ABSTRACT
A growing body of literature examines the potential benefits of a time-based diet strategy referred to as time-restricted eating (TRE). TRE, a type of intermittent fasting, restricts the time of eating to a window of 4–12 h/d but allows ad libitum intake during eating windows. Although TRE diets do not overtly attempt to reduce energy intake, preliminary evidence from small studies suggests that TRE can lead to concomitant reduction in total energy, improvements in metabolic health, and weight loss. Unique features of the TRE diet strategy may facilitate adherence and long-term weight loss maintenance. In this Perspective, we explore the potential multilevel (i.e., biological, behavioral, psychosocial, environmental) facilitators and barriers of TRE for long-term weight loss maintenance in comparison with the more commonly used diet strategy, caloric restriction (CR). Compared with CR, TRE may facilitate weight loss maintenance by counteracting physiological adaptations to weight loss (biological), allowing for usual dietary preferences to be maintained (behavioral), preserving executive functioning (psychosocial), and enabling individuals to withstand situational pressures to overeat (environmental). However, TRE may also pose unique barriers to weight loss maintenance, particularly for individuals with poor baseline diet quality, internal or social pressures to eat outside selected windows (e.g., grazers), and competing demands that interfere with the scheduling of eating. Future studies of TRE in free-living individuals should consider the multiple levels of influence impacting long-term adherence and weight loss maintenance. Ultimately, TRE could be one strategy in a toolkit of tailored diet strategies to support metabolic health and weight loss maintenance.
Keywords: TRE, CR, intermittent fasting, adherence, obesity, ADOPT Framework, chronomedicine
Introduction
An estimated 72% of US adults have overweight or obesity (1), and it is projected that by the year 2030, the prevalence of adult obesity (BMI ≥30 kg/m2) will increase from 40% to nearly 50% (2). Additionally, the vast majority (87%) of US adults exhibit metabolic dysfunction (3), which is strongly linked to obesity. The increasing prevalence of obesity and metabolic dysfunction is concerning, because obesity is linked to chronic diseases such as diabetes, cardiovascular disease (4), and several cancers (5). Although a variety of behavioral strategies can effectively promote initial weight loss, long-term weight loss maintenance remains a challenge (6) because of difficulties with long-term adherence (7, 8). It is estimated that only 1 in 5 individuals with overweight or obesity who lose ≥10% of their body weight successfully maintain their reduced weight status for ≥1 y (9). In contrast, most individuals go on to regain or surpass initial weight, leading to further compromised metabolic health (10). Therefore, identifying behavioral strategies to improve weight loss maintenance is an important public health priority (6).
The standard diet strategy for weight loss and maintenance is caloric restriction (CR), defined as a sustained daily reduction of calories. Despite its utility for initial weight loss, long-term CR is not a successful weight loss maintenance strategy for many, due to low long-term adherence rates even among motivated individuals. Difficulty sustaining weight loss over time can be attributed to multiple influences, including biological (e.g., hormonal changes), behavioral (e.g., increased caloric intake), psychosocial (e.g., elevated food reward), and environmental (e.g., ubiquity of high-calorie foods) (6, 11). Because one of the best predictors of long-term weight loss maintenance is shorter-term adherence to a chosen weight loss strategy (8), additional approaches are needed to improve adherence for those unable or unwilling to follow the CR diet strategy.
A small but growing body of literature examines the potential benefits of a time-based diet strategy referred to as time-restricted eating (TRE). TRE, a type of intermittent fasting, consists of a primary behavioral change to restrict the time of eating to a daily window of 4–12 h. Compared with CR, TRE allows for ad libitum intake during eating windows, with no overt restrictions on total energy or diet quality (12). TRE attempts to improve circadian rhythmicity and optimize metabolic function through a consistent daily eating schedule that is consolidated and restricted to the biological day (12). TRE can reduce energy intake and promote weight loss even without overt attempts to restrict energy (13–15). Several studies also suggest that TRE leads to improvements in metabolic health (15, 16) that are independent of weight loss (17). Existing studies of TRE in humans are characterized by small sample sizes, lack of a control group, a focus on overweight/obese populations, and relatively short study durations; because of this, the research community has called for rigorous trials to assess the efficacy and sustainability of TRE as a long-term behavioral strategy (12). The growing focus on understanding how TRE and other time-based diet strategies can impact health is also highlighted by the recently released 2020–2030 Strategic Plan for NIH Nutrition Research (18).
This Perspective explores the key features and potential facilitators and barriers of TRE compared with CR to consider its viability as a sustainable strategy for weight loss maintenance. To do so, this article considers the interrelated constructs associated with both diet strategies across multiple domains (e.g., biological to environmental) that might lead to differential effects on adherence and long-term weight loss maintenance. We conclude with a summary of practical considerations and recommendations for future research to investigate how TRE diet strategies might be used independently or as a complementary approach to facilitate long-term weight loss maintenance.
Key Features of TRE
Key features of the TRE diet strategy can be perceived as simpler or more flexible compared with CR (Table 1). These features can equip many individuals to successfully adhere to a TRE diet for a longer time period compared with a CR diet, facilitating long-term weight loss maintenance. For example, whereas CR requires individuals to change their usual diet (i.e., caloric intake and/or diet quality), TRE allows individuals to maintain their usual diet within eating windows. As a result, a TRE diet may require relatively lower cognitive effort compared with a CR diet, because individuals do not have to monitor what they are eating each day. Conversely, another feature of the TRE diet is that individuals are required to change their usual timing of eating. Compared with a CR diet, this may result in relatively greater cognitive effort to monitor and restrict eating during the fasting windows, which might present conflicts for scheduling meals. These features and their expected impact on adherence are discussed in more detail in the following sections.
TABLE 1.
