ABSTRACT
Various global public health agencies recommend minimizing exposure to sweet-tasting foods or beverages. The underlying rationale is that reducing exposure to the perception of sweet tastes, without regard to the source of sweetness, may reduce preferences for sweetness, added sugar intake, caloric intake, and body weight. However, the veracity of this sequence of outcomes has yet to be documented, as revealed by findings from recent systematic reviews on the topic. Efforts to examine and document the effects of sweetness exposure are needed to support evidence-based recommendations. They require a generally agreed-upon methodology for measuring sweetness in foods, beverages, and the overall diet. Although well-established sensory evaluation techniques exist for individual foods in laboratory settings, they are expensive and time-consuming, and agreement on the optimal approach for measuring the sweetness of the total diet is lacking. If such a measure could be developed, it would permit researchers to combine data from different studies and populations and facilitate the design and conduct of new studies to address unresolved research questions about dietary sweetness. This narrative review includes an overview of available sensory techniques, their strengths and limitations, recent efforts to measure the sweetness of foods and diets across countries and cultures, and a proposed future direction for improving methods for measuring sweetness toward developing the data required to support evidence-based recommendations around dietary sweetness.
Keywords: sweetness, sensory measure, taste, diet, nutrition, sweetness exposure
Introduction
The WHO currently recommends that the intake of free sugars (defined as monosaccharides and disaccharides added to foods, plus sugars that are naturally present in honey, syrups, and fruit juices) be reduced to <10% of total energy intake (1, 2). This recommendation is based on moderate evidence from observational studies on risk of dental caries. However, overconsumption of sugar, a high-calorie, low-nutrient-density food ingredient, is widely assumed to contribute to obesity and associated health conditions through the energy it provides directly and, perhaps more important, also through enhancing the overall appeal of a broader portion of the diet. Thus, weight control is another presumed benefit of reduced sugar intake.
To assist in reducing sugar consumption, various governmental organizations currently recommend reducing the consumption of sweet-tasting foods and beverages, regardless of the source of the sweet taste (i.e., caloric and low-/no-calorie sweeteners) (2–6). The Cambridge English Dictionary (7) defines sweet as having a taste similar to that of sugar (not bitter or salty). The Oxford Learner's Dictionary (8) defines sweetness as the quality of being pleasant, or the quality of tasting or smelling sweet. Both a specific ingredient and a quality or sensation are elements of these various definitions, and both aspects appear to be important. From a physiological perspective, one could consider sweetness to be the generally appetitive sensation that arises when sugars or other sweet compounds stimulate specialized receptor proteins expressed in a subset of cells in taste buds. The rationale for these recommendations is the hypothesis that reduced exposure to sweetness will lead to reduced preferences for sweet-tasting foods and beverages, reduced preferences will lead to reduced consumption of sweet-tasting foods and beverages, and ultimately reduced consumption will decrease caloric intake and favor weight management. However, many links in this presumed causal chain still require empirical confirmation. Elsewhere, a substantial body of research demonstrates that a reduction in dietary sodium intake for a period of several months reduces the preferred saltiness of foods, facilitating the reduction of subsequent sodium intake in the entire diet (9, 10). Interestingly, exposure altered liking but not sensitivity ratings. These observations are referenced in support of the current CDC (11) and FDA (12) policies recommending gradual reductions in the sodium content of foods and the overall food supply. By analogy, it is assumed that parallel adjustments may also occur for sweetness, but empirical evidence to support this assumption is lacking. The situation is more complicated with sweet ingredients than with sodium, an ingredient that does not contribute to energy intake. Energy-yielding sweeteners may contribute positive energy balance via 2 primary mechanisms. First, processed sweet food may be energy dense, not so much because of the sugar itself but, rather, because sugar is often combined with fat, a primary driver of energy density. Small quantities of these products can contribute a disproportionate amount of energy. Second, in children and adults, 41% and 33%, respectively, of added sugars are obtained from sugar-sweetened beverages (13, 14), which are extremely energy dilute but are consumed in high quantities. Reductions in the preferred sweetness level of foods and beverages in the diet could modify the appeal of both types of products and help moderate energy intake and body weight.
It has been hypothesized that dietary exposure to sweetness influences the way individuals perceive foods and beverages, what and how much is consumed, and/or how the body processes and reacts to what is consumed (15–18). Indeed, an ability to detect and savor simple sugars, the major source of sweetness in nature, reflects the importance of glucose as the major energy source in humans and many other species (19). For example, a strong attraction to honey as a food source in many primates, particularly humans, is consistent with its high levels of glucose and fructose and hence honey's intense sweetness (20, 21).
Appleton et al. (15) conducted a systematic review of published data on the influence of dietary exposure to sweet-tasting foods or beverages on the subsequent generalized acceptance, preference, or choice of sweet foods and beverages in the diet. These studies provided no consistent support for a relation between sweet taste exposure and subsequent preferences or subsequent sweet food intake (15). Public Health England (4) reached a similar conclusion based on a literature review. Thus, empirical evidence about the relation between consumption of sweet-tasting foods and subsequent preferences for or consumption of sweet foods is lacking, as is evidence for the assumption that consumption of lesser amounts of sweet foods (apart from all other intake) will bring about decreased caloric intake and decreased body weight. Whether the incongruous responses to salt and sweet exposure reflect a true mechanistic difference is not known. However, it is notable that unlike the case with salt, preliminary evidence suggests that exposure effects for sweetness alter intensity ratings but not hedonic responses (22). If sweet intensity perception does not result in a shift in the preferred sweetness of foods, it is not clear that food choice will be altered.
Numerous studies over several decades have found that overweight and obese individuals have similar sweetness perception and preferences compared with normal-weight individuals (23–26), but WHO (1) found evidence from observational studies conducted in adults and children (moderate and low strength, respectively) for an association between body weight and intake of free sugars, primarily from sugar-sweetened beverages. Less is known about the association between the sweetness of the whole diet and energy intake and health-related outcomes.
Clearly, further work is needed to elucidate putative effects of dietary sweetness on preference for sweet foods, intake of sweet foods, body weight, and associated health outcomes. Critically, the perception of sweetness cannot be measured simply by quantifying concentrations of sugars in the diet and therefore must be measured by the experiences of human observers. First, a generally agreed-upon and validated measure of dietary sweetness is needed. Methods to measure the effects of sweetness on consumption have generally used a combination of measures including sweetness intensity or preference ratings of individual food items with a measure of food intake.
Given clear evidence that dietary patterns are relevant to long-term health, an effective measure would need to capture the sweetness of an entire diet or dietary pattern, not merely the sweetness of specific foods or food categories. The 2015–2020 Dietary Guidelines for Americans (27), as well as the Scientific Report of the 2020 Dietary Guidelines Advisory Committee (28), emphasize the importance of overall eating patterns for health compared with a focus on individual foods. This narrative review sets the stage for examination of 1) the current approaches for measuring perceived sweetness of individual foods and diets and 2) the extant databases related to the sweetness of whole diets. Based on this review, research questions are identified for improving the measurement of the perception of sweetness.
Available Methods to Measure Perception of Sweetness of Individual Foods and Diets
To support the collection of data on the relations between sweetness and diet, validated measures of sweetness are needed that are well accepted across the scientific and public health communities. The most relevant parameters of exposure remain unknown but may include mean sweetness across foods in the diet, maximum sweetness among all items consumed, and frequency with which people consume items of some minimum level of sweetness. Because the most important parameters are unknown, an ideal method would capture as much information as possible about the sensory properties of the diet. Ideally, the measurement method should 1) be easy for participants, experimenters, and possibly clinicians to complete; 2) be low cost; and 3) allow valid comparisons between groups of people with different cultures, numeracy, and literacy.
Sweetness measurement theory
Before discussing specific techniques to measure sweetness, it may be useful to consider a general concept from theory of measurement—namely that some measures provide more information than others. In 1946, Stevens (29) proposed a hierarchical framework to describe various levels of measurement and types of data: nominal, ordinal, interval, and ratio (Table 1). Ordered data (ordinal scale) provide a ranking but no indication of spacing between items. Interval data are equally spaced, but the zero value may not have a real meaning (e.g., 20°F is not twice as warm as 10°F). The most informative approach to measure perceived sweetness intensity of sweet substances is to use a ratio-level measurement with a true zero because this allows for meaningful ratios to be constructed and for more powerful statistical evaluation. For example, a liquid at 310 K does in fact have 14% more heat than a 273-K liquid because the zero is meaningful on a Kelvin scale. Only with ratio-level measurement can it be determined that, for example, a food assigned a number twice as high has twice the perceived sweetness. Why might this matter? As discussed previously, the most relevant parameters of exposure to sweetness remain unknown. The ability, for example, to perform valid calculations of the ratio of minimum to maximum sweetness for foods consumed within a meal or over the course of some period of time may not prove important for understanding the impact of exposure to sweetness. However, all else being equal, a measure that provides more information will allow more options for valid analyses.
