Abstract
Background
Households studies reflect the natural spread of SARS-CoV-2 in immunologically naive populations with limited preventive measures to control transmission.
We hypothesise that seropositivity provides more accurate household attack rates than RT-PCR. Here, we investigated the importance of age in household transmission dynamics.
Methods
We enroled 112 households (291 participants) in a case-ascertained study in Bergen, Norway from 28th February to 4th April 2020, collecting demographic and clinical data from index patients and household members. SARS-CoV-2-specific antibodies were measured in sera collected 6–8 weeks after index patient nasopharyngeal testing to define household attack rates.
Findings
The overall attack rate was 45% (95% CI 38–53) assessed by serology, and 47% when also including seronegative RT-PCR positives. Serology identified a higher number of infected household members than RT-PCR. Attack rates were equally high in children (48%) and young adults (42%). The attack rate was 16% in asymptomatic household members and 42% in RT-PCR negative contacts. Older adults had higher antibody titres than younger adults. The risk of household transmission was higher when the index patient had fever (aOR 3.31 [95% CI 1.52–7.24]; p = 0.003) or dyspnoea (aOR 2.25 [95% CI 1.80–4.62]; p = 0.027) during acute illness.
Interpretation
Serological assays provide more sensitive and robust estimates of household attack rates than RT-PCR. Children are equally susceptible to infection as young adults. Negative RT-PCR or lack of symptoms are not sufficient to rule out infection in household members.
Funding
Helse Vest (F-11628), Trond Mohn Foundation (TMS2020TMT05).
Abstrakt
Bakgrunn
Studier av husstander gjenspeiler den naturlige spredningen av SARS-CoV-2 blant ikke-immune populasjoner med begrensede tiltak for å forebygge smittespredning. Vår hypotese er at antistoff-påvisning gir mer nøyaktige angrepsrater i husstander sammenliknet med RT-PCR. Her undersøker vi betydnignenngen betydningen av alder i smittespredningen.
Metoder
Vi rekrutterte 112 husstander (291 studiedeltakere) i en indeks kasus-bekreftet studie i Bergen, Norge fra 28.02.2020 til 04.04.2020, og samlet inn demografiske og kliniske data fra indekspasienter og deres husstandsmedlemmer. Angrepsrate i husstander ble beregnet ved å måle SARS-CoV-2-spesifikke antistoffer i sera samlet 6–8 uker etter nasofarynksprøve av indekspasienten.
Funn
Den totale angrepsraten var 45% (95% KI 38–53) vurdert ved serologi, og 47% ved å inkludere antistoff negative, RT-PCR positive husstandsmedlemmer. Spesifikke antistoffer identifiserer en høyere andel infiserte husstandsmedlemmer sammenliknet med RT-PCR. Angrepsraten var like høy hos barn (48%) og unge voksne (42%). Angrepsraten var 16% hos personer uten symptomer og 42% hos RT-PCR negative husstandsmedlemmer. Eldre voksne hadde høyere antistoff titre enn yngre voksne. Risiko for smitte i husstander var høyere når indekspasienten hadde feber (aOR 3.31 [95% KI 1.52–7.24]; p = 0.003) eller dyspne (aOR 2.25 [95% KI 1.80–4.62]; p = 0.027) under akuttfasen.
Tolkning
Serologiske analyser gir mer sensitive og robuste estimater av angrepsrate i husstander sammenliknet med RT-PCR. Barn er like utsatt for infeksjon som voksne. Negativ RT-PCR eller fravær av symptomer er ikke tilstrekkelige for å utelukke infeksjon blant husstandsmedlemmer.
Finansiering
Helse Vest (F-11628), Trond Mohn Stiftelse (TMS2020TMT05).
Research in context.
Evidence before this study
Studies of household transmission of SARS-CoV-2 provide valuable information about transmission dynamics of the virus. As of 3rd November 2020, our search in PubMed, medRxiv, bioRxiv and other relevant sources using the terms COVID-19, SARS-CoV-2, household secondary attack rate, household transmission, and similar terms, yielded 25 articles out of which ten were relevant. The majority of studies directly targeting household secondary attack rates were conducted in countries with high levels of community transmission. All studies used RT-PCR in the acute phase as the sole diagnostic tool, with attack rates varying from 7% to 38%. Children were reported to have the lowest attack rates (4% to 23.1%), although the definition of children varied greatly across studies. Older age in household members was consistently reported to be a significant risk factor for transmission. Household transmission risks have also been indirectly estimated from large serosurveillance studies. These studies were in high community transmission areas and were not specifically designed to estimate the household secondary attack rate, and children were under-represented in the study samples.
Added value of this study
This case-ascertained study was designed to estimate household attack rates based on the detection of SARS-CoV-2 specific antibodies in household members of RT-PCR confirmed index cases, in a low community spread setting. Households of COVID-19 patients were consecutively recruited, starting with the first case identified in Bergen, Norway during the start of the pandemic. We demonstrate that serological assays show higher attack rates than RT-PCR, especially in children. Attack rates were equally high in children and adults. Surprisingly, in household members who were RT-PCR tested when they were symptomatic, the attack rate was as high as 42% in RT-PCR negatives. In asymptomatic household members, 16% developed virus specific antibodies. Clinical risk factors amongst index cases for household transmission included fever and dyspnoea during acute illness, but not having cough.
Implications of all the available evidence
Whereas RT-PCR is ideal for the timely diagnosis of COVID-19, serological assays are more sensitive in estimating the attack rates in household members of RT-PCR positive cases. Serological assays can also be used to confirm infection in RT-PCR negative and asymptomatic household members. Our results support previous studies showing high attack rates amongst the elderly. Our finding of high attack rate amongst children contrasts with previous findings of low prevalence in this age group, and the true infection rate of SARS-CoV-2 in children requires further investigation.
Alt-text: Unlabelled box
1. Introduction
Since first being identified in Wuhan, China in December 2019, the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has rapidly emerged into a global pandemic affecting over 180 countries. As of 10th December 2020, there were more than 69 million confirmed cases of coronavirus disease 2019 (COVID-19) with over 1.5 million deaths globally, and 39.768 confirmed cases and 382 deaths in Norway ([1, 2]). In Norway, reverse transcription polymerase chain reaction (RT-PCR) testing of SARS-CoV-2 commenced on 23rd January 2020 and the first confirmed case was identified on 26th February 2020 [3]. Quarantine of suspected cases and isolation of confirmed cases was practised from late February. To combat further spread of the virus in the community, the government implemented comprehensive infection control measures on 12th March 2020 [4].