Key features of time-restricted eating (TRE) and caloric restriction (CR) diet strategies and hypothesized implications for adherence to TRE
| Key features of diet strategies | Hypothesized implications for adherence | |
|---|---|---|
| TRE | CR | Compared with CR, TRE might result in |
| Maintain usual diet within eating windows | Change usual diet (caloric intake and/or diet quality) |
|
| Track and limit daily eating window | Track and limit daily caloric intake |
|
| Change usual timing of eating | Maintain usual timing of eating |
|
| Alternate periods of ad libitum eating with fasting | Maintain continuous caloric deficit |
|
Conceptual model
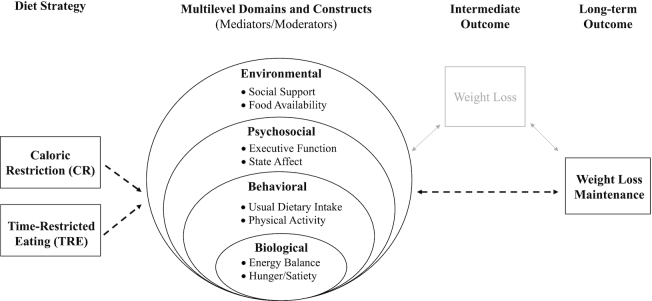
Weight loss maintenance is affected by a complex interplay of factors. We propose that the unique features of the TRE diet can influence and be influenced by a variety of constructs (e.g., executive function) within multilevel domains (e.g., psychosocial) and those constructs can interact with one another to impact long-term weight loss maintenance (Figure 1). The selected domains and constructs, inspired by the ADOPT (Accumulating Data to Optimally Predict Obesity Treatment) Core Measures Project (11), are not meant to be exhaustive, but are used to highlight constructs known or hypothesized to be differentially related to the 2 diet strategies.
FIGURE 1.
Conceptual model of multilevel domains (e.g., psychosocial) known to influence weight loss maintenance, and highlighted constructs (e.g., executive function) within each domain. Caloric restriction (CR) and time-restricted eating (TRE) diet strategies intersect (i.e., as mediators or moderators) with multiple domains of influence to act as facilitators or barriers of long-term weight loss maintenance.
Biological Domain
Energy balance
A key driver of initial weight loss is negative energy balance, which results from decreased energy intake and maintained/increased energy expenditure. Sustained reduction of energy intake often results in short-term weight loss (i.e., in the initial 6 mo of intervention) (6) followed by diminished weight loss or weight regain, due to physiological adaptations (e.g., homeostatic controls) and changes to body composition (19).
In individuals following a CR diet, energy expenditure decreases over time due to changes in metabolic function, meaning that even with stable energy intake, energy expenditure declines (9, 20, 21). This decline can occur through decreases in both resting energy expenditure (e.g., adaptive thermogenesis) and nonresting energy expenditure (e.g., reductions in energy use for daily living) (20). Additionally, CR results in changes to body composition (22), and more restrictive CR can lead to more weight lost in the form of fat-free mass (i.e., muscle), which decreases metabolic rate and energy expenditure. Together, loss of fat-free mass and homeostatic responses to the reduced weight state require increased efforts to maintain weight loss over time, countering long-term weight loss maintenance.
TRE may have unique effects on both sides of the energy balance equation. Alternating periods of energy restriction (i.e., fasting) with periods of energy balance (i.e., eating) may counteract the homeostatic physiological adaptations to weight loss that favor weight regain (23). This might be partially driven by changes in body composition, such as reductions in fat mass and preservation of fat-free mass (16, 24–27). For example, in a small randomized trial (n = 34) of weight training in young men, a shorter eating window (8 compared with 12 h) resulted in decreased fat mass and preserved fat-free mass (26). Additionally, despite ad libitum intake during eating windows, many individuals following TRE have been observed to unintentionally decrease energy intake relative to baseline levels (13, 14, 24). In general, individuals following a TRE diet have been observed to reduce their energy intake by ∼500 kcal/d (28). Yet, because TRE does not dictate total energy or diet quality, it is possible that individuals who increase their energy intake from baseline levels might gain weight using TRE. Additionally, it is unknown how a restricted eating window might impact nutrient absorption in humans, which could have important implications for energy balance and/or nutrient deficiency. Taken together, additional research is needed to understand the effects of TRE on biological processes underlying energy balance and its ability to promote more sustainable weight loss maintenance.
Hunger/satiety
Biological hunger and satiety are key components affecting weight loss maintenance. In the context of a diet, elevated biological hunger cues can lead to feelings of deprivation and urges to eat, which can undermine dietary adherence (20, 29, 30).
One hallmark of continuous CR is increased biological appetitive signals and decreased satiety signals (20, 30). Specifically, CR reduces the satiety hormone leptin and increases the hunger hormone ghrelin, thereby increasing hunger. This increased biological hunger in the presence of restricted energy intake can create a physiological barrier to weight loss maintenance by increasing the likelihood of dietary lapse (i.e., overconsumption of calories). There is evidence that these metabolic hormonal changes can persist for >1 y (19), posing a significant barrier to long-term weight loss maintenance.
Preliminary evidence from small observational studies and randomized controlled trials suggests that TRE may favorably impact hunger and satiety hormones. Prolonged daily fasting can promote lower and more stable biological hunger levels compared with eating throughout an extended period of the day. For example, compared with a 12-h control condition, 6-h TRE (eating from 08:00 to 14:00) led to reduced mean ghrelin concentrations, higher daytime satiety hormone expression, and lower diurnal amplitude (variability) in hunger (31). Biological hunger/satiety cues that favor a negative or neutral energy balance might make long-term weight loss maintenance more sustainable with TRE compared with CR.
