Abstract
Background
In patients with type 2 diabetes, hyperglycaemia is an independent risk factor for COVID-19-related mortality. Associations between pre-infection prescription for glucose-lowering drugs and COVID-19-related mortality in people with type 2 diabetes have been postulated but only investigated in small studies and limited to a few agents. We investigated whether there are associations between prescription of different classes of glucose-lowering drugs and risk of COVID-19-related mortality in people with type 2 diabetes.
Methods
This was a nationwide observational cohort study done with data from the National Diabetes Audit for people with type 2 diabetes and registered with a general practice in England since 2003. Cox regression was used to estimate the hazard ratio (HR) of COVID-19-related mortality in people prescribed each class of glucose-lowering drug, with covariate adjustment with a propensity score to address confounding by demographic, socioeconomic, and clinical factors.
Findings
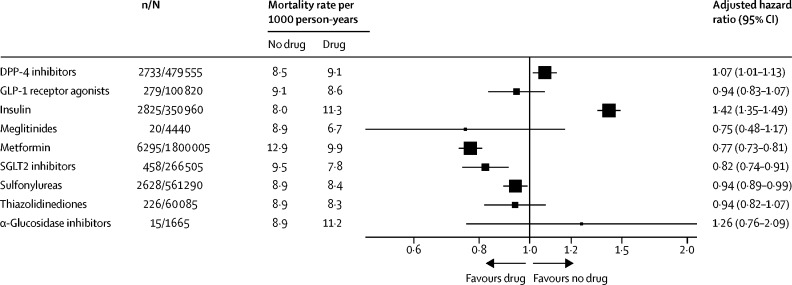
Among the 2 851 465 people with type 2 diabetes included in our analyses, 13 479 (0·5%) COVID-19-related deaths occurred during the study period (Feb 16 to Aug 31, 2020), corresponding to a rate of 8·9 per 1000 person-years (95% CI 8·7–9·0). The adjusted HR associated with recorded versus no recorded prescription was 0·77 (95% CI 0·73–0·81) for metformin and 1·42 (1·35–1·49) for insulin. Adjusted HRs for prescription of other individual classes of glucose-lowering treatment were as follows: 0·75 (0·48–1·17) for meglitinides, 0·82 (0·74–0·91) for SGLT2 inhibitors, 0·94 (0·82–1·07) for thiazolidinediones, 0·94 (0·89–0·99) for sulfonylureas, 0·94 (0·83–1·07) for GLP-1 receptor agonists, 1·07 (1·01–1·13) for DPP-4 inhibitors, and 1·26 (0·76–2·09) for α-glucosidase inhibitors.
Interpretation
Our results provide evidence of associations between prescription of some glucose-lowering drugs and COVID-19-related mortality, although the differences in risk are small and these findings are likely to be due to confounding by indication, in view of the use of different drug classes at different stages of type 2 diabetes disease progression. In the context of the COVID-19 pandemic, there is no clear indication to change prescribing of glucose-lowering drugs in people with type 2 diabetes.
Funding
None.
Introduction
Among the risk factors for COVID-19 mortality, type 2 diabetes has emerged as one of the most important and common.1 In a recent population-based cohort study, the risk of COVID-19 mortality was twice as high in people with type 2 diabetes as in those without;2 studies have also shown an association between hyperglycaemia and increased COVID-19-related mortality in people with type 2 diabetes.3, 4
Although potential direct therapeutic benefits in relation to COVID-19 have been proposed, the safety of some glucose-lowering therapies in people with type 2 diabetes and COVID-19 has been questioned.1 The DPP-4 inhibitors have been hypothesised to potentially modify the biological activity of various substrates involved in the immune response to the infection, with suggestions of potential benefit or harm.1, 5 It has been proposed that the SGLT2 inhibitors could increase COVID-19-related risk via increased kidney expression of angiotensin-converting enzyme 2 (ACE2),6 a receptor of the SARS-CoV-2 virus.7 Similarly, GLP-1 receptor agonists have been associated with increased ACE2 expression in lungs and heart tissue and with beneficial effects in acute lung injury, with suggestions of possible helpful and harmful effects in COVID-19.8, 9 Recent studies have suggested a lower risk of COVID-19-related hospital mortality in people with type 2 diabetes who were prescribed metformin before hospital admission10 or sitagliptin on admission.11
Notwithstanding calls for ongoing scrutiny to understand the use, risks, and benefits of individual classes of glucose-lowering drugs in people with type 2 diabetes and COVID-19, there are no comprehensive, comparative data on differential effects of glucose-lowering drugs on risk for severe COVID-19.5 Expert groups have made consensus recommendations for glucose-lowering drugs, such as to avoid metformin and SGLT2 inhibitors, because of putative risks of lactic acidosis with metformin and diabetic ketoacidosis with SGLT2 inhibitors in patients admitted to hospital with COVID-19.12, 13, 14, 15, 16, 17, 18 However, withdrawing these medications could not only result in hyperglycaemia, a risk factor for poorer outcomes in COVID-19,3 but also increase the long-term risk of major cardiovascular events and progression of chronic kidney disease.19
Research in context.
Evidence before this study
From March 1 to Nov 30, 2020, we did weekly searches of PubMed and medRxiv using the search terms “COVID-19”, “SARS-CoV-2”, “coronavirus”, “SARS virus”, and “diabetes”, restricted to English-language publications. Smaller retrospective studies from the USA, China, and France have all reported a lower or neutral risk of COVID-19-related mortality in people previously or currently prescribed metformin. A recent meta-analysis of five observational studies showed that the use of metformin before hospital admission in people with diabetes and sepsis (non-COVID-19 related) was associated with lower mortality. In a small French multicentre observational study, investigators reported no association between use of sulfonylureas, meglitinides, DPP-4 inhibitors, or GLP-1 receptor agonists and COVID-19-related mortality, but higher mortality associated with insulin therapy. Most previous studies were done at a single centre with a small number of people with type 2 diabetes, and most studies investigated a small number of glucose-lowering drugs.
Added value of this study
To our knowledge, this is the largest COVID-19-related population study, covering almost the entire population of people with type 2 diabetes in England. We assessed the association of prescriptions for the following glucose-lowering drugs or drug classes with COVID-related mortality: DPP-4 inhibitors, GLP-1 receptor agonists, insulin, meglitinides, metformin, SGLT2 inhibitors, sulfonylureas, thiazolidinediones, and α-glucosidase inhibitors. People with type 2 diabetes prescribed metformin, SGLT2 inhibitors, and sulfonylureas had a lower risk of COVID-19-related mortality and those prescribed insulin and DPP-4 inhibitors had a higher risk of COVID-19 related mortality (compared with those not prescribed these drugs), although these findings are likely to be due to confounding by indication, in view of the use of different drug classes in the early and late stages of the type 2 diabetes disease trajectory.
Implications of all the available evidence
Our results suggest that there is no evidence to change prescribing of glucose-lowering drugs in people with type 2 diabetes in clinical practice in the context of the COVID-19 pandemic.
In view of the high prevalence of type 2 diabetes worldwide, its higher prevalence in people with COVID-19, and the heightening of COVID-19 mortality risk by hyperglycaemia, investigating the safety of glucose-lowering drugs is important for people with diabetes, clinicians, and policy makers. We therefore investigated the relation between the prescription of commonly used glucose-lowering drugs and COVID-19 mortality in a national cohort of people with type 2 diabetes. In particular, we aimed to identify any unexpected large effect sizes that might have potential for recommendations about routinely used glucose-lowering drugs in clinical practice.
Methods
Study design and data sources
This was a nationwide observational cohort study done with data from England. The study was done in line with the RECORD-PE guidelines for conducting and reporting a pharmacoepidemiological study with routinely collected data (appendix pp 7–9).
The National Diabetes Audit (NDA) has collated data on people with diagnosed diabetes registered with a health-care provider in England since 2003.20 These data were linked via unique National Health Service (NHS) number to hospital episode statistics, a record of all hospital admissions in England; and to civil death registrations, collated by the Office for National Statistics (ONS). Information on data approval and permissions is in the appendix (pp 1–2).
