Abstract
In clinical practice, tegafur, gimeracil, and oteracil potassium (S-1) therapy is commonly administered to treat nasopharyngeal carcinoma (NPC). However, its efficacy and safety remain controversial in both randomized controlled trials (RCTs) and non-RCTs. We aimed to evaluate the efficacy and safety of S-1 treatment for NPC. We searched PubMed, Ovid, EMBASE, the Cochrane Library, China National Knowledge Infrastructure, Wanfang Database, and VIP databases for RCTs of chemotherapy with or without S-1 for NPC, from 2001 to 2020. A meta-analysis was performed using RevMan5.3 and Stata15. Randomized controlled trials published in journals were included irrespective of blinding and language used. Patients were diagnosed with NPC through a clinicopathological examination; patients of all cancer stages and ages were included. Overall, 25 trials and 1858 patients were included. There were significant differences in the complete remission (OR = 2.42, 95% CI (1.88–3.10), P < 0.05) and overall response rate (OR = 2.68, 95% CI (2.08–3.45), P < 0.05) between the S-1 and non-S-1 groups. However, there was no significant difference in partial remission (OR = 1.10, 95% CI (0.87–1.39), P=0.42) and seven adverse reactions (leukopenia, thrombocytopenia, nausea and vomiting, diarrhea, dermatitis, oral mucositis, and anemia) between the S-1 and non-S-1 groups. Additionally, statistical analyses with six subgroups were performed. S-1 was found to be a satisfactory chemotherapeutic agent combined with radiotherapy, intravenous chemotherapy, or chemoradiotherapy for NPC. As an oral medicine, the adverse reactions of S-1, especially gastrointestinal reactions, can be tolerated by patients, thereby optimizing their quality of life. S-1 may be a better choice for the treatment of NPC. This trial is registered with CRD42019122041.
1. Introduction
Nasopharyngeal carcinoma (NPC) is a malignant tumor prevalent in Southeast Asia and South China. Most patients with NPC are in an advanced stage at diagnosis, as it is asymptomatic in the early stages, resulting in a high mortality rate [1, 2]. Although advanced radiotherapy [3] and concurrent chemotherapy can improve progression-free survival and overall survival [4] in patients with NPC, there is a high risk of local recurrence, distant metastasis, and mortality [5]. Thus, to optimize their quality of life, the efficacy of chemotherapy must be evaluated. However, studies on the efficacy of chemotherapy in randomized controlled trials (RCTs) for NPC have received limited attention [6, 7].
The combination of tegafur, gimeracil, and oteracil potassium (S-1) was approved as a treatment for progressive or recurrent head and neck malignancies in 2001, and it is widely used in the treatment of gastric, esophageal, colorectal, pancreatic, and nonsmall cell lung cancers, and other malignant tumors [8–13]. As a second-generation fluorouracil and oral chemotherapy compound, S-1 has been widely used in clinical applications. Owing to its short half-life and few adverse reactions, S-1 is easy to administer and reduces the pain of intravenous fluids, thereby making patients more receptive, more tolerant, and less likely to develop drug resistance [14–18]. S-1 combined with chemotherapy or radiotherapy for head and neck tumors could have a good effect [19–21]. Moreover, when used during radiotherapy, S-1 has been associated with a low recurrence rate, satisfactory long treatment effects, and improved survival quality [9, 20]. In contrast, its efficacy with chemotherapy is not satisfactory [22] and remains controversial. Blanchard [4] confirmed that chemotherapy combined with radiotherapy significantly improves the survival of patients with locally advanced NPC. Recently, despite the efficacy of S-1 for NPC, as reported by several studies [2, 18, 23], there have been only a few systemic reviews and meta-analyses because of some research limitations. First, the number of patients treated with S-1 is small because the studies are mostly from endemic areas. Second, systematic reviews of RCTs determining the efficacy of S-1 for NPC are limited. The effects of S-1 combined with radiotherapy have been discussed in a previous meta-analysis [24], but those of chemoradiotherapy or chemotherapy alone have not been investigated. Additionally, the databases used [24] only contain data up to 2015; thus, they may have missed data for the past 7 years. It is crucial to systematically analyze the efficacy to improve available chemotherapy approaches that use S-1. Therefore, to objectively evaluate the efficacy and safety of S-1 in the treatment of NPC, we conducted a systematic review and meta-analysis of RCTs of S-1 treatment combined with radiotherapy, intravenous chemotherapy, or chemoradiotherapy in patients with NPC.
2. Materials and Methods
2.1. Literature Selection
We searched PubMed, Ovid, EMBASE, the Cochrane Library, China National Knowledge Infrastructure, Wanfang Database, and VIP databases. The search language was not restricted, and the retrieval time spanned from January 1, 2001 (S-1 was approved for the treatment of progressive or recurrent head and neck malignancies in 2001) to February 24, 2020. We used the following keywords: S-1, tegafur, gimeracil, oteracil potassium, nasopharyngeal cancer, nasopharyngeal carcinoma, nasopharyngeal cancers, and the Chinese terms for S-1 and NPC.
2.2. Eligibility Criteria
RCTs published in journals were included irrespective of blinding. Patients were diagnosed with NPC through a clinicopathological examination, and patients of all cancer stages and ages were included. The experimental group patients were treated with S-1, whereas the control group patients were treated with non-S-1 (e.g., 5-fluorouracil (5-Fu) + cisplatin (DDP), intensity-modulated radiotherapy (IMRT), chemotherapy, or docetaxel (TXT) + DDP) (Table S1 in Supplementary Materials). The baseline characteristics were matched and were comparable between the experimental and control groups in 25 RCTs.
The following studies were excluded: (1) non-RCTs, (2) nonpublished studies, (3) studies with incomplete or unavailable data, and (4) retrospective trials, animal studies, meeting abstracts, letters, comments, editorials, reviews, those not described as RCTs, or systematic reviews and meta-analysis. The retrieved literature was independently screened by two authors (Deng and Ma) using the inclusion and exclusion criteria.
