Abstract
Aims:
To test whether a long-acting GLP-1 receptor agonist would improve glucose control in patients with type 1 diabetes (T1D) and to determine whether the presence of residual beta cell function would affect the response. In addition, we sought to determine whether the drug would affect beta cell function.
Methods:
We performed a randomized placebo-controlled trial of exenatide extended release (ER) in participants with T1D with and without detectable levels of C-peptide. Seventy-nine participants were randomized to exenatide ER 2 mcg weekly, or placebo, stratified by the presence or absence of detectable C-peptide levels. The primary outcome was the difference in glycated haemoglobin (HbA1c) levels at 24 weeks. Participants were followed for another 6 months off study drug.
Results:
At week 24, the time of the primary outcome, the least squares (LS) mean HbA1c level was 7.76% (95% confidence interval [CI] 7.42, 8.10) in the exenatide ER group versus 8.0% (95% CI 7.64, 8.35) in the placebo group (P = 0.08). At week 12 the LS mean HbA1c levels were 7.71% (95% CI 7.37, 8.05) in the exenatide ER group versus 8.05% (95% CI 7.7, 8.4) in the placebo group (P = 0.01). The improvement at week 12 was driven mainly by those with detectable levels of C-peptide. Those treated with exenatide ER lost weight at 12 and 24 weeks compared to those treated with placebo (P <0.001 and P = 0.007). The total insulin dose was lower, but not when corrected for body weight, and was not affected by residual insulin production. Adverse events were more frequent with exenatide ER, but hypoglycaemia was not increased.
Conclusion:
Treatment with exenatide ER may have short-term benefits in some individuals with T1D who are overweight or who have detectable levels of C-peptide, but short-term improvements were not sustained.
Keywords: adjunctive therapy, C-peptide, glucagon-like peptide-1 receptor agonist, type 1 diabetes
1 ∣. INTRODUCTION
Glucagon-like peptide-1 receptor agonists (GLP-1RAs) have become widely used for the treatment of type 2 diabetes.1-4 Their metabolic actions involve augmenting glucose-stimulated insulin release, inhibition of glucagon secretion, and slowed gastric emptying. The drug class has been found to have additional therapeutic benefits such as weight loss and reduced major cardiovascular disease events in several large randomized controlled trials.5
The metabolic properties of these agents might also be of value for patients with type 1 diabetes (T1D), particularly those with residual insulin production. Many patients, even those with long-standing T1D, may have detectable levels of C-peptide well beyond the new-onset period.6,7 Tropic effects of exendin-4 on β cells were shown in rodents after partial pancreatectomy, and synergy with immune therapy at the time of diabetes onset enhanced the insulin content of β cells.8,9 Data from human studies have identified impaired function of residual β cells in patients with T1D, thus further supporting a potential use of GLP-1RAs in these patients.10
The results from previous clinical trials of GLP-1RAs in patients with T1D, however, were inconclusive. Sarkar et al11 reported that exenatide treatment given four times daily for 6 months in adults with T1D improved insulin sensitivity, assessed by hyperinsulinaemic-euglycaemic clamp, and reduced postprandial glucose levels, although fasting glucose levels were increased. In the ADJUNCT ONE study, liraglutide, administered once daily at three dosing levels, added to insulin therapy in patients with T1D, reduced glycated haemoglobin (HbA1c) levels, total daily insulin dose and body weight but increased the rates of hypoglycaemia and hyperglycaemia with ketosis.12 Similar data were reported in the ADJUNCT TWO study, evaluating 1.2 or 1.8 mg/d of liraglutide added to capped insulin therapy.13 Recently, short-acting exenatide did not improve HbA1c levels when given for 26 weeks as add-on therapy to insulin-treated patients with T1D.14
A possible reason for these inconclusive data is that the metabolic effects of GLP-1RAs, particularly the augmentation of insulin production, might only be of value to patients with residual insulin production. In the ADJUNCT ONE trial, those with detectable C-peptide at baseline had improved responses to liraglutide compared to those without.12 In an earlier study, we analysed the acute metabolic effects of exenatide in patients with T1D during mixed-meal tolerance tests and observed a marked improvement in glucose excursion in response to oral but not to intravenous glucose.15 In those with residual insulin production, there was a relative increase in insulin secreted in response to glucose, most likely related to the reduced glucose excursion because the total amount of insulin secreted did not change with exenatide. To date, the metabolic effects of GLP-1RAs specifically comparing patients with T1D with and without residual insulin production, have not been directly studied. In addition, newer agents with weekly dosing may have a greater impact on fasting blood sugars and decreased burden of use.
We therefore conducted a randomized placebo-controlled trial to determine whether the long-acting GLP-1RA, exenatide extended release (ER), affected metabolic control in patients with stable management of T1D and whether there were differences in the responses in patients with and without detectable levels of endogenous insulin production, that is, with detectable C-peptide levels.
2 ∣. RESEARCH DESIGN AND METHODS
2.1 ∣. Trial design
A randomized double-blind phase 2b study of 2 mg exenatide ER subcutaneously weekly or matched placebo for 24 weeks in patients with T1D was conducted at seven academic sites in the United States between September 2013 and November 2017. The clinical trial was approved by the institutional review boards at each of the clinical sites and the participants signed written consent. The trial was registered at ClinicalTrials.gov: NCT01928329.
Eligible patients were 18 years and older with “stable” T1D of at least 2 years' duration (defined as insulin requirement <0.9 U/kg/d, an HbA1c of <9.0% and absence of diabetic ketoacidosis in the past 6 months; Table S1). Exclusion criteria included pregnancy, a personal or family history of multiple endocrine neoplasia type 2, history of pancreatitis, gastroparesis or other gastrointestinal disturbances, abnormal liver function tests, renal impairment, active infection, use of other antidiabetic medications other than insulin, or a history of severe hypoglycaemia.
