Abstract
Purpose:
To determine if fibroids or their characteristics are associated with birthweight and/or gestational age, and to assess the impact of race or ethnicity.
Methods:
Right from the Start (2000–2012) is a prospective cohort that enrolled women from the southern US in early pregnancy. Transvaginal ultrasounds were used to measure fibroid characteristics and confirm gestational age. Date of birth and birthweight were obtained from vital or medical records. We assessed whether fibroid presence, number, type, and volume were associated with birthweight and/or gestational age using multivariate analysis of covariance, accounting for a priori confounders.
Results:
Among 3926 women, 416 had one or more fibroids. Mean infant birthweight and gestational age were similar among women with and without fibroids. When adjusting for race or ethnicity, all associations were attenuated. Overall, women with and without fibroids had infants of similar birthweight (−20 grams, 95% confidence interval [CI] −77, 36) and gestational age (0.4 days, 95% CI −0.9, 1.8). Women with three or more fibroids were more likely to have lighter infants (−201 grams, 95% CI −345, −58).
Conclusions:
Race or ethnicity substantially confounds the associations. The clinical belief that uterine fibroids impair fetal growth is supported only by a significant decrease in birthweight for women with multiple fibroids.
Keywords: Leiomyoma, Pregnancy, Prospective studies, Gestational age, Birthweight, Confounding factors
Capsule:
Our prospective pregnancy cohort suggests women with three or more fibroids are more likely to have lighter infants than those without. No meaningful fibroid-related differences were observed in gestational age.
Background
Infant weight and gestational age at birth are strong indicators for maternal and newborn health. As birthweight decreases and gestational age shortens, an infant’s risk of mortality increases [1]. Low birthweight (<2500 grams) and preterm birth (<37 weeks’ gestation) affect 8% and 10% of all births in the United States, respectively [2]. Long-term consequences of reduced birthweight and decreased gestational age include poor growth in childhood and higher incidence of adult diseases such as type 2 diabetes, hypertension, and cardiovascular disease [3,4].
Uterine fibroids occur in 70%–80% of women over their lifetime and are found in 10–20% of pregnancies [5,6]. Fibroids are suspected to influence birthweight and length of gestation by distorting the uterine cavity and interfering with optimal uterine-placental perfusion and fetal nutrition [7–11]. However, prior literature has reported conflicting results on the association of fibroids and birth outcomes. While some studies found no difference in birthweight or gestational age at delivery among women with and without fibroids [9,10,12], other studies suggested that women with fibroids were more likely to have infants with lower birthweight or earlier gestational age at birth [8,9,11,13].
The lack of uniform screening and characterization of fibroids, in addition to the handling of the contribution of gestational age to birthweight and confounding by race or ethnicity, are all likely to bias the results and lead to inconsistency across studies [14,15]. We aimed to determine if fibroids or their characteristics are associated with birthweight and/or gestational age using a large prospective cohort of pregnant women who underwent systematic ultrasound screening during early pregnancy. We also used a regression model that addresses the correlation between birthweight and/or gestational age without dichotomizing birthweight based on gestational age, while assessing the impact of race or ethnicity on these associations.
Methods
Cohort selection
Right from the Start is a community-based pregnancy cohort that enrolled women in early pregnancy across the southeastern US from 2000 to 2012 [16]. Women who were pregnant or trying to become pregnant, 18 years or older, spoke English, planned to carry to term, and did not use assisted reproductive technologies were eligible. Informed consent was obtained from all study participants at enrollment. For women who participated in Right from the Start for more than one pregnancy (n = 325), we only included information from the first study pregnancy to maintain independence between observations. Among 5780 enrolled participants, we excluded women who had an induced abortion, spontaneous abortion, stillbirth, or other pregnancy outcome that did not result in a live birth, women with multiple gestation, and women with no first-trimester ultrasound or recorded infant birthweight (Fig. 1). The resulting population of 3926 women had uniform assessment of fibroids. This study was approved by the Institutional Review Board of Vanderbilt University Medical Center (070037).
Fig. 1.