Study population
The study population consisted of individuals with type 2 diabetes included in the NDA data extraction from Jan 1, 2018, to March 31, 2019, whose most recent general practice was in England and provided data on prescriptions to the NDA, and who were alive on Feb 16, 2020 (index date). People with an unknown date of birth or recorded date of birth giving an age of 110 years or older were excluded from the analysis. Diabetes was diagnosed in routine clinical care and type 2 diabetes was deduced from the clinical codes recorded in electronic patient records and collated by the NDA. Where there were codes for multiple types of diabetes, the type identified by a specialist diabetes service was given precedence over that defined in primary care; where there was a discrepancy between codes used in specialist services or within primary care, the latest recorded type of diabetes was assumed to be the most accurate.
Exposure and outcome
The primary exposure of interest in this analysis was prescription for the following glucose-lowering drugs or drug classes: DPP-4 inhibitors, GLP-1 receptor agonists, insulin, meglitinides, metformin, SGLT2 inhibitors, sulfonylureas, thiazolidinediones, and α-glucosidase inhibitors. Individuals were classed as being prescribed the drug or drug class if they had received two or more prescriptions between July 1, 2019, and Dec 31, 2019. Deaths registered with ONS that occurred between Feb 16, 2020, and Aug 31, 2020, were included in the analysis. Deaths were defined as COVID-19 related if the International Classification of Diseases (ICD; version 10) codes U07.1 (COVID-19, virus identified) or U07.2 (COVID-19, virus not identified) were recorded as either the primary underlying or secondary cause of death.
Covariates
Age and duration of diagnosed diabetes were calculated for the index date of Feb 16, 2020. Home postcode was used to identify region of residence and social deprivation status, as defined by the Indices of Multiple Deprivation 2019.21 Ethnicity was obtained from records of self-reported ethnic group during the course of routine clinical care. The most recent measurements of HbA1c, systolic blood pressure, total serum cholesterol, and creatinine recorded between Jan 1, 2019, and Dec 31, 2019, were identified. The Modification of Diet in Renal Disease formula was used to calculate the estimated glomerular filtration rate (eGFR). People who had received one or more prescriptions for antihypertensive drugs or statins between Jan 1, 2019, and Dec 31, 2019, were identified from general practice prescribing records. BMI and smoking status were identified from the latest recorded measurement between Jan 1, 2017, and Dec 31, 2019. A history of cardiovascular disease was defined as a hospital admission for myocardial infarction (ICD-10 codes I21–22), heart failure (I50), or angina (I20) between April 1, 2009, and Dec 31, 2019, where the relevant diagnosis codes were included as either the primary or one of up to 20 secondary diagnoses, or a diagnosis of ischaemic heart disease recorded in the primary care record. Individuals were classed as having a history of cardiovascular disease if they had one or more admissions for any of these three conditions or their primary care records included a diagnosis of ischaemic heart disease.
Statistical analysis
Individuals with missing information on sex were excluded from the analysis. All numbers taken directly from the NDA were rounded to the nearest five people to protect confidentiality; mortality data from the ONS were unrounded. Descriptive statistics were used to summarise the characteristics of the included patients. For each drug class, the association with COVID-19-related mortality was estimated by comparing the two groups of people prescribed and not prescribed the specific drug class by use of Cox regression models, with a timescale from index date to COVID-19-related mortality or censoring. A doubly robust Cox model included several a-priori defined potential confounders and a propensity score variable estimated from the same confounders; this approach can compensate for residual imbalance of confounders and has been suggested to offer more robustness to model mis-specification.22, 23, 24, 25
Propensity score was calculated with a logistic regression including the following variables: age (5-year bands), sex (male and female), ethnicity (White, Asian, Black, mixed, other, and unknown), deprivation (quintiles of Indices of Multiple Deprivation 2019), region (London, South West, South East, Midlands, East of England, North West, North East and Yorkshire, and unknown), diabetes duration (<1, 1–2, 3–4, 5–9, 10–14, 15–19, or ≥20 years), smoking status (current smoker; ex-smoker; current non-smoker, history unknown; never smoked; and unknown), BMI (<20, 20 to <25, 25 to <30, 30 to <35, 35 to <40, ≥40 kg/m2, and unknown), HbA1c (<48, 48 to <54, 54 to <59, 59 to <75, 75 to <86, and ≥86 mmol/mol, and unknown), eGFR (≥90, 60 to <90, 45 to <60, 30 to <45, 15 to <30, and <15 mL/min × 1·73 m2, and unknown), systolic blood pressure (≤140 mm Hg, >140 mm Hg, and unknown), total serum cholesterol (<5 mmol/L, ≥5 mmol/L, and unknown), cardiovascular disease history (yes and no), and prescribed medications (statins, antihypertensives, and glucose-lowering drugs [none, one, two, and three or more classes]). For missing data, we used the missing category approach.
The proportional hazards assumption was assessed and confirmed. Exploratory subgroup analyses, unadjusted for multiple comparisons, were done by age, sex, previous cardiovascular disease, and eGFR. All statistical analyses were done in SAS version 9.4 and results are reported as hazard ratios (HRs) with 95% CIs for the main analyses and 99% CIs for subgroup analyses.
Role of the funding source
There was no funding source for this study.
Results
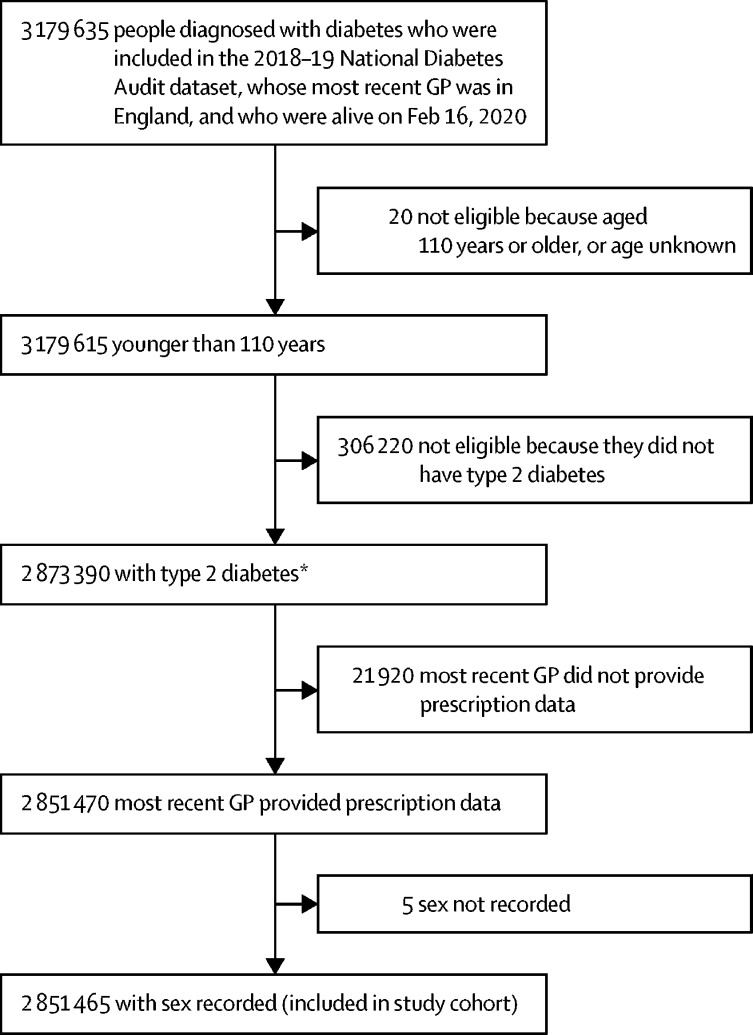
2 851 465 people were included in this analysis, of whom 1 593 730 (55·9%) were men; 1 884 675 (66·1%) were White, 399 540 (14·0%) were Asian, and 135 860 (4·8%) were Black; and the median age was 67 years (IQR 57–77; figure 1 , table ). The most commonly prescribed glucose-lowering drug or drug class was metformin (1 800 005 [63·1%] people), followed by sulfonylureas (561 290 [19·7%]), DPP-4 inhibitors (479 555 [16·8%]), insulin (350 960 [12·3%]), SGLT2 inhibitors (266 505 [9·3%]), GLP-1 receptor agonists (110 820 [3·9%]), thiazolidinediones (60 085 [2·1%]), meglitinides (4440 [0·2%]), and α-glucosidase inhibitors (1665 [0·2%]; table). Across participants prescribed different classes of glucose-lowering drugs, differences were present for most of the characteristics investigated, including HbA1c level, number of glucose-lowering drugs prescribed, diabetes duration, and BMI (table).