Current testing for SARS-CoV-2 relies on amplification of the viral RNA genome from respiratory specimens, which can generally only be detected during acute infection. Whereas serological assays can determine exposure or infection over a longer time period. Furthermore, with a high proportion of asymptomatic and mild illness [5], it is highly likely that data restricted to RT-PCR provide an underestimate of the infection rate of SARS-CoV-2.
The SARS-CoV-2 is a novel virus in humans, and there are negligible levels of pre-existing antibodies in the population ([6, 7]). SARS-CoV-2-specific antibodies appear in the early convalescent phase approximately two weeks after infection and are maintained for at least four months ([8, 9]). Therefore, serological assays can provide valuable information on the real infection rate in a community. SARS-CoV-2 binds to the surface receptors of cells in the respiratory tract through the receptor-binding domain (RBD) on its spike protein, and neutralising antibodies prevent infections by blocking viral entry.
The household attack rate of SARS-CoV-2 from index patients to household members reflects the natural spread of infection in immunologically naive populations with limited preventive measures to control transmission. Respiratory tract infections have been documented to give varying attack rates in families, particularly in influenza, where previous pandemics have reported attack rates from 4% to 20% or higher [10]. With no pre-existing immunity in the population, a higher household attack rate would be expected with SARS-CoV-2.
Previous studies on the household transmission of SARS-CoV-2 have reported attack rates, ranging from 6% to 38%, based on RT-PCR of either single or repeated respiratory samples from household members of confirmed cases in different study settings [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21]. However, sensitive serological assays are likely to give more accurate estimates of attack rates [22], regardless of whether household members are asymptomatic or RT-PCR negative.
Here, we estimated the household attack rate of SARS-CoV-2 and identified the determinants of household transmission by measuring SARS-CoV-2-specific antibodies in household members of RT-PCR confirmed cases during the start of the COVID-19 pandemic in Norway.
2. Methods
2.1. Study design, setting and participants
A case-ascertained study was conducted in Bergen, Norway. Testing for SARS-CoV-2 by RT-PCR from nasopharyngeal swabs was centralized at Bergen Municipality Emergency Clinic for the city. The main outcome was seroconversion for SARS-CoV-2 spike antibodies 6–8 weeks after inclusion. All outpatient RT-PCR confirmed cases (termed index patient) tested at the clinic during the first 35 days of the outbreak (28th February–4th April 2020), and their household members were eligible for inclusion after written informed consent. Household members were defined as individuals who resided in the same household as an index patient. Index patients and their household members were contacted by telephone and asked to participate in the study. In households with >1 case, the member first diagnosed by RT-PCR was defined as the index patient, and cases with a later date of laboratory confirmation (non-primary cases) were defined as household members. Households where a case resided alone or no household members were willing to participate in the study, were excluded from the analysis. The study was approved by the Regional Ethics Committee (#118664).
2.2. Clinical information
Electronic case report forms (eCRF) were developed using REDCap® (Research Electronic Data Capture) (Vanderbilt University, Nashville, Tennessee). The eCRF for index patients contained demographics, COVID-19-like symptoms, recent travel history, recent close contact with confirmed COVID-19 cases, as well as household size and number of household members that had been ill with similar symptoms. Household members were contacted individually to register information on gender, age, RT-PCR test result (if available), and COVID-19-like symptoms.
2.3. Serological assays
Serum samples were collected from index patients and household members 6–8 weeks after nasopharyngeal sampling of the index patient at Bergen Municipality Emergency Clinic, mainly during the shutdown period with low community transmission. Sera were stored at −80 °C and heat-inactivated for one hour at 56 °C before use in serological assays.
2.4. Enzyme-linked immunosorbent assay (ELISA)
A two-step ELISA was used for detecting SARS-CoV-2-specific antibodies, initially by screening with receptor-binding domain (RBD) and then confirming seropositivity by spike IgG [6]. Endpoint titres were calculated as the reciprocal of the serum dilution giving an optical density (OD) value of 3 standard deviations above the mean of historical pre-pandemic serum samples (n = 128) (supplementary figures 1 and 2). Individuals with titres ≥100 were defined as positive and those with no antibodies were assigned a titre of 50 for calculation purposes. Since the historical serum samples were defined as seronegative, and recruitment was initiated from the first case in the region, we assume that all participants were seronegative at baseline and the term seroconversion is used to define attack rate for participants with seropositive spike-specific IgG.
3. Neutralisation assays
The neutralisation assays were used to quantify SARS-CoV-2-specific functional antibodies. The assays were performed in a certified Biosafety Level 3 Laboratory using a local clinical isolate hCoV-19/Norway/Bergen-01/2020 (GISAID accession ID EPI_ISL_541,970) at 2000 tissue culture infectious dose 50% (TCID50)/ml. In the microneutralisation (MN) assay, virus infectivity was measured by detecting nucleoprotein after 24 h incubation in Vero cells. The MN titre (IC50) was determined as the reciprocal of the serum dilution giving 50% inhibition of virus infectivity. In the virus neutralisation (VN) assay, the cytopathic effect (CPE) in Vero cells was recorded after 4–5 days. VN titres were determined as the reciprocal of the highest serum dilution giving no CPE. Negative titres (<20) were assigned a value of 10 for calculation purpose.
3.1. Statistical methods
Risk factors for seroconversion, including household size and characteristics of index patients and household members, were presented as percentages. In univariable analysis, categorical explanatory variables were assessed by Fisher's exact test and by logistic regression for variables with multiple levels using the level with most observations as reference. Numeric variables, such as antibody titres, were compared by Mann-Whitney test. Multivariable analysis was performed using generalized estimating equation (GEE) to account for the potential correlation of outcomes within households. Analyses were performed in R 4.0.3 (www.R-project.org). Graphs were drawn in Prism 7 (GraphPad Software, San Diego, CA).
See Supplementary Methods for further details of inclusion and laboratory methods.
3.2. Role of the funding sources
The funding bodies had no role in study design, collection, analysis and interpretation of data, in writing the manuscript, and in the decision to submit this paper for publication.
