Abstract
This work aimed to study the effect of some light spectra on the growth, oxidative state, and stress of einkorn wheatgrass (Triticum monococcum L. ssp. monococcum). To this end, six light treatments, having the same total incident photon flux density (PFD) of 200 μmol m–2 s–1, were applied to einkorn and compared: only blue light; only red; three blue:red combinations, at different proportions of total PFD (75:25%, 50:50%, and 25:75%, respectively); and a wide spectrum, taken as a control treatment, composed of blue (18% of PFD), red (18%), and intermediate wavelengths (64%). Light treatments affected the contents of pigments (chlorophylls and carotenes), hydrogen peroxide (H2O2), and malondialdehyde (MDA). These results revealed the changes in the oxidative status of wheatgrass, in response to the different light treatments. However, the dichromatic light with blue ≥50% of the total PFD appeared to be the best combination, guarantying good wheatgrass yield, increasing pigment content, and reducing H2O2 and MDA when compared to the other light treatments. Our findings also contribute to explaining the available literature on the effect of these kinds of light on the increase in phenolic compounds and antioxidant activity in einkorn wheatgrass.
Keywords: LED, wavelength, chlorophylls, carotenoids, hydrogen peroxide, malondialdehyde
Introduction
Wheatgrass is currently recognized by scientific literature and consumers as an important source of many health-promoting compounds (e.g., phenolic compounds, carotenoids, etc.).1,2 In particular, the wheatgrass obtained from einkorn (T. monococcumL. ssp. monococcum) shows a high content of polyphenols, phenolic acids, and other antioxidants.3−5 Recently, Benincasa et al.6 demonstrated that the amount and composition of antioxidants in einkorn wheatgrass can be sharply affected by the light spectrum. In particular, the total polyphenol content can be increased by the blue radiation, and the total phenolic acid content by both the blue and red radiations, when compared to the white radiation used as the control treatment. The authors did not include the combinations of blue and red lights, but it is known that this may further increase the synthesis of certain compounds, differently from the monochromatic lights.7 In general, the use of specific light spectra, in place of the white light, is justified by the fact that sprout production is more and more carried out indoor with artificial light, both for the homemade and specialized production, and can be easily obtained by LED lamps, which have a long life span, low heat emission, and low power consumption.8
Blue and red lights are the major wavelengths perceived by plant photoreceptors (i.e., phototropins or cryptochromes for blue light and phytochromes for red light). The photoresponses are wavelength-dependent reactions,9 which take place with blue light in the region of 400–500 nm and with red light in that of 600–700 nm. Furthermore, it is noteworthy that blue and red lights can affect the plant morphology, physiology and development, photosynthesis, and primary and secondary metabolism (i.e., the synthesis of some phytochemicals).7 Although there is a large literature on the effect of blue:red light on the nutritional traits of plants, its role on the physiological, biochemical, and nutritional traits of wheatgrass, sprouts, and microgreens still remains unclear or unavailable for most plant species.7,10
Chlorophylls and carotenoids are key molecules operating in the photosynthetic pathway; the content of these pigments in plants is very responsive to light spectra and intensity to such an extent that their content can be increased or decreased by slight differences in the light.11 In general, it is not possible to depict a general trend of how species respond to different light spectra; there is a wide variability in the modulation of the plant pigments with the quality and quantity of the light.12,13 This evidence suggests that red and blue light should be investigated case by case, evaluating for each species the different sensitivity of its photoreceptors. On the other hand, variations in the content of these pigments with the light spectra should be carefully considered as they could indicate that the light treatments could also determine the insurgence of oxidative perturbations, affecting the cell, plant health status, as well as modifying the antioxidant activities.14 Particular attention should also be paid to the effect of light spectra on carotenoid contents for their pivotal role as light-harvesting pigments and scavengers of reactive oxygen species (ROS).7 Generally, when abiotic factors give rise to oxidative perturbations, an overproduction of ROS is observed. Among the ROS, increases of hydrogen peroxide (H2O2) can be recorded in response to different light spectra, as well as the accumulation of malondialdehyde (MDA). MDA is routinely used as an index of lipid peroxidation, as it is related to the oxidative damages to membranes, thus representing an indicator of the oxidative stress in plants.14
These premises show the intriguing perspective of studying the effects of blue:red LED lights on einkorn wheatgrass, with the aim to find suitable combinations capable of maximizing the content of pigments and minimizing that of oxidants. Therefore, some experiments were planned and carried out on einkorn wheatgrass grown with different light treatments, with the scope to evaluate the effect of the blue and red lights, alone or combined in different proportions, on chlorophyll a and b, and carotenoids, assessing whether the different light treatments caused oxidative perturbations, as revealed by the changes in H2O2 and MDA contents.
Materials and Methods
Plant Material and Sprouting
Einkorn grains (T. monococcum L. ssp. monococcum, cv. Monlis, TMoM) were incubated on a filter paper laid over sterile cotton contained in plastic trays (15 g of seeds per tray) and wetted with distilled water (150 mL) to guarantee constant water availability throughout the incubation period and prevent anoxia.4 The trays were placed in a growth chamber, in the dark, for 3 days after sowing (DAS) when most of the seeds germinated. Six different light treatments were then applied, all having the same total incident photon flux density (PFD) of 200 μmol m–2 s–1 (Table 1): only blue light (B100); only red light (R100); blue by 75% + red by 25% of total PFD (B75R25); blue by 50% + red by 50% of total PFD (B50R50); blue by 25% + red by 75% of total PFD (B25R75); and a wide spectrum (WIDE), composed of blue by 18%, red by 18%, and intermediate wavelengths by the remaining 64% of total PFD. In all the treatments, a light/dark photoperiod of 10/14 h was imposed. Three replicates per light treatment were performed. The light treatments were performed using the same LED lamps (DSA3 lamps) used by Tosti et al.15 The combination of wavelengths and the corresponding PFD of each light treatment are listed in Table 1. The growth chamber was maintained at 20 ± 1 °C and at a relative humidity of 70 ± 5%.