Behavioral Domain
Usual dietary intake
Dietary intake patterns are often habitual and ingrained (32, 33) The overall complexity of a new diet strategy, including the degree of change required to conform, can impact adherence and weight loss maintenance (34).
In CR, the rule rigidity and potential complexity of restricting calories and/or changing diet quality can be a barrier to adherence (35). CR often requires calorie counting and specialized low-calorie foods or meal replacements. During weight loss initiation, an individual might elect to purchase prepackaged low-calorie foods to consume at home. During the maintenance phase of weight loss, adherence to CR can diminish, as individuals become increasingly exposed to foods outside their controlled diet strategy regimen or controlled eating environment (36). CR diets that require individuals to avoid habitually consumed foods, maintain a high degree of dietary complexity, or significantly alter their usual macronutrient profile can limit adherence and negatively impact long-term weight loss maintenance.
Conversely, TRE can be conceptualized as a relatively less complex diet strategy. It consists of 1 primary behavior change principle (i.e., limiting daily eating time), requires relatively fewer decision points, and promotes a consistent daily behavioral pattern, which can be important for encouraging behavior change maintenance (8, 9). By minimizing complexity and allowing usual intake to persist within eating windows, TRE can address many of the behavioral challenges of CR and promote both dietary satisfaction and long-term adherence (8). However, scheduling of meals within a limited window of time can present an additional barrier unique to TRE, especially for individuals who habitually eat during a longer daily window. Additionally, it is important to note that poor diet quality can limit the effectiveness of the TRE diet, if individuals consume the same types and amounts of low-nutrient, high-fat foods as prior to beginning the TRE diet.
Physical activity
An individual's ability to adhere to a diet strategy for weight loss maintenance can differ based on habitual levels of physical activity. Regular participation in planned physical activity is a predictor of long-term weight loss maintenance. For example, a group of >4000 individuals maintaining >30 lb (13.6 kg) weight loss for a minimum of 1 y in the National Weight Control Registry reported an average of 1 h/d of moderate to vigorous physical activity, suggesting that regular engagement in physical activity supports long-term weight loss maintenance (9).
One influence on adherence to a diet strategy for weight loss maintenance can be energy needs. CR can lead to compensatory reductions in energy expenditure in the form of lower physical activity levels (20). Because of this, CR can be more challenging for an active person to adhere to in the long term.
Conversely, TRE may be easier to adhere to, given that eating and fasting windows can shift to accommodate an individual's physical activity schedule. Energy intake and diet quality can also be tailored to support energy needs. Studies by Tinsley et al. (27, 37) suggest that TRE might not adversely affect physical activity if adequate protein and total energy are consumed within designated eating windows. Thus, compared with CR, the flexibility of TRE can allow for the compensation of macronutrients as needed to facilitate and/or maintain an individual's physical activity levels.
Psychosocial Domain
Executive functioning
Executive function is an important predictor of goal-oriented behaviors requiring planning, self-control, and inhibition (38–40). Adequate executive function is essential for self-monitoring and problem solving (38), 2 skills necessary for successful weight loss, whereas low levels of executive function predict impulsivity and poor self-control (39). Executive function is also theorized to be associated with long-term weight loss maintenance (41), because reduced executive function can decrease dietary control (42) and lead to lapses in dietary adherence.
CR requires consistent executive function engagement to plan meals, track calories, self-monitor, and inhibit excess caloric intake (43). Given the complexity of many CR diets, adherence can come with associated costs to executive function such as decreased attention and self-control in domains unrelated to diet (e.g., work, relationships, physical exercise regimens) (41).
Although TRE also requires consistent planning, self-monitoring, and inhibitory behaviors, these may be less cognitively taxing given the flexibility of ad libitum intake of usual diet during eating windows. An individual following TRE does not have to monitor what or how much is consumed during an eating window, thus requiring relatively lower levels of executive function compared with CR. (43) Additionally, there is evidence that extended fasting can lead to an evolutionarily adaptive response of increased brain-derived neurotrophic factor expression (44), which is associated with improved executive function (45). Thus, adherence to TRE might be less dependent on executive function, whereas insufficient executive function for those engaging in CR can lead to dietary lapses, ultimately limiting weight-loss maintenance.
State affect
Emotions, or transient affective states, both influence and are influenced by dietary intake (46). Experimentally induced positive affect leads to increased eating, particularly for emotional eaters (47). Additionally, momentary negative affect (48) predicts dietary lapses, and negative mood is linked to binge eating (49). Among overweight and obese individuals in particular, both positive and negative affective states are associated with dietary lapses (50). Therefore, affective states are important to consider in the contexts of CR and TRE diet strategies and long-term weight loss maintenance.
In CR diets, positive and negative affect have been demonstrated to predict dietary lapses (47, 50). However, the impact of CR on state affect is less clear. Although 1 study found that negative mood states decreased after 24 mo of CR in adults without obesity (51), another study implementing a CR intervention in older adult women with obesity did not find any changes to negative or positive affect at weeks 8 and 12 of follow-up (52). More acute episodes of hunger resulting from the onset of CR diets have the potential to result in increased negative affect. Thus, depending on the length of a CR diet intervention and length of time following the diet, state affect can differ.
Although allowing ad libitum intake during eating windows can obviate the idea of dietary lapses in a traditional sense (i.e., overconsumption of calories), specific types of negative affect (e.g., boredom and stress) associated with eating at unintended times (53) might make TRE dieters uniquely vulnerable to dietary lapses. Additionally, affective states resulting from acute hunger induced by the onset of a CR diet compared with hunger induced from a fasting episode in a TRE diet might also differ. Preliminary evidence suggests that negative side effects (e.g., irritability), which have been observed to persist for the first few weeks of initiating an intermittent fasting diet (23), might not occur in the context of a TRE diet (28). Nevertheless, it can be beneficial to inform individuals of potential transient negative side effects in an effort to encourage adherence and satisfaction with a TRE diet. The clear boundaries of self-selected eating and fasting times with TRE might protect against such dietary lapses resulting from affective changes and enable individuals to remain resilient to emotional cues to eat. Overall, additional research is needed to better determine the impact of TRE on state affect.