TABLE 1.
Hierarchical levels of measurement
| Level of measurement | Data description |
|---|---|
| Nominal | Named variable |
| No specific order | |
| A = B or A ≠ B | |
| Ordinal | Named + ordered |
| A > B | |
| Interval | Named + ordered + proportionate interval between variables |
| A is 2.3 units greater than B | |
| Ratio | Named + ordered + proportionate interval between variables + can accommodate absolute zero |
| A is 35% higher than B |
Examples of techniques for measuring sweetness: direct and indirect methods
Perception is an internalized experience that cannot be directly observed by another individual. Some indirect methods infer perceived sweetness from observable behaviors (e.g., how well people can discriminate between different concentrations of a sweetener). Despite the advantage of being based on objective data, such techniques are not practical for assessing the sweetness of a large number of foods and beverages, due in large part to their intensive nature. For example, indirect measurement of an intensity compared with concentration function for sucrose in water required 3600 judgments collected over months (30). In contrast, direct methods rely on the observer to report the intensity of a sensation. A number of such techniques for measuring sweetness are discussed next, and a general summary of each, including the limitations, is provided in Table 2.
TABLE 2.
Available methods to measure perceptions of sweetness of individual foods and diets
| Method | Direct, indirect, objective, or subjective | Easy to implement and score | Requires a large number of judgments | Other features, including challenges and limitations |
|---|---|---|---|---|
| Discrimination | Indirect | No | Yes | For foods, fixed standard of comparison impossible |
| Objective | Can be unclear about what cues people use to make the judgment | |||
| Slow and labor-intensive | ||||
| Magnitude estimation | Direct | Yes | No | Considered the “gold standard” for ratio-level measurement of intensity |
| Subjective | Obtained values are only meaningful in relation to a fixed concentration (e.g., modulus) and provide no semantic information about absolute intensity. | |||
| Difficult for some raters to use, particularly those with low numeracy | ||||
| Category scale | Direct | Yes | No | Only provides rank-order data |
| Subjective | Provides semantic information about absolute intensity (but is easy and intuitive for raters) | |||
| Visual analog scale | Direct | Yes | No | Provides no semantic information about absolute intensity |
| Subjective | Usually does not provide ratio-level data (but is relatively easy and intuitive to use) | |||
| Spectrum method of descriptive analysis and quantitative descriptive analysis | Direct | No | No, once panelists are trained | Panelists are typically screened for sensory acuity and ability to make sensory judgments and undergo many hours of rigorous training before data collection begins. |
| Subjective | A concern is whether highly trained panels, selected and coached to uniformity, provide sensory profiles that reflect differences in the perception of randomly selected naïve consumers. | |||
| No effort is made to check or ensure ratio properties of ratings. | ||||
| Labeled magnitude scale | Direct | Yes | No | Provides ratio-level data and semantic information about absolute intensity |
| Subjective | Can require more extensive instructions and practice compared to other methods | — | Translating the verbal intensity descriptors between languages and cultures might prove difficult. | |
| Magnitude matching | Direct | Easy to score | No | Less ambiguous language to communicate results |
| Subjective; can include an objective component | Relatively more difficult to implement | — | Accuracy of the judgments depends in part on how well participants have been trained.Ordinal measurement | |
Magnitude estimation (ME) is perhaps the “gold standard” direct method for ratio-level measurement. In various forms of ME, subjects assign numbers to sensations proportional to perceived intensity. Thus, a sugar solution that tastes twice as sweet as another would be assigned a number twice as large, whereas a solution that tastes half as sweet would be assigned a number half as large (31). ME has been cross-validated, in part, via comparisons across different sensory modalities and to neural activity recorded from taste nerves (32, 33). ME has played an important role in developing and validating other indirect methods [including the general labeled magnitude scale (gLMS), discussed later]. However, ME is probably not ideal for measuring sweetness in the diet because it provides only relative information regarding perceived intensity (no semantic information regarding intensity is provided) and it can be difficult for participants with low numeracy.
Because researchers often desire semantic information that ME cannot provide, another widely used direct method is category scaling (CS). In CS, participants select 1 of a fixed number of responses; the response options may or may not have numbers visible to participants, but researchers typically code these with integers to provide ordinal data. For taste intensity, various CS approaches have been used (e.g., a 9-point scale with labels “no sweetness” at 1, “slightly sweet” at 3, “moderately sweet” at 5, “strongly sweet” at 7, and “extremely sweet” at 9) (34, 35). For measurements of sweetness using a CS, one can reasonably conclude that a sample scored 4 tastes sweeter than a sample scored 2. Critically, however, it cannot be assumed that the intensity is twice as large, unlike with a ratio-level measure such as ME. However, labels associated with the categories provide some useful semantic information about the absolute level of sweetness, and category scales tend to be easy and intuitive for participants to use, so they remain popular despite this limitation. Also, note that the semantic labels may not indicate the same level of sensation across individuals or between groups that differ systematically (e.g., by age, dietary exposure, or genetics) (36–38).
The line scale/visual analog scale (VAS) is another widely used direct scaling method. In this method, participants rate sensation by marking a line segment with anchored endpoints at the extremes (e.g., minimum and maximum). These differ from CS in that participants can mark at any point on the line, resulting in the data being roughly continuous rather than being discrete. These scales may yield better resolution, and some empirical work is consistent with this idea (39–41), although other work finds that a 9-point category scale, a VAS, and ME are comparable in their reliability and ability to resolve small differences among stimuli (42, 43). Regardless, participants are not usually instructed to make ratio judgments, and therefore ratio-level data are not typically assumed. However, some variant of the VAS may yield ratio-level data with appropriate construction and orientation (44, 45).
Another direct scaling technique, the labeled magnitude scale (LMS), attempts to combine the semantic information of CS, the continuous response properties of the VAS, and the ratio measurement of ME. This scale has semantic intensity labels [barely detectable and strongest imaginable (taste or oral) sensation at the extremes, with intermediate labels such as moderate]. Unlike CS, spacing of descriptors is empirically determined according to ME ratings for these labels and therefore nonlinear (46–48). Slopes of intensity compared with concentration (“psychophysical functions”) measured using LMS agree well with slopes measured using ME (44, 47, 49), suggesting that LMS yields ratio-level data. A common variant called gLMS anchors the top of the scale with “the strongest imaginable sensation of any kind,” which was intended in part to allow valid comparisons between groups of people who differ in sensitivity to taste or oral sensations (36, 38). Thus, gLMS combines the strengths of various other direct methods outlined previously, although there is a tendency for participants to cluster ratings near the verbal labels (44), suggesting that some participants may use it in a manner similar to CS. Furthermore, gLMS requires more extensive training and instructions compared with CS.
All of the methods mentioned previously have originated in the academic literature and have been used largely in studies using naïve participants. These methods will now be contrasted with another set of methods that first arose in industrial practice, discussed next, which rely on highly trained observers.
Descriptive analysis methods for measurement of sweetness
As a family of methods, descriptive analysis (DA) techniques were initially created by practitioners to meet the needs of food and consumer goods companies [e.g., (50, 51)] that, unlike academic psychophysicists, had a practical need to quantify the sensations from foods and consumer products (52). The basic approach includes 1) selection of panelists, 2) development of a common language that comprehensively and accurately describes product attributes, 3) training panelists to align use of these common product attributes and the use of intensity scales in the products being tested, and 4) blinded evaluation of the products (52).
To quantify sweetness, a simplified version of DA is sometimes used in which participants are trained to rate sweetness relative to standard reference solutions of sucrose, often from 0% to 20% sucrose by weight (or “brix,” a measure of the sugar content of an aqueous solution). Thus, a sample that tastes as sweet as 8% sucrose is rated as 8, a sample that tastes as sweet as 12% sucrose is rated as 12, and so forth [e.g., (53)]. Using such physical referents instead of semantic intensity labels provides an unambiguous means to communicate results and, within the context of a particular product, can provide simple direct information to guide concentration adjustments to match a target level of sweetness. Repeated resampling of the references during blind testing may reduce the amount of training needed beforehand, but their addition might also cause sensory adaptation that alters the accuracy of the rating; with practice, participants are able to rely less on references. However, even if performance was functionally perfect, using a physical concentration as a reference would not result in ratio properties in regard to perceived sweetness because intensity is usually a nonlinear function of concentration (i.e., doubling the concentration of sucrose does not double perceived sweetness) (49).
