Abstract
Rift Valley fever (RVF) is a zoonotic, vector-borne infectious disease of ruminants and camels transmitted mainly by the Aedes and Culex mosquito species. Contact with the blood or organs of infected animals may infect humans. Its etiological factor is the Rift Valley fever virus (RVFV) of the Phlebovirus genus and Bunyaviridae family. Sheep and goats are most susceptible to infection and newborns and young individuals endure the most severe disease course. High abortion rates and infant mortality are typical for RVF; its clinical signs are high fever, lymphadenitis, nasal and ocular secretions and vomiting. Conventional diagnosis is done by the detection of specific IgM or IgG antibodies and RVFV nucleic acids and by virus isolation. Inactivated and live-attenuated vaccines obtained from virulent RVFV isolates are available for livestock. RVF is endemic in sub-Saharan Africa and the Arabian Peninsula, but in the last two decades, it was also reported in other African regions. Seropositive animals were detected in Turkey, Tunisia and Libya. The wide distribution of competent vectors in non-endemic areas coupled with global climate change threaten to spread RVF transboundarily. The EFSA considers the movement of infected animals and vectors to be other plausible pathways of RVF introduction into Europe. A very low risk both of introduction of the virus through an infected animal or vector and of establishment of the virus, and a moderate risk of its transmission through these means was estimated for Poland. The risk of these specific modes of disease introduction into Europe is rated as very low, but surveillance and response capabilities and cooperation with the proximal endemic regions are recommended.
Keywords: Rift Valley fever virus, vector-borne disease, mosquitoes, haemorrhagic fever
Introduction
Rift Valley fever (RVF) is a zoonotic, vector-borne infectious disease classified as a haemorrhagic fever. The aetiological factor of the disease is the Rift Valley fever virus (RVFV), considered one of the most important pathogens in Africa (15, 16). RVFV belongs to the Bunyaviridae family and Phlebovirus genus with nine other species including Punta Toro virus, sandfly fever virus, and severe fever with thrombocytopenia syndrome virus (72). RVF is a lethal illness, with high rates of mortality and abortion, causing economic losses in affected regions. RVFV infects cattle, sheep, goats and camels in an age-dependent manner, where young animals are significantly more likely to suffer than adults. RVFV primarily affects animals but can also cause sickness in humans. The virus is transmitted by mosquitoes (mainly of the Aedes and Culex genera). Humans usually get RVF through contact with infected livestock, but also can through bites from infected mosquitoes (48, 62). As other arboviral infections (e.g. dengue, chikungunya and Zika), RVF may emerge worldwide as a result of globalisation’s distribution effect on arthropod vectors, mainly mosquitoes, which transmit an increasing number of unrecognised and new viruses efficiently. The risk is real of arbovirus introduction to continents including Europe and North America with the possibility of co-circulation (53). The World Organisation for Animal Health (OIE) has designated RVF a notifiable disease (78), and it is also classified as an overlap select agent by the Centers for Disease Control and Prevention (CDC) and the United States Department of Agriculture (USDA). In 2020, the European Food Safety Agency (EFSA) published a report on the risk of RVFV’s introduction to Europe due to the emergence of the fever in new territories and reported seropositivity in animals in Turkey and Tunisia (60). Evidence of seropositivity in sheep and goats that have not lived through an RVF outbreak suggests that low-level circulation of the virus can occur in livestock (64).
Epidemiology
RVFV was first isolated in 1931 during an epidemic among sheep in the Rift Valley in Kenya (16). Since then, major outbreaks affecting livestock and humans have occurred in several countries in Africa, including Egypt, Kenya, South Africa, Madagascar, Mauritania, Senegal, and Gambia (29, 40, 59, 66, 68). The occurrence of severe epidemic RVF has been considered to be related to climate conditions, periodic flooding, a higher greenness of vegetation index, and the consequent emergence of mosquito vectors infecting susceptible ruminant hosts (59). Nevertheless, in the last decade, RVF epidemics have been occurring more frequently in West Africa and in other sub-Saharan countries. In 2000 and 2001, a major outbreak of RVF was reported for the first time outside the African continent, in Saudi Arabia and Yemen (67).
In Mayotte, an overseas department of France and part of the Comoros archipelago in the Indian Ocean, human cases of RVF were detected for the first time in 2007. Retrospective serological studies demonstrated the presence of RVFV in livestock since 2004. RVF re-emerged in 2018, when 142 human cases and several clusters of ruminants were reported (3, 81).
Recent studies carried out in the countries surrounding the Mediterranean (Turkey, Tunisia, Iran, Iraq, and Algeria) and in the Western Sahara, where neither a human nor animal case of the disease has ever been reported, indicate the presence of a certain level of seropositivity in animals and in humans (2, 8, 18, 28, 35, 57, 80). In most of these studies, the sample size and areas of origin of the tested animals were limited, so inference from the results may be difficult.
In January 2020, two RVF outbreaks were notified in the south-eastern Libyan region of Al Kufrah. In each outbreak (one in sheep and the other in sheep and goats), one case was declared, and regarded only serological positivity. No deaths were reported (60).
Virion structure
RVFV is an enveloped virus with the genome in the form of segmented negative-sense RNA, comprising large (L), medium (M), and small (S) segments. The L segment encodes viral RNA polymerase and the M segment encodes the Gc and Gn envelope glycoproteins and the NSm1 and NSm2 nonstructural proteins (10). The S segment shows an ambisense strategy and encodes the nucleoprotein (N protein) and the NSs nonstructural protein (32). Genomic segments make up the viral ribonucleoprotein complexes and are associated with multiple copies of the N protein and RNA-dependent RNA polymerase (10). RVFV has an envelope composed of a lipid bilayer and the Gn and Gc glycoproteins forming heterodimers, which further assemble into pentamers and hexamers with icosahedral symmetry (37).
RVFV attaches to cells via interaction between the Gn and Gc viral glycoproteins and DC-SIGN and I-SIGN cellular C-type lectins (79). Being exposed on the outer surface of the virus, the Gn and Gc glycoproteins are recognised by the host immune system and induce neutralising antibody production. Cells are infected by receptor-mediated endocytosis of the virus, followed by pH-mediated fusion of viral and endosomal membranes resulting in the release of nucleocapsids into the cytoplasm, where transcription, translation, and genome replication occur. The non-structural protein NSs is known to be a major virulence factor allowing the virus to escape the host innate immune response through suppression of the type I interferon response (43).
