Abstract
Purpose:
The RET proto-oncogene encodes a receptor tyrosine kinase which is activated by gene fusion in 1-2% of non-small cell lung cancers (NSCLC) and rarely in other cancer types. Selpercatinib is a highly selective RET kinase inhibitor that has recently been approved by the FDA in lung and thyroid cancers with activating RET gene fusions and mutations. Molecular mechanisms of acquired resistance to selpercatinib are poorly understood.
Patients and Methods:
We studied patients treated on the first-in-human clinical trial of selpercatinib (NCT03157129) who were found to have MET amplification associated with resistance to selpercatinib. We validated MET activation as a targetable mediator of resistance to RET-directed therapy, and combined selpercatinib with the MET/ALK/ROS1 inhibitor crizotinib in a series of single patient protocols (SPPs).
Results:
MET amplification was identified in post-treatment biopsies in four patients with RET fusion-positive NSCLC treated with selpercatinib. In at least one case, MET amplification was clearly evident prior to therapy with selpercatinib. We demonstrate that increased MET expression in RET fusion-positive tumor cells causes resistance to selpercatinib, and this can be overcome by combining selpercatinib with crizotinib. Using SPPs, selpercatinib with crizotinib were given together generating anecdotal evidence of clinical activity and tolerability, with one response lasting 10 months.
Conclusions:
Through the use of SPPs we were able to offer combination therapy targeting MET-amplified resistance identified on the first-in-human study of selpercatinib. These data provide suggest that MET dependence is a recurring and potentially targetable mechanism of resistance to selective RET inhibition in advanced NSCLC.
Keywords: RET fusion, RET inhibitor, Drug resistance, MET amplification, NSCLC
INTRODUCTION
Combination targeted therapy represents a compelling strategy for overcoming drug resistance in metastatic cancer. However, the clinical development of combination approaches has been challenging due to toxicity from combining two agents and the need for appropriate patient selection. While several combination therapies are approved (e.g. MEK inhibition with BRAF inhibition in BRAF-mutant melanoma; CDK4/6 inhibition with endocrine therapy in ER+ breast cancer)1-4, no drug combination has yet met the standard of regulatory approval for effective treatment of resistance to targeted kinase inhibitors (TKIs) in genotype-selected patients. Since resistance to targeted TKIs is universal, effective strategies to overcome acquired resistance are key to prolonging clinical benefit. To that end, combination approaches remain a compelling investigational strategy in oncogene-dependent non-small cell lung cancer (NSCLC) with several clinical trials ongoing5-8.
The RET proto-oncogene encodes a receptor tyrosine kinase which is activated by gene fusion in 1-2% of NSCLC and rarely in many other tumor types. RET gene fusions are bona fide cancer drivers and they display the key characteristics of oncogene addiction preclinically9. Selpercatinib is a highly selective and potent anti-RET tyrosine kinase inhibitors (TKI) which has recently reported durable responses in lung and thyroid cancers, and these responses were maintained regardless of the specific RET alteration or prior TKI use, and also in the setting of the RET V804 “gatekeeper” mutation10. Selpercatinib was recently approved by the FDA for use in these cancers. Mechanisms of acquired resistance to treatment with selective RET inhibitors are not well understood. While a secondary mutation in the RET kinase domain has recently been reported11, activation of bypass tracts, such as MET amplification, also represent a recurring mechanism of resistance to driver genotypes in NSCLC12,13. Here we piloted combination therapy to target MET amplification detected in four RET-positive NSCLC patients (of a total 79 patients with NSCLC enrolled at all three sites) with resistance to selpercatinib. This was made possible through the use of multiple SPPs which allowed for the quick delivery of potentially effective combination therapy to patients with clear unmet clinical need.
METHODS
Analysis of resistance to selpercatinib
Patients were included in this analysis if they had received selpercatinib (LOXO-292) for RET-positive NSCLC on the first-in-human study of selpercatinib (NCT03157128) and exhibited evidence of MET amplification following drug resistance. All patients provided written informed consent wherever necessary. Genomic analysis of tumor and plasma cfDNA was performed independently at each participating site. All specimens were studied at each participating institution with IRB approval and were analyzed in accordance with the Declaration of Helsinki.
Preclinical RET-dependent models
HBEC-RET cell lines expressing a CCDC6-RET fusion were engineered to overexpress MET and a patient derived organoid was also established. These models were subsequently studied to investigate the role of MET in selpercatinib resistance (see Supplemental Methods).
Single patient protocols of selpercatinib and crizotinib
Each single-patient protocol was sponsored by LOXO and drafted in collaboration between LOXO and the site primary investigator. Each protocol enrolled a single patient after review by the FDA and approval by the site IRB. Dosing was individualized per patient and dose escalation was permitted as tolerated up to the established tolerable dose for each drug. Patients initiated combination therapy directly after demonstrating resistance to prior targeted therapy (Table 1, Supplemental Figure 1).
Table 1.