4. Results
4.1. Participants
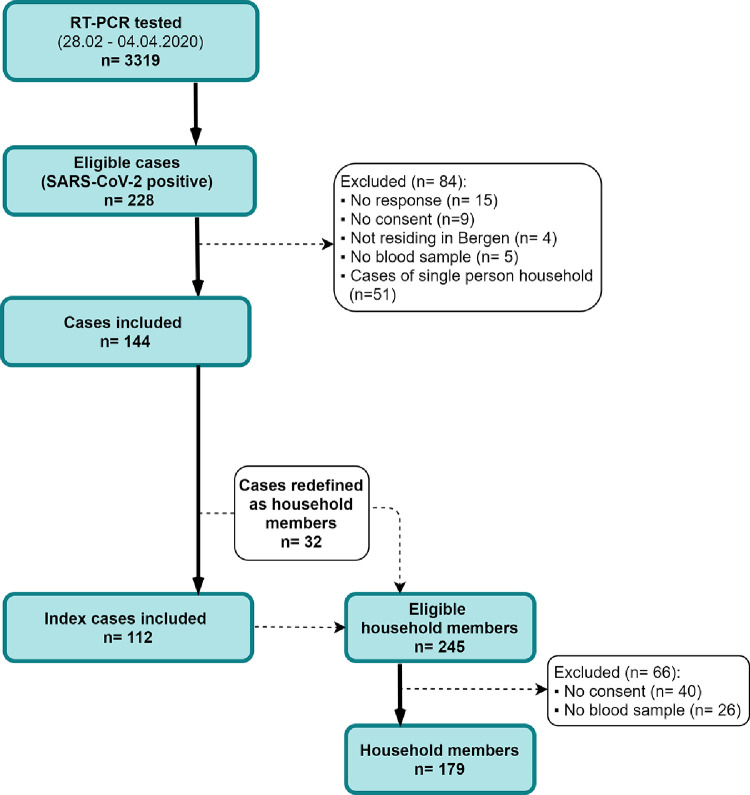
Between 28th February and 4th April 2020, 228 out of 3319 RT-PCR tested individuals were identified as SARS-CoV-2 positive in Bergen (Fig. 1). All positive cases were contacted, of which 144 cases were enroled in the study. In households where more than one case resided, the primary case was defined as the index patient and 32 non-primary cases were redefined as household members. amongst 245 eligible household members, 179 were enroled in the study (see supplementary methods). The final cohort for analysis consisted of 112 index patients and 179 household members (Fig. 1 and 2B). Index patients were home isolated and their household members were instructed to quarantine. Overall, there was an equal distribution of males and females, but household members were younger than the index patients (supplementary Table 1). A large proportion (73%, 130/179) of household members reported having COVID-19 compatible symptoms.
Fig. 1.
Recruitment procedure of study participants.
Between 28.02.2020 and 04.04.2020, 223 SARS-CoV-2 cases were identified amongst 3319 SARS-CoV-2 suspected cases that were RT-PCR-tested (1.1% of population tested), out of which 194 were included in the study. There were 245 eligible household members, out of which 148 were included. In households with more than one case, 31 non-primary cases were redefined as household members, giving a total of 179 household members. Possible household clusters of six or nine co-primary cases had symptom onset within 24 or 48 h, respectively, after symptom onset in the index patient. Forty household members did not consent, 3 of whom were RT-PCR positive, 18 were children under 10 years old and 12 were under 20 years old. In total, we included 291 people comprising 112 index patients living with others and their 179 household members. Fifty-one cases were not included in the analyses as they lived alone or were defined as single-person households because they did not have household members who wished to participate in the study.
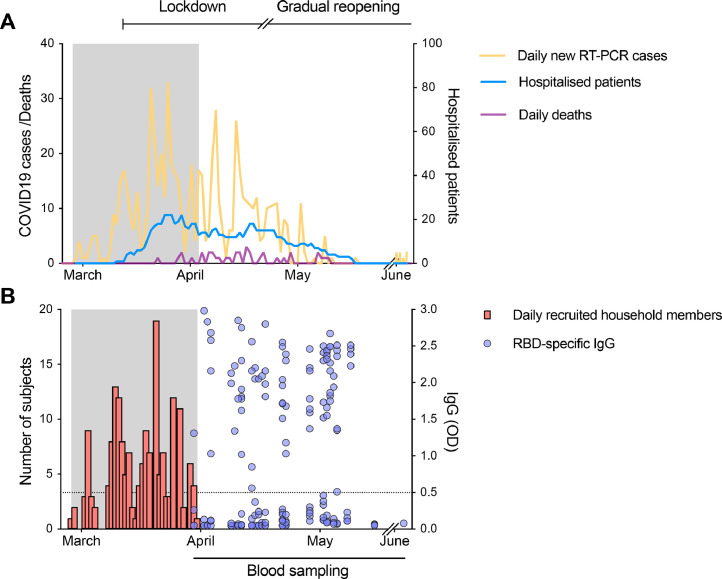
Fig. 2.
The course of the first wave of the pandemic in Bergen and period of recruitment of index patients and household members.
(A) The daily number of SARS-CoV-2 RT-PCR positive cases (shown in orange) from the centralised testing centre at Bergen Municipality Emergency Clinic covering a population of 284.000 people and the daily number of COVID-19 deaths (shown in purple) in Bergen, Norway (left Y-axis). The number of hospitalised patients from SARS-CoV-2 infection in Bergen (shown in blue, right Y-axis). Lockdown was initiated in Norway on 12th March, and a gradual reopening starting on 20th April 2020. (B) The number of household members recruited (shown in red) during the recruitment period (grey shaded area). Clinical information was collected from the index patient and their household members at the time of recruitment. Blood samples were collected 6–8 weeks after the date of nasopharyngeal samples (blue dots), at which time there was low transmission in Bergen reducing the likelihood of community infection. Sera from all household members were tested against the receptor-binding domain (RBD) of spike protein in screening ELISA. RBD-specific IgG are shown as the optical density (OD) at 1/100 dilution of sera (shown in blue, right Y-axis). Each symbol represents one subject. The horizontal dotted line indicates OD 0.5 as the cut-off defined by a panel of 128 pre-pandemic sera. Duplicates were performed in ELISA.
During the study period, 1.1% of the population of Bergen was tested (3319/284000), with positive RT-PCR results in 6.9% (228/3319) of those tested (Fig. 2). This corresponds to a minimal daily incidence of 2.2 RT-PCR-confirmed cases per 100000 population, but the minimal daily incidence of probable cases is 3.9 per 100000 considering the lower sensitivity of RT-PCR. We expect low prevalence amongst non-tested population, since there was high general awareness in the population and since the strict lockdown efficiently curbed the epidemic within a few weeks. We found there was little clustering of cases in specific districts of the city, with a variation in detected cases between 15/100 000 and 36/100 000 inhabitants.
