ABSTRACT
Sugar-sweetened beverage (SSB) and artificially sweetened beverage (ASB) intakes have been reported to be associated with mortality; however, conclusions have been inconsistent. This review synthesized the evidence on the associations of SSB and ASB intakes with mortality from all causes, cardiovascular disease (CVD), and cancer among all populations (including general, diseased, or occupational populations, etc.). PubMed, EMBASE, Web of Science, Cochrane Library, ProQuest, ClinicalTrials.gov, and the International Clinical Trials Registry Platform were searched up to March 2020. Fifteen studies including 17 cohorts were included in meta-analyses. Each serving (12 fluid ounces or 355 mL) increase in daily SSB consumption was associated with higher risks of all-cause (HR: 1.08; 95% CI: 1.04, 1.12; 11 cohorts with 965,851 participants) and CVD (HR: 1.08; 95% CI: 1.04, 1.12; 13 cohorts with 898,005 participants) mortality. The associations of ASB intakes with all-cause and CVD mortality were J-shaped, and HRs (95% CI) across different doses (0, 1, 1.5, 2, and 2.5 servings/d) were 1.00, 1.01 (0.99, 1.03), 1.04 (1.02, 1.07), 1.08 (1.05, 1.11), and 1.13 (1.09, 1.18) for all-cause mortality and 1.00, 1.01 (0.96, 1.07), 1.07 (1.01, 1.13), 1.15 (1.08, 1.23), and 1.25 (1.14, 1.37) for CVD mortality. No significant association was found for cancer mortality. According to the NutriGrade scoring system, the quality of evidence on the associations of SSB intakes with all-cause and CVD mortality was high, and the quality of evidence on other associations was low to moderate. In summary, higher SSB and ASB intakes were associated with higher risks of all-cause mortality and CVD mortality. Given the limited evidence, future studies should further investigate the association between ASB intakes and cause-specific mortality.
Keywords: sugar-sweetened beverage, artificially sweetened beverage, mortality, cardiovascular disease, meta-analysis
Higher sugar–sweetened beverage and artificially sweetened beverage intakes were associated with higher risks of all-cause mortality and cardiovascular mortality.
Introduction
Sugar-sweetened beverages (SSBs) include the complete spectrum of soft drinks, fruit drinks, energy drinks, and vitamin-water drinks containing added sugars (e.g., high-fructose corn syrup, sucrose, and fruit juice concentrates) (1). Although consumption of SSBs has been decreasing annually among US adults in the past 2 decades (2), still half of US adults consume at least 1 serving of an SSB on a given day (3). In addition, urbanization and beverage marketing have prompted the increasing consumption of SSBs in low- and middle-income countries (4). The health hazards associated with higher intakes of SSBs have been widely studied, and higher risks have been reported for weight gain, type 2 diabetes, cardiovascular disease (CVD), and other cardiometabolic diseases (5, 6). Additionally, increasing evidence has suggested a positive association between intakes of SSBs and all-cause mortality (7–13); however, some studies found no significant associations (12–18). In addition, evidence for CVD mortality and cancer mortality has been sparse and inconsistent (9–12, 18–21).
Artificially sweetened beverages (ASBs) have been viewed as a replacement to reduce SSB intakes, provided that substituting SSBs with ASBs might reduce energy intake and body weight (22). However, the long-term health impacts of ASBs remain unclear. Although some randomized controlled trials found that low-calorie sweeteners might facilitate weight reduction (23), some cohort studies implied that higher ASB intakes might be associated with higher risks of obesity, type 2 diabetes, and stroke (24–27). Furthermore, emerging cohort studies investigated the associations of ASB intakes with all-cause and cause-specific mortality, but the results remain controversial (10, 11, 16, 17, 19, 28).
To comprehensively summarize the updated evidence on the association of SSB and ASB intakes with all-cause and cause-specific mortality, we conducted a systematic review and dose–response meta-analysis of prospective cohort studies.
Methods
We followed the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (29). Two of the authors (Y-BZ and Y-WJ) independently performed study selection, data extraction, assessment of study quality, and analysis, and divergences were solved by discussion or by consulting a third author (AP).
Data sources and searches
We searched PubMed, EMBASE, Web of Science, Cochrane Library, ProQuest, ClinicalTrials.gov, and the International Clinical Trials Registry Platform through March 2019, and we updated the search in March 2020. Supplemental Table 1 shows the strategies used for each database. Briefly, keywords and medical subject headings related to 1) ASB OR SSB AND 2) mortality AND 3) prospective observational studies OR intervention studies were used. We planned to include intervention studies; however, no eligible intervention studies were identified. No language restriction was applied. References of related studies were also screened.