Figure 1.
Cohort profile
Numbers are rounded to the nearest five people to protect confidentiality. GP=general practice. *Difference of five from expected value because number rounded to nearest five after calculation from raw (unrounded) data.
Table.
Characteristics of people with diabetes included in the cohort
| DPP-4 inhibitors (n=479 555) | GLP-1 receptor agonists (n=110 820) | Insulin (n=350 960) | Meglitinides (n=4440) | Metformin (n=1 800 005) | SGLT2 inhibitors (n=266 505) | Sulfonylureas (n=561 290) | Thiazolidinediones (n=60 085) | α-glucosidase inhibitors (n=1665) | Whole cohort (n=2 851 465) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | |||||||||||
| Male | 279 500 (58·3%) | 57 250 (51·7%) | 191 170 (54·5%) | 2505 (56·4%) | 1 044 920 (58·1%) | 162 070 (60·8%) | 339 715 (60·5%) | 38 095 (63·4%) | 945 (56·8%) | 1 593 730 (55·9%) | |
| Female | 200 055 (41·7%) | 53 570 (48·3%) | 159 790 (45·5%) | 1935 (43·6%) | 755 085 (41·9%) | 104 435 (39·2%) | 221 575 (39·5%) | 21 990 (36·6%) | 720 (43·2%) | 1 257 735 (44·1%) | |
| Age, years | |||||||||||
| <40 | 7140 (1·5%) | 3630 (3·3%) | 8385 (2·4%) | 55 (1·2%) | 43 870 (2·4%) | 8770 (3·3%) | 9160 (1·6%) | 720 (1·2%) | 20 (1·2%) | 77 260 (2·7%) | |
| 40–44 | 10 560 (2·2%) | 4120 (3·7%) | 7710 (2·2%) | 90 (2·0%) | 54 105 (3·0%) | 11 345 (4·3%) | 13 290 (2·4%) | 1170 (1·9%) | 30 (1·8%) | 83 830 (2·9%) | |
| 45–49 | 21 505 (4·5%) | 8265 (7·5%) | 15 080 (4·3%) | 180 (4·1%) | 100 995 (5·6%) | 22 955 (8·6%) | 26 815 (4·8%) | 2610 (4·3%) | 50 (3·0%) | 148 230 (5·2%) | |
| 50–54 | 36 535 (7·6%) | 14 100 (12·7%) | 26 215 (7·5%) | 310 (7·0%) | 162 155 (9·0%) | 37 325 (14·0%) | 45 660 (8·1%) | 4740 (7·9%) | 85 (5·1%) | 231 885 (8·1%) | |
| 55–59 | 52 005 (10·8%) | 18 825 (17·0%) | 37 865 (10·8%) | 480 (10·8%) | 221 185 (12·3%) | 48 770 (18·3%) | 64 010 (11·4%) | 7185 (12·0%) | 140 (8·4%) | 310 740 (10·9%) | |
| 60–64 | 61 805 (12·9%) | 19 775 (17·8%) | 46 435 (13·2%) | 625 (14·1%) | 250 810 (13·9%) | 49 045 (18·4%) | 75 980 (13·5%) | 8860 (14·7%) | 205 (12·3%) | 353 870 (12·4%) | |
| 65–69 | 66 560 (13·9%) | 17 460 (15·8%) | 49 995 (14·2%) | 635 (14·3%) | 256 350 (14·2%) | 40 240 (15·1%) | 80 275 (14·3%) | 9490 (15·8%) | 235 (14·1%) | 373 030 (13·1%) | |
| 70–74 | 73 860 (15·4%) | 14 495 (13·1%) | 54 185 (15·4%) | 725 (16·3%) | 265 195 (14·7%) | 29 625 (11·1%) | 86 025 (15·3%) | 9850 (16·4%) | 245 (14·7%) | 410 275 (14·4%) | |
| 75–79 | 62 345 (13·0%) | 6990 (6·3%) | 44 720 (12·7%) | 600 (13·5%) | 203 020 (11·3%) | 13 060 (4·9%) | 69 440 (12·4%) | 7445 (12·4%) | 245 (14·7%) | 341 090 (12·0%) | |
| ≥80 | 87 240 (18·2%) | 3155 (2·8%) | 60 380 (17·2%) | 740 (16·7%) | 242 325 (13·5%) | 5360 (2·0%) | 90 630 (16·1%) | 8010 (13·3%) | 415 (24·9%) | 521 255 (18·3%) | |
| Ethnicity | |||||||||||
| White | 314 015 (65·5%) | 84 525 (76·3%) | 249 615 (71·1%) | 2320 (52·3%) | 1 160 760 (64·5%) | 178 095 (66·8%) | 357 320 (63·7%) | 38 175 (63·5%) | 940 (56·5%) | 1 884 675 (66·1%) | |
| Asian | 75 275 (15·7%) | 8800 (7·9%) | 43 170 (12·3%) | 1205 (27·1%) | 288 450 (16·0%) | 40 600 (15·2%) | 96 625 (17·2%) | 11 080 (18·4%) | 390 (23·4%) | 399 540 (14·0%) | |
| Black | 22 730 (4·7%) | 3680 (3·3%) | 16 380 (4·7%) | 390 (8·8%) | 85 750 (4·8%) | 9650 (3·6%) | 28 330 (5·0%) | 2200 (3·7%) | 125 (7·5%) | 135 860 (4·8%) | |
| Mixed | 4800 (1·0%) | 1010 (0·9%) | 3500 (1·0%) | 60 (1·4%) | 19 700 (1·1%) | 2875 (1·1%) | 5945 (1·1%) | 550 (0·9%) | 20 (1·2%) | 30 480 (1·1%) | |
| Other | 8780 (1·8%) | 1835 (1·7%) | 6065 (1·7%) | 140 (3·2%) | 31 855 (1·8%) | 4965 (1·9%) | 11 415 (2·0%) | 1090 (1·8%) | 50 (3·0%) | 46 920 (1·6%) | |
| Unknown | 53 960 (11·3%) | 10 965 (9·9%) | 32 235 (9·2%) | 330 (7·4%) | 213 490 (11·9%) | 30 320 (11·4%) | 61 650 (11·0%) | 6990 (11·6%) | 135 (8·1%) | 353 990 (12·4%) | |
| Deprivation quintile | |||||||||||
| First (most deprived) | 120 530 (25·1%) | 30 145 (27·2%) | 92 890 (26·5%) | 1065 (24·0%) | 459 675 (25·5%) | 70 740 (26·5%) | 143 840 (25·6%) | 17 150 (28·5%) | 405 (24·3%) | 695 215 (24·4%) | |
| Second | 109 875 (22·9%) | 24 940 (22·5%) | 79 730 (22·7%) | 1265 (28·5%) | 410 585 (22·8%) | 59 475 (22·3%) | 130 730 (23·3%) | 13 265 (22·1%) | 450 (27·0%) | 636 065 (22·3%) | |
| Third | 96 405 (20·1%) | 21 820 (19·7%) | 68 800 (19·6%) | 775 (17·5%) | 355 830 (19·8%) | 52 145 (19·6%) | 110 850 (19·7%) | 11 425 (19·0%) | 355 (21·3%) | 570 380 (20·0%) | |
| Fourth | 83 805 (17·5%) | 18 905 (17·1%) | 59 990 (17·1%) | 715 (16·1%) | 311 140 (17·3%) | 45 890 (17·2%) | 95 460 (17·0%) | 9850 (16·4%) | 265 (15·9%) | 510 220 (17·9%) | |
| Fifth (least deprived) | 68 685 (14·3%) | 14 940 (13·5%) | 49 380 (14·1%) | 625 (14·1%) | 261 875 (14·5%) | 38 095 (14·3%) | 80 145 (14·3%) | 8360 (13·9%) | 185 (11·1%) | 438 095 (15·4%) | |