Table 1. Incident PFD for Each Radiation Wavelength in Each Light Treatment.
| PFD (μmol m–2 s–1) of each wavelengtha |
||||
|---|---|---|---|---|
| light treatment | blue | intermediate | red | total |
| B100 | 200 | 0 | 0 | 200 |
| B75R25 | 150 | 0 | 50 | 200 |
| B50R50 | 100 | 0 | 100 | 200 |
| B25R75 | 50 | 0 | 150 | 200 |
| R100 | 0 | 0 | 200 | 200 |
| WIDE | 36 | 128 | 36 | 200 |
Blue: range from 400 to 500 nm, peak at 460 nm; red: range from 600 to 700 nm, peak at 660; intermediate: range from 500 to 600 nm, peak at 520 nm.
Wheatgrass was harvested at 9 DAS, collecting only the shoots. The sampled material was stored at −20 °C until analytical determinations, performed in triplicates. The fresh and oven-dried weights of shoots were measured on a subsample of 10 individuals per replicate.
Photosynthetic Pigments
Einkorn seedlings were collected at 9 DAS, and the contents of chlorophyll a, chlorophyll b, and carotenoids were assessed. To this aim, the plant samples (1.5 g) were extracted, in a mortar and a pestle, with 85% acetone in water (v/v), adding small amounts of quartz sand to disrupt the tissues. The resulting suspensions were filtered, and the absorbance was determined spectrometrically at 452.5, 644 and 663 nm. The following equation was used to ascertain the content of the photosynthetic pigments:16
H2O2 Assay
Plant tissues (0.5 g) were extracted, with a mortar and a pestle, in 4 mL of a buffer 50 mM KH2PO4/K2HPO4 (pH 6.5) and 1 mM hydroxylamine. Then, the extracts were centrifuged, and the H2O2 contents were assessed using a xylenol orange-based method.17 In detail, to 0.1 mL of the plant extract, 0.45 mL of a solution containing 200 μM (NH4)2Fe(SO4)2·6H2O and 50 mM H2SO4, and 0.45 mL of a solution containing 500 μM xylenol orange and 200 mM sorbitol, were added. These mixtures were then left to react for 30 min in the dark, and hydrogen peroxide was quantified spectrometrically at 560 nm according to Gay and Gebicki.17
MDA Content
The level of lipid peroxidation was determined in einkorn seedlings, quantifying the plant concentration of MDA. To this scope, the seedlings (0.25 g) were homogenized in a solution containing 10% (w/v) trichloroacetic acid and 0.25% (w/v) thiobarbituric acid. The resulting suspensions were centrifuged for 15 min at 10,000g. Then, the supernatants were transferred into a water bath (95 °C) and warmed for 20 min. After quick cooling, the absorbance of the samples was determined spectrophotometrically at 532 and 600 nm, and the MDA content was calculated according to the study of Panfili et al.18
Statistical Analysis
All data were analyzed by one-way ANOVA according to a randomized block design with three replicates. The average values of triplicate determinations ± standard errors are depicted. The means were compared by Fisher’s least significant difference (LSD) at P value < 0.05. The R statistical environment was used to perform the analysis.19
Results
Growth Parameters
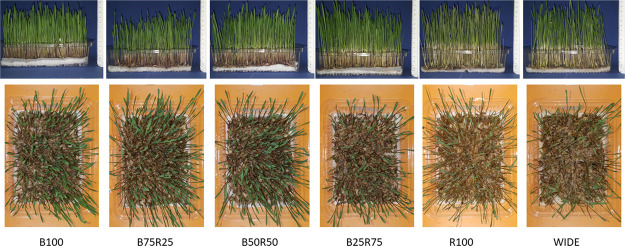
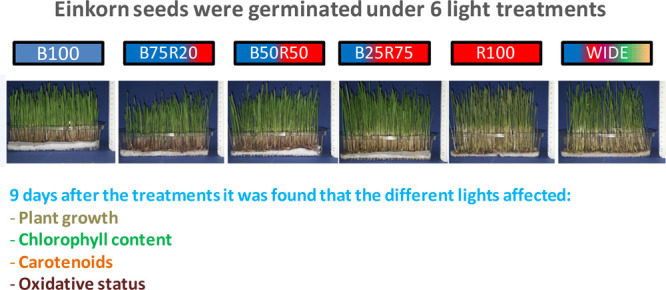
The growth parameters of the einkorn samples grown under the six different light treatments are reported in Table 2 and Figure 1, whereas Figure 2 well testifies the different vegetative statuses of einkorn wheatgrass at harvest. Both the average height and the fresh weight of 10 individuals of wheatgrass tended to increase with decreasing proportions of the blue radiation. The expansion of the leaves was greater and the green was the more intense the higher the percentage of the blue radiation. While the wheat grass grown only with the red radiation was thin, tall, and pale green. Einkorn wheatgrass grown under WIDE was the tallest, but the fresh weight was intermediate between that shown by the samples developed under blue- and red-only monochromatic lights. The dry matter concentration was not significantly affected by light treatments, with an average of 14.2%.
