonic nozzle and discharged at a speed of 510 m/s in the lower region of the plasma arc. The exposure time of HA particles to the plasma flame is reduced by utilizing the supersonic nozzle, and the temperature is relatively lower than the core of the plasma zone. With this shorter duration in high-temperature processing, HA shows little to no phase decomposition. The X-ray diffraction plots of plasma-sprayed MgO/SiO2-HA and HA, as shown in Figure 2, indicate little HA decomposition in all samples, which is in line with our previous studies.9,19
Figure 2.
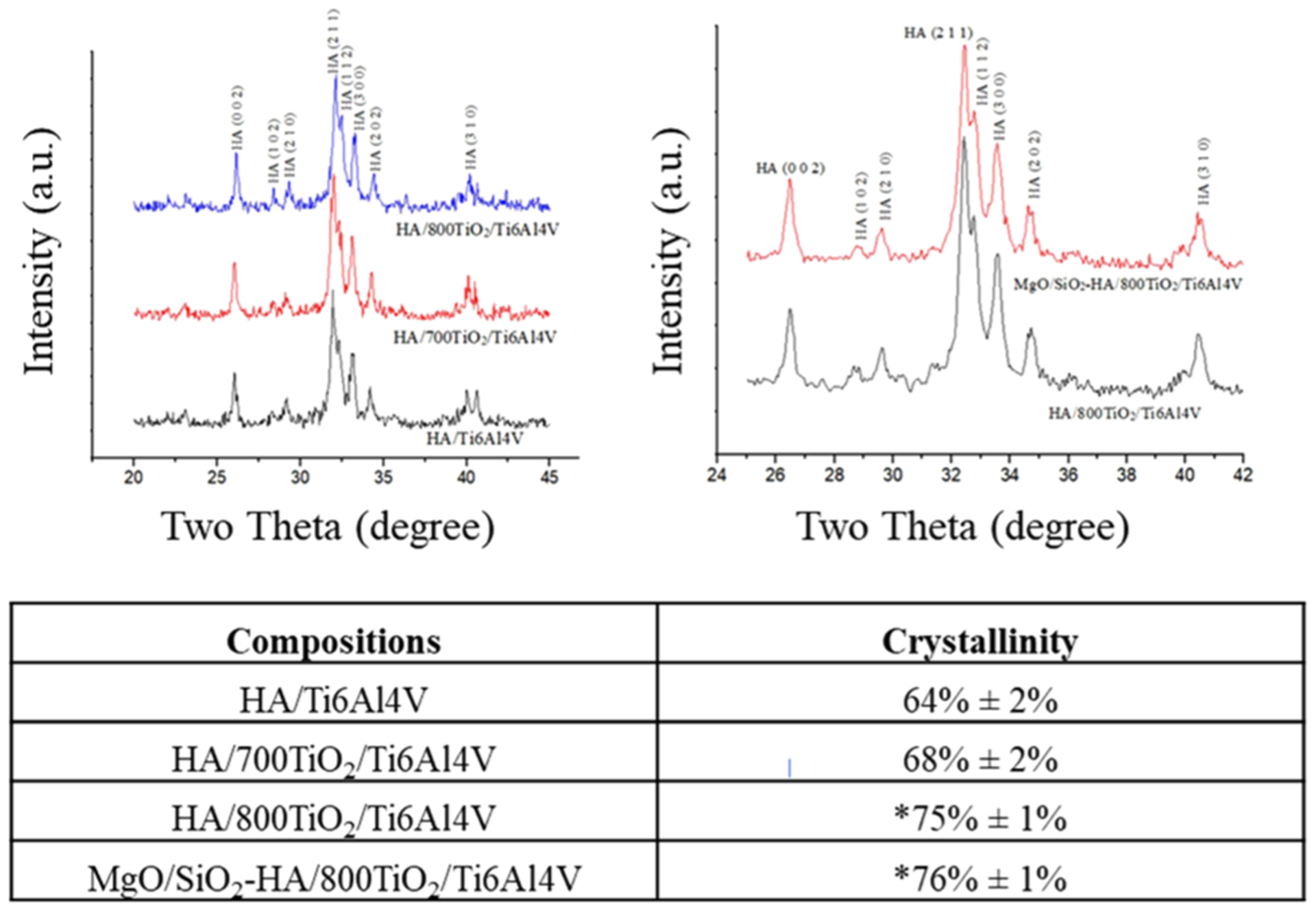
X-ray diffraction plots of plasma-sprayed HA and MgO/SiO2-HA coatings on Ti6Al4V, as well as calculations of crystallinity of these coatings showcasing the improvements in crystallinity for coatings deposited on oxide layers. Significance is indicated by * (n = 3, p < 0.05).