Study selection
The following studies were excluded: 1) unrelated to SSBs, ASBs, or mortality; 2) not a prospective design; 3) not from a peer-reviewed publication; 4) duplicate publications or reporting from the same cohort (the one with shorter follow-up duration would be excluded); and 5) not sufficient data (i.e., unable to get or estimate HRs or median SSB/ASB intakes across all groups). We did not select studies according to participants’ characteristics; thus, studies conducted in certain occupational groups or patients could be included. We did not include unpublished conference abstracts, but we contacted the authors at least twice to inquire whether the full text was accepted by peer-reviewed journals.
Data extraction and assessment of study quality
Predesigned tables were used to extract information including cohort name, baseline year, median/mean follow-up duration, sex, age, race/ethnicity, mean BMI, health status, mean SSB or ASB intakes, definition and acquisition of SSB or ASB intakes, assessment of outcome, covariates included in final models, rate of loss to follow-up, HR with its CI, mean/median or range of intakes in each category, and the number of participants and deaths in each group.
The Newcastle-Ottawa scale was used to assess study quality, which evaluated the representativeness of the exposed cohort, the selection of the nonexposed cohort, ascertainment of exposure, demonstration that the outcome of interest was not present at the start of study, comparability of cohort, assessment of outcome, whether the follow-up was long enough for the outcome to occur, and adequacy of follow-up of cohorts (details are reported in Supplemental Table 2). A study would be viewed as high quality when it received ≥6 points out of 9 points (30).
Data synthesis
A dose–response meta-analysis was conducted using the Greenland and Longnecker method (31). In brief, the analysis tested both the linear and nonlinear dose–response associations between SSB or ASB intakes and mortality. For linear associations, HRs associated with each serving increase in daily SSB or ASB consumption were synthesized by random-effects model. When a study did not report the HR associated with each serving increase in daily SSB or ASB consumption but reported the HRs comparing at least 3 groups, each group's dose amount, the HR with its CI, person-years or number of participants, and number of deaths were used to estimate the HR associated with each serving increase in daily SSB or ASB consumption. The mean, median, or midpoint of each group was used as the dose amount. If the dose range was open-ended, half of the range of the adjacent group was used to estimate the midpoint (32). For example, if a study reported HRs in group 1 (daily consuming 1–1.9 servings of SSBs) and group 2 (daily consuming ≥2 servings of SSBs) but did not report the mean or median intakes of each group, then the midpoint in group 1 was 1.45 servings/d and the midpoint in group 2 was set at 2.45 servings/d (2 plus half of the range of group 1). Consistent with most included studies, 1 serving of SSB or ASB equaled 12 fluid ounces or 355 mL (10, 15, 17, 19, 28, 33). We also repeated the main analysis by using the 8-fluid-ounce (237-mL) serving, which was also commonly used (18). Nonlinear dose–response relations were estimated through restricted cubic splines (RCSs) using 3 knots at the 5th, 50th, and 95th percentiles of the distribution of SSB or ASB intakes.
Heterogeneity across studies was assessed using I2 statistics, with small values indicating less heterogeneity. It was suggested that I2 statistics of 0–25%, 25–50%, 50–75%, and 75–100%, respectively, indicated modest, modest-to-moderate, moderate-to-high, and high heterogeneity (34). Prespecified subgroup analyses according to the studies’ characteristics (i.e., study location, median follow-up duration, and whether mutually controlled for SSB and ASB intakes) and participants’ characteristics (i.e., mean age, sex, and median SSB or ASB intakes) were conducted to explore sources of heterogeneity. P values for differences between subgroups were tested by meta-regression. We also conducted sensitivity analyses by respectively excluding studies from cancer survivors, those of low quality, or those not controlling for total energy intakes. All included studies controlled for BMI in the analyses; thus, we could not perform the sensitivity analysis with and without BMI adjustment.
Funnel plots were depicted to detect publication bias visually. Publication bias was also tested using Begg's test, Egger's test, and Duval and Tweedie's trim-and-fill method (34). All analyses were performed by using STATA version 14 (StataCorp).
The NutriGrade scoring system was used to evaluate the quality of our meta-analyses. This scoring system was adapted from the Grading of Recommendations Assessment, Development, and Evaluation and was more suitable for nutrition research. The scoring system evaluated the risk of bias, precision, heterogeneity, directness, publication bias, funding bias, effect size, and dose–response for meta-analyses of cohort studies (details are reported in Supplemental Table 3), and the meta-analysis would be viewed as high-, moderate-, low-, or very-low-quality when it received 8 to 10, 6 to <8, 4 to <6, or 0 to <4 points (35).