Disease Course and Treatment History Summary.
| Case 1 | Case 2 | Case 3 | Case 4 | |
|---|---|---|---|---|
| Clinical presentation | 36 yo former smoker (18 py), multiple prior lines of therapy, recent alectinib | 48 yo former light smoker (<1 py) s/p first-line pembrolizumab | 69 yo never-smoker, second-line | 61 yo never-smoker, s/p first-line pembrolizmab |
| Pretreatment genotype | EML4-RET fusion on plasma NGS, prior MET CNG | KIF5B-RET fusion on tumor NGS | KIF5B-RET fusion on tumor NGS, also high MET amplification | KIF5B-RET fusion on tumor NGS, also MET CNG |
| Treatment duration on selpercatinib monotherapy and dosing | 6.5 months (40mg BID → 160mg BID) | 11 months (20mg BID → 80mg BID) | 3 months (80mg BID) | 6 months (160mg BID → 120mg BID) |
| Treatment duration on selpercatinib & crizotinib and dosing | 3.5 months (80mg BID → 160mg BID; 250mg QD → BID) | 10 months (160mg BID; 250mg BID) | 4 months (80mg BID → 160mg BID; 250mg BID) | 4 months (160mg BID; 250mg BID) |
| Outcome of combination therapy | Mixed response | Durable response | Brief response | Brief response |
| Adverse Events | Nausea | Lower extremity edema, reflux | Myocardial infarction (not attributed to study drugs) | Severe colitis (not attributed to study drugs) |
RESULTS
MET-dependent resistance to selpercatinib in patients with advanced NSCLC
Case 1:
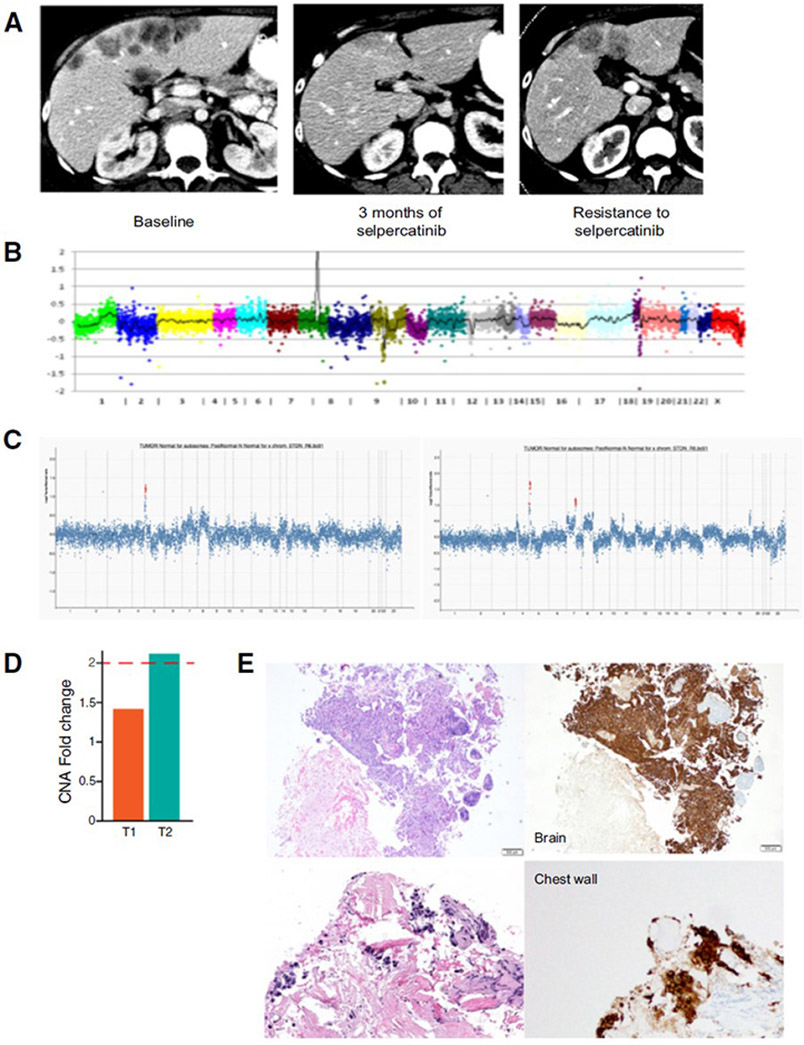
The first patient was a 36-year-old female former smoker with stage IV NSCLC metastatic to bone, heavily pretreated. Molecular testing identified a RET rearrangement by break-apart fluorescence in situ hybridization (FISH; 83% of cells) as well as MET copy number gain (CNG) by FISH (6 copies). She initiated treatment with alectinib, an ALK TKI with some degree of anti-RET activity preclinically14, and progressed in less than 2 months. Next-generation sequencing (NGS) analysis of plasma circulating cell-free tumor DNA (cfDNA) then identified an EML4-RET fusion (AF 14%)15. She started treatment with selpercatinib and experienced a clinical response (decreased tumor-related pain and anorexia) with radiographic tumor reduction on imaging (−21% decrease in tumor burden after 16 weeks, Figure 1A, Table 1). She progressed after 4.5 months with growth of liver metastases and a new skin nodule on the neck. Biopsy of the skin nodule revealed metastatic adenocarcinoma and molecular analysis by next-generation sequencing (NGS) re-identified the EML4-RET fusion as well as increased MET copy number (56 copies, Figure 1B). Given ongoing clinical benefit, she continued on selpercatinib post-progression for a total treatment time of 6.5 months.