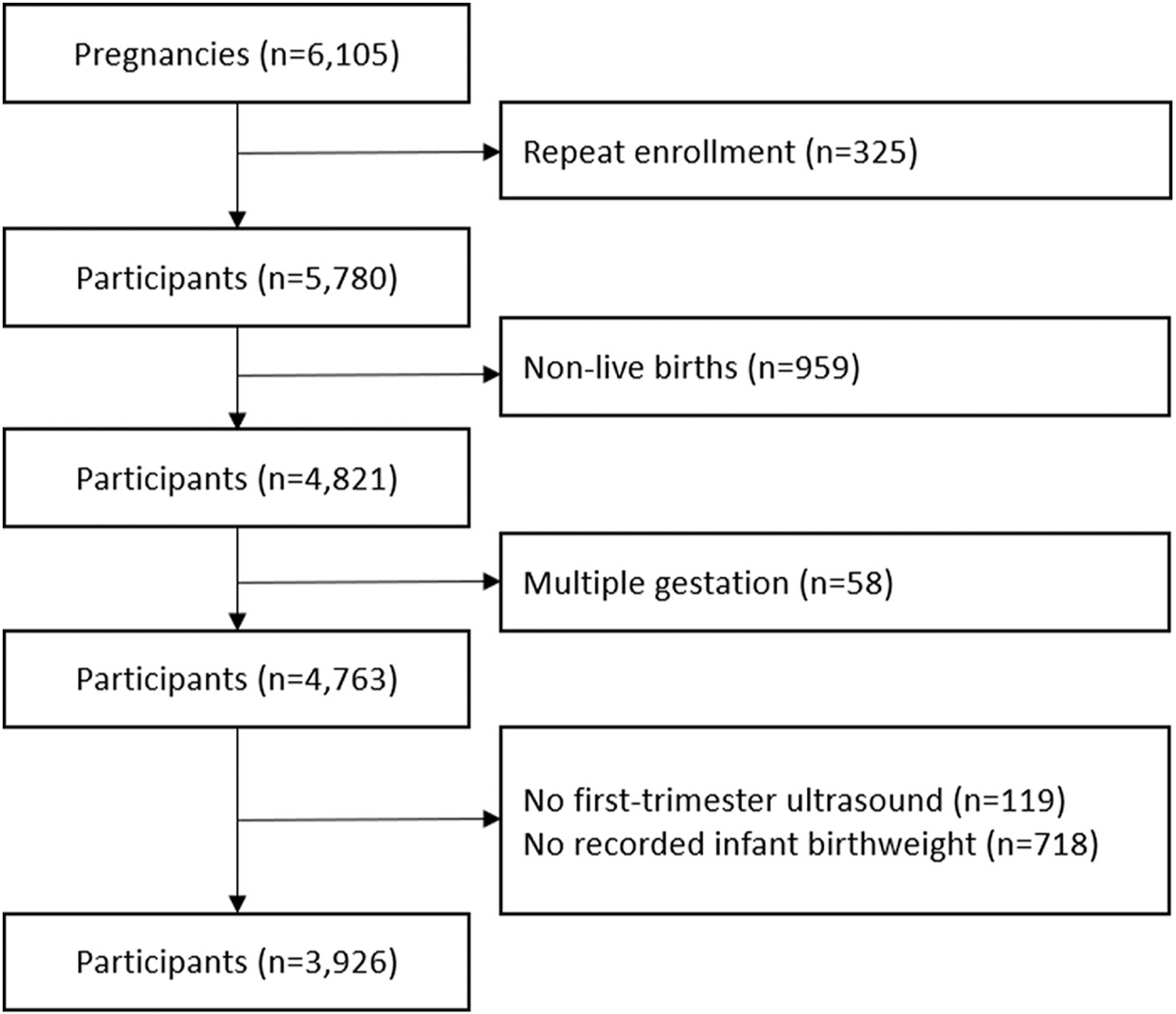
Flow chart of study subject exclusion criteria.
Exposure
The presence of fibroids and their characteristics, including fibroid number, type (any submucosal vs. any subserosal vs. any intramural), and total volume (cm3), were uniformly assessed during the first-trimester research ultrasound visit [5]. Experienced clinical sonographers followed a standardized protocol taking repeated measures to decrease misclassification of fibroids. The presence of a fibroid was defined by the Muram criteria with modifications to include masses of maximum diameter at least 0.5 cm [17]. Fibroid type was determined by sonographers and investigators reviewing study images, which has been described [5]. The median time of ultrasound visit was 8 weeks’ gestation.