Results
Study selection and characteristics
We identified 22,805 unique citations and excluded 22,785 citations after screening titles and abstracts. Five articles were further excluded after full-text reading (reasons are shown in Supplemental Table 4), and 17 cohorts from 15 studies were included in the meta-analysis (Figure 1). The characteristics of eligible studies are shown in Table 1. Twelve cohorts were from the United States, 3 from Europe, and 2 from Asia. Four cohorts recruited females only (10, 17, 19, 33), 3 cohorts recruited males only (10, 33), and 10 cohorts (8,9,11, 13,14,15,16, 18, 20,21,28) reported results in females and males together [among which, 1 study also conducted subgroup analysis according to sex (14)]. Most cohorts were conducted among middle-aged or elderly participants (the mean age ranged between 42.8 and 74.0 y), and most cohorts were from generally healthy populations, except for the Cancer and Leukemia Group B 89803, which was from patients with stage III colon cancer (15, 28). The sample size ranged between 1018 and 451,743, and the median follow-up period ranged between 5.9 and 31.0 y. Food-frequency questionnaires were used for data collection in most cohorts, except for the UK Biobank where 24-h dietary recalls (38%, 23%, 21%, and 18% of participants completed 1, 2, 3, and 4–5 dietary recalls) were applied (8). The median daily SSB intakes among each cohort ranged between 0.03 servings and 0.73 servings, and the median daily ASB intakes ranged between 0.04 servings and 0.45 servings. All analyses controlled for BMI in the models, and only 2 analyses did not control for total energy intake (16, 17). Only 3 studies mutually controlled for SSB and ASB intakes (10, 11, 19). Twelve studies were of high quality (Newcastle-Ottawa Scale score ≥6 points; Supplemental Table 2).
FIGURE 1.
Flow chart of the study selection. ASB, artificially sweetened beverage; CVD, cardiovascular disease; SSB, sugar-sweetened beverage.
TABLE 1.
Characteristics of studies included in the meta-analysis1
| Study, year (ref) | Cohort | Country | Participants,2n | Deaths, n | Median follow-up, y | Male, % | Age,3 y | Race/ethnicity (%) | Definition of SSB/ASB4 | Median intakes,5 servings/d | Adjustment6 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Anderson et al., 2020 (8) | UK Biobank | UK | 161,415 | 2311 All causes | <7 | 44.0 | 42–71 (56.1) | White (predominant) | The definition of SSB or ASB was not reported. | SSB, 0.18; ASB, 0.14 | E + D |
| Barrington and White, 2016 (9) | Vitamins and Lifestyle study | USA | 69,582 | 4187 All causes, 1066 CVD, 1933 cancer | 6.9 | 48.8 | 50–76 (61.4) | White (93.1) | SSBs included sugar-sweetened soda, fruit drinks not including juice, and cranberry juice. | SSB, 0.29 | E |
| Collin et al., 2019 (14)7 | Reasons for Geographic and Racial Differences in Stroke study | USA | 13,440 | 1000 All causes, 168 CHD | 5.9 | 59.3 | 45–≥65 (63.6) | White (68.9) | SSBs included sodas, soft drinks, or fruit-flavored drinks. | SSB, 0.49 | E |
| Fuchs et al., 2014 (15) | Cancer and Leukemia Group B 89803 | USA | 1011 | 305 All causes | 7.3 | 56.3 | NA (60.2) | White (88.9) | SSBs included sugar-sweetened caffeinated colas, caffeine-free colas, other carbonated sugar-sweetened beverages, and noncarbonated sugar-sweetened beverages. | SSB, 0.57 | E |
| Guercio et al., 2018 (28) | Cancer and Leukemia Group B 89803 | USA | 1018 | 309 All causes | 7.3 | 56.4 | 21–85 (60.4) | White (89.0) | ASBs included caffeinated colas, caffeine-free colas, and other carbonated beverages. | ASB, 0.35 | E |
| Keller et al., 2020 (33) | Atherosclerosis Risk in Communities Study (men) | USA | 5238 | 52 CHD | 9.2 | 100 | 35–NA (54.6) | White (predominant) | SSBs included carbonated/noncarbonated and caffeinated/noncaffeinated sodas, sports drinks, and fruit drinks with any type of added sugar. | SSB, 0.39 | E |
| Keller et al., 2020 (33) | Alpha-Tocopherol and Beta-Carotene Cancer Prevention Study | USA | 21,141 | 534 CHD | 6.0 | 100 | 35–NA (57.3) | White (predominant) | SSBs included carbonated/noncarbonated and caffeinated/noncaffeinated sodas, sports drinks, and fruit drinks with any type of added sugar. | SSB, 0.39 | E |