Environmental Domain
Social support
Social support is associated with adherence to weight-loss interventions (54), and evidence suggests that dietary behaviors and BMI can be transmissible among individuals who share a social network (55–57). Therefore, diet strategies that incur support from friends and family and that promote adherence long enough to diffuse to other network members are more likely to be adopted and sustained by individuals and populations.
An essential barrier to CR is a lack of social norms supporting reduced energy intake (57). For example, research indicates that eating in the presence of others results in greater energy intake (58), indicating that the social setting can act as a barrier to adherence in individuals following a CR diet. Furthermore, evidence of increased average portion sizes over time showcases the ability for consumption norms to diffuse throughout social networks, leading to increased energy intake at a population level (59, 60).
Social support is also an important component for adherence to TRE diets. For example, whereas individuals following a TRE diet might prefer to schedule their eating window earlier in the day, parents or caregivers might find it challenging to fast in the evenings when their dependents or other family members need to eat. Furthermore, a recent pilot study found that evening social activities were perceived as conflicting with the TRE diet (14). However, social support can also provide unique facilitators to TRE adherence. For example, whereas CR requires maintaining a continuous energy deficit, the comparatively flexible characteristics of TRE can allow an individual to plan eating windows and meals around family needs or social activities, with unrestricted ad libitum intake permitted during these events.
Food availability
An individual's ability to maintain a healthy weight is influenced by their built environment (61, 62). For example, supermarket access and use is correlated with greater fruit and vegetable consumption (62), and perceived food availability has been consistently shown to correlate with healthy diet (e.g., fruit and vegetable intake, diet quality indices) (62). It is hypothesized that omnipresent food cues, particularly high-fat and high-calorie foods, stimulate psychological desires for food in the absence of hunger (63), requiring individuals to exert continuous cognitive effort to adhere to a diet strategy.
CR diets often require specific foods, such as fresh produce, low-calorie foods, or meal replacements, and in order to adhere, individuals must locate, purchase, prepare, and consistently consume these items. Objective and perceived lack of availability of these foods can challenge CR adherence. Individuals residing in resource-poor neighborhoods are likely to be particularly vulnerable to adherence issues, because such neighborhoods often have lower availability of fresh produce. Simultaneously, the perceived availability of “forbidden” (e.g., high-calorie) foods in an individual's environment can negatively impact adherence, because environmental food cues can trigger food cravings and result in dietary lapse (64).
The flexibility of the TRE diet might lend itself to dietary adherence in individuals who live or work in environments with salient food cues. In TRE, because ad libitum intake is prescribed during eating windows, an individual's usual food environment is not in conflict with TRE during eating windows. However, food availability can still play a role and even become a barrier to adherence during fasting windows, when an overabundance of salient food cues can contribute to lapses (64, 65). This emphasizes the potential utility of a simple decision rule (“I'm not eating now”) as a strategy to decrease reliance on executive function while navigating obesogenic food environments. Alternatively, an individual practicing TRE could choose to avoid salient food environments during the non-eating window or shift the fasting window to coincide with times when food cues are less abundant or when hunger is diminished. It is important to note that not all individuals are able to make such adjustments to their daily schedule (e.g., fast-food workers) and some might experience a greater cognitive challenge during the fasting window.
Discussion
In this article we have explored how a TRE diet might uniquely facilitate or act as a barrier to long-term weight loss maintenance compared with a CR diet. We examined each diet strategy in relation to 4 domains of influence (i.e., biological, behavioral, psychosocial, and environmental), highlighting constructs known or hypothesized to differentially influence long-term weight loss maintenance. Taken together, many of the constructs discussed suggest that a TRE diet strategy can pose considerable advantages over CR for promoting long-term weight loss maintenance. However, there are also several potential barriers to adherence using a TRE diet strategy that should be considered in future studies. The preceding sections call forth important considerations and recommendations for future research, which are described below.
Gradients of intensity of intervention
The inherently varying degrees of intensity within CR and TRE diets can act as facilitators or barriers to adherence. For example, in CR, the target caloric goal can range widely (e.g., 800 to 2000 kcal/d) depending on baseline weight, activity levels, and weight maintenance goals. With increasingly lower caloric intake goals, adherence can become increasingly challenging. Similarly, in TRE, the eating window can range from as short as 4 h to as long as 10–12 h. It is likely that the more restrictive the eating window, the more challenging it might be to adhere to a TRE diet; however the 1 published study in this area found no differences in adherence or weight loss between 4-h and 6-h TRE groups (28). A gradual, scaled approach to TRE, in which the eating window decreases over time, might help to optimize long-term adherence (23, 66). For individuals following a TRE diet for weight loss maintenance, the eating window can be adjusted in response to fluctuations in weight status, such as temporarily decreasing the eating window from 10 h to 8 h if significant weight gain has occurred. Future research should consider how varying the intensity of the eating window might help to improve adherence to a TRE diet.
Balancing competing factors for optimized adherence
An individualized approach could be required to understand the relative importance and challenges of following a TRE diet and to balance restrictiveness with feasibility to promote weight loss maintenance. For example, evidence suggests that an early TRE window (e.g., 10:00–18:00) can be more beneficial for weight loss and metabolic health than a late TRE window (e.g., 12:00–20:00) due in part to alignment with circadian rhythms in metabolism. However, for many individuals, early TRE can be difficult to adhere to, whereas late TRE might be more feasible for adherence due to alignment of eating with family and social needs. This represents a tension between biological and environmental domains that can ultimately impact an individual's ability to adhere to the TRE diet long term. The balance of these competing demands is an important topic for future research.