| Unknown | 255 (0·1%) | 75 (0·1%) | 175 (<0·1%) | 0 | 900 (<0·1%) | 160 (0·1%) | 270 (<0·1%) | 40 (0·1%) | 5 (0·3%) | 1485 (0·1%) | |
| Region | |||||||||||
| London | 85 280 (17·8%) | 13 325 (12·0%) | 51 195 (14·6%) | 1830 (41·2%) | 308 860 (17·2%) | 37 050 (13·9%) | 103 345 (18·4%) | 6625 (11·0%) | 505 (30·3%) | 460 960 (16·2%) | |
| South West | 41 905 (8·7%) | 11 795 (10·6%) | 33 470 (9·5%) | 180 (4·1%) | 155 180 (8·6%) | 25 455 (9·6%) | 49 800 (8·9%) | 5500 (9·2%) | 100 (6·0%) | 268 085 (9·4%) | |
| South East | 65 920 (13·7%) | 18 535 (16·7%) | 53 050 (15·1%) | 475 (10·7%) | 248 260 (13·8%) | 41 490 (15·6%) | 69 740 (12·4%) | 9145 (15·2%) | 195 (11·7%) | 398 155 (14·0%) | |
| Midlands | 97 185 (20·3%) | 21 970 (19·8%) | 75 830 (21·6%) | 715 (16·1%) | 368 405 (20·5%) | 53 240 (20·0%) | 109 210 (19·5%) | 11 430 (19·0%) | 230 (13·8%) | 581 070 (20·4%) | |
| East of England | 53 075 (11·1%) | 12 215 (11·0%) | 38 650 (11·0%) | 385 (8·7%) | 197 005 (10·9%) | 28 005 (10·5%) | 65 915 (11·7%) | 6325 (10·5%) | 245 (14·7%) | 310 140 (10·9%) | |
| North West | 68 340 (14·3%) | 15 175 (13·7%) | 42 145 (12·0%) | 560 (12·6%) | 236 670 (13·1%) | 39 105 (14·7%) | 69 595 (12·4%) | 9640 (16·0%) | 195 (11·7%) | 371 060 (13·0%) | |
| North East and Yorkshire | 67 600 (14·1%) | 17 730 (16·0%) | 56 445 (16·1%) | 295 (6·6%) | 284 725 (15·8%) | 42 000 (15·8%) | 93 410 (16·6%) | 11 380 (18·9%) | 185 (11·1%) | 460 515 (16·2%) | |
| Unknown | 255 (0·1%) | 75 (0·1%) | 175 (<0·1%) | 0 | 900 (<0·1%) | 160 (0·1%) | 270 (<0·1%) | 40 (0·1%) | 5 (0·3%) | 1485 (0·1%) | |
| Diabetes duration, years | |||||||||||
| <1 | 730 (0·2%) | 85 (0·1%) | 340 (0·1%) | 5 (0·1%) | 10 965 (0·6%) | 460 (0·2%) | 935 (0·2%) | 25 (<0·1%) | 5 (0·3%) | 25 595 (0·9%) | |
| 1–2 | 19 525 (4·1%) | 3005 (2·7%) | 6740 (1·9%) | 80 (1·8%) | 180 825 (10·0%) | 13 210 (5·0%) | 18 075 (3·2%) | 810 (1·3%) | 35 (2·1%) | 375 145 (13·2%) | |
| 3–4 | 35 195 (7·3%) | 6100 (5·5%) | 10 025 (2·9%) | 130 (2·9%) | 209 400 (11·6%) | 23 390 (8·8%) | 30 695 (5·5%) | 1755 (2·9%) | 40 (2·4%) | 371 805 (13·0%) | |
| 5–9 | 123 830 (25·8%) | 25 100 (22·6%) | 42 700 (12·2%) | 635 (14·3%) | 502 950 (27·9%) | 77 575 (29·1%) | 128 480 (22·9%) | 8955 (14·9%) | 155 (9·3%) | 786 040 (27·6%) | |
| 10–14 | 137 200 (28·6%) | 33 270 (30·0%) | 79 995 (22·8%) | 1110 (25·0%) | 436 870 (24·3%) | 78 525 (29·5%) | 170 575 (30·4%) | 17 575 (29·3%) | 305 (18·3%) | 624 330 (21·9%) | |
| 15–19 | 104 120 (21·7%) | 27 720 (25·0%) | 102 560 (29·2%) | 1380 (31·1%) | 299 280 (16·6%) | 51 050 (19·2%) | 139 690 (24·9%) | 19 825 (33·0%) | 430 (25·8%) | 424 060 (14·9%) | |
| ≥20 | 58 955 (12·3%) | 15 535 (14·0%) | 108 605 (30·9%) | 1100 (24·8%) | 159 715 (8·9%) | 22 295 (8·4%) | 72 835 (13·0%) | 11 140 (18·5%) | 700 (42·0%) | 244 490 (8·6%) | |
| Smoking status | |||||||||||
| Current smoker | 56 030 (11·7%) | 14 045 (12·7%) | 43 315 (12·3%) | 430 (9·7%) | 235 240 (13·1%) | 36 275 (13·6%) | 70 620 (12·6%) | 7145 (11·9%) | 155 (9·3%) | 364 855 (12·8%) | |
| Ex-smoker | 171 565 (35·8%) | 43 220 (39·0%) | 131 280 (37·4%) | 1295 (29·2%) | 626 170 (34·8%) | 91 900 (34·5%) | 195 115 (34·8%) | 20 685 (34·4%) | 540 (32·4%) | 999 840 (35·1%) | |
| Current non-smoker, history unknown | 8540 (1·8%) | 2105 (1·9%) | 7065 (2·0%) | 70 (1·6%) | 29 825 (1·7%) | 4365 (1·6%) | 9070 (1·6%) | 940 (1·6%) | 25 (1·5%) | 49 955 (1·8%) | |
| Never smoked | 243 180 (50·7%) | 51 385 (46·4%) | 169 015 (48·2%) | 2645 (59·6%) | 907 670 (50·4%) | 133 830 (50·2%) | 286 170 (51·0%) | 31 280 (52·1%) | 945 (56·8%) | 1 434 475 (50·3%) | |
| Unknown | 245 (0·1%) | 60 (0·1%) | 285 (0·1%) | 5 (0·1%) | 1100 (0·1%) | 135 (0·1%) | 310 (0·1%) | 35 (0·1%) | 5 (0·3%) | 2340 (0·1%) | |
| BMI, kg/m2 | |||||||||||
| <20 | 5620 (1·2%) | 60 (0·1%) | 4355 (1·2%) | 90 (2·0%) | 18 240 (1·0%) | 900 (0·3%) | 6405 (1·1%) | 320 (0·5%) | 25 (1·5%) | 41 780 (1·5%) | |
| 20 to <25 | 66 450 (13·9%) | 1780 (1·6%) | 39 145 (11·2%) | 930 (20·9%) | 232 510 (12·9%) | 21 170 (7·9%) | 78 635 (14·0%) | 5400 (9·0%) | 310 (18·6%) | 395 530 (13·9%) | |
| 25 to <30 | 160 740 (33·5%) | 15 535 (14·0%) | 98 855 (28·2%) | 1525 (34·3%) | 572 160 (31·8%) | 75 925 (28·5%) | 184 785 (32·9%) | 16 600 (27·6%) | 505 (30·3%) | 899 635 (31·5%) | |
| 30 to <35 | 130 015 (27·1%) | 34 570 (31·2%) | 97 435 (27·8%) | 1025 (23·1%) | 487 320 (27·1%) | 80 775 (30·3%) | 150 640 (26·8%) | 17 170 (28·6%) | 390 (23·4%) | 738 590 (25·9%) | |
| 35 to <40 | 59 790 (12·5%) | 28 925 (26·1%) | 55 040 (15·7%) | 470 (10·6%) | 245 045 (13·6%) | 45 780 (17·2%) | 71 010 (12·7%) | 9910 (16·5%) | 190 (11·4%) | 364 950 (12·8%) | |
| ≥40 | 35 740 (7·5%) | 26 360 (23·8%) | 39 215 (11·2%) | 230 (5·2%) | 162 435 (9·0%) | 32 205 (12·1%) | 42 690 (7·6%) | 7830 (13·0%) | 135 (8·1%) | 240 000 (8·4%) | |
