Abstract
Introduction
While the current tools to assess canine postoperative pain using physiological and behavioural parameters are reliable, an objective method such as the parasympathetic tone activity (PTA) index could improve postoperative care. The aim of the study was to determine the utility of the PTA index in assessing postoperative analgaesia.
Material and Methods
Thirty healthy bitches of different breeds were randomly allocated into three groups for analgaesic treatment: the paracetamol group (GPARAC, n = 10) received 15 mg/kg b.w., the carprofen group (GCARP, n = 10) 4 mg/kg b.w., and the meloxicam group (GMELOX, n = 10) 0.2 mg/kg b.w. for 48 h after surgery. GPARAC was medicated orally every 8 h, while GCARP and GMELOX were medicated intravenously every 24 h. The PTA index was used to measure the analgaesia–nociception balance 1 h before surgery (baseline), and at 1, 2, 4, 6, 8, 12, 16, 20, 24, 36, and 48 h after, at which times evaluation on the University of Melbourne Pain Scale (UMPS) was made.
Results
The baseline PTA index was 65 ± 8 for GPARAC, 65 ± 7 for GCARP, and 62 ± 5 for GMELOX. Postoperatively, it was 65 ± 9 for GPARAC, 63 ± 8 for GCARP, and 65 ± 8 for GMELOX. No statistically significant difference existed between baseline values or between values directly after treatments (P = 0.99 and P = 0.97, respectively). The PTA index showed a sensitivity of 40%, specificity of 98.46% and a negative predictive value of 99.07%.
Conclusion
Our findings suggest that the PTA index measures comfort and postoperative analgaesia objectively, since it showed a clinical relationship with the UMPS.
Keywords: analgaesia, dogs, ovariohysterectomy, PTA index
Introduction
The state of general anaesthesia is one in which various behavioural end points can be distinguished, including amnaesia, hypnosis (defined as lack of perceptive awareness to non-noxious stimuli), analgaesia, immobility, and blunting of autonomic reflexes produced by general anaesthetics acting on the neuronal loci (20). When a subject is put into this state, a volatile anaesthetic acts on the central nervous system to produce two irreversible conditions: immobility and amnaesia. These drugs may produce some other reversible and clinically useful conditions, like unconsciousness, relaxation, suppression of autonomic reflexes, or analgaesia, but none of these are essential to the definition of the anaesthetic state since they are merely side effects (10).
The hypnogenic centre in the preoptic area of the hypothalamus is responsible for the sleep-promoting neurons. The state of rapid eye movement (REM) sleep is characterised by a high-frequency, low-amplitude rhythm on the electroencephalogram (EEG) but minimal or absent electromyogram (EMG) activity. The effect of amnaesia is produced by abolishing hippocampal neurons and basolateral nucleus of the amygdala. While consciousness is modulated by thalamus, midbrain reticular formation and thalamocortical system. To do this, tools such as BIS (Bispectral Index) allow assessing the consciousness and depth of anaesthesia (20).
Analgaesia is difficult to measure precisely during general anaesthesia, but haemodynamic reactivity, such as increased heart rate and blood pressure, may suggest failure in achieving it in an anaesthetised patient. However, these parameters do not show adequate specificity for an independent measurement of the intensity or magnitude of pain during the perioperative period (14, 30, 35). The analgaesic component of anaesthesia can be indirectly monitored for variations in sympathetic and parasympathetic tone as an objective way of evaluating the balance between nociception and antinociception (3, 12).
Several methods have been implemented to quantify the nociception–antinociception balance in the anaesthetised patient in a more reliable way, e.g. the analysis of reflex pathways, pulse photoplethysmography, skin vasomotor reflexes via laser Doppler flowmetry, pupillometry, cerebral evoked potentials, and heart rate variability (HRV) (1, 5, 7, 11, 12). In human anaesthesiology, the analgaesia–nociception index (ANI), a recent development derived from HRV, has been validated for intraoperative nociception detection (3, 5, 8, 15, 24), as it reflects the relative parasympathetic tone (11). This technology was adapted for the study of acute pain in dogs, cats, and horses in the form of a parasympathetic tone activity (PTA) monitor, using an algorithm similar to that of the ANI. Both indices assess intraoperative nociception based on the analysis of HRV to measure relative parasympathetic tone, sympathetic balance (26), and analgaesia–nociception balance during a painful stimulus (23, 26, 27).
PTA index values range from 0 to 100. An index of 50–70 suggests the absence of nociception; values close to 100 correspond to a predominant parasympathetic tone (low level of stress) or opioid overdose; and values below 50 correspond to a predominant sympathetic tone (anticipating haemodynamic responses) associated with a high level of stress or nociceptive pain in dogs undergoing surgical procedures. The PTA index can also be used to predict immediate postoperative analgaesia (4, 6).