Table 2. Height (mm), Fresh Weight of 10 Individuals, and dry Matter Concentration (%) of Wheatgrass Grown with Different Light Treatments, all Having the Same Total Incident PFD of 200 μmol m–2 s–1a,b.
| light treatment | height (mm) | fresh weight (g) of 10 individuals | dry matter concentration (%) |
|---|---|---|---|
| B100 | 145 (±0.0)d | 1.00 (±0.036)cd | 14.2 (±0.17) |
| B75R25 | 172 (±7.3)c | 1.05 (±0.C83)bcd | 14.9 (±0.13) |
| B50R50 | 175 (±2.9)c | 0.94 (±0.066)d | 15.0 (±0.37) |
| B25R75 | 193 (±1.7)b | 1.16 (±0.015)bc | 14.2 (±0.45) |
| R100 | 202 (±6.0)ab | 1.45 (±0.075)a | 13.5 (±0.58) |
| WIDE | 212 (±3.3)a | 1.20 (±0.052)b | 13.6 (±0.39) |
| F test | |||
| significance | ** | ** | n.s. |
| LSD | 1.3 | 0.182 | 1.17 |
Standard errors in the brackets. LSD: least significance difference for P = 0.05; n.s.: not significant.
B100: only blue light; R100: only red light; B75R25: blue 75% + red 25% of total PFD; B50R50: blue 50% + red 50% of total PFD; B25R75: blue 25% + red 75% of total PFD; WIDE = wide spectrum, composed of blue 18% + red 18% + intermediate wavelengths for the remaining 64% of total PFD.
Figure 1.
Side and top views of one tray of einkorn wheatgrass from each light treatment. B100: only blue light; R100: only red light; B75R25: blue 75% + red 25% of total PFD; B50R50: blue 50% + red 50% of total PFD; B25R75: blue 25% + red 75% of total PFD; WIDE = wide spectrum, composed of blue 18% + red 18% + intermediate wavelengths of the remaining 64% of total PFD.
Figure 2.
Relationships between either individual wheatgrass height (A) or fresh weight of 10 individuals (B) and percent fraction of total PFD for blue (white squares) and red (black diamonds) radiation in einkorn.
Pigments in Einkorn
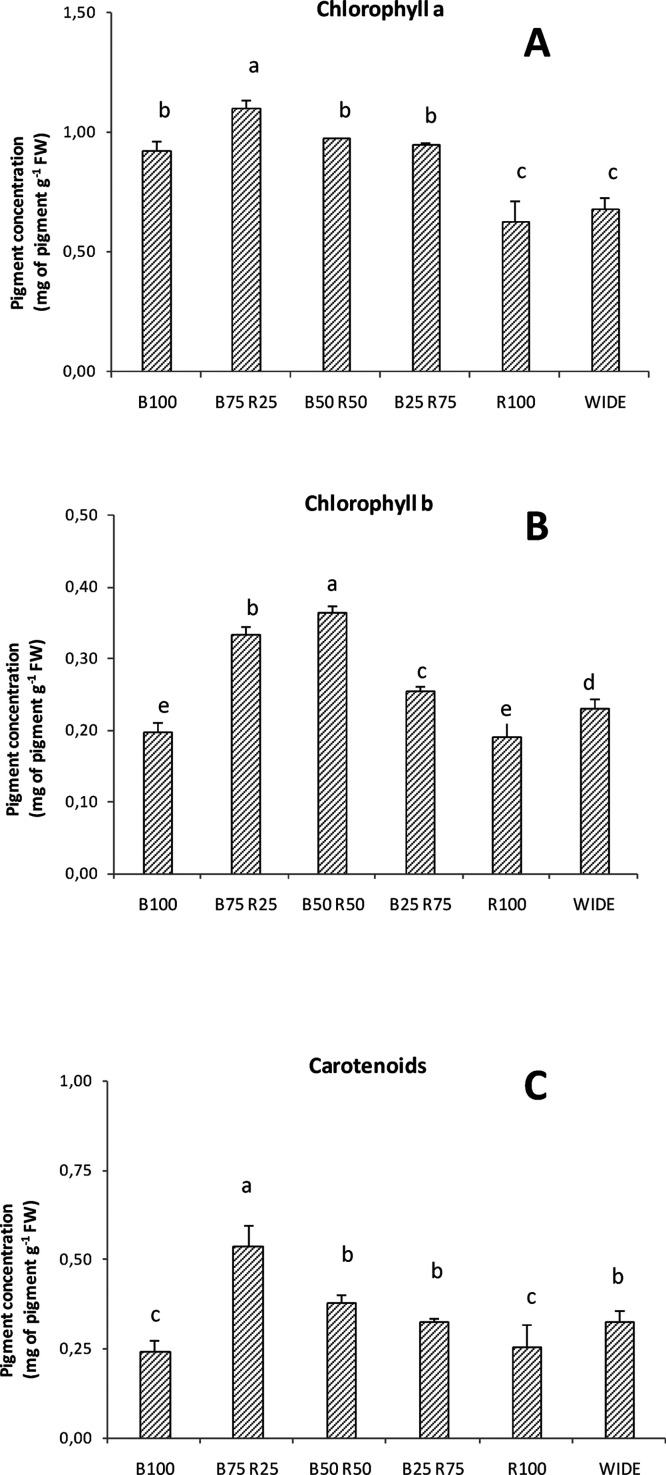
Figure 3a reports the contents of chlorophyll a found in the einkorn samples grown with the six different light treatments. Plants grown under B75R25 showed the highest pigment content, which was significantly different from all the other samples investigated, and reached the value of 1.10 mg g–1 FW. Plants grown with B50R50, B25R75, and B100 showed lower Chl-a contents of 0.97, 0.94, and 0.93 mg g–1 FW, respectively. These three values did not statistically differ among them. Einkorn wheatgrass grown under WIDE and R100 exhibited the lowest Chl-a contents, which were 0.68 and 0.63 mg g–1 FW, respectively.