Combining TRE with other diet strategies
Given that there is a great degree of interindividual variability in responses to obesity treatments (11), it is likely that there will also be a large degree of interindividual variability in how specific domains and constructs impact adherence to both TRE and CR diets. Combining multiple behavioral strategies for weight management to address varying stages of change (e.g., weight loss, weight maintenance) could yield beneficial outcomes. Because CR has been demonstrated to be highly effective for initial weight loss yet less effective for long-term weight loss maintenance, CR could be recommended or prescribed for initial weight loss and TRE could be incorporated when a goal weight is achieved. For example, initial (first 1–6 mo) weight loss could focus on reducing energy intake and improving diet quality, whereas later stages (6–12 mo) could build upon this new baseline diet by gradually restricting the daily eating window while decreasing reliance on calorie counting. This tailored, 2-stage approach that combines varying gradients of TRE and CR across weight loss and maintenance could leverage both diet strategies to support weight goals. The layering of TRE with existing behavioral strategies for obesity treatment is an area meriting future study. Ultimately, TRE could be one strategy in a toolkit of personalized approaches to nutrition (67), which can be used alone or in conjunction with other strategies to support weight maintenance and overall health.
Measurement priorities
Given the relative youth of the body of literature examining TRE in humans, future studies should attempt to identify and empirically test constructs within the biological, behavioral, psychosocial, and environmental domains that might influence long-term adherence to this diet strategy. These multilevel domains of influence should be considered in the design and implementation of time-based dietary interventions for weight management to facilitate cross-study comparisons and improve measurement methodologies. Identification of stable (i.e., trait) constructs (e.g., eating in anticipation of future hunger) determined to be important facilitators or barriers to a TRE diet could be used to screen individuals for likelihood of adherence. This information can be used to tailor TRE diet features, such as the duration and timing of the eating window, to produce the greatest health benefits while minimizing the degree of conflict between TRE and an individual's psychological, social, and environmental needs and preferences. Additionally, the use of ecological momentary assessment or other real-time data capture strategies to identify dynamic, time-varying (i.e., state) constructs that facilitate or impede adherence to TRE can help to identify and address contextual factors linked to diminished adherence.
Scalability and public health utility
A primary advantage of the TRE diet is its relative simplicity as a dietary intervention. TRE does not require specialized equipment or knowledge and can be successfully administered within primary care or clinic settings (23). These features can improve the scalability and feasibility of using TRE for weight management and metabolic health in low-income and low-resource populations, who are disproportionately impacted by obesity and metabolic disease yet have the poorest access to preventive services. Overall, there appears to be growing interest among public health practitioners and the general public for chronomedicine or circadian medicine (68) approaches to health promotion such as TRE. Future studies should consider how TRE could be implemented within high-risk populations in a cost- and resource-effective manner.
A deeper understanding of the multilevel factors influencing adherence to the TRE diet can inform interventions leveraging TRE for weight-loss maintenance (66). Ultimately, time-based diet strategies such as TRE represent a novel and potentially sustainable diet strategy, which can be used individually or in combination with existing strategies to promote long-term weight loss maintenance.
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—SGO'C, PB, CPB, and MMS-W: conceptualized the article and drafted the manuscript; TA-C, KH, JR, ERS, and SMC: provided critical feedback in the development and refinement of the manuscript, including revision and editing of the manuscript; and all authors: read and approved the final manuscript.
Notes
The authors reported no funding received for this study.
Author disclosures: The authors report no conflicts of interest.
The opinions expressed in this article are the authors’ own and do not necessarily reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the US government.
Perspective articles allow authors to take a position on a topic of current major importance or controversy in the field of nutrition. As such, these articles could include statements based on author opinions or point of view. Opinions expressed in Perspective articles are those of the author and are not attributable to the funder(s) or the sponsor(s) or the publisher, Editor, or Editorial Board of Advances in Nutrition. Individuals with different positions on the topic of a Perspective are invited to submit their comments in the form of a Perspectives article or in a Letter to the Editor.
Contributor Information
Sydney G O'Connor, Behavioral Research Program, Division of Cancer Control & Population Sciences, National Cancer Institute, National Institutes of Health, Rockville, MD, USA.
Patrick Boyd, Behavioral Research Program, Division of Cancer Control & Population Sciences, National Cancer Institute, National Institutes of Health, Rockville, MD, USA.
Caitlin P Bailey, Behavioral Research Program, Division of Cancer Control & Population Sciences, National Cancer Institute, National Institutes of Health, Rockville, MD, USA.
Marissa M Shams-White, Epidemiology and Genomics Research Program, Division of Cancer Control & Population Sciences, National Cancer Institute, National Institutes of Health, Rockville, MD, USA.
Tanya Agurs-Collins, Behavioral Research Program, Division of Cancer Control & Population Sciences, National Cancer Institute, National Institutes of Health, Rockville, MD, USA.
Kara Hall, Behavioral Research Program, Division of Cancer Control & Population Sciences, National Cancer Institute, National Institutes of Health, Rockville, MD, USA.
Jill Reedy, Epidemiology and Genomics Research Program, Division of Cancer Control & Population Sciences, National Cancer Institute, National Institutes of Health, Rockville, MD, USA.
Edward R Sauter, Breast and Gynecologic Cancer Research Group, Division of Cancer Prevention, National Cancer Institute, National Institutes of Health, Rockville, MD, USA.