The ANI index has been validated for use in human medicine as a non-invasive tool to assess pain during the immediate postoperative period, as it correlates significantly with pain intensity (6). It has been evaluated during general anaesthesia in adults and children as an intraoperative tool (17) and for labour pain evaluation, and exhibited significant changes between periods with and without pain (22). However, equivalent studies in animal medicine using the PTA index have not been undertaken.
The objective of this study was to rectify this deficiency in knowledge by determining the utility of the PTA index for assessment of analgaesia during the postoperative period in female dogs undergoing ovariohysterectomy. We hypothesised that the PTA index could be used to assess pain response, as its dynamic variation can be used as a signal of a haemodynamic response associated with pain.
Material and Methods
This was a randomised, prospective, blinded clinical study.
Animals. For the present study, we selected 30 bitches of different breeds which were scheduled for elective ovariohysterectomy. We obtained prior written informed consent from the owners for their animals’ participation in the study. The patients had a weight of 11.2 ± 6.2 kg (mean ± standard deviation), and an age of 2.7 ± 1.7 years. All dogs were clinically healthy as determined by physical examination, complete blood count, urinalysis, and serum biochemical analysis. Brachycephalic breeds and patients prescribed antiarrhythmic treatment were excluded.
Experimental design and anaesthetic and surgical procedure. Animals were received 48 h prior to the surgical procedure and had access to commercial feed and water ad libitum in that time. All animals were fasted for 8 h before surgery. On the day of the surgery, the animals were randomly assigned to one of three treatment groups: the GPARAC group administered paracetamol/acetaminophen (15 mg/kg b.w., intravenously (IV); Tempra (Reckitt Benckiser S.A. De C.V., Ciudad de México, Mexico); the GCARP group treated with carprofen (4 mg/kg b.w., IV; Rimadyl, Zoetis Inc., Kalamazoo, MI, USA); and the GMELOX group receiving meloxicam (0.2 mg/kg b.w., IV; Meloxi-Jet NRV, NorVet, Torreón, Mexico). A venous catheter was inserted and the selected analgaesic was administered 30 min before surgery. An isotonic fluid solution (0.9% sodium chloride solution, PiSA Farmacéutica, Ciudad de México, Mexico) was administered at a flow rate of 10 mL/kg b.w./h. Anaesthesia was induced by administration of propofol (2–6 mg/kg b.w. IV; Recofol, PiSA Farmacéutica) to allow intubation, and maintained with an initial end-tidal isoflurane concentration of 1.3% (Forane, Baxter International Inc., Deerfield, IL, USA).
During surgery, this concentration was increased or decreased based on the depth of anaesthesia required for surgery based on clinical signs, including absence of the palpebral reflex, relaxed jaw tone, and mean arterial pressure of 60–90 mmHg. Immediately after induction, fentanyl was administered (5 μg/kg b.w. IV; Fenodid, PiSA Farmacéutica) and constantly infused at 5 μg/kg b.w./h. At the end of surgery, fentanyl infusion was discontinued. The dogs were mechanically ventilated at an initial respiratory rate (RR) of 15 breaths per minute and a tidal volume of 12–15 mL/kg b.w. adjusted to maintain end-tidal carbon dioxide tension (ETCO2) of 35–45 mmHg, which was facilitated by administration of rocuronium (0.6 mg/kg b.w. IV; Lufcuren, PiSA Farmacéutica). All anaesthetic and surgical procedures were performed by the same anaesthetist and surgeon. Postoperatively for 48 h, paracetamol was administered to the designated group dogs every 8 h in doses of 15 mg/kg b.w. orally, and carprofen (4 mg/kg b.w.) and meloxicam were administered to the appropriate subjects every 24 h, IV. In this period, the dose of meloxicam was reduced to 0.1 mg/kg b.w.
During anaesthesia, heart rate (HR), ETCO2 and pulse oximetry were monitored. A 22-gauge catheter was aseptically placed in the dorsal metatarsal artery and attached to a transducer (DTX plus DT 4812; Becton Dickinson Critical Care Systems Pte. Ltd., Singapore) for direct monitoring of arterial blood pressure (systolic blood pressure (SBP), diastolic and mean). Thermal support was provided throughout the surgery to maintain the temperature in physiological ranges (36–38°C) (Equator Convective Warming Device, Smiths Medical, Inc., Minneapolis, MN, USA). Neuromuscular function was monitored via acceleromyography (Stimpod, Xavant Technology Pty, Pretoria, South Africa), stimulating the ulnar nerve in a train-of-four pattern and calculating the train-of-four ratio T4 : T1. The dogs were extubated only after T4 : T1 was > 0.90.
Evaluation of postoperative analgaesia using the PTA index. Analgaesia assessments were completed by the same investigator, using a Physio Doloris PTA monitor (MDoloris Medical Systems, Loos, France), and the investigator was blinded to the treatment group. The baseline assessment was performed 60 min before surgery in a quiet and calm environment. Further assessments were performed postoperatively at 1, 2, 4, 6, 8, 12, 16, 20, 24, 36, and 48 h, likewise in a stress-free environment.