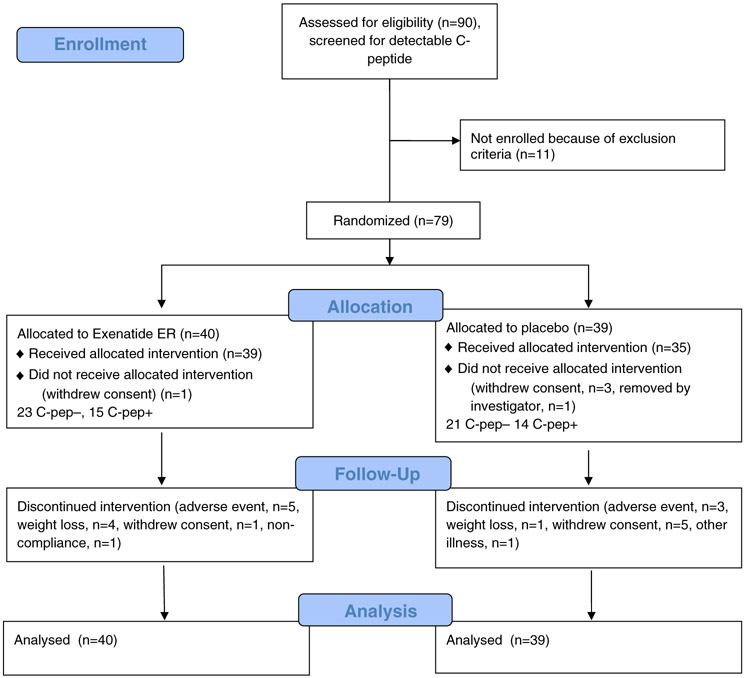
A total of 79 patients were enrolled. They were screened for detectable levels of C-peptide in response to a mixed-meal tolerance test (MMTT), performed with a liquid meal (Boost) using described methods16: 33 patients had a level during the test of ≥0.017 nmol/L and 46 had levels <0.017 nmol/L (0.05 ng/mL), the lower limit of detection in the C-peptide assay (Figure 1, Table S1). The patients were randomized 1:1 to treatment arms within the two strata. The two treatment arms were subcutaneous exenatide ER 2 mcg/wk or matched subcutaneous placebo. The patients were asked to reduce insulin by half after initiating study drug and then to change the dosing in discussion with their physician. At week 24, the patients discontinued the study drug and were followed for another 24 weeks.
FIGURE 1.
CONSORT diagram showing flow through the clinical study. C-pep−, without detectable C-peptide; C-pep+, with detectable C-peptide
Compliance was assessed through query by the study staff at each visit. Diabetes management was left to the patients' care providers; all received “intensive” management of their diabetes in line with the current American Diabetes Association (ADA) standards.17
Drug discontinuation was specified in the study protocol for the following reasons: nausea or vomiting that precluded adherence to diet; three severe hypoglycaemic reactions on separate days (requiring assistance from another individual); weight loss of ≥5 kg from baseline; or any grade 3 or higher adverse event that prevented completion of the treatments.
2.2 ∣. Assessments
After the screening visit, the patients were seen at weeks 2, 4, 12, 24, 38 and 52. C-peptide and glucose levels were measured during the 120-minute MMTT at weeks 12, 24 and 52. The average insulin use per day was determined from patient diaries that recorded insulin use for 3 days prior to a study visit. Insulin use was expressed as the total units or units/kg/d. Hypoglycaemia was graded according to the Common Terminology Criteria for Adverse Events (version 4). Hypoglycaemia was captured from patient diaries, with glucose measurements up to six times daily for 3 days prior to study visits or with symptoms. Severe hypoglycaemia was designated if assistance from others was required for recovery, or if it resulted in hospitalization or seizure.
Two-hour MMTTs were performed at each study visit. HbA1c and C-peptide (Tosoh assay) levels were measured at the Northwest Lipid Research Laboratory. In a subgroup of patients, glucagon levels were measured with the Millipore assay (n = 29) and glucagon-like peptide (GLP) and gastric inhibitory peptide (GIP) by ELISA (n = 35) in the Yale Diabetes Center Core Laboratory.
2.3 ∣. Outcome measures and statistical analysis
The primary outcome was a comparison of the HbA1c levels, corrected for the baseline, between the two treatment arms at 24 weeks. Prespecified secondary outcomes at 24 weeks included: change in weight; change in total daily insulin dose; C-peptide and glucose responses during the MMTTs; frequency of hypoglycaemia; and other adverse events, with a comparison within and between patients with and without detectable C-peptide at entry.
The original target sample size calculation was based on repeated measures of HbA1c in patients with T1D in our clinic in which the standard deviation (SD) of the HbA1c level was 1.25% and the correlation between measurements of HbA1c, performed 24 weeks apart, was 0.88. A sample size of 54 patients per group would have provided 90% power to detect a difference of HbA1c of 0.4% between the study arms. Because of rates of enrolment, the original planned 120 participants was reduced to 79 participants. This gave us 79% power to detect a difference of 0.40% in HbA1c.