2.3. Outcome Measures
The primary outcomes were complete remission (CR), partial remission (PR), and overall response rate (RR) of the short-term effects, whereas the secondary outcomes were adverse reactions. Additionally, statistical analyses with six subgroups were performed. The RR was calculated as follows:
| (1) |
2.4. Data Collection and Quality Assessment
The data extracted included the first authors' name, time of publication, patient status, trial design, intervention, sample size, dose, outcome indicator, and last follow-up (missing). The extracted information was then cross-verified. Any indeterminate difference or disagreement was resolved through a discussion with the third author who made the final decision.
The quality of the studies was evaluated independently by two authors using the bias risk assessment tool from the RCT evaluation manual in the Cochrane Handbook (version 5.1.0., 2011) [25] with the following parameters: (1) random allocation methods, (2) allocation concealment, (3) blinding of research subjects and researchers, (4) blinding of outcome assessment, (5) incomplete outcome data, (6) selective report outcomes, and (7) other sources of bias. Each included study was evaluated according to the criteria as “high risk,” “low risk,” or “unclear.” When there were disputes regarding the evaluations, a third author made the final decision.
2.5. Data Analysis
RevMan (version 5.3, Cochrane Collaboration, Copenhagen, Denmark) and STATA (version 15.0, StataCorp LLC, College Station, TX, USA) software were used for the meta-analysis. If the results were statistically homogeneous (P > 0.05, I2 <50%), a fixed-effect model was selected. If the data were statistically heterogeneous, a random effect model was used. Descriptive analysis was conducted for the data that could not be combined. The Mantel–Haenszel test was used to calculate 95% confidence interval (CIs), and odds ratio (OR) was used to combine the effect. We used the corresponding data model to combine effect sizes across studies and implement a sensitivity analysis for assessing the potential effects of individual datasets on the results and pooled data. Publication bias was identified and was visually inspected using Begg's rank correlation method and the shear complement test.
2.6. Protocol and Registration
The protocol was fully implemented in accordance with the requirements of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses [26] (Table S2. PRISMA 2009 checklist in the Supplementary Materials). The review protocol is available on the PROSPERO official website (registration number: CRD42019122041).
3. Results
3.1. Literature Selection and Study Characteristics
One hundred and eighty-one articles were initially retrieved, and the selection process is shown in Figure 1. After removing the duplicates, 93 studies remained. After screening the titles and abstracts, 32 studies remained. After examining the full text of 32 articles, 25 studies [1, 9, 14–16, 19, 22, 27–44] were included in this meta-analysis, and seven were excluded. Twenty-three studies reported three main outcomes (CR, PR, and RR). Patients included in the studies were over 14 years of age; the total number of patients in each study ranged from 40 to 120. Furthermore, 13 studies described patients with NPC in locally advanced stages, 8 in advanced stages, and 3 in early stages. The characteristics of the included studies are summarized in Table S1 in the Supplementary Materials. The 25 RCTs [1, 9, 14–16, 19, 22, 27–44] included involved 1858 patients. Of the studies, only one study used a single-blind method, whereas the others did not describe their allocation concealment or the blinding method. Data from 22 patients were incomplete or lost to follow-up. The outcome data were complete, and most studies did not selectively report results. Two studies had a random sequence generation bias, and allocation concealment in 25 studies was unclear. Five studies had incomplete main outcome data, and one study had a selective reporting bias, although the bias risk was not high. The bias risk assessment is shown in Figure S1 in the Supplementary Materials.
Figure 1.

PRISMA flow diagram of literature screening.
3.2. Outcome Measures
3.2.1. Primary Outcome Measures
CR was reported by 24 studies involving 1798 patients [1, 9, 14–16, 22, 27–44]. The heterogeneity test indicated that the fixed-effect model could be selected (P=0.33, I2 = 10%). The pooled analysis indicated a significant difference between the treatment and control groups (OR = 2.42, 95% CI (1.88–3.10), P < 0.05) (Figure 2(a)).
Figure 2.

Forest plots of the comparison between the experimental (S-1 treatment) and control (non-S-1 treatment) groups in terms of (a) complete remission (CR), (b) partial remission (PR), and (c) response rate (RR).
Twenty-three studies involving 1737 patients [1, 9, 14–16, 22, 27, 28, 30–44] reported PR. Based on the heterogeneity test results (P=0.24, I2 = 16%), a fixed-effect model was selected. There was no significant difference between the treatment and control groups (OR = 1.10, 95% CI (0.87–1.39), P > 0.05) (Figure 2(b)).
RR was reported by 24 studies involving 1792 patients [1, 9, 14–16, 19, 22, 27, 28, 30–44]. The fixed-effect model was used because there was no statistical heterogeneity (P=0.32, I2 = 11%). There was a significant difference between the treatment and control groups (OR = 2.68, 95% CI (2.08–3.45), P < 0.05) (Figure 2(c)).
3.2.2. Secondary Outcome Measures
Twelve studies involving 838 patients reported leukopenia [1, 9, 14–16, 19, 30, 32, 38, 41, 42, 44]. The forest plot (Figure 3(a)) revealed no significant difference between the groups (OR = 1.03, P > 0.05). As there was statistical heterogeneity (P < 0.05, I2 = 70%), the random-effect model was selected.
Figure 3.

Forest plots of the comparison of adverse reactions between the experimental (S-1 treatment) and control (non-S-1 treatment) groups. (a) Leukopenia, (b) thrombocytopenia, (c) nausea and vomiting, (d) gastrointestinal reactions, (e) diarrhea, (f) oral mucositis, (g) dermatitis, and (h) anemia.