5. Household attack rate
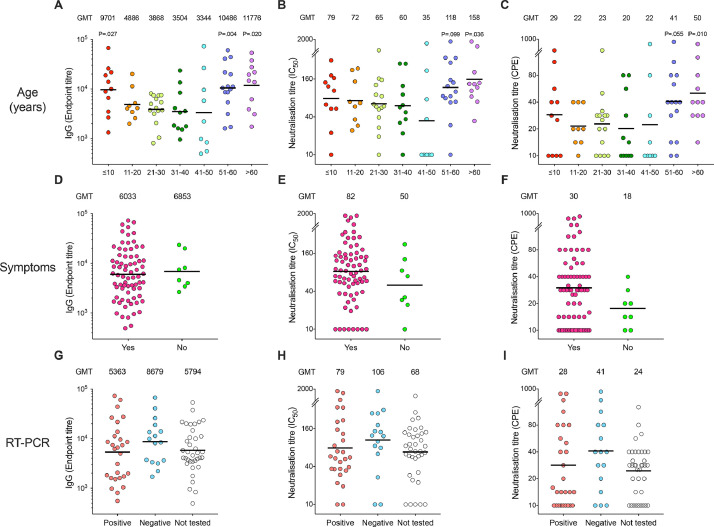
We measured SARS-CoV-2-spike- specific IgG in household members to calculate the household attack rate. The overall attack rate in households was 45% (95% CI 38–53), with no significant gender difference (table 1). Attack rates varied between 26% and 73% amongst the 10-year age cohorts. Interestingly, the attack rate in children aged 0–10 years (48%) was similar to that of adults (aOR 1.51 [95% CI 0.42–5.41]; p = 0.529). Titres of spike-specific IgG amongst seropositive children ≤10 years old were significantly higher (p = 0.03) than in adults (21–31 years old) (Fig. 3A). As expected, symptoms were related to COVID-19 infection, and seroconversion occurred in 56% (95% CI 48–64) of symptomatic and 16% (95% CI 9–29) of asymptomatic household members (table 1). Household size was not convincingly associated with household transmission. Attack rates were higher in two-person households (57% [95% CI 42–70]) (table 1), but household size varied between age groups, with the majority of the oldest household members living in two-person households. There was no significant correlation of attack rate between families with children and only spouses, (data not shown). When potential co-primary cases were removed in a sensitivity analysis, the household attack rate remains largely unchanged (supplementary Table 2).
Table 1.
Household attack rates amongst 179 household members of 112 index patients in Bergen, Norway, and odds ratios for association with SARS-CoV-2 seropositivity by characteristics of household members, index patients, and household size.
| Number of index patients | Number of household contacts |
Number of secondary seropositive household contacts | Attack rate (95% CI) | Univariable analysis | Multivariable analysis* | |||
|---|---|---|---|---|---|---|---|---|
| Crude odds ratio (95% CI) | p | Adjusted odds ratio (95%CI) | p | |||||
| Overall | 112 | 179 | 81 | 45% (0.38 – 0.53) | ||||
| Household member sex | ||||||||
| Male | 80 | 38 | 48% (0.37 - 0.58) | 1.18 (0.62 – 2.22) | 0.651 | 1.02 (0.48 - 2.15) | 0.963 | |
| Female | 99 | 43 | 43% (0.34 - 0.53) | 1 (ref) | – | |||
| Household member age, years | ||||||||
| 0–10 | 23 | 11 | 48% (0.29 - 0.67) | 1.28 (0.45 – 3.71) | 0.642 | 1.31 (0.33 - 5.22) | 0.706 |
|
| 11–20 | 34 | 9 | 26% (0.15 - 0.43) | 0.50 (0.18 – 1.36) | 0.183 | 0.59 (0.20 - 1.70) | 0.326 | |
| 21–30 | 36 | 15 | 42% (0.27 - 0.58) | 1 (ref) | 1 (ref) | – | ||
| 31–40 | 18 | 11 | 61% (0.39 - 0.80) | 2.20 (0.70 – 7.27) | 0.181 | 3.16 (0.80 - 12.51) | 0.101 | |
| 41–50 | 24 | 9 | 38% (0.21 - 0.57) | 0.84 (0.29 – 2.41) | 0.747 | 1.01 (0.30 - 3.47) | 0.984 | |
| 51–60 | 29 | 15 | 52% (0.34 - 0.69) | 1.50 (0.56 – 4.07) | 0.420 | 1.62 (0.47 - 5.65) | 0.446 | |
| >60 | 15 | 11 | 73% (0.48 - 0.89) | 3.85 (1.09 – 16.10) | 0.046 | 2.58 (0.49 – 13.54) | 0.262 | |
| Household member symptomatic | ||||||||
| Yes | 130 | 73 | 56% (0.48 - 0.64) | 6.50 (2.73 – 17.34) | <0.001 | Omitted | ||
| No | 49 | 8 | 16% (0.09 - 0.29) | 1 (ref) | ||||
| Household member RT-PCR result | ||||||||
| Positive | 32 | 28 | 88% (0.72 - 0.95) | 9.29 (2.55 – 43.82) | <0.001 | Omitted | ||
| Negative | 38 | 16 | 42% (0.28 - 0.58) | 1 (ref) | ||||
| Not tested | 109 | 37 | 34% (0.26 - 0.43) | omitted | ||||
| Index patient sex | ||||||||
| Male | 55 | 96 | 41 | 43% (0.33 - 0.53) | 0.80 (0.43 – 1.51) | 0.547 | 0.65 (0.28 - 1.53) | 0.322 |
| Female | 57 | 83 | 40 | 48% (0.38 - 0.59) | 1 (ref) | 1 (ref) | – | |
| Index patients age, years | ||||||||
| < 20 | 2 | 6 | 2 | 33% (0.10 - 0.70) | 0.65 (0.56 – 1.05) | 0.630 | 0.64 (0.21 - 1.92) | 0.423 |
| 20–60 | 95 | 157 | 68 | 43% (0.36 - 0.51) | 1 (ref) | ref | 1 (ref) | – |
| > 60 | 15 | 16 | 11 | 69% (0.44 - 0.86) | 2.88 (0.997 – 9.50) | 0.060 | 1.46 (0.36 – 5.92) | 0.593 |
| Index patient cough | ||||||||
| Yes | 72 | 114 | 52 | 46% (0.37 - 0.55) | 1.04 (0.54 – 2.01) | 1.000 | 0.93 (0.46 - 1.87) | 0.834 |
| No | 40 | 65 | 29 | 45% (0.33 - 0.57) | 1 (ref) | 1 (ref) | – | |
| Index patient fever | ||||||||
| Yes | 79 | 127 | 67 | 53% (0.44 – 0.61) | 3.01 (1.43 – 6.64) | 0.002 | 3.62 (1.63 - 8.03) | 0.002 |
| No | 33 | 52 | 14 | 27% (0.17 – 0.40) | 1 (ref) | 1 (ref) | – | |
| Index patient dyspnoea | ||||||||
| Yes | 58 | 100 | 53 | 53% (0.43 – 0.62) | 2.05 (1.07 – 3.94) | 0.023 | 2.30 (1.14 - 4.68) | 0.021 |
| No | 54 | 79 | 28 | 35% (0.26 - 0.46) | 1 (ref) | 1 (ref) | – | |
| Household size, no. of persons | ||||||||
| 2 | 44 | 25 | 57% (0.42 - 0.70) | 1.94 (0.95 – 4.02) | 0.07 | 1.40 (0.49 - 3.98) | 0.527 | |
| 3 | 36 | 16 | 44% (0.30 - 0.60) | 1.18 (0.54 – 2.55) | 0.7 | 1.32 (0.42 - 4.19) | 0.639 | |
| ≥4 | 99 | 40 | 40% (0.31 - 0.50) | 1 (ref) | 1 (ref) | ref |
Abbreviations: CI: confidence interval; RT-PCR: reverse transcription polymerase chain reaction.