The final analysis involved all enrolled patients. A likelihood-based ignorable analysis using a linear mixed model was used to compare HbA1c between groups.18,19 The analysis assumed that missing data occurred at random. Fixed effects for treatment arm, time (12, 24 and 52 weeks), and the interaction of treatment with time were tested with additional fixed effects for baseline covariates (baseline HbA1c, detectable/non-detectable baseline C-peptide, site, gender, race, body mass index). A linear model compared the least squares (LS) means of exenatide ER to placebo at 24 weeks between groups at the two-sided 0.05 significance level. In subgroup analysis to determine whether the presence of residual insulin production affected treatment response, two- and threeway interactions of that stratification factor with treatment and time were evaluated using a multiple degree of freedom likelihood ratio test at the 0.10 significance level. Linear mixed effect models similar to those described above were used to evaluate continuous secondary outcomes. For hypoglycaemic events, the number of months that an individual was on and off study drug was used to calculate an event rate (rate = total events/total months). To compare these rates between treatment arms, the Mann-Whitney test was used. The number of patients that experienced severe adverse events on and off study drug were compared using Fisher's exact test. Adjustments were not made for multiple comparisons for secondary outcomes.
2.3.1 ∣. Role of the funding source
The funders were not involved in the design or execution of the study, collection of the data, writing of the manuscript, participation in the Data Safety Monitoring Board, or the decision to submit the manuscript for publication.
3 ∣. RESULTS
3.1 ∣. Study participants
The baseline characteristics and flow of patients in the trial are shown in Figure 1 and Table S1. Of the 79 enrolled patients, five were randomized but never received study drug. (Four withdrew consent and one was withdrawn by the study team.) Twenty-one patients (28%) discontinued the study drug during the first 6 months. The reasons cited for discontinuation were: adverse events (n=8), withdrawn consent or ineligibility (n=6), weight loss >5 kg (n=5), unrelated illness (n=1) and non-compliance with insulin regimen (n=1).
The study participants' age at T1D diagnosis ranged from 2 to 50 years. Their mean (SD) baseline body weight was 83.7 (21.7) kg in the exenatide ER group and 84.13 (22.6) in the placebo group. There were no significant differences in baseline characteristics between those randomized to exenatide ER versus those randomized to placebo treatment. The mean (SD) baseline HbA1c (7.60 [±0.82]%), and daily insulin use (0.59 [0.18] U/kg/d) were consistent with features of individuals with long-standing T1D. Insulin delivery methods were similar in the two treatment arms: 20 patients (25.3%) were using multiple daily injections and 58 (73.4%) were using pumps.
Overall, 42% (33 patients) had detectable C-peptide levels at screening (Table S1B). These patients had shorter duration of T1D (mean [SD] 14.9 [10.9] years) compared to those without detectable C-peptide levels (mean [SD] 22.8 [10.3] years; P = 0.002). Among those with detectable C-peptide, those randomized to exenatide ER treatment had a significantly lower HbA1c at baseline (P = 0.03), but otherwise there were no significant differences between the subgroups.
3.2 ∣. Primary outcome
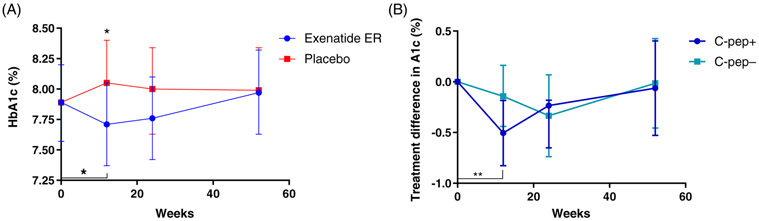
At the primary endpoint, week 24, the effects of exenatide ER were not statistically different compared to placebo (group differences −0.237 [95% CI 0.50, 0.03], LS mean 7.76% [95% CI 7.42, 8.10] in the exenatide ER group vs. 8.0% [95% CI 7.64, 8.35] in the placebo group; P = 0.08 [Figure 2A]). Exenatide ER treatment did have a rapid initial effect on the HbA1c. There was a significant decline in the active drug arm from baseline to 12 weeks (an LS mean difference −0.179% [95% CI −0.352, −0.004]), and a difference in the HbA1c levels of 7.71% (95% CI 7.37, 8.05) versus 8.05% (95% CI 7.7, 8.4) between exenatide ER and placebo, respectively (P = 0.01). In the observational follow-up at week 52, 24 weeks after study drug discontinuation, the HbA1c level increased in the exenatide ER group to the pretreatment levels.
FIGURE 2.
Effects of exenatide extended release (ER) treatment on glycated haemoglobin (HbA1c) levels. A, HbA1c levels in the two treatment arms at each study visit. There was a significant reduction in the HbA1c level in the exenatide ER group at 12 weeks (P = 0.045) and the levels were significantly different from the placebo group (P = 0.01). However, at 24 weeks, the differences between the groups were not statistically significant (P = 0.08). B, In those with a detectable level of C-peptide (C-pep+) at baseline (C-peptide ≥0.017 nmol/L), there was a significant reduction, compared to baseline, in the HbA1c level at 12 weeks (P = 0.0025) but not in those with undetectable C-peptide levels (C-pep−; C-peptide < 0.017 nmol/L). The treatment changes in each subgroup taken from the linear mixed model are shown. All data shown are from the linear mixed model (mean ± 95% confidence interval)
The decline in HbA1c at 12 weeks was largely driven by those with detectable C-peptide (mean −0.51% [95% CI −0.827, −0.184]; P = 0.0025 vs. baseline) versus those without detectable C-peptide (mean −0.143% [95% CI −0.447, 0.162]), but the differences between those with and without detectable C-peptide were not statistically significant at that time (mean 0.363 [95% CI −0.08, 0.806]; P = 0.107) or at 24 weeks (mean −0.101% [95% CI −0.68, 0.479]; P = 0.73 [Figure 2B]). At 52 weeks, the declines in HbA1c from the baseline were <0.1% in both subgroups.