In addition to phase purity, another vital factor that can alter coating stability is crystallinity. A highly crystalline HA coating can aid in decreased dissolution kinetics compared to an amorphous HA coating. One study reported that a highly crystalline HA coating enabled a decreased Ca2+ release when immersed in a buffer solution.25 The crystallinity of plasma-sprayed HA coating is generally controlled by the crystallization of the molten HA during plasma-spray processing; however, metallic substrates, such as Ti6Al4V, have high thermal conductivity leading to a large amount of amorphous HA formation. There are three major titanium dioxide phases: rutile, anatase, and brookite. Anatase and brookite can transform irreversibly to rutile when heated at temperatures between 600 and 800 °C, which indicates that the rutile phase should be the most stable titanium dioxide phase under high temperatures.11,12,29 Temperatures of 600, 700, and 800 °C are used in this study to create an oxide layer. A much larger amount of the rutile phase is formed at 700 and 800 °C compared to 600 °C, as shown in Figure 2, which led to the decision to only perform further testing with higher temperature oxidation samples. The crystallinity of HA coating increased from 64 to 68 and 75% when deposited on oxide layers at 700 and 800 °C, respectively. Compared to 700 °C, 800 °C results in higher rutile phase formation on the oxide layer producing higher crystallinity of the plasma-sprayed HA coating. It is important to note that rather than the thickness of the oxide layer being the major contributor, this increase in the rutile phase caused by the higher temperature oxidation processing is the driving factor for the enhanced crystallinity seen in the plasma-spray HA coating. MgO and SiO2 have negligible effects on the crystallinity of plasma-sprayed HA coating. Both are substituted into the HA lattice structure without altering the original structure, evident by the lack of peak shift in the XRD plots, as shown in Figure 2. All coatings correspond well with standard HA peaks (JCPDS #09–0432), however, plasma-sprayed HA coatings deposited on the oxide layer are more crystalline compared to without an oxide layer barrier. In addition, the crystallinity of HA coating increases with the increase of thermal oxidation temperature.
The adhesive bond strength is also crucial to clinical applications and in vivo lifetime of HA-coated implants. The clinical minimum requirement of the adhesive bond strength for HA-coated implants is 15 MPa.9,19,30 In this study, the adhesive bond strength for all samples is higher than the requirement, indicating adequate mechanical stability for load-bearing applications. Control HA/Ti6Al4V coating adhesive bond strength is 25.9 ± 2.3 MPa. After oxidation at 700 °C, the adhesive bond strength decreases to 16.5 ± 3.3 MPa. On increasing the temperature of oxidation to 800 °C, the adhesive bond strength increases to 30.7 ± 1.0 MPa, equating to a nearly 20% increase. The adhesive bond strength for MgO/SiO2-HA/800TiO2/Ti6Al4V is 29.5 ± 2.3 MPa, statistically insignificant compared to both control and undoped. HA/800TiO2/Ti6Al4V and MgO/SiO2-HA/800TiO2/Ti6Al4V coatings are strongly bonded to the metal as observed by the gap-free interfaces, as shown in Figure 3b. During the HA deposition on bare Ti6Al4V, the partially molten HA particles cool down quickly upon impingement on the Ti6Al4V substrates. Knowing this, it can be inferred that by the time new molten HA is deposited, the prior layer has solidified. In the presence of the oxide layer, prior layers are likely to retain a molten state comparatively to an unoxidized sample, therefore allowing enhanced surface interaction between ceramic layers. This strategy can result in higher bonding energy than HA directly deposited on the Ti6Al4V surface, which increases its adhesive bond strength. In addition, the needle-like structures that form in the oxide layer, as seen in Figure 4, showcase a topographical change from the Ti6Al4V sandblasted surface. This surface modification indicates a higher surface area for interfacial bonding with the HA coating. Moreover, the addition of MgO and SiO2 has negligible effects on the adhesive bond strength of plasma-sprayed HA coating since the plasma coating forms more of a physical or mechanical bond, rather than a chemical bond.9
Figure 3.