Vectors
RVFV has been isolated from more than 53 species in eight genera within the Culicidae family, where the Aedes and Culex genera are considered to be the main vectors (48, 50). Primary vectors such as floodwater Aedes (e.g Ae. mcintoshi, Ae. ochraceus, Ae. sudanensis, and Ae. dentatus) maintain the virus’ viability in their eggs in dry soil even during dry periods. Vertical transmission (adult to egg) is hypothesised to allow the virus to persist during inter-epidemic and overwintering periods (47, 55). When the rainy season begins, infected eggs hatch and infected adult female mosquitoes initiate transmission to nearby animals, and then disease outbreaks may occur. Another group of secondary vector mosquitoes mainly from the Culex (Culex pipiens, Culex poicilipes, and Culex univittatus), Anopheles, and Mansonia species may move into sites with infected animals, and thus virus transmission continues, which may result in wider geographical coverage of the disease (66). Secondary vectors of RVFV transmit the virus horizontally from highly viraemic animals to humans. Epidemic transmission of RVFV has been related to heavy and prolonged rainfall primarily due to the El Niño Oscillation. Secondary vectors are highly abundant when stagnant floodwaters are colonised by Culex and Mansonia species and this increases transmission to domestic animals and humans (36).
Transmission
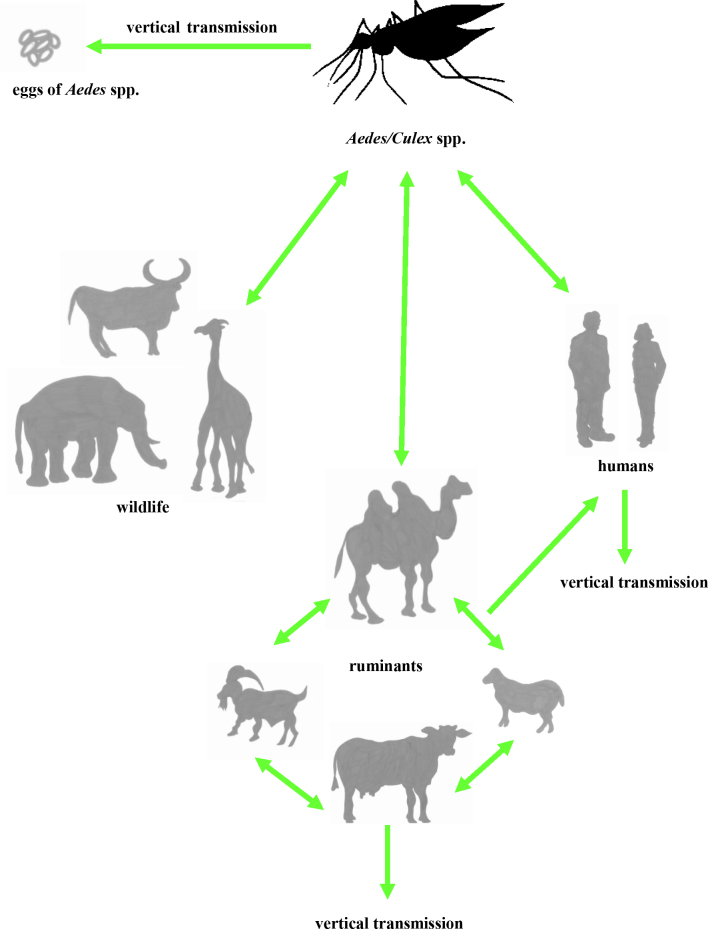
Direct transmission is possible among animals and from animals to humans, and its vertical mode has been described in humans, animals and vectors (Fig. 1). The main route of human infection is contact with blood, body fluids, or tissues and organs of infected animals and aborted animal foetuses. Infection can occur by inhaling aerosols of infectious body fluids. Therefore, various forms of employment cannot avoid exposure as an occupational hazard and in particular skinning or slaughtering of infected animals and veterinary practices are among the activities the least safe from RVFV infection risk. Consumption of raw or unpasteurised milk has also been identified as a risk factor for RVFV infection (77). Grossi-Soyster et al. (34) suggest that exposure to raw milk might contribute to a significant number of cases of RVF, especially during outbreaks and in endemic areas. Mosquito bites are also considered a risk factor for RVFV infection in humans. There is no documented direct horizontal human-to-human transmission, although sporadic RVFV vertical transmission from mothers to their newborns has been reported (1). Considering wild animals as an RVFV reservoir, the possibility of African buffalo (Syncerus caffer) or other wild native or endemic ruminants, wild rodents or bats participating in the spread of the virus is not proven but also not discounted (4, 27, 42, 76).
Fig. 1.
The scheme of RVF transmission. Vectorial transmission is to humans, ruminants, and wildlife; direct transmission is between ruminants and from ruminants to humans; and vertical transmission is in humans, ruminants, and Aedes spp.
Pathogenesis
Although infection shows unique pathogenesis in each animal model (39), generally three types of infection pattern are observed (in both naturally infected and experimentally infected animals), depending on the sensitivity or resistance of the animals. It can be a severe acute infection with uncontrolled viraemia, when high blood viral load is significantly associated with fatal outcome and the infected animal dies quickly. The second pattern, with mild to asymptomatic infection, is one in which the viraemia decreases quickly. The third pattern, with delayed onset of infection complications, has fever and viraemia in the first phase, and in the second phase an additional fever episode may occur. The virus may spread to other organs in this pattern, especially to those of the central nervous system after crossing the blood-brain barrier and to the retina, which is often associated with serious long-term consequences. In both animals and humans, RVFV-induced lesions occur predominantly in the liver. This finding is consistent among severe cases and has been clearly demonstrated by histopathological examination of tissues of experimentally infected sheep (14, 62). The tropism of the virus is limited to hepatocytes and monocytes. Infection can lead to hepatocellular changes progressing to necrosis, which is manifested by increased levels of liver enzymes, leukopaenia or thrombocytopaenia (30).