Thrombocytopenia was reported by 11 studies involving 739 patients [1, 9, 14–16, 22, 30, 32, 41, 43, 44]. The random effect model was selected based on the heterogeneity test results (P < 0.05, I2 = 71%). There was no significant difference between the groups (OR = 0.74, P > 0.05) (Figure 3(b)).
Fourteen studies [1, 9, 14–16, 19, 22, 30, 32, 36, 41–44] involving 1001 patients reported nausea and vomiting. The random-effect model was selected based on the heterogeneity test results (P < 0.05, I2 = 60%). The forest plot (Figure 3(c)) revealed that there was no significant difference between the groups (OR = 0.86, P > 0.05).
Five studies involving 373 patients reported gastrointestinal reactions [27, 29, 34, 37, 38]. The fixed-effect model was selected based on the heterogeneity test results (P > 0.05, I2 = 8%). There was a significant difference between the groups (OR = 2.51, P < 0.05) (Figure 3(d)).
Eight studies [1, 14, 16, 22, 32, 36, 41, 43] involving 538 patients reported diarrhea. The random-effect model was selected based on the heterogeneity test results (P < 0.05, I2 = 59%). There was no significant difference between the treatment and control groups (OR = 0.72, P > 0.05) (Figure 3(e)).
Ten studies involving 721 patients reported oral mucositis [14, 15, 22, 27, 29, 32, 37, 38, 40, 41]. The random-effect model was selected based on the heterogeneity test results (P < 0.05, I2 = 79%). There was no significant difference between the treatment and control groups (OR = 0.72, P > 0.05) (Figure 3(f)).
Eleven studies involving 862 patients [1, 9, 14, 15, 22, 27, 29, 32, 34, 37, 40] reported dermatitis. The random-effect model was selected based on the heterogeneity test results (P < 0.05, I2 = 68%). There was no significant difference between the groups (OR = 0.77, P > 0.05) (Figure 3(g)).
Anemia was reported by 12 studies [1, 9, 14–16, 22, 32, 38, 41–44] involving 851 patients. The random effect model was selected based on the heterogeneity test results (P < 0.05, I2 = 66%). The forest plot (Figure 3(h)) revealed no significant difference between the groups (OR = 0.78, P > 0.05).
3.2.3. Subgroup Outcome Measures
Statistical analyses with six subgroups were performed, and the outcome measure was RR. These subgroups were as follows: (1) locally advanced NPC (LANPC), (2) advanced NPC (ANPC), (3) treatment of the experimental and control groups with chemotherapy, (4) treatment with chemotherapy concomitant with radiotherapy (S-1 treatment) and radiotherapy alone (non-S-1 treatment) (RC vs. R), (5) treatment of the experimental and control groups with chemoradiotherapy (RC), and (6) treatment of the experimental group with S-1 and the control group with 5-Fu (S-1 vs. 5-Fu). The sites of locally advanced NPC were different from advanced NPC. Locally advanced NPC is not associated with distant metastasis to the lung and liver, whereas advanced NPC can have distant metastasis to these sites.
Thirteen studies involving 1016 patients reported RR to LANPC [1, 9, 14, 15, 19, 22, 27, 32–34, 36, 37, 40]. The fixed-effect model was selected based on the heterogeneity test results (P=0.21, I2 = 23%). There was a significant difference between the treatment and control groups (OR = 3.22, 95% CI (2.28–4.56), P < 0.05) (Figure 4(a)).
Figure 4.
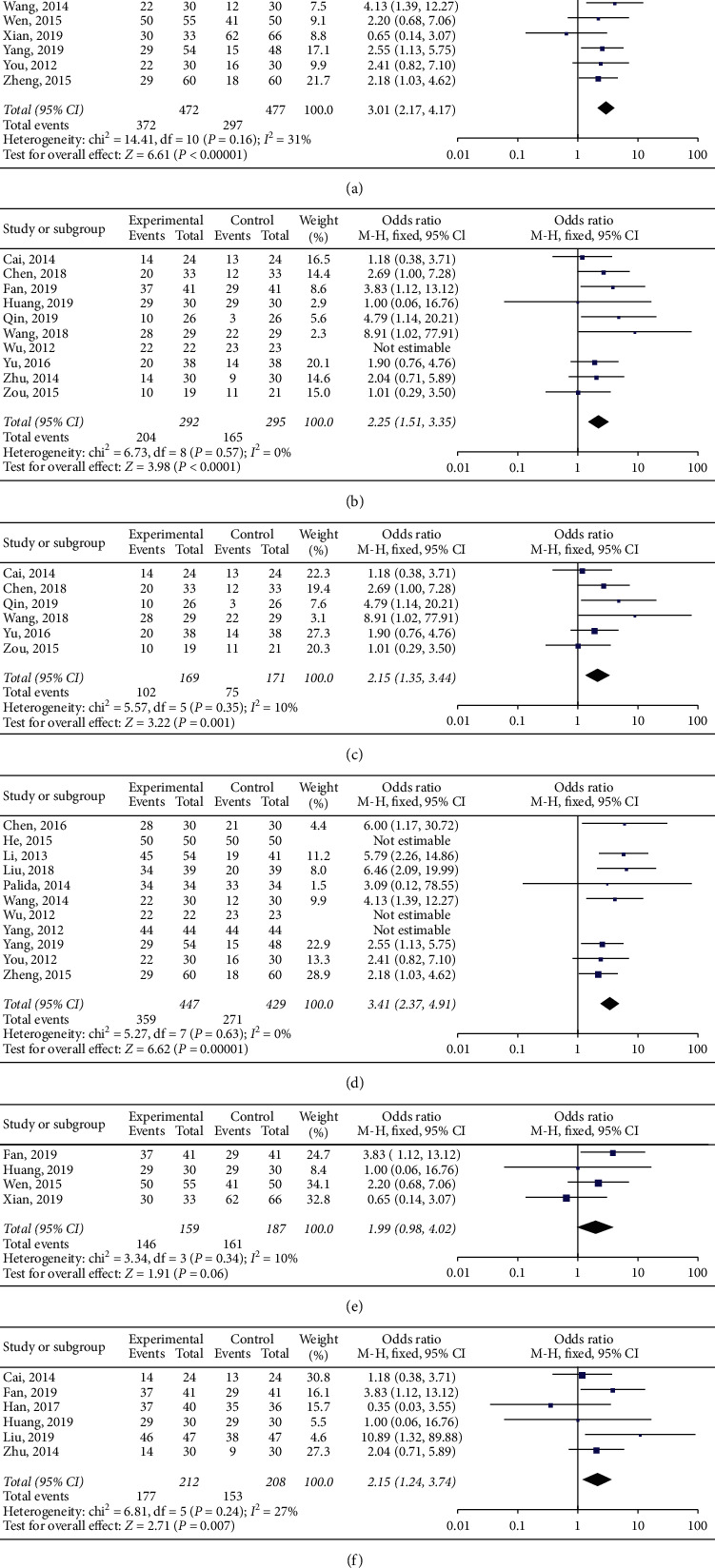
Forest plots of the comparison between S-1 treatment and non-S-1 treatment in the subgroups: (a) locally advanced NPC RR, (b) advanced NPC RR, (c) chemotherapy RR, (d) RC vs R RR, (e) RC RR, and (f) S-1 vs. 5-Fu RR. RR, response rate; chemotherapy, treatment of the experimental and control groups with chemotherapy; RC vs. R, chemotherapy concomitant with radiotherapy (S-1 treatment) and radiotherapy alone (non-S-1 treatment); RC, treatment of the experimental and control groups with chemoradiotherapy; 5-Fu, 5-fluorouracil; S-1 vs. 5-Fu, treatment of the experimental group with S-1 and the control group with 5-Fu.