More broadly, 2 major approaches to DA emerged in the 1970s: quantitative descriptive analysis (QDA) and spectrum descriptive analysis (Spectrum). These 2 methods use the same general approach—trained panelists are aligned on descriptors, and then products are rated blind for intensity of each descriptor—but they have some nuanced differences in their implementation (54). In QDA, all intensity ratings are considered relative to the other items tested, whereas Spectrum uses a “universal scale.” Typically, this is a 150-point scale (classically 15 cm, measured to 1 decimal point) meant to encompass the entire range of sensations one might encounter in commercial food products. Participants then receive extensive training on use of this scale, with intensity anchors tied back to specific references, which may be simple model systems or real commercial products because this method arose from industrial practice (54). For sweetness, a 2 would be 2% sucrose, a 7 would be the sweetness of lemonade, and a 9 would be a specific national brand of soft drink. In Spectrum scaling, a 6 for intensity is meant to be equal across qualities, so a 6 for sourness should equal the intensity of a 6 for sweetness. A putative strength of this approach is that training with references that evoke multiple qualities (i.e., lemonade is both sweet and sour) should encourage an analytic mindset while rating but also clarify distinctions between attributes (55). Regardless of whether Spectrum or QDA is used, heavy use of exemplars during training helps reduce conceptual ambiguity of what panelists are rating in perceptually complex foods. Information on multiple sensation qualities can also allow analyses of how other flavors modulate perceived sweetness and how well the overall flavor profile predicts nutrient content. The scales used in QDA and Spectrum are rarely discussed within Stevens’ (29) measurement typology, but ratio-level data cannot be assumed.
Factors Affecting Ratings of Perceived Intensity
Numerous factors affect the perceived intensity of sweetness. Three important factors are discussed here. In addition to these, it may be useful to characterize color because there is evidence (albeit inconsistent) for an effect of color on sweetness perception (56).
Sweetener concentration
Most obviously, sweetener concentration is a major factor in the sensation of sweetness, and it is routinely changed to alter sweetness intensity of products (57). Rated sweetness increases with sweetener concentration over a wide range of concentrations (22, 58–60). Ratings made using all the methods outlined previously increase with sweetener concentration to some point, suggesting that all offer at least ordinal measurements of sweetness. It is critical to distinguish between the amount of sugar in a product and the rated sweetness it evokes. As shown in Figure 1, the same amount of sucrose (920 mg) tastes roughly half as sweet when presented in twice the amount of water. Nutritionally, these samples are functionally identical from the amount of sugars and calories they provide, but perceptually, they are not.
FIGURE 1.
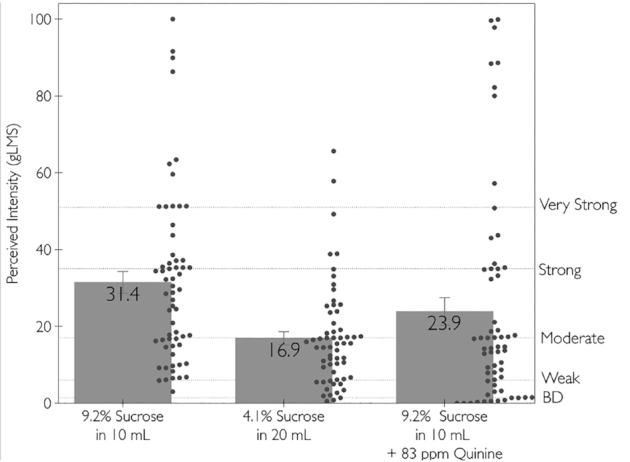
Sweetness ratings of 3 samples that each contain 920 mg of sucrose. Bars are group means and SEs, and dots are the individual ratings. All samples were presented in a counterbalanced Williams design; ratings were obtained using a gLMS by 61 participants. BD, barely detectable; gLMS, general labeled magnitude scale.
Range of presented stimuli (context)
There are also other factors that affect ratings of intensity sensation. One important factor concerns the concentration range of stimuli that are experienced within a test session. For ME, exponents of rated intensity compared with concentration functions are flatter when the range of presented stimuli is wide and steeper when the range of presented stimuli is narrow (35). Lawless and colleagues (61) showed that category scales, VASs, and 2 forms of LMS showed range bias as well. Participants also tend to rate a given stimulus as less intense in the presence of stronger stimuli and more intense in the presence of weaker stimuli (a contrast effect) (61). Sensory adaptation, or fatigue, could contribute to such effects, but they still occur when adaptation is controlled (61). Thus, intensity ratings ultimately cannot be regarded as simple reflections of underlying sensation outside the context of a particular method (58, 62). A key question here is whether these contexts affect how participants mentally map responses into sensations, or how the sensations are actually perceived. For example, if someone rates a 10% by weight sucrose solution as “moderate” when presented after a 5% solution but “weak” when presented after a 20% solution, did the perceived sweetness of the solution really change, or did the rater simply choose a different response?
Food matrix
The same amount of a sweetener might taste very different depending on the food matrix. Taste differences may be due to physical effects, such as effectively sequestering sweeteners so that they are less able to dissolve in saliva and interact with sweet receptors. Although sweetness is clearly related to concentrations of sugars in foods, this relation can be decoupled in several ways: Sweetness is imparted by nonsugar ingredients (e.g., low-calorie sweeteners), or sweetness can be masked by other ingredients. Sweetness can be inhibited centrally (in the brain), as exemplified by mutual suppression of sweetness and bitterness, such that adding a bitter compound to a fixed concentration of sweetener makes the solution taste less sweet (63, 64). This is the reason, for example, why people might underestimate the sugar content of tonic water containing bitter quinine (Figure 1). Enhancement effects, including synergy between different sweeteners, also occur (65), and such effects are part of what shapes rated sweetness during the consumption of foods and beverages. Assuming that rated sweetness reflects sweetness sensation, food matrix and enhancement effects are not problematic for measuring the sweetness of the diet. Such effects, however, may affect the influence of dietary sweetness on food choice, intake, and physiological responses to foods. Furthermore, these interactions highlight the point that added sugars and perceived sweetness are not interchangeable.
Individual differences and their effects on the measurement of sweetness
Even within a particular context, people differ markedly in how they rate sensations (35). If one person rates a given concentration of sugar as sweeter than does a second person, do the people differ in what they actually taste, differ in how they rate what they taste, or both? At least some individual differences in sensitivity to the sweetness of sugar are associated with differences in genes that encode sweet receptors expressed in taste buds (66–68). Thus, people probably do differ in what they actually taste. However, people are often consistent in their tendency to assign relatively high or low ratings across sensory modalities (69). Accordingly, researchers have proposed various ways to use cross-modal information to adjust for such individual idiosyncrasies (70, 71). An important assumption is that individual differences in the modality that is used to “control” for differences in how people use scales (e.g., the loudness of tones or the brightness of lights) are at least partially independent of individual differences in the modality of interest (in the current context, sweet taste). Regardless, measuring the intensity of sensation in more than one kind of sensory modality on the same scale can offer potential advantages in understanding individual differences (72).
The subject's concept of sweetness
Ratings of intensity depend, in part, on the raters’ concept of the sensation to be rated. Few people with an otherwise normal sense of taste would fail to recognize sucrose (table sugar) as sweet. However, in sensory experiments, adding some nominally tasteless aromas, such as fruity esters or vanilla, can cause subjects to rate both simple sugar solutions and real foods as sweeter (73–76). In many studies, sweetness enhancement by aroma is less likely if the participants rate both sweetness and aroma (e.g., “fruitiness”) than if they just rate sweetness (77–79). Clark and Lawless (77) have framed such enhancement effects as a scaling bias called “dumping.” In this explanation, participants perceive a distinct sensation from the added aroma but, lacking an appropriate option to rate this aroma, they assign the sensation to ratings of sweetness. Notably, however, odors less congruent (or compatible) with sweetness, such as that of peanut butter, do not enhance sweetness (80). Furthermore, training raters to adopt an analytic approach (analyze sensation into components) rather than a synthetic approach (respond to flavor as a whole, a natural tendency for untrained consumers) (81) also makes it less likely for aroma to influence ratings of taste intensity, even if subjects rate sweetness but not aroma (82, 83). Accordingly, odor enhancement of sweetness is not purely an artifact of the rating task (84). Rather, it appears that the concept of “sweetness” can include compatible nontaste sensations, depending on how experimenters ask the question and how raters approach the task (83). Although overall flavor is widely believed to be important for food choice and eating behavior (83), the relative importance of odor and taste in determining food choice and satiety is unclear (85); thus, additional research is needed. Ideally, ratings of sweetness would be obtained from both naïve consumers and more trained, analytically oriented panels because both may convey important and potentially complementary information.