Clinical symptoms of RVF include high fever, lymphadenitis, nasal and ocular secretions in adult animals, haemorrhagic diarrhoea, vomiting, abdominal colic, lasting prostration, dysgalactia, jaundice, increased abortion frequency (“abortion storms”) and a high mortality rate in young animals (39). A clear distinction is observed regarding the susceptibility to and progression of RVF between young animals and adults.
Sheep and goats are the most susceptible animals to RVFV infection and show similar clinical symptoms. In sheep, the incubation period of the disease is 24–36 h. Post-mortem analysis revealed multi-focal liver necrosis and occasional mild splenomegaly (24). Adult sheep mortality after experimental infection is between 20 and 30%, while in newborn lambs it is much greater at 95–100% (22); the risk of abortion in pregnant ewes is close to 100% (39). In goats, the course of the disease can be somewhat more variable, with viraemia and symptoms inconsistent after infection. Peak viraemia in the blood of goats is also significantly lower than in lambs of identical age after experimental infection. Unlike lambs, goats do not always experience febrile illness. In goats, the formation of hepatic lesions, which are more focal in adults, is followed by necrotic hepatitis.
Cattle are less susceptible to disease than sheep and goats. RVFV infections in adults are usually asymptomatic but can also manifest as an acute disease with mortality rate up to 5 % (39, 75). A recent study has demonstrated that the onset and duration of fever, in addition to other aspects of the disease including viraemia and liver pathology, are less consistent than in sheep (75).
Camels in turn seem to be less susceptible than cattle, with the vast majority of infections being asymptomatic. However, acute RVFV infection may still result in a severe disease course and death. Symptoms can include ocular discharge, haemorrhages, foot lesions and abortions (29).
Infection in humans primarily causes a self-limiting febrile illness. Approximately 50% of infected humans have no clinical signs, while others may experience flu-like symptoms. A small percentage may develop severe clinical forms, involving haemorrhagic fever with hepatic disease, encephalitis or ocular complications (22, 39).
Diagnosis
The techniques used for the diagnosis of RVF include detection of specific IgM or IgG antibodies, virus isolation, and detection of RVFV nucleic acids. The enzyme-linked immunosorbent assay (ELISA) is a reliable and sensitive test to detect antibodies against RVFV. A number of ELISAs of different formats are commercially available or under development (26, 41). They are used routinely in many countries for single case diagnosis, outbreak management, and surveillance. Both IgG and IgM ELISAs are available for most species. Depending on the type of ELISA used, it is possible to distinguish between recent (presence of IgM) and past (presence of IgG) infection and to differentiate between vaccinated and infected animals (DIVA). In naturally occurring infections, an antibody response against both N and NSs would be expected, whereas in individuals vaccinated with the attenuated vaccines only an antibody response to the N protein would be observed (54). Therefore, a dual-target ELISAs has been developed with two viral proteins, N and NSs, for DIVA.
Virus isolation or detection of viral RNA is performed from whole blood or serum samples taken during the acute (febrile) stage of the disease, or various post-mortem organs such as the brain, liver or spleen taken from fresh carcasses or aborted foetuses. The virus can be detected in milk, although tests are not specifically designed for this material (unpublished results from the COOPADEM farmers’ association in Mayotte). Different highly sensitive molecular tests have been developed for RVFV: nested RT-PCR (65), quantitative real-time PCR (6, 20, 31), multiplex PCR-based microarray assay (70), RT loop-mediated isothermal amplification (46), and recombinase polymerase amplification (23). Molecular tests have also been used for the detection of RVFV in mosquitoes in the Kenyan surveillance program (44, 73). Conventional and real-time RT-PCR assays are currently the most rapid and sensitive tests for the detection and quantification of RVFV during outbreaks (31). Methods based on next-generation sequencing approaches, colorimetry, or TaqMan array cards have been developed recently, but most of these techniques are expensive and require dedicated trained personnel (11, 49, 82).
Isolation of RVFV is usually conducted by inoculation of suckling mice or various susceptible mammalian or invertebrate cell cultures (African green monkey kidney of Vero lineage, BHK-21 baby hamster kidney, and AP61 mosquito cells). A cytopathic effect is usually observed within 5 days post-inoculation, the presence of RVFV being confirmed by immunostaining (19).
A pen-side test or lateral flow immuno-chromatographic strip test for detection of the nucleoprotein of RVFV was developed. This type of assay helps to better manage the early diagnosis and control of RVF in the case of ongoing outbreaks (13).
Vaccine
In endemic and non-endemic areas, vaccination of livestock against RVF is an important disease control tool. Different strategies have been used to develop RVF vaccines. The earliest vaccines, such as inactivated vaccines and the live-attenuated Smithburn preparation, were developed from virulent RVFV isolates (25). The first RVF vaccine was formalin-inactivated and was based on the NDBR103 Entebbe strain isolated from a mosquito in Uganda (63). This was further developed into TSI-GSD200, a new generation of formalin-inactivated RVFV vaccine manufactured by the U.S. Army Medical Research Institute of Infectious Diseases (USAMRIID). The RVFV vaccine inactivated with formalin or ethyleneamine has been prepared for veterinary use by passage in BHK-21 hamster kidney cells. Repeated immunisation schedules and high costs are the main disadvantages of inactivated vaccines (25).
The live attenuated Smithburn vaccine is one of the oldest and most widely used vaccines for the control of RVF. The strain was isolated in 1944 in Uganda from Eretmapodites spp. (38). Despite its potency and low cost, the Smithburn vaccine has several disadvantages like residual pathogenicity, causing abortion and foetal malformations and not precluding its possible return to full virulence. Live attenuated vaccines are prohibited in pregnant animal prophylaxis and restricted to use in RVFV-free countries. There is also a possibility of reassortment when the vaccine is used during outbreaks, leading to increased diversity of the virus (9).
The next live attenuated vaccine, MP-12, was developed by the USAMRIID for both human and veterinary use exploiting the virulent ZH548 and ZH501 strains isolated from Egyptian patients and passaging them serially 12 times in MRC-5 cells in the presence of 5-fluorouracil as a mutagen. The MP-12 strain is temperature-sensitive and carries redundant mutations in all three genomic segments. Generally, MP-12 has the ability to produce antibody titres sufficient to protect vaccinated animals against RVFV and there is a potential beneficial effect of immunising pregnant animals, which is the protection of newborns (12).
