Randomised controlled trials on convalescent plasma in patients with COVID-19 have given conflicting results with regards to therapeutic benefit.1, 2 Possible factors include previous seroconversion in recipients1 and late treatment when proinflammatory factors dominate tissue damage.2 Patients with impaired immune function due to B-cell depletion might develop chronic SARS-CoV-2 infections, which can be controlled by convalescent plasma transfusions.3
Here, we report our experience with convalescent plasma administered to 14 patients with COVID-19 (seven [50%] were female, and median age was 65 years [IQR 58–70]) with acquired immunodeficiencies due to: solid organ transplantation (eight patients), allogeneic stem cell transplantation (four patients), or active haematological malignancy (two patients). All patients had no detectable IgG against SARS-CoV-2 at the time of transfusion (LIAISON SARS-CoV-2 S1/S2 IgG, DiaSorin, Stillwater, MN, USA). Mean time from positive SARS-CoV-2 PCR to transfusion was 5·14 days (SD 5·14). Median initial disease severity on the 10-point WHO Clinical Progression Scale was 5 (range 4–6). Convalescent plasma preparations were subjected to pathogen inactivation (Intercept Blood System, Cerus, Concord, CA USA), as reported previously.4 All plasma preparations had plaque reduction neutralisation test 50 (PRNT50) values of 40 or higher. 11 patients received three transfusions, two received two transfusions, and one patient received one transfusion, each of 200 mL.
Transfusion of convalescent plasma was well tolerated. 13 patients developed detectable anti-SARS-CoV-2 IgG 24–48 h after the last transfusion. Eight (57%) of 14 patients showed clinical improvement on day 5 after the last transfusion, defined as an improvement of 1 point or more on the WHO Clinical Progression Scale. 12 (86%) patients were discharged from hospital. Two (14%) patients died due to a secondary infection. Interestingly, we found a significant correlation between the serum level of anti-SARS-CoV-2 IgG after the last transfusion and the degree of clinical improvement on day 5 (figure ). Because an early intrinsic antibody response to SARS-CoV-2 infection in severely immunocompromised patients seems unlikely,5 we assume that the IgG titres reflected transfused anti-SARS-CoV-2 IgG from the convalescent plasma. More importantly, our data suggest that anti-SARS-CoV-2 IgG serum titres of more than 20 IU/mL are able to confer a more than 2-point improvement in the WHO Clinical progression Scale (figure) and could act to guide the use of convalescent plasma transfusions.
Figure.
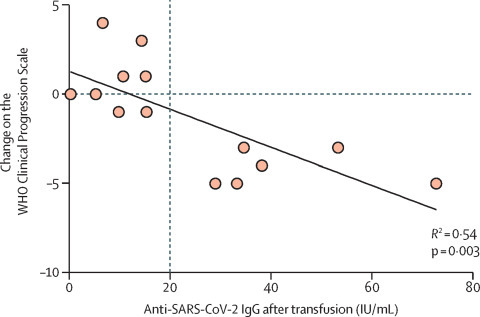
Correlation between anti-SARS-CoV-2 IgG titres 24–48 h after the last transfusion and improvement in clinical status in patients with COVID-19 (n=14)
Datapoints represent each patient. Clinical improvement was defined as an improvement of 1 point or more on the 10-point WHO Clinical Progression Scale for COVID-19 5 days after the last transfusion and the clinical status before transfusion.
In summary, our pilot study, which is limited by the small number of participants, suggests patients who are immunosuppressed with early stage SARS-CoV-2 infection and no detectable anti-SARS-CoV-2 IgG are potential candidates for treatment with convalescent plasma, and the IgG titre after transfusion could be used as a potential predictive parameter for treatment success.
We declare no competing interests. RNR and AB contributed equally.
References
- 1.Simonovich VA, Burgos Pratx LD, Scibona P. A randomized trial of convalescent plasma in COVID-19 severe pneumonia. N Engl J Med. 2020 doi: 10.1056/NEJMoa2031304. published online Nov 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libster R, Pérez Marc G, Wappner D. Early high-titer plasma therapy to prevent severe COVID-19 in older adults. N Engl J Med. 2021 doi: 10.1056/NEJMoa2033700. published online Jan 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hueso T, Pouderoux C, Péré H. Convalescent plasma therapy for B-cell-depleted patients with protracted COVID-19. Blood. 2020;136:2290–2295. doi: 10.1182/blood.2020008423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tonn T, Corman VM, Johnsen M. Stability and neutralising capacity of SARS-CoV-2-specific antibodies in convalescent plasma. Lancet Microbe. 2020;1:e63. doi: 10.1016/S2666-5247(20)30037-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aydillo T, Gonzalez-Reiche AS, Aslam S. Shedding of viable SARS-CoV-2 after immunosuppressive therapy for cancer. N Engl J Med. 2020;383:2586–2588. doi: 10.1056/NEJMc2031670. [DOI] [PMC free article] [PubMed] [Google Scholar]