| Unknown | 21 200 (4·4%) | 3595 (3·2%) | 16 915 (4·8%) | 175 (3·9%) | 82 290 (4·6%) | 9745 (3·7%) | 27 125 (4·8%) | 2855 (4·8%) | 105 (6·3%) | 170 980 (6·0%) | |
| HbA1c, mmol/mol (%) | |||||||||||
| <48 | 49 675 (10·4%) | 10 500 (9·5%) | 22 640 (6·5%) | 500 (11·3%) | 348 420 (19·4%) | 15 110 (5·7%) | 54 565 (9·7%) | 8525 (14·2%) | 260 (15·6%) | 721 855 (25·3%) | |
| 48 to <54 (6·5% to <7·1%) | 81 295 (17·0%) | 13 305 (12·0%) | 32 835 (9·4%) | 690 (15·5%) | 383 730 (21·3%) | 32 745 (12·3%) | 82 850 (14·8%) | 11 805 (19·6%) | 280 (16·8%) | 591 760 (20·8%) | |
| 54 to <59 (7·1% to <7·5%) | 78 775 (16·4%) | 14 065 (12·7%) | 39 490 (11·3%) | 665 (15·0%) | 274 865 (15·3%) | 40 740 (15·3%) | 84 115 (15·0%) | 10 015 (16·7%) | 265 (15·9%) | 365 925 (12·8%) | |
| 59 to <75 (7·5% to <9·0%) | 150 635 (31·4%) | 35 950 (32·4%) | 120 840 (34·4%) | 1440 (32·4%) | 423 835 (23·5%) | 100 880 (37·9%) | 177 575 (31·6%) | 16 340 (27·2%) | 445 (26·7%) | 551 480 (19·3%) | |
| 75 to <86 (9·0% to <10·0%) | 43 745 (9·1%) | 14 180 (12·8%) | 49 815 (14·2%) | 400 (9·0%) | 117 115 (6·5%) | 31 835 (11·9%) | 58 515 (10·4%) | 4315 (7·2%) | 125 (7·5%) | 157 015 (5·5%) | |
| ≥86 | 43 860 (9·1%) | 15 855 (14·3%) | 58 470 (16·7%) | 440 (9·9%) | 122 515 (6·8%) | 29 365 (11·0%) | 63 930 (11·4%) | 4810 (8·0%) | 145 (8·7%) | 174 815 (6·1%) | |
| Unknown | 31 575 (6·6%) | 6965 (6·3%) | 26 865 (7·7%) | 300 (6·8%) | 129 520 (7·2%) | 15 830 (5·9%) | 39 740 (7·1%) | 4270 (7·1%) | 140 (8·4%) | 288 615 (10·1%) | |
| eGFR, mL/min per 1·73 m2 | |||||||||||
| ≥90 | 167 690 (35·0%) | 51 120 (46·1%) | 110 645 (31·5%) | 1615 (36·4%) | 756 755 (42·0%) | 146 240 (54·9%) | 217 250 (38·7%) | 21 570 (35·9%) | 545 (32·7%) | 1 065 540 (37·4%) | |
| 60 to <90 | 188 485 (39·3%) | 43 055 (38·9%) | 129 570 (36·9%) | 1725 (38·9%) | 788 790 (43·8%) | 107 210 (40·2%) | 226 340 (40·3%) | 25 760 (42·9%) | 675 (40·5%) | 1 218 455 (42·7%) | |
| 45 to <60 | 61 320 (12·8%) | 10 230 (9·2%) | 49 395 (14·1%) | 580 (13·1%) | 177 890 (9·9%) | 9710 (3·6%) | 65 835 (11·7%) | 7495 (12·5%) | 240 (14·4%) | 305 995 (10·7%) | |
| 30 to <45 | 41 320 (8·6%) | 4535 (4·1%) | 37 320 (10·6%) | 340 (7·7%) | 54 125 (3·0%) | 1135 (0·4%) | 35 240 (6·3%) | 3610 (6·0%) | 150 (9·0%) | 144 705 (5·1%) | |
| 15 to <30 | 13 620 (2·8%) | 880 (0·8%) | 15 210 (4·3%) | 100 (2·3%) | 3275 (0·2%) | 85 (<0·1%) | 8920 (1·6%) | 825 (1·4%) | 40 (2·4%) | 39 920 (1·4%) | |
| <15 | 3045 (0·6%) | 80 (0·1%) | 4445 (1·3%) | 35 (0·8%) | 145 (<0·1%) | 10 (<0·1%) | 1360 (0·2%) | 100 (0·2%) | 5 (0·3%) | 10 460 (0·4%) | |
| Unknown | 4080 (0·9%) | 920 (0·8%) | 4370 (1·2%) | 50 (1·1%) | 19 025 (1·1%) | 2115 (0·8%) | 6345 (1·1%) | 725 (1·2%) | 20 (1·2%) | 66 390 (2·3%) | |
| Systolic blood pressure, mm Hg | |||||||||||
| ≤140 | 378 280 (78·9%) | 88 240 (79·6%) | 264 235 (75·3%) | 3550 (80·0%) | 1 416 115 (78·7%) | 220 035 (82·6%) | 436 215 (77·7%) | 47 240 (78·6%) | 1310 (78·7%) | 2 163 325 (75·9%) | |
| >140 | 74 180 (15·5%) | 16 170 (14·6%) | 66 250 (18·9%) | 635 (14·3%) | 268 680 (14·9%) | 30 300 (11·4%) | 88 410 (15·8%) | 8735 (14·5%) | 250 (15·0%) | 449 595 (15·8%) | |
| Unknown | 27 100 (5·7%) | 6410 (5·8%) | 20 475 (5·8%) | 255 (5·7%) | 115 205 (6·4%) | 16 175 (6·1%) | 36 665 (6·5%) | 4115 (6·8%) | 105 (6·3%) | 238 545 (8·4%) | |
| Total serum cholesterol, mmol/L (mg/dL) | |||||||||||
| <5 (193 mg/dL) | 360 345 (75·1%) | 81 615 (73·6%) | 251 225 (71·6%) | 3385 (76·2%) | 1 326 140 (73·7%) | 192 115 (72·1%) | 418 855 (74·6%) | 45 750 (76·1%) | 1290 (77·5%) | 1 935 545 (67·9%) | |
| ≥5 (193 mg/dL) | 67 360 (14·0%) | 17 030 (15·4%) | 53 815 (15·3%) | 600 (13·5%) | 274 535 (15·3%) | 47 150 (17·7%) | 77 290 (13·8%) | 7645 (12·7%) | 165 (9·9%) | 508 140 (17·8%) | |
| Unknown | 51 850 (10·8%) | 12 170 (11·0%) | 45 920 (13·1%) | 455 (10·2%) | 199 330 (11·1%) | 27 240 (10·2%) | 65 140 (11·6%) | 6690 (11·1%) | 210 (12·6%) | 407 785 (14·3%) | |
| Disease history and medications | |||||||||||
| History of cardiovascular disease | 131 585 (27·4%) | 25 815 (23·3%) | 124 635 (35·5%) | 1105 (24·9%) | 405 290 (22·5%) | 48 605 (18·2%) | 140 755 (25·1%) | 9680 (16·1%) | 480 (28·8%) | 699 390 (24·5%) | |
| Antihypertensive drugs | 391 510 (81·6%) | 92 110 (83·1%) | 298 885 (85·2%) | 3710 (83·6%) | 1 403 145 (78·0%) | 200 830 (75·4%) | 453 100 (80·7%) | 48 350 (80·5%) | 1455 (87·4%) | 2 185 685 (76·7%) | |
| Statins | 395 195 (82·4%) | 93 010 (83·9%) | 285 620 (81·4%) | 3675 (82·8%) | 1 444 545 (80·3%) | 221 070 (83·0%) | 459 485 (81·9%) | 51 750 (86·1%) | 1415 (85·0%) | 2 099 315 (73·6%) | |
| Glucose-lowering drugs | |||||||||||
| None | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 755 045 (26·5%) | |
