ABSTRACT
Protein supplementation is an attractive strategy to prevent loss of muscle mass in older adults. However, it could be counterproductive due to adverse effects on appetite. This systematic review and meta-analysis aimed to determine the effects of protein supplementation on appetite and/or energy intake (EI) in healthy older adults. MEDLINE, The Cochrane Library, CINAHL, and Web of Science were searched up to June 2020. Acute and longitudinal studies in healthy adults ≥60 y of age that reported effects of protein supplementation (through supplements or whole foods) compared with control and/or preintervention (for longitudinal studies) on appetite ratings, appetite-related peptides, and/or EI were included. Random-effects model meta-analysis was performed on EI, with other outcomes qualitatively reviewed. Twenty-two studies (9 acute, 13 longitudinal) were included, involving 857 participants (331 males, 526 females). In acute studies (n = 8), appetite ratings were suppressed in 7 out of 24 protein arms. For acute studies reporting EI (n = 7, n = 22 protein arms), test meal EI was reduced following protein preload compared with control [mean difference (MD): −164 kJ; 95% CI: −299, −29 kJ; P = 0.02]. However, when energy content of the supplement was accounted for, total EI was greater with protein compared with control (MD: 649 kJ; 95% CI: 438, 861 kJ; P < 0.00001). Longitudinal studies (n = 12 protein arms) showed a higher protein intake (MD: 0.29 g ⋅ kg−1 ⋅ d−1; 95% CI: 0.14, 0.45 g ⋅ kg−1 ⋅ d−1; P < 0.001) and no difference in daily EI between protein and control groups at the end of trials (MD: −54 kJ/d; 95% CI: −300, 193 kJ/d; P = 0.67). While appetite ratings may be suppressed with acute protein supplementation, there is either a positive effect or no effect on total EI in acute and longitudinal studies, respectively. Therefore, protein supplementation may represent an effective solution to increase protein intakes in healthy older adults without compromising EI through appetite suppression. This trial was registered at PROSPERO as https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42019125771 (CRD42019125771).
Keywords: aging, appetite, energy intake, gut hormones, hunger, protein, older adults
Introduction
In 2050, 2 billion of the world's population will be >60 years of age (1). This increase in numbers of older adults, although an achievement, incurs little value if a good health trajectory is not maintained. Frailty and sarcopenia, including loss of muscle mass and function with aging, is a key issue, associated with loss of independence, reduced quality of life, and increased mortality (2). Exercise and appropriate nutrition, especially adequate protein and energy intake (EI), are considered the optimal strategies to limit age-related declines in muscle mass and function (3, 4). For healthy older adults, consumption of 1.0–1.2 g/kg body weight of protein is recommended, with higher daily intakes recommended for those with malnutrition or at risk of malnutrition or severe illness or injury (3, 4). However, many older adults, ≤46% (5), do not meet these requirements.
Protein supplementation could have an important role in addressing deficiencies in older adults but could also be counterproductive by increasing satiety and, consequently, compromising total daily EI. Protein has been shown to be the most satiating macronutrient in the general population (6). However, there is some evidence that the appetite response to protein may differ in younger compared with older adults (7, 8).
Understanding the effects of protein supplementation on appetite and EI in older adults is crucial when investigating the potential efficacy for improving health outcomes in this population. The effects of oral protein and energy supplementation on a range of outcomes in older adults have previously been systematically reviewed (9). However, the latter review is now >10 y old, most individuals included were hospital inpatients, and appetite was not examined.
The aims of the present systematic review and meta-analysis were to determine the effects of protein supplementation (through supplements or whole foods) on appetite (ratings and related peptides) and EI in healthy older adults.
Methods
We conducted a systematic review with meta-analysis to investigate the effects of protein supplementation on appetite ratings, appetite-related peptides, and/or EI in older adults. The review was conducted according to the Primary Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and registered in PROSPERO (ID: CRD42019125771).
Study selection and inclusion criteria
Major search databases [MEDLINE via PubMed; the Cochrane Central Register of Controlled Trials (CENTRAL), CINAHL via EBSCO; and Web of Science] were searched up to June 2020. There was no limitation on publication dates and all searches were completed in the English language. Keyword searches were performed for “protein supplementation” AND (“energy intake” OR “appetite”) AND “older adults” (Supplemental Tables 1 and 2). The review was limited to randomized controlled trials and cross-sectional and longitudinal studies that involved human participants only. Following the initial data search and removal of duplicates, 2 independent reviewers (SB-H and KMH) screened titles and/or abstracts to identify studies that potentially met the inclusion criteria outlined. Full texts of potentially eligible studies were then retrieved and independently assessed for eligibility.
Defining the term “older adults” varies but, for the purpose of this review, it was ascribed to adults ≥60 y of age, as outlined by the United Nations (10). Protein supplementation refers to supplementing dietary intake with protein through either protein supplements or specific foods targeting an increase in protein intake. Selection criteria were not limited by study duration; therefore, both acute (effects of intake of a single supplement) and longitudinal study (effects of repeated protein supplementation with participants followed over time) results were included with outcomes analyzed separately. The comparator control criterion was no supplementary protein or placebo. Studies must have reported effects of protein supplementation compared with control (acute studies) and control/preintervention (longitudinal studies) on appetite ratings, appetite-related peptides, and/or EI in healthy older adults. To be as broad and inclusive as possible, studies that involved protein supplementation combined with other interventions, such as exercise or as part of a supplement along with additional ingredients, were also included. Where studies involved an exercise intervention, the control condition used in meta-analysis was exercise alone or exercise with placebo. There were no inclusion criteria regarding measurement method for appetite ratings or time duration relative to the supplement. Therefore, all questionnaires and parameters that related to measurement of subjective appetite were included. Similarly, for appetite-related peptides, there were no inclusion criteria regarding specific outcomes or parameters reported. For EI, in acute studies, data were included as those from an ad libitum test meal served to the participant alongside or after consumption of the protein supplement. For longitudinal studies, there were no prespecified EI inclusion criteria regarding measurement method used (i.e., data from food diary, food-frequency questionnaire, and diet recall were all included). The study selection process is shown in Figure 1.
FIGURE 1.
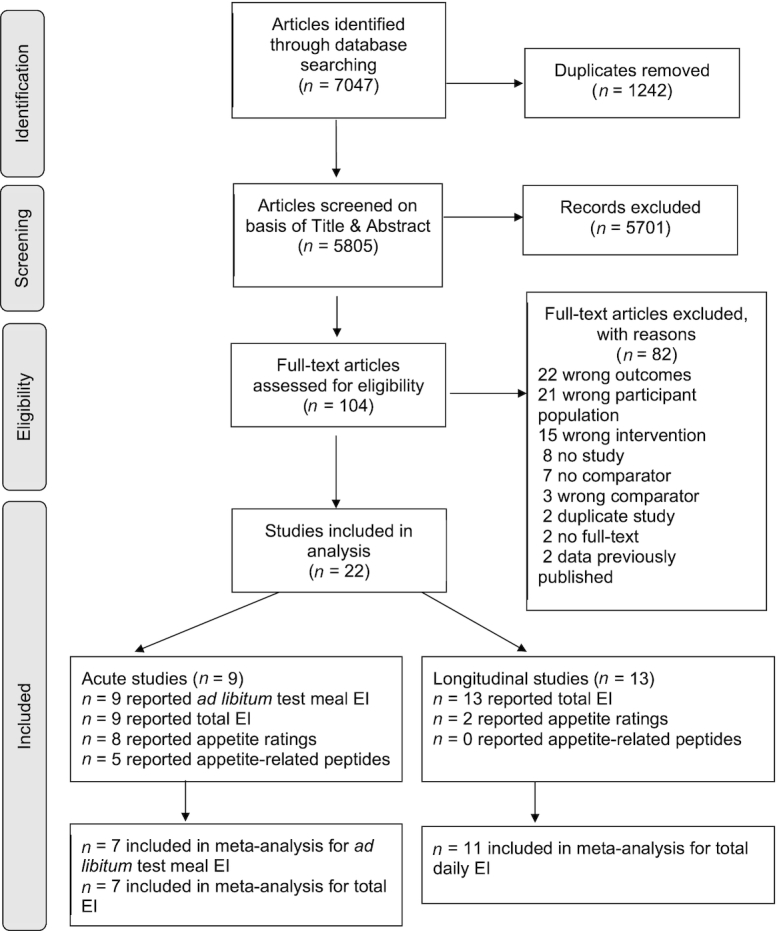
Flowchart of the methodology used to identify studies for inclusion. Total EI for acute studies refers to energy content of the supplement plus energy consumed at the ad libitum meal. EI, energy intake.