Conclusions and Future Directions Regarding Measurement of Sweetness
Key features, strengths, and weaknesses of the various techniques discussed previously are presented in Table 2, and some directions for future work are presented in Box 1. Regarding the various subjective scales used (ME, category, VAS, and gLMS), it is important to remember that all meet the most important criterion for validity—namely that rated sweetness increases over a broad range of concentrations of sweetener. Thus, all provide potentially useful information. ME and gLMS may provide more information in Stevens’ (29) theoretical framework of level of measurement and thus allow a broader array of valid analyses of parameters of dietary sweetness. However, ME might prove difficult for people of low numeracy to use, and gLMS labels might be difficult to translate for use across various cultures. VAS and the closely related scales used in the Spectrum-derived techniques do not have these limitations, but they may or may not provide ratio-level measurement. However, relatively little effort would be required to validate a particular VAS for sweetness against ME to establish level of measurement.
Box 1. Key research questions about the measurement of sweetness to support evidence-based recommendations on dietary sweetness
How should sweetness be defined?
What are appropriate methods for judging sweetness intensity in individual foods?
What are the key food matrix and processing effects that affect sweetness ratings of individual foods?
How can quantitative data on sweetness from individual foods be translated to the sweetness of a meal, entire diet, or dietary pattern?
For profiling dietary sweetness, what current or additional databases are available or needed?
Does experience with different levels of sweetness influence subsequent sweet preferences? Does this influence operate similarly based on sweetness level, regardless of food type/category?
Do preferences for the sweetness of individual foods and beverages predict long-term preferences for sweetness levels of foods and beverages?
Regardless of the particular scale used, ratings of sweetness will depend on factors such as context (crucially, the overall range of sweetness intensity among presented samples) and panelists’ concept of the sensations they rate (e.g., how completely they separate “sweet” aroma from sweet taste). Because these factors are in turn methods dependent, perhaps the most important consideration in developing a technique that various laboratories can use to build a joint database is to establish a more comprehensive set of overall procedures for training and testing panelists. The Spectrum-derived techniques embody these principles, even if they have not been perfectly standardized to date. A possible area for further development is to determine if comparable data can be obtained with less intensive, long-term training of panelists.
Current Taste Databases for the Measurement of Sweetness in Diets
Development of sensory databases
Three studies developed taste databases that measured the sweetness intensities of foods in Australia (86), France (87), and the Netherlands and Malaysia (88). These studies all used a modified Spectrum approach for measuring the perception of sweetness (89) using trained sensory panelists for developing the taste databases for each country. These standardized scales allow for a comparison of the data collected across studies, with multiple panels, and even allow for a comparison of the rated intensities of sensory attributes across the different types of foods (90, 91). These rigorous approaches have potential utility for evaluating the sweetness of diets on a population level but are likewise resource-intensive. Note that sucrose and food standards, even if they were entirely consistent across panels, might not allow valid between-group comparisons if groups differ systematically in how they perceive the standards. Granted, the Spectrum-inspired techniques also include ratings of other sensation qualities, but all tend to be focused on taste or oral sensation, which might in turn be correlated with sweetness (92). A possible modification would be to include some uncorrelated sensations, such as loudness of sounds or brightness of lights, as in the method of magnitude matching.
Lease et al. (86) developed a Sensory-Diet database using an Australian children's national nutrition survey. Foods were selected as representing the total diet based on frequency, food grouping, nutritional, and/or sensory differences. Database development involved measuring basic taste intensities (sweet, salt, sour, bitter, and umami) and texture profiles of 377 single foods based on the Australian food consumption survey. From the 377 foods, the researchers imputed the sensory profile of 3758 foods.
A similar approach was used by Teo et al. (88) for the Netherlands and Malaysia, where 469 Dutch and 423 Malaysian foods were profiled for the 5 basic taste intensities and fat sensation. In the Netherlands, mostly single foods were characterized, whereas in Malaysia, mixed dishes were profiled. The profiles of the measured intensities were used to create a taste database of 1407 Malaysian foods and 1346 Dutch foods, representative of 97% and 99% of energy intakes in Malaysia and the Netherlands, respectively.
Martin et al. (87) created a food “taste” database following intensive panel training similar to that conducted by Lease and colleagues (86). A slightly different approach was used, in that a trained panel profiled the 5 basic taste intensities and fat sensation of predominantly mixed dishes as eaten at home. In total, 590 foods/dishes were profiled. Using cluster analyses, the foods were categorized into 6 taste clusters: 1) salty, umami, fatty (253 foods; 43%); 2) sweet (155 foods); 3) sweet, sour, bitter (57 foods); 4) bitter (24 foods); 5) salt, umami, bitter, sour (58 foods); and 6) salt (43 foods).
The Dutch and Australian taste databases are based on single foods that are frequently consumed as reported in the Dutch and Australian food consumption surveys. The Dutch database has also been validated using an FFQ and biomarkers of nutrient exposure (93) and therefore would be useful in evaluating sweetness exposure and health outcomes in large prospective cohort studies. The French database is based on composite foods consumed by a group of ∼15 trained subjects, which makes it more difficult to make a connection with the overall sweetness exposure of the French diet. The critical gap with all 3 databases is that they have not yet been used in relation to the large prospective cohort studies.
Panel training and performance
Teo et al. (88) aimed to assess the extent to which an extensive training procedure with 2 panels from different cultures yields similar results with respect to the taste profiles of 15 reference taste solutions and a selection of 19 identical control foods. Both taste panels were monitored for their discriminatory power, explanatory power agreement within the group, and repeatability. Panelists were checked on whether they used the same range of scale, scored the product in the same magnitude, discriminated the products, perceived the same taste attributes, and scored the products similarly to the rest of the panel during each training session. Figure 2 shows the mean sweetness intensity ratings of 19 identical products of the Malaysian panel as a function of the mean sweetness ratings of the Dutch panel. The 2 panels yielded similar sweetness ratings for each food. This study demonstrates that extensive panel training resulted in similar taste evaluation results, regardless of cultural and geographical backgrounds. Whether such performance will be achievable with untrained consumers is not known.
FIGURE 2.
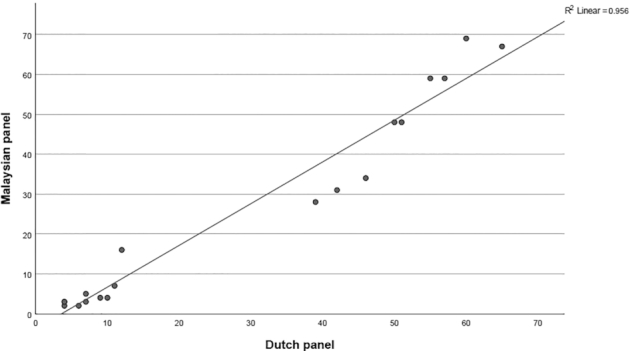
Mean sweetness intensity of 19 identical products rated by the trained Malaysian panel as a function of the mean sweetness intensity of the trained Dutch panel. Figure is based on data provided in Teo et al. (88).
Relation between sweetness and nutrient content of foods
The taste database developed by Martin et al. (87) and a French food composition table were used to obtain a data set combining sensory and nutritional information for 365 foods (94). The sweet taste intensity ratings correlated with the carbohydrate content (r = 0.57, P < 0.0001) and strongly correlated with the mono- and disaccharide content (r = 0.84, P < 0.0001). No strong correlations were observed with other nutrients.