The natural deletion of 549 nucleotides (70%) of the NSs gene led to the development of the Clone 13 attenuated vaccine. This vaccine is based on the 74HB59 plaque-purified clone isolated in the Central African Republic. Clone 13-vaccinated animals can be easily differentiated from naturally infected animals using the DIVA test. The mutant virus is unable to replicate efficiently in vaccinated animals and does not produce a long-term immune response, which is the main disadvantage of the vaccine (58).
A group of new vaccines, produced using recombinant nucleic acid technology, includes subunit protein vaccines, DNA vaccines (45, 69), virus-like particles (VLPs) (51), virus replicon particle vaccines (61), virus vectored vaccines (74), and genetically modified live vaccines (developed from recombinant viruses engineered using reverse genetics) (5). The solutions with most promise seem to be the recombinant protein-based vaccines, VLPs, and DNA vaccines that are DIVA-compatible, because they are highly safe and a minimal environmental risk (25). Currently, no vaccines have been authorised for use in the European Union (EU) and none of the vaccines have been made available for human use.
Risk of the introduction of RVFV into the EU
The mechanisms by which RVF has moved to new geographic areas are unknown. Mosquitoes involved in RVFV transmission do not appear to migrate or move appreciable distances (47). Migrating or windblown vectors, movements of viraemic mammals, phoresy of RVFV-infected ticks on migratory birds, or movement of infected humans or vectors by aircraft have come under consideration. Studies on birds have demonstrated that they do not become infected or develop antibodies (17).
The wide distribution of competent vectors, the availability of susceptible ruminants, and global climate change combine to seriously threaten the rest of the world with the transboundary spread of RVFV. At least nine vector species able to transmit RVFV are known to be present in Europe and neighbouring countries. Among them, Aedes caspius and Culex pipiens are assumed to be the most efficient vectors (7, 21, 52, 56, 71). This should be taken into consideration in the design of surveillance and control programs which should operate in Europe (33).
According to an EFSA report (60), movements of infected animals and vectors (shipped by air, sea container or road transport) are considered other plausible pathways for the introduction of RVFV into Europe. The risk of its introduction into the EU was assessed using a model called MINTRISK (Method to INTegrate all relevant RISK aspects). MINTRISK risk estimates derive from the combination of pathogen entry rate, level of transmission (as the basic reproductive number) and the probability of the establishment of RVF in the EU (associated with the presence of susceptible hosts and conditions) along appropriate pathways for disease introduction. For Poland, the authors of the report estimated a very low risk of introduction of the virus through an infected animal or vector, a moderate risk of virus transmission through an infected animal or vector and a very low risk of establishment of the virus (60).
The overall risk of introduction of RVF into the EU through the movement of infected animals is very low in all the EU regions (assessed as less than one outbreak every 500 years). The same level of risk of introduction in all regions through the movement of infected vectors was also found, with the highest level for Belgium, Greece, Malta, and the Netherlands (one epidemic every 228–700 years), mainly linked to the number of connections by air and sea transport with African RVF-infected countries. However, taking into account the risk of the spread of RVF in countries neighbouring the EU and the potential introduction of infected vectors, the EFSA suggests that EU authorities should strengthen their surveillance and response capabilities, as well as cooperate with the countries of North Africa and the Middle East (60).
Footnotes
Conflict of Interest
Conflict of Interests Statement: The authors declare that there is no conflict of interests regarding the publication of this article.
Financial Disclosure Statement: This study was funded by the statutory activity of the National Veterinary Research Institute in Puławy.
Animal Rights Statement: Not applicable.
References
- 1.Adam I., Karsany M.S.. Case report: Rift Valley Fever with vertical transmission in a pregnant Sudanese woman. J Med Virol. 2008;80:929–929. doi: 10.1002/jmv.21132. [DOI] [PubMed] [Google Scholar]
- 2.Aghaa O.B.S., Rhaymah M.S.. Seroprevelance study of Rift Valley fever antibody in sheep and goats in Ninevah governorate. Iraqi J Vet Sci. 2013;27:53–61. doi: 10.33899/ijvs.2013.82778. [DOI] [Google Scholar]