The PTA monitor displays a graphic recording of the derivative II of the electrocardiogram (ECG), via three electrodes attached to the skin with conductive gel. The red and yellow electrodes were placed on the right and left forelimbs, respectively, at the level of the olecranon on the caudal aspect of the limb. The black electrode was placed on the right hindlimb, over the patellar ligament at the cranial face of the pelvic limb (28). The device’s algorithm was used to calculate the PTA index.
The PTA index was calculated according to the following formula:
PTA = (100*[α *AUCmin + β] / 12.8)*100/161 where α and β values have been empirically determined as 5.1 and 1.2, respectively, to maintain the consistency of the respiratory influence on the R–R interval series of the ECG; AUCmin is the minimum area under the curve; and 100/12.8 and 100/161 are coefficients for different species determined to obtain PTA values between 0 and 100, with 100/161 being specific to the dog (7, 28).
HRV was evaluated via the PTA monitor as a non-invasive method to measure the activity of the autonomic nervous system. HRV was based on two main components of the ECG: low frequency variations (0.004–0.15 Hz) as an indicator of sympathetic activity, and high frequency variations (0.15–0.5 Hz) as an indicator of parasympathetic activity. The latter are mainly influenced by respiratory sinus arrhythmia (30).
The PTA monitor continuously shows the instantaneous/immediate PTA index, as well as the average/mediate PTA index values collected over 120 s and 240 s. The PTA index was continuously measured through a window of 64 s after each measurement (4, 6, 22, 27, 28). The PTA monitor was calibrated with the canine-specific coefficients already described. Once the ECG electrodes were placed, the criteria for considering a PTA index measurement valid was the monitor recording good signal quality. For each postoperative analgaesia assessment interval, HRV was recorded for 5 min with the patient standing, at which time the average/mediate PTA index value was recorded. At the same postoperative examination times, the validated University of Melbourne Pain Scale (UMPS) score was evaluated (34).
Rescue analgaesic medication. When dogs showed a PTA < 50 and a score > 10 on the UMPS scale, rescue analgaesia was administered. For this, tramadol was used (2 mg/kg b.w. IV; Tramadol Jet NRV injectable solution; NorVet, Torreón, Mexico). Animals which received rescue analgaesia were reported but not included in the study. The same investigator performed all the measurements.
Statistical analysis. Statistical analyses were performed using Prism version 8.3.1 (GraphPad Software, LLC, San Diego, CA, USA). The Shapiro–Wilk test was used for the assessment of data normality. Data were reported as the mean value ± standard deviation. The PTA index data were analysed via a repeated measures ANOVA test, followed by a Holm–Šídák post-hoc test to account for multiple comparisons. The Friedman non-parametric ANOVA and Dunn’s tests were used to analyse postoperative pain as measured by UMPS. Sensitivity, specificity and negative predictive value for the PTA index were calculated. Values were considered statistically different when P < 0.05.
In this study, we estimated that nine dogs for any group were sufficient to assert that a difference of 20 PTA index scores (65 ± 15 versus 45 ± 15) indicated absence or presence of nociception with a power of 0.8 (Type II error) and alpha level of 0.05 (Type I error).
Results
Fig. 1 depicts the PTA indices measured for the three study groups. It should be noted that the mean PTA indices measured for all three study groups and at all assessment intervals were similar. The baseline PTA index for GPARAC was 65 ± 8, for GCARP it was 65 ± 7, and for GMELOX it was 62 ± 5. During the postoperative period, the PTA index was 65 ± 9 for GPARAC, 63 ± 8 for GCARP, and 65 ± 8 for GMELOX (Fig. 1). We detected no statistically significant difference between either baseline values or between treatments (P = 0.99 and P = 0.97), respectively.
Fig. 1.