| Keller et al., 2020 (33) | Iowa Women's Health Study | USA | 29,528 | 291 CHD | 10.0 | 0 | 35–NA (61.4) | White (predominant) | SSBs included carbonated/noncarbonated and caffeinated/noncaffeinated sodas, sports drinks, and fruit drinks with any type of added sugar. | SSB, 0.33; ASB, 0.41 | E |
| Keller et al., 2020 (33) | Nurses' Health Study II | USA | 61,700 | 208 CHD | 10.0 | 0 | 35–NA (52.6) | White (predominant) | SSBs included carbonated/noncarbonated and caffeinated/noncaffeinated sodas, sports drinks, and fruit drinks with any type of added sugar. | SSB, 0.33; ASB, 0.45 | E |
| Liu et al., 2018 (20)7 | Mr. and Ms. OS of Hong Kong | China | 3339 | 174 CVD | 11.1 | 49.9 | 65–≥75 (72.5) | Asian (predominant) | SSB included soda, fruit juice, fruit flavored beverages, energy drinks, vitamin water drinks, soy drink, and sweetened tea drink, etc. | SSB, 0.18 | E |
| Malik et al., 2019 (10) | Nurses' Health Study | USA | 80,647 | 23,432 All causes, 4139 CVD, 8318 cancer | 31.0 | 0 | 34–59 (45.9) | White (97.5) | SSBs included caffeinated colas, caffeine-free colas, other carbonated sugar-sweetened beverages, and noncarbonated sugar-sweetened beverages; ASBs included caffeinated, caffeine-free, and noncarbonated low-calorie or diet beverages. | SSB, 0.28 | E + D |
| Malik et al., 2019 (10) | Health Professionals Follow-Up Study | USA | 37,716 | 13,004 All causes, 3757 CVD, 4062 cancer | 24.2 | 100 | 40–75 (52.4) | White (95.0) | SSBs included caffeinated colas, caffeine-free colas, other carbonated sugar-sweetened beverages, and noncarbonated sugar-sweetened beverages; ASBs included caffeinated, caffeine-free, and noncarbonated low-calorie or diet beverages. | SSB, 0.27 | E + D |
| Mossavar-Rahmani et al., 2019 (17) | Women's Health Initiative Observational Study | USA | 71,926 | 12,978 All causes | 11.9 | 0 | 53–82 (66.6) | White (85.2) | ASB included any Diet Coke or diet fruit drinks. | SSB, 0.32 | None |
| Mullee et al., 2019 (11) | European Prospective Investigation into Cancer and Nutrition | Europe | 451,743 | 41,963 All causes, 18,003 cancer, 9106 CVD | 16.4 | 28.9 | NA (50.8) | White (predominant) | Total soft drinks referred to a combination of soft drinks, carbonated and isotonic drinks, and diluted syrups. Total soft drink consumption was subdivided into sugar-sweetened and artificially sweetened soft drink consumption. | SSB, 0.30; ASB, 0.25 | E + D |
| Odegaard et al., 2015 (18) | Singapore Chinese Health Study | Singapore | 52,584 | 10,029 All causes, 4092 cancer, 3097 CVD | 16.3 | 44.0 | 45–74 (55.8) | Asian (predominant) | SSB was defined as soft drinks. | SSB, 0.06 | E |
| Paganini-Hill et al., 2007 (16)7 | Leisure World Cohort Study | USA | 13,624 | 11,386 All causes | 13.2 | 36.6 | 44–101 (74.0) | White (predominant) | SSB and ASB were divided into cola and other soft drinks. | SSB, 0.03; ASB, 0.04 | None |
| Ramne et al., 2019 (13)8 | Northern Swedish Health and Disease Study | Sweden | 24,475 | 2881 All causes | 19.4 | 46.3 | 36–64 (48.6) | White (predominant) | SSBs included carbonated soft drinks, noncarbonated sweetened drinks, and fruit drinks (not pure fruit juices). | SSB, 0.11 | E |
| Vyas et al., 2014 (19) | Women's Health Initiative | USA | 59,614 | 4437 All causes, 942 CVD | 8.7 | 0 | 50–79 (62.8) | White (85.7) | The definition of SSB or ASB was not reported. | SSB, 0.21; ASB, 0.31 | E + D |
| Yang et al., 2014 (21) | NHANES III | USA | 11,733 | 831 CVD | 13.9 | 48.1 | 20–>80 (42.8) | White (76.3) | SSBs included soda or energy and sports drinks with added sugar. | SSB, 0.73 | E |
ASB, artificially sweetened beverage; CHD, coronary heart disease; CVD, cardiovascular disease; NA, not available; ref, reference; SSB, sugar-sweetened beverage.
All studies were from general populations except for 2 studies from the Cancer and Leukemia Group B 89803 (15, 28).
Values are ranges (means).
All studies used food-frequency questionnaires for dietary information collection except for the UK Biobank, which used 24-h dietary recalls (8).
One serving equals 12 fluid ounces or 355 mL.
“E” denotes that the study controlled for total energy intake. “D” denotes that the study controlled for SSB and ASB intakes mutually. “None” denotes that the study neither controlled for total energy intake nor mutually controlled for SSB and ASB intakes.
The median intakes of beverage among each group were not reported in articles, which was provided by authors.