Figure 3.
Chlorophyll a (A), chlorophyll b (B), and carotenoid (C) concentration (mg g–1 FW) found in einkorn wheatgrass grown with the different light treatments. Data are means + SD, and significant differences among samples are indicated by different letters (P < 0.05) (n = 3).
Concerning Chl-b, a different trend was found in the light-treated einkorn (Figure 3b). Samples grown under B50R50 raised the content of this pigment at 0.36 mg g–1 FW, which was the highest value found in the samples treated with the six different lights. Plants grown under B75R25 showed a Chl-b content of 0.33 mg g–1 FW. Differently, the plants grown with the other light treatments showed a decreased Chl-b content. In particular, einkorn grown under B25R75, WIDE, B100, and R100 light treatments had pigment contents of 0.25, 0.23, 0.19, and 0.19 mg g–1 FW, respectively.
The content of carotenoids ascertained in the einkorn samples (Figure 3c), subjected to the six different light treatments, exhibited a trend more similar to that of Chl-b than Chl-a. However, the highest content of these pigments was found in the samples grown under B75R25 (0.53 mg g–1 FW). Wheatgrass grown under B50R50, B25R75, and WIDE light treatments showed the amounts of carotenoids of 0.37, 0.32, and 0.32 mg g–1 FW, respectively. These values did not statistically differ. The lowest content of pigments was found in the samples grown under B100 and R100, which showed the carotenoid contents of 0.24 and 0.26 mg g–1 FW, respectively.
H2O2 Contents
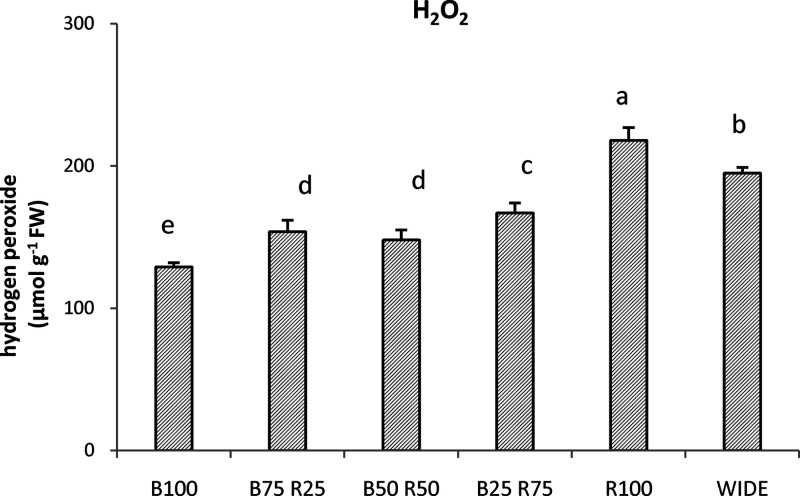
Hydrogen peroxide, a product very indicative of oxidative perturbation which can determine the stress to plants, was assessed in einkorn treated with the six different light combinations. Figure 4 shows the results of the H2O2 quantifications. The plants grown under R100 light treatment elevated the content of this oxidant to 218 μmol g–1 FW, representing the highest value following the different light treatments. The use of the WIDE light treatment showed a slightly lower H2O2 concentration, which, however, was significantly higher than that with the other remaining treatments. The content of H2O2 progressively decreased in einkorn treated with B25R75, B75R25, B50R50, and B100. The last treatment showed the lowest value (148 μmol g–1 FW).
Figure 4.
Hydrogen peroxide (H2O2) concentration (μmol g–1 FW) ascertained in einkorn wheatgrass grown with the different light treatments. Data are means + SD, and significant differences among samples are indicated by different letters (P < 0.05) (n = 3).
MDA Contents
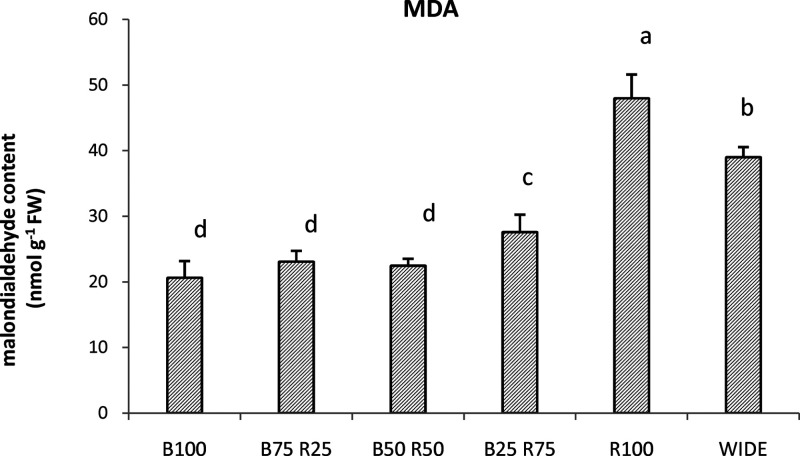
Figure 5 shows the data of the MDA content, assessed in the einkorn seedling subjected to the six different light treatments. The highest concentration of this product of lipid oxidation was found in samples treated with R100 (48 nmol g–1 FW). Plants grown under the other light treatments showed MDA values significantly lower than the plants treated with WIDE (39 nmol g–1 FW). In particular, plants treated with B25R75, B75R25, B50R50, and B100 showed MDA values of 28, 23, 22, and 21 nmol g–1 FW, respectively.