Outcomes
Infant birthweights (in grams) were obtained from vital records or abstracted from medical records when vital records were unavailable. Gestational age at birth was determined using date of birth from vital or medical records and date of self-reported last menstrual period (LMP). When self-reported LMP was unavailable or differed from ultrasound-confirmed LMP by more than 7 days, ultrasound LMP was used.
Covariates
During first-trimester computer-assisted telephone interviews, women self-reported their age (years), race or ethnicity (mutually exclusive groups: non-Hispanic white, non-Hispanic black, Hispanic, and other non-Hispanic), parity, education level (high school or less, some college, college or more), household income, smoking status (never, current or recent quit, and quit prior to four months before first trimester interview), and alcohol use (never, current or recent quit, and quit prior to four months before first trimester interview). Body mass index (BMI) was calculated from standardized measures of height and weight obtained at the ultrasound visit and entered all models as a continuous variable.
Statistical analysis
Maternal characteristics were compared among women with and without fibroids (Table 1). Considering the correlated nature of birthweight and gestational age and the role of potential risk factors, a multivariate analysis of variance (MANOVA) was performed to assess whether the presence, number, type, and total volume of fibroids were associated with birthweight and/or gestational age. Multivariate analysis of covariance was used when adjusting for maternal age, BMI, and race or ethnicity, which were selected as a priori confounders using a directed acyclic graph based on the literature (Supplemental Fig. 1). Participants missing any confounder data were excluded from the analysis (n = 81). We compared models with and without adjustment for race or ethnicity to evaluate the impact of race or ethnicity on the association of fibroids and birth outcomes (birthweight and gestational age, Table 2).
Table 1.
Maternal characteristics by fibroid status: Right from the Start, 2000–2012 (n = 3926)
| Characteristic | With fibroids n = 416 (10.6%)* |
Without fibroids n = 3510 (89.4%)* |
|---|---|---|
| Maternal age, y | ||
| <25 | 33 (7.9) | 690 (19.7) |
| 25–29 | 100 (24.0) | 1303 (37.2) |
| 30–34 | 181 (43.6) | 1126 (32.1) |
| ≥35 | 102 (24.5) | 388 (11.1) |
| Missing | 0 | 3 |
| Race or ethnicity | ||
| White non-Hispanic | 238 (57.2) | 2615 (74.6) |
| Black non-Hispanic | 130 (31.3) | 498 (14.2) |
| Hispanic | 24 (5.8) | 251 (7.2) |
| Other non-Hispanic | 24 (5.8) | 143 (4.1) |
| Missing | 0 | 3 |
| Body mass index† | ||
| Underweight | 9 (2.2) | 89 (2.6) |
| Normal weight | 179 (43.3) | 1935 (56.0) |
| Overweight | 115 (27.9) | 785 (22.7) |
| Obese | 110 (26.6) | 645 (18.7) |
| Missing | 3 | 56 |
| Education level | ||
| High school or less | 47 (11.3) | 592 (16.8) |
| Some college | 65 (15.6) | 643 (18.3) |
| College or more | 304 (73.1) | 2275 (64.8) |
| Marital status | ||
| Married | 374 (89.9) | 3142 (89.5) |
| Other | 42 (10.1) | 368 (10.5) |
| Household income | ||
| ≤$40,000 | 96 (24.1) | 946 (29.1) |
| $40,001–80,000 | 152 (38.2) | 1257 (38.7) |
| ≥$80,001 | 150 (37.7) | 1047 (32.2) |
| Missing | 18 | 260 |
| Parity | ||
| 0 | 199 (49.1) | 1587 (47.6) |
| 1 | 134 (33.1) | 1184 (35.5) |