3.3 ∣. Secondary outcomes
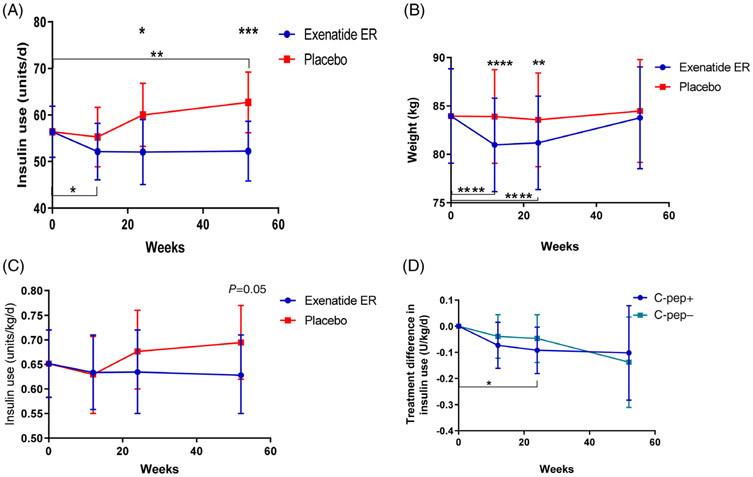
Daily insulin dose declined significantly at 12 weeks in the exenatide ER group compared to baseline (P = 0.04; Figure 3A). At the same time, the total daily insulin dose increased in the placebo group, leading to a significant difference at week 24 when the two treatment arms were compared (P = 0.025). The difference between the treatment arms continued even at 52 weeks due to an increase in insulin use among those originally assigned to placebo treatment (P <0.001).
FIGURE 3.
Effects of exenatide extended release (ER) treatment on insulin use and weight. A, The total daily insulin use (U/d) in the two treatment arms is shown. There was a reduction in the use of insulin in the exenatide ER group at 12 weeks compared to baseline (P = 0.038). At 24 weeks insulin use in the exenatide group was significantly less than in the placebo group (P = 0.025). At 52 weeks insulin use in the placebo group was increased compared to baseline (P = 0.008) and was significantly greater than in the patients that were treated with exenatide ER during the first 6 months (P = 0.0009). B, There was significant loss in weight in the exenatide ER- versus the placebo-treated patients at 12 weeks (P = 0.003) and 24 weeks (P = 0.017). C, Insulin use corrected for body weight (U/kg/d). D, A comparison of the treatment difference (vs. placebo) in the use of insulin in those with (C-pep+) and without detectable C-peptide (C-pep−) at baseline. All data shown are from the linear mixed models (mean [95% CI])
At both weeks 12 and 24, those treated with exenatide ER lost more weight from baseline than those treated with placebo (group differences mean −2.93 kg [95% CI −4.33, −1.5]; P <0.0001 and −2.38 kg [95% CI −4.11, −0.644]; P = 0.0078, respectively [Figure 3B]). At 52 weeks, the median weight loss in both treatment arms was <1 kg compared to baseline weight (exenatide ER −0.176 kg [95% CI −2,1.65] and placebo 0.52 kg [95% CI −1.45, 2.49]).
When the insulin dose was corrected for body weight, there was no significant difference with exenatide ER treatment compared to placebo (Figure 3C). The presence or absence of detectable C-peptide did not have a significant effect on the change in insulin dose, either total U/d or U/kg/d, with treatment at 12, 24 or 52 weeks (Figure 3D). In contrast to that reported for patients with T2D,3 we did not find a significant relationship between the change in weight and the change in HbA1c (Figure 1A,B).
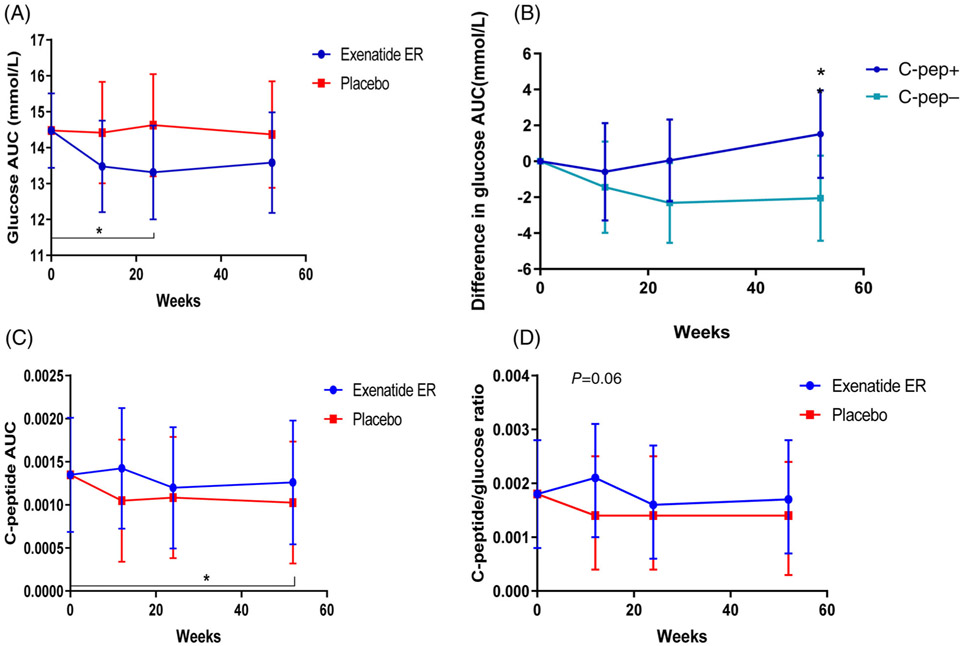
The glucose response during the MMTT improved significantly from baseline at 24 weeks in the exenatide ER-treated patients (P = 0.04, Figure 4A). However, there was no significant difference from the glucose areas under the curve (AUCs) in the exenatide ER- versus the placebo-treated patients at any of the time points. Among those without detectable C-peptide, the glucose AUC had declined from baseline at 24 weeks (P = 0.04) and was significantly lower than among those with detectable C-peptide at 52 weeks (P = 0.04; Figure 4B). To determine whether the exenatide ER treatment improved insulin secretory responses, we analysed the effects of exenatide ER on C-peptide responses during the MMTT in those with detectable levels at baseline. The differences between the two treatment arms were not significantly different at baseline or three time points, but there was a trend for improvement in the C-peptide at 12 weeks between the treatment groups (group difference = 0.000374 [−0.00004, 0.000791]; P = 0.08). This was attributable primarily to a decline in the placebo group from baseline (−0.0003 [−0.00061, 0.000012]; P = 0.06) and there was a significant decline at week 52 (P = 0.04) in the placebo group (Figure 4C). Our previous studies had suggested an improvement in the C-peptide:glucose ratio with short-acting exenatide, but we did not find a significant change or difference between treatment arms with exenatide ER treatment (Figure 4D).