Figure 5.
MDA content (nmol g–1 FW) found in einkorn wheatgrass grown with the different light treatments. Data are means + SD, and significant differences among samples are indicated by different letters (P < 0.05) (n = 3).
Discussion
Plants adapt morphological and photosynthetic responses as a consequence of the light quality and quantity, and this is pivotal for their survival in a variety of dynamic environments.20 The same mechanisms are activated by crops subjected to the different light spectra, as obtained by LEDs,21 in a way that can be completely different among species with blue and red lights, and of their combinations. In our experiments, the differential effect of B100 and R100 on individual wheatgrass height and dry matter content was in line with the study of Benincasa et al.6 (Table 2), although the blue and red spectra of the two experiments were not exactly the same. Under the combinations of blue and red lights, wheatgrass appeared increasingly slim and pale green as the proportion of red light increased (Figure 1). A negative linear correlation was found between blue PFD and the fresh weight and height of wheatgrass. Such an effect was expected, based on the work by Hernández and Kubota,22 which demonstrated that the fresh shoot mass decreased by increasing the fraction of blue light. A possible explanation to this phenomenon was the decrease of the leaf area, associated with a reduction of the photosynthetic activity, because of the lowered plant capacity of intercepting the light. Pennisi et al.10 explained a similar trend, consisting in the reduction of basil yield under a higher fraction of blue light, as a consequence of a smaller leaf area and shortened internode length which worsen the light distribution within the canopy. However, it is worth to notice that, in our case, the dry matter accumulation (i.e., the product between the fresh weight and the dry matter concentration) was not affected; thus, the differences mainly concerned the tissue water status and the related cell expansion. Blue light is widely reported to reduce the cell wall extensibility and increase the cell turgor pressure and the rigidity of the hypocotyls.23 On the other hand, red light affects the stem elongation because of the phytochrome regulation.24 The effect on the seedling water status and fresh weight might also be the consequence of light treatments on stomatal functioning. Blue light has been reported to induce stomatal opening in a short-term exposure and to increase stomata number, as well as chloroplast functionality, in a long-term exposure.23 Similarly, red light is reported to stimulate the stomatal opening.24 Anyway, in the literature, the effect of blue and red light is not always univocal because of the differences in: (i) plant species; (ii) PFD values; and (iii) spectral composition (e.g., different R/B ratios or other wavelengths included).23,24 The slim and pale green appearance of wheatgrass obtained under the WIDE treatment was somehow not expected, with all the wavelengths being included. However, the blue and red portions of the total PFD were lesser than that in the typical white light, whereas intermediate wavelengths were more represented, and the overall light color tended to green.
At the whole plant level, different light spectra can decrease growth, condition the contents of photosynthetic pigments and antioxidants, and affect the nutritional status. These effects can be due to the plant’s capacity to perceive the differences in light quality through its photoreceptors, which can be active or inactive, with the composition of the light spectra in the range 300–800 nm.25 However, our experiments indicated a significant effect of the light treatments on chlorophyll a, b, and carotenoids (Figure 3). As a general trend, the monochromatic red and blue lights resulted in being less effective in stimulating the pigment contents, particularly those of chlorophyll b and carotenoids. In the case of dichromatic light treatments, depending on the relative blue:red ratio, the amount of pigments increased, and the best combination was found to be B75R25.
It is well known that chlorophylls a and b show strong absorption in the red (at 633 and 642 nm, respectively) and blue (430 and 453, respectively) regions.26 Plants modify their content of chlorophylls with the light spectrum. The controversial effect of red and blue light on the pigment contents is reported in the literature, indicating that plant responses are very different among species.11 In general, the monochromatic light alone (blue or red) has been shown to decrease the chlorophyll content in plants often.11 Furthermore, some authors reported for cucumber, spinach, radish, and lettuce, that, when blue light is present with other wavelengths, the chlorophyll content in the investigated species tends to increase with the given amount of blue light.13,22,27,28 Another interesting finding that emerged by comparing the relative content of chlorophylls a and b (Chl a/Chl b) is that passing from the blue to the red light this ratio significantly decreased (4.70 and 2.94 with B100 and R100, respectively). The monochromatic blue light tends, therefore, to actively stimulate the content of chlorophyll a, whereas the opposite effect was caused by the monochromatic red light, which, vice versa, positively affected the content of chlorophyll b. In general, the photosynthetic activity seems to proportionally increase with the amount of blue light present in the treatments.13,22 It is to be mentioned that the monochromatic lights alone, regardless of their wavelength, are unable to sustain an adequate photosynthetic process. However, some authors have evidenced the importance of the presence of blue light in the spectrum, as its absence can exert a substantial negative impact on the photosynthetic activity.13,27,28 Finally, the content of chlorophyll, if counted as the sum of chlorophylls a and b, further confirms that dichromatic light enhanced the chlorophyll content as higher as that with the proportion of blue. These effects can also be explained by the documented stimulatory action of the blue light, which can induce the relocation of chloroplasts which move to the cell surface with the scope to increase the photosynthetic efficiency.29 In this sense, chloroplasts showed a larger area in birch leaves (starch-free part of the chloroplast), and this effect was attributed to the blue fraction of the light. This reorganization of the chloroplasts prevented thylakoids from being too pressed against each other.30 Regarding the WIDE treatment, einkorn wheatgrass showed the chlorophyll content generally lower than that with dichromatic blue:red treatment and higher than those of samples grown with monochromatic lights. These differences reflected, as previously discussed, the spectral characteristics of the WIDE light, having a lower portion of red and blue (18% for each fraction), with the color tending to green, and further highlight the effectiveness of using specific spectra to increase the content of such pivotal molecules.