Susan M Czajkowski, Behavioral Research Program, Division of Cancer Control & Population Sciences, National Cancer Institute, National Institutes of Health, Rockville, MD, USA.
References
- 1. National Center for Health Statistics . Obesity and overweight. National Center for Health Statistics. 2016 [Internet] [cited 2020 April 17]. Available from: https://www.cdc.gov/nchs/fastats/obesity-overweight.htm. [Google Scholar]
- 2. Ward ZJ, Bleich SN, Cradock AL, Barrett JL, Giles CM, Flax C, Long MW, Gortmaker SL. Projected U.S. state-level prevalence of adult obesity and severe obesity. N Engl J Med. 2019;381(25):2440–50. [DOI] [PubMed] [Google Scholar]
- 3. Araujo J, Cai J, Stevens J. Prevalence of optimal metabolic health in American adults: National Health and Nutrition Examination Survey 2009–2016. Metab Syndr Relat Disord. 2019;17(1):46–52. [DOI] [PubMed] [Google Scholar]
- 4. Scherer PE, Hill JA. Obesity, diabetes, and cardiovascular diseases: a compendium. Circ Res. 2016;118(11):1703–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lauby-Secretan B, Scoccianti C, Loomis D, Grosse Y, Bianchini F, Straif K. Body fatness and cancer—viewpoint of the IARC working group. N Engl J Med. 2016;375(8):794–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. MacLean PS, Wing RR, Davidson T, Epstein L, Goodpaster B, Hall KD, Levin BE, Perri MG, Rolls BJ, Rosenbaum Met al. NIH working group report: innovative research to improve maintenance of weight loss. Obesity (Silver Spring). 2015;23(1):7–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sacks F, Bray GA, Carey VJ, Smith SR, Ryan DH, Anton SD, McManus K, Champagne CM, Bishop LM, Laranjo Net al. Comparison of weight-loss diets with different compositions of fat, protein, and carbohydrates. N Engl J Med. 2009;360(9):859–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Middleton KR, Anton SD, Perri MG. Long-term adherence to health behavior change. Am J Lifestyle Med. 2013;7(6):395–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wing RR, Phelan S. Long-term weight loss maintenance. Am J Clin Nutr. 2005;82:222S. [DOI] [PubMed] [Google Scholar]
- 10. Dandanell S, Skovborg C, Praest CB, Kristensen KB, Nielsen MG, Lionett S, Jorgensen SD, Vigelso A, Dela F, Helge JW. Maintaining a clinical weight loss after intensive lifestyle intervention is the key to cardiometabolic health. Obes Res Clin Pract. 2017;11(4):489–98. [DOI] [PubMed] [Google Scholar]
- 11. MacLean PS, Rothman AJ, Nicastro HL, Czajkowski SM, Agurs-Collins T, Rice EL, Courcoulas AP, Ryan DH, Bessesen DH, Loria CM. The Accumulating Data to Optimally Predict Obesity Treatment (ADOPT) core measures project: rationale and approach. Obesity (Silver Spring). 2018;26(Suppl 2):S6–S15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chaix A, Manoogian ENC, Melkani GC, Panda S. Time-restricted eating to prevent and manage chronic metabolic diseases. Annu Rev Nutr. 2019;39:291–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gill S, Panda S. A smartphone app reveals erratic diurnal eating patterns in humans that can be modulated for health benefits. Cell Metab. 2015;22(5):789–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Antoni R, Robertson TM, Robertson MD, Johnston JD. A pilot feasibility study exploring the effects of a moderate time-restricted feeding intervention on energy intake, adiposity and metabolic physiology in free-living human subjects. J Nutr Sci. 2018;7. [Google Scholar]
- 15. Kesztyus D, Cermak P, Gulich M, Kesztyus T. Adherence to time-restricted feeding and impact on abdominal obesity in primary care patients: results of a pilot study in a pre-post design. Nutrients. 2019;11(12):2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wilkinson MJ, Manoogian ENC, Zadourian A, Lo H, Fakhouri S, Shoghi A, Wang X, Fleischer JG, Navlakha S, Panda Set al. Ten-hour time-restricted eating reduces weight, blood pressure, and atherogenic lipids in patients with metabolic syndrome. Cell Metab. 2020;31(1):92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Sutton EF, Beyl R, Early KS, Cefalu WT, Ravussin E, Peterson CM. Early time-restricted feeding improves insulin sensitivity, blood pressure, and oxidative stress even without weight loss in men with prediabetes. Cell Metab. 2018;27(6):1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Institutes of Health . 2020–2030 Strategic plan for NIH nutrition research: a report of the NIH Nutrition Research Task Force. [Internet]. NIH; 2020; [cited 2020 Jun 15]. Available from: https://www.niddk.nih.gov/about-niddk/strategic-plans-reports/strategic-plan-nih-nutrition-research. [Google Scholar]
- 19. Sumithran P, Prendergast LA, Delbridge E, Purcell K, Shulkes A, Kriketos A, Proietto J. Long-term persistence of hormonal adaptations to weight loss. N Engl J Med. 2011;365(365):1597–604. [DOI] [PubMed] [Google Scholar]
- 20. Anastasiou CA, Karfopoulou E, Yannakoulia M. Weight regaining: from statistics and behaviors to physiology and metabolism. Metabolism. 2015;64(11):1395–407. [DOI] [PubMed] [Google Scholar]
- 21. Sainsbury K, Evans EH, Pedersen S, Marques MM, Teixeira PJ, Lahteenmaki L, Stubbs RJ, Heitmann BL, Sniehotta FF. Attribution of weight regain to emotional reasons amongst European adults with overweight and obesity who regained weight following a weight loss attempt. Eat Weight Disord. 2019;24(2):351–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rosenbaum M, Agurs-Collins T, Bray MS, Hall KD, Hopkins M, Laughlin M, MacLean PS, Maruvada P, Savage CR, Small DMet al. Accumulating Data to Optimally Predict Obesity Treatment (ADOPT): recommendations from the biological domain. Obesity. 2018;26(Suppl 2):S25–S34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. de Cabo R, Mattson MP. Effects of intermittent fasting on health, aging, and disease. N Engl J Med. 2019;381(26):2541–51. [DOI] [PubMed] [Google Scholar]