Eight studies involving 520 patients reported RR to ANPC [16, 28, 30, 38, 41–44]. The fixed-effect model was used because the data were not statistically heterogeneous (P=0.59, I2 = 0%). There was a significant difference between the treatment and control groups (OR = 2.15, 95% CI (1.48–3.11), P < 0.05) (Figure 4(b)).
The RR to chemotherapy was reported by six studies involving 340 patients [28, 30, 38, 41, 43, 44]. The fixed-effect model was used because the data were not statistically heterogeneous (P=0.35, I2 = 10%). There was a significant difference between the treatment and control groups (OR = 2.15, 95% CI (1.35–3.44), P < 0.05) (Figure 4(c)).
Eleven studies involving 876 patients reported RR to RC vs. R [14, 15, 19, 22, 27, 31, 33, 35, 37, 40, 42]. The fixed-effect model was used because the data were not statistically heterogeneous (P=0.63, I2 = 0%). There was a significant difference between the treatment and control groups (OR = 3.28, 95% CI (2.29–4.69), P < 0.05) (Figure 4(d)).
The experimental and control groups' RR to chemoradiotherapy was reported by four studies with 346 patients [9, 22, 32, 36]. The fixed-effect model was used because there was no statistical heterogeneity (P=0.34, I2 = 10%), and there was no significant difference between the treatment and control groups (OR = 1.99, 95% CI (0.98, 4.02), P=0.06 > 0.05) (Figure 4(e)).
Six studies involving 420 patients reported RR to S-1 vs. 5-Fu [1, 16, 28, 32, 34, 36]. The fixed-effect model was selected based on the heterogeneity test results (P=0.24, I2 = 27%). There was a significant difference between the treatment and control groups (OR = 2.15, 95% CI (1.24–3.74), P < 0.05) (Figure 4(f)).
3.3. Sensitivity Analysis
By removing one study at a time, the sensitivity analysis was performed to assess the potential effect of individual datasets on the results and pooled data. For CR (Figure 5(a), Table 1, and Table S3 in Supplementary Materials), the OR value in the pooled analysis became stable (I2 = 0%) after removing the study of Xian [22]. This indicated that the results of the other 24 studies were relatively consistent and that the efficacy of S-1 was certain. For PR and RR (Figures 5(b) and 5(c)), the results were consistent with those of the forest plot; thus, these studies can be considered homogeneous.
Figure 5.

Sensitivity analysis of the primary outcomes: (a) complete remission, (b) partial remission, and (c) response rate. Funnel plot analysis: Begg's test for (d) complete remission and (e) response rate. (f) Trim-and-fill method for complete remission.
Table 1.
Sensitivity analysis of complete remission.
| Criterion | Included studies (n) | Experimental groups (n) | Control groups (n) | ORa (95% CI) | P value | I 2 (%) |
|---|---|---|---|---|---|---|
| Before exclusion | 24 | 896 | 902 | 2.42 (1.88–3.10) | 0.33 | 10 |
| After exclusion | 23 | 863 | 836 | 2.72 (2.09–3.54) | 0.75 | 0 |
aOdds ratio.
3.4. Publication Bias
Using Begg's funnel plot and the trim-and-fill method, publication bias was analyzed (Figures 5(d)–5(f)). There was evidence of publication bias in the meta-analysis of CR. After adding the six missing studies (two of which overlapped) according to the trim-and-fill plot (Figure 5(f), Table 2), the results of the meta-analysis did not change significantly. The publication bias was small, indicating that the conclusions were robust. There was no publication bias in the analysis of RR because Begg's funnel plots and the results of the trim-and-fill method were similar (Figure 5(e)) [45].
Table 2.