As far as the total carotenoids are concerned (Figure 3), their content was low with monochromatic light, whereas it increased with dichromatic light, particularly when the blue light was predominant with respect to the red light (B75R25). Carotenoids are photosensitizers and act as scavengers of ROS.7 As light-harvesting pigments, they collect light to pass the energy to the chlorophylls and protect them from higher energy forms.7 It is not possible to define a general trend among the amount of carotenoids produced by species and the treatment with monochromatic (red or blue light) and the dichromatic red and blue lights. The responses are species-specific; however, some studies have highlighted that blue light can induce the production of these pigments proportionally to their fractions in dichromatic treatments (red and blue). In particular, a positive correlation with the blue light was found in green leaves.31,32 The effectiveness of the combination of blue:red LED light in inducing the content of carotenoids was imputed to its capacity to regulate the carotenoid biosynthetic genes in Tartary buckwheat.33 Furthermore, it is to be mentioned that the content of carotenoids was lower in samples treated with WIDE light than in those treated with the dichromatic light B75R25, not significantly different from the other treatments with dichromatic lights, and higher than the monochromatic treatment with blue and red. This effect underlines one more time that the inductive effect of blue:red light on carotenoids, with a higher content of blue, deserves attention as other combinations of light could be ineffective in specifically stimulating a similar beneficial effect on wheatgrass.
Taking into account the effects exerted by the light treatments on the pigments, we investigated the content of hydrogen peroxide and MDA. This is with the scope to give evidence on the impact of light treatments on plants as these molecules can accumulate in response to oxidative perturbations. Many factors, for example, abiotic and biotic stresses, can give rise to the overproduction of H2O2, a harmful oxidant to cells for its capacity to progressively damage a series of molecules, even causing cellular death.34 This molecule is particularly reactive toward chlorophylls, proteins, lipids, DNA, and so forth.34 In our experimentation, the evaluation of the cellular amount of H2O2 produced by einkorn, following the different monochromatic or dichromatic light treatments, was functional to select the most suitable LED light treatment for the species, capable of avoiding oxidative perturbations and explaining the drop in pigments caused by some light treatments. The data from Figure 4 indicate that the blue light generally did not increase the amount of this oxidant, whereas the monochromatic red light was capable of inducing it. Consequently, the entity of damages caused by light treatments to cells was estimated by assessing the cellular MDA content. This molecule, a product of lipid peroxidation, is an important indicator of lipid degradation in response to various abiotic factors.18 The results of MDA evidenced, according to the amount of hydrogen peroxide found following the different light treatments, that the blue light, monochromatic or in combination with red light, did not cause oxidative perturbations to einkorn. In contrast, the treatment with red light alone raised the value of this lipid peroxidation product. Our findings on the pigment content, hydrogen peroxide, and MDA are in line with the results by Benincasa et al.,6 which demonstrated that a 2 day exposition of einkorn sprout to light was a too short period to record any relevant effect on antioxidant activities (expressed as DPPH and FRAP), whereas a week of exposition of this species to monochromatic LED lights modulated them. In particular, the stimulatory effect was ascertained in samples exposed to the blue light, whereas those grown with the red light showed decreasing antioxidant activities. The WIDE light, according to the findings reported here on pigments, was ascertained to be the second treatment most capable of increasing the H2O2 content and MDA accumulation. It can be reasonably postulated that a similar effect was the consequence of the low content of the blue fraction in the light composition, making the spectra ineffective in inducing antioxidant activity.
In conclusion, this work demonstrated that different proportions of blue and red lights could affect the pigment contents and the relative ratios and interfere with the oxidative status of einkorn wheatgrass. This fact is relevant and deserves attention as the ascertained reductions in the content of hydrogen peroxide and MDA, in einkorn wheatgrass, are the consequence of the increased content of some protective molecules, likely phenolic acids and other antioxidants, which are reported in the literature to be preferentially induced by the light spectra with a high proportion of blue light.
Acknowledgments
We gratefully acknowledge GNC s.r.l. for providing the LED lamps, Massimo Fiorani (Prometeo s.r.l.) for providing grains, and Silvano Locchi and Rossano Cortona for technical assistance in the seed lab.
Glossary
Abbreviation
- B25R75
light treatment with blue by 25% + red by 75% of total PFD
- B50R50
light treatment with blue by 50% + red by 50% of total PFD
- B75R25
light treatment with blue by 75% + red by 25% of total PFD
- B100
light treatment with only blue light
- Chl-a
chlorophyll a
- Chl-b
chlorophyll b
- DAS
days after sowing
- DPPH
2,2-diphenyl-1-picrylhydrazyl
- FW
fresh weight
- FRAP
ferric reducing antioxidant power
- MDA
malondialdehyde
- LED
light-emitting diode
- LSD
least significant difference
- PFD
photon flux density
- R100
light treatment with only red light
- ROS
reactive oxygen species
- WIDE
light treatment composed of blue by 18%, red by 18%, and intermediate wavelengths by the remaining 64% of total PFD
This research was supported by the Project “Ricerca di Base 2018–2020” of the Department of Agricultural, Food, and Environmental Sciences of the University of Perugia (Coordinator: Paolo Benincasa).