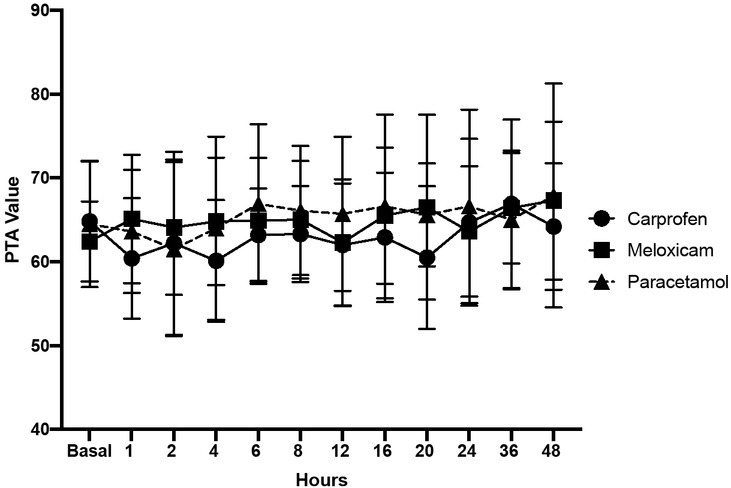
Postoperative PTA values during different treatment times with carprofen, meloxicam, and paracetamol
The P value comparing the PTA indices between treatments was 0.97 and the P value comparing baseline values to post-operative values was 0.99
For 26 dogs, postoperative PTA indices were within normal parameters. Four dogs (one dog in GPARAC and GMELOX and two dogs in GCARP) required rescue analgaesia as subjects with PTA indices between 40 and 49 (moderate pain) and a score > 10 in UMPS. The scores on this scale for the three study groups during the postoperative period are listed in Table 1. These results were used in this investigation as a reference for the evaluation and validation of the clinical utility of the PTA index in conscious animals. The sensitivity, specificity and negative predictive value are presented in Table 2, which shows that PTA index has a greater capacity to recognize pain-free states associated with comfort and postoperative analgaesia. Therefore, with the determination of these diagnostic characteristics, it was possible to establish that individuals who manifested pain could be recognised through the measurement of PTA index and by clinical observation performed by UMPS.
Table 1.
UMPS scores during the postoperative period in GPARAC, GCARP, and GMELOX
| Postoperative period (hours) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 4 | 6 | 8 | 12 | 16 | 20 | 24 | 36 | 48 | |
| GPARAC | 5.5±0.8 | 4.5±0.8 | 3.0±0.6 | 3.0±0.4 | 2.0±0.4 | 2.0±0.4 | 1.5±0.3 | 1.0±0.3 | 1.0±0.3 | 0.0±0.2* | 0.0±0.2* |
| GCARP | 4.5±1.4 | 4.0±0.6 | 3.0±0.3 | 2.0±0.3 | 2.0±0.3 | 1.0±0.4 | 1.0±0.4 | 0.5±0.4 | 0.0±0.4* | 0.0±0.1* | 0.0±0.1* |
| GMELOX | 5±0.9 | 3.5±0.8 | 3.0±0.4 | 2.5±0.3 | 1.5±0.5 | 0.5±0.3 | 0.5±0.3 | 0.0±0.1* | 0.0±0.1* | 0.0±0.0* | 0.0±0.0* |
(*) Statistically significant differences from the first evaluation (P = 0.001)
No statistically significant differences were found between treatments (P = 0.99)
Table 2.
Intrinsic diagnostic characteristics of the PTA index during the evaluation of postoperative analgaesia
| Percentage | |
|---|---|
| Sensitivity | 40% |
| Specificity | 98.46% |
| Negative predictive value | 99.07% |
Discussion
The process of pain assessment during the postoperative period is affected by factors in the assessor such as their age and sex, pharmacological knowledge of analgaesic medication, attitude toward pain, clinical experience of its identification and ability to quantify and manage it. Therefore, observations of pain should be made consistently by the same person (14, 31). These human factors have led to tools that evaluate the sympathetic–parasympathetic tone balance attracting special interest (18, 32), as one of the first responses of an organism to surgical stress is an increase in sympathetic tone and a decrease in parasympathetic tone, which influences HRV (23, 29).
In the case of a predominant parasympathetic tone, each inhalation briefly increases HR and concomitantly decreases the R–R interval of the ECG, which can cause a wide variation in the R–R pattern. Conversely, in the case of a predominant sympathetic tone, the HR increases, but the effect of respiratory arrhythmia on the R–R pattern diminishes, which results in a filtered band with little variation that allows the evaluation of the analgaesia–nociception balance (7, 16, 17). Thus, in the present study, the PTA index calculated for the GPARAC, GCARP, and GMELOX groups indicated that patients exhibited postoperative analgaesia.
Mansour et al. (28) reported the measurement of the PTA index, as well as HR, SBP, and haemodynamic response (defined as a > 20% increase in HR and/or SBP within 5 min of a stimulus) after various surgical stimuli. The authors detected a significant decrease in PTA (P < 0.002) 1 min after the stimuli, followed by a significant increase in HR and/or SBP within 5 min (P < 0.01). Therefore, they concluded that in the veterinary clinical context, the PTA index is a measure of the analgaesia–nociception balance, and can signal a haemodynamic response in anaesthetised dogs. Based on these results, the PTA index could be used to detect perioperative nociception and optimise the administration of analgaesics during or after canine surgery. This corresponded with the present study, where the PTA index ranged from 62 to 65 during the postoperative period in the three study groups, indicating the analgaesia–nociception balance.