Adjusted odds ratio and p value were calculated by generalized estimating equation (GEE) analysis including household member characteristics (sex and age), index patient characteristics (sex, age, history of cough, fever, dyspnoea, any comorbidities, inhalation steroid medication, and smoking status), and household size. P values < 0.05 marked in bold were considered statistically significant.
Fig. 3.
The SARS-CoV-2 antibody responses in seropositive household members.
Clinical symptoms of COVID-19 illness and SARS-CoV-2 RT-PCR results were collected from household members at the time of recruitment, blood samples were collected 6–8 weeks later. Only symptomatic household members were tested by RT-PCR depending on the testing capacity at the centralized testing centre, therefore results are not available (NA) from all subjects. Sera from all household members were tested against the receptor-binding domain (RBD) of spike protein by screening ELISA. The positive samples from screening RBD IgG ELISA (OD>0.555) were confirmed by spike ELISA, microneutralisation and virus neutralisation assays with live virus hCoV-19/Norway/Bergen-01/2020 (GISAID accession ID EPI_ISL_541,970) in a certified Biosafety Level 3 Laboratory. Household members with spike-specific IgG endpoint titre ≥100 were defined as seropositive, and were divided into 10-year age cohorts (A-C), clinical symptoms of COVID-19 illness (D-F) and SARS-CoV-2 RT-PCR (G-I). Spike-specific IgG (A, D, G), microneutralisation (B, E, H) and virus neutralisation (C, F, I) titres from all seropositive household members. Clinical symptoms are plotted against symptoms (n = 73 in “Yes”, n = 8 in “No” in d-F and RT-PCR results (n = 28 in “Positive”, n = 16 for “Negative” and n = 37 in “NA” in G-I). The geometric mean titres (GMT) are noted above the graphs for each column, and indicated by a horizontal line. Each symbol represents one subject. Mann-Whitney test was used in comparing antibody titres between household member age cohorts, 21–30 years as the reference group, (A-C) symptomatic and asymptomatic subjects (D-F) and RT-PCR positive and negative subjects (G-I).P<0.05 were considered significant. All P<0.10 are noted. Two or more replicates were performed in all experiments. IC50, 50% inhibitory concentration. CPE, cytopathic effect. No significant difference was found. Two or more replicates were performed in all experiments. IC50, 50% inhibitory concentration. CPE, cytopathic effect.
6. Comparison of RT-PCR and seroconversion
The seroconversion and RT-PCR positivity rates were further compared in the 70 household members who were RT-PCR tested during acute illness. We found that spike IgG detected a higher number of infected household members (44/70 vs. 32/70), with a lower variation amongst different age cohorts (ranging from 27 to 73% vs 14 to 86%) than RT-PCR, and thus are more sensitive and robust in detecting infected individuals (table 2 and supplementary figure 3). Of the 32 household members who tested positive by RT-PCR during acute illness, twenty-eight (88%) did seroconvert (table 2), in contrast, 111/112 (99%) index patients seroconverted (supplementary Table 1). If infection is defined by either seroconversion or RT-PCR positivity, the overall attack rate was 47% amongst household members.
Table 2.
RT-PCR positivity in 70 household members and associated characteristics, with SARS-CoV-2 spike IgG positivity for comparison.
| N | RT-PCR positive HM n /N (%)* |
Adjusted odds ratio (95% CI)⁎⁎ |
p⁎⁎ | N | Spike IgG positive HM n/N (%)* |
Adjusted odds ratio (95% CI)⁎⁎ |
p⁎⁎ | |
|---|---|---|---|---|---|---|---|---|
| Overall | 70 | 32 /70 (46%) | 179 | 81/179 (45%) | ||||
| Sex | ||||||||
| Male | 34 | 15/34 (44%) | 0.96 (0.24 - 3.91) | 0.95 | 80 | 38/80 (48%) | 1.02 (0.48 - 2.15) | 0.963 |
| Female | 36 | 17/36 (47%) | 1 (ref) | – | 99 | 43/99 (43%) | 1 (ref) | – |
| Age, years | ||||||||
| 0–10 | 7 | 1/7 (14%) | 0.28 (0.02 - 3.28) | 0.31 | 23 | 11/23 (48%) | 1.31 (0.33 - 5.22) | 0.706 |
| 11–20 | 9 | 2/9 (22%) | 0.41 (0.05 - 3.02) | 0.38 | 34 | 9/34 (26%) | 0.59 (0.20 - 1.70) | 0.326 |
| 21–30 | 19 | 8/19 (42%) | 1 (ref) | – | 36 | 15/36 (42%) | 1 (ref) | – |
| 31–40 | 8 | 4/8 (50%) | 2.20 (0.27 - 18.17) | 0.46 | 18 | 11/18 (61%) | 3.16 (0.80 - 12.51) | 0.101 |
| 41–50 | 9 | 5/9 (56%) | 3.23 (0.31 - 33.26) | 0.32 | 24 | 9/24 (38%) | 1.01 (0.30 - 3.47) | 0.984 |
| 51–60 | 11 | 6/11 (55%) | 2.42 (0.31 - 19.17) | 0.40 | 29 | 15/29 (52%) | 1.62 (0.47 - 5.65) | 0.446 |
| >60 | 7 | 6/7 (86%) | 8.86 (0.62 - 126.23) | 0.11 | 15 | 11/15 (73%) | 2.58 (0.49 - 13.54) | 0.262 |
| Symptoms | ||||||||
| Yes | 66 | 32/66 (48%) | - | – | 130 | 73/130 (56%) | – | – |
| No | 4 | 0/4 (0.0%) | - | – | 49 | 8/49 (16%) | – | – |
| RT-PCR | ||||||||
| Positive | – | - | - | – | 32 | 28/32 (88%) | – | – |
| Negative | – | - | - | – | 38 | 16/38 (42%) | – | – |
| Household size | ||||||||
| 2 | 17 | 11/17 (65%) | 1.66 (0.25 - 10.99) | 0.60 | 44 | 25/44 (57%) | 1.40 (0.49 - 3.98) | 0.527 |
| 3 | 15 | 6/15 (40%) | 068 (0.13 - 3.49) | 0.65 | 36 | 16/36 (44%) | 1.32 (0.42 - 4.19) | 0.639 |
| ≥4 | 38 | 15/38 (39%) | 1 (ref) | – | 99 | 40/99 (40%) | 1 (ref) | – |
Abbreviations: CI: confidence interval; RT-PCR: reverse transcription polymerase chain reaction.
n SARS-CoV-2 (RT-PCR and spike IgG) positive household members. N total sample tested.