Figure 1. MET amplification identified in RET fusion-positive lung cancers treated with a selective RET inhibitor.
A. Patient 1 had a symptomatic response to selpercatinib with radiographic tumor reduction on imaging (−21% decrease in tumor burden) after 16 weeks, but eventually developed resistance to drug. B. Tumor NGS at time of resistance showed, in addition to the original EML4-RET fusion, high amplification of MET (56 copies). C, D. In patient 4, NGS showed MET amplification in the post-treatment sample (T2), with lower level gain below threshold for amplification in the pre-treatment biopsy (T1)). E. The presence of MET overexpression (right) was confirmed with MET IHC both pre-treatment (top) and at time of resistance (bottom).
Case 2:
The second patient was a 48-year-old male former light smoker with stage IV PD-L1-positive NSCLC that harbored a KIF5B-RET fusion identified on tumor NGS. After progression on first-line pembrolizumab, he initiated selpercatinib and achieved a partial response after 3 months of therapy (best response of 49% decrease in target lesions, Table 1). He eventually developed disease progression after 11 months on selpercatinib. NGS analysis of paired pre-treatment and post-progression tumor samples identified acquired MET amplification (9 copies) completely absent from the pre-treatment sample.
Case 3:
The third patient was a 69 year-old Asian male nonsmoker diagnosed with stage IV NSCLC, and after developing a solitary brain metastasis he underwent craniotomy. Tumor NGS (Foundation One) identified a KIF5B-RET fusion and MET amplification (18 copies) in brain. He initiated treatment with selpercatinib which resulted in shrinkage of pulmonary nodules and improvement in clinical symptoms, but he also developed a new left adrenal nodule, indicating disease progression. RNA sequencing was performed on the previously resected brain metastasis (Illumina TST-170) which confirmed high-level MET mRNA expression. He then initiated crizotinib monotherapy given his MET gene amplification and overexpression, and he again demonstrated a mixed response, with decrease in size of the left adrenal nodule but growth of a right adrenal nodule and left hilar adenopathy; he remained on crizotinib for 3 months.
Case 4:
The fourth patient was a 61-year old woman, never smoker, with stage IV lung adenocarcinoma metastatic to brain, PD-L1 positive. After progression on first-line pembrolizumab, tumor NGS identified a KIF5B-RET fusion and selpercatinib was initiated. The patient had a best response of stable disease (1% increase in the sum of tumor diameters at six weeks) but subsequently progressed after 6 months and discontinued treatment. NGS analysis of post-treatment tumor showed MET amplification (fold change 2.1), while the pre-treatment biopsy showed low-level MET CNG without frank amplification (Figure 1C-D). By FISH, the post-treatment biopsy showed MET amplification (66% of cells) while the pretreatment biopsy noted MET polysomy (3-6 copies) in 78% of cells. The presence of MET overexpression was subsequently confirmed by immunohistochemistry at both timepoints (Figure 1E).
MET overexpression causes acquired resistance to selpercatinib preclinically and may be overcome by combining selpercatinib with the MET inhibitor crizotinib.
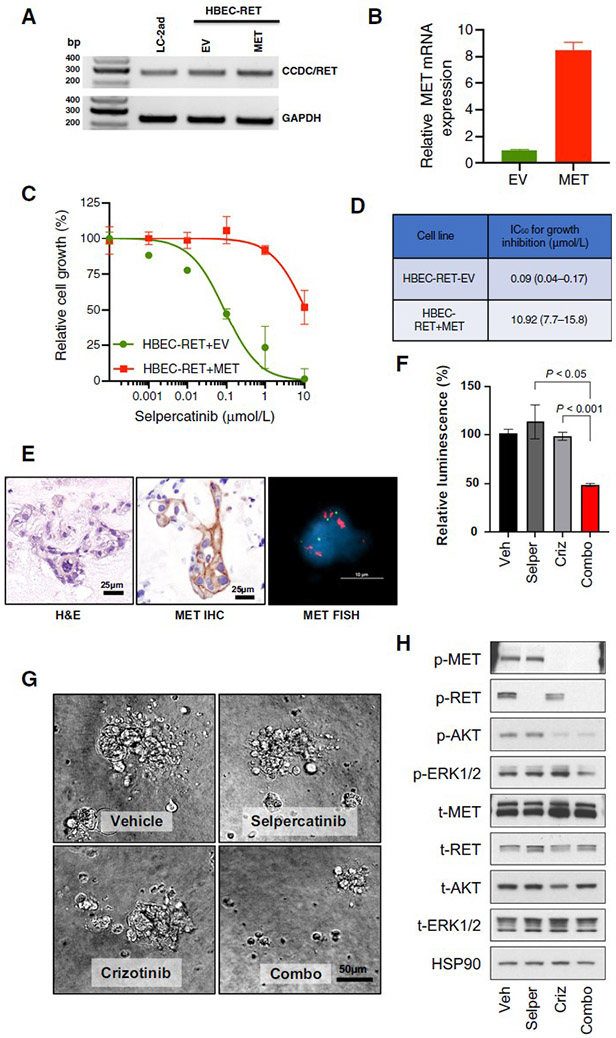
To determine the potential effect of MET overexpression on sensitivity of RET fusion-positive tumor cells to selpercatinib, we overexpressed MET in RET fusion-positive human bronchioepithelial cells (HBEC-RET). HBEC-RET cells were designed to express a CCDC6-RET fusion cDNA and are sensitive to RET inhibitors (Figure 2A, 2B). HBEC-RET+MET cells were far less sensitive to selpercatinib (Figure 2C, 2D, IC50=10.92 μM) compared to the isogenic control cells (IC50=0.09 μM), with a more than a hundred-fold shift in the IC50 values for growth inhibition in the presence of MET overexpression, suggesting that overexpression of MET drives resistance to selective RET inhibition.