The PTA index during the intraoperative period has also been evaluated in male dogs subjected to castration and females undergoing ovariohysterectomy, where a significant difference was observed and the PTA values were higher (indicative of a lower degree of nociception) in males (61 ± 19) than in females (50 ± 17), as well as in patients where an epidural block was used (57 ± 19 versus 48 ± 18; P = 0.003) (33). In that same study, a statistically null correlation was reported between the PTA index values and mean arterial pressure (P = 0.045). Thus, the authors concluded that the PTA monitor can be useful to evaluate the degree of intraoperative nociception. In the present study, a monitor of this type was used to measure postoperative nociception under the same precept, where the treatments used provided an adequate level of analgaesia.
Recently, Aguado et al. (1) applied electrical nociceptive stimuli of different intensities to research dogs and demonstrated that at low intensity, the PTA monitor was able to detect nociceptive responses before cardiovascular changes in HR and mean arterial pressure were elicited. In our study, the PTA values were not significantly different between baselines or between treatments and HR and HRV ranges measured in the PTA monitor were within normal ranges. These authors also demonstrated that changes in the cardiovascular constants were only detected with high intensity nociceptive electrical stimulation, along with low values for the PTA (between 0 and 39).
Given that the studies or reports carried out so far in dogs are scarce, articles related to the postoperative period in humans where the ANI has been used as a tool to measure patients’ pain will be used in the discussion. In a prospective observational study of 200 individuals undergoing orthopaedic surgery, Boselli et al. (4) assessed the ANI upon patients’ awakening from general anaesthesia to predict immediate postoperative pain upon reaching the recovery room (post-anaesthesia care unit), reporting a negative correlation between the ANI measured immediately before extubation and the simple numerical scale upon arrival at the care unit. This same behaviour was observed by Boselli et al. (6) in another study in which they concluded that the ANI monitor is a useful tool during the immediate postoperative period in human patients undergoing scheduled surgery or endoscopy under general anaesthesia, where this index is significantly correlated with pain intensity. Thus, with the results obtained it can be inferred that the measurement of the ANI or the PTA is a simple and non-invasive method to evaluate immediate postoperative analgaesia.
This usefulness is consistent with that described by Ledowski et al. (25), who mentioned that the ANI index based on HRV is a parameter proposed in postoperative monitoring to reflect different levels of acute pain, presenting a situation similar to what was observed in the present study. However, unlike our research, these authors reported a statistically significant negative correlation between the ANI index scores and the numerical rating scale (0, 1, 2, 3, 4, 5, 6, 7, 8, 9, 10) based on the assessment of pain in the recovery room after general anaesthesia with sevoflurane in adult patients.
In the present study, four analgaesic rescues were performed where the values in the PTA index resolved to moderate pain. These patients also presented a score > 10 in UMPS. In this regard, the ability of the PTA index to detect pain in patients who manifest it clinically (the index’s sensitivity) has been reported by other researchers (3, 28) where it fluctuated between 77% and 86%; specificity ranged from 72% to 86%, and the negative predictive value was 92% (3). These results were similar to those calculated in the present study. Therefore, the PTA index appears to be a reliable tool to measure the degree of analgaesia.
In human medicine, there are more studies where the activity of the parasympathetic tone is evaluated through the capacity of the ANI monitor to detect nociceptive stimulation (16, 17), but there are questions regarding the use of a similar index in animals due to species variations of the sympathovagal balance. Nevertheless, HRV measurements that were described in dogs had similar values to those reported in humans, with a low frequency of 0.004–0.15 Hz and a high frequency of 0.15–0.5 Hz (30), which allows comparison of the results obtained in this study with those cited by various researchers in human studies.
In practice, there are factors external to nociception that can influence the reading of the PTA index, such as age, species, the increased vagal tone in brachycephalic breeds, arrhythmia, apnoea, fewer than 8 respiratory cycles/min, the operation of a pacemaker, pathologies (e.g. epilepsy or cerebrovascular accident) or medications (e.g. atropine or vasopressors such as dobutamine and sedatives such as medetomidine or dexmedetomidine) that interrupt the regulation of the autonomic nervous system (3, 5, 9, 13, 37). However, at least in humans, there is evidence that intravenous ketamine microdose infusion contributes to analgaesia without affecting the ANI index under clinical conditions (2). The PTA index therefore helps to assess whether there is an analgaesic sub or overdose condition during the perioperative period (24, 26).
The PTA index as applied in this study could also have been misrepresentative during the first minutes of postoperative evaluation after extubation, since the evaluation of nociception with this type of tool that assesses the autonomous cardiovascular control of HRV in real time has previously aggregated data related to negative emotional states (including pain, stress, anxiety and aggressiveness) in dogs (19, 21, 38). The dogs in this study were assessed by the anaesthetist during recovery and deemed to be calm and comfortable, which should have minimised autonomic responses that affect the PTA index; this was also evidenced by the low number of dogs that exhibited a PTA index indicative of pain.