Exclusion criteria
Animal studies were excluded. Studies in adults <60 y of age or older adults with known medical conditions reported in the study manuscript or who were hospitalized were excluded.
Type of outcome measures
Primary outcome measures included the following: EI, appetite ratings, and appetite-related peptides. EI was included in the meta-analysis, while appetite ratings and appetite-related peptide outcomes were qualitatively reviewed due to the variability in methods and outcomes reported. Protein intake was also assessed in meta-analysis for longitudinal studies.
Data synthesis
Included studies were extracted into Microsoft Excel (Microsoft Corporation). The following data were extracted: study reference, design, participant information, details of the intervention, outcome measures, and results for each outcome measure. Outcome measures included the following: ad libitum test meal EI (in kilocalories or kilojoules), total EI (energy content of supplement added to ad libitum test meal EI), daily EI, subjective appetite ratings, and appetite-related peptides. Protein intakes (grams per day and grams per kilogram per day) were also assessed for longitudinal studies. Kilocalories were converted to kilojoules and SEM was converted to SD.
Meta-analysis procedures
After data extraction, EI data were entered into Review Manager software (Revman version 5.3.5; The Cochrane Collaboration, Copenhagen, Denmark). The data imputed included sample size, ad libitum EI and total EI with respective SDs. For protein intake, sample size, grams/day, and grams/kilogram per day with respective SDs were included. Estimates of mean difference were synthesized using a random-effects meta-analysis model, based on the assumption that clinical and methodological heterogeneity was likely to exist and to have an effect on the results. Heterogeneity was assessed using the chi-square test and the I2 statistic, with ≥75% classified as considerable heterogeneity (11). If studies reported incomplete data, the authors were contacted to provide missing information. All summarized effects in meta-analysis compared protein supplementation with control. No crossover trials reported correlation coefficients between testing arms. Although the review was not limited to crossover trials, all acute studies retrieved that met the inclusion criteria had crossover designs. All acute studies also included multiple arms (receiving different protein supplement interventions) and each arm was considered independently in analysis, similar to other meta-analyses on EI (12–15). For acute and longitudinal studies, only randomized controlled trials were included in meta-analysis. For longitudinal studies, 1 study (16) had 2 protein parallel subgroups/arms, which were treated separately in analyses. However, as it was the only study with multiple subgroups, the sample size of the control group was halved to reduce bias (11). For longitudinal studies reporting multiple time points, only final time points were used in analysis to reduce bias.
Subgroup and sensitivity analysis
In order to explore potential factors in study-level heterogeneity, subgroup analyses were subsequently undertaken for acute studies based on protein quantity (providing ≤30 g or >30 g), protein type (whey protein versus other (whole food/diet/gel), protein timing (alongside a meal vs. ≥60 min before an ad libitum test meal), protein form (solid/semi-solid vs. liquid), and type of control (flavored water/water, saline, or nothing). For longitudinal studies, EI and protein intake were assessed and a subgroup analysis was subsequently completed for study duration (≤12 wk and >12 wk), for intervention type (protein supplementation alone vs. protein supplementation combined with exercise intervention) and protein type (whey protein drinks or mixed with other ingredients, protein-enriched milk/milk protein concentrate drinks, or protein through whole foods). To examine the effects of gender, subgroup analysis was also conducted in both acute and longitudinal studies to investigate any differences between female-only studies, male-only studies, and studies that included both genders. For sensitivity analysis, the impact of each study on the combined effect was assessed by leaving 1 study out at a time. Funnel plots were generated to investigate any differences in study effects and publication bias. All analyses were completed using Review Manager software (Revman version 5.3.5; The Cochrane Collaboration) and forest plots graphed using GraphPad Prism version 8.0 for Mac (GraphPad Software, San Diego, CA).
Risk of bias
Trials were assessed using Cochrane's tool for assessing risk of bias (17). The tool includes the following domains: random-sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other sources of bias. Each domain was rated as low risk, unclear risk, or high risk of bias.
Results
Overview
The 22 included studies (n = 9 acute, n = 13 longitudinal) were peer-reviewed journal articles with publication dates spanning between 2007 and 2019 and are summarized in Supplemental Table 3.
Participant demographics
A total of 857 participants (331 males, 526 females) were included across all studies in the review. The mean study sample size was n = 38 (range: 8–114). Participants were healthy free-living older adults who had a mean (SD) age of 71 (3.8) y and a BMI (in kg/m2) (SD) of 25.7 (2.6). Two acute studies (18, 19) and 4 longitudinal studies (16, 20–22) were restricted to female participants only, while 4 acute studies (7, 8, 23, 24) and 3 longitudinal studies were restricted to male participants only (25–27).
Description of included studies
Acute
Out of the 9 acute studies (n = 24 protein arms) included, all were randomized crossover designs (7, 8, 18, 19, 23, 24, 28–30). For supplementing protein, whey protein drinks providing 15 to 70 g protein (n = 10 arms) or whey protein infusions providing between 8 and 48 g protein (n = 3 arms) were used in 6 studies (7, 8, 18, 23, 24, 28). One study provided whey protein as part of a mixed-macronutrient drink providing 14 and 70 g protein, respectively (n = 2 arms) (23). Two studies used a gel including commercially available essential amino acids (EAAs) together with other macronutrients providing 7.5 to 15 g protein (n = 3 arms) (18, 19). One of those studies in separate arms used a bar including commercially available EAAs together with other macronutrients providing 25 g protein (n = 2 arms) (19). One study used a soy protein drink (n = 1 arm), milk (n = 1 arm), Greek yogurt (n = 1 arm), and cheddar cheese (n = 1 arm), providing 12, 15, 23, and 13 g protein, respectively (29). Energy content of the supplements ranged from 126 to 1172 kJ, respectively (see Supplemental Table 3).
Seven studies involved oral administration (8, 18, 19, 23, 24, 28, 29), and 2 publications of the same study but with different appetite-related outcomes in each used intraduodenal infusion (7, 30). For the control arm, water/flavored water (n = 5 studies, n = 15 arms) (8, 23, 24, 28, 29), saline infusion (n = 2 studies, n = 3 arms) (7, 30), or nothing (n = 2 studies, n = 6 arms) (18, 19) were used. Ad libitum test meal EI was assessed either immediately (n = 3 arms) (19, 24), 1 h after the supplement (n = 8 arms) (7, 18, 19, 24), 2 h after the supplement (n = 1 arm) (24), or 3 h after the supplement (n = 12 arms) (8, 23, 24, 28, 29). All acute studies that measured appetite ratings (n = 8, n = 22 arms) used visual analog scales (VAS) (7, 8, 18, 19, 23, 24, 28, 29), with 1 study reporting results for 2 protein arms for men and women separately (28)—therefore, totaling 24 comparisons. Results were reported for hunger (n = 16 arms), fullness (n = 16 arms), desire to eat (n = 16 arms), prospective food consumption (n = 16 arms), and composite appetite scores (n = 8 arms).