FIGURE 4.
Effects of exenatide extended release (ER) treatment on glucose and C-peptide responses. A, There was a significant reduction in the glucose area under the curve (AUC) at week 24 in the exenatide ER group compared to baseline (P = 0.04), but not compared to placebo (P = 0.1). B, There was a significant improvement, compared to baseline vs. placebo, in the group without detectable C-peptide at week 24 (P = 0.04). At 52 weeks there was a greater effect on the glucose AUC in the patients that did not have detectable C-peptide (C-pep−) compared to the patients that did (C-pep+; P = 0.04). C, C-peptide AUC (in pmol/mL/min) was compared between the exenatide ER- and placebo-treated patients by linear mixed model at each of the study time points in those with detectable C-peptide levels at baseline. Data are shown as ln(AUC/120 min + 1). There was a significant decline at 52 weeks, compared to the baseline, in patients treated with placebo for the first 24 weeks (P = 0.04). D, C-peptide:glucose ratio was compared in the two treatment arms for those with detectable C-peptide levels at baseline. There was a modest effect of exenatide ER on the C-peptide/glucose ratio at 12 weeks in comparison with placebo (P = 0.06). Units of nmol/L and mg/dl were used for calculation of the C-peptide/glucose ratio
Plasma glucagon levels were measured before and after therapy with exenatide ER in nine patients. The glucagon levels did not show a clear pattern of response, either in the AUC during the MMTT or in the peak value. In these same patients we did not detect a change in plasma GIP or GLP levels (Figure S2).
The frequencies of hypoglycaemic events are summarized in Table 1. Hypoglycaemia was classified using ADA criteria.17 To standardize these measures, the number of months that an individual was on and off study drug was used to calculate an event rate (rate = total events/total months). While on drug, the placebo treatment arm had higher mean and median rates of minor, major and total hypoglycaemic events compared to the intervention arm, but the frequency of these events was not significantly different. While off study drug (months 6–12), the placebo treatment arm continued to have higher mean and median rates of minor, major and total hypoglycaemic events, but were again not significantly different. The frequency and severity of hypoglycaemic events were not evenly distributed among the patients. One individual, treated with exenatide ER, had 115 events. Another exenatide ER-treated patient had a grade 3 major event with loss of consciousness.
TABLE 1.
Hypoglycaemic eventsa
| Timeframe | Arm | Variable | N | Mean | SE | Median | Minimumb | Maximumb |
|---|---|---|---|---|---|---|---|---|
| On drug | Exenatide ER | Level 1 hypoglycaemia event rate | 39 | 2.07 | 0.44 | 1.00 | 0 | 11.33 |
| Level 2 hypoglycaemia event rate | 39 | 1.82 | 0.50 | 0.67 | 0 | 13.00 | ||
| Total event rate | 39 | 3.89 | 0.88 | 1.33 | 0 | 21.67 | ||
| Placebo | Level 1 hypoglycaemia event rate | 35 | 2.60 | 0.54 | 1.60 | 0 | 13.00 | |
| Level 2 hypoglycaemia event rate | 35 | 2.00 | 0.43 | 0.80 | 0 | 10.50 | ||
| Total event rate | 35 | 4.59 | 0.94 | 2.33 | 0 | 23.50 | ||
| Off drug | Exenatide ER | Level 1 hypoglycaemia event rate | 37 | 1.09 | 0.29 | 0.17 | 0 | 8.33 |
| Level 2 hypoglycaemia event rate | 37 | 0.74 | 0.19 | 0.33 | 0 | 5.00 | ||
| Total event rate | 37 | 1.83 | 0.45 | 0.50 | 0 | 11.17 | ||
| Placebo | Level 1 hypoglycaemia event rate | 26 | 1.81 | 0.53 | 0.50 | 0 | 12.50 | |
| Level 2 hypoglycaemia event rate | 26 | 1.62 | 0.55 | 0.50 | 0 | 13.17 | ||
| Total event rate | 26 | 3.43 | 1.05 | 1.00 | 0 | 25.67 |
Hypoglycaemia was defined using American Diabetes Association criteria (level 1 between 55 mg/dL (3.05 mmol/L) and 70 mg/dL (3.89 mmol/L), level 2 hypoglycaemia ≤55mg/dL (3.05 mmol/L).17
Events/study month/person.