This study offers some significant benefits for pain assessment in conscious patients or in the postoperative period, however, this research has some limitations that require discussion. For example, the dogs included in this study have a potentially different variability of nervous system activity due to size, age and breed, so future studies should consider a homologation of these factors. Additional studies are needed to validate the use of the PTA index to rate postoperative pain when considering other forms of nociceptive stimuli, since the present study only examined healthy dogs after elective surgery. Also, it is recommended that in future investigations, the use of this device should be considered against other analgaesic options, such as the new opioid agonist related to the selective activation of the β-arrestin signalling pathway called oliceridine (36), a drug that has recently been approved for perioperative pain control as an alternative to morphine or fentanyl, and opioids that influence HRV and have shown properties that can modify the PTA index values. Likewise, the PTA monitor also has some limitations in conscious patients, since the electrocardiogram signal can be altered by the animal’s movements (28). Finally, the PTA monitor does not show the dynamic value of the HRV, and consequently it must be calculated based on the static values provided by the same monitor. In this study, this restricted PTA reporting to only the median/average values.
In conclusion, our findings suggest that the PTA index represents an objective measurement of comfort and analgaesia during the postoperative period, since it showed a clinical relationship with the UMPS; therefore, it is a tool that could help monitor the haemodynamic responses associated with pain or stress.
Acknowledgements
We wish to thank the Mexican College of Veterinary Anaesthesiology and Analgaesia for its support of this study with the necessary equipment and materials.
Footnotes
Conflict of Interest
Conflict of Interests Statement. The authors declare that there is no conflict of interests regarding the publication of this article.
Financial Disclosure Statement. This research was fully funded by the Mexican College of Veterinary Anaesthesiology and Analgesia, considering materials, equipment and infrastructure necessary for its development. The research project from which this manuscript emerges is registered in the Directorate of Advanced Studies of the Universidad Autónoma del Estado de México with registration number DICARM-1717. The funders did not play any role in the study design, the collection and analysis of data, the decision to publish or the preparation of the paper.
Animal Rights Statement. The study was approved by the Ethics Committee for Experimental Animals (COBYBA by its acronym in Spanish) of the Faculty of Veterinary Medicine, Universidad Autónoma del Estado de México, in accordance with the ARRIVE guidelines to improve the design, analysis and publication of animal research.
References
- 1.Aguado D., Bustamante R., García-Sanz V., González-Blanco P., Gómez de Segura I.A.. Efficacy of the Parasympathetic Tone Activity monitor to assess nociception in healthy dogs anaesthetized with propofol and sevoflurane. Vet Anaesth Analg. 2020;47:103–110. doi: 10.1016/j.vaa.2019.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Bollag L., Ortner C.M., Jelacic S., Rivat C., Landau R., Richebé P.. The effects of low-dose ketamine on the analgesia nociception index (ANI) measured with the novel PhysioDolorisTM analgesia monitor: a pilot study. J Clin Monit Comput. 2015;29:291–295. doi: 10.1007/s10877-014-9600-8. [DOI] [PubMed] [Google Scholar]
- 3.Boselli E., Bouvet L., Allaouchiche B.. Analgesia monitoring using Analgesia/Nociception Index: results of clinical studies in awake and anesthetized patients. Praticien Anesthésie Réanim. 2015;19:78–86. doi: 10.1016/j.pratan.2015.03.006. [DOI] [Google Scholar]
- 4.Boselli E., Bouvet L., Bégou G., Dabouz R., Davidson J., Deloste J.Y., Rahali N., Zadam A., Allaouchiche B.. Prediction of immediate postoperative pain using the Analgesia/Nociception Index: a prospective observational study. Br J Anaesth. 2014;112:715–721. doi: 10.1093/bja/aet407. [DOI] [PubMed] [Google Scholar]