Longitudinal
Thirteen longitudinal studies (n = 14 protein arms) were included (16, 20–22, 25–27, 31–36). All were randomized controlled trials; one had a crossover design (25) and 12 had a parallel-group design. Six studies (n = 7 protein arms) used between 20 and 35 g of whey protein drinks (16, 21, 22, 26, 27, 33), daily in 3 of these studies (21, 26, 27) and on resistance-training days only (3 d/wk) in 3 studies (16, 22, 33). One study used a milk protein concentrate drink providing 31 g protein daily (36), and 3 studies used protein-enriched milk (∼20% whey and 80% casein) providing 15 g protein daily (31), 10.5 g daily (34), and 5 g twice daily (32). Three studies used high-protein diets as interventions. One study prepared the diet using different protein sources to provide 1 g ⋅ kg−1 ⋅ d−1 protein, including but not limited to, dairy, grains, and legumes (25); and 1 study added two 80-g servings of lean red meat to the overall diet providing overall ∼45g protein consumed 6 d/wk (20). Another study provided 5 protein-enriched readymade meals (7 g more protein than control) and protein-enriched bread (2.2 g more protein than control with, on average, 3 slices consumed/d) for 5 d/wk (35). Three studies included vitamin D: in 1 study for both protein and control groups (20) and in 2 studies for the protein groups only (26, 27). Eight studies (n = 9 protein arms) included resistance training as part of the intervention alongside protein supplementation, which ranged between 2 to 3 times/wk (16, 20–22, 26, 31, 33, 34); and 1 study (n = 1 protein arm) included walking but did not specify range or duration (36).
For the control group, 2 studies used an isocaloric maltodextrin drink (16, 22), 1 study used an isocaloric and calcium-matched carbohydrate drink (32), and 1 study used a mixed-macronutrient isocaloric flavor-matched placebo drink (containing 1.1 g protein, 5.2 g fat, and 36 g carbohydrate) (36). One study used a maltodextrin drink that was lower in energy content compared with the protein supplement (26) and 1 study used a nonprotein placebo (containing 7.13 g lactose, 0.42 g calcium) that was lower in energy content compared with the protein supplement (31). One study used habitual diet (34) and 1 study habitual diet with carbohydrate of ≥1 serving added (20). Two studies provided nothing for the control arm (25, 33). One study provided regular meals comparable to the intervention without protein enrichment matched for weight but not energy (35), and 1 study used flavored water (27). One study asked participants in the control group to maintain at least 1.2 g ⋅ kg−1 ⋅ d−1 of protein while consuming a normal diet (21).
Longitudinal studies had a mean intervention duration of 14 wk (range: 2–26 wk). Six studies assessed EI using 24-h recall (16, 20, 25, 32, 35, 36), and 7 by food diary [3 d (n = 5) (22, 26, 27, 33, 34), 4 d (n = 1) (31), 5 d (n = 1) (21)]. Two longitudinal studies assessed appetite ratings, one used VAS (33), while the other study used generalized labeled magnitude scales (gLMS) (25). Appetite ratings were reported for hunger and fullness (n = 2 studies) (25, 33), desire to eat (n = 1) (25), “how much you can eat” (n = 1) (33) and a combined satiety score (n = 1) (33).
Qualitative review
Acute interventions: impact on appetite measures
Eight acute studies (n = 24 comparisons) reported results for appetite ratings (see Supplemental Table 3). Four studies reported protein arms (n = 7) in which appetite was suppressed compared with control (7, 19, 28, 29), with composite appetite score lower after a whey protein drink versus control (nothing) (18), after a protein bar versus control (nothing) (19), and after milk, soy beverage, Greek yogurt, and cheddar cheese versus control (water) (29). In 1 study in older women only, prospective food consumption was lower after 70 g protein (1172 kJ) versus control (flavored water), but there was no difference in other appetite sensations (hunger, fullness, or desire to eat) and no differences in older men (28).
Composite appetite score did not differ after a protein gel versus control (nothing) in 2 studies (18, 19). Similarly, 3 studies reported no difference in hunger, fullness, desire to eat, or prospective food consumption between a 70-g whey protein drink (1172 kJ), a 14-g whey protein mixed-macronutrient drink (1172 kJ), or a 70-g whey protein mixed-macronutrient drink (2109 kJ) compared with control (water) (23) between 30 g whey protein (502 kJ) and control (water) served at 4 different time intervals in relation to a subsequent meal (24) or between 30 g whey protein (502 kJ) and 70 g whey protein (1172 kJ) and control (water) in another study (8).
One study reported that prospective food consumption was higher after a 126-kJ protein intraduodenal infusion versus control (saline), but no differences were found for 377 kJ or 753 kJ loads or for any other sensations (hunger, fullness, desire to eat) compared with control (7).
Five studies (n = 12 protein arms) reported appetite-related peptide results (18, 19, 23, 28, 30), including results for total or acylated ghrelin (n = 4 studies), peptide YY (tyrosine tyrosine) (PYY; n = 4 studies), cholecystokinin (CCK; n = 2 studies), glucagon-like peptide 1 (GLP-1; n = 3 studies), and gastric inhibitory polypeptide (GIP; n = 2 studies) (see Supplemental Table 3). Three studies (n = 7 out of 7 arms) reported that ghrelin was decreased after protein supplementation compared with control (flavored water or saline) (23, 28, 30), and 1 study showed a trend towards a reduction in acylated ghrelin after consumption of a bar and gel compared with control (nothing) (n = 2 out of 2 arms) (19). In contrast, CCK (n = 2 studies, n = 5 out of 5 arms) (23, 28), GLP-1 (n = 3 studies, n = 7 out of 8 arms) (23, 28, 30), GIP (n = 2 studies, n = 5 out of 5 arms) (23, 28), and PYY (n = 4 studies, n = 8 out of 11 arms) (18, 19, 23, 28) were reported to increase after a protein supplement versus control (nothing, flavored water or saline). There was no difference in GLP-1 or PYY after an 8-g protein infusion and no difference in PYY after a 48-g infusion versus saline in 1 study (23). There was also no difference in PYY in 1 study after a protein gel versus control (nothing) (18).
Longitudinal studies: impact on appetite measures
Two studies reported effects on appetite ratings. One showed hunger to be suppressed on higher-protein (1.0 g ⋅ kg−1 ⋅ d−1) compared with lower-protein (0.75 g ⋅ kg−1 ⋅ d−1 and 0.5 g ⋅ kg−1 ⋅ d−1) diets and desire to eat also decreased after the higher-protein (1.0 g ⋅ kg−1 ⋅ d−1) compared with the lowest-protein (0.5 g ⋅ kg−1 ⋅ d−1) diets (25). The other showed that hunger after whey consumption was higher after 11 wk of whey protein supplementation compared with preintervention (33). No longitudinal studies investigated effects on appetite-related peptides.
EI
Thirteen studies reported effects on EI. Compared with control conditions, 12 reported no effect of protein supplementation compared with control on daily EI (16, 20–22, 25–27, 31, 32, 34–36). One study with no control-group data reported no difference between baseline and week 11 of supplementation (33).
Meta-analysis
Acute studies—ad libitum test meal EI
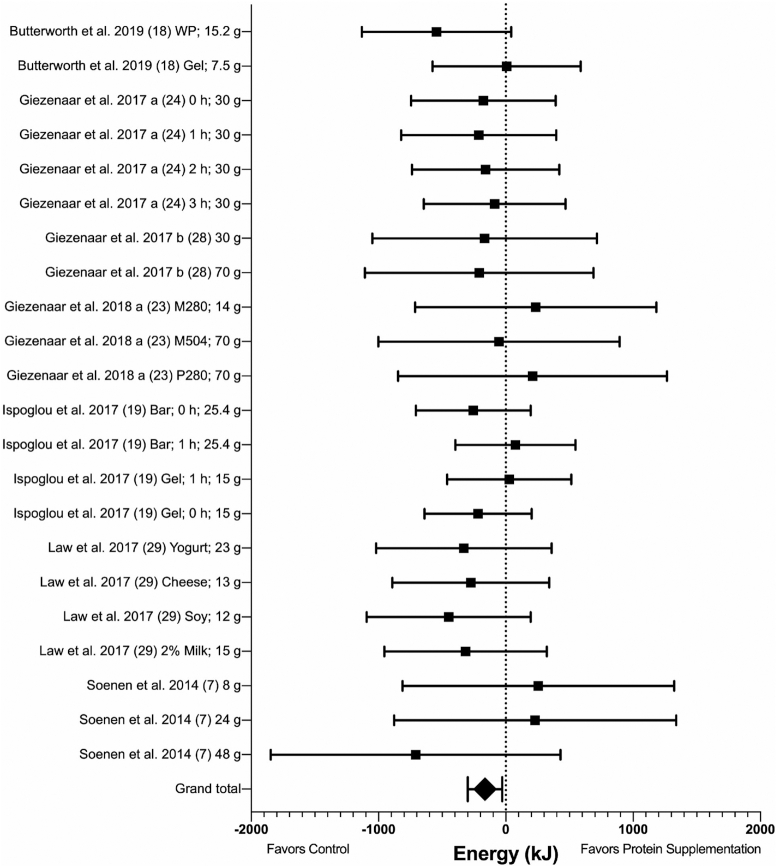
While a total of 9 studies included EI as an outcome, ad libitum EI data for n = 7 (n = 22 arms) studies were included in meta-analysis. Two studies were excluded for the following reasons: one reported previously published results (23), and one lacked usable outcome data (8). However, a study that incorporated these data was subsequently published with usable outcome data and included in the meta-analysis (28). The 7 acute studies included involved a total of 116 participants (61 males, 55 females) with a mean (SD) age of 71 (3.5) y and BMI of 25.3 (2.1).