Trim-and-fill method for complete remission.
| Step 1a | ||||||
|
| ||||||
| Model | Pooled estimate | 95% CI | Asymptotic | |||
| Lower limit | Upper limit | z value | P value | Studies (n) | ||
| Fixed | 0.853 | 0.595 | 1.111 | 6.483 | 0 | 23 |
| Random | 0.866 | 0.587 | 1.144 | 6.096 | 0 | |
|
| ||||||
| Test for heterogeneity: Q = 24.331 on 22 degrees of freedom (P=0.330) | ||||||
| Moment-based estimate of between-studies variance = 0.043 | ||||||
|
| ||||||
| Step 2b | ||||||
| Trimming estimator: linear | ||||||
| Meta-analysis type: random effect model | ||||||
|
| ||||||
| Iteration | Estimate | Tn | # to trim | Diff | ||
| 1 | 0.866 | 184 | 4 | 276 | ||
| 2 | 0.796 | 197 | 5 | 26 | ||
| 3 | 0.79 | 200 | 6 | 6 | ||
| 4 | 0.784 | 200 | 6 | 0 | ||
|
| ||||||
| Step 3c | ||||||
| Filled meta-analysis | ||||||
|
| ||||||
| Model | Pooled | 95% CI | Asymptotic | Studies (n) | ||
| Estimate | Lower limit | Upper limit | z value | P value | ||
| Fixed | 0.78 | 0.529 | 1.03 | 6.104 | 0 | 29 |
| Random | 0.783 | 0.515 | 1.051 | 5.726 | 0 | |
aMeta-analysis was performed by combined results of all effects with a fixed model and random model. bThe trimming estimator of the random-effect model (through four iterations). cMeta-analysis was performed again after the inclusion of the missing studies.
4. Discussion
In this study, 25 RCTs were reviewed to evaluate the efficacy of S-1 treatment for NPC. The control (non-S-1) groups were treated with radiotherapy alone, intravenous chemotherapy, or chemoradiotherapy. Of the 25 studies, 24 indicated that S-1 is more effective than other non-S-1 treatments for NPC. As an oral drug, S-1 is also easier to administer than intravenous chemotherapy regimens and could reduce the incidence of adverse reactions. Xian [22] included three chemotherapeutic control groups (radiotherapy + S-1, radiotherapy + TXT, and radiotherapy + DDP) and concluded that treatment with S-1 is not as effective for NPC; this contradicted the findings of the other 24 RCTs.
We then analyzed the clinical efficacy of S-1 in NPC. There were significant differences in CR and RR but not in PR, indicating that the three primary outcomes are not statistically heterogeneous. Therefore, S-1 is active against NPC.
Just five studies included 1-year [27, 29, 37, 40, 43] and 2-year [9, 19, 27, 37, 40] survival rates. The results of these studies showed that S-1 effectively improved the survival rates of patients with NPC. However, the survival and recurrence rates were low and inconsistent among these studies. For example, Yang [40] reported 1-year and 2-year survival rates, but the recurrence and metastasis rates were not indicated. Yu [41] found that the survival rate, distant transfer rate, and recurrence rate in the S-1 group were significantly higher than those in the control group, whereas the distant metastasis rate and recurrence rate in the study were opposite. The survival rate, distant metastasis rate, and recurrence rate were not specified in several studies. Therefore, some results could not be merged, and the data quality was not optimal. More rigorous studies with a higher number of patients should be conducted to analyze the survival rates.
To analyze the nine adverse reactions of S-1 treatment, we used forest plots. Only the adverse reactions reported in at least five studies were analyzed. Data on the adverse reactions contained four levels (I–IV). There were no significant differences in the occurrence rate of leukopenia, thrombocytopenia, nausea and vomiting, diarrhea, oral mucositis, dermatitis, and anemia between the S-1 and non-S-1 groups, indicating that these adverse reactions are tolerable. However, gastrointestinal reactions were more severe in the S-1 group than in the control group in five RCTs. Nausea, vomiting, and diarrhea are considered gastrointestinal reactions, and some studies reported these adverse reactions. However, these studies only included gastrointestinal reactions without separate detailed items (nausea and vomiting in 14 studies, diarrhea in 8 studies, and gastrointestinal reactions in 5 studies), and therefore, the analyses of nausea and vomiting and diarrhea were probably different from those of gastrointestinal reactions. Importantly, there was heterogeneity in all adverse reactions except for gastrointestinal reactions, which may be related to the stage of NPC, patient age, or the classification of adverse reactions.
Furthermore, we performed statistical analyses with six subgroups. The forest plots revealed significant differences in five subgroups, namely, LANPC, ANPC, chemotherapy, RC vs. R, and S-1 vs. 5-Fu, but not in the subgroup RC. This indicated that the outcomes of the six subgroups are not statistically heterogeneous. Among the 25 RCTs, 22 RCTs had discussed the treatment of S-1 for locally advanced NPC and advanced NPC, and 17 articles had analyzed the treatment of experimental and control groups with chemotherapy and chemotherapy concomitant with radiotherapy (S-1 arms) and radiotherapy alone (non-S-1 arms). The use of S-1 was reported effective in the studies involving these subgroups.
For the chemotherapy subgroup, six studies [28, 30, 38, 41, 43, 44] were included, and all of them were on advanced NPC. This subgroup analysis showed the combined treatment with S-1 for advanced NPC: improved the clinical efficacy [30, 41, 44], toxicities and adverse effects were tolerable [28, 38, 41, 43], safe [30, 41], prolonged survival of patients with NPC, and improved their quality of life [38, 41, 44].
Additionally, for treatment with chemoradiotherapy [9, 22, 32, 36], there were only four studies with the S-1 and control groups. Wen et al. [9] concluded that the efficacy of S-1 and control was similar but superior in terms of toxicity over the control.