The study also reported the results in the Malmö Diet and Cancer Study, which was not included in the meta-analysis, because the Malmö Diet and Cancer Study was included in the European Prospective Investigation into Cancer and Nutrition study.
Association between SSB intakes and mortality
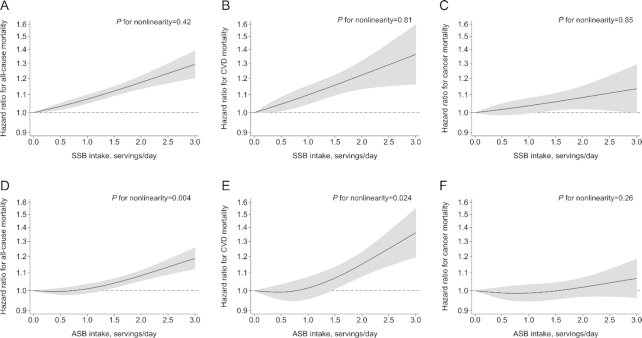
The P values for nonlinearity between SSB intakes and mortality outcomes were all nonsignificant (P ≥ 0.42; Figure 2), indicating linear relations between SSB intakes and mortality. Figure 3 shows that each serving increase in daily SSB consumption was associated with a higher risk of all-cause mortality (HR: 1.08; 95% CI: 1.04, 1.12; I2 = 70.5%; 11 cohorts with 965,851 participants and 114,935 deaths) and CVD mortality (HR: 1.08; 95% CI: 1.04, 1.12; I2 = 16.4%; 13 cohorts with 898,005 participants and 24,365 deaths). No significant association was found between SSB intakes and cancer mortality.
FIGURE 2.
Nonlinear dose–response relations of SSB (A–C) and ASB (D–F) intakes with all-cause (A, D), CVD (B, E), and cancer (C, F) mortality in adults. The x axes show the intakes of beverages (12-fluid-ounce or 355-mL servings/d), and the y axes show the HRs for mortality risk where the reference level was the nonconsumer. The solid curves show HRs compared with the reference levels from nonlinear dose–response meta-analyses, and the gray zones show 95% CIs of HRs. Restricted cubic spline functions were estimated with 3 knots located at the 5th, 50th, and 95th percentiles of daily SSB or ASB intakes. ASB, artificially sweetened beverage; CVD, cardiovascular disease; SSB, sugar-sweetened beverage.
FIGURE 3.
Forest plot of the linear dose–response meta-analysis of the association between intakes of sugar-sweetened beverages and mortality in adults. The black circles and horizontal lines indicate HRs and 95% CIs of the mortality associated with each 12-fluid-ounce or 355-mL serving increase in daily sugar-sweetened beverages in original articles. The rhombuses indicate pooled HRs and 95% CIs. The left arrow indicates that the lower bound of the CI of the HR is out of the lower bound of the x axis (i.e., 0.50). CVD, cardiovascular disease.
Subgroup analyses found no evidence of the different associations between SSB intakes and all-cause or cause-specific mortality among different subgroups (Supplemental Figure 1); however, the association between SSB intakes and all-cause or CVD mortality was not statistically significant among participants from Asia, female participants, and populations with generally low SSB intakes. Study location and follow-up duration might partially explain the heterogeneity of studies investigating the association between SSB intakes and all-cause mortality. The results remained consistent after excluding low-quality studies, studies in cancer survivors, or studies not controlling for total energy intakes. In addition, the HR (95% CI) associated with each 8-fluid-ounce/d increase in SSB intakes was 1.05 (1.02, 1.08) for all-cause mortality or CVD mortality (Supplemental Figure 2).
Funnel plots, Begg's test, and Egger's test indicated a small possibility of publication bias (Supplemental Table 5, Supplemental Figure 3). According to the NutriGrade scoring system, the quality of evidence on the associations of SSB intakes with all-cause and CVD mortality was high and the quality of evidence on the association of SSB intakes with cancer mortality was low (Supplemental Table 3).
Association between ASB intakes and mortality
The associations of ASB intakes with all-cause mortality and CVD mortality were J-shaped (P values for nonlinearity ≤0.024), and according to the results of RCSs, HRs (95% CIs) across different doses (0, 1, 1.5, 2, and 2.5 servings/d) were 1.00, 1.01 (0.99, 1.03), 1.04 (1.02, 1.07), 1.08 (1.05, 1.11), and 1.13 (1.09, 1.18) for all-cause mortality and 1.00, 1.01 (0.96, 1.07), 1.07 (1.01, 1.13), 1.15 (1.08, 1.23), and 1.25 (1.14, 1.37) for CVD mortality (Figure 2). No significant association was found for cancer mortality.
Associations were largely consistent among subgroups (Supplemental Figure 4). The results also remained consistent after excluding low-quality studies, studies in cancer survivors, or studies not controlling for total energy intakes. When using the 8-fluid-ounce serving, the associations between ASB intakes and mortality were comparable to those when using the 12-fluid-ounce serving (Supplemental Figure 2).