| ≥2 | 72 (17.8) | 561 (16.8) |
| Missing | 11 | 178 |
| Prior preterm birth | ||
| None | 367 (90.6) | 3074 (92.3) |
| Any | 38 (9.4) | 258 (7.7) |
| Missing | 11 | 178 |
| Smoking | ||
| Never | 308 (75.7) | 2445 (72.9) |
| Current or recent quit | 28 (6.9) | 412 (12.3) |
| Distant quit‡ | 71 (17.4) | 498 (14.8) |
| Missing | 9 | 155 |
| Alcohol | ||
| Never | 52 (12.8) | 455 (13.6) |
| Current or recent quit | 211 (51.8) | 1917 (57.2) |
| Distant quit‡ | 144 (35.4) | 982 (29.3) |
| Missing | 9 | 156 |
| Diabetes | ||
| None | 391 (96.1) | 3269 (97.6) |
| Type I | 1 (0.3) | 10 (0.3) |
| Type II | 3 (0.7) | 7 (0.2) |
| Gestational diabetes | 12 (3.0) | 64 (1.9) |
| Multiple types | 0 (0.0) | 1 (0.0) |
| Missing | 9 | 159 |
| Caffeine consumption | ||
| None | 134 (32.9) | 1032 (30.8) |
| Any | 273 (67.1) | 2324 (69.3) |
| Missing | 9 | 154 |
| Prenatal vitamins | ||
| None | 8 (2.0) | 85 (2.5) |
| Any | 399 (98.0) | 3262 (97.5) |
| Missing | 9 | 163 |
| Folic acid | ||
| None | 8 (2.0) | 85 (2.5) |
| Any | 399 (98.0) | 3261 (97.5) |
| Missing | 9 | 164 |
| Infant sex | ||
| Male | 178 (49.9) | 1436 (52.2) |
| Female | 179 (50.1) | 1313 (47.8) |
| Missing | 59 | 761 |
| Study site | ||
| North Carolina | 288 (69.2) | 2067 (58.9) |
| Tennessee | 119 (28.6) | 1138 (32.4) |
| Texas | 9 (2.2) | 305 (8.7) |
Data are counts and column percentages for each characteristic. Percentages exclude missing.
Body mass index was calculated as weight (kg)/height (m)2 and was categorized as underweight: <18.5; normal weight: 18.5–24.9; overweight: 25.0–29.9; or obese: ≥30.
Distant quit defined as cessation before four months before first-trimester interview.
Table 2.
Fibroid and birth outcomes adjusting for age and BMI (n = 3848)*
| Fibroid characteristic | n (%)† | Birthweight (grams) | Gestational age (d) | ||
|---|---|---|---|---|---|
| Model 1 Coefficient (95% CI) | Model 2 Coefficient (95% CI) | Model 1 Coefficient (95% CI) | Model 2 Coefficient (95% CI) | ||
| Fibroids present | |||||
| No | 3510 (89.4) | 0 (Reference) | 0 (Reference) | 0 (Reference) | 0 (Reference) |
| Yes | 416 (10.6) | −86 (−143, −29) | −20 (−77, 36) | −0.2 (−1.6, 1.1) | 0.4 (−0.9, 1.8) |
| Fibroid number‡ | |||||
| 1 | 294 (70.7) | −33 (−99, 33) | 12 (−53, 77) | 0.6 (−1.0, 2.2) | 1.1 (−0.6, 2.7) |
| 2 | 65 (15.6) | −104 (−239, 31) | −22 (−155, 111) | −1.2 (−4.4, 2.0) | −0.4 (−3.5, 2.8) |
| ≥3 | 57 (13.7) | −353 (−497, −208) | −201 (−345, −58) | −3.8 (−7.3, −0.4 | −2.1 (−5.5, 1.3) |
| Fibroid type‡,§ | |||||
| Any subserosal | 173 (41.6) | −115 (−200, −30) | −39 (−122, 45) | −1.1 (−3.1, 0.9) | −0.2 (−2.2, 1.8) |
| Any intramural | 186 (44.7) | −119 (−201, −38) | −47 (−128, 33) | −0.1 (−2.0, 1.8) | 0.7 (−1.2, 2.6) |
| Any submucosal | 82 (19.7) | −86 (−205, 32) | 24 (−93, 141) | −0.1 (−2.9, 2.7) | 1.0 (−1.8, 3.8) |
| Missing | 13 | ||||
| Total volume‡,∥ | |||||
| Lowest quartile | 102 (24.5) | 44 (−63, 151) | 72 (−33, 177) | 1.5 (−1.1, 4.0) | 1.7 (−0.8, 4.2) |
| 2nd quartile | 102 (24.5) | −162 (−271, −54) | −109 (−215, −3) | −0.9 (−3.5, 1.7) | 0.0 (−2.8, 2.3) |
| 3rd quartile | 106 (25.5) | −32 (−139, 75) | 31 (−74, 136) | 0.2 (−2.3, 2.7) | 0.9 (−1.7, 3.4) |
| Top quartile | 105 (25.2) | −190 (−298, −83) | −73 (−179, 34) | −1.7 (−4.3, 0.8) | −0.5 (−3.0, 2.1) |
| Missing | 1 | ||||
Abbreviations: BMI = body mass index; CI = confidence interval.