- 3.Balenghien T., Cardinale E., Chevalier V., Elissa N., Failloux A.B., Nipomichene T.N.J.J., Nicolas G., Rakotoharinome V.M., Roger M., Zumbo B.. Towards a better understanding of Rift Valley fever epidemiology in the south-west of the Indian Ocean. Vet Res. 2013;44:78. doi: 10.1186/1297-9716-44-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balkema-Buschmann A., Rissmann M., Kley N., Ulrich R., Eiden M., Groschup M.H.. Productive Propagation of Rift Valley Fever Phlebovirus Vaccine Strain MP-12 in Rousettus aegyptiacus Fruit Bats. Viruses. 2018;10:681. doi: 10.3390/v10120681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bird B.H., Albariño C.G., Hartman A.L., Erickson B.R., Ksiazek T.G., Nichol S.T.. Rift valley fever virus lacking the NSs and NSm genes is highly attenuated, confers protective immunity from virulent virus challenge, and allows for differential identification of infected and vaccinated animals. J Virol. 2008;82:2681–2691. doi: 10.1128/JVI.02501-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bird B.H., Bawiec D.A., Ksiazek T.G., Shoemaker T.R., Nichol S.T.. Highly sensitive and broadly reactive quantitative reverse transcription-PCR assay for high-throughput detection of Rift Valley fever virus. J Clin Microbiol. 2007;45:3506–3513. doi: 10.1128/JCM.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Birnberg L., Talavera S., Aranda C., Núñez A.I., Napp S., Busquets N.. Field-captured Aedes vexans (Meigen. 1830) is a competent vector for Rift Valley fever phlebovirus in Europe. Parasit Vectors. 2019;12:484. doi: 10.1186/s13071-019-3728-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bosworth A., Ghabbari T., Dowall S., Varghese A., Fares W., Hewson R., Zhioua E., Chakroun M., Tiouiri H., Ben Jemaa M., Znazen A., Letaief A.. Serologic evidence of exposure to Rift Valley fever virus detected in Tunisia. New Microbes New Infect. 2016;9:1–7. doi: 10.1016/j.nmni.2015.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Botros B., Omar A., Elian K., Mohamed G., Soliman A., Salib A., Salman D., Saad M., Earhart K.. Adverse response of non-indigenous cattle of European breeds to live attenuated Smithburn Rift Valley fever vaccine. J Med Virol. 2006;78:787–791. doi: 10.1002/jmv.20624. [DOI] [PubMed] [Google Scholar]
- 10.Bouloy M., Weber F.. Molecular Biology of Rift Valley Fever Virus. Open Virol J. 2010;4:8–14. doi: 10.2174/1874357901004020008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brinkmann A., Ergünay K., Radonić A., Kocak Tufan Z., Domingo C., Nitsche A.. Development and preliminary evaluation of a multiplexed amplification and next generation sequencing method for viral hemorrhagic fever diagnostics. PLoS Negl Trop Dis. 2017;11:e0006075. doi: 10.1371/journal.pntd.0006075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caplen H., Peters C.J., Bishop D.H.. Mutagen-directed attenuation of Rift Valley fever virus as a method for vaccine development. J Gen Virol. 1985;66:2271–2277. doi: 10.1099/0022-1317-66-10-2271. [DOI] [PubMed] [Google Scholar]
- 13.Cêtre-Sossah C., Pédarrieu A., Juremalm M., Jansen van Vuren P., Brun A., El Mamy A.B.O., Héraud J.M., Filippone C., Ravalohery J.P., Chaabihi H., Albina E., Dommergues L., Paweska J.T., Cardinale E.. Development and validation of a pen side test for Rift Valley fever. PLoS Negl Trop Dis. 2019;13:e0007700. doi: 10.1371/journal.pntd.0007700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Coetzer J.A.W., Ishak K.G.. Sequential development of the liver lesions in new-born lambs infected with Rift Valley fever virus. I. Macroscopic and microscopic pathology. Onderstepoort J Vet Res. 1982;49:103–108. [PubMed] [Google Scholar]
- 15.Coetzer J.A.W., Tustin R.C.. Infectious diseases of livestock, 2nd edition, edited by J.A.W. Coetzer, R.C. Tustin, Oxford University Press Southern Africa, Cape Town. 2005;95:1037–1070. [Google Scholar]
- 16.Daubney R., Hudson J.R.. Rift Valley fever. Lancet. 1932;219:611–612. doi: 10.1016/S0140-6736(01)24634-0. [DOI] [Google Scholar]
- 17.Davies F.G., Linthicum K.J.. The Sudan dioch Quelea quelea aethiopica and Rift Valley fever. Trans R Soc Trop Med Hyg. 1986;80:171–172. doi: 10.1016/0035-9203(86)90233-6. [DOI] [PubMed] [Google Scholar]
- 18.Di Nardo A., Rossi D., Saleh S.M.L., Lejlifa S.M., Hamdi S.J., Di Gennaro A., Savini G., Thrusfield M.V.. Evidence of rift valley fever seroprevalence in the Sahrawi semi-nomadic pastoralist system, Western Sahara. BMC Vet Res. 2014;10:92. doi: 10.1186/1746-6148-10-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Digoutte J.P., Peters C.J.. General aspects of the 1987 Rift Valley fever epidemic in Mauritania. Res Virol. 1989;140:27–30. doi: 10.1016/S0923-2516(89)80081-0. [DOI] [PubMed] [Google Scholar]
- 20.Drosten C., Göttig S., Schilling S., Asper M., Panning M., Schmitz H., Günther S.. Rapid detection and quantification of RNA of Ebola and Marburg viruses, Lassa virus, Crimean-Congo hemorrhagic fever virus, Rift Valley fever virus, dengue virus, and yellow fever virus by real-time reverse transcription-PCR. J Clin Microbiol. 2002;40:2323–2330. doi: 10.1128/JCM.40.7.2323-2330.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ducheyne E., Versteirt V., Hendrickx G.. Abundance of Rift Valley Fever vectors in Europe and the Mediterranean Basin. EFSA Supporting Publications. 2013;10:420E. doi: 10.2903/sp.efsa.2013.EN-420. [DOI] [Google Scholar]
- 22.Easterday B.C.. Rift Valley fever. Adv Vet Sci. 1965;10:65–127. [PubMed] [Google Scholar]