Figure 2. MET amplification drives resistance to selpercatinib and responds to MET inhibition in RET fusion-positive models.
A, B. RET fusion confirmed by RT-PCR using primers targeting CCDC6 (exon 1, forward) and RET (exon 12, reverse), and MET expression was confirmed by qPCR. C. Cells were treated with the indicated concentrations of selpercatinib for 96 hours and then the relative number of cells determined using proliferation dye. D. Viability data was analyzed and estimated IC50 values with the 95% confidence interval are shown. HBEC: bronchiolar epithelial cells. EV: empty vector. E. Patient-derived organoid from KIF5B-RET fusion-positive NSCLC (Case 2) shows MET gain by both IHC and FISH. F, G. Cell viability of dissociated cells from cultured organoids treated with either selpercatinib (0.3 μM) or crizotinib (1 μM) has little effect, but the combination is cytotoxic. H. Selpercatinib alone blocked RET activity whereas pAKT and pERK were retained, while combination treatment successfully led to inactivation of both AKT and ERK.
Additionally, we derived an organoid culture from tumor cells isolated from pleural fluid of the patient described in Case 2. Analysis of the organoid by MET FISH confirmed high level MET amplification, and IHC confirmed high-level MET protein expression, consistent with the post-selpercatanib NGS analyses (Figure 2E). In vitro treatment with selpercatinib or crizotinib monotherapy was ineffective, but combined treatment with selpercatinib and crizotinib showed a cytotoxic effect (Figure 2F, 2G). As expected, only combined treatment resulted in decreased phospho-RET and phospho-MET levels concomitantly (Figure 2H). Selpercatinib alone blocked RET activity whereas the activity of AKT and ERK were retained possibly demonstrating a mechanism of resistance in this patient. Interestingly, crizotinib alone inhibited MET and AKT signaling but not pERK (Figure 2H). Lastly, the combination treatment successfully led to inactivation of both ERK and AKT, suggesting a potential mechanism for the utility of this drug combination in this RET fusion / MET amplification patient. These data demonstrate that MET amplification causes resistance to selpercatinib in RET fusion-positive NSCLC patients, which can be overcome preclinically by combined treatment with selpercatinib and crizotinib.
Combination treatment with selpercatinib and crizotinib overcomes MET dependent resistance in patients.
We were motivated by the high selectivity and clean safety profile of selpercatinib and by the known feasibility of adding crizotinib to other targeted TKIs in other biomarker-selected subsets of NSCLC patients. Therefore, we treated the above four patients with the combination of selpercatinib and crizotinib, each using an FDA-allowed, independent review board (IRB)-approved SPP.
Case 1 (patient with minor response to selpercatinib, MET CNG before treatment and high MET amplification after treatment) started treatment at one-half the recommended phase 2 (RP2D) of selpercatinib (80 mg BID) and one-half the approved dose of crizotinib (250 mg QD). After tolerating these doses for 4 weeks, the patient was escalated sequentially until reaching RP2D/approved doses of 160 mg BID/250 mg BID of selpercatinib and crizotinib, respectively. Treatment was tolerated with only mild nausea. Real-time pharmacokinetic (PK) analysis indicated selpercatinib exposure remained consistent with the patient’s exposure during selpercatinib monotherapy, while crizotinib exposure remained consistent with published exposures when used as monotherapy (Supplemental Figure 2A). She experienced clinical improvement in bone pain after 1 month of combination therapy; however, scans after 2.5 months revealed a mixed response with improvement in liver metastases but progressive pulmonary disease. Due to ongoing improvement in bone pain she continued on study therapy for a total of 3.5 months before dying of her cancer.