The authors declare no competing financial interest.
References
- Benincasa P.; Falcinelli B.; Lutts S.; Stagnari F.; Galieni A. Sprouted grains: a comprehensive review. Nutrients 2019, 11, 421. 10.3390/nu11020421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan R. Y.; Chan C. L.; Yang Q. Q.; Li H. B.; Zhang D.; Ge Y. Y.; Gunaratne A.; Ge J.; Corke H.. Bioactive compounds and beneficial functions of sprouted grains. In Sprouted Grains, 1ed.; Feng H., Nemzer B., De Vries J. W., Eds.; Woodhead Publishing and AACC International Press, 2019; pp 191–246. [Google Scholar]
- Benincasa P.; Galieni A.; Manetta A. C.; Pace R.; Guiducci M.; Pisante M.; Stagnari F. Phenolic compounds in grains, sprouts and wheatgrass of hulled and non-hulled wheat species. J. Sci. Food Agric. 2015, 95, 1795–1803. 10.1002/jsfa.6877. [DOI] [PubMed] [Google Scholar]
- Falcinelli B.; Benincasa P.; Calzuola I.; Gigliarelli L.; Lutts S.; Marsili V. Phenolic content and antioxidant activity in raw and denatured aqueous extracts from sprouts and wheatgrass of einkorn and emmer obtained under salinity. Molecules 2017, 22, 2132. 10.3390/molecules22122132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagnari F.; Galieni A.; D’Egidio S.; Falcinelli B.; Pagnani G.; Pace R.; Pisante M.; Benincasa P. Effects of sprouting and salt stress on polyphenol composition and antiradical activity of einkorn, emmer and durum wheat. Ital. J. Agron. 2017, 12, 848. 10.4081/ija.2017.848. [DOI] [Google Scholar]
- Benincasa P.; Tosti G.; Farneselli M.; Maranghi S.; Bravi E.; Marconi O.; Falcinelli B.; Guiducci M. Phenolic content and antioxidant activity of einkorn and emmer sprouts and wheatgrass obtained under different radiation wavelengths. Ann. Agric. Sci. 2020, 10.1016/j.aoas.2020.02.001. [DOI] [Google Scholar]
- Alrifai O.; Hao X.; Marcone M. F.; Tsao R. Current review of the modulatory effects of led lights on photosynthesis of secondary metabolites and future perspectives of microgreen vegetables. J. Agric. Food Chem. 2019, 67, 6075–6090. 10.1021/acs.jafc.9b00819. [DOI] [PubMed] [Google Scholar]
- Taulavuori E.; Taulavuori K.; Holopainen J. K.; Julkunen-Tiitto R.; Acar C.; Dincer I. Targeted use of LEDs in improvement of production efficiency through phytochemical enrichment. J. Sci. Food Agric. 2017, 97, 5059–5064. 10.1002/jsfa.8492. [DOI] [PubMed] [Google Scholar]
- van Ieperen W. Plant morphological and developmental responses to light quality in a horticultural context. Acta Hortic. 2012, 956, 131–139. 10.17660/actahortic.2012.956.12. [DOI] [Google Scholar]
- Pennisi G.; Blasioli S.; Cellini C.; Maia L.; Crepaldi A.; Braschi I.; Spinelli F.; Nicola S.; Fernandez J. A.; Stanghellini C.; Marcelis L. F. M.; Orsini F.; Gianquinto G. Unraveling the role of red:blue LED lights on resource use efficiency and nutritional properties of indoor grown sweet basil. Front. Plant Sci. 2019, 10, 305. 10.3389/fpls.2019.00305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abidi F.; Girault T.; Douillet O.; Guillemain G.; Sintes G.; Laffaire M.; Ahmed H. B.; Smiti S.; Huché-Thélier L.; Leduc N. Blue light effects on rose photosynthesis and photomorphogenesis. Plant Biol. 2013, 15, 67–74. 10.1111/j.1438-8677.2012.00603.x. [DOI] [PubMed] [Google Scholar]
- Hernández R.; Kubota C. Growth and morphological response of cucumber seedlings to supplemental red and blue photon flux ratios under varied solar daily light integrals. Sci. Hortic. 2014, 173, 92–99. 10.1016/j.scienta.2014.04.035. [DOI] [Google Scholar]
- Hogewoning S. W.; Trouwborst G.; Maljaars H.; Poorter H.; van Ieperen W.; Harbinson J. Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. 10.1093/jxb/erq132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Causin H. F.; Jauregui R. N.; Barneix A. J. The effect of light spectral quality on leaf senescence and oxidative stress in wheat. Plant Sci. 2006, 171, 24–33. 10.1016/j.plantsci.2006.02.009. [DOI] [Google Scholar]
- Tosti G.; Benincasa P.; Cortona R.; Falcinelli B.; Farneselli M.; Guiducci M.; Onofri A.; Pannacci E.; Tei F.; Giulietti M. Growing lettuce under multispectral light-emitting diodes lamps with adjustable light intensity. Ital. J. Agron. 2018, 13, 57–62. [Google Scholar]
- Metzner H.; Rau H. r.; Senger H. Untersuchungen zur synchronisierbar kein einzelner pigment-mangelmutanten von chlorella. Planta 1965, 65, 186–194. 10.1007/bf00384998. [DOI] [Google Scholar]
- Gay C.; Gebicki J. M. A critical evaluation of the effect of sorbitol on the ferric-xylenol orange hydroperoxide assay. Anal. Biochem. 2000, 284, 217–220. 10.1006/abio.2000.4696. [DOI] [PubMed] [Google Scholar]