Critically, as a kind of 5-Fu derivative oral anticancer drug, S-1 is comprised of tegafur, gimeracil, and oteracil potassium [1, 15, 30, 40]. Tegafur can be transformed into 5-Fu in the human body to exert antitumor activity [1, 30, 38], and the catabolism of 5-Fu is inhibited by gimeracil [15, 29]; thus, a relatively constant blood drug concentration has to be maintained [27, 36]. Oteracil potassium can reduce gastrointestinal adverse reactions [1, 28, 29].
Especially for 5-Fu, among the 25 RCTs, six studies [1, 16, 28, 32, 34, 36] analyzed the effects of S-1 for NPC, four studies were for LANPC [1, 32, 34, 36], two studies were for ANPC [16, 28], two evaluated chemoradiotherapy [32, 36], two evaluated neoadjuvant chemotherapy [1, 34], and two evaluated chemotherapy [16, 28]. Han et al. [1, 28] reported that S-1 and 5-Fu have equal efficacies. Liu et al. [16, 32, 34, 36] indicated that S-1 was more effective. Cai et al. [28] reported S-1 was better for patients with deep vein catheterization. Furthermore, Zhu et al. reported that there was no phlebitis in the S-1 group [16]. Studies in this subgroup reported the following: toxic and adverse effects were tolerated by patients [28, 32], adverse reactions did not increase [16, 34], and most adverse reactions were at the 0–2 levels [1, 32]. In particular, the studies pointed out that the 1–4 level toxicities of S-1 were lower than those of 5-Fu [32], neoadjuvant chemotherapy of S-1 had effectively improved the immune function of patients with locally advanced NPC [34], and S-1 had reduced the total proportion of adverse reactions [36]. Therefore, S-1 was better than 5-Fu for NPC, and S-1 was safe for treatment [32, 34].
Based on the outcome measures and the above discussion, it can be concluded that treatment with S-1 was more active than that without S-1 for NPC. S-1 was a satisfactory chemotherapeutic agent combined with radiotherapy or intravenous chemotherapy for NPC, importantly. As an oral medicine, the adverse reactions, especially gastrointestinal reactions, associated with S-1 can be tolerated by patients, thereby optimizing the quality of life of patients. S-1 may be a better choice of treatment for NPC.
From the sensitivity analysis, the OR value of CR was stable after removing the study of Xian et al. [22], and the I2 changed from 10% to 0%. The results indicated that the other 23 studies were relatively consistent. For RR, the included studies were homogeneous. This confirmed the efficacy of S-1. Xian [22] evaluated the efficacy and toxic adverse effects of chemotherapy concurrent with radiotherapy in the treatment of locally advanced NPC in three groups administered TXT, DDP, or S-1. The S-1 treatment presented a slightly lower efficacy than the others, but the incidence of oral mucositis was significantly lower. Except in the study of Xian [22], the efficacy of S-1 for NPC in 23 studies was consistent with the finding of our meta-analysis.
There are some weaknesses to our meta-analysis. We found publication bias in CR; thus, six studies had to be added to the meta-analysis. Additionally, our study used published trials rather than individual patient data, which could lower the accuracy of the estimates. Hence, the results obtained from the literature review and meta-analysis suggest the need to confirm the noninferiority in efficacy of S-1 in different settings with prospective randomized, controlled clinical trials, in which toxicity and quality of life are also evaluated, to better define the role of this drug in NPC treatment. In addition, there is the potential limitation of late toxicities.
5. Conclusions
In summary, our study showed that S-1 for NPC treatment has satisfactory activity; moreover, the adverse effects were tolerated by patients. As S-1 is an oral medication, it is convenient for clinical use. S-1 may be a standard regimen for NPC; especially, it is suitable for patients who cannot tolerate the intravenous chemotherapy. Furthermore, it can reduce the pain caused by intravenous administration and thereby improve the quality of life of patients. To confirm the effectiveness of S-1 treatment, more rigorous studies with a higher number of patients should be conducted, and later toxicities in patients, such as the reaction in the gastrointestinal tract after long-term use of S-1, should be analyzed, especially to verify the efficacy in advanced NPC patients with recurrence and metastasis. Additionally, the outcome indicators of these efficacy and toxicities should be further unified.
Acknowledgments
The authors would like to thank Editage (http://www.editage.cn) for English language editing. This work was funded by the National Nature Science Foundation of China (grant no. 81771891).
Data Availability
The data used to support the findings of this study are included within the article and supplementary information files.
Disclosure
Ximing Zhang and Xiumei Tian are the co-first authors.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
Ximing Zhang and Xiumei Tian contributed equally to this work.
Supplementary Materials
Table S1. Basic characteristics of the included studies. Table S2. PRISMA checklist. Table S3. Sensitivity analysis for complete remission. Table S4. Search strategies. Figure S1. Results of the risk bias assessment of 25 RCTs (EPS 4462 kb).