| One class | 45 515 (9·5%) | 4435 (4·0%) | 88 210 (25·1%) | 340 (7·7%) | 842 515 (46·8%) | 9380 (3·5%) | 47 850 (8·5%) | 2710 (4·5%) | 140 (8·4%) | 1 041 095 (36·5%) | |
| Two classes | 207 515 (43·3%) | 30 805 (27·8%) | 141 270 (40·3%) | 1550 (34·9%) | 557 100 (30·9%) | 85 710 (32·2%) | 232 145 (41·4%) | 19 665 (32·7%) | 290 (17·4%) | 638 025 (22·4%) | |
| Three classes or more | 226 525 (47·2%) | 75 580 (68·2%) | 121 480 (34·6%) | 2555 (57·5%) | 400 390 (22·2%) | 171 410 (64·3%) | 281 295 (50·1%) | 37 710 (62·8%) | 1230 (73·9%) | 417 300 (14·6%) | |
| Outcome | |||||||||||
| Alive | 467 325 (97·4%) | 109 650 (98·9%) | 338 180 (96·4%) | 4345 (97·9%) | 1 770 855 (98·4%) | 264 850 (99·4%) | 549 305 (97·9%) | 59 175 (98·5%) | 1620 (97·3%) | 2 784 520 (97·7%) | |
| Deaths in people with COVID-19* | 2733 (0·6%) | 279 (0·3%) | 2825 (0·8%) | 20 (0·5%) | 6295 (0·3%) | 458 (0·2%) | 2628 (0·5%) | 226 (0·4%) | 15 (0·9%) | 13 479 (0·5%) | |
| Mortality rate (per 1000 person-years) | 10·7 (10·3–11·1) | 4·7 (4·1–5·2) | 15·2 (14·7–15·8) | 8·4 (4·7–12·2) | 6·5 (6·4–6·7) | 3·2 (2·9–3·5) | 8·8 (8·4–9·1) | 7·0 (6·1–7·9) | 17·0 (8·4–25·5) | 8·9 (8·7–9·0) | |
| Deaths in people without COVID-19* | 9499 (2·0%) | 887 (0·8%) | 9953 (2·8%) | 76 (1·7%) | 22 855 (1·3%) | 1200 (0·5%) | 9359 (1·7%) | 684 (1·1%) | 27 (1·6%) | 53 469 (1·9%) | |
| Mortality rate (per 1000 person-years) | 37·3 (36·5–38·0) | 14·9 (13·9–15·9) | 53·7 (52·6–54·7) | 32·1 (24·9–39·3) | 23·8 (23·4–24·1) | 8·4 (7·9–8·9) | 31·3 (30·7–31·9) | 21·3 (19·7–22·9) | 30·5 (19·0–42·0) | 35·2 (34·9–35·5) | |
Data are n (%), apart from mortality rates (95% CI). Data taken directly from the National Diabetes Audit are rounded to the nearest five people to protect confidentiality. eGFR=estimated glomerular filtration rate.
Death data from the Office for National Statistics and associated mortality rates are unrounded; whole-cohort data also include people who were prescribed none of the listed glucose-lowering drugs (4132 deaths with COVID-19).
During 1 517 762 person-years of follow-up, 13 479 (0·5%) of the 2 851 465 people in the cohort had COVID-19-related deaths, corresponding to a crude mortality rate of 8·9 per 1000 person-years (95% CI 8·7–9·0). Rates per 1000 person-years ranged from 3·2 (95% CI 2·9–3·5) in patients prescribed SGLT2 inhibitors to 17·0 (8·4–25·5) in those prescribed α-glucosidase inhibitors (table).
Unadjusted associations between each variable and the risk COVID-19-related death are reported in the appendix (pp 3–4). Accounting for differences in the characteristics of the patients, the adjusted HR for COVID-19-related death was 0·77 (95% CI 0·73–0·81) for those prescribed metformin (compared with those not prescribed metformin), 0·75 (0·48–1·17) for meglitinides, 0·82 (0·74–0·91) for SGLT2 inhibitors, 0·94 (0·82–1·07) for thiazolidinediones, 0·94 (0·89–0·99) for sulfonylureas, 0·94 (0·83–1·07) for GLP-1 receptor agonists, 1·07 (1·01–1·13) for DPP-4 inhibitors, 1·26 (0·76–2·09) for α-glucosidase inhibitors, and 1·42 (1·35–1·49) for insulin (figure 2 ; appendix pp 5–6).
Figure 2.
Association between prescription of glucose-lowering drugs and COVID-19-related mortality
Numbers of people are rounded to the nearest five to protect confidentiality. Rates of COVID-19 death in patients prescribed the specific drug are obtained by multiplying the hazard ratio by the rate in patients without the prescription of the drug. The size of the box is proportional to the inverse of the variance and the error bars show 95% CIs. n=number of events (deaths). N=total number of people.
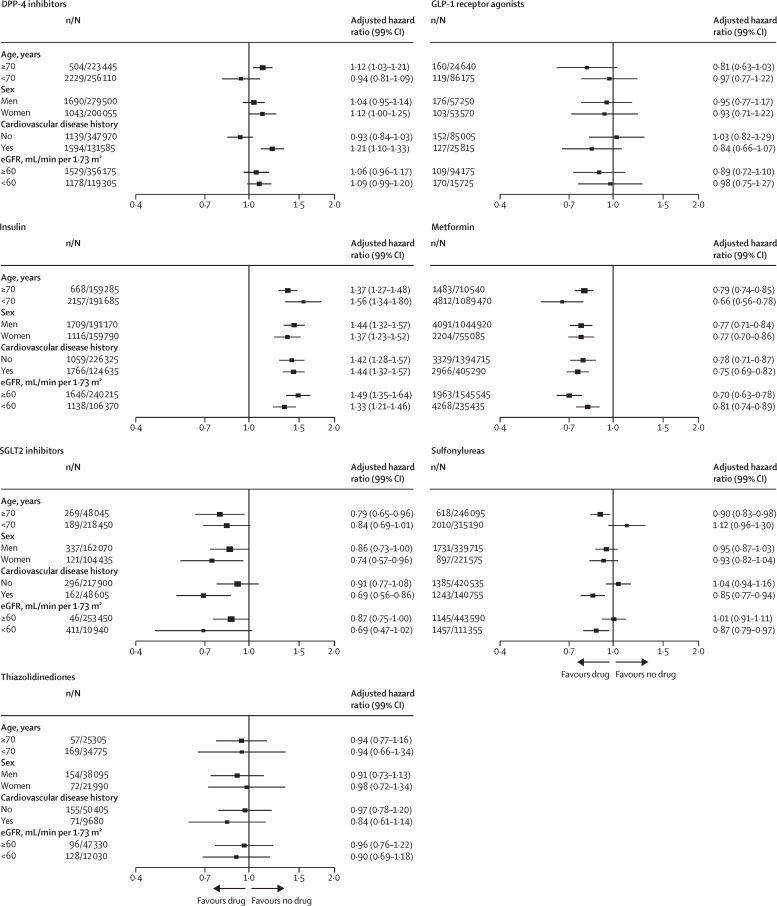
Associations were mainly consistent in explorative subgroup analyses by age, sex, cardiovascular disease history, and eGFR, with only a few small deviations from neutrality apart from for metformin, for which the risks were lower, and insulin, for which the risks were higher, in all investigated subgroups (figure 3 ). There were possible effects of age (in people aged ≥70 years, mortality was higher with DPP-4 inhibitors and lower with sulfonylureas) and cardiovascular disease history (a higher risk with DPP-4 inhibitors and lower with sulfonylureas).
Figure 3.
Subgroup analysis for association between prescription of glucose-lowering drugs and COVID-19-related mortality
Numbers of people are rounded to the nearest five to protect confidentiality. The size of the boxes is proportional to the inverse of the variance and the error bars show 99% CIs. Subgroup analysis for prescription of meglitinides and α-glucosidase inhibitors are not shown because the numbers of COVID-19-related deaths were too low for robust analysis. n=number of events (deaths). N=total number of people. eGFR=estimated glomerular filtration rate.