Model 1 adjusts for age and BMI; 78 participants missing confounder data were excluded. Model 2 adjusts for age, BMI, and race or ethnicity; 81 participants missing confounder data were excluded.
Data are counts and column percentages for each characteristic for model 1. Percentages exclude missing data.
Only available for women with fibroids.
Type is mutually exclusive for each fibroid, but a woman with multiple fibroids can have more than one type of fibroid.
Quartiles for total fibroid volume (cm3): <0.92, 0.92–4.54, 4.55–19.47, >19.47.
Several additional analyses were performed. First, we used logistic regression to test if any a priori confounders were predictive of missing birthweight. Second, we compared maternal characteristics by fibroids status among the selected population and the excluded group (no recorded infant birthweight [n = 718], Supplemental Table 1). Both were performed to gauge the missing pattern for birthweight. We decided to not to impute the few covariates missing (n = 81, 2%) and not to impute missing birthweight. Assuming birthweight is missing at random, complete case analysis with covariate adjustment should provide unbiased results with similar precision compared with imputing the outcome [18]. In addition, we repeated the analysis excluding women who had preterm births due to medical indications including induction of labor for maternal fetal indications or placental abruption, previa, and bleeding (n = 65). This was performed because the influence of preterm births due to medical indications on birth outcomes may be different from that among spontaneous preterm births. Lastly, we repeated the analysis and compared results restricting to white non-Hispanics with results restricting to black non-Hispanics (Supplemental Table 2). While race or ethnicity is likely a confounder (Supplemental Figure 1), we wanted to explore if any associations differed by race or ethnicity (Hispanics and Other were excluded due to small numbers). We suspect an unknown variable may serve as an indirect effect modifier through race or ethnicity [19]. All statistical analyses were performed at a 2-sided significance level of 0.05 using Stata 14.2 (StataCorp, Texas). Stata codes are available upon request.
Results
Among 3926 women, 416 (10.6%) had at least one fibroid. Compared to women without fibroids, women with fibroids were more likely to be older (mean age: 31 years vs. 29 years), black non-Hispanic (31.3% vs. 14.2%), and have higher BMI (mean BMI: 27.2 vs. 25.5 kg/m2, Table 1). The number of fibroids among study participants ranged from zero to seven. Among women with fibroids, the most common type was intramural (44.7%) followed by subserosal (41.6%) and then submucosal (19.7%). The median total fibroid volume was 4.8 cm3 (interquartile range [IQR]: 1.1, 20.0 cm3). Neither infant birthweight nor gestational age at birth was significantly different among women with and without fibroids based on MANOVA (3386 ± 604 grams vs. 3430 ± 541 grams; 274.8 ± 14 days vs. 275.3 ± 12.8 days).
When adjusting for age and BMI but not race or ethnicity, women with fibroids gave birth to infants that tended to have lower birthweights than women without fibroids (mean difference, −86 grams, 95% confidence interval [CI] −143, −29). Compared with women without fibroids, women with any subserosal or any intramural fibroids were more likely to have infants that weighted less (−115 grams, 95% CI −200, −30 and −119 grams, 95% CI −201, −38, respectively). Women with three or more fibroids gave birth to infants who had lower birthweight (−353 grams, 95% CI −497, −208) and shorter gestational age (−3.8 days, 95% CI −7.3, −0.4), than women without fibroids (model 1, Table 2).
When adjusting for age, BMI, and race or ethnicity, all associations of fibroid characteristics and birth outcomes shifted toward the null (model 2, Table 2). Comparing women with and without fibroids, the birthweights were similar (−20 grams, 95% CI −77, 36) as were the lengths of gestation (0.4 days, 95% CI −0.9, 1.8). However, women with three or more fibroids and those with total fibroid volume in the second quartile were more likely to have infants with lower birthweights (−201 grams, 95% CI −345, −58 and −109 grams, 95% CI −215, −3, respectively) but not infants with shorter gestational age (−2.1 days, 95% CI −5.5, 1.3 and 0.3 days, 95% CI −2.8, 2.3, respectively). Birthweight and gestational age did not significantly differ by fibroid type.