Coating adhesive bond strength results show statistically improved strength with thermal oxidation at 800 °C compared to control. Interface microstructures of HA/800TiO2/Ti6Al4V (a) and MgO/SiO2-HA/800TiO2/Ti6Al4V (b) show strong gap-free bonding interfaces.
Figure 4.

Surface topography FESEM images of Ti6Al4V and 800TiO2/Ti6Al4V. Images show the oxide layer created microstructure formations resembling needle-like structures.
3.2. Ca2+ Release.
To support our finding of increased crystallinity of HA coatings on thermally oxidized Ti6Al4V, a dissolution study was conducted with samples submerged in SBF. Coated samples normally experience two separate release kinetics: (a) calcium dissolution from coatings to SBF or (b) calcium precipitation from SBF onto coatings.31,32 The accumulative state of calcium is dependent on the coating composition and crystallinity. MgO/SiO2-HA/Ti6Al4V shows overall dissolution during 12 weeks in SBF, however, HA/Ti6Al4V, HA/800TiO2/Ti6Al4V, and MgO/SiO2-HA/800TiO2/Ti6Al4V show initial dissolution in the first 12 days and continuous precipitation till 12 weeks in SBF, as shown in Figure 5a. The presence of MgO/SiO2 increases the Ca2+ release, while the addition of a thermal oxide layer decreases the Ca2+ release. FESEM images depict these findings as well, showcasing the Ca2+ precipitation on all samples except MgO/SiO2-HA/Ti6Al4V and higher precipitation on samples consisting of an oxide layer, as shown in Figure 5b. The SEM micrographs before release were further analyzed to quantify the porosity of the coatings. Black color represents “holes”, and the coating and coating particles are shown in the gray scale. This analysis yielded approximately 20% porosity with HA/Ti6Al4V, 30% porosity with MgO/SiO2-HA/Ti6Al4V, 20% porosity with HA/800TiO2/Ti6Al4V, and 20% porosity with MgO/SiO2-HA/800TiO2/Ti6Al4V. Figure 6 shows a schematic to explain the release data from Figure 5. In this study, HA/Ti6Al4V showed overall Ca2+ precipitation in SBF. On adding an oxide layer, the coating crystallinity is enhanced, which decreases the Ca2+ release rate and increases the overall Ca2+ precipitation compared to HA/Ti6Al4V. When additives, MgO and SiO2, are added to HA prior to coating, the change of crystalline HA is negligible, however, the addition of Mg2+ and Si4+ in the HA lattice increases the Ca2+ release rate and shows an overall Ca2+ dissolution. The substitution of Si4+ with Ca2+ likely causes crystal defects and increases the solubility of the calcium phosphate material.33 In a previous study, it was also reported that the presence of Si4+ enhanced the release kinetics of Si-TCP in vivo.34 These findings were further supported by the microstructural images before and after release, as shown in Figure 5. Images of microstructure show Ca2+ precipitation on all samples except MgO/SiO2-HA/Ti6Al4V and higher precipitation on samples consisting of an oxide layer.
Figure 5.

(a) Accumulative Ca2+ release of HA/Ti6Al4V, MgO/SiO2-HA/Ti6Al4V, HA/800TiO2/Ti6Al4V, and MgO/SiO2-HA/800TiO2/Ti6Al4V in SBF (n = 3). (b) Images of microstructure show Ca2+ precipitation on all samples except MgO/SiO2-HA/Ti6Al4V and higher precipitation on samples consisting of an oxide layer.
Figure 6.

Possible schematic of the release mechanism to showcase Figure 5 results with (a) HA/Ti6Al4V, (b) MgO/SiO2-HA/Ti6Al4V, (c) HA/800TiO2/Ti6Al4V, and (d) MgO/SiO2-HA/800TiO2/Ti6Al4V. HA coatings show an overall Ca2+ precipitation; however, with the additives, crystallinity is not affected but there is an overall Ca2+ dissolution. The oxide layer increases the crystallinity of the HA plasma coating and prevents Ca2+ precipitation from converting to dissolution with the presences of additives.