References
- 1.Han Y. Q., He Q., Liu F., et al. A study of TPS1 and TPF neoadjuvant chemotherapy on curative effect for treatment of patients with locally advanced nasopharyngeal carcinoma. Anti-Tumor Pharmacy. 2017;7:425–430. [Google Scholar]
- 2.Zong J. F., Xu H. C., Chen B. J., et al. Maintenance chemotherapy using S-1 following definitive chemoradiotherapy in patients with N3 nasopharyngeal carcinoma. Radiation Oncology. 2019;14:p. 182. doi: 10.1186/s13014-019-1387-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang M.-X., Li J., Shen G.-P., et al. Intensity-modulated radiotherapy prolongs the survival of patients with nasopharyngeal carcinoma compared with conventional two-dimensional radiotherapy: a 10-year experience with a large cohort and long follow-up. European Journal of Cancer. 2015;51(17):2587–2595. doi: 10.1016/j.ejca.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 4.Blanchard P., Lee A., Marguet S., et al. Chemotherapy and radiotherapy in nasopharyngeal carcinoma: an update of the MAC-NPC meta-analysis. The Lancet Oncology. 2015;16(6):645–655. doi: 10.1016/s1470-2045(15)70126-9. [DOI] [PubMed] [Google Scholar]
- 5.Wu F., Wang R., Lu H., et al. Concurrent chemoradiotherapy in locoregionally advanced nasopharyngeal carcinoma: treatment outcomes of a prospective, multicentric clinical study. Radiotherapy and Oncology. 2014;112(1):106–111. doi: 10.1016/j.radonc.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Chen W., Zheng R., Baade P. D., et al. Cancer statistics in China, 2015. CA: A Cancer Journal for Clinicians. 2016;66(2):115–132. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 7.Huang T.-R., Zhang S.-W., Chen W.-Q., et al. Trends in nasopharyngeal carcinoma mortality in China, 1973-2005. Asian Pacific Journal of Cancer Prevention. 2012;13(6):2495–2502. doi: 10.7314/apjcp.2012.13.6.2495. [DOI] [PubMed] [Google Scholar]
- 8.Kou W. Z., Yang L. Z., Yang L. Z., et al. Therapeutic effect of tegafur, gimeracil and oteracil capsule combined with celecoxib on patients with advanced gastric cancer. Journal of Xinxiang Medical University. 2015;32:336–339. [Google Scholar]
- 9.Wen L., You C., Lu X., Zhang L. Phase II trial of concurrent chemoradiotherapy with S-1 versus weekly cisplatin for locoregionally advanced nasopharyngeal carcinoma. Molecular and Clinical Oncology. 2015;3(3):687–691. doi: 10.3892/mco.2015.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Emi M., Yamaguchi Y., Hihara J., Hironaka K., Okada M. Phase I trial of oxaliplatin plus S-1 chemotherapy in patients with metastatic colorectal cancer. Oncology Letters. 2010;1(1):95–98. doi: 10.3892/ol_00000017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aupérin A., Le Péchoux C., Rolland E., et al. Meta-analysis of concomitant versus sequential radiochemotherapy in locally advanced non-small-cell lung cancer. Journal of Clinical Oncology. 2010;28(13):2181–2190. doi: 10.1200/jco.2009.26.2543. [DOI] [PubMed] [Google Scholar]
- 12.Tsushima T., Hironaka S., Boku N., et al. Safety and efficacy of S-1 monotherapy in elderly patients with advanced gastric cancer. Gastric Cancer. 2010;13(4):245–250. doi: 10.1007/s10120-010-0566-z. [DOI] [PubMed] [Google Scholar]
- 13.Kim H. M., Bang S., Park J. Y., et al. Phase II trial of S-1 and concurrent radiotherapy in patients with locally advanced pancreatic cancer. Cancer Chemotherapy and Pharmacology. 2009;63(3):535–541. doi: 10.1007/s00280-008-0836-1. [DOI] [PubMed] [Google Scholar]
- 14.Wu H. L., Deng J. J., Jiang Z. M., et al. A clinical observation of S-1 chemotherapy combined with radiotherapy for the treatment of advanced nasopharyngeal carcinoma. Journal of Modern Oncology. 2012;20:2498–2500. [Google Scholar]
- 15.Chen M., Su Z. Clinical observation of combination lobaplatin and S-1 concurrent chemoradiotherapy for treatment of loco-regionally advanced nasopharyngeal carcinoma. World Latest Medicine Information. 2016;16:16–19. [Google Scholar]
- 16.Zhu M. Q., Xia J. X., Xu M., et al. Efficacy and safety of S-1 in elderly patients with advanced nasopharyngeal carcinoma. Chinese Journal of Medical Guide. 2014;16:107–110. [Google Scholar]
- 17.Fang W. D., Chen C. N., Zhang C., et al. Effect of oral low-dose gimeracil and oteracil potassium capsules on advanced colorectal cancer giving up intravenous chemotherapy. Journal of Modern Oncology. 2011;19:1823–1825. [Google Scholar]
- 18.Lv T., Wang Y., Ou D., et al. IMRT combined with S-1 concurrent chemoradiotherapy in locally advanced nasopharyngeal carcinoma: a prospective phase II study. Investigational New Drugs. 2019;37(2):352–359. doi: 10.1007/s10637-018-00720-0. [DOI] [PubMed] [Google Scholar]
- 19.You C. W., Wen L. C. S-1 combined with concurrent radiotherapy for elderly patients with locally advanced nasopharyngeal carcinoma. Chinese Journal of Clinical Oncology and Rehabilitation. 2012;19:443–445. [Google Scholar]
- 20.Guo Q., Chen M., Xu H., et al. Oral maintenance chemotherapy using S-1/capecitabine in metastatic nasopharyngeal carcinoma patients after systemic chemotherapy: a single-institution experience. Cancer Management and Research. 2020;12:1387–1396. doi: 10.2147/cmar.s234271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tahara M., Kiyota N., Mizusawa J., et al. Phase II trial of chemoradiotherapy with S‐1 plus cisplatin for unresectable locally advanced head and neck cancer ( JCOG 0706) Cancer Science. 2015;106(6):726–733. doi: 10.1111/cas.12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Xian F., Hu X., Zhang Q., et al. Comparison of radiotherapy respectively combined with docetaxel, cisplatin, or S-1 in the treatment of locally advanced nasopharyngeal carcinoma. Journal of Modern Oncology. 2019;27:2852–2855. [Google Scholar]