- Panfili I.; Bartucca M. L.; Del Buono D. The treatment of duckweed with a plant biostimulant or a safener improves the plant capacity to clean water polluted by terbuthylazine. Sci. Total Environ. 2019, 646, 832–840. 10.1016/j.scitotenv.2018.07.356. [DOI] [PubMed] [Google Scholar]
- R Core Team . R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2019. http://www.r-project.org/. [Google Scholar]
- Wager H. The perception of light in plants. Ann. Bot. 1909, 23, 459–489. 10.1093/oxfordjournals.aob.a089231. [DOI] [Google Scholar]
- Grahama T.; Yoriob N.; Zhang P.; Massa G.; Wheeler R. Early seedling response of six candidate crop species to increasing levels of blue light. Life Sci. Space Res. 2019, 21, 40–48. 10.1016/j.lssr.2019.03.001. [DOI] [PubMed] [Google Scholar]
- Hernández R.; Kubota C. Physiological responses of cucumber seedlings under different blue and red photon flux ratios using LEDs. Environ. Exp. Bot. 2016, 121, 66–74. 10.1016/j.envexpbot.2015.04.001. [DOI] [Google Scholar]
- Huché-Thélier L.; Crespel L.; Gourrierec J. L.; Morel P.; Sakr S.; Leduc N. Light signalling and plant responses to blue and UV radiations-Perspectives for applications in horticulture. Environ. Exp. Bot. 2016, 121, 22–38. 10.1016/j.envexpbot.2015.06.009. [DOI] [Google Scholar]
- Demotes-Mainard S.; Péron T.; Corot A.; Bertheloot J.; Le Gourrierec J.; Pelleschi-Travier S.; Crespel L.; Morel P.; Huché-Thélier L.; Boumaza R.; Vian A.; Guérin V.; Leduc N.; Sakr S. Plant responses to red and far-red lights, applications in horticulture. Environ. Exp. Bot. 2016, 121, 4–21. 10.1016/j.envexpbot.2015.05.010. [DOI] [Google Scholar]
- Kopsell D. A.; Sams C. E.; Morrow R. C. Blue wavelengths from LED lighting increase nutritionally important metabolites in specialty crops. HortScience 2015, 50, 1285–1288. 10.21273/hortsci.50.9.1285. [DOI] [Google Scholar]
- Wright S. W.; Shearer J. D. Rapid extraction and high-performance liquid chromatography of chlorophylls and carotenoids from marine phytoplankton. J. Chromatogr. A 1984, 294, 281–295. 10.1016/s0021-9673(01)96134-5. [DOI] [Google Scholar]
- Matsuda R.; Ohashi-Kaneko K.; Fujiwara K.; Kurata K. Analysis of the relationship between blue-light photon flux density and the photosynthetic properties of spinach (Spinacia oleracea L.) leaves with regard to the acclimation of photosynthesis to growth irradiance. Soil Sci. Plant Nutr. 2007, 53, 459–465. 10.1111/j.1747-0765.2007.00150.x. [DOI] [Google Scholar]
- Yorio N. C.; Goins G. D.; Kagie H. R.; Wheeler R. M.; Sager J. C. Improving spinach, radish, and lettuce growth under red light-emitting diodes (LEDs) with blue light supplementation. HortScience 2001, 36, 380–383. 10.21273/hortsci.36.2.380. [DOI] [PubMed] [Google Scholar]
- Kasahara M.; Swartz T. E.; Olney M. A.; Onodera A.; Mochizuki N.; Fukuzawa H.; Asamizu E.; Tabata S.; Kanegae H.; Takano M.; Christie J. M.; Nagatani A.; Briggs W. R. Photochemical properties of the flavin mononucleotidebinding domains of the phototropins from Arabidopsis, rice, and Chlamydomonas reinhardtii. Plant Physiol. 2002, 129, 762–773. 10.1104/pp.002410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondada B. R.; Oosterhuis D. M. Morphometric analysis of chloroplasts of cotton leaf and fruiting organs. Biol. Plant. 2003, 46, 281–284. 10.1023/b:biop.0000022266.67097.3d. [DOI] [Google Scholar]
- Lefsrud M. G.; Kopsell D. A.; Sams C. E. Irradiance from distinct wavelength light-emitting diodes affect secondary metabolites in kale. HortScience 2008, 43, 2243–2244. 10.21273/hortsci.43.7.2243. [DOI] [Google Scholar]
- He J.; Qin L.; Chong E. L.; Choong T. W.; Lee S. K. Plant growth and photosynthetic characteristics of Mesembryanthemumcrystallinum grown aeroponically under different blue-and red-LEDs. Front. Plant Sci. 2017, 8, 361. 10.3389/fpls.2017.00361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuan P. A.; Thwe A. A.; Kim Y. B.; Kim J. K.; Kim S.-J.; Lee S.; Chung S.-O.; Park S. U. Effects of white, blue, and red light emitting diodes on carotenoid biosynthetic gene expression levels and carotenoid accumulation in sprouts of tartary buckwheat (Fagopyrum tataricum Gaertn.). J. Agric. Food Chem. 2013, 61, 12356–12361. 10.1021/jf4039937. [DOI] [PubMed] [Google Scholar]
- Del Buono D.; Ioli G.; Nasini L.; Proietti P. A comparative study on the interference of two herbicides in wheat and Italian ryegrass and on their antioxidant activities and detoxification rates. J. Agric. Food Chem. 2011, 59, 12109–12115. 10.1021/jf2026555. [DOI] [PubMed] [Google Scholar]