3.3. Biological Properties: In Vitro and In Vivo.
Finally, the last important factor for orthopedic implants is the biological properties, which determine the osseointegration and fixation time. Although the focus of this work emphasizes the benefits of an oxide layer between HA coatings and metal substrates, this is not the direct layer in contact with cells and surrounding host tissues. It is still imperative to understand the biological effects of this system in vitro followed by in vivo. If the cell culture yields positive results, the oxide layer would not be the direct cause, but rather directly affects coating crystallinity. This work seeks to understand how the enhanced coating would perform. In this study, the human fetal osteoblast cell line is used to characterize the coating in vitro. The osteoblast lifecycle typically stages from proliferation to differentiation, and finally termination. First, osteoblasts increase in number through proliferating substantially followed by differentiation to mature osteoblasts with decreases in proliferation activity.35 Mature osteoblasts then change their phenotype to osteocytes, bone lining cells, or apoptosis during the termination stage. The presence of MgO and SiO2 enhance osteoblast proliferation and differentiation by the observation of higher MTT and ALP expression compared to HA-coated samples, as shown in Figure 7. At all time points, the MTT assay identifies the presence of an HA coating showing significantly enhanced cell viability of MgO and SiO2 in HA compared to Ti6Al4V, as shown in Figure 7a. The presence of MgO and SiO2 also enhance osteoblast proliferation by the observation of the increase in the MTT value of MgO/SiO2-HA/800TiO2/Ti6Al4V at days 3 and 7. At day 11, the optical density of MgO/SiO2-HA/800TiO2/Ti6Al4V is similar compared to its value at day 7, which follows a different trend compared to Ti6Al4V control and HA/800TiO2/Ti6Al4V. As previously mentioned, mature osteoblasts may undergo apoptosis during the termination stage, which can reduce cell viability. Hence, this different trend indicates apoptosis on MgO/SiO2-HA/800TiO2/Ti6Al4V during the termination state, which demonstrates that the presence of MgO and SiO2 also expedites osteoblast differentiation. At days 3 and 7, the ALP expressions are insignificantly different between all compositions, as shown in Figure 7b. By day 11, the ALP expression is significantly enhanced through the use of an HA coating and even more significantly enhanced by the presence of MgO and SiO2. From the low level of ALP expression at days 3 and 7, it is evident that osteoblasts are still in their proliferation stage for all compositions. At day 11, all compositions show higher ALP expression compared to previous time points, indicating osteoblast differentiation. In a previous study, we reported that the addition of 1 wt % MgO enhanced the osteoblastic attachment and ALP production of tricalcium phosphate scaffolds.36 It was also reported that the presence of silicon enhanced osteoblast proliferation, extracellular matrix, osteocalcin synthesis, and ALP activity.37 In addition, coated samples show significantly higher osteoblast proliferation and differentiation compared to uncoated samples, which provides further evidence for the significance of plasma-sprayed HA coating on Ti6Al4V.
Figure 7.

MTT assay (a) and ALP assay (b) of Ti6Al4V, HA/800TiO2/Ti6Al4V, and MgO/SiO2-HA/800TiO2/Ti6Al4V after 3, 7, and 11 days. Osteoblast cell viability is enhanced by in the presence of MgO and SiO2 and plasma-sprayed HA coatings compared to Ti6Al4V control (n = 3, *p < 0.05). Osteoblast cell differentiation is enhanced at day 11 in the presence of MgO and SiO2 and plasma-sprayed HA coatings compared to Ti6Al4V control (n = 3, *p < 0.05).
Following the osteoblast in vitro, it is identified that the oxidation layer did not negatively affect the inherent naturally positive biological ability of the HA coating; however, the true biological enhancement stems from the additives within the HA coating. This led to the decision to focus on the additive effect in vivo. The importance of the presence of additives in the HA coating on enhancing biological properties, discovered by the in vitro study, is again supported by the results of the rat distal femur model. Osseointegration is not only reinforced by the MgO/SiO2-HA coating compared to uncoated Ti6Al4V but is also improved compared to the HA coating alone, as shown in Figure 8. Large gaps are observed between the Ti6Al4V implant surface with surrounding osseous tissue, whereas no large gaps are observed between HA and MgO/SiO2-HA coating with surrounding osseous tissue. Enhanced maturation of bone is observed by the MgO/SiO2-HA/Ti6Al4V implants from the greater prominence of blue color at the implant/tissue interface compared to just HA/Ti6Al4V implants in both time points. Surface modifications prior to plasma-spray HA coatings, including oxidation of Ti6Al4V and addition of MgO and SiO2, can enhance implant viability, decrease dissolution behavior, and improve osseointegration.