- 5.Boselli E., Bouvet L., Bégou G., Torkmani S., Allaouchiche B.. Prediction of hemodynamic reactivity during total intravenous anaesthesia for suspension laryngoscopy using Analgesia/ Nociception Index (ANI): a prospective observational study. Minerva Anestesiol. 2015;81:288–297. [PubMed] [Google Scholar]
- 6.Boselli E., Daniela-Ionescu M., Bégou G., Bouvet L., Dabouz R., Magnin C., Allaouchiche B.. Prospective observational study of the non-invasive assessment of immediate postoperative pain using the analgesia/nociception index (ANI) Br J Anaesth. 2013;111:453–459. doi: 10.1093/bja/aet110. [DOI] [PubMed] [Google Scholar]
- 7.De Jonckheere J., Bonhomme V., Jeanne M., Boselli E., Gruenewald M., Logier R., Richebé P.. Physiological signal processing for individualized anti-nociception management during general anaesthesia: a review. IMIA Yearb Med Inform. 2015;10:95–101. doi: 10.15265/IY-2015-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.De Jonckheere J., Delecroix M., Jeanne M., Keribedj A., Couturier N., Logier R. Automated analgesic drugs delivery guided by vagal tone evaluation: interest of the Analgesia Nociception Index (ANI) Conf Proc 35th Annual International Conference of the IEEE Eng Med Biol Soc. 2013. pp. 1952–1955. [DOI] [PubMed]
- 9.Doxey S., Boswood A.. Differences between breeds of dog in a measure of heart rate variability. Vet Rec. 2004;154:713–717. doi: 10.1136/vr.154.23.713. [DOI] [PubMed] [Google Scholar]
- 10.Eger E.I., Sonner J.M.. Anaesthesia defined (Gentlemen, this is no humbug) Best Pract Res Clin Anaesthesiol. 2006;20:23–29. doi: 10.1016/j.bpa.2005.07.01. [DOI] [PubMed] [Google Scholar]
- 11.Gruenewald M., Ilies C.. Monitoring the nociception – anti-nociception balance. Best Pract Res Clin Anaesthesiol. 2013;27:235–247. doi: 10.1016/j.bpa.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 12.Guignard B.. Monitoring analgesia. Best Pract Res Clin Anaesthesiol. 2006;20:161–180. doi: 10.1016/j.bpa.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 13.Hezzell M.J., Ferrari J., Arndt J., Sleeper M.. Sample size determination for evaluation of time domain heart rate variability indices in canine lameness. J Am Anim Hosp Assoc. 2018;54:235–238. doi: 10.5326/JAAHA-MS-6533. [DOI] [PubMed] [Google Scholar]
- 14.Hugonnard M., Leblond A., Keroack S., Cadoré J.L., Troncy E.. Attitudes and concerns of French veterinarians towards pain and analgesia in dogs and cats. Vet Anaesth Analg. 2004;31:154–163. doi: 10.1111/j.1467-2987.2004.00175.x. [DOI] [PubMed] [Google Scholar]
- 15.Jeanne M., Delecroix M., De Jonckheere J., Keribedj A., Logier R., Tavernier B.. Variations of the analgesia nociception index during propofol anaesthesia for total knee replacement. Clin J Pain. 2014;30:1084–1088. doi: 10.1097/AJP.0000000000000083. [DOI] [PubMed] [Google Scholar]
- 16.Jeanne M., Logier R., De Jonckheere J., Tavernier B.. Heart rate variability during total intravenous anaesthesia: effects of nociception and analgesia. Auton Neurosci Basic Clinical. 2009;147:91–96. doi: 10.1016/j.autneu.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 17.Jeanne M., Logier R., De Jonckheere J., Tavernier B. Validation of a graphic measurement of heart rate variability to assess analgesia / nociception balance during general anaesthesia. Conf Proc 31st IEEE Eng Med Biol Soc. 2009. pp. 1840–1843. [DOI] [PubMed]
- 18.Kang H.. Intraoperative nociception monitoring. Anesth Pain Med. 2015;10:227–234. doi: 10.17085/apm.2015.10.4.227. [DOI] [Google Scholar]
- 19.Katayama M., Kubo T., Mogi K., Ikeda K., Nagasawa M., Kikusui T.. Heart rate variability predicts the emotional state in dogs. Behav Proc. 2016;128:108–112. doi: 10.1016/j.beproc.2016.04.015. [DOI] [PubMed] [Google Scholar]
- 20.Kelz M.B., Mashour G.A., Abel T.G., Maze M. Miller R.D., Eriksson L.I., Fleisher L.A., Wiener-Kronish J.P., Young W.L. Miller’s Anaesthesia. Churchill Livingstone Elsevier; Philadelphia: 2010. Chapter 11: Sleep, memory, and consciousness; pp. 237–259. edited by. [Google Scholar]
- 21.Kuhne F., Hößler J.C., Struwe R.. Emotions in dogs being petted by a familiar or unfamiliar person: validating behavioural indicators of emotional states using heart rate variability. Applied Anim Behav Sci. 2014;161:113–120. doi: 10.1016/j.applanim.2014.09.020. [DOI] [Google Scholar]