Results favored suppression of EI following protein supplementation compared with control [mean difference (MD): −164 kJ; 95% CI: −299, −29 kJ; P = 0.02; I2 = 0%) (Figure 2 and Supplemental Figure 1). The exclusion of Soenen et al. (7), in which protein was administered intraduodenally, did not alter the significance of results. No heterogeneity was observed between studies for ad libitum test meal EI, and subgroup analyses showed no significant differences based on protein form, protein type, protein timing, protein quantity, control types, and gender analysis (see Supplemental Table 4).
FIGURE 2.
Forest plot of the effects of protein supplementation compared with control on EI at an ad libitum test meal in healthy older adults. Results of random-effects meta-analysis (n = 7 studies, n = 22 comparisons) are shown as MDs with 95% CIs. MD: −164 kJ; 95% CI: −299, −29 kJ; P = 0.02; I2 = 0%. EI, energy intake; MD, mean difference; M280, mixed macronutrient 280 kcal (1172 kJ); M504, mixed macronutrient 504 kcal (2109 kJ); P280, whey protein 280 kcal (1172 kJ); WP, whey protein.
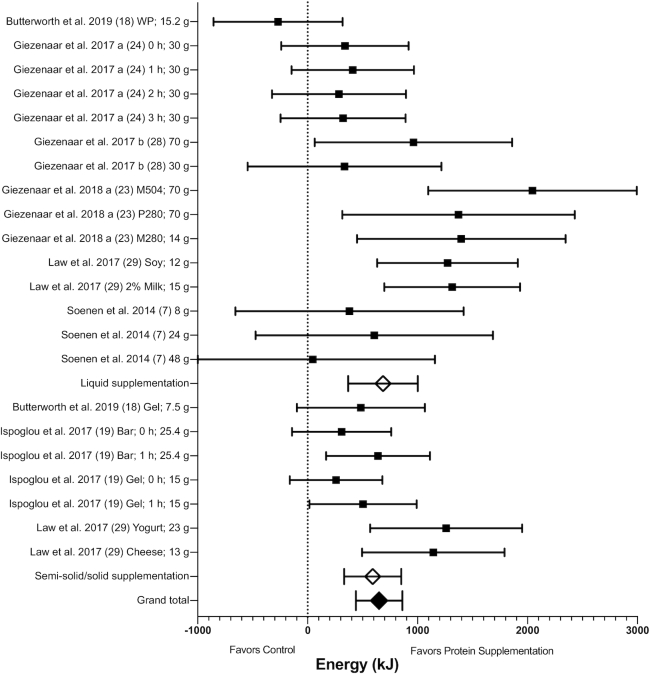
Total EI
Total EI (combining energy content of the supplement with EI at the test meal) data were available for n = 7 studies (n = 22 arms) for meta-analysis. Results showed that there was an increase in EI with protein supplementation compared with control (MD: 649 kJ; 95% CI: 438, 861 kJ; P < 0.00001) (Figure 3 and Supplemental Figure 2). The exclusion of Soenen et al. (7), where protein was administered intraduodenally, did not alter the significance of results.
FIGURE 3.
Forest plot of the effects of protein supplementation compared with control on total EI (energy content of the supplement plus energy consumed at an ad libitum test meal) in healthy older adults. Results of random-effects meta-analysis (n = 7 studies, n = 22 comparisons) are shown as MDs with 95% CIs. Overall effect—MD: 649 kJ; 95% CI: 438, 861 kJ; P < 0.00001; I2 = 56%. Subgroup differences are also shown for protein supplementation provided in liquid and semi-solid/solid form. EI increased with supplementation provided in both liquid (MD: 685 kJ; 95% CI: 369, 1002 kJ; P < 0.0001, I2 = 62%) and in semi-solid/solid form (MD: 592 kJ; 95% CI: 332, 852 kJ; P < 0.00001; I2 = 42%), with no subgroup difference (P = 0.66). EI, energy intake; MD, mean difference; M280, mixed macronutrient 280 kcal (1172 kJ); M504, mixed macronutrient 504 kcal (2109 kJ); P280, whey protein 280 kcal (1172 kJ); WP, whey protein.
Given the observed heterogeneity (I2 = 56%), subgroup analysis was performed for protein form, type, quantity of protein provided, timing of protein intake, the control type used, and gender (Table 1). There were no significant subgroup differences, except for protein timing, control type, and gender. Energy intake was higher when the protein supplement was served ≥60 min prior to the meal compared with when served alongside or immediately prior to the meal (P = 0.02; Table 1). However, EI was still significantly higher in both groups compared with control. EI was also higher with protein supplementation in studies when water/flavored water was used as the control arm compared with saline infusion or nothing being used (P = 0.01; Table 1). In addition, although EI was higher with protein supplementation versus control in studies that involved females only, males only, and studies that included both genders, there were significant subgroup differences, with a higher EI with protein supplementation in male-only and mixed-gender studies (P = 0.0002; Table 1).
TABLE 1.