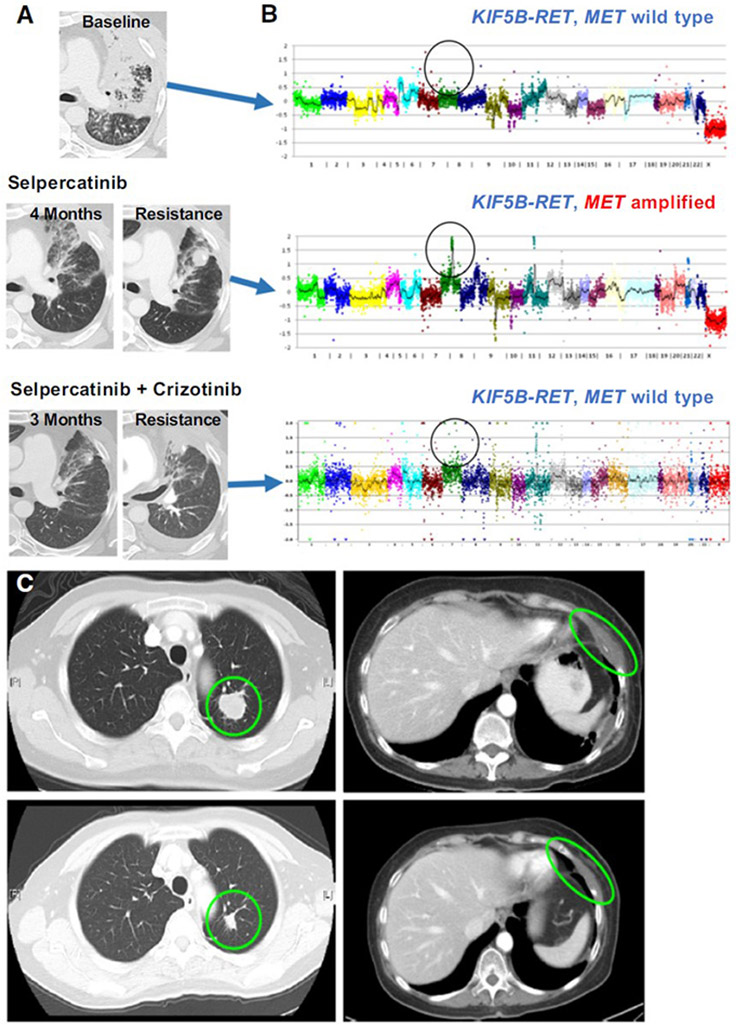
Case 2 (patient with partial response to selpercatinib lasting 11 months and acquired MET amplification) initiated treatment with the combination of selpercatinib and crizotinib, and pharmacokinetic analyses revealed the expected levels of each drug when used as monotherapy (Supplemental Figure 2B). The patient experienced a clinical and radiographic tumor response to combination treatment, with resolution of shortness of breath and maximal tumor diameter reduction of −38%. He responded for 10 months before discontinuing treatment for progression in the lungs and increase in ascites (Figure 3A). He tolerated treatment well, with AEs of lower extremity edema, possibly related to crizotinib, and reflux. NGS of a resistance biopsy showed persistence of the RET fusion but loss of the MET gene amplification (Figure 3B). Notably, the only other alteration detected was the ATM splice variant (c.8988-1G>C (splice)).
Figure 3. Response to Selective Dual RET Inhibition and RET Inhibition.
A. In patient 2, combination treatment yielded a clinical and radiological response, until he eventually developed disease progression. B. NGS showing acquired amplification of MET at time of resistance to selpercatinib, then at time of resistance the loss of the MET amplification but with continued presence of RET fusion. C. Patient 4 pre-treatment (top) and on-treatment (bottom) imaging showing a partial response at 4 weeks to selpercatinib and crizotinib.
Case 3 (patient with mixed response on selpercatinib followed by mixed response on crizotinib, with pretreatment MET amplification) continued treatment with crizotinib while restarting treatment with selpercatinib at 80 mg BID which was subsequently dose escalated. He experienced a partial response by RECIST 1.1 (maximum tumor reduction −42% below baseline) after 1.5 months of combination therapy; he died unexpectedly of an unrelated cardiac event after 4 months. Combination treatment was otherwise well tolerated without AEs.
Case 4 (patient with best response of stable disease on selpercatinib, with pretreatment MET CNG and post-treatment MET amplification) initiated treatment at the full doses of selpercatinib at 160mg BID and crizotinib at 250mg BID. A brisk partial response (−40%) was achieved at 4 weeks (Figure 3C) with disease regression in a left lung mass and a left chest wall mass. Although the patient tolerated combination treatment well without drug-related AEs, she developed colitis (determined by the investigator to be unrelated to treatment) that ultimately required treatment interruption and surgery, and she elected to transition to hospice care.
DISCUSSION
We describe MET amplification as a targetable mechanism of resistance to selpercatinib in RET-rearranged NSCLC. As greater number of patients develop resistance to selpercatinib, it will be important to systematically quantify the prevalence of MET amplification and other potentially targetable resistance mechanisms, such as the secondary RET mutation that was recently described11. We do note that there are 79 NSCLC patients enrolled at our three centers, which does offer the reader a rough estimate of the rarity of this type of resistance. While the level of MET gene amplification clearly increased during selpercatinib monotherapy, in 3 of 4 cases, some degree of MET gain was already present prior to exposure to selpercatinib. This is reminiscent of EGFR-mutant NSCLC, in which rare clones with high level MET amplification may be detected at baseline, prior to EGFR inhibitor therapy16,17. It is notable that a recent early phase EGFR mutant / MET amplified NSCLC trial showed an ORR of 44% to osimertinib (EFGR-TKI) and savolitinib (MET-TKI)8.
While the median progression free survival has been reported at 18 months for selpercatinib in RET-positive NSCLC18, our patients in contrast had an unusually short benefit from selpercatinib. The cause of this modest PFS benefit is unknown, but this may be due to some degree of MET amplification present at baseline in these patients or may be related to the aggressive nature of the MET oncogene19. Additionally, these brief responses may be due to the presence of additional driver mutations, either through heterogeneity of resistance to selpercatinib at distinct metastatic sites, or by means of additional subclonal drivers not detected on NGS.