Discussion
In view of the disproportionately high contribution of people with diabetes to overall COVID-19-related deaths (up to a third of all deaths in some countries),2 the relation between glucose-lowering drugs and COVID-19 is an important issue for people with diabetes, clinicians, and policy makers. To our knowledge, this is the largest study to examine the association of several commonly prescribed classes of glucose-lowering drugs with COVID-19-related mortality. In this nationally representative, population-based study of 2·85 million people with type 2 diabetes, there was statistical evidence that people prescribed metformin, SGLT2 inhibitors, and sulfonylureas had a lower mortality risk than those not prescribed these drugs. Conversely, the risk was higher in those prescribed insulin and DPP-4 inhibitors than those not prescribed these drugs. These finding are likely to be related in part to confounding by indication, because metformin is used early in the disease trajectory of type 2 diabetes whereas insulin is typically initiated later. Furthermore, the absolute increases and decreases in risk were very small. National recommendations for use of DPP-4 inhibitors include older people and particularly those with frailty.26 The observed lower risks in people prescribed SGLT2 inhibitors and sulfonylureas are likely to be related to confounding by indication and their lower use in older people, particularly those with frailty, in view of increase risk of volume depletion for SGLT2 inhibitors and hypoglycaemia for sulfonylureas. NDA data do not include information on frailty; we were therefore unable to adjust for this variable. In exploratory subgroup analyses, we identified some differences by age and history of cardiovascular disease, although these results should be interpreted with caution in view of a lower statistical power and residual confounding.27 The lower risk of mortality in patients who were prescribed metformin and the higher risk in those prescribed insulin, have been observed previously and might be related to residual confounding by the burden of cardiorenal comorbidities and historical glycaemic burden, factors that cannot be fully addressed by statistical adjustments.10 Associations between insulin prescription and mortality have also been reported in previous observational studies and were related to commencement of insulin at a late stage of the disease.28, 29
Smaller retrospective studies from the USA, China, and France have all reported a lower or neutral risk of COVID-19-related mortality in people previously or currently prescribed metformin.10, 18, 30, 31, 32 In a meta-analysis of five observational studies including 1282 patients, use of metformin before hospital admission in people with diabetes and sepsis (non-COVID-19 related) was associated with lower mortality.33 In a French multicentre observational study that included 1166 people with type 2 diabetes, investigators reported no association between use of sulfonylureas, meglitinides, DPP-4 inhibitors, or GLP-1 receptor agonists and COVID-19-related mortality; however, there was higher mortality associated with insulin therapy (odds ratio 1·71 [95% CI 1·20–2·43]).10 In one small observational study in Northern Italy of 338 patients with type 2 diabetes who were admitted to hospital with COVID-19, a lower risk of mortality was seen in patients on sitagliptin than in those who received standard of care (HR 0·44 [95% CI 0·29–0·66]).11 Although some of these findings were confirmed in our analyses, we also observed several differences probably related to dissimilarities in sample size or confounding adjustment.
Our study has several limitations. Because the medications included were prescribed in the last 6 months of 2019, there is the possibility that some of these agents had been suspended before or on admission to hospital for COVID-19. There was no measure of medication adherence in this study; however, a previous report of overall adherence for most glucose-lowering therapies using an objective measure (urine liquid chromatography) showed adherence rates for glucose-lowering medications of about 90%.34 Moreover, the NDA does not collect data on the dose of medications. In our analysis, we adjusted for several important potential confounders, including regional differences, to reduce the risk of confounding; however, we cannot exclude residual confounding due to imperfect adjustment for those factors we have considered, nor unmeasured confounding by factors that we have not included. Furthermore, during the pandemic, the reporting of COVID-19 as either the underlying cause of death or a significant contributory factor might have been inconsistent; although it is difficult to quantify the extent or direction of any bias created by the omission of deaths possibly related to COVID-19, it is unlikely that this bias would be differential with respect to class of glucose-lowering therapy. We reported associations for the prescription of all classes of glucose-lowering medications in people with type 2 diabetes in England; the number of events was low for meglitinides and α-glucosidase inhibitors because these drugs are not widely used in England, although they are used more extensively in some other countries. Given the many possible combinations of glucose-lowering drug classes that can be used by patients with type 2 diabetes, it was not possible to estimate the HRs for these combinations; however, this was not the main purpose of the analysis. We used the missing-category approach to maximise the size of the cohort and the statistical power; although other approaches could be applied to account for missing data, their relative strengths and limitations in the context of doubly robust adjustment with the use of propensity score as a variable are uncertain.
A strength of our study is the whole-population inclusion of nearly all people diagnosed with type 2 diabetes in England, including comprehensive prepandemic information on risk factors; the results are therefore probably generalisable to the general population of people with type 2 diabetes. A further strength is the comprehensive assessment of the risk across all classes of glucose-lowering drugs commonly used in clinical practice—previous studies were largely limited to single drugs. Because of provision of universal health care, with all medicines for people with diabetes available without charge in England, our results should not be biased by access to medications. Many studies of the risks of therapies in patients with type 2 diabetes and COVID-19 have focused on people in hospital,10 whereas our cohort included all people with type 2 diabetes, whether or not they were admitted to hospital with COVID-19. Furthermore, our outcome of all deaths with COVID-19 identified as a cause, in-hospital or outside of hospital, provides a robust outcome independent of clinical decisions, administrative arrangements, and resource availability, which might influence decisions related to hospital or intensive care unit admissions.
Given the nature of this study, we cannot infer causality from our observations. Randomised clinical trials assessing the role of glucose-lowering therapies would be necessary to assess any causal effect of glucose-lowering drugs on COVID-19 outcomes in patients with type 2 diabetes. The DARE-19 study (NCT04350593) is investigating the effect of the SGLT2 inhibitor dapagliflozin versus placebo on the risk of death or organ dysfunction in patients admitted to hospital with COVID-19. In the MET-COVID trial (NCT04510194), the effect of metformin is being compared with placebo for both the prevention of SARS-COV-2 infection and the risk of COVID-19 disease. Because neither of these studies is specifically including patients with type 2 diabetes, their results will generate little evidence on the safety of these glucose-lowering drugs in this patient population. However, several studies with DPP-4 inhibitors (NCT04365517, NCT04371978, and NCT04341935) are being conducted in patients with type 2 diabetes and COVID-19.
In summary, in this study of a national cohort of 2·85 million people with type 2 diabetes, we identified a statistically lower risk of COVID-19-related mortality in patients prescribed metformin and a higher risk of COVID-19-related mortality in patients prescribed insulin, supporting findings from previous smaller studies. We also identified a lower risk with sulfonylureas and SGLT2 inhibitors and a slightly higher risk with DPP-4 inhibitors. The lower and higher risks associated with these drugs are likely to be due to residual confounding rather than direct drug effects. Cardiorenal comorbidities and frailty are probable contributors to our observations and are also important COVID-19 risk factors. We interpret these findings to suggest that there is, as yet, no clear indication to jeopardise a modifiable risk factor—glucose control—or other potential glucose-independent benefits of specific drugs by stopping or changing diabetes medications in people with type 2 diabetes in daily practice. Additional studies to validate these findings in large national datasets in other countries are warranted.
Data sharing
Data from the NDA can be requested through the NHS Digital Data Access Request Service process. For further information, contact diabetes@nhs.net.
Declaration of interests
KK, NH, PKn, PKa, NS, BY, and JV are members of the NDA research committee. KK has been a consultant and speaker for Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, and Merck Sharp & Dohme (MSD); has received grants in support of investigator-initiated studies from Novartis, Novo Nordisk, Sanofi-Aventis, Lilly, MSD, Pfizer, and Boehringer Ingelheim; and has served on advisory boards for Novo Nordisk, Sanofi-Aventis, Lilly, and MSD. FZ has been a speaker for Napp Pharmaceuticals and Boehringer Ingelheim. CB is an adviser to the NHS England and NHS Improvement Diabetes Prevention Programme. NH is funded by Diabetes UK and NHS England and NHS Improvement. PKa is national specialty adviser for diabetes and obesity at NHS England and NHS Improvement. NS has consulted for Amgen, AstraZeneca, Boehringer Ingelheim, Eli Lilly, MSD, Novartis, Novo Nordisk, Pfizer, and Sanofi; and has received grant support from Boehringer Ingelheim. BY is clinical lead for the NDA and a trustee of Diabetes UK. JV is the national clinical director for diabetes and obesity at NHS England and NHS Improvement. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
NHS Digital provided the infrastructures for the data repository and data linkages for these analyses and salaries for the data analysts conducting this work. KK and FZ are supported by the UK National Institute for Health Research (NIHR) Applied Research Collaboration East Midlands and the NIHR Leicester Biomedical Research Centre. NJW and SS are supported by the UK Medical Research Council (MC_UU_00006/1).