Among women who had a singleton live birth and a first-trimester ultrasound (n = 4644), 718 (15.5%) did not have a recorded infant birthweight. Factors significantly associated with missing birthweight include age (OR = 0.97, 95% CI 0.95, 0.98) and being black non-Hispanic (OR = 1.57, 95% CI 1.27, 1.93). This can also be observed when comparing maternal characteristics by fibroid status among the study population and the excluded group with missing birthweight. Having any fibroids was not significantly associated with missing birthweight (8.6% vs. 10.6%, Table 1 and Supplemental Table 1). The results were similar when we restricted to women who had spontaneous births (results not shown). In our exploratory analysis, results were generally similar among white non-Hispanics (n = 2853) and black non-Hispanics (n = 628, Supplemental Table 2). However, women with any intramural fibroids were more likely to have lower infant birthweight among white non-Hispanics (−114 grams, 95% CI −216, −12).
Discussion
Principal findings
Fibroids have been hypothesized to interfere with pregnancy through poor placental implantation or decreased uterine distensibility, presenting mechanical obstructions that restrict fetal growth [7–11]. However, our study suggests women with and without fibroids have similar birthweights and gestational ages at birth. Only women with three or more fibroids were more likely to have infants with lower birthweights. Although we observed that an association between total fibroid volume in the second quartile and lower birthweight, we suspect this is due to chance because no dose response pattern is present.
Interpretation
Previous studies had inconsistent results for the association of fibroids with birthweight and gestational age. Among four retrospective studies that examined the presence of fibroids and birth outcomes, two studies (n = 6706 and n = 153, respectively) found birthweight or gestational age at birth did not differ by the presence of fibroids [10,12]. The other two studies (n = 6308 and n = 33,762, respectively) found women with fibroids were more likely to have low birthweight and preterm births [8,13]. All four studies were retrospective and used existing medical records or databases, which are not designed to consistently identify fibroids [8,10,12,13]. Existing databases often missed asymptomatic fibroids, evidenced by the low prevalence; the only two retrospective cohort studies reported the prevalence of 1.2% and 1.4% [10,13]. In comparison, all women in our prospective cohort study underwent a systematic ultrasound screening in the first trimester and had a measured prevalence of 10.6% using research ultrasounds.
In addition to different study designs, our results may also be explained by different statistical methods and analytical decisions. Two previous studies compared the mean or proportions of birth outcomes without accounting for gestational age [10,12]. A third study modeled birthweight and gestational age as separate outcomes [8]. While the last study removed the contribution of gestational age to birthweight by using a multivariable regression [13], they should not have adjusted for gestational age because it may be an intermediate on the pathway between fibroids and birthweight [15]. We used MANOVA and multivariate analysis of covariance to address the correlation between gestational age and birthweight. In addition, most studies did not address confounding by race or ethnicity despite low birthweight and preterm birth disproportionately affecting racial or ethnic minorities. Only one study attempted to address confounding by race or ethnicity by matching cases and controls [10]. Of the three studies that did not account for race or ethnicity, two had homogeneous populations (82% black and 100% Taiwanese, respectively), which decreases possible confounding by race or ethnicity and resulted in similar conclusions to ours [12,13]. Positive results in the remaining study may be driven by unaccounted confounding by race or ethnicity [8].
Whether race or ethnicity modifies the association of fibroids with birthweight and gestational age is unknown. When exploring the plausibility of race or ethnicity (or an ancestor variable of race or ethnicity) as an effect modifier, we found having any intramural fibroids was associated with lower infant birthweight among white non-Hispanics but not among black non-Hispanics. Whether this association is truly different among racial or ethnic groups deserves future research. The large confidence interval and absence of a significant association between having three or more fibroids and birthweight among black non-Hispanics (−191 grams, 95% CI −426, 44) are likely due to small numbers. Small numbers may have also contributed to the significant association between total volume in the lowest quartile and gestation among black non-Hispanics (Supplemental Table 2).