To better understand the clinical effect of this combination, prospective efforts will be needed to study combination therapy with selpercatinib plus MET inhibition. Additionally, treatment tolerability is difficulty to assess in individual SPPs – while these patients did not complain of intolerable toxicity while under treatment, one patient died of an apparently unrelated cardiac event, and a second patient experienced severe colitis. Both of these adverse events were thought to be unrelated to this drug combination, but the potential toxicities of such drug combinations will need to be studied in future prospective cohorts of patients with appropriate performance status and comorbidities. Lastly, we are hopeful that the use of newer, more specific MET inhibitors including capmatinib7,20 (which is FDA approved) and tepotinib21 in combination with selpercatinib may result in increased efficacy and better tolerability of this drug combination.
In these SPPs, the expeditious delivery of a potentially effective combination therapy to patients with high unmet clinical need was enabled by the availability of an approved second agent, the willingness of the sponsor to permit early use of combination therapy with an investigational therapy still being studied in a first-in-human-trial, and the rapid review and allowance of each SPP by the FDA and by local IRBs. Our experience provides further evidence for the importance of robust, multi-cancer gene panel-based molecular analysis in patients with resistance to targeted therapies to enable the identification of potentially targetable acquired resistance mechanisms in a time frame that can help each patient. These cases provide evidence for the feasibility of this approach, and this may enable other potentially effective combination therapies with a clear biologic rationale to be offered immediately to individual patients without alternative treatment options, while also providing clinical proof-of-concept that may be validated in subsequent, prospective clinical trials.
Supplementary Material
Supplemental Figure 1. Treatment History Summary.
Supplemental Figure 2. Pharmacokinetic Curves for Cases 1 and 2. A, B. Pharmacokinetic curves showing stable expected drug levels of both selpercatinib and crizotinib in both case 1 (panel A) and case 2 (panel B).
Translational Relevance.
Molecular mechanisms of acquired resistance to selpercatinib, a highly selective and potent RET kinase inhibitor, are poorly understood. We identified MET amplification as a recurrent mechanism of resistance to targeted therapy in NSCLC patients treated with selpercatinib. We show that MET amplification is sufficient to cause selpercatinib resistance in vitro, and that the addition of the MET/ALK/ROS1 inhibitor crizotinib can rescue this phenotype. We then utilize a series of single patient protocols to treat these patients with combination therapy, and this combination treatment showed clinical activity, with one response lasting 10 months. These data suggest that MET dependence is a recurring and potentially targetable mechanism of resistance to selective RET inhibition in advanced NSCLC. Prospective clinical trials are needed to validate these findings and to identify effective combination therapies to treat acquired resistance to selpercatinib.
Acknowledgments
Financial support:
This work was supported by the National Cancer Institute grants R01CA222823 (P.A.J.) and R35CA220497 (P.A.J.), NIH 2T32 CA009512-29A1 (E.Y.R.), NIH Cancer Center Grant P30CA008748 to Memorial Sloan Kettering Cancer Center, and The Expects Miracles Foundation (E.V.I.) and Robert and Renée Belfer Foundation (E.V.I.).
Footnotes
Conflicts of interest disclosure statement:
E.Y.R. reports research support from Bayer. M.L.J. reports research funds to her institution from Loxo Oncology, Inc., a wholly owned subsidiary of Eli Lilly and Company (hereafter referred to as Loxo Oncology); AbbVie; Acerta; Adaptimmune; Apexigen; Array BioPharma; AstraZeneca; Atreca; BeiGene; Birdie; Boehringer Ingelheim; Checkpoint Therapeutics; Corvus Pharmaceuticals; CytomX; Daiichi Sankyo; Dynavax; Eli Lilly and Company; EMD Serono; Genentech/Roche; Genmab; Genocea Biosciences; GlaxoSmithKline; Gritstone Oncology; Guardant Health; Hengrui Therapeutics; Immunocore; Incyte; Janssen; Jounce Therapeutics; Kadmon Pharmaceuticals; Lycera; Merck; Mirati