- 23.Euler M., Wang Y., Nentwich O., Piepenburg O., Hufert F.T., Weidmann M.. Recombinase polymerase amplification assay for rapid detection of Rift Valley fever virus. J Clin Virol. 2012;54:308–312. doi: 10.1016/j.jcv.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 24.Faburay B., Gaudreault N.N., Liu Q., Davis A.S., Shivanna V., Sunwoo S.Y., Lang Y., Morozov I., Ruder M., Drolet B., McVey D.S., Ma W., Wilson W.C., Richt J.A.. Development of a sheep challenge model for Rift Valley fever. Virology. 2016;489:128–140. doi: 10.1016/j.virol.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Faburay B., LaBeaud A.D., McVey D.S., Wilson W.C., Richt J.A.. Current Status of Rift Valley Fever Vaccine Development. Vaccines (Basel) 2017;5:E29. doi: 10.3390/vaccines5030029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fafetine J.M., Tijhaar E., Paweska J.T., Neves L.C.B.G., Hendriks J., Swanepoel R., Coetzer J.A.W., Egberink H.F., Rutten V.P.M.G.. Cloning and expression of Rift Valley fever virus nucleocapsid (N) protein and evaluation of a N-protein based indirect ELISA for the detection of specific IgG and IgM antibodies in domestic ruminants. Vet Microbiol. 2007;121:29–38. doi: 10.1016/j.vetmic.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 27.Fagre A.C., Kading R.C.. Can Bats Serve as Reservoirs for Arboviruses? Viruses. 2019;11:215. doi: 10.3390/v11030215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fakour S., Naserabadi S., Ahmadi E.. The first positive serological study on Rift Valley fever in ruminants of Iran. J Vector Borne Dis. 2017;54:5. doi: 10.4103/0972-9062.225840. [DOI] [PubMed] [Google Scholar]
- 29.Fawzy M., Helmy Y.A.. The One Health Approach is Necessary for the Control of Rift Valley Fever Infections in Egypt: A Comprehensive Review. Viruses. 2019;11:E139. doi: 10.3390/v1102013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Findlay G.M.. Rift Valley fever or enzootic hepatitis. Trans R Soc Trop Med Hyg. 1932;25:229–248. doi: 10.1016/S0035-9203(32)90042-X. [DOI] [Google Scholar]
- 31.Garcia S., Crance J.M., Billecocq A., Peinnequin A., Jouan A., Bouloy M., Garin D.. Quantitative real-time PCR detection of Rift Valley fever virus and its application to evaluation of antiviral compounds. J Clin Microbiol. 2001;39:4456–4461. doi: 10.1128/JCM.39.12.4456-4461.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gerrard S.R., Bird B.H., Albariño C.G., Nichol S.T.. The NSm proteins of Rift Valley fever virus are dispensable for maturation, replication and infection. Virology. 2007;359:459–465. doi: 10.1016/j.virol.2006.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gliński Z.. Gorączka Doliny Rift zagraża Europie. Życie Weter. 2019;94:547–553. [Google Scholar]
- 34.Grossi-Soyster E.N., Lee J., King C.H., LaBeaud A.D.. The influence of raw milk exposures on Rift Valley fever virus transmission. PLoS Negl Trop Dis. 2019;13:e0007258. doi: 10.1371/journal.pntd.0007258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gür S., Kale M., Erol N., Yapici O., Mamak N., Yavru S.. The first serological evidence for Rift Valley fever infection in the camel, goitered gazelle and Anatolian water buffaloes in Turkey. Trop Anim Health Prod. 2017;49:1531–1535. doi: 10.1007/s11250-017-1359-8. [DOI] [PubMed] [Google Scholar]
- 36.Hartman A.. Rift Valley Fever. Clin Lab Med. 2017;37:285–301. doi: 10.1016/j.cll.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huiskonen J.T., Overby A.K., Weber F., Grünewald K.. Electron cryo-microscopy and single-particle averaging of Rift Valley fever virus: evidence for GN-GC glycoprotein heterodimers. J Virol. 2009;83:3762–3769. doi: 10.1128/JVI.02483-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ikegami T., Makino S.. Rift valley fever vaccines. Vaccine. 2009;27S4:D69–D72. doi: 10.1016/j.vaccine.2009.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ikegami T., Makino S.. The Pathogenesis of Rift Valley Fever. Viruses. 2011;3:493–519. doi: 10.3390/v3050493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jäckel S., Eiden M., El Mamy A.B.O., Isselmou K., Vina‐Rodriguez A., Doumbia B., Groschup M.H.. Molecular and Serological Studies on the Rift Valley Fever Outbreak in Mauritania in 2010. Transbound Emerg Dis. 2013;60:31–39. doi: 10.1111/tbed.12142. [DOI] [PubMed] [Google Scholar]
- 41.Jansen van Vuren P., Paweska J.T.. Laboratory safe detection of nucleocapsid protein of Rift Valley fever virus in human and animal specimens by a sandwich ELISA. J Virol Methods. 2009;157:15–24. doi: 10.1016/j.jviromet.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 42.Kading R.C., Kityo R.M., Mossel E.C., Borland E.M., Nakayiki T., Nalikka B., Nyakarahuka L., Ledermann J.P., Panella N.A., Gilbert A.T., Crabtree M.B., Peterhans J.K., Towner J.S., Amman B.R., Sealy T.K., Nichol S.T., Powers A.M., Lutwama J.J., Miller B.R.. Neutralizing antibodies against flaviviruses, Babanki virus, and Rift Valley fever virus in Ugandan bats. Infect Ecol Epidemiol. 2018;8:1439215. doi: 10.1080/20008686.2018.1439215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kainulainen M., Lau S., Samuel C.E., Hornung V., Weber F.. NSs Virulence Factor of Rift Valley Fever Virus Engages the F-Box Proteins FBXW11 and β-TRCP1 To Degrade the Antiviral Protein Kinase PKR. J Virol. 2016;90:6140–6147. doi: 10.1128/JVI.00016-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.LaBeaud A.D., Sutherland L.J., Muiruri S., Muchiri E.M., Gray L.R., Zimmerman P.A., Hise A.G., King C.H.. Arbovirus prevalence in mosquitoes, Kenya. Emerg Infect Dis. 2011;17:233–241. doi: 10.3201/eid1702.091666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lagerqvist N., Näslund J., Lundkvist A., Bouloy M., Ahlm C., Bucht G.. Characterisation of immune responses and protective efficacy in mice after immunisation with Rift Valley Fever virus cDNA constructs. Virol J. 2009;6:6. doi: 10.1186/1743-422x-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le Roux C.A., Kubo T., Grobbelaar A.A., Jansen van Vuren P.J., Weyer J., Nel L.H., Swanepoel R., Morita K., Paweska J.T.. Development and evaluation of a real-time reverse transcription-loop-mediated isothermal amplification assay for rapid detection of Rift Valley fever virus in clinical specimens. J Clin Microbiol. 2009;47:645–651. doi: 10.1128/JCM.01412-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Linthicum K.J., Bailey C.L., Davies F.G., Kairo A.. Observations on the Dispersal and Survival of a Population of Aedes-Lineatopennis (Ludlow) (Diptera, Culicidae) in Kenya. Bull Entomol Res. 1985;75:661–670. doi: 10.1017/S0007485300015923. [DOI] [Google Scholar]