Figure 8.

Microscope images of Ti6Al4V control, HA/Ti6Al4V, and MgO/SiO2-HA/Ti6Al4V-sectioned implants stained by a modified Masson–Goldner trichrome stain. Larger gaps were observed between the Ti6Al4V implant surface with surrounding osseous tissue. Osseointegration between the implant surface and osseous tissue was enhanced by the HA coating and was further enhanced by the MgO/SiO2-HA coating. Osteoid formation is depicted in red/orange and mineralized bone is depicted in blue/green.
4. CONCLUSION
A thermal oxidation method is used to increase the crystallinity of HA coatings during plasma-spray processing on Ti6Al4V substrates. The resulting oxide layer at 800 °C further improves the crystallinity of plasma-sprayed HA coating from 64 to 75%, thus decreasing the dissolution, which is observed via a lower quantity of Ca2+ release in SBF. The adhesive bond strength of plasma-sprayed HA coating is improved from 25.9 ± 2.3 to 30.7 ± 1.1 MPa due to the addition of an oxide layer. The presence of MgO and SiO2 has negligible effects on the crystallinity and adhesive bond strength of plasma-sprayed HA coating. The additives improve osteoblast proliferation and differentiation as well as significantly enhances osseointegration and bone mineralization in a rat distal femur model. This study presents a practical approach to improve physical, mechanical, and osteogenic properties of plasma-sprayed HA coatings, which are vital for the development of coated orthopedic load-bearing implants.
ACKNOWLEDGMENTS
Authors acknowledge financial support from the National Institute of Arthritis and Musculoskeletal and Skin Diseases of the National Institutes of Health under award number 1R01AR066361. Authors also thank the Franceschi Microscopy & Imaging Center at the Washington State University. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Complete contact information is available at: https://pubs.acs.org/10.1021/acsami.0c05035
The authors declare no competing financial interest.
Contributor Information
Susmita Bose, W. M. Keck Biomedical Materials Research Laboratory, School of Mechanical and Materials Engineering, Washington State University, Pullman, Washington 99163, United States;.
Dongxu Ke, W. M. Keck Biomedical Materials Research Laboratory, School of Mechanical and Materials Engineering, Washington State University, Pullman, Washington 99163, United States;.
Ashley A. Vu, W. M. Keck Biomedical Materials Research Laboratory, School of Mechanical and Materials Engineering, Washington State University, Pullman, Washington 99163, United States;.
Amit Bandyopadhyay, W. M. Keck Biomedical Materials Research Laboratory, School of Mechanical and Materials Engineering, Washington State University, Pullman, Washington 99163, United States;.
Stuart B. Goodman, Department of Orthopaedic Surgery, Stanford Medicine Outpatient Center, Redwood City, California 94063, United States
REFERENCES
- (1).Kurtz S; Ong K; Lau E; Mowat F; Halpern M Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Joint Surg. Am 2007, 89, 780. [DOI] [PubMed] [Google Scholar]
- (2).Khan M; Osman K; Green G; Haddad FS The epidemiology of failure in total knee arthroplasty: avoiding your next revision. Bone Joint J. 2016, 98, 105–112. [DOI] [PubMed] [Google Scholar]
- (3).Morais LS; Serra GG; Muller CA; Andrade LR; Palermo EFA; Elias CN; Meyers M Titanium alloy mini-implants for orthodontic anchorage: immediate loading and metal ion release. Acta Biomater. 2007, 3, 331–339. [DOI] [PubMed] [Google Scholar]