Adjusted odds ratio and P values was calculated by generalized estimating equation (GEE) analysis including household member characteristics (sex and age), index patient charachterisitics (sex, age, history of cough, fever, dyspnoea, any comorbidities, inhalation steroid medication, and smoking status), and household size. P values < 0.05 marked in bold were considered statistically significant.
As only symptomatic people were tested, RT-PCR positivity amongst asymptomatic household members was not assessed. Interestingly, of the 38 household members who were RT-PCR tested negative, sixteen (42%, [95% CI 28–58]) seroconverted (table 2). Amongst the 38 household members who were RT-PCR negative, 16 were seropositive, and 15/16 (94%) reported COVID-19 related symptoms. Median time between date of symptom onset and date of RT-PCR test was five days for seropositive, RT-PCR negative household members. We found no significant difference in the antibody titres in seroconverters who tested RT-PCR positive or negative (Fig. 3G). Intriguingly, asymptomatic but seroconverted household members had similar spike specific antibody titre with symptomatic and seroconverted ones (Fig. 3D).
Interestingly, amongst children (0–10 years) with symptoms compatible with COVID-19, only 14% (1/7) of those tested with RT-PCR were positive, while 48% (11/23) seroconverted (table 2). In contrast, amongst symptomatic persons aged >60 years, 86% (6/7) of those tested were RT-PCR positive, while 73% (11/15) seroconverted.
6.1. Neutralising antibody responses
We further analysed the neutralising antibody response by using the sensitive microneutralisation assay and the virus neutralisation assay which measures sterilising immunity. Significantly higher neutralisation titres were found in adults >60 years old than in younger adults (Fig. 3B-C). Although children had higher spike-specific IgG than adults (21–30 years), they had similar titres of neutralising antibodies. Furthermore, household members who seroconverted developed comparable levels of neutralising antibodies regardless of RT-PCR result (Fig. 3HI). There was a trend of higher neutralising antibodies amongst symptomatic seroconverted household members, although not statistically significant (Fig. 3EF).
6.2. Risk factors for transmission
The risk factors for household transmission are presented in table 1 and supplementary Table 3. Whereas attack rates did not increase when the index patient had a cough (aOR 1.05 [95% CI 0.51–2.16]; p = 0.09), transmission was more likely when the index patients had fever (aOR 3.31 [95% CI 1.52–7.24]; p = 0.003) or dyspnoea (aOR 2.25 [95% CI 1.80–4.62]; p = 0.027) (table 1). Neutralising antibodies were also significantly associated with fever and dyspnoea (supplementary Table 3).
7. Discussion
Studies on household transmission of SARS-CoV-2 provide crucial knowledge about the transmission dynamics of the virus in immunologically naïve individuals in a home environment characterized by limited personal protection. Norway contained community transmission at an early phase of the pandemic's first wave by prompt lockdown of the society. This ensured that our study was conducted with low levels of community transmission and negligible baseline immunity amongst the participants, which can otherwise confound household transmission studies. To investigate household transmission, we recruited the initial 112 households of RT-PCR confirmed SARS-CoV-2 index patients during the first 35 days of the outbreak in Bergen, in a case-ascertained study. Our study was explicitly designed to measure household attack rates based on the serological evaluation of SARS-CoV-2-specific antibodies. We found higher rates of transmission within households than previously reported [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], particularly in young children.
The overall household attack rate as measured by seroconversion amongst household members of RT-PCR confirmed, home-isolated patients was 45%. Currently, there are only two other studies that have estimated household attack rates based on seropositivity, 37% in Spain [7] and 35% in Brazil [23]. Although both studies had large sample sizes in the early phase of the pandemic, they are population-based serosurveillance surveys with high levels of community transmission and they cannot confirm that subjects were infected by a household member. Our study was specifically designed to assess household attack rates as measured by seropositivity in household members 6–8 weeks after nasopharyngeal sampling in index patients, at a time of low prevalence of SARS-CoV-2 virus in the community. Thus, our data provide a more accurate estimate of attack rates.
We calculated attack rates based on SARS-CoV-2-specific antibodies in household members, whereas most previous studies have ascertained transmission based on RT-PCR, with estimates of 6% to 38% [11], [12], [13], [14], [15], [16], [17], [18], [19], [20]. RT-PCR can only detect SARS-CoV-2 during the acute phase and has been reported to have an unsatisfactory positivity rate ([24, 25]). Thus, the household attack rates are likely underestimated in RT-PCR-based studies. This is supported by our finding that the seropositivity rate amongst RT-PCR negative household members (42%) was as high as the overall seropositivity rate amongst all household members (45%), despite testing at a median of 5 days after symptom onset, an optimal time for RT-PCR positivity. Although we have a relatively small subgroup of RT-PCR negative household members during the inclusion period, our findings have two major implications. Firstly, using RT-PCR amongst household members of confirmed cases has a low predictive value, and solely relying on RT-PCR could consequently cause further transmission from false-negative cases to new individuals in both the household and the community. Secondly, our findings highlight that serological testing is equally or more effective than RT-PCR in confirming a final diagnosis of COVID-19, especially amongst household members. This is supported by several studies demonstrating the importance of serological testing to confirm cases ([26, 27]). However, amongst the household members in our study who had a positive RT-PCR test at the time of symptoms, 13% did not seroconvert, whereas 99% of index patients seroconverted. If we extrapolate an 87% sensitivity of our assay to our whole cohort, the total attack rate would be 51%. Thus, the true attack rate in our study is likely higher than estimated solely by seroconversion.
According to a recent meta-analysis [5], an average of 15% of RT-PCR confirmed cases are asymptomatic, and importantly asymptomatic children have lower viral loads [28]. We found that 16% of asymptomatic household members seroconverted, and in addition, 42% of RT-PCR negative household members seroconverted. Thus, our findings show that close contacts of confirmed cases are potentially contagious, irrespective of being asymptomatic or having a negative RT-PCR result.