Therapeutics; Neovia Oncology; Novartis; OncoMed Pharmaceuticals; Pfizer; Regeneron Pharmaceuticals; Sanofi; Shattuck Labs; Stem CentRx; Syndax Pharmaceuticals; Takeda Pharmaceuticals; Tarveda; University of Michigan; WindMIL; TCR2 Therapeutics; Arcus Biosciences; Ribon Therapeutics; and Amgen. M.L.J. also reports consulting/advisory roles through her institution for AbbVie, Achilles Therapeutics, AstraZeneca, Atreca, Boehringer Ingelheim, Calithera Biosciences, Genentech, GlaxoSmithKline, Gritstone Oncology, Guardant Health, Incyte, Janssen, Eli Lilly and Company, Loxo Oncology, Merck, Mirati Therapeutics, Novartis, Pfizer, Ribon Therapeutics, Sanofi, and Association of Community Cancer Centers; fees for food/beverage/travel from Abbvie, Astellas, AstraZeneca, Boehringer Ingelheim, Clovis, Daiichi Sankyo, EMD Serono, Bristol Myers Squibb, Exelixis, Genentech/Roche, Incyte, Merck, Pfizer, Sysmex Inostics, Vapotherm, Janssen, Eli Lilly and Company, Novartis, and Sanofi; and Contract Lobbyist roles of her spouse for Astellas and Otsuka Pharmaceuticals. S.E.C. reports no conflicts of interest. R.S. reports research funding from Loxo Oncology, Helsinn Healthcare, Merus, and Elevation Oncology. J.F.K. is an employee of Loxo Oncology. J.S., A.A.B., M.A.D., and E.G. report no conflicts of interest. E.V.I. reports being an inventor on a pending patent related to manipulating, culturing, and evaluating tumor organotypic spheroids in 3D microfluidic devices. D.N.H. is an employee of Loxo Oncology. E.M.K., M. Lin, and M.S.D.M. report no conflicts of interest. B.C.N. and E.A.O. are employees of Loxo Oncology. J.E.S. and M.V. report no conflicts of interest. K.E. is an employee and stockholder of Loxo Oncology. J.F.H. reports a research grant from Eli Lilly and Company and an honorarium from Illumina. B.T.L. reports two pending institutional patents at Memorial Sloan Kettering Cancer Center (US62/685,057, US62/514,661); consultant/advisory board member roles for Roche/Genentech, Biosceptre International, Thermo Fisher Scientific, Mersana Therapeutics, Hengrui Therapeutics, Guardant Health, Eli Lilly and Company; and research funds to his institution from Roche/Genentech, Daiichi Sankyo, Hengrui Therapeutics, Illumina, Guardant Health, BioMed Valley Discoveries, AstraZeneca, GRAIL, MORE Health, Amgen, and Eli Lilly and Company. L.M.S. reports consulting fees from EMD Serono, scientific advisory board roles for Loxo Oncology and Foghorn Therapeutics, and honorarium from Astra Zeneca. B.S.T. reports honoraria and research funding from Genentech and discovery board activities for Boehringer Ingelheim and Loxo Oncology; all stated activities were outside of the work described herein. M. Ladanyi reports ad hoc advisory board roles for Eli Lilly and Company and Loxo Oncology, and research support from Helsinn Healthcare and Loxo Oncology. P.A.J. reports consulting fees from AstraZeneca, Boehringer-Ingelheim, Pfizer, Roche/Genentech, Takeda Oncology, ACEA Biosciences, Eli Lilly and Company, Araxes Pharma, Ignyta, Mirati Therapeutics, Novartis, Loxo Oncology, Daiichi Sankyo, Sanofi Oncology, Voronoi, SFJ Pharmaceuticals and Biocartis; receiving post-marketing royalties from DFCI owned intellectual property on EGFR mutations licensed to Lab Corp; sponsored research agreements with AstraZeneca, Daichi-Sankyo, PUMA, Boehringer Ingelheim, Eli Lilly and Company, Revolution Medicines and Astellas Pharmaceuticals; and stock ownership in Loxo Oncology and Gatekeeper Pharmaceuticals. S.M.R., a former employee of Loxo Oncology, was employed by Loxo Oncology at the time of this work. A.E.D. reports consulting/advisory roles for Ignyta, Loxo Oncology, TP Therapeutics, AstraZeneca, Pfizer, Blueprint Medicines, Genentech/Roche, Helsinn Therapeutics, BeiGene, Hengrui Therapeutics, Exelixis, Bayer, Tyra Biosciences, Verastem, Takeda/Millennium, BerGenBio, MORE Health, Eli Lilly and Company, and Verastem; royalties for Pocket Oncology; honoraria from Medscape, OncLive, PeerVoice, Physician’s Education Resource, Targeted Oncology, MORE Health, Research to Practice, Foundation Medicine, and Peerview; and research funding from Foundation Medicine. G.R.O. reports consulting fees from Abbvie, AstraZeneca, Blueprint, Dropworks, GRAIL, Janssen, Illumina, Inivata, Loxo Oncology, Sysmex, and Takeda; and honoraria from Foundation Medicine and Guardant.