Sweetness exposure, energy intake, and diet quality
Van Langeveld et al. (94) assessed dietary taste patterns in the Netherlands by sex, BMI, age, and education. Six taste clusters were identified among 476 profiled foods: neutral (27%), sweet and sour (14%), sweet and fat (23%), bitter (4%), salt/umami/fat (24%), and fat (8%). Two population-based cohorts [Dutch National Food (DNF), n = 1351; and the Nutrition Questionnaires plus (NQ+) study, n = 944] were used to calculate the contribution of each of these taste clusters to the overall energy intake of the diet. Women consumed a higher percentage of energy from sweet and fat–tasting [15% (DNF) and 15% (NQ+)] and sweet and sour–tasting [13% (DNF) and 12% (NQ+)] foods compared with men [sweet and fat: 13% (DNF) and 12% (NQ+), P < 0.001; sweet and sour: 13% (DNF) and 10% (NQ+), P < 0.001]. Notably, energy intake from sweet and sour– and sweet and fat–tasting foods was relatively higher during snacking occasions compared with main meals, which corresponds with reported intakes of monosaccharides and disaccharides with snack consumption. The conclusion was that taste can be related to macronutrient intake of individual foods, as well as the total diet. The data also showed that the contribution of sweet-tasting foods to energy intake in the diet is generally similar among people with normal weight, overweight, and obesity (Figure 3).
FIGURE 3.
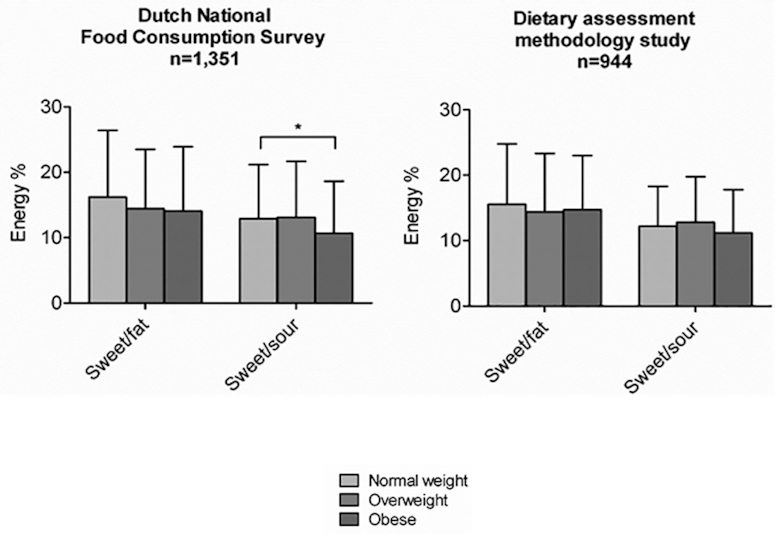
Contribution of sweet-tasting foods to energy intake in the diet among individuals with normal weight, overweight, and obesity. *, Significant difference between weight-status subgroups. Data adapted from van Langeveld et al. (94).
Cox et al. (95) quantified the sensory profiles of different food groups (e.g., fruits, vegetables, grain, and meats) in Australia using the validated Sensory-Diet tool database (89), representing the specific foods covered in each question of the Commonwealth Scientific and Industrial Research Organisation's (CSIRO) Healthy Diet Score survey used to estimate food intake. The CSIRO Healthy Diet Score survey was also used to calculate a diet quality score. Average sensory scores (weighted by frequency of consumption) were calculated for each grouping of food covered per survey question. Reported intake of each food group was multiplied by the sensory scores for each food group. To determine the total sensory value of an individual's diet, sensory values of each food group were then summed to give a total dietary sensory score. Sweetness of the diet was quantified by multiplying grams of each food consumed by the sweetness intensity of that food. By dividing the sweetness value by the total energy intake, the sweetness density of the diet was calculated for ∼10,000 adults and 2700 children. Higher diet quality was associated with higher sweet and bitter scores, but a greater proportion of this sweetness was from healthy core foods (e.g., fruit, vegetables, grains, and dairy) rather than discretionary foods (e.g., chocolate and confectionary, cakes and biscuits, pies and pastries).
Conclusions
Some governments and influential health organizations recommend diets low in sweetness based on a widespread and long-standing belief in a causal chain: A highly sweet diet leads to changes in perception of sweet foods and beverages, which in turn leads to overconsumption of sugar, which finally leads to negative health outcomes. However, no link in this proposed causal chain has strong empirical support. Empirical evaluation of at least the first 2 links will require measures of human perception of sweetness. Some of the important research questions that have been identified are provided in Box 1.
There are important challenges in measuring perceptions of sweetness of individual foods and beverages. The challenges are even more daunting when attempting to measure the sweetness of entire diets. Ratings of sweetness depend on the scale one uses, the context (i.e., the range of intensities presented in a test session and perhaps the level of sweetness raters experience in daily life), and how raters approach their task (e.g., whether their concept of sweetness includes “sweet” aromas such as vanilla). In short, ratings of sweetness are not independent of the set of procedures that are used. Furthermore, it is not known if differences are due to how a person perceives sweetness and/or how the person uses the rating tool/scale. Accordingly, agreement on a standard set of procedures to facilitate comparisons across studies toward an integrated database is one priority.
With a reliable method, studies related to the effects of sweetness on health-related outcomes could be evaluated. Although well-established sensory evaluation techniques in laboratory settings exist for individual foods, agreement on the optimal approach for measuring the sweetness of the total diet is lacking, particularly in settings other than in the laboratory. The development of such measures would permit researchers to combine data from different studies and populations. This would facilitate the design and conduct of new studies to address unresolved research questions about dietary sweetness in foods and diets and relations to health outcomes. This is a second priority.
Future research, including longitudinal research, is needed to understand 1) the role, if any, of sweet-tasting foods, beverages, and diets, as well as sweetness intensity, in food preferences, energy intake, dietary intake, and health-related outcomes such as obesity and dental caries; and 2) if so, in what way these factors operate. Findings from the Netherlands using a taste database (94) indicate that it may be possible to profile diets based on their taste characteristics. If this is accomplished, the association between sweetness in the diet and food preferences and health-related outcomes could be evaluated, along with the role of sociodemographic/cultural variables. Addressing these is a third priority.
ACKNOWLEDGEMENTS
We thank Prof. France Bellisle (University of Paris) for her valuable contributions and Suzanne Pecore (P&D Consulting) for her contributions provided at the “Think Tank: Measuring Sweetness in Foods, Beverages and Diets” meeting, organized by ILSI North America and held on 12 December 2019 in Washington, DC. The authors also thank the Penn State Sensory Evaluation Center for collecting the data shown in Figure 1, especially Shannon Alshouse and Jennifer Brodock. The authors’ responsibilities were as follows—PRT, KMA, KdG, JEH, PMW, and RDM: wrote the manuscript; DJB, GKB, JTD, JDF, and DMK: had roles in the conceptualization, review, and editing; and all authors: read and approved the final manuscript.
Notes
This work was supported by the North American Branch of the International Life Sciences Institute (ILSI North America) Low-Calorie Sweeteners Committee. ILSI North America is a public, nonprofit science foundation that provides a forum to advance understanding of scientific issues related to the nutritional quality and safety of the food supply. ILSI North America receives support primarily from its industry membership. This review, in part, includes information shared in a meeting, “Think Tank: Measuring Sweetness in Foods, Beverages and Diets,” organized by ILSI North America and held on 12 December 2019 in Washington, DC (see https://ilsina.org/event/think-tank-measuring-sweetness-in-foods-beverages-and-diets/). PRT, KMA, KdG, JEH, GKB, JDF, RDM, and PMW received travel funding to participate in the December 2019 ILSI North America meeting. PRT received funding support to prepare the manuscript. DJB, JTD, and DMK confirm that no financial support was received for this work.