Children have been reported to be less affected by COVID-19 [29] and previous studies have reported a secondary attack rate of 4% to 23% amongst children ([13, 17, 19, 21]). We found that a large proportion of children aged 0–10 years were infected (48%) in a household setting. The lower attack rates in previous studies may be due to the use of RT-PCR as a diagnostic method, consequently underestimating the number of secondary cases amongst children. Indeed, our data show a lower positivity rate on RT-PCR amongst symptomatic children compared to serological testing, which contrasts with older age cohorts. Due to the low number of RT-PCR tested children, the robustness of our observation needs to be confirmed in larger studies but suggests careful consideration of negative RT-PCR results in children. Estimates of transmission to children are also likely to be lower since children often present with milder symptoms, possibly resulting in lower testing rates. We conducted serological testing of all children in the household, regardless of symptoms. With an attack rate of almost 50% as measured by seroconversion, our results show that children may have higher infection rates than has been previously reported ([7, 13, 17, 21]).
In our study, household members >60 years old had a high attack rate (73%, table 1), confirming findings from other studies on household transmission amongst older age groups ([12, 14]). Moreover, we found sterilising neutralising antibodies in all but one of the seropositive participants aged >60 years (Fig. 3).
The finding that index patients with fever and dyspnoea were more likely to transmit infection to others, is not surprising as patients with more severe symptoms may require closer follow-up and care, incurring increased risk of transmission. It may appear counter-intuitive that cough in the index patient was not a significant risk factor for transmission. A likely explanation for this would be that, due to widespread awareness of this transmission route, cough would trigger household members to use precautions such as distancing and masque use, while a person with other symptoms such as fever and dyspnoea may not be perceived as equally infectious.
Whilst self-isolation of cases and good hygiene may prevent infection within a household, pre-existing immunity may also be important. Recently, pre-existing cross-reactive T-cell immunity derived from infection with human coronaviruses has been speculated to protect from infection [30]. Although, the immune response to the SARS-CoV-2 virus is multifaceted and the correlates of protection from COVID-19 disease have yet to be defined. The presence of spike-specific antibodies does not directly correlate with protective immunity and therefore we used stringent serological assays measuring both microneutralising antibodies, which may prevent re-infection, and virus neutralising antibodies which provide sterilizing immunity. No neutralising antibodies were found in the household members who did not seroconvert (supplementary figure 4). When comparing the different assays, we found the highest attack rates measured by spike-specific antibodies, and lower numbers of household members developed neutralising antibodies.
The strengths of our study are the centralized testing facility which allowed for the identification of all RT-PCR test positive cases in Bergen, the low levels of community spread and the stringent use of serological assays to define infected people, firstly by screening all subjects for RBD-specific antibodies, then confirming infection by SARS-CoV-2 spike ELISA and by two neutralisations assays. Further strengths are the inclusion of families with children, as well as a subgroup of subjects who were both tested by RT-PCR and serology, detailed interviews to define the true index case and risk factors for infection. The study was specifically designed to identify household attack rate, and inclusion started with the first RT-PCR positive case in the city, followed by detailed interviews to differentiate between index patients and household members.
The interpretation of our findings has some limitations, which may influence our estimation of attack rates. Despite high participation rates, there may have been a bias in who consented to participate, limiting and influencing our interpretation of results. During our study inclusion period, all individuals were RT-PCR tested at a single time during acute illness and more accurate diagnosis may have been provided by repeated RT-PCR testing. There was also a risk that the index patient was not correctly identified, although to minimize this, extensive telephone interviews were conducted once a positive case was identified. Likewise, we cannot exclude the possibility that some cases and household members had a common source of exposure outside the household, although a sensitivity analysis excluding possible co-primary results did not change results.
In conclusion, we found a higher household attack rate of SARS-CoV-2 than previous studies, and show that serological testing is more sensitive and robust than RT-PCR-testing in assessing attack rates. Children are far more susceptible to household transmission than previously reported, and relying on RT-PCR for diagnosis may miss the majority of infected children. This highlights the importance of including children when considering measures to reduce spread of SARS-CoV-2 virus. The risk of transmission was highest from index patients with dyspnoea and fever, both potential surrogate markers for severity of disease.
Author contributions
NL, BB, RJC, KAB, KGIM, and CT designed the study. KK and DL recruited the participants. SL, KAB, MCT, and AM conducted laboratory analysis and developed the assays. FZ developed and ran all the neutralisation assays. CT recruited all the children from households. FK developed the two-step ELISA. KK, FZ, AB, BB, RJC, and NL analysed the data and wrote the manuscript. All authors have read and approved the final version of the manuscript.
Bergen Covid-19 Research Group
Annette Corydon j, Francisco Real c, Geir Bredholt c, Hauke Bartsch g, Helene Heitmann Sandnes c, Juha Vahokoski a, Kjerstin Jacobsen b, Marianne Eidsheim b, Marianne Sævik e, Nina Urke Ertesva°g a, Synnøve Ygre Hauge g, and Therese Bredholt Onyango a.
Declaration of Competing Interest
An ELISA assay used to screen for seroconversion was developed in Florian Krammer´s laboratory. Mount Sinai has filed patent applications to protect that assay and has licensed its use to several companies. Mount Sinai is also commercializing the assay. All other authors declare no conflict of interest.
Acknowledgments
Acknowledgments
We wish to thank all the participants who altruistically contributed to this study. We especially thank Bergen Municipality Emergency Clinic for excellent collaboration.
Data sharing statement
The data presented in this paper are available from the corresponding authors upon reasonable request.
Footnotes
Editor's disclaimer:
The following translation in Norwegian was submitted by the authors and we reproduce them as supplied. They have not been peer reviewed. Our editorial processes have only been applied to the original abstract in English, which should serve as reference for this manuscript.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lanepe.2020.100014.
Contributor Information
Rebecca J. Cox, Email: rebecca.cox@uib.no.
Nina Langeland, Email: nina.langeland@uib.no.
Bergen COVID-19 research group:
Annette Corydon, Francisco Real, Geir Bredholt, Hauke Bartsch, Helene Heitmann Sandnes, Juha Vahokoski, Kjerstin Jacobsen, Marianne Eidsheim, Marianne Sævik, Nina Urke Ertesvåg, Synnøve Ygre Hauge, and Therese Bredholt Onyango
Appendix. Supplementary materials
References
- 1.Organization WHO. WHO Coronavirus disease (COVID-19) dashboard 2020 [Available from: https://covid19.who.int/.
- 2.Folkehelseinstituttet. COVID-19 Ukerapport - uke 43 2020 [updated 28th October 2020. Available from: https://www.fhi.no/contentassets/8a971e7b0a3c4a06bdbf381ab52e6157/vedlegg/andre-halvar-2020/2020.10.28-ukerapport-uke-43-covid-19.pdf.
- 3.Folkehelseinstituttet. En person har testet positivt på koronavirus 2020 [Available from: https://www.fhi.no/nyheter/2020/en-person-har-testet-positivt-pa-koronavirus/.