Subgroup analyses for total energy intake (energy content of the supplement plus energy consumed at an ad libitum test meal), according to protein form, type, timing, quantity, type of control used, and gender analysis in healthy older adults
| Number of comparisons | Mean difference, kJ | 95% CI | Heterogeneity (I2), % | P | P value for subgroup difference | |
|---|---|---|---|---|---|---|
| Total energy intake | 22 | 649 | 438, 861 | 56 | <0.00001 | |
| Protein form | 0.66 | |||||
| Liquid | 15 | 685 | 369, 1002 | 62 | <0.0001 | |
| Semi-solid/solid | 7 | 592 | 332, 852 | 42 | <0.00001 | |
| Protein type | 0.40 | |||||
| Whey protein | 13 | 561 | 242, 880 | 54 | <0.001 | |
| Protein through diet/whole foods/other | 9 | 745 | 463, 1027 | 59 | <0.00001 | |
| Protein timing | 0.02 | |||||
| Alongside meal | 3 | 295 | 23, 567 | 0 | 0.03 | |
| ≥60 min before ad libitum test meal | 19 | 730 | 485, 976 | 57 | <0.00001 | |
| Protein quantity | 0.18 | |||||
| ≤30 g | 18 | 576 | 371, 782 | 50 | <0.00001 | |
| >30 g | 4 | 1133 | 340, 1925 | 60 | <0.01 | |
| Control types | 0.01 | |||||
| Water/flavored water | 13 | 899 | 600, 1197 | 57 | <0.00001 | |
| Saline | 3 | 350 | −270, 971 | 0 | 0.27 | |
| Nothing | 6 | 340 | 110, 569 | 23 | <0.01 | |
| Gender analysis | <0.001 | |||||
| Female-only studies | 6 | 340 | 110, 569 | 23 | <0.01 | |
| Male-only studies | 10 | 650 | 297, 1002 | 50 | <0.001 | |
| Both genders | 6 | 1122 | 834, 1410 | 0 | <0.00001 | |
Longitudinal studies
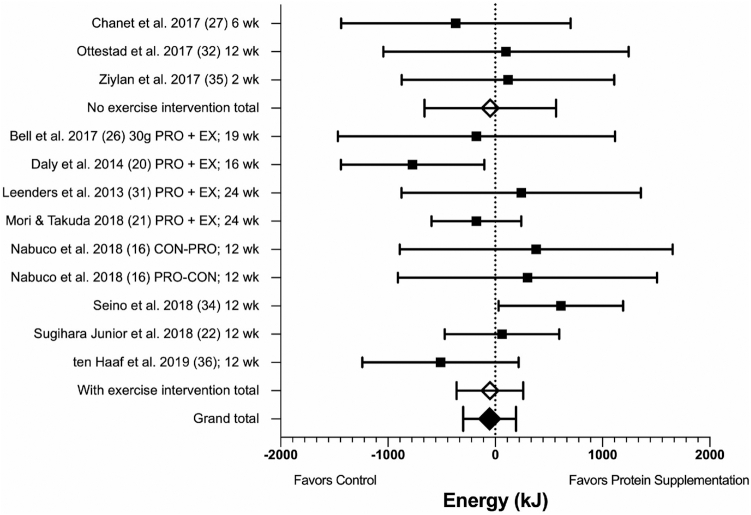
Eleven longitudinal studies (n = 12 protein arms), all with a parallel-group design, involving 687 participants (236 males, 451 females) with a mean (SD) age of 70 (3.9) y and BMI of 25.9 (2.8) were included in meta-analysis. Two studies were excluded from meta-analysis: one because it was the only study with a different design than all others [crossover design (25)] and one due to data being available for the intervention group only (33). Although 1 study lacked usable outcome data in the published manuscript, the relevant data were provided by the authors upon request and therefore included (31).
There was no difference in self-reported daily EI between those assigned to protein supplementation compared with control at the end of trials (MD: −54 kJ/d; 95% CI: −300, 193 kJ/d; P = 0.67, I2 = 16%; Figure 4). With regard to the type of intervention, a subgroup analysis showed no significant difference between studies involving protein supplementation only compared with those combining protein supplementation with exercise training (P = 0.99; Figure 4, Supplemental Figure 3). In addition, there were no significant subgroup differences based on protein type (P = 0.68) or gender of participants (P = 0.52). There was a trend (P = 0.08) towards a difference between shorter-duration (≤12 wk) and longer-duration studies with a lower EI in longer-duration studies (Supplemental Figures 4–6).
FIGURE 4.
Forest plot for longitudinal studies showing the mean difference in EI between control and protein supplement groups, at the end of trials, in healthy older adults. Results of random-effects meta-analysis (n = 11 studies, n = 12 comparisons) are shown as MDs with 95% CIs. Overall effect: MD: −54 kJ/d; 95% CI: −300, 193; P = 0.67; I2 = 16%. Subgroup analysis is shown for interventions involving protein supplementation only (MD: −47 kJ/d; 95% CI: −660, 567 kJ/d; P = 0.88; I2 = 0%) compared with those combining protein supplementation with exercise intervention (MD: −50 kJ/d; 95% CI: −362, 261 kJ/d; P = 0.75; I2 = 36%), with no subgroup difference (P = 0.99). EI, energy intake; MD, mean difference.
Protein intake was significantly higher in the protein intervention compared with control arms at the end of trials (available in grams per day for n = 9 out of 12 arms, and grams/kilogram per day for n = 11 out of 12 arms; MD: 18.21 g/d; 95% CI: 7.39, 29.04 g/d; P = 0.001; I2 = 96%; MD: 0.29 g ⋅ kg−1 ⋅ d−1; 95% CI: 0.14, 0.45 g ⋅ kg−1 ⋅ d−1; P = 0.0003; I2 = 95%) (Supplemental Figures 7–10), illustrating the interventions were effective overall in increasing protein intake. Subgroup analyses were performed to explore the heterogeneity, with no differences between interventions with or without exercise or between shorter- and longer-duration interventions (Supplemental Figures 7–10).
Risk-of-bias assessment, publication bias, and sensitivity analysis
Full details of the risk-of-bias assessment for clinical trials are provided in Supplemental Table 5. Among the 22 trials, the main issues were the high risk of bias due to lack of blinding of participants and/or study personnel, and lack of blinding of outcome assessment. However, >50% of trials still had low bias for both of these items. Information on allocation concealment and selective reporting was unclear for most trials. A summary of the proportion of trials that were at low, unclear, and high risk of bias for each domain is shown in Figure 5.
FIGURE 5.
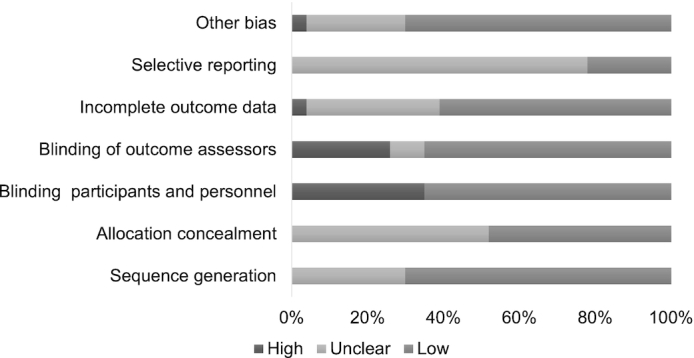
Review of authors’ judgements about each risk-of-bias item, presented as percentages across all included studies.
No evidence of asymmetry was observed in funnel plots for acute and longitudinal studies (Supplemental Figures 11–13), implying no publication bias. In addition, sensitivity analysis showed that no single study altered the outcomes for acute or longitudinal study analyses.
Discussion
The review and meta-analysis provide evidence that protein supplementation may suppress appetite under some conditions in older adults. However, there is either a positive effect or no effect on EI in both acute and longitudinal studies, respectively. This contrasts with findings in younger adults showing appetite and EI suppression following a protein preload (7). Moreover, longitudinal studies showed a higher protein intake in protein-supplementation groups compared with control groups at the end of trials. This highlights an important gap in knowledge wherein protein supplementation may be a promising strategy to address protein deficiencies in older adults, without compromising appetite and total daily EI. Clearly the interrelation between protein and appetite may be skewed according to age, although when that age-dependent threshold affects appetite is not defined.
Acute studies
Although acute studies showed a lower EI at a test meal after protein supplementation compared with control, once the energy content of the supplement was accounted for, supplementation increased total EI. This illustrates the positive effect that protein supplementation can have on overall EI following intake of a single supplement. Interestingly, total EI increased in studies providing quantities ≤30 g and >30 g, with a higher EI in those studies providing >30 g, although this difference was not significant. Two acute studies directly compared different protein loads (30 g or 70 g) on EI (8, 28). One study showed that total EI was increased by 32% for the highest 70-g (1172 kJ) protein load compared with 12% for the 30-g (502 kJ) protein load (28). Elsewhere, no difference in total EI was observed following a mixed-macronutrient drink containing 14 g whey protein compared with a 70-g whey protein drink matched for energy content (1172 kJ) (23). Overall, these observations are consistent with the current literature that older adults are less responsive to quantity of protein load in terms of effects on subsequent EI compared with younger adults (8).
The quality of or form in which that protein is provided is also important to consider. Five acute studies used whey protein drinks administered orally (8, 18, 23, 24, 28). Whey protein has a high leucine content, which plays an important role in muscle protein synthesis (19). Our findings from subgroup analysis suggest that whey protein, protein provided through whole foods, diets, and other forms including commercially available amino acids incorporated into gels and bars are all effective for acutely increasing EI in older adults.