Begg's test and Egger's test indicated a small possibility of publication bias; however, funnel plots seemed asymmetrical (Supplemental Table 4, Supplemental Figure 3). According to the NutriGrade scoring system, the quality of evidence on the association between ASB intakes and CVD mortality was moderate and the quality of evidence on the associations of ASB intakes with all-cause and cancer mortality was low (Supplemental Table 3).
Discussion
Our systematic review and meta-analysis found that each serving increase in daily SSB consumption was associated with an 8% higher risk of all-cause mortality or CVD mortality. The associations of ASB intakes with all-cause and CVD mortality were J-shaped, and compared with nonconsumers, those who daily consumed 1.5 or 2 servings of ASBs were associated with a 4% or 8% higher risk of all-cause mortality and a 7% or 15% higher risk of CVD mortality. No significant associations were found for cancer mortality.
The current evidence on the association between SSB intakes and all-cause mortality was largely consistent; however, most studies observed a nonsignificant positive association between SSB intakes and CVD mortality (reported P values for trend ranged between 0.074 and 0.54, except for the Nurses' Health Study, Health Professionals Follow-Up Study, and NHANES III) due to small sample sizes (particularly the limited number of CVD deaths). A recent meta-analysis of 8 cohort studies also found that each 250-mL increase in SSB intakes was associated with a 4% higher risk of all-cause mortality (36). However, the study failed to include analyses from the UK Biobank (8), Cancer and Leukemia Group B 89803 (15), and the Women's Health Initiative (19). Instead, the study included an analysis from a Chinese elderly population, which actually investigated the association between dietary added sugar and all-cause mortality (37), and the study simultaneously included the analyses from the European Prospective Investigation into Cancer and Nutrition (11) and the Malmö Diet and Cancer Study (13), although the latter study is a subset of the former one. These issues resulted in a relatively weaker association between SSB intakes and all-cause mortality even compared with our analysis when using the 8-fluid-ounce serving. Additionally, among the studies included in our meta-analysis, 2 Asian studies in Chinese populations found nonsignificant inverse associations of SSB intakes with all-cause and CVD mortality (P values for trend ranged between 0.074 and 0.54) (18, 20), which might be due to residual confounding. In these 2 studies, participants consuming SSBs were more likely to be well educated, indicating that SSB consumers were more likely to be of advantageous socioeconomic status. Although educational attainment and income levels were controlled, residual confounding from other socioeconomic factors (such as occupation) was possible. In addition, participants from the Hong Kong study (20) were aged >65 y, and consuming SSBs in the elderly might be associated with better appetite and health status. In addition, the consumption levels in these 2 studies were very low [the median daily SSB intakes in the Singapore Chinese Health Study (18) and the study from Hong Kong (20) were 0.06 and 0.18 servings, respectively] compared with the studies in Western populations (the median daily SSB intakes were >0.27 servings in most studies).
The mechanisms whereby higher SSB intakes were related to increased risks of all-cause and CVD mortality are well grounded. As a beverage with high energy, moderate-to-high glycemic index value, and inefficiency in increasing satiety, higher SSB intakes contribute to excessive weight gain, insulin resistance, inflammation, and atherogenic dyslipidemia (6). Previous meta-analyses also found each additional serving/day of SSBs was associated with a 13% higher risk of type 2 diabetes and a 16% higher risk of coronary artery disease (24, 38).
The evidence regarding the association between SSB intakes and cancer mortality is sparse. Five cohorts were included in our meta-analysis and the pooled results indicated no significant association. Three out of 5 cohorts found a null association (9, 11, 18); however, the other 2 cohorts (the Nurses’ Health Study and the Health Professionals Follow-Up Study) found each serving increase in daily SSB consumption was associated with a 5% higher risk of cancer mortality (10). When it comes to site-specific cancer mortality, SSB intakes were only associated with a higher risk of breast cancer mortality (10). Further studies are needed to investigate the relations with morbidity and mortality of site-specific cancer, rather than just a combination of all types of cancer.
There is limited evidence on the association between ASB intakes and mortality. Our meta-analysis included 7 cohorts and found a J-shaped association between ASB intakes and all-cause or CVD mortality, which was also observed in most original studies (8, 10, 11, 16, 17). Of note, participants in the highest levels of ASB intake were more likely to be overweight/obese, hypertensive, and hypercholesterolemic in most studies (8, 10, 11, 17, 28), and thus reverse causation was possible. However, all analyses adjusted for BMI, and some studies performed sensitivity analyses by excluding deaths that occurred during the first 2 to 8 y of follow-up and found the associations were attenuated but still significant (8, 10, 11, 17), indicating the significant association between ASB intakes and all-cause or CVD mortality could not simply be explained by reverse causation. A recent meta-analysis included 4 cohort studies and also found a J-shaped association between ASB intakes and all-cause mortality (36). However, the study mainly reported the results from their linear dose–response meta-analysis and failed to give HRs associated with different levels of ASB intake. Our analysis showed that daily consumption of 1.5, 2, and 2.5 servings of ASBs was associated with 4%, 8%, and 13% higher risks of all-cause mortality, providing quantitative evidence for dietary guidelines.