Strengths of the study
Our study has several strengths. First, fibroids and fibroid characteristics are uniformly assessed. Having experienced sonographers follow a standardized protocol and take repeated measures decreases the likelihood of fibroid misclassification. The assessment also took place during the first trimester (on average around 8 weeks gestation), before the occurrence of many pregnancy-related hormonal changes that could influence the fibroid size. For cases where fibroid misclassification existed, it would not result in differential assessment of gestational age or birthweight because women were followed prospectively. In addition, we tried to minimize selection bias by constructing a community-based cohort of women planning or carrying a pregnancy. Finally, we used continuous birthweight and gestational age, as opposed to using outcomes such as small for gestational age which dichotomizes birthweight based on gestational age. Modeling continuously allows us to estimate the difference in grams of birthweight and the days of gestational age, which is arguably more clinically meaningful.
Limitations of the data
Our study also has several limitations to consider. First, we did not have the sample size to further examine fibroid characteristics such as number and fibroid volume stratified by fibroid type, especially for submucosal fibroids which may have influenced the extent of invasion into the uterine cavity. Because women often have one large solitary fibroid or multiple small fibroids, the number and volume of submucosal fibroids may have contributed to the observed association between fibroid volume in the second quartile and birthweight. Second, we do not know if first-trimester fibroid status reflects fibroid status throughout pregnancy because fibroids can change in size due to increasing progesterone levels and influence birthweight and gestational age [9]. In addition, we did not account for gestational weight gain or maternal nutrition, which may impact fetal nutrition and thus birthweight and gestational age. While we did not collect data on daily nutrition, mothers with and without fibroids have similar intake of folic acid and vitamins (Table 1). It should be noted that our cohort consists of women with similar economic status, which makes low birthweight due to malnutrition unlikely. In addition to unmeasured confounding, our study may also be susceptible to selection bias. If fibroids influenced fetal survival and measured risk factors confounded the relationship between fibroids and fetal survival, restricting our analysis to live births is effectively conditioning on a collider. Earlier work from our group found that fibroids have little effect on time to pregnancy in our cohort and indicated no increase in the risk of miscarriage [20,21]. Furthermore, a meta-analysis of prior literature also found no effect of fibroids on miscarriage [22]. Assuming fibroids do not influence birthweight through fetal survival, fetal survival could still be a collider if there is unmeasured common cause between fetal survival and birthweight. Finally, 15.5% (n = 718) of confirmed live births have no reported birthweight after all available vital and medical records were obtained and reviewed. This is due to women giving birth out of state or other unknown reasons. Although age and race or ethnicity are associated with missing birthweight, missing is likely to occur at random within categories of young women and/or black non-Hispanic women, which should not bias our results.
Conclusions
Our findings from a prospective community-based cohort study suggest that race or ethnicity substantially confounds the association of fibroids and birth outcomes. The clinical belief that fibroids impair fetal growth and cause lower birthweight and earlier gestational age at birth is supported only by a decrement in birthweight for women with multiple fibroids. Most pregnancies with fibroids will not influence birthweight or gestational age at birth; most women with fibroids do not require intensive perinatal and intrapartum surveillance. However, women with three or more fibroids are more likely to have infants with reduced birthweight and may warrant some level of surveillance. Our results add to the evidence for targeted antenatal counseling based on number of fibroids. Future research might consider studying women with multiple fibroids while computing the extent of invasion into the uterine cavity and obtaining information on gestational weight gain and/or maternal nutrition.
Supplementary Material
Acknowledgment
Supported by grants from the Eunice Kennedy Shriver National Institute of Child and Human Development (R01HD043883 and R01HD049675) and the American Water Works Association Research Foundation (2579). Additional infrastructure resources were supported by Clinical and Translational Science Awards (UL1TR000445) from the National Center for Advancing Translational Sciences. Presented at the Society for Reproductive Investigation 65th Annual Meeting, March 6-10, 2018, San Diego, CA.