3.4 ∣. Adverse events
While on study drug, 38 out of 39 patients in the active drug (exenatide ER) group (97.4%) experienced at least one adverse event, while in the placebo group, a total of 28 out of 35 patients (80.0%) experienced at least one adverse event (Table S2; P = 0.02). There was a significant difference between the drug group (n = 22, 56.4%) and the placebo group (n = 8, 22.9%) with respect to gastrointestinal disorders. Skin manifestations were more frequent in the exenatide ER group. However overall, there were no significant differences between the treatment groups in the other organ class adverse event categories nor with respect to grade 3 and grade 4 events.
While off study drug, 29 out of 37 original exenatide ER-treated patients (78.4%) experienced at least one adverse event, while in those originally treated with placebo, 25 out of 26 patients (96.2%) experienced at least one adverse event (P = 0.069). A greater proportion of those in the placebo group (n = 22, 84.6%) compared to the active treatment group (n = 23, 62.2%) experienced adverse events related to metabolism and nutrition disorders. There were no significant differences between the treatment groups on other organ class-specific adverse events, nor on grade 3 or grade 4 adverse events during the off study drug phase.
There were a total of eight serious adverse events, six in the exenatide ER arm while taking study drug and two in the placebo group. One of the events in the exenatide ER arm involved ketoacidosis. The most frequent serious event was hypoglycaemia. These events are shown in Table S3. Of the serious adverse events, all except for the hypoglycaemia were considered unrelated to study drug.
4 ∣. DISCUSSION
We tested whether treatment with exenatide ER for 24 weeks would improve glycaemic control in patients with T1D on stable insulin regimens, and examined the role of residual C-peptide in determining the responses. Because GLP-1RAs improve endogenous glucose-stimulated insulin secretion, we postulated that the effects of the drug would be greater in those with residual insulin production compared to those in whom C-peptide was undetectable. We found that the primary endpoint of the trial, change in HbA1c levels at 24 weeks, was not significantly different when exenatide ER-treated patients with T1D were compared to placebo treatment, but we did find improvement between the treatment arms in the HbA1c levels at 12 weeks after starting drug therapy. There was no lasting effect on HbA1c, as levels 6 months after the study drug was discontinued were similar in the two study arms, suggesting that the continued presence of drug was needed for metabolic effects. The drug treatment caused weight loss which resolved when the treatment was discontinued. Total insulin use declined but, when corrected for weight, there was no significant difference either between the groups or compared to baseline, suggesting that the exenatide ER treatment did not improve insulin sensitivity. Hypoglycaemia was common in all study participants and there were more severe hypoglycaemic events in the exenatide ER-treated patients, although the rate was low overall. The frequency of skin manifestations with exenatide ER injections was higher than with placebo. There was one episode of diabetic ketoacidosis and three episodes of hypoglycaemia that were classified as serious adverse events in the exenatide ER arm. Other adverse events were similar in the two treatment arms.
The improvement in HbA1c level at 12 weeks was observed in those with and without residual insulin production at study entry, but the effect was greater in those with detectable C-peptide. Other measures, such as insulin use or glucose AUC during the MMTTs, were not different in those with and without residual insulin production. Because GLP-1RAs are known to augment insulin production we predicted a greater treatment effect in those with residual insulin secretion but, similar to our acute studies, the metabolic effects of the drug were not limited to those with residual insulin secretion.2,15 Our findings were similar even when we separately analysed those with the highest levels of C-peptide at baseline (not shown). Interestingly, we found a trend in improved C-peptide responses in the exenatide ER-treated versus the placebo-treated patients in terms of stimulated responses in the treatment group but also compared to the decline in the placebo group. This is most likely explained by the relatively short duration of diabetes in those with detectable C-peptide and the ongoing decline over 1 year, reflective of the natural history of the disease. Therefore, together with the HbA1c data, these findings suggest but do not conclusively indicate, that the drug may have additional benefit in those with residual β-cell function.
The adverse events were consistent with the experience of GLP-1RAs in T2D, but the rates of hypoglycaemia overall were higher.3,4,20 We observed other differences compared to the described effects in patients with T2D. First, exenatide ER had been shown to reduce glucagon levels in patients with T2D, but we did not observe this during the provocative studies.3 This may reflect a relative insensitivity or dysregulation of α cells in patients with T1D to the effects of the agonist, which had been observed in acute studies.15 In addition, we did not find a relationship between weight loss and the improvement in HbA1c or insulin use. There may be additional effects of the drug on insulin sensitivity in patients with both forms of diabetes, as suggested by Rother et al.21
Our findings differ from other studies of GLP-1RAs in patients with T1D. In the ADJUNCT ONE trial, addition of liraglutide to insulin therapy reduced HbA1c levels, total insulin dose and body weight, but also increased the rates of symptomatic hypoglycaemia and hyperglycaemia with ketosis.12 In the ADJUNCT TWO trial, liraglutide, added to capped insulin, reduced HbA1c levels, body weight and insulin requirements, but with higher rates of hypoglycaemia and ketosis.13 The differences between the adverse events in the present study and the ADJUNCT trials may reflect our reduction in exogenous insulin treatment when the study drug was initiated, or possibly the differing pharmacokinetics of the GLP-1RAs given once weekly versus daily. Indeed, with acute administration of exenatide we found a flattening of the glucose response during an MMTT, which was not seen in the present study.15 Our findings suggest a more robust response of HbA1c than was seen in the recently reported trial of exenatide, given three times daily to patients with T1D.14
The observed rates of hypoglycaemia were high but not higher in the exenatide ER versus the placebo arm, but there were six severe hypoglycaemic events in three exenatide ER-treated patients. The rates were higher in the exenatide ER-treated group on versus off study drug. This suggests that the reduced need for exogenous insulin may not affect the rates of hypoglycaemia overall, but there may be particular individuals at high risk for hypoglycaemia when exenatide ER is given in addition to insulin.