Author disclosures: PRT serves as an independent consultant on projects supported by the ILSI North America Carbohydrate Committee. She has served as a consultant to PepsiCo, Ocean Spray, Bioneutra, Lantmännen, Hayashibara, GlaxoSmithKline, and 8Greens, and she received speaker travel expenses from the Council for Responsible Nutrition that are not related to sweetness or sweeteners. In connection with research on sweetness, KMA has received funding from Unilever R&D Vlaardingen, Netherlands; has current funding from TIFN, Netherlands (in collaboration with Arla Foods, Denmark; American Beverage Association, United States; Cargill, United States; Dutch Knowledge Centre for Sugar, Netherlands; Firmenich, Switzerland; the International Sweeteners Association, Belgium; SinoSweet, China; and Unilever, Netherlands) and from the International Sweeteners Association; and has received speaker's expenses from the International Sweeteners Association and PepsiCo. KdG has current funding from TIFN (in collaboration with Arla Foods, American Beverage Association, Cargill, Dutch Knowledge Centre for Sugar, Firmenich, International Sweeteners Association, SinoSweet, and Unilever), and he received speaker's expenses from the International Sweeteners Association for a symposium held in 2018. In relation to his research on taste perception, JEH has received travel expenses from Kerry Health & Nutrition and ILSI North America; he has also received research support from the Sugar Association and the World Sugar Research Organisation for unrelated research on sweetness, and funding from PepsiCo for research not related to sweetness. Also, JEH is Director of the Sensory Evaluation Center at Penn State, which routinely conducts taste tests for industrial clients to facilitate experiential learning opportunities for undergraduate and graduate students. PMW has received travel and/or research funding from various companies in the food, beverage, ingredient, and pharmaceutical industries, including past research related to sweetness from PepsiCo. The only current research funding for PMW related to sweetness is a competitive grant from the US NIH (National Institute on Deafness and Other Communication Disorders). GKB receives no personal funds, including speaker fees, from any commercial entity. Ajinomoto provides a consulting fee to the Monell Chemical Senses Center that is used to support a small portion of his research. His research on sweetness is supported by a competitive grant from the US NIH (National Institute on Deafness and Other Communication Disorders). GKB is a member of the Board of Directors of ILSI North America. JTD is a member of the scientific advisory boards of the Mushroom Council, McCormick Science Institute, and Bay State Milling. She serves as a nonpaid advisor to the Low-Calorie Sweeteners and Bioactives Committees of ILSI North America. She served on the Conagra scientific advisory board until 2018 and on a scientific advisory group for Gerber Nestle until 2019, and she advised Motif Foodworks in 2019. She holds stock in several food and drug companies. She is editor of Nutrition Today, a nutrition journal, and is a professor at Tufts University School of Medicine and Senior Scientist at the Jean Mayer USDA Human Nutrition Research Center on Aging. JDF is a scientific advisor to the International Glutamate Technical Committee (Brussels, Belgium) and to ILSI North America (Washington, DC). RDM has received research and travel support as well as honoraria from various sources related to sweeteners but has no current support relevant to the topic of this article. DJB and DMK serve as government liaisons to the ILSI North America Lipids and Carbohydrates Committees, respectively, and DJB serves as an unpaid member of the Sabra Wellness and Nutrition Advisory Board, Avocado Nutrition Science Advisory Group, and the California Walnut Commission Health Research Advisory Group, and funding for sweetness research supported by a competitive grant from the US NIH (National Institute on Deafness and Other Communication Disorders) (other funding is unrelated to sweetness or other topics of this article).
Perspective articles allow authors to take a position on a topic of current major importance or controversy in the field of nutrition. As such, these articles could include statements based on author opinions or point of view. Opinions expressed in Perspective articles are those of the author and are not attributable to the funder(s) or the sponsor(s) or the publisher, Editor, or Editorial Board of Advances in Nutrition. Individuals with different positions on the topic of a Perspective are invited to submit their comments in the form of a Perspectives article or in a Letter to the Editor.
Abbreviations used: CS, category scaling; CSIRO, Commonwealth Scientific and Industrial Research Organisation; DA, descriptive analysis; DNF, Dutch National Food; gLMS, general labeled magnitude scale; LMS, labeled magnitude scale; ME, magnitude estimation; NQ+, Nutrition Questionnaires plus; QDA, quantitative descriptive analysis; VAS, visual analog scale.
Contributor Information
Paula R Trumbo, Department of Food Science and Nutrition, University of Minnesota, St. Paul, MN, USA.
Katherine M Appleton, Department of Psychology, Bournemouth University, Dorset, United Kingdom.
Kees de Graaf, Division of Human Nutrition and Health, Wageningen University, Wageningen, Netherlands.
John E Hayes, Department of Food Science, Pennsylvania State University, University Park, PA, USA.
David J Baer, US Department of Agriculture Agricultural Research Service, Beltsville, MD, USA.
Gary K Beauchamp, Monell Chemical Senses Center, Philadelphia, PA, USA.
Johanna T Dwyer, School of Medicine and Friedman School of Nutrition Science and Policy, Tufts University, Boston, MA, USA.
John D Fernstrom, School of Medicine, University of Pittsburgh, Pittsburgh, PA, USA.
David M Klurfeld, US Department of Agriculture Agricultural Research Service, Beltsville, MD, USA.
Richard D Mattes, Purdue University, West Lafayette, IN, USA.
Paul M Wise, Monell Chemical Senses Center, Philadelphia, PA, USA.
References
- 1. WHO . Guideline: sugars intake for adults and children. Geneva (Switzerland): WHO; 2015. [PubMed] [Google Scholar]
- 2. WHO Regional Office for the Eastern Mediterranean . Policy statement and recommended actions for lowering sugar intake and reducing prevalence of type 2 diabetes and obesity in the Eastern Mediterranean Region. [Internet]. 2016[cited April 21, 2020]. Available from: http://www.emro.who.int/nutrition/strategy/policy-statement-and-recommended-actions-for-lowering-sugar-intake-and-reducing-prevalence-of-type-2-diabetes-and-obesity-in-the-eastern-mediterranean-region.html. [Google Scholar]
- 3. Government of Canada . Canada's food guide: sugar substitutes and healthy eating. [Internet]. 2019[cited April 21, 2020]. Available from: https://food-guide.canada.ca/en/tips-for-healthy-eating/sugar-substitutes-and-healthy-eating. [Google Scholar]
- 4. Public Health England . Sugar reduction: the evidence for action. Annexe 5: food supply[Internet]. 2015[cited April 21, 2020]. Available from: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/470176/Annexe_5._Food_Supply.pdf. [Google Scholar]
- 5. European Union . Framework for national initiatives on selected nutrients. Annex II: added sugars. [Internet]. 2011[cited 21 April 2020]. Available from: https://ec.europa.eu/health/sites/health/files/nutrition_physical_activity/docs/added_sugars_en.pdf. [Google Scholar]
- 6. Pan American Health Organization . Pan American Health Organization nutrient profile model. [Internet]. 2016[cited April 21, 2020]. Available from: https://iris.paho.org/bitstream/handle/10665.2/18621/9789275118733_eng.pdf?sequence=9&isAllowed=y. [Google Scholar]
- 7. Cambridge English Dictionary . Sweet. [Internet]. 2020[cited July 28, 2020]. Available from: https://dictionary.cambridge.org/dictionary/english/sweet. [Google Scholar]
- 8. Oxford Learner's Dictionary . Sweetness. [Internet]. 2020[cited July 28, 2020]. Available from: https://www.oxfordlearnersdictionaries.com/us/definition/english/sweetness?q=sweetness. [Google Scholar]