- 24. Gabel K, Hoddy KK, Haggerty N, Song J, Kroeger CM, Trepanowski JF, Panda S, Varady KA. Effects of 8-hour time restricted feeding on body weight and metabolic disease risk factors in obese adults: a pilot study. Nutr Healthy Aging. 2018;4(4):345–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Stote KS, Baer DJ, Spears K, Paul DR, Harris GK, Rumpler WV, Strycula P, Najjar SS, Ferrucci L, Ingram DKet al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;85:981–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Moro T, Tinsley G, Bianco A, Marcolin G, Pacelli QF, Battaglia G, Palma A, Gentil P, Neri M, Paoli A. Effects of eight weeks of time-restricted feeding (16/8) on basal metabolism, maximal strength, body composition, inflammation, and cardiovascular risk factors in resistance-trained males. J Transl Med. 2016;14(1):290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tinsley GM, Forsse JS, Butler NK, Paoli A, Bane AA, La Bounty PM, Morgan GB, Grandjean PW. Time-restricted feeding in young men performing resistance training: a randomized controlled trial. Eur J Sport Sci. 2017;17(2):200–7. [DOI] [PubMed] [Google Scholar]
- 28. Cienfuegos S, Gabel K, Kalam F, Ezpeleta M, Wiseman E, Pavlou V, Lin S, Oliveira ML, Varady KA. Effects of 4- and 6-h time-restricted feeding on weight and cardiometabolic health: a randomized controlled trial in adults with obesity. Cell Metab. 2020;32:366–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Hawks SR, Madanat HN, Christley HS. Behavioral and biological associations of dietary restraint: a review of the literature. Ecol Food Nutr. 2008;47(5):415–49. [Google Scholar]
- 30. Greenway FL. Physiological adaptations to weight loss and factors favouring weight regain. Int J Obes. 2015;39(8):1188–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ravussin E, Beyl RA, Poggiogalle E, Hsia DS, Peterson CM. Early time-restricted feeding reduces appetite and increases fat oxidation but does not affect energy expenditure in humans. Obesity. 2019;27(8):1244–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kumanyika SK, Van Horn L, Bowen D, Perri MG, Rolls BJ, Czajkowski SM, Schron E. Maintenance of dietary behavior change. Health Psychol. 2000;19(1):42–56. [DOI] [PubMed] [Google Scholar]
- 33. Leung AWY, Chan RSM, Sea MMM, Woo J. An overview of factors associated with adherence to lifestyle modification programs for weight management in adults. Int J Environ Res Public Health. 2017;14(8):922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Coelho do Vale R, Pieters R, Zeelenberg M. The benefits of behaving badly on occasion: successful regulation by planned hedonic deviations. J Consum Psychol. 2016;26(1):17–28. [Google Scholar]
- 35. Donnelly LS, Shaw RL, Pegington M, Armitage CJ, Evans DG, Howell A, Harvie MN. 'For me it's about not feeling like I'm on a diet': a thematic analysis of women's experiences of an intermittent energy restricted diet to reduce breast cancer risk. J Hum Nutr Diet. 2018;31(6):773–80. [DOI] [PubMed] [Google Scholar]
- 36. Moreira EA, Most M, Howard J, Ravussin E. Dietary adherence to long-term controlled feeding in a calorie-restriction study in overweight men and women. Nutr Clin Pract. 2011;26(3):309–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Tinsley GM, Moore ML, Graybeal AJ, Paoli A, Kim Y, Gonzales JU, Harry JR, VanDusseldorp TA, Kennedy DN, Cruz MR. Time-restricted feeding plus resistance training in active females: a randomized trial. Am J Clin Nutr. 2019;110(3):628–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Foster GD, Makris AP, Bailer BA. Behavioral treatment of obesity. Am J Clin Nutr. 2005;34(4):841–59. [DOI] [PubMed] [Google Scholar]
- 39. Lavagnino L, Arnone D, Cao B, Soares JC, Selvaraj S. Inhibitory control in obesity and binge eating disorder: a systematic review and meta-analysis of neurocognitive and neuroimaging studies. Neurosci Biobehav Rev. 2016;68:714–26. [DOI] [PubMed] [Google Scholar]
- 40. Crochiere RJ, Mangubat CJ, Manasse SM, Forman EM. Does executive function moderate the relation between momentary affective and physical states and subsequent dietary lapse? An EMA investigation. J Behav Med. 2019;42(6):1148–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Gettens KM, Gorin AA. Executive function in weight loss and weight loss maintenance: a conceptual review and novel neuropsychological model of weight control. J Behav Med. 2017;40(5):687–701. [DOI] [PubMed] [Google Scholar]
- 42. Hofmann W, Rauch W, Gawronski B. And deplete us not into temptation: automatic attitudes, dietary restraint, and self-regulatory resources as determinants of eating behavior. J Exp Soc Psychol. 2007;43(3):497–504. [Google Scholar]
- 43. Rothman AJ, Sheeran P, Wood W. Reflective and automatic processes in the initiation and maintenance of dietary change. Ann Behav Med. 2009;38(Suppl 1):S4–17. [DOI] [PubMed] [Google Scholar]