- 23.Zhang S., Zhou L., Huang X., Lin S. A retrospective study of concurrent chemoradiotherapy plus S-1 adjuvant chemotherapy on curative effect for treatment of patients with N3 stage nasopharyngeal carcinoma. Cancer Management and Research. 2018;10:1705–1711. doi: 10.2147/cmar.s165804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fengtong J., Jiangtao F., Yating W., Lili W., Jianbo C., Xiaofei W. Effects of S-1 combined with radiotherapy in the treatment of nasopharyngeal cancer: a meta-analysis based on randomized controlled trials. Open Medicine. 2017;12(1):107–114. doi: 10.1515/med-2017-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins J., Green S. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0. Hoboken, NJ, USA: Wiley; 2011. http://www.cochrane.org/resources/handbook. [Google Scholar]
- 26.Moher D., Liberati A., Tetzlaff J., Altman D. G. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA Statement. BMJ. 2009;339(jul21 1):p. b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Li Z. Y., Sha D., Liu S. X., Han Y. C. S-1 joint synchronization efficacy of radiation therapy for patients with locally advanced nasopharyngeal carcinoma. Chinese Journal of Cancer Prevention and Treatment. 2013;20:1825–1827. [Google Scholar]
- 28.Cai Y. P., Xu S., Lin S. J. Comparative analyses of curative effect of SP and FP regimen chemotherapy in the treatment of advanced nasopharyngeal carcinoma. China Practical Medical. 2014;9:40–41. [Google Scholar]
- 29.Chen L. J., Ren M. Z., Yuan D. F. Curative effect of intensity-modulated radiotherapy combined with tegafur, gimeracil and oteracil potassium capsule for treatment of elderly patients with localized advanced nasopharyngeal carcinoma. Journal of Xinxiang Medical University. 2017;34:532–534. [Google Scholar]
- 30.Chen H. Effect of S-1 combined with docetaxel in the treatment of patients with advanced nasopharyngeal carcinoma. Medical Equipment. 2018;31 [Google Scholar]
- 31.He B. Y. Effect observation of radical radiotherapy combined with S-1 and radical radiotherapy alone in the treatment of early nasopharyngeal carcinoma. Modern Diagnosis and Treatment. 2015;26:5329–5330. [Google Scholar]
- 32.Huang N. X., Guo H. Z., Tang Z. M. Recent curative effect and adverse reaction of S-1 and cisplatin concurrent chemoradiotherapy in the treatment of IMRT III and IV for stage a nasopharyngeal carcinoma. Heilongjiang Medical Journal. 2019;32:264–266. [Google Scholar]
- 33.Liu P. J. Effect of intensity modulated radiotherapy combined with S-1 on local advanced nasopharyngeal carcinoma in the elderly. The Journal of Psychology. 2018;238 [Google Scholar]
- 34.Liu L., Xiao F., Zhang L., et al. The short-term clinical efficacy and safety of docetaxel combined with cisplatin and S-1 in locally advanced nasopharynx cancer. Anti-Tumor Pharmacy. 2019;9:72–75. [Google Scholar]
- 35.Mutaxi P. Short-term efficacy and toxicity of S-1 combined with radical radiotherapy and radical radiotherapy for early nasopharyngeal carcinoma. World Latest Medicine Information. 2014;18:125–128. [Google Scholar]
- 36.Fan Q., Zhou J. L., Zhang Y. X., Wu J. The short-terms effects and adverse reactions of IMRT chemotherapy with S-1 and cisplatin in the treatment of III, IV a phase nasopharyngeal cancer. Electronic Journal of Clinical Medical Literature. 2019;697:39–40. [Google Scholar]
- 37.Wang Z. W., Ma X. L., Wang B. N., Ren H. T., Wang Y. L. Effect of S-1 concurrent chemoradiotherapy on locally advanced nasopharyngeal cancer. Journal of Modern Oncology. 2014;22:2827–2829. [Google Scholar]
- 38.Wang Y. Application of S-1 in maintenance therapy after first-line treatment in patients with advanced nasopharyngeal carcinoma. Shenzhen Journal of Integrated Traditional Chinese and Western Medicine. 2018;28:175–176. [Google Scholar]
- 39.Yang J., Li X. J., Ren Y. M., Li L. Comparison of S-1 combined with radical radiation therapy and single radical radiation therapy in treatment of early nasopharyngeal carcinoma. Journal of Kunming Medical College. 2012;33:93–96. [Google Scholar]
- 40.Yang F., Jiang X. P., Chen T. B., Hao P. Effect of tegafur combined with simultaneous integrated boost intensity modulated radiotherapy on local advanced nasopharyngeal carcinoma in the elderly and on immune function. Geriatrics Health Care. 2019;25:658–664. [Google Scholar]
- 41.Yu H. Q., Wu J., Pang S. Q. Clinical observation of docetaxel combined with S-1 in the treatment of 3 cases in advanced nasopharyngeal carcinoma. Chinese Journal of Ethnomedicine and Ethnopharmacy. 2016;25:72–73. [Google Scholar]
- 42.Zheng Z. H., Luo W. J., Liang S. B. The clinical effect of S-1 reverse intensity modulated radiotherapy on advanced nasopharyngeal carcinoma in the elderly. Contemporary Medicine. 2015;21 [Google Scholar]
- 43.Zou Y. Clinical observation of nedaplatin combined with S-1 in the treatment of patients with distant metastatic nasopharyngeal carcinoma. Chinese Journal of Primary Medicine and Pharmacy. 2015;10 [Google Scholar]
- 44.Qin A. Y., Ren T. J. The effect of S-1 combined with docetaxel in the treatment of patients with advanced chronic nasopharyngeal carcinoma. Chronic Pathematology Journal. 2019;2007:1078–1079. [Google Scholar]
- 45.Zheng M. H. Meta-analysis Software Application and Case Analysis. 1st. Beijing, China: People’s Medical Publishing House; 2013. pp. 95–101. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Basic characteristics of the included studies. Table S2. PRISMA checklist. Table S3. Sensitivity analysis for complete remission. Table S4. Search strategies. Figure S1. Results of the risk bias assessment of 25 RCTs (EPS 4462 kb).
Data Availability Statement
The data used to support the findings of this study are included within the article and supplementary information files.