Contributors
KK, FZ, CB, EB, PKa, CM, NS, SS, NJW, AW, BY, and JV conceived the study. PKn, NH, and EW managed the data and did statistical analyses. NH and PKn had full access to the data and can verify the data presented. All authors had access to all the data in the study and had final responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Apicella M, Campopiano MC, Mantuano M, Mazoni L, Coppelli A, Del Prato S. COVID-19 in people with diabetes: understanding the reasons for worse outcomes. Lancet Diabetes Endocrinol. 2020;8:782–792. doi: 10.1016/S2213-8587(20)30238-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barron E, Bakhai C, Kar P, et al. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8:813–822. doi: 10.1016/S2213-8587(20)30272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhu L, She Z-G, Cheng X, et al. Association of blood glucose control and outcomes in patients with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;31:1068. doi: 10.1016/j.cmet.2020.04.021. 77.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holman N, Knighton P, Kar P, et al. Risk factors for COVID-19-related mortality in people with type 1 and type 2 diabetes in England: a population-based cohort study. Lancet Diabetes Endocrinol. 2020;8:823–833. doi: 10.1016/S2213-8587(20)30271-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Drucker DJ. Coronavirus infections and type 2 diabetes—shared pathways with therapeutic implications. Endocr Rev. 2020;41:457–470. doi: 10.1210/endrev/bnaa011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pal R, Bhadada SK. Should anti-diabetic medications be reconsidered amid COVID-19 pandemic? Diabetes Res Clin Pract. 2020;163 doi: 10.1016/j.diabres.2020.108146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Monteil V, Kwon H, Prado P, et al. Inhibition of SARS-CoV-2 infections in engineered human tissues using clinical-grade soluble human ACE2. Cell. 2020;181:905. doi: 10.1016/j.cell.2020.04.004. 13.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng Y, Wang L, Ma X, et al. Effect of hCMSCs and liraglutide combination in ALI through cAMP/PKAc/β-catenin signaling pathway. Stem Cell Res Ther. 2020;11:2. doi: 10.1186/s13287-019-1492-6. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 9.Monda VM, Porcellati F, Strollo F, Gentile S. ACE2 and SARS-CoV-2 infection: might GLP-1 receptor agonists play a role? Diabetes Ther. 2020;11:1909–1914. doi: 10.1007/s13300-020-00898-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cariou B, Hadjadj S, Wargny M, et al. Phenotypic characteristics and prognosis of inpatients with COVID-19 and diabetes: the CORONADO study. Diabetologia. 2020;63:1500–1515. doi: 10.1007/s00125-020-05180-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solerte SB, D'Addio F, Trevisan R, et al. Sitagliptin treatment at the time of hospitalization was associated with reduced mortality in patients with type 2 diabetes and COVID-19: a multicenter, case-control, retrospective, observational study. Diabetes Care. 2020;43:2999–3006. doi: 10.2337/dc20-1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta R, Ghosh A, Singh AK, Misra A. Clinical considerations for patients with diabetes in times of COVID-19 epidemic. Diabetes Metab Syndr. 2020;14:211–212. doi: 10.1016/j.dsx.2020.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bornstein SR, Rubino F, Khunti K, et al. Practical recommendations for the management of diabetes in patients with COVID-19. Lancet Diabetes Endocrinol. 2020;8:546–550. doi: 10.1016/S2213-8587(20)30152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Singh AK, Gupta R, Ghosh A, Misra A. Diabetes in COVID-19: prevalence, pathophysiology, prognosis and practical considerations. Diabetes Metab Syndr. 2020;14:303–310. doi: 10.1016/j.dsx.2020.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sinclair A, Dhatariya K, Burr O, et al. Guidelines for the management of diabetes in care homes during the Covid-19 pandemic. Diabet Med. 2020;37:1090–1093. doi: 10.1111/dme.14317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Puig-Domingo M, Marazuela M, Giustina A. COVID-19 and endocrine diseases. A statement from the European Society of Endocrinology. Endocrine. 2020;68:2–5. doi: 10.1007/s12020-020-02294-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kaiser UB, Mirmira RG, Stewart PM. Our response to COVID-19 as endocrinologists and diabetologists. J Clin Endocrinol Metab. 2020;105:1299–1301. doi: 10.1210/clinem/dgaa148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng X, Liu Y-M, Li H, et al. Metformin is associated with higher incidence of acidosis, but not mortality, in individuals with COVID-19 and pre-existing type 2 diabetes. Cell Metab. 2020;32:537. doi: 10.1016/j.cmet.2020.08.013. 47.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Buse JB, Wexler DJ, Tsapas A, et al. 2019 Update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2020;43:487–493. doi: 10.2337/dci19-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.NHS Digital National Diabetes Audit Programme. Dec 11, 2020. https://digital.nhs.uk/data-and-information/clinical-audits-and-registries/national-diabetes-audit
- 21.Ministry of Housing. Communities & Local Government English indices of deprivation 2019. Sept 26, 2019. https://www.gov.uk/government/statistics/english-indices-of-deprivation-2019
- 22.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34:3661–3679. doi: 10.1002/sim.6607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 24.Kang JDY, Schafer JL. Demystifying double robustness: a comparison of alternative strategies for estimating a population mean from incomplete data. Stat Sci. 2007;22:523–539. doi: 10.1214/07-STS227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 26.Strain WD, Hope SV, Green A, Kar P, Valabhji J, Sinclair AJ. Type 2 diabetes mellitus in older people: a brief statement of key principles of modern day management including the assessment of frailty. A national collaborative stakeholder initiative. Diabet Med. 2018;35:838–845. doi: 10.1111/dme.13644. [DOI] [PubMed] [Google Scholar]
- 27.VanderWeele TJ, Knol MJ. A tutorial on interaction. Epidemiol Methods. 2014;3:33–72. [Google Scholar]
- 28.Currie CJ, Poole CD, Evans M, Peters JR, Morgan CL. Mortality and other important diabetes-related outcomes with insulin vs other antihyperglycemic therapies in type 2 diabetes. J Clin Endocrinol Metab. 2013;98:668–677. doi: 10.1210/jc.2012-3042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Anyanwagu U, Mamza J, Mehta R, Donnelly R, Idris I. Cardiovascular events and all-cause mortality with insulin versus glucagon-like peptide-1 analogue in type 2 diabetes. Heart. 2016;102:1581–1587. doi: 10.1136/heartjnl-2015-309164. [DOI] [PubMed] [Google Scholar]
- 30.Luo P, Qiu L, Liu Y, et al. Metformin treatment was associated with decreased mortality in COVID-19 patients with diabetes in a retrospective analysis. Am J Trop Med Hyg. 2020;103:69–72. doi: 10.4269/ajtmh.20-0375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bramante CT, Ingraham NE, Murray TA, et al. Metformin and risk of mortality in patients hospitalised with COVID-19: a retrospective cohort analysis. Lancet Healthy Longev. 2021;2:e34–e41. doi: 10.1016/S2666-7568(20)30033-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crouse AB, Grimes T, Li P, Might M, Ovalle F, Shalev A. Metformin use is associated with reduced mortality in a diverse population with COVID-19 and diabetes. Front Endocrinol. 2021;11 doi: 10.3389/fendo.2020.600439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liang H, Ding X, Li L, et al. Association of preadmission metformin use and mortality in patients with sepsis and diabetes mellitus: a systematic review and meta-analysis of cohort studies. Crit Care. 2019;23:50. doi: 10.1186/s13054-019-2346-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patel P, Gupta P, Burns A, et al. Biochemical urine testing of adherence to cardiovascular medications reveals high rates of nonadherence in people attending their annual review for type 2 diabetes. Diabetes Care. 2019;42:1132–1135. doi: 10.2337/dc18-1453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data from the NDA can be requested through the NHS Digital Data Access Request Service process. For further information, contact diabetes@nhs.net.