The health effects of ASBs are intensely discussed. Meta-analyses of randomized controlled trials found that low-calorie sweeteners modestly but significantly reduced body weight, BMI, fat mass, and waist circumference (23), but had no effects on blood glucose and blood lipids compared with saccharides (39). No clinical trial is currently available to investigate the long-term effects of low-calorie sweeteners on cardiometabolic diseases, while some meta-analyses of cohort studies found that low-calorie sweetener or ASB intakes were associated with slightly higher risks of diabetes and CVD (24, 25). Although biological mechanisms remain inconclusive, some studies indicated detrimental effects of low-calorie sweeteners on the regulatory mechanisms of appetite and satiety, release of gastrointestinal hormones, gastric motility, and balance and diversity of gut microbiota, which may further increase energy intake and disrupt blood glucose homeostasis (40). Taken together, ASBs might be optional alternatives for SSBs only when they are consumed in small quantities for weight management, and the long-term adverse associations of high amounts of ASBs with cardiometabolic diseases and mortality should be considered.
This is the largest systematic review and meta-analysis summarizing up-to-date evidence of the associations of SSB and ASB intakes with mortality, which also summarizes the evidence of the associations of SSB and ASB intakes with cause-specific mortality for the first time. The results of our dose–response meta-analyses could provide important evidence for policymaking and dietary guidelines. In addition, the quality of evidence on the association between SSB intakes and all-cause or CVD mortality was high. However, several limitations should be acknowledged. First, there might be the possibility of publication bias in the analysis of the association between ASB intakes and mortality. Second, except for the analysis of the association between SSB intakes and all-cause or CVD mortality, other analyses only included limited studies and were of low-to-moderate quality. Thus, the results should be interpreted cautiously, and more studies are warranted. Third, there was moderate or high heterogeneity in some analyses, which could result from different populations and definitions of SSBs or ASBs. In addition, most studies did not consider sweetened dairy beverages or dairy alternatives, powdered beverages, and beverages with sweeteners added by consumers; thus, future studies should comprehensively and reasonably evaluate sweetened beverages (41).
In conclusion, higher SSB intakes were associated with higher risks of all-cause mortality and CVD mortality, while the association between ASB intakes and all-cause or CVD mortality was J-shaped. Based on current evidence, SSB intakes should be avoided, and if ASBs are considered as optional alternatives for SSBs, they should be consumed in small quantities (i.e., <1.5 servings/d). Nevertheless, further high-quality studies are still warranted, particularly on the long-term impact of ASB intakes, because of limited studies and low-to-moderate quality of the current evidence.
Supplementary Material
ACKNOWLEDGEMENTS
The authors thank Annlia Paganini-Hill (University of Southern California), Jean A Welsh (Emory University), Lindsay Collin (Emory University), Stina Ramne (Lund University), Yasmin Mossavar-Rahmani (Albert Einstein College of Medicine), and Zhao-min Liu (Sun Yat-sen University) for providing extra data. The authors’ responsibilities were as follows—Y-BZ and AP: conceived and designed the study; Y-WJ, JC, and PX: rechecked and analyzed the data and provided input for the initial and final drafts of the manuscript; Y-BZ: performed the independent literature search, analyzed the data, and wrote the first and final drafts of the manuscript with guidance from AP; Y-BZ and Y-WJ: selected the studies and extracted the data; and all authors: read and approved the final manuscript.
Notes
This work was funded by the National Key Research and Development Program of China (2017YFC0907504) and National Nature Science Foundation of China (81930124).
Author disclosures: The authors report no conflicts of interest.
The funders had no role in the study selection, quality assessment, data analysis, or writing of the manuscript.
Supplemental Tables 1–5 and Supplemental Figures 1–4 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: ASB, artificially sweetened beverage; CVD, cardiovascular disease; RCS, restricted cubic spline; SSB, sugar-sweetened beverage.