- 9. Institute of Medicine . Strategies to reduce sodium intake in the United States. Washington (DC): National Academies Press; 2010. [Google Scholar]
- 10. Bertino M, Beauchamp GK, Engelman K. Long-term reduction in dietary sodium alters the taste of salt. Am J Clin Nutr. 1982;36:1134–44. [DOI] [PubMed] [Google Scholar]
- 11. CDC . CDC sodium reduction initiative. [Internet]. 2018[cited April 21, 2020]. Available from: https://www.cdc.gov/salt/sodium_reduction_initiative.htm. [Google Scholar]
- 12. FDA . Sodium reduction. [Internet]. 2018[cited April 21, 2020]. Available from: https://www.fda.gov/food/food-additives-petitions/sodium-reduction. [Google Scholar]
- 13. Moshfegh AJ, Garceau AO, Parker EA, Clemens JC. Beverage choices among children: What We Eat in America, NHANES 2015–2016. Food Surveys Research Group Dietary Data Brief no. 22 (May 2019).[Internet]. 2019[cited August 2, 2020]. Available from: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/DBrief/22_Beverage_choices_children_1516.pdf. [PubMed] [Google Scholar]
- 14. Moshfegh AJ, Garceau AO, Parker EA, Clemens JC. Beverage choices among adults: What We Eat in America, NHANES 2015–2016. Food Surveys Research Group Dietary Data Brief no. 21 (May 2019).[Internet]. 2019[cited August 2, 2020]. Available from: https://www.ars.usda.gov/ARSUserFiles/80400530/pdf/DBrief/21_Beverage_choices_adults_1516.pdf. [PubMed] [Google Scholar]
- 15. Appleton KM, Tuorila H, Bertenshaw EJ, de Graaf C, Mela DJ. Sweet taste exposure and the subsequent acceptance and preference for sweet taste in the diet: systematic review of the published literature. Am J Clin Nutr. 2018;107:405–19. [DOI] [PubMed] [Google Scholar]
- 16. Veldhuizen MG, Babbs RK, Patel B, Fobbs W, Kroemer NB, Garcia E, Yeomans MR, Small DM. Integration of sweet taste and metabolism determines carbohydrate reward. Curr Biol. 2017;27:2476–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. May CE, Vaziri A, Lin YQ, Grushko O, Khabiri M, Wang QP, Holme KJ, Pletcher SD, Freddolino PL, Neely GGet al. High dietary sugar reshapes sweet taste to promote feeding behavior in Drosophila melanogaster. Cell Rep. 2019;27:1675–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Liauchonak I, Qorri B, Dawoud F, Riat Y, Szewczuk MR. Non-nutritive sweeteners and their implications on the development of metabolic syndrome. Nutrients. 2019;11:644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Beauchamp GK. Why do we like sweet taste: a bitter tale?. Physiol Behav. 2016;164:432–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. DeCasien AR, Williams SA, Higham JP. Primate brain size is predicted by diet but not sociality. Nat Ecol Evol. 2017;1:112. [DOI] [PubMed] [Google Scholar]
- 21. Marlowe FW, Berbesque JC, Wood B, Crittenden A, Porter C, Mabulla A. Honey, Hadza, hunter–gatherers, and human evolution. J Hum Evol. 2014;71:119–28. [DOI] [PubMed] [Google Scholar]
- 22. Wise PM, Nattress L, Flammer LJ, Beauchamp GK. Reduced dietary intake of simple sugars alters perceived sweet taste intensity but not perceived pleasantness. Am J Clin Nutr. 2016;103:50–60. [DOI] [PubMed] [Google Scholar]
- 23. Pangborn RM, Simone M. Body size and sweetness preference. J Am Diet Assoc. 1958;34:924–8. [PubMed] [Google Scholar]
- 24. Witherly SA, Pangborn RM, Stern JS. Gustatory responses and eating duration of obese and lean adults. Appetite. 1980;1:53–63. [Google Scholar]
- 25. Bobowski N, Mennella JA. Personal variation in preference for sweetness: effects of age and obesity. Child Obes. 2017;13:369–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cox DN, Hendrie GA, Carty D. Sensitivity, hedonics and preferences for basic tastes and fat amongst adults and children of differing weight status: a comprehensive review. Food Qual Prefer. 2016;48:359–67. [Google Scholar]
- 27. US Department of Health and Human Services, US Department of Agriculture . 2015–2020 dietary guidelines for Americans. 8th ed. [Internet]. 2015[cited July 28, 2020]. Available from: https://health.gov/our-work/food-nutrition/2015-2020-dietary-guidelines. [Google Scholar]
- 28. Dietary Guidelines Advisory Committee . Scientific report of the 2020 Dietary Guidelines Advisory Committee: advisory report to the Secretary of Agriculture and the Secretary of Health and Human Services. [Internet]. 2020[cited July 28, 2020]. Available from: https://www.dietaryguidelines.gov/sites/default/files/2020-07/ScientificReport_of_the_2020DietaryGuidelinesAdvisoryCommittee_first-print.pdf. [Google Scholar]
- 29. Stevens SS. On the theory of scales of measurement. Science. 1946;103:677–80. [DOI] [PubMed] [Google Scholar]
- 30. McBride RL. A JND-scale/category-scale convergence in taste. Percept Psychophys. 1983;34:77–83. [DOI] [PubMed] [Google Scholar]
- 31. Moskowitz HR. Magnitude estimation: notes on what, how, when, and why to use it. J Food Qual. 1977;1:195–227. [Google Scholar]
- 32. Stevens JC, Mack JD, Stevens SS. Growth of sensation on seven continua as measured by force of handgrip. J Physiol. 1960;59:60–7. [DOI] [PubMed] [Google Scholar]
- 33. Borg G, Diamant H, Ström L, Zotterman Y. The relation between neural and perceptual intensity: a comparative study on the neural and psychophysical response to taste stimuli. J Physiol. 1967;192:13–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kamen JM, Pilgrim FJ, Gutman NJ, Kroll BJ. Interactions of suprathreshold taste stimuli. J Exp Psychol. 1961;62:348–56. [DOI] [PubMed] [Google Scholar]
- 35. Lawless HT, Heymann H. Scaling in sensory evaluation of food: principles and practices. New York: Springer; 2010. [Google Scholar]
- 36. Bartoshuk LM, Duffy VB, Fast K, Green BG, Prutkin J, Snyder DJ. Labeled scales (e.g., category, Likert, VAS) and invalid across-group comparisons: what we have learned from genetic variation in taste. Food Qual Prefer. 2003;14:125–38. [Google Scholar]
- 37. Snyder DJ, Fast K, Baroshuk LM. Valid comparisons of suprathreshold sensations. In: Roepstorff JA, editor. Trusting the subject: the use of introspective evidence in cognitive science. London: Academic Press; 2004. Vol. 2, p. 96–112. [Google Scholar]
- 38. Bartoshuk LM, Duffy VB, Chapo AK, Fast K, Yiee JH, Hoffman HJ, Ko C-W, Snyder DJ. From psychophysics to the clinic: missteps and advances. Food Qual Prefer. 2004;15:617–32. [Google Scholar]
- 39. Keele KD. The pain chart. Lancet. 1948;252:6–8. [DOI] [PubMed] [Google Scholar]
- 40. Sriwatanakul K, Kelvie W, Lasagna L, Calimlim JF, Weis OF, Mehta G. Studies with different types of visual analog scales for measurement of pain. Clin Pharmacol Ther. 1983;34:234–9. [DOI] [PubMed] [Google Scholar]
- 41. Zdilla MJ, Starkey LD, Saling JR. A taste-intensity visual analog scale: an improved zinc taste-test protocol. Integr Med (Encinitas). 2015;14:34–8. [PMC free article] [PubMed] [Google Scholar]
- 42. Lawless HT, Malone GJ. Comparison of rating scales: sensitivity, replicates and relative measurement. J Sens Stud. 1986;1:155–74. [Google Scholar]
- 43. Lawless HT, Malone GJ. The disriminative efficiency of common scaling methods. J Sens Stud. 1986;1:85–98. [Google Scholar]
- 44. Hayes JE, Allen AL, Bennett SM. Direct comparison of the generalized visual analog scale (gVAS) and general labeled magnitude scale (gLMS). Food Qual Prefer. 2013;28:36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45–56. [DOI] [PubMed] [Google Scholar]
- 46. Stevens SS. Sensory scales of taste intensity. Percept Psychophys. 1969;6:302–8. [Google Scholar]
- 47. Green BG. Psychophysical measurement of oral chemesthesis. In: Simon SA, Nicolelis MA, eds. Methods in chemosensory research. Boca Raton (FL): CRC Press; 2002. p.3–20. [Google Scholar]
- 48. Schifferstein HNJ. Labeled magnitude scales: a critical review. Food Qual Prefer. 2012;26:151–8. [Google Scholar]
- 49. Green BG, Dalton P, Cowart B, Shaffer G, Rankin K, Higgins J. Evaluating the “labeled magnitude scale” for measuring sensations of taste and smell. Chem Senses. 1996;21:323–34. [DOI] [PubMed] [Google Scholar]