The findings also indicate protein supplementation in both liquid and semi-solid/solid forms is effective for increasing EI. Although the mean difference in EI was higher when provided in liquid form compared with semi-solid/solid form, the difference was not statistically significant. Ispoglou et al. (19) investigated the effects of a protein gel (477 kJ) compared with bar (565 kJ) matched for EAA content and control (nothing). The bar suppressed appetite ratings compared with control and gel. However, when total EI was examined, there was a significant increase in total EI with both the bar and gel, when compared with control. One study investigated effects of supplementing protein in the diet through different high-protein whole foods (29). Protein intake in solid/semi-solid form (cheese, yogurt) reduced appetite ratings to a greater extent than liquid intake (soy beverage or 2% milk) (29). However, there was no difference in EI between the different protein arms. This supports previous findings that supplementation in liquid form has less of a suppressive effect on appetite ratings compared with solids (37–39), but both forms appear to be effective for increasing EI.
Co-ingestion of protein with other nutrients may also impact the appetite and EI response. However, few acute studies have directly compared the effects of combining protein with other nutrients. Giezenaar et al. (23) nevertheless showed no difference in appetite ratings or EI whether protein was provided as a whey protein drink only compared with when provided as part of a mixed-macronutrient drink matched for energy content or when provided as part of a mixed-macronutrient drink matched for protein but not energy content. These findings indicate the addition of other macronutrients to protein had no impact on appetite or EI.
The timing of the protein preload could be another factor influencing subsequent EI. Subgroup analysis showed that total EI increased regardless of whether it was consumed ≥1 h before the meal or alongside the meal. However, EI increased to a lesser extent when served alongside the meal, as could be expected from existing literature in the general population (39, 40). In contrast, Giezenaar et al. (24) directly compared ingestion of whey protein loads at 3, 2, and 1 h or immediately before an ad libitum buffet meal and found that there was no effect of timing of protein ingestion. As the use of a buffet meal may produce different results compared with a single-course test meal (40), further studies are warranted to investigate the impact of protein timing relative to meals on EI in older adults. Overall, the results suggest that both protein supplementation prior to a meal or alongside a meal is effective for increasing EI.
Gender differences in response to protein supplementation have been examined previously in healthy young adults (41), showing that total EI increased in women more than in men. The present meta-analysis showed that, in older adults, total EI was increased following protein supplementation in studies involving men only and women only, as well as in studies involving both. However, the increase was greater in studies of men only or studies combining both genders compared with studies of women only. This illustrates that protein supplementation is effective for increasing EI in both men and women, but the extent to which this occurs could be influenced by gender and warrants further study.
Longitudinal studies
Two longitudinal studies reported appetite ratings. One study found that hunger and desire to eat were higher when subjects consumed diets containing 63% and 94% of the recommended daily intake (RDI) for protein, compared with 125% of the RDI (25). Elsewhere, Ridge et al. (33) found that, in response to whey protein ingestion, hunger was higher following 11 wk of whey protein supplementation and exercise compared with preintervention. The latter finding could also be influenced by the exercise intervention as there was no control group for comparison. Given these limited and mixed results, further studies are needed to examine the effects of longer-term protein supplementation on subjective appetite ratings. Similarly, given that no studies reported effects on appetite-related gut peptides, this warrants further investigation.
With regard to protein and EI, meta-analysis showed the interventions were successful in increasing protein intake compared with control with no significant effects on daily EI. However, there was a trend towards a higher EI in shorter-term studies (≤12 wk) compared with longer-duration studies (>12 wk), suggesting the duration of supplementation may influence the EI response. As longer-duration studies were limited and there was significant variability in responses, further studies are needed to determine whether the duration of intervention may potentially influence the appetite and EI response.
Exercise is another essential aspect for the prevention of loss of muscle mass. Several studies (n = 9) combined protein supplementation with exercise intervention (16, 20–22, 26, 31, 33, 34, 36). However, no significant effect on EI was reported compared with control (16, 20–22, 26, 31, 33, 34, 36). Similarly, meta-analysis showed no difference in responses between interventions that included protein supplementation only or those that combined protein supplementation with exercise compared with control.
Longitudinal studies showed similar effects regardless of the type of protein used, with findings of no effects of protein-enriched meals on daily EI (20, 25, 32, 34–36), or when supplementing with whey protein alone (16, 22, 33), compared with control. Others similarly showed that, when whey was combined with other nutrients and micronutrients, there was no effect on EI (21, 26, 27). These findings were supported by meta-analysis of effects of protein type on EI and indicate no effect of protein supplementation on EI regardless of the type of supplement used in longitudinal studies.
Several methodological aspects of the present review should be considered. The study excluded those <60 y old and those with any medical conditions. This limits the generalizability of the findings. The inclusion of crossover studies with multiple study arms raises the issue of “double counting” (11) for the acute-study meta-analysis. We followed a similar approach to several meta-analyses examining appetite and energy intake (12–15, 42) by considering the separate comparisons from multiple subgroups independently. This may impact estimates of variance; however, this is difficult to eradicate in appetite studies when studies are of this design (42). In addition, as all acute studies had a crossover design and multiple subgroups this should not bias any single study. The timing of intake was not controlled and, therefore, whether protein supplementation has different effects based on time of day warrants further investigation. It should also be recognized that appetite sensations do not provide a complete representation of appetite control and other processes also contribute (43). The individual contributions to satiety of some gut hormones are unclear (44), and this also needs to be considered in interpreting the findings. To provide a more comprehensive overview, the current review aimed to provide insight into both subjective and objective measures of appetite and EI. The limitations of assessing EI by self-report in longitudinal studies should also be recognized. Change in body weight can also be studied as an objective proxy measure to determine whether EI changed over time. Eight studies reported body mass changes, with mixed results and the majority favoring no weight change [n = 7 no weight change (21, 26, 27, 31, 32, 34, 36), n = 1 body weight increase postintervention (33)].
Further studies are needed to investigate different types of protein: for example, only 1 study included a plant protein (29). The effects of combining protein supplementation with other key nutrients such as fiber, which may be deficient in older adults (45), on appetite and EI also warrant further study. The impact of pre-sleep protein intake is another area that needs further investigation, although recent work suggests no effect on next-day appetite in older adults (46). Although gut hormones are proposed as a potential underlying mechanism behind the reason that protein may suppress appetite to a lesser extent in older compared with younger adults (8), the influence of psychological aspects of the “anorexia of aging” on the appetite response to protein also warrants further investigation (47). Interindividual variability in appetite and EI responses should also be considered further in future studies (43, 48). The present review focused on healthy older adults; however, further studies are needed to investigate effects on appetite and EI in other older-adult populations: for example, those with diagnosed medical conditions such as sarcopenia or frail older adults in whom protein supplementation has been shown to have beneficial effects on physical performance (49) and muscle mass when combined with exercise (50).
Overall, the findings have implications for increasing protein intakes in healthy older adults, highlighting that, while appetite ratings and related peptides may be suppressed under some conditions, protein supplementation does not compromise EI in this population. Protein supplements in all forms appear to be effective in increasing EI acutely in healthy older adults. This can be achieved through providing protein supplementation before the meal or alongside a meal, although supplementation ≥1 h before a meal may be particularly effective. Protein supplementation can also be used effectively in the longer term to increase protein intake, without suppressing EI. This supports the use of protein supplementation in healthy older adults without compromising EI.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—SB-H, HMR, CAC, and KMH: contributed to the conception of the research; KMH and SB-H: designed the research, performed the systematic review, and wrote the manuscript; SBH: performed meta-analysis; KMH, HMR, and CAC: reviewed and revised the manuscript; KMH: had primary responsibility for final content; and all authors: read and approved the final manuscript.
Notes
SB-H is funded by an Irish Research Council postgraduate scholarship. No grant was received from a funding agency for this review. Work pertaining to this article was supported by the Irish Department of Agriculture, Food and Marine “Nutrimal Programme” 14/F/822 (HMR and CAC) and Irish Health Research Board “ONSPres programme” RCQPS-2017-4 (CAC).
Author disclosures: The authors report no conflicts of interest.
Supplemental Tables 1–5 and Supplemental Figures 1–13 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances.