In the absence of clear enhancement of insulin secretion, reduced glucagon release, and change in insulin sensitivity with exenatide ER, the basis for the improvement in HbA1c after 12 weeks remains unexplained. It is possible that the metabolic effects that we had seen with the acute administration of exenatide also occurred with the long-acting formulation of the drug, but were more modest, and that our assays to detect these effects were limited by the sample size or that there was tachyphylaxis to the long-term GLP-1RA exposure. It is also possible that other mechanisms are involved, such as slowing gastric emptying or changes in dietary patterns in response to the gastrointestinal adverse events that resulted in improved glycaemic control, in the short term. Finally, GLP-1RAs have been found to have anti-inflammatory effects which could account for improved metabolic control.22 However, we did not find changes in immune cells (CD4, CD8) or markers of cell activation (RAGE expression) with exenatide ER treatment (not shown).23
The present study has a number of limitations. The total sample size was insufficient to detect the difference in the HbA1c level that we had originally planned. In addition, not all of the patients completed the 6-month follow-up visit to determine whether any effects of the drug treatment may have persisted. Furthermore, our study design did not entail a treat-to-target regimen or capped insulin dose that had been used in the ADJUNCT trials and, therefore, the management of the patients may have varied based on the care provider. Finally, the patients were heterogeneous, as reflected by the shorter duration of disease in those with residual insulin production, which may have affected the management patterns or the responses to the drug. Nonetheless, the patients are representative of those seen in practice, with clinical features such as increased body mass index and residual insulin production that might suggest the appropriateness of a GLP-1RA for treatment.
In conclusion, in this clinical trial of exenatide ER in patients with T1D, we did not find a significant improvement in HbA1c after 6 months of treatment, but HbA1c levels were significantly reduced after 12 weeks. The effects of the drug treatment in the short term were more pronounced in those with residual insulin production but not significantly different from those without detectable C-peptide. Weight loss was common, but the rates of hypoglycaemia were similar in the two treatment arms. We conclude that adjunctive treatment with exenatide ER may have value in some individuals with T1D, mainly those with obesity and in whom there is residual insulin production, but the short-term improvements were not sustained. The reduced dependence on exogenous insulin without increased rates of hypoglycaemia may make this adjunctive therapy attractive, but caution should be exercised in view of the higher rates of hypoglycaemia in some patients. In addition, the emerging beneficial effects of GLP-1RAs on cardiovascular and renal disease suggest there may be additional benefits of these agents, but further studies will be needed to determine whether these other beneficial effects are common to T1D.24-29
Supplementary Material
ACKNOWLEDGMENTS
We wish to thank Drs Barbara Gulanski, Richard Kibbey, Tassos Kyriakides and LaToya Howard, who served on the Data Safety and Monitoring Board. We also thank Julie Holub and Alyssa Gateman for their administrative assistance.
Support was obtained by JDRF grant 17-2013-504 and 5-ECR-2016-186-A-N, as well as the following grants from the National Institute of Health: P30DK045735, P30DK02059, UL1TR000142, UL1 TR001872, UL1TR000004, P30DK036836, R01DK107956-01, R01DK057846, and U01DK119083.
Footnotes
CONFLICTS OF INTEREST
None declared.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
Peer Review
The peer review history for this article is available at https://publons.com/publon/10.1111/dom.14121.
REFERENCES
- 1.Drucker DJ. The ascending GLP-1 road from clinical safety to reduction of cardiovascular complications. Diabetes. 2018;67(9):1710–1719. [DOI] [PubMed] [Google Scholar]
- 2.Drucker DJ. Mechanisms of action and therapeutic application of glucagon-like Peptide-1. Cell Metab. 2018;27(4):740–756. [DOI] [PubMed] [Google Scholar]
- 3.Drucker DJ, Buse JB, Taylor K, et al. Exenatide once weekly versus twice daily for the treatment of type 2 diabetes: a randomised, open-label, non-inferiority study. Lancet. 2008;372(9645):1240–1250. [DOI] [PubMed] [Google Scholar]
- 4.Kolterman OG, Buse JB, Fineman MS, et al. Synthetic exendin-4 (exenatide) significantly reduces postprandial and fasting plasma glucose in subjects with type 2 diabetes. J Clin Endocrinol Metab. 2003; 88(7):3082–3089. [DOI] [PubMed] [Google Scholar]
- 5.Husain M, Donsmark M, Bain SC. Oral Semaglutide and cardiovascular outcomes in type 2 diabetes. Reply. N Engl J Med. 2019;381(21): 2076–2077. [DOI] [PubMed] [Google Scholar]
- 6.Oram RA, Jones AG, Besser RE, et al. The majority of patients with long-duration type 1 diabetes are insulin microsecretors and have functioning beta cells. Diabetologia. 2014;57(1):187–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Keenan HA, Sun JK, Levine J, et al. Residual insulin production and pancreatic ss-cell turnover after 50 years of diabetes: Joslin Medalist Study. Diabetes. 2010;59(11):2846–2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Xu G, Stoffers DA, Habener JF, Bonner-Weir S. Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes. 1999;48(12):2270–2276. [DOI] [PubMed] [Google Scholar]