- 48.Linthicum K.J., Britch S.C., Anyamba A.. Rift Valley fever: an emerging mosquito-borne disease. Annu Rev Entomol. 2016;61:395–415. doi: 10.1146/annurev-ento-010715-023819. [DOI] [PubMed] [Google Scholar]
- 49.Liu J., Ochieng C., Wiersma S., Ströher U., Towner J.S., Whitmer S., Nichol S.T., Moore C.C., Kersh G.J., Kato C., Sexton C., Petersen J., Massung R., Hercik C., Crump J.A., Kibiki G., Maro A., Mujaga B., Gratz J., Jacob S.T., Banura P., Scheld W.M., Juma B., Onyango C.O., Montgomery J.M., Houpt E., Fields B.. Development of a TaqMan Array Card for Acute-Febrile-Illness Outbreak Investigation and Surveillance of Emerging Pathogens, Including Ebola Virus. J Clin Microbiol. 2016;54:49–58. doi: 10.1128/JCM.02257-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lumley S., Horton D.L., Hernandez-Triana L.L., Johnson N., Fooks A.R., Hewson R.. Rift Valley fever virus: strategies for maintenance, survival and vertical transmission in mosquitoes. J GenVirol. 2017;98:875–887. doi: 10.1099/jgv.0.000765. [DOI] [PubMed] [Google Scholar]
- 51.Mandell R.B., Koukuntla R., Mogler L.J., Carzoli A.K., Freiberg A.N., Holbrook M.R., Martin B.K., Staplin W.R., Vahanian N.N., Link C.J., Flick R.. A replication-incompetent Rift Valley fever vaccine: Chimeric virus-like particles protect mice and rats against lethal challenge. Virology. 2010;397:187–198. doi: 10.1016/j.virol.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mansfield K.L., Banyard A.C., McElhinney L., Johnson N., Horton D.L., Hernández-Triana L.M., Fooks A.R.. Rift Valley fever virus: A review of diagnosis and vaccination, and implications for emergence in Europe. Vaccine. 2015;33:5520–5531. doi: 10.1016/j.vaccine.2015.08.020. [DOI] [PubMed] [Google Scholar]
- 53.Martinet J.P., Ferté H., Failloux A.B., Schaffner F., Depaquit J.. Mosquitoes of North-Western Europe as potential vectors of arboviruses: a review. Viruses. 2019;11:E1059. doi: 10.3390/v11111059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.McElroy A.K., Albariño C.G., Nichol S.T.. Development of a RVFV ELISA that can distinguish infected from vaccinated animals. Virol J. 2009;6:125. doi: 10.1186/1743-422X-6-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mohamed R.A.E.H., Abdelgadir D.M., Bashab H.M.. Transovarian transmission of Rift Valley fever virus by two species of mosquitoes in Khartoum state (Sudan): Aedes vexans (Meigen) and Culex quinquefasciatus (Say) Sudan J Public Health. 2013;8:164–170. [Google Scholar]
- 56.Moutailler S., Krida G., Schaffner F., Vazeille M., Failloux A.B.. Potential vectors of Rift Valley fever virus in the Mediterranean region. Vector Borne Zoonotic Dis. 2008;8:749–754. doi: 10.1089/vbz.2008.0009. [DOI] [PubMed] [Google Scholar]
- 57.Muhsen R.K.. Seroepidemiology of Rift Valley Fever in Basrah. Kufa J Vet Med Sci. 2012;3:91–95. [Google Scholar]
- 58.Muller R., Saluzzo J.F., Lopez N., Dreier T., Turell M., Smith J., Bouloy M.. Characterization of clone 13, a naturally attenuated avirulent isolate of Rift Valley fever virus, which is altered in the small segment. Am J Trop Med Hyg. 1995;53:405–411. doi: 10.4269/ajtmh.1995.53.405. [DOI] [PubMed] [Google Scholar]
- 59.Nanyingi M.O., Munyua P., Kiama S.G., Muchemi G.M., Thumbi S.M., Bitek A.O., Bett B., Muriithi R.M., Njenga M.K.. A systematic review of Rift Valley Fever epidemiology 1931–2014. Infect Ecol Epidemiol. 2015;5:28024. doi: 10.3402/iee.v5.28024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nielsen S.S., Alvarez J., Bicout D.J., Calistri P., Depner K., Drewe J.A., Garin‐Bastuji B., Gonzales Rojas J.L., Gortàzar Schmidt C., Michel V., Miranda Chueca M.A., Roberts H.C., Sihvonen L.H., Stahl K., Velarde Calvo A., Viltrop A., Winckler C., Bett B., Cêtre-Sossah C., Chevalier V., Devos C., Gubbins S., Monaco F., Sotiria-Eleni A., Broglia A., Cortiñas Abrahantes J., Dhollander S., Van der Stede Y., Zancanaro G.. Rift Valley Fever – epidemiological update and risk of introduction into Europe. EFSA Journal. 2020;18:e06041. doi: 10.2903/j.efsa.2020.6041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Oreshkova N., van Keulen L., Kant J., Moormann R.J., Kortekaas J.. A single vaccination with an improved nonspreading Rift Valley fever virus vaccine provides sterile immunity in lambs. PLoS ONE. 2013;8:e77461. doi: 10.1371/journal.pone.0077461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Pepin M., Bouloy M., Bird B.H., Kemp A., Paweska J.T.. Rift Valley fever virus Bunyaviridae Phlebovirus an update on pathogenesis, molecular epidemiology, vectors, diagnostics and prevention. Vet Res. 2010;41:61. doi: 10.1051/vetres/2010033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Randall R., Gibbs C.J. Jr., Aulisio C.G., Binn L.N., Harrison V.R.. The development of a formalin-killed Rift Valley fever virus vaccine for use in man. J Immunol. 1962;89:660–671. [PubMed] [Google Scholar]
- 64.Rostal M.K., Evans A.L., Sang R., Gikundi S., Wakhule L., Munyua P., Macharia J., Feikin D.R., Breiman R.F., Njenga M.K.. Identification of potential vectors of and detection of antibodies against Rift Valley fever virus in livestock during interepizootic periods. Am J Vet Res. 2010;71:522–526. doi: 10.2460/ajvr.71.5.522. [DOI] [PubMed] [Google Scholar]