Data described in the manuscript are available in the Supplemental Material Table 3, Table 4, Figures 1–10 and available upon request.
Abbreviations used: CCK, cholecystokinin; EAA, essential amino acid; EI, energy intake; GIP, gastric inhibitory polypeptide; GLP-1, glucagon-like peptide 1; MD, mean difference; PYY, peptide YY (tyrosine tyrosine); RDI, recommended daily intake; VAS, visual analog scale.
Contributor Information
Sana Ben-Harchache, School of Public Health, Physiotherapy and Sport Science, University College Dublin, Belfield, Dublin, Ireland; Institute of Food and Health, University College Dublin, Belfield, Dublin, Ireland.
Helen M Roche, School of Public Health, Physiotherapy and Sport Science, University College Dublin, Belfield, Dublin, Ireland; Institute of Food and Health, University College Dublin, Belfield, Dublin, Ireland; Nutrigenomics Research Group, UCD Conway Institute, University College Dublin, Belfield, Dublin, Ireland; Institute for Global Food Security, Queen's University Belfast, Belfast, Northern Ireland.
Clare A Corish, School of Public Health, Physiotherapy and Sport Science, University College Dublin, Belfield, Dublin, Ireland; Institute of Food and Health, University College Dublin, Belfield, Dublin, Ireland.
Katy M Horner, School of Public Health, Physiotherapy and Sport Science, University College Dublin, Belfield, Dublin, Ireland; Institute of Food and Health, University College Dublin, Belfield, Dublin, Ireland.
References
- 1. World Health Organization . Global strategy and action plan on ageing and health. Geneva (Switzerland): World Health Organization; 2017. [Google Scholar]
- 2. Cruz-Jentoft AJ, Landi F, Schneider SM, Zúñiga C, Arai H, Boirie Y, Chen LK, Fielding RA, Martin FC, Michel JPet al. Prevalence of and interventions for sarcopenia in ageing adults: a systematic review. Report of the International Sarcopenia Initiative (EWGSOP and IWGS). Age Ageing. 2014;43(6):748–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bauer J, Biolo G, Cederholm T, Cesari M, Cruz-Jentoft AJ, Morley JE, Phillips S, Sieber C, Stehle P, Teta Det al. Evidence-based recommendations for optimal dietary protein intake in older people: a position paper from the PROT-AGE Study Group. J Am Med Dir Assoc. 2013;14(8):542–59. [DOI] [PubMed] [Google Scholar]
- 4. Deutz NEP, Bauer JM, Barazzoni R, Biolo G, Boirie Y, Bosy-Westphal A, Cederholm T, Cruz-Jentoft A, Krznaric Z, Nair KSet al. Protein intake and exercise for optimal muscle function with aging: recommendations from the ESPEN Expert Group. Clin Nutr. 2014;33(6):929–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Krok-Schoen JL, Archdeacon Price A, Luo M, Kelly OJ, Taylor CA. Low dietary protein intakes and associated dietary patterns and functional limitations in an aging population: a NHANES analysis. J Nutr Health Aging. 2019;23(4):338–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Paddon-Jones D, Westman E, Mattes RD, Wolfe RR, Astrup A, Westerterp-Plantenga M. Protein, weight management, and satiety. Am J Clin Nutr. 2008;87(5):1558s–61s. [DOI] [PubMed] [Google Scholar]
- 7. Soenen S, Giezenaar C, Hutchison AT, Horowitz M, Chapman I, Luscombe-Marsh ND. Effects of intraduodenal protein on appetite, energy intake, and antropyloroduodenal motility in healthy older compared with young men in a randomized trial. Am J Clin Nutr. 2014;100(4):1108–15. [DOI] [PubMed] [Google Scholar]
- 8. Giezenaar C, Trahair LG, Rigda R, Hutchison AT, Feinle-Bisset C, Luscombe-Marsh ND, Hausken T, Jones KL, Horowitz M, Chapman Iet al. Lesser suppression of energy intake by orally ingested whey protein in healthy older men compared with young controls. Am J Physiol Regul Integr Comp Physiol. 2015;309(8):R845–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Milne AC, Potter J, Vivanti A, Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst Rev. 2009;2:CD003288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. United Nations . World population ageing 2019: highlights. [cited 2020 Apr 10] [Internet]. Available from: https://www.un.org/en/development/desa/population/publications/pdf/ageing/WorldPopulationAgeing2019-Highlights.pdf. [Google Scholar]
- 11. Higgins JPT, Green S. Cochrane handbook for systematic reviews of interventions. Hoboken (NJ): Wiley-Blackwell; 2008. [Google Scholar]
- 12. Schubert MM, Desbrow B, Sabapathy S, Leveritt M. Acute exercise and subsequent energy intake: a meta-analysis. Appetite. 2013;63:92–104. [DOI] [PubMed] [Google Scholar]
- 13. Schwartz C, King NA, Perreira B, Blundell JE, Thivel D. A systematic review and meta-analysis of energy and macronutrient intake responses to physical activity interventions in children and adolescents with obesity. Pediatr Obes. 2017;12(3):179–94. [DOI] [PubMed] [Google Scholar]
- 14. Thivel D, Rumbold PL, King NA, Pereira B, Blundell JE, Mathieu ME. Acute post-exercise energy and macronutrient intake in lean and obese youth: a systematic review and meta-analysis. Int J Obes. 2016;40(10):1469–79. [DOI] [PubMed] [Google Scholar]
- 15. Chapman CD, Benedict C, Brooks SJ, Schiöth HB. Lifestyle determinants of the drive to eat: a meta-analysis. Am J Clin Nutr. 2012;96(3):492–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Nabuco HCG, Tomeleri CM, Sugihara Junior P, Fernandes RR, Cavalcante EF, Antunes M, Ribeiro AS, Teixeira DC, Silva AM, Sardinha LBet al. Effects of whey protein supplementation pre- or post-resistance training on muscle mass, muscular strength, and functional capacity in pre-conditioned older women: a randomized clinical trial. Nutrients. 2018;10(5):563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JA. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Butterworth M, Lees M, Harlow P, Hind K, Duckworth L, Ispoglou T. Acute effects of essential amino acid gel-based and whey protein supplements on appetite and energy intake in older women. Appl Physiol Nutr Metab. 2019;44(11):1141–9. [DOI] [PubMed] [Google Scholar]
- 19. Ispoglou T, Deighton K, King RF, White H, Lees M. Novel essential amino acid supplements enriched with L-leucine facilitate increased protein and energy intakes in older women: a randomised controlled trial. Nutr J. 2017;16(1):75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Daly RM, O'Connell SL, Mundell NL, Grimes CA, Dunstan DW, Nowson CA. Protein-enriched diet, with the use of lean red meat, combined with progressive resistance training enhances lean tissue mass and muscle strength and reduces circulating IL-6 concentrations in elderly women: a cluster randomized controlled trial. Am J Clin Nutr. 2014;99(4):899–910. [DOI] [PubMed] [Google Scholar]
- 21. Mori H, Tokuda Y. Effect of whey protein supplementation after resistance exercise on the muscle mass and physical function of healthy older women: a randomized controlled trial. Geriatr Gerontol Int. 2018;18(9):1398–404. [DOI] [PubMed] [Google Scholar]
- 22. Sugihara Junior P, Ribeiro AS, Nabuco HCG, Fernandes RR, Tomeleri CM, Cunha PM, Venturini D, Barbosa DS, Schoenfeld BJ, Cyrino ES. Effects of whey protein supplementation associated with resistance training on muscular strength, hypertrophy, and muscle quality in preconditioned older women. Int J Sport Nutr Exerc Metab. 2018;28(5):528–35. [DOI] [PubMed] [Google Scholar]
- 23. Giezenaar C, van der Burgh Y, Lange K, Hatzinikolas S, Hausken T, Jones KL, Horowitz M, Chapman I, Soenen S. Effects of substitution, and adding of carbohydrate and fat to whey-protein on energy intake, appetite, gastric emptying, glucose, insulin, ghrelin, CCK and GLP-1 in healthy older men—a randomized controlled trial. Nutrients. 2018;10(2):113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Giezenaar C, Coudert Z, Baqeri A, Jensen C, Hausken T, Horowitz M, Chapman I, Soenen S. Effects of timing of whey protein intake on appetite and energy intake in healthy older men. J Am Med Dir Assoc. 2017;18(10):898, e9–13. [DOI] [PubMed] [Google Scholar]