- (4).Lin X; Xiao X; Wang Y; Gu C; Wang C; Chen J; Liu H; Luo J; Li T; Wang D; Fan S Biocompatibility of Bespoke 3D-Printed Titanium Alloy Plates for Treating Acetabular Fractures. BioMed. Res. Int 2018, 2018, No. 2053486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Pilliar RM; Lee JM; Maniatopoulos C Observations on the effect of movement on bone ingrowth into porous-surfaced implants. Clin. Orthop. Relat. Res 1986, 108–113. [PubMed] [Google Scholar]
- (6).Søballe K; Hansen ES; Rasmussen H; Jorgensen PH; Bunger C Tissue ingrowth into titanium and hydroxyapatite-coated implants during stable and unstable mechanical conditions. J. Orthop. Res 1992, 10, 285–299. [DOI] [PubMed] [Google Scholar]
- (7).Dumbleton J; Manley MT Hydroxyapatite-coated prostheses in total hip and knee arthroplasty. J. Bone Joint Surg. Am 2004, 86-A, 2526–2540. [DOI] [PubMed] [Google Scholar]
- (8).Sun L; Berndt CC; Gross KA; Kucuk A Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: a review. J. Biomed. Mater. Res 2001, 58, 570–592. [DOI] [PubMed] [Google Scholar]
- (9).Roy M; Bandyopadhyay A; Bose S Induction plasma sprayed nano hydroxyapatite coatings on titanium for orthopaedic and dental implants. Surf. Coat. Technol 2011, 205, 2785–2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (10).Wu J-M; Liu J-F; Hayakawa S; Tsuru K; Osaka A Low-temperature deposition of rutile film on biomaterials substrates and its ability to induce apatite deposition in vitro. J. Mater. Sci. Mater. Med 2007, 18, 1529–1536. [DOI] [PubMed] [Google Scholar]
- (11).Kumar S; Sankara Narayanan TSN; Ganesh Sundara Raman S; Seshadri SK Thermal oxidation of Ti6Al4V alloy: Microstructural and electrochemical characterization. Mater. Chem. Phys 2010, 119, 337–346. [Google Scholar]
- (12).Guleryuz H; Cimenoglu H Surface modification of a Ti–6Al–4V alloy by thermal oxidation. Surf. Coat. Technol 2005, 192, 164–170. [Google Scholar]
- (13).Ke D; Bose S Doped tricalcium phosphate bone tissue engineering scaffolds using sucrose as template and microwave sintering: enhancement of mechanical and biological properties. Mater. Sci. Eng. C 2017, 78, 398–404. [DOI] [PubMed] [Google Scholar]
- (14).Park K-D; Lee B-A; Piao X-H; Lee K-K; Park S-W; Oh H-K; Kim Y-J; Park H-J Effect of magnesium and calcium phosphate coatings on osteoblastic responses to the titanium surface. J. Adv. Prosthodont 2013, 5, 402–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).Landi E; Logroscino G; Proietti L; Tampieri A; Sandri M; Sprio S Biomimetic Mg-substituted hydroxyapatite: from synthesis to in vivo behaviour. J. Mater. Sci. Mater. Med 2008, 19, 239–247. [DOI] [PubMed] [Google Scholar]
- (16).Hing KA; Revell PA; Smith N; Buckland T Effect of silicon level on rate, quality and progression of bone healing within silicate-substituted porous hydroxyapatite scaffolds. Biomaterials 2006, 27, 5014–5026. [DOI] [PubMed] [Google Scholar]
- (17).Ke D; Dernell W; Bandyopadhyay A; Bose S Doped tricalcium phosphate scaffolds by thermal decomposition of naphthalene: Mechanical properties and in vivo osteogenesis in a rabbit femur model. J. Biomed. Mater. Res., Part B 2015, 103, 1549–1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Patel N; Best SM; Bonfield W; Gibson IR; Hing KA; Damien E; Revell PA A comparative study on the in vivo behavior of hydroxyapatite and silicon substituted hydroxyapatite granules. J. Mater. Sci. Mater. Med 2002, 13, 1199–1206. [DOI] [PubMed] [Google Scholar]
- (19).Vu AA; Robertson SF; Ke D; Bandyopadhyay A; Bose S Mechanical and biological properties of ZnO, SiO2, and Ag2O doped plasma sprayed hydroxyapatite coating for orthopaedic and dental applications. Acta Biomater. 2019, 92, 325–335. [DOI] [PubMed] [Google Scholar]
- (20).Su Y-F; Lin C-C; Huang T-H; Chou M-Y; Yang J-J; Shie M-Y Osteogenesis and angiogenesis properties of dental pulp cell on novel injectable tricalcium phosphate cement by silica doped. Mater. Sci. Eng.: C 2014, 42, 672–680. [DOI] [PubMed] [Google Scholar]