- 4.Regjeringen.no . 2020. Omfattende tiltak for å bekjempe koronaviruset 2020. [updated 12th March. [Google Scholar]
- 5.Byambasuren O., Cardona M., Bell K., Clark J., McLaws M.-.L., Glasziou P. Estimating the extent of asymptomatic COVID-19 and its potential for community transmission: systematic review and meta-analysis. medRxiv. 2020 doi: 10.3138/jammi-2020-0030. 2020.05.10.20097543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Amanat F., Stadlbauer D., Strohmeier S., Nguyen T.H.O., Chromikova V., McMahon M. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat Med. 2020;26(7):1033–1036. doi: 10.1038/s41591-020-0913-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pollan M., Perez-Gomez B., Pastor-Barriuso R., Oteo J., Hernan M.A., Perez-Olmeda M. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): a nationwide, population-based seroepidemiological study. Lancet. 2020;396(10250):535–544. doi: 10.1016/S0140-6736(20)31483-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao J., Yuan Q., Wang H., Liu W., Liao X., Su Y. Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gudbjartsson D.F., Norddahl G.L., Melsted P., Gunnarsdottir K., Holm H., Eythorsson E. Humoral immune response to SARS-CoV-2 in Iceland. N Engl J Med. 2020 doi: 10.1056/NEJMoa2026116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Salzberger B., Gluck T., Ehrenstein B. Successful containment of COVID-19: the WHO-report on the COVID-19 outbreak in China. Infection. 2020;48(2):151–153. doi: 10.1007/s15010-020-01409-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bi Q., Wu Y., Mei S., Ye C., Zou X., Zhang Z. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect Dis. 2020;20(8):911–919. doi: 10.1016/S1473-3099(20)30287-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jing Q.L., Liu M.J., Zhang Z.B., Fang L.Q., Yuan J., Zhang A.R. Household secondary attack rate of COVID-19 and associated determinants in Guangzhou, China: a retrospective cohort study. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li W., Zhang B., Lu J., Liu S., Chang Z., Cao P. The characteristics of household transmission of COVID-19. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wu J., Huang Y., Tu C., Bi C., Chen Z., Luo L. Household transmission of SARS-CoV-2, Zhuhai, China, 2020. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grijalva CG R.M., Zhu Y. Transmission of SARS-COV-2 infections in households — Tennessee and Wisconsin, april–september 2020. MMWR Morb Mortal Wkly Rep. 2020 doi: 10.15585/mmwr.mm6944e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bohmer M.M., Buchholz U., Corman V.M., Hoch M., Katz K., Marosevic D.V. Investigation of a COVID-19 outbreak in Germany resulting from a single travel-associated primary case: a case series. Lancet Infect Dis. 2020;20(8):920–928. doi: 10.1016/S1473-3099(20)30314-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yung C.F., Kam K.Q., Chong C.Y., Nadua K.D., Li J., Tan N.W.H. Household transmission of severe acute respiratory syndrome coronavirus 2 from adults to children. J Pediatr. 2020;225:249–251. doi: 10.1016/j.jpeds.2020.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo L., Liu D., Liao X., Wu X., Jing Q., Zheng J. Contact settings and risk for transmission in 3410 close contacts of patients with COVID-19 in Guangzhou, China: a prospective cohort study. Ann Intern Med. 2020 doi: 10.7326/M20-2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez Bernal J., Panagiotopoulos N., Byers C., Garcia Vilaplana T., Boddington N.L., Zhang X. Transmission dynamics of COVID-19 in household and community settings in the United Kingdom. medRxiv. 2020 doi: 10.2807/1560-7917.ES.2022.27.15.2001551. 2020.08.19.20177188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lyngse F.P., Kirkeby C.T., Halasa T., Andreasen V., Skov R.L., Møller F.T. COVID-19 transmission within Danish households: a nationwide study from lockdown to reopening. medRxiv. 2020 doi: 10.2807/1560-7917.ES.2022.27.6.2001800. 2020.09.09.20191239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rosenberg E.S., Dufort E.M., Blog D.S., Hall E.W., Hoefer D., Backenson B.P. COVID-19 testing, epidemic features, hospital outcomes, and household prevalence, New York State—march 2020. Clinical Infectious Diseases. 2020 doi: 10.1093/cid/ciaa549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cox R.J., Brokstad K.A., Krammer F., Langeland N., Bergen C.-.R.G. Seroconversion in household members of COVID-19 outpatients. Lancet Infect Dis. 2020 doi: 10.1016/S1473-3099(20)30466-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Silveira M.F., Barros A.J.D., Horta B.L., Pellanda L.C., Victora G.D., Dellagostin O.A. Population-based surveys of antibodies against SARS-CoV-2 in Southern Brazil. Nat Med. 2020;26(8):1196–1199. doi: 10.1038/s41591-020-0992-3. [DOI] [PubMed] [Google Scholar]
- 24.Di Paolo M., Iacovelli A., Olmati F., Menichini I., Oliva A., Carnevalini M. False-negative RT-PCR in SARS-CoV-2 disease: experience from an Italian COVID-19 unit. ERJ Open Res. 2020;6(2) doi: 10.1183/23120541.00324-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang Y., Yang M., Shen C., Wang F., Yuan J., Li J. Evaluating the accuracy of different respiratory specimens in the laboratory diagnosis and monitoring the viral shedding of 2019-nCoV infections. medRxiv. 2020 2020.02.11.20021493. [Google Scholar]
- 26.Zhang J., Zhang X., Liu J., Ban Y., Li N., Wu Y. Serological detection of 2019-nCoV respond to the epidemic: a useful complement to nucleic acid testing. Int Immunopharmacol. 2020;88 doi: 10.1016/j.intimp.2020.106861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu Y., Xiao M., Liu X., Xu S., Du T., Xu J. Significance of serology testing to assist timely diagnosis of SARS-CoV-2 infections: implication from a family cluster. Emerg Microbes Infect. 2020;9(1):924–927. doi: 10.1080/22221751.2020.1752610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kociolek L.K., Muller W.J., Yee R., Dien Bard J., Brown C.A., Revell P. Comparison of upper respiratory viral load distributions in asymptomatic and symptomatic children diagnosed with SARS-CoV-2 infection in pediatric hospital testing programs. J Clin Microbiol. 2020 doi: 10.1128/JCM.02593-20. JCM.02593-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludvigsson J.F. Children are unlikely to be the main drivers of the COVID-19 pandemic – a systematic review. Acta Paediatr. 2020;109(8):1525–1530. doi: 10.1111/apa.15371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grifoni A., Weiskopf D., Ramirez S.I., Mateus J., Dan J.M., Moderbacher C.R. Targets of T cell responses to SARS-CoV-2 coronavirus in humans with COVID-19 disease and unexposed individuals. Cell. 2020;181(7) doi: 10.1016/j.cell.2020.05.015. 1489-501 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.