References
- [1].Wilcox AJ. On the importance–and the unimportance–of birthweight. Int J Epidemiol 2001;30(6):1233–41. [DOI] [PubMed] [Google Scholar]
- [2].Martin JA, Hamilton BE, Osterman MJ, Driscoll AK, Drake P. Births: final data for 2016. National Vital Stat Rep 2018;67(1):1–55. [PubMed] [Google Scholar]
- [3].United Nations Children’s Fund, World Health Organization. Low birthweight: country, regional and global estimates. New York: UNICEF; 2004. [Google Scholar]
- [4].Rogers LK, Velten M. Maternal inflammation, growth retardation, and preterm birth: insights into adult cardiovascular disease. Life Sci 2011;89(13–14): 417–21. [DOI] [PubMed] [Google Scholar]
- [5].Laughlin SK, Baird DD, Savitz DA, Herring AH, Hartmann KE. Prevalence of uterine leiomyomas in the first trimester of pregnancy: an ultrasound-screening study. Obstet Gynecol 2009;113(3):630–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM. High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynecol 2003;188(1):100–7. [DOI] [PubMed] [Google Scholar]
- [7].Lai J, Caughey AB, Qidwai GI, Jacoby AF. Neonatal outcomes in women with sonographically identified uterine leiomyomata. J Matern Fetal Neonatal Med 2012;25(6):710–3. [DOI] [PubMed] [Google Scholar]
- [8].Coronado GD, Marshall LM, Schwartz SM. Complications in pregnancy, labor, and delivery with uterine leiomyomas: a population-based study. Obstet Gynecol 2000;95(5):764–9. [DOI] [PubMed] [Google Scholar]
- [9].Davis JL, Ray-Mazumder S, Hobel CJ, Baley K, Sassoon D. Uterine leiomyomas in pregnancy: a prospective study. Obstet Gynecol 1990;75(1):41–4. [PubMed] [Google Scholar]
- [10].Rice JP, Kay HH, Mahony BS. The clinical significance of uterine leiomyomas in pregnancy. Am J Obstet Gynecol 1989;160(5):1212–6. [DOI] [PubMed] [Google Scholar]
- [11].Ciavattini A, Clemente N, Delli Carpini G, Di Giuseppe J, Giannubilo SR, Tranquilli AL. Number and size of uterine fibroids and obstetric outcomes. J Matern Fetal Neonatal Med 2015;28(4):484–8. [DOI] [PubMed] [Google Scholar]
- [12].Roberts WE, Fulp KS, Martin JN Jr. The impact of leiomyomas on pregnancy. Aust N Z J Obstet Gynaecol 1999;39(1):43–7. [DOI] [PubMed] [Google Scholar]
- [13].Chen YH, Lin HC, Chen SF, Lin HC. Increased risk of preterm births among women with uterine leiomyoma: a nationwide population-based study. Hum Reprod 2009;24(12):3049–56. [DOI] [PubMed] [Google Scholar]
- [14].Frisbie WP, Biegler M, De Turk P, Forbes D, Pullum SG. Racial and ethnic differences in determinants of intrauterine growth retardation and other compromised birth outcomes. Am J Public Health 1997;87(12):1977–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ananth CV, Schisterman EF. Confounding, causality, and confusion: the role of intermediate variables in interpreting observational studies in obstetrics. Am J Obstet Gynecol 2017;217(2):167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Promislow JH, Makarushka CM, Gorman JR, Howards PP, Savitz DA, Hartmann KE. Recruitment for a community-based study of early pregnancy: the Right from the Start study. Paediatr Perinat Epidemiol 2004;18(2): 143–52. [DOI] [PubMed] [Google Scholar]
- [17].Muram D, Gillieson M, Walters JH. Myomas of the uterus in pregnancy: ultrasonographic follow-up. Am J Obstet Gynecol 1980;138(1):16–9. [DOI] [PubMed] [Google Scholar]
- [18].Groenwold RH, Donders ART, Roes KC, Harrell FE Jr, Moons KG. Dealing with missing outcome data in randomized trials and observational studies. Am J Epidemiol 2011;175(3):210–7. [DOI] [PubMed] [Google Scholar]
- [19].VanderWeele TJ, Robins JM. Four types of effect modification: a classification based on directed acyclic graphs. Epidemiology 2007;18:561–8. [DOI] [PubMed] [Google Scholar]
- [20].Hartmann KE, Velez Edwards DR, Savitz DA, Jonsson-Funk ML, Wu P, Sundermann AC, et al. Prospective cohort study of uterine fibroids and miscarriage risk. Am J Epidemiol 2017;186(10):1140–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Johnson G, MacLehose RF, Baird DD, Laughlin-Tommaso SK, Hartmann KE. Uterine leiomyomata and fecundability in the Right from the Start study. Hum Reprod 2012;27(10):2991–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Sundermann AC, Velez DE, Bray MJ, Jones SH, Latham SM, Hartmann KE. Leiomyomas in pregnancy and spontaneous abortion: a systematic review and meta-analysis. Obstet Gynecol 2017;130(5):1065–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