- 65.Sall A.A., Macondo E.A., Sène O.K., Diagne M., Sylla R., Mondo M., Girault L., Marrama L., Spiegel A., Diallo M., Bouloy M., Mathiot C.. Use of reverse transcriptase PCR in early diagnosis of Rift Valley fever. Clin Diagn Lab Immunol. 2002;9:713–715. doi: 10.1128/CDLI.9.3.713-715.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sang R., Arum S., Chepkorir E., Mosomtai G., Tigoi C., Sigei F., Lwande O.W., Landmann T., Affognon H., Ahlm C., Evander M.. Distribution and abundance of key vectors of Rift Valley fever and other arboviruses in two ecologically distinct counties in Kenya. PLoS Negl Trop Dis. 2017;11:e0005341. doi: 10.1371/journal.pntd.0005341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Shoemaker T., Boulianne C., Vincent M.J., Pezzanite L., Al-Qahtani M.M., Al-Mazrou Y., Khan A.S., Rollin P.E., Swanepoel R., Ksiazek T.G., Nichol S.T.. Genetic analysis of viruses associated with emergence of Rift Valley fever in Saudi Arabia and Yemen, 2000-01. Emerg Infect Dis. 2002;8:1415–1420. doi: 10.3201/eid0812.020195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sow A., Faye O., Faye O., Diallo D., Sadio B.D., Weaver S.C., Diallo M., Sall A.A.. Rift Valley fever in Kedougou. southeastern Senegal. 2012;20:504–506. doi: 10.3201/eid2003.131174. Emerg Infect Dis 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Spik K., Shurtleff A., McElroy A.K., Guttieri M.C., Hooper J.W., Schmaljohn C.. Immunogenicity of combination DNA vaccines for Rift Valley fever virus, tick-borne encephalitis virus, Hantaan virus, and Crimean Congo hemorrhagic fever virus. Vaccine. 2006;24:4657–4666. doi: 10.1016/j.vaccine.2005.08.034. [DOI] [PubMed] [Google Scholar]
- 70.Venter M., Zaayman D., van Niekerk S., Stivaktas V., Goolab S., Weyer J., Paweska J.T., Swanepoel R.. Macroarray assay for differential diagnosis of meningoencephalitis in southern Africa. J Clin Virol. 2014;60:50–56. doi: 10.1016/j.jcv.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 71.Vloet R.P.M., Vogels C.B.F., Koenraadt C.J.M., Pijlman G.P., Eiden M., Gonzales J.L., van Keulen L.J.M., Wichgers Schreur P.J., Kortekaas J.. Transmission of Rift Valley fever virus from European-breed lambs to Culex pipiens mosquitoes. PLoS Negl Trop Dis. 2017;11:e0006145. doi: 10.1371/journal.pntd.0006145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Walter C.T., Barr J.N.. Recent advances in the molecular and cellular biology of bunyaviruses. J Gen Virol. 2011;92:2467–2484. doi: 10.1099/vir.0.035105-0. [DOI] [PubMed] [Google Scholar]
- 73.Wanja E., Parker Z., Rowland T., Turell M.J., Clark J.W., Davé K., Dave S., Sang R.. Field Evaluation of A Wicking Assay for the Rapid Detection of Rift Valley Fever Viral Antigens In Mosquitoes. J Am Mosq Control Assoc. 2011;27:370–376. doi: 10.2987/11-6176.1. [DOI] [PubMed] [Google Scholar]
- 74.Warimwe G.M., Gesharisha J., Carr B.V., Otieno S., Otingah K., Wright D., Charleston B., Okoth E., Lopez-Gil E., Lorenzo G., El-Behiry A., Alharbi N.K., Al-Dubaib M.A., Brun A., Gilbert S.C., Nene V., Hill A.V.S.. Chimpanzee Adenovirus Vaccine Provides Multispecies Protection against Rift Valley Fever. Sci Rep. 2016;6:20617. doi: 10.1038/srep20617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilson W.C., Davis A.S., Gaudreault N.N., Faburay B., Trujillo J.D., Shivanna V., Sunwoo S.Y., Balogh A., Endalew A., Ma W., Drolet B.S., Ruder M.G., Morozov I., McVey D.S., Richt J.A.. Experimental infection of calves by two genetically-distinct strains of Rift Valley fever virus. Viruses. 2016;8:145. doi: 10.3390/v8050145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wilson W.C., Kim I.J., Trujillo J.D., Sunwoo S.Y., Noronha L.E., Urbaniak K., McVey D.S., Drolet B.S., Morozov I., Faburay B., Schirtzinger E.E., Koopman T., Indran S.V., Balaraman V., Richt J.A.. Susceptibility of white-tailed deer to Rift Valley fever virus. Emerg Infect Dis. 2018;24:1717–1719. doi: 10.3201/eid2409.180265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.World Health Organization: Rift Valley Fever. 2018. https://www.who.int/news-room/fact-sheets/detail/rift-valley-fever 19 February.
- 78.World Organisation for Animal Health (OIE): List of Notifiable Diseases. 2017. http://www.oie.int/animal-health-in-the-world/oie-listed-diseases-2017/ (accessed on 25 August.
- 79.Wright D., Kortekaas J., Bowden T.A., Warimwe G.M.. Rift Valley fever: biology and epidemiology. J Gen Virol. 2019;100:1187–1199. doi: 10.1099/jgv.0.001296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Yilmaz A., Yilmaz H., Faburay B., Karakullukçu A., Kasapçopur A., Barut K., Çizmecigil U.Y., Aydin O., Tekelioglu B.K., Kasapçopur O., Ozkul A.A., LaBeaud D., Kocazeybek B., Richt J.A., Turan N.. Presence of antibodies to Rift Valley fever virus in children, cattle and sheep in Turkey. J Virol Antivir Res. 2017;6:29. [Google Scholar]
- 81.Youssouf H., Subiros M., Dennetière G., Collet L., Dommergues L., Pauvert A., Rabarison P., Vauloup-Fellous C., Le Godais G., Jaffar-Bandjee M.C., Jean M., Paty M.C., Noel H., Oliver S., Filleul L., Larsen C.. Rift Valley Fever Outbreak, Mayotte, France, 2018–2019. Emerg Infect Dis. 2020;26:769–772. doi: 10.3201/eid2604.191147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zaher M.R., Ahmed H.A., Hamada K.E.Z., Tammam R.H.. Colorimetric Detection of Unamplified Rift Valley Fever Virus Genetic Material Using Unmodified Gold Nanoparticles. Appl Biochem Biotechnol. 2018;184:898–908. doi: 10.1007/s12010-017-2592-3. [DOI] [PubMed] [Google Scholar]