- 9.Sherry NA, Chen W, Kushner JA, et al. Exendin-4 improves reversal of diabetes in NOD mice treated with anti-CD3 monoclonal antibody by enhancing recovery of beta-cells. Endocrinology. 2007;148(11): 5136–5144. [DOI] [PubMed] [Google Scholar]
- 10.Sims EK, Bahnson HT, Nyalwidhe J, et al. Proinsulin secretion is a persistent feature of type 1 diabetes. Diabetes Care. 2019;42(2): 258–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sarkar G, Alattar M, Brown RJ, Quon MJ, Harlan DM, Rother KI. Exenatide treatment for 6 months improves insulin sensitivity in adults with type 1 diabetes. Diabetes Care. 2014;37(3):666–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mathieu C, Zinman B, Hemmingsson JU, et al. Efficacy and safety of liraglutide added to insulin treatment in type 1 diabetes: the ADJUNCT ONE treat-to-target randomized trial. Diabetes Care. 2016; 39(10):1702–1710. [DOI] [PubMed] [Google Scholar]
- 13.Ahren B, Hirsch IB, Pieber TR, et al. Efficacy and safety of Liraglutide added to capped insulin treatment in subjects with type 1 diabetes: the ADJUNCT TWO randomized trial. Diabetes Care. 2016;39(10):1693–1701. [DOI] [PubMed] [Google Scholar]
- 14.Johansen NJ, Dejgaard TF, Lund A, et al. Efficacy and safety of meal-time administration of short-acting exenatide for glycaemic control in type 1 diabetes (MAG1C): a randomised, double-blind, placebo-controlled trial. Lancet Diabetes Endocrinol. 2020;8(4):313–324. [DOI] [PubMed] [Google Scholar]
- 15.Ghazi T, Rink L, Sherr JL, Herold KC. Acute metabolic effects of exenatide in patients with type 1 diabetes with and without residual insulin to oral and IV glucose challenges. Diabetes Care. 2014;37(1): 210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Herold KC, Gitelman SE, Ehlers MR, et al. Teplizumab (anti-CD3 mAb) treatment preserves C-peptide responses in patients with new-onset type 1 diabetes in a randomized controlled trial: metabolic and immunologic features at baseline identify a subgroup of responders. Diabetes. 2013;62:3766–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Professional Practice Committee. Standards of medical care in diabetes-2019. Diabetes Care. 2019;42(Suppl 1):S3. [DOI] [PubMed] [Google Scholar]
- 18.Lachin JL. Statistical considerations in the intent-to-treat principle. Control Clin Trials. 2000;21(5):526. [DOI] [PubMed] [Google Scholar]
- 19.Molenberghs G, Thijs H, Jansen I, et al. Analyzing incomplete longitudinal clinical trial data. Biostatistics. 2004;5(3):445–464. [DOI] [PubMed] [Google Scholar]
- 20.Buse JB, Henry RR, Han J, Kim DD, Fineman MS, Baron AD. Effects of exenatide (exendin-4) on glycemic control over 30 weeks in sulfonylurea-treated patients with type 2 diabetes. Diabetes Care. 2004;27(11):2628–2635. [DOI] [PubMed] [Google Scholar]
- 21.Rother KI, Spain LM, Wesley RA, et al. Effects of exenatide alone and in combination with daclizumab on beta-cell function in long-standing type 1 diabetes. Diabetes Care. 2009;32(12):2251–2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yaribeygi H, Maleki M, Sathyapalan T, Jamialahmadi T, Sahebkar A. Anti-inflammatory potentials of incretin-based therapies used in the management of diabetes. Life Sci. 2020;241:117152. [DOI] [PubMed] [Google Scholar]
- 23.Durning SP, Preston-Hurlburt P, Clark PR, Xu D, Herold KC. Type 1 diabetes TrialNet study G. the receptor for advanced glycation end-products drives T cell survival and inflammation in type 1 diabetes mellitus. J Immunol. 2016;197(8):3076–3085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elnour AA, Al Hajri N, El Khidir IY, Al Amoodi A, Ahmed SA, Sadeq A. Glucagon-like peptide-1 receptor agonists cardio-protective effects: an umbrella review. Curr Diabetes Rev. 2020;16. 10.2174/1573399816666200522214554. [DOI] [PubMed] [Google Scholar]
- 25.van der Aart-van der Beek AB, van Raalte DH, Guja C, et al. Exenatide once weekly decreases urinary albumin excretion in patients with type 2 diabetes and elevated albuminuria: pooled analysis of randomized active controlled clinical trials. Diabetes Obes Metab. 2020. 10.1111/dom.14067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bethel MA, Patel RA, Merrill P, et al. Cardiovascular outcomes with glucagon-like peptide-1 receptor agonists in patients with type 2 diabetes: a meta-analysis. Lancet Diabetes Endocrinol. 2018;6(2):105–113. [DOI] [PubMed] [Google Scholar]
- 27.Thomas MC. The potential and pitfalls of GLP-1 receptor agonists for renal protection in type 2 diabetes. Diabetes Metab. 2017;43(Suppl 1):2S20–2S27. [DOI] [PubMed] [Google Scholar]
- 28.Rawshani A, Sattar N, Franzen S, et al. Excess mortality and cardiovascular disease in young adults with type 1 diabetes in relation to age at onset: a nationwide, register-based cohort study. Lancet. 2018; 392(10146):477–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Livingstone SJ, Levin D, Looker HC, et al. Estimated life expectancy in a Scottish cohort with type 1 diabetes, 2008-2010. JAMA. 2015;313(1):37–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.