- 50. Sjostrom LB. The descriptive analysis of flavor. In: Food acceptance testing methodology. Chicago (IL): Quartermaster Food and Container Institute; 1954. p. 4–20. [Google Scholar]
- 51. Heymann H. A personal history of sensory science. Food Cult Soc. 2019;22:203–23. [Google Scholar]
- 52. Murray JM, Delahunty CM, Baxter IA. Descriptive sensory analysis: past, present and future. Food Res Int. 2001;34:461–71. [Google Scholar]
- 53. DuBois GE, Walters DE, Schiffman SS, Warwick ZS, Booth BJ, Pecore SD, Gibes K, Carr BT, Brands LM. Concentration–response relationships of sweeteners. In: Walters DE, Orthoefer FT, DuBois GE, eds. Sweeteners: discovery, molecular design, and chemoreception. Washington (DC): ACS Publications; 1991. p. 261–76. [Google Scholar]
- 54. Hootman RC. Manual on descriptive analysis testing for sensory evaluation. Philadelphia (PA): ASTM International; 1992. [Google Scholar]
- 55. Civille GV, Lawless HT. The importance of language in describing perceptions. J Sens Stud. 1986;1:203–15. [Google Scholar]
- 56. Spence C. On the relationship(s) between color and taste/flavor. Exp Psychol. 2019;66:99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Li B, Hayes JE, Ziegler GR. Interpreting consumer preferences: physicohedonic and psychohedonic models yield different information in a coffee-flavored dairy beverage. Food Qual Prefer. 2014;36:27–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Riskey DR, Parducci A, Beauchamp GK. Effects of context in judgments of sweetness and pleasantness. Percept Psychophys. 1979;26:171–6. [Google Scholar]
- 59. Wee M, Tan V, Forde C. A comparison of psychophysical dose–response behaviour across 16 sweeteners. Nutrients. 2018;10:1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Schutz HG, Pilgrim FJ. Sweetness of various compounds and its measurement. J Food Sci. 1957;22:206–13. [Google Scholar]
- 61. Lawless HT, Horne J, Spiers W. Contrast and range effects for category, magnitude and labeled magnitude scales in judgements of sweetness intensity. Chem Senses. 2000;25:85–92. [DOI] [PubMed] [Google Scholar]
- 62. Parducci A. The relativism of absolute judgements. Sci Am. 1968;219:84–90. [DOI] [PubMed] [Google Scholar]
- 63. Kroeze JHA, Bartoshuk LM. Bitterness suppression as revealed by split-tongue taste stimulation in humans. Physiol Behav. 1985;35:779–83. [DOI] [PubMed] [Google Scholar]
- 64. Lawless HT. Evidence for neural inhibition in bittersweet taste mixtures. J Comp Physiol Psychol. 1979;93:538–47. [DOI] [PubMed] [Google Scholar]
- 65. Reyes MM, Gravina SA, Hayes JE. Evaluation of sweetener synergy in humans by isobole analyses. Chem Senses. 2019;44:571–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Fushan AA, Simons CT, Slack JP, Manichaikul A, Drayna D. Allelic polymorphism within the TAS1R3 promoter is associated with human taste sensitivity to sucrose. Curr Biol. 2009;19:1288–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Haznedaroğlu E, Koldemir-Gündüz M, Bakır-Coşkun N, Bozkuş HM, Çağatay P, Süsleyici-Duman B, Menteş A. Association of sweet taste receptor gene polymorphisms with dental caries experience in school children. Caries Res. 2015;49:275–81. [DOI] [PubMed] [Google Scholar]
- 68. Hwang LD, Cuellar-Partida G, Ong JS, Breslin PA, Reed DR, MacGregor S, Gharahkhani P, Martin NG, Rentería ME. Sweet taste perception is associated with body mass index at the phenotypic and genotypic level. Twin Res Hum Genet. 2016;19:465–71. [DOI] [PubMed] [Google Scholar]
- 69. Jones FN, Marcus MJ. The subject effect in judgements of subjective magnitude. J Exp Psychol. 1961;61:40–4. [DOI] [PubMed] [Google Scholar]
- 70. Gescheider GA. Psychophysics: the fundamentals. Mahwah (NJ): Erlbaum; 1997. [Google Scholar]
- 71. Marks LE, Stevens JC, Bartoshuk LM, Gent JF, Rifkin B, Stone VK. Magnitude-matching: the measurement of taste and smell. Chem Senses. 1988;13:63–87. [Google Scholar]
- 72. Coldwell SE, Mennella JA, Duffy VB, Pelchat ML, Griffith JW, Smutzer G, Cowart BJ, Breslin PA, Bartoshuk LM, Hastings Let al. Gustation assessment using the NIH Toolbox. Neurology. 2013;80(Suppl 3):S20–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Isogai T, Wise PM. The effects of odor quality and temporal asynchrony on modulation of taste intensity by retronasal odor. Chem Senses. 2016;41:557–66. [DOI] [PubMed] [Google Scholar]
- 74. Prescott J. The neural bases of multisensory processes. Boca Raton (FL): CRC Press; 2012. [PubMed] [Google Scholar]
- 75. Prescott J, Stevenson R. Chemosensory integration and the perception of flavor. In: Handbook of olfaction and gustation. London: Blackwell; 2015. p. 1007–26. [Google Scholar]
- 76. Wang G, Hayes EJ, Zeigler RG, Roberts FR, Hopfer H. Dose–response relationships for vanilla flavor and sucrose in skin milk: evidence of synergy. Beverages. 2018;4:73. [Google Scholar]
- 77. Clark CC, Lawless HT. Limiting response alternatives in time-intensity scaling: an examination of the halo-dumping effect. Chem Senses. 1994;19:583–94. [DOI] [PubMed] [Google Scholar]
- 78. Green BG, Nachtigal D, Hammond S, Lim J. Enhancement of retronasal odors by taste. Chem Senses. 2012;37:77–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. van der Klaauw NJ, Frank RA. Scaling component intensities of complex stimuli: the influence of response alternatives. Environ Int. 1996;22:21–31. [Google Scholar]
- 80. Frank RA, Byram J. Taste–smell interactions are tastant and odorant dependent. Chem Senses. 1988;13:445–55. [Google Scholar]
- 81. Lawless HT, Heymann H. Sensory evaluation of food: principles and practices. New York: Springer; 2010. [Google Scholar]
- 82. Bingham AF, Birch GG, de Graaf C, Behan JM, Perring KD. Sensory studies with sucrose-maltol mixtures. Chem Senses. 1990;15:447–56. [Google Scholar]
- 83. Prescott J. Flavor perception. London: Blackwell; 2004. [Google Scholar]
- 84. Wang G, Bakke AJ, Hayes JE, Hopfer H. Demonstrating cross-modal enhancement in a real food with a modified ABX test. Food Qual Prefer. 2019;77:206–13. [Google Scholar]
- 85. Boesveldt S, de Graaf K. The differential role of smell and taste for eating behavior. Perception. 2017;46:307–19. [DOI] [PubMed] [Google Scholar]
- 86. Lease H, Hendrie GA, Poelman AAM, Delahunty C, Cox DN. A sensory-diet database: a tool to characterise the sensory qualities of diets. Food Qual Prefer. 2016;49:20–32. [Google Scholar]
- 87. Martin C, Visalli M, Lange C, Schlich P, Issanchou S. Creation of a food taste database using an in-home “taste” profile method. Food Qual Prefer. 2014;36:70–80. [Google Scholar]
- 88. Teo PS, van Langeveld AWB, Pol K, Siebelink E, de Graaf C, Martin C, Issanchou S, Yan SW, Mars M. Training of a Dutch and Malaysian sensory panel to assess intensities of basic tastes and fat sensation of commonly consumed foods. Food Qual Prefer. 2018;65:49–59. [Google Scholar]
- 89. Meilgaard MC, Carr BT, Civille GV. Sensory evaluation techniques. Boca Raton (FL): CRC Press; 2006. [Google Scholar]
- 90. Martin C, Maire A, Chabanet C, Issanchou S. Equi-intensity across the Spectrum taste scales. Food Qual Prefer. 2015;44:75–83. [Google Scholar]
- 91. Martin C, Issanchou S. Nutrient sensing: what can we learn from different tastes about the nutrient contents in today's foods?. Food Qual Prefer. 2019;71:185–96. [Google Scholar]
- 92. Bartoshuk LM, Duffy VB, Green BG, Hoffman HJ, Ko CW, Lucchina LA, Marks LE, Snyder DJ, Weiffenbach JM. Valid across-group comparisons with labeled scales: the gLMS versus magnitude matching. Physiol Behav. 2004;82:109–14. [DOI] [PubMed] [Google Scholar]
- 93. van Langeveld AWB, Teo PS, Mars M, Feskens EJM, de Graaf C, de Vries JHM. Evaluation of dietary taste patterns as assessed by FFQ against 24-h recalls and biomarkers of exposure. Eur J Clin Nutr. 2019;73:132–40. [DOI] [PubMed] [Google Scholar]
- 94. van Langeveld AWB, Teo PS, de Vries JHM, Feskens EJM, de Graaf C, Mars M. Dietary taste patterns by sex and weight status in the Netherlands. Br J Nutr. 2018;119:1195–206. [DOI] [PubMed] [Google Scholar]
- 95. Cox DN, Hendrie GA, Lease HJ. Do healthy diets differ in their sensory characteristics?. Food Qual Prefer. 2018;68:12–8. [Google Scholar]