- 22.Le Guen M., Jeanne M., Sievert K., Al Moubarik M., Chazot T., Laloë P.A., Dreyfus J.F., Fischler M.. The analgesia nociception index: a pilot study to evaluation of a new pain parameter during labour. Int J Obstetric Anesth. 2012;21:146–151. doi: 10.1016/j.ijoa.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 23.Ledowski T.. Objective monitoring of nociception: a review of current commercial solutions. Br J Anaesth. 2019;123:312–321. doi: 10.1016/j.bja.2019.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ledowski T., Averhoff L., Tiong W.S., Lee C.. Analgesia nociception index (ANI) to predict intraoperative haemodynamic changes: results of a pilot investigation. Acta Anaesthesiol Scand. 2014;58:74–79. doi: 10.1111/aas.12216. [DOI] [PubMed] [Google Scholar]
- 25.Ledowski T., Tiong W.S., Lee C., Wong B., Fiori T., Parker N.. Analgesia nociception index: evaluation as a new parameter for acute postoperative pain. Br J Anaesth. 2013;111:627–629. doi: 10.1093/bja/aet111. [DOI] [PubMed] [Google Scholar]
- 26.Logier R., Jeanne M., De Jonckheere J., Dassonneville A., Delecroix M., Tavernier B. PhysioDoloris: a monitoring device for analgesia/nociception balance evaluation using Heart Rate Variability analysis. Conf Proc 32nd Annual International Conference of the IEEE Eng Med Biol Soc. 2010. pp. 1194–1197. [DOI] [PubMed]
- 27.Logier R., Jeanne M., Tavernier B., De Jonckheere J.. Pain/Analgesia evaluation using heart rate variability analysis. Conf Proc 28th IEEE Eng Med Biol Soc. 2006;1:4303–4306. doi: 10.1109/IEMBS.2006.260494. [DOI] [PubMed] [Google Scholar]
- 28.Mansour C., Merlin T., Bonnet-Garin J.M., Chaaya R., Mocci R., Conde Ruiz C., Allaouchiche B., Boselli E., Junot S.. Evaluation of the Parasympathetic Tone Activity (PTA) index to assess the analgesia/nociception balance in anaesthetised dogs. Res Vet Sci. 2017;115:271–277. doi: 10.1016/j.rvsc.2017.05.009. [DOI] [PubMed] [Google Scholar]
- 29.Mansour C., Merlin T., Boselli E., Allaouchiche B., Bonnet-Garin J.M., Junot S.. Performance du Parasympathetic Tone Activity (PTA) pour prédire la réactivité hémodynamique chez le chien anesthésié. Anesth Réanim. 2015;1(S1):A269–A270. doi: 10.1016/j.anrea.2015.07.412. [DOI] [Google Scholar]
- 30.Manzo A., Ootaki Y., Ootaki C., Kamohara K., Fukamachi K.. Comparative study of heart rate variability between healthy human subjects and healthy dogs, rabbits and calves. Lab Anim. 2009;43:41–45. doi: 10.1258/la.2007.007085. [DOI] [PubMed] [Google Scholar]
- 31.Morton C.M., Reid J., Scott E.M., Holton L.L., Nolan A.M.. Application of a scaling model to establish and validate an interval level pain scale for assessment of acute pain in dogs. Am J Vet Res. 2005;66:2154–2166. doi: 10.2460/ajvr.2005.66.2154. [DOI] [PubMed] [Google Scholar]
- 32.Murrell J.C., Johnson C.B.. Neurophysiological techniques to assess pain in animals. J Vet Pharmacol Therap. 2006;29:325–335. doi: 10.1111/j.1365-2885.2006.00758.x. [DOI] [PubMed] [Google Scholar]
- 33.Pedrosa-López S., Expósito-García de la Mora A., Aguado-Domínguez D., Cediel-Algovia R., Canfran-Arrabé S., Álvarez-Gómez de Segura I.. Intraoperative nociception monitoring with a parasympathetic tone activity monitor: a pilot study in dogs. Lab Anim. 2015;49:26–94. doi: 10.1177/0023677215609551. [DOI] [Google Scholar]
- 34.Reid J., Nolan A.M., Scott E.M.. Measuring pain in dogs and cats using structured behavioural observation. Vet J. 2018;236:72–79. doi: 10.1016/j.tvjl.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 35.Tallant A., Ambros B., Freire C., Sakals S.. Comparison of intraoperative and postoperative pain during canine ovariohysterectomy and ovariectomy. Can Vet J. 2016;57:741–746. [PMC free article] [PubMed] [Google Scholar]
- 36.Urits I., Viswanath O., Orhurhu V., Gress K., Charipova K., Kaye A.D., Ngo A.. The utilization of Mu-Opioid Receptor Biased Agonists: Oliceridine, an opioid analgesic with reduced adverse effects. Curr Pain Headache Rep. 2019;23:31. doi: 10.1007/s11916-019-0773-1. [DOI] [PubMed] [Google Scholar]
- 37.Voigt A.M., Bergfeld C., Beyerbach M., Kästner S.B.R.. Effect of isoflurane with and without dexmedetomidine or remifentanil on heart rate variability before and after nociceptive stimulation at different multiples of minimum alveolar concentration in dogs. Am J Vet Res. 2013;74:665–671. doi: 10.2460/ajvr.74.5.665. [DOI] [PubMed] [Google Scholar]
- 38.Wormald D., Lawrence A.J., Carter G., Fisher A.D.. Reduced heart rate variability in pet dogs affected by anxiety-related behaviour problems. Physiol Behav. 2017;168:122–127. doi: 10.1016/j.physbeh.2016.11.003. [DOI] [PubMed] [Google Scholar]