- 25. Apolzan JW, Carnell NS, Mattes RD, Campbell WW. Inadequate dietary protein increases hunger and desire to eat in younger and older men. J Nutr. 2007;137(6):1478–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bell KE, Snijders T, Zulyniak M, Kumbhare D, Parise G, Chabowski A, Phillips SM. A whey protein-based multi-ingredient nutritional supplement stimulates gains in lean body mass and strength in healthy older men: a randomized controlled trial. PLoS One. 2017;12(7):e0181387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chanet A, Verlaan S, Salles J, Giraudet C, Patrac V, Pidou V, Pouyet C, Hafnaoui N, Blot A, Cano Net al. Supplementing breakfast with a vitamin D and leucine-enriched whey protein medical nutrition drink enhances postprandial muscle protein synthesis and muscle mass in healthy older men. J Nutr. 2017;147(12):2262–71. [DOI] [PubMed] [Google Scholar]
- 28. Giezenaar C, Trahair LG, Luscombe-Marsh ND, Hausken T, Standfield S, Jones KL, Lange K, Horowitz M, Chapman I, Soenen S. Effects of randomized whey-protein loads on energy intake, appetite, gastric emptying, and plasma gut-hormone concentrations in older men and women. Am J Clin Nutr. 2017;106(3):865–77. [DOI] [PubMed] [Google Scholar]
- 29. Law M, Lee YT, Vien S, Luhovyy BL, Anderson GH. The effect of dairy products consumed with high glycemic carbohydrate on subjective appetite, food intake, and postprandial glycemia in older adults. Appl Physiol Nutr Metab. 2017;42(11):1210–6. [DOI] [PubMed] [Google Scholar]
- 30. Giezenaar C, Luscombe-Marsh ND, Hutchison AT, Standfield S, Feinle-Bisset C, Horowitz M, Chapman I, Soenen S. Dose-dependent effects of randomized intraduodenal whey-protein loads on glucose, gut hormone, and amino acid concentrations in healthy older and younger men. Nutrients. 2018b;10(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Leenders M, Verdijk LB, Van der Hoeven L, Van Kranenburg J, Nilwik R, Wodzig WK, Senden JM, Keizer HA, Van Loon LJ. Protein supplementation during resistance-type exercise training in the elderly. Med Sci Sports Exerc. 2013;45(3):542–52. [DOI] [PubMed] [Google Scholar]
- 32. Ottestad I, Lovstad AT, Gjevestad GO, Hamarsland H, Saltyte Benth J, Andersen LF, Bye A, Biong AS, Retterstol K, Iversen POet al. Intake of a protein-enriched milk and effects on muscle mass and strength. a 12-week randomized placebo controlled trial among community-dwelling older adults. J Nutr Health Aging. 2017;21(10):1160–9. [DOI] [PubMed] [Google Scholar]
- 33. Ridge A DA, Lyons-wall P, Conlon J, Lo J. The impact of whey protein supplementation in older adults on nutrient intakes and satiety over an 11-week exercise intervention. Food Qual Prefer. 2018;68:72–9. [Google Scholar]
- 34. Seino S, Sumi K, Narita M, Yokoyama Y, Ashida K, Kitamura A, Shinkai S. Effects of low-dose dairy protein plus micronutrient supplementation during resistance exercise on muscle mass and physical performance in older adults: a randomized, controlled trial. J Nutr Health Aging. 2018;22(1):59–67. [DOI] [PubMed] [Google Scholar]
- 35. Ziylan C, Haveman-Nies A, Kremer S, de Groot LC. Protein-enriched bread and readymade meals increase community-dwelling older adults' protein intake in a double-blind randomized controlled trial. J Am Med Dir Assoc. 2017;18(2):145–51. [DOI] [PubMed] [Google Scholar]
- 36. ten Haaf DSM, Eijsvogels TMH, Bongers C, Horstman AMH, Timmers S, de Groot L, Hopman MTE. Protein supplementation improves lean body mass in physically active older adults: a randomized placebo-controlled trial. J Cachexia Sarcopenia Muscle. 2019;10(2):298–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stull AJ, Apolzan JW, Thalacker-Mercer AE, Iglay HB, Campbell WW. Liquid and solid meal replacement products differentially affect postprandial appetite and food intake in older adults. J Am Diet Assoc. 2008;108(7):1226–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Tieken SM, Leidy HJ, Stull AJ, Mattes RD, Schuster RA, Campbell WW. Effects of solid versus liquid meal-replacement products of similar energy content on hunger, satiety, and appetite-regulating hormones in older adults. Horm Metab Res. 2007;39(5):389–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Almiron-Roig E, Palla L, Guest K, Ricchiuti C, Vint N, Jebb SA, Drewnowski A. Factors that determine energy compensation: a systematic review of preload studies. Nutr Rev. 2013;71(7):458–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Blundell J, De Graaf C, Hulshof T, Jebb S, Livingstone B, Lluch A, Mela D, Salah S, Schuring E, Van Der Knaap H. Appetite control: methodological aspects of the evaluation of foods. Obes Rev. 2010;11(3):251–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Giezenaar C, Luscombe-Marsh ND, Hutchison AT, Lange K, Hausken T, Jones KL, Horowitz M, Chapman I, Soenen S. Effect of gender on the acute effects of whey protein ingestion on energy intake, appetite, gastric emptying and gut hormone responses in healthy young adults. Nutr Diabetes. 2018;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Douglas JA, Deighton K, Atkinson JM, Sari-Sarraf V, Stensel DJ, Atkinson G. Acute exercise and appetite-regulating hormones in overweight and obese individuals: a meta-analysis. J Obes. 2016;2016:2643625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gibbons C, Hopkins M, Beaulieu K, Oustric P, Blundell JE. Issues in interpreting and measuring human appetite (satiety/satiation) and its contribution to obesity. Curr Obes Rep. 2019;8:77–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Mars M, Stafleu A, de Graaf C. Use of satiety peptides in assessing the satiating capacity of foods. Physiol Behav. 2012;105(2):483–8. [DOI] [PubMed] [Google Scholar]
- 45. van der Meij BS, Wijnhoven HA, Lee JS, Houston DK, Hue T, Harris TB, Kritchevsky SB, Newman AB, Visser M. Poor appetite and dietary intake in community‐dwelling older adults. J Am Ger Soc. 2017;65(10):2190–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Morehen S, Smeuninx B, Perkins M, Morgan P, Breen L. Pre-sleep casein protein ingestion does not impact next-day appetite, energy intake and metabolism in older individuals. Nutrients. 2020;12(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cox NJ, Morrison L, Ibrahim K, Robinson SM, Sayer AA, Roberts HC. New horizons in appetite and the anorexia of ageing. Age Ageing. 2020;49(4):526–34. [DOI] [PubMed] [Google Scholar]
- 48. Horner K, Hopkins M, Finlayson G, Gibbons C, Brennan L. Biomarkers of appetite: is there a potential role for metabolomics?. Nutr Res Rev. Published online 2020. Mar 6. doi: 10.1017/S0954422420000062. [DOI] [PubMed] [Google Scholar]
- 49. Tieland M, van de Rest O, Dirks ML, van der Zwaluw N, Mensink M, van Loon LJ, de Groot LC. Protein supplementation improves physical performance in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2012;13(8):720–6. [DOI] [PubMed] [Google Scholar]
- 50. Tieland M, Dirks ML, van der Zwaluw N, Verdijk LB, Van De Rest O, de Groot LC, Van Loon LJ. Protein supplementation increases muscle mass gain during prolonged resistance-type exercise training in frail elderly people: a randomized, double-blind, placebo-controlled trial. J Am Med Dir Assoc. 2012;13(8):713–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.