- (21).Mladenović Ž; Johansson A; Willman B; Shahabi K; Björn E; Ransjö M Soluble silica inhibits osteoclast formation and bone resorption in vitro. Acta Biomater. 2014, 10, 406–418. [DOI] [PubMed] [Google Scholar]
- (22).Li H; Chang J Bioactive silicate materials stimulate angiogenesis in fibroblast and endothelial cell co-culture system through paracrine effect. Acta Biomater. 2013, 9, 6981–6991. [DOI] [PubMed] [Google Scholar]
- (23).Tsui YC; Doyle C; Clyne TW Plasma sprayed hydroxyapatite coatings on titanium substrates. Part 1: Mechanical properties and residual stress levels. Biomaterials 1998, 19, 2015–2029. [DOI] [PubMed] [Google Scholar]
- (24).McPherson R; Gane N; Bastow TJ Structural characterization of plasma-sprayed hydroxylapatite coatings. J. Mater. Sci. Mater. Med 1995, 6, 327–334. [Google Scholar]
- (25).Sun L; Berndt CC; Khor KA; Cheang HN; Gross KA Surface characteristics and dissolution behavior of plasma-sprayed hydroxyapatite coating. J. Biomed. Mater. Res 2002, 62, 228–236. [DOI] [PubMed] [Google Scholar]
- (26).Wang YM; Tian H; Guo LX; Ouyang JH; Zhou Y; Jia DC Amorphous AlPO4 coating formed on titanium alloy for high temperature oxidation protection: oxidation kinetics and microstructure. Surf. Coat. Technol 2014, 252, 134–141. [Google Scholar]
- (27).Vojtěch D; Bártová B; Kubatı’k T High temperature oxidation of titanium–silicon alloys. Mater. Sci. Eng.: A 2003, 361, 50–57. [Google Scholar]
- (28).de Groot K; Wolke JG; Jansen JA Calcium phosphate coatings for medical implants. Proc. Inst. Mech. Eng 1998, 212, 137–147. [DOI] [PubMed] [Google Scholar]
- (29).Luo Y; Chen W; Tian M; Teng S Thermal oxidation of Ti6Al4V alloy and its biotribological properties under serum lubrication. Tribol. Int 2015, 89, 67–71. [Google Scholar]
- (30).Søballe K Hydroxyapatite ceramic coating for bone implant fixation. Mechanical and histological studies in dogs. Acta Orthop. Scand. Suppl 1993, 255, 1–58. [DOI] [PubMed] [Google Scholar]
- (31).Bandyopadhyay A; Bernard S; Xue W; Bose S Calcium Phosphate-Based Resorbable Ceramics: Influence of MgO, ZnO, and SiO2 Dopants. J. Am. Ceram. Soc 2006, 89, 2675–2688. [Google Scholar]
- (32).Seeley Z; Bandyopadhyay A; Bose S Influence of TiO2 and Ag2O addition on tricalcium phosphate ceramics. J. Biomed. Mater. Res. A 2007, 82, 113–121. [DOI] [PubMed] [Google Scholar]
- (33).Bose S; Fielding G; Tarafder S; Bandyopadhyay A Trace element doping in calcium phosphate ceramics to Understand osteogenesis and angiogenesis. Trends Biotechnol. 2013, 31, No. 6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Mastrogiacomo M; Papadimitropoulos A; Cedola A; Peyrin F; Giannoni P; Pearce SG; Alini M; Giannini C; Guagliardi A; Cancedda R Engineering of bone using bone marrow stromal cells and a silicon-stabilized tricalcium phosphate bioceramic: evidence for a coupling between bone formation and scaffold resorption. Biomaterials 2007, 28, 1376–1384. [DOI] [PubMed] [Google Scholar]
- (35).Donahue HJ; Li Z; Zhou Z; Yellowley CE Differentiation of human fetal osteoblastic cells and gap junctional intercellular communication. Am. J. Physiol. - Cell Physiol 2000, 278, C315–C322. [DOI] [PubMed] [Google Scholar]
- (36).Xue W; Dahlquist K; Banerjee A; Bandyopadhyay A; Bose S Synthesis and characterization of tricalcium phosphate with Zn and Mg based dopants. J. Mater. Sci. Mater. Med 2008, 19, 2669–2677. [DOI] [PubMed] [Google Scholar]
- (37).Keeting PE; Oursler MJ; Wiegand KE; Bonde SK; Spelsberg TC; Riggs BL Zeolite A increases proliferation, differentiation, and transforming growth factor beta production in normal adult human osteoblast-like cells in vitro. J. Bone Miner. Res 1992, 7, 1281–1289. [DOI] [PubMed] [Google Scholar]