- 44. Mattson MP, Arumugam TV. Hallmarks of brain aging: adaptive and pathological modification by metabolic states. Cell Metab. 2018;27(6):1176–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Leckie RL, Oberlin LE, Voss MW, Prakash RS, Szabo-Reed A, Chaddock-Heyman L, Phillips SM, Gothe NP, Mailey E, Vieira-Potter VJet al. BDNF mediates improvements in executive function following a 1-year exercise intervention. Front Hum Neurosci. 2014;8:985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Macht M, Mueller J. Immediate effects of chocolate on experimentally induced mood states. Appetite. 2007;49(3):667–74. [DOI] [PubMed] [Google Scholar]
- 47. Bongers P, Jansen A, Havermans R, Roefs A, Nederkoorn C. Happy eating: the underestimated role of overeating in a positive mood. Appetite. 2013;67:74–80. [DOI] [PubMed] [Google Scholar]
- 48. Forman EM, Schumacher LM, Crosby R, Manasse SM, Goldstein SP, Butryn ML, Wyckoff EP, Graham Thomas J. Ecological momentary assessment of dietary lapses across behavioral weight loss treatment: characteristics, predictors, and relationships with weight change. Ann Behav Med. 2017;51(5):741–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Razzoli M, Pearson C, Crow S, Bartolomucci A. Stress, overeating, and obesity: insights from human studies and preclinical models. Neurosci Biobehav Rev. 2017;76(Pt A):154–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Carels RA, Douglass OM, Cacciapaglia HM, O'Brien WH. An ecological momentary assessment of relapse crises in dieting. J Consult Clin Psychol. 2004;72(2):341–8. [DOI] [PubMed] [Google Scholar]
- 51. Martin CK, Bhapkar M, Pittas AG, Pieper CF, Das SK, Williamson DA, Scott T, Redman LM, Stein R, Gilhooly CHet al. Effect of calorie restriction on mood, quality of life, sleep, and sexual function in healthy nonobese adults: the CALERIE 2 randomized clinical trial. JAMA Intern Med. 2016;176(6):743–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Prehn K, Jumpertz von Schwartzenberg R, Mai K, Zeitz U, Witte AV, Hampel D, Szela AM, Fabian S, Grittner U, Spranger Jet al. Caloric restriction in older adults—differential effects of weight loss and reduced weight on brain structure and function. Cereb Cortex. 2017;27(3):1765–78. [DOI] [PubMed] [Google Scholar]
- 53. Goldstein SP, Dochat C, Schumacher LM, Manasse SM, Crosby RD, Thomas JG, Butryn ML, Forman EM. Using ecological momentary assessment to better understand dietary lapse types. Appetite. 2018;129:198–206. [DOI] [PubMed] [Google Scholar]
- 54. Greaves CJ, Sheppard KE, Abraham C, Hardeman W, Roden M, Evans PH, Schwarz P, IMAGE Study Group . Systematic review of reviews of intervention components associated with increased effectiveness in dietary and physical activity interventions. BMC Public Health. 2011;11:119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Pachucki MA, Jacques PF, Christakis NA. Social network concordance in food choice among spouses, friends, and siblings. Am J Public Health. 2011;101(11):2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Christakis NA, Fowler JH. The spread of obesity in a large social network over 32 years. N Engl J Med. 2007;357(4):370. [DOI] [PubMed] [Google Scholar]
- 57. Higgs S, Thomas J. Social influences on eating. Curr Opin Behav Sci. 2016;9:1–6. [Google Scholar]
- 58. Patel KA, Schlundt DG. Impact of moods and social context on eating behavior. Appetite. 2001;36(2):111–8. [DOI] [PubMed] [Google Scholar]
- 59. Wansink B, Wansink CS. The largest Last Supper: depictions of food portions and plate size increased over the millennium. Int J Obes. 2010;34(5):943–4. [DOI] [PubMed] [Google Scholar]
- 60. Peter Herman C, Polivy J, Pliner P, Vartanian LR. Mechanisms underlying the portion-size effect. Physiol Behav. 2015;144:129–36. [DOI] [PubMed] [Google Scholar]
- 61. Travert AS, Sidney Annerstedt K, Daivadanam M. Built environment and health behaviors: deconstructing the black box of interactions—a review of reviews. Int J Environ Res Public Health. 2019;16(8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Caspi CE, Sorensen G, Subramanian SV, Kawachi I. The local food environment and diet: a systematic review. Health Place. 2012;18(5):1172–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Lowe MR, Levine AS. Eating motives and the controversy over dieting: eating less than needed versus less than wanted. Obes Res. 2005;13(5):797–806. [DOI] [PubMed] [Google Scholar]
- 64. Meule A, Lutz A, Vogele C, Kubler A. Food cravings discriminate differentially between successful and unsuccessful dieters and non-dieters. Validation of the Food Cravings Questionnaires in German. Appetite. 2012;58(1):88–97. [DOI] [PubMed] [Google Scholar]
- 65. Rejeski WJ, Burdette J, Burns M, Morgan AR, Hayasaka S, Norris J, Williamson DA, Laurienti PJ. Power of food moderates food craving, perceived control, and brain networks following a short-term post-absorptive state in older adults. Appetite. 2012;58(3):806–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Dashti HS, Scheer F, Saxena R, Garaulet M. Timing of food intake: identifying contributing factors to design effective interventions. Adv Nutr. 2019;10(4):606–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Adams SH, Anthony JC, Carvajal R, Chae L, Khoo CSH, Latulippe ME, Matusheski NV, McClung HL, Rozga M, Schmid CHet al. Perspective: guiding principles for the implementation of personalized nutrition approaches that benefit health and function. Adv Nutr. 2020;11(1):25–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Panda S. The arrival of circadian medicine. Nat Rev Endocrinol. 2019;15(2):67–9. [DOI] [PubMed] [Google Scholar]