REFERENCES
- 1.Long GV et al. Combined BRAF and MEK inhibition versus BRAF inhibition alone in melanoma. N. Engl. J. Med (2014). doi: 10.1056/NEJMoa1406037 [DOI] [PubMed] [Google Scholar]
- 2.Flaherty KT et al. Combined BRAF and MEK inhibition in melanoma with BRAF V600 mutations. N. Engl. J. Med (2012). doi: 10.1056/NEJMoa1210093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sherr CJ, Beach D & Shapiro GI Targeting CDK4 and CDK6: From discovery to therapy. Cancer Discovery (2016). doi: 10.1158/2159-8290.CD-15-0894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O’Leary B, Finn RS & Turner NC Treating cancer with selective CDK4/6 inhibitors. Nature Reviews Clinical Oncology (2016). doi: 10.1038/nrclinonc.2016.26 [DOI] [PubMed] [Google Scholar]
- 5.Oxnard GR et al. Assessment of Resistance Mechanisms and Clinical Implications in Patients with EGFR T790M-Positive Lung Cancer and Acquired Resistance to Osimertinib. JAMA Oncol. (2018). doi: 10.1001/jamaoncol.2018.2969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yu HA et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin. Cancer Res (2013). doi: 10.1158/1078-0432.CCR-12-2246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wu YL et al. Phase Ib/II Study of Capmatinib (INC280) Plus Gefitinib after Failure of Epidermal Growth Factor Receptor (EGFR) Inhibitor Therapy in Patients with EGFR-Mutated, MET Factor-Dysregulated Non-Small-Cell Lung Cancer. J. Clin. Oncol (2018). doi: 10.1200/JCO.2018.77.7326 [DOI] [PubMed] [Google Scholar]
- 8.Oxnard GR et al. TATTON: a multi-arm, phase Ib trial of osimertinib combined with selumetinib, savolitinib, or durvalumab in EGFR-mutant lung cancer. Ann. Oncol (2020). doi: 10.1016/j.annonc.2020.01.013 [DOI] [PubMed] [Google Scholar]
- 9.Mulligan LM Progress and potential impact of RET kinase targeting in cancer. Expert Review of Proteomics (2016). doi: 10.1080/14789450.2016.1205491 [DOI] [PubMed] [Google Scholar]
- 10.Subbiah V et al. Selective RET Kinase Inhibition for Patients with RET-Altered Cancers. Ann. Oncol (2018). doi: 10.1093/annonc/mdy137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon BJ et al. RET Solvent Front Mutations Mediate Acquired Resistance to Selective RET Inhibition in RET-driven malignancies. J. Thorac. Oncol (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turke AB et al. Preexistence and Clonal Selection of MET Amplification in EGFR Mutant NSCLC. Cancer Cell (2010). doi: 10.1016/j.ccr.2009.11.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dagogo-Jack I et al. MET Alterations Are a Recurring and Actionable Resistance Mechanism in ALK-Positive Lung Cancer. Clin. Cancer Res (2020). doi: 10.1158/1078-0432.ccr-19-3906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lin JJ et al. Clinical activity of alectinib in advanced RET-rearranged non-small cell lung cancer. J. Thorac. Oncol (2016). doi: 10.1016/j.jtho.2016.08.126 [DOI] [PubMed] [Google Scholar]
- 15.Supplee JG et al. Sensitivity of next-generation sequencing assays detecting oncogenic fusions in plasma cell-free DNA. Lung Cancer (2019). doi: 10.1016/j.lungcan.2019.06.004 [DOI] [PubMed] [Google Scholar]
- 16.Scagliotti G et al. A Randomized-Controlled Phase 2 Study of the MET Antibody Emibetuzumab in Combination with Erlotinib as First-Line Treatment for EGFR Mutation–Positive NSCLC Patients. J. Thorac. Oncol (2020). doi: 10.1016/j.jtho.2019.10.003 [DOI] [PubMed] [Google Scholar]
- 17.Cappuzzo F et al. MET increased gene copy number and primary resistance to gefitinib therapy in non-small-cell lung cancer patients. Ann. Oncol (2009). doi: 10.1093/annonc/mdn635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Drilon A et al. PL02.08 Registrational Results of LIBRETTO-001: A Phase 1/2 Trial of LOXO-292 in Patients with RET Fusion-Positive Lung Cancers. J. Thorac. Oncol (2019). doi: 10.1016/j.jtho.2019.08.059 [DOI] [Google Scholar]
- 19.Tong JH et al. MET amplification and exon 14 splice site mutation define unique molecular subgroups of non-small cell lung carcinoma with poor prognosis. Clin. Cancer Res (2016). doi: 10.1158/1078-0432.CCR-15-2061 [DOI] [PubMed] [Google Scholar]
- 20.Baltschukat S et al. Capmatinib (INC280) is active against models of non–small cell lung cancer and other cancer types with defined mechanisms of MET activation. Clin. Cancer Res (2019). doi: 10.1158/1078-0432.CCR-18-2814 [DOI] [PubMed] [Google Scholar]
- 21.Paik PK et al. Tepotinib in Non–Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations . N. Engl. J. Med (2020). doi: 10.1056/nejmoa2004407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ross DS et al. Next-Generation Assessment of Human Growth Factor Receptor 2 (ERBB2) Amplification Status: Clinical Validation in the Context of a Hybrid Capture-Based, Comprehensive Solid Tumor Genomic Profiling Assay. J. Mol. Diagnostics (2017). doi: 10.1016/j.jmoldx.2016.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bi WL et al. Clinical identification of oncogenic drivers and copy-number alterations in pituitary tumors. Endocrinology (2017). doi: 10.1210/en.2016-1967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Carter SL et al. Absolute quantification of somatic DNA alterations in human cancer. Nat. Biotechnol (2012). doi: 10.1038/nbt.2203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schrock AB et al. Characterization of 298 patients with lung cancer harboring MET Exon 14 skipping alterations. J. Thorac. Oncol (2016). doi: 10.1016/j.jtho.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 26.Li GG et al. Antitumor activity of RXDX-105 in multiple cancer types with RET rearrangements or mutations. Clin. Cancer Res (2017). doi: 10.1158/1078-0432.CCR-16-1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Treatment History Summary.
Supplemental Figure 2. Pharmacokinetic Curves for Cases 1 and 2. A, B. Pharmacokinetic curves showing stable expected drug levels of both selpercatinib and crizotinib in both case 1 (panel A) and case 2 (panel B).