Contributor Information
Yan-Bo Zhang, Department of Epidemiology and Biostatistics, Key Laboratory of Environment and Health, Ministry of Education and Ministry of Environmental Protection, State Key Laboratory of Environmental Health (Incubating), School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Yi-Wen Jiang, Department of Epidemiology and Biostatistics, Key Laboratory of Environment and Health, Ministry of Education and Ministry of Environmental Protection, State Key Laboratory of Environmental Health (Incubating), School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Jun-Xiang Chen, Department of Epidemiology and Biostatistics, Key Laboratory of Environment and Health, Ministry of Education and Ministry of Environmental Protection, State Key Laboratory of Environmental Health (Incubating), School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
Peng-Fei Xia, Department of Epidemiology and Biostatistics, Key Laboratory of Environment and Health, Ministry of Education and Ministry of Environmental Protection, State Key Laboratory of Environmental Health (Incubating), School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
An Pan, Department of Epidemiology and Biostatistics, Key Laboratory of Environment and Health, Ministry of Education and Ministry of Environmental Protection, State Key Laboratory of Environmental Health (Incubating), School of Public Health, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China.
References
- 1. Hu FB. Resolved: there is sufficient scientific evidence that decreasing sugar-sweetened beverage consumption will reduce the prevalence of obesity and obesity-related diseases. Obes Rev. 2013;14(8):606–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Marriott BP, Hunt KJ, Malek AM, Newman JC. Trends in intake of energy and total sugar from sugar-sweetened beverages in the United States among children and adults, NHANES 2003–2016. Nutrients. 2019;11(9):2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rosinger A, Herrick K, Gahche J, Park S. Sugar-sweetened beverage consumption among U.S. adults. NCHS Data Brief. 2017;(270):1–8. [PubMed] [Google Scholar]
- 4. Popkin BM, Hawkes C. Sweetening of the global diet, particularly beverages: patterns, trends, and policy responses. Lancet Diabetes Endocrinol. 2016;4(2):174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wijarnpreecha K, Thongprayoon C, Edmonds PJ, Cheungpasitporn W. Associations of sugar- and artificially sweetened soda with nonalcoholic fatty liver disease: a systematic review and meta-analysis. QJM. 2016;109(7):461–6. [DOI] [PubMed] [Google Scholar]
- 6. Malik VS, Hu FB. Sugar-sweetened beverages and cardiometabolic health: an update of the evidence. Nutrients. 2019;11(8):1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Miles FL, Chang SC, Morgenstern H, Tashkin D, Rao JY, Cozen W, Mack T, Lu QY, Zhang ZF. Association of sugary beverages with survival among patients with cancers of the upper aerodigestive tract. Cancer Causes Control. 2016;27(11):1293–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Anderson JJ, Gray SR, Welsh P, Mackay DF, Celis-Morales CA, Lyall DM, Forbes J, Sattar N, Gill JMR, Pell JP. The associations of sugar-sweetened, artificially sweetened and naturally sweet juices with all-cause mortality in 198,285 UK Biobank participants: a prospective cohort study. BMC Med. 2020;18(1):97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Barrington WE, White E. Mortality outcomes associated with intake of fast-food items and sugar-sweetened drinks among older adults in the Vitamins and Lifestyle (VITAL) study. Public Health Nutr. 2016;19(18):3319–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malik VS, Li Y, Pan A, De Koning L, Schernhammer E, Willett WC, Hu FB. Long-term consumption of sugar-sweetened and artificially sweetened beverages and risk of mortality in US adults. Circulation. 2019;139(18):2113–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mullee A, Romaguera D, Pearson-Stuttard J, Viallon V, Stepien M, Freisling H, Fagherazzi G, Mancini FR, Boutron-Ruault MC, Kuhn Tet al. Association between soft drink consumption and mortality in 10 European countries. JAMA Intern Med. 2019;179(11):1479–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tasevska N, Park Y, Jiao L, Hollenbeck A, Subar AF, Potischman N. Sugars and risk of mortality in the NIH-AARP Diet and Health Study. Am J Clin Nutr. 2014;99(5):1077–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ramne S, Alves Dias J, Gonzalez-Padilla E, Olsson K, Lindahl B, Engstrom G, Ericson U, Johansson I, Sonestedt E. Association between added sugar intake and mortality is nonlinear and dependent on sugar source in 2 Swedish population-based prospective cohorts. Am J Clin Nutr. 2019;109(2):411–23. [DOI] [PubMed] [Google Scholar]
- 14. Collin LJ, Judd S, Safford M, Vaccarino V, Welsh JA. Association of sugary beverage consumption with mortality risk in US adults: a secondary analysis of data from the REGARDS study. JAMA Netw Open. 2019;2(5):e193121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Fuchs MA, Sato K, Niedzwiecki D, Ye X, Saltz LB, Mayer RJ, Mowat RB, Whittom R, Hantel A, Benson Aet al. Sugar-sweetened beverage intake and cancer recurrence and survival in CALGB 89803 (Alliance). PLoS One. 2014;9(6):e99816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Paganini-Hill A, Kawas CH, Corrada MM. Non-alcoholic beverage and caffeine consumption and mortality: the Leisure World Cohort Study. Prev Med. 2007;44(4):305–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Mossavar-Rahmani Y, Kamensky V, Manson JE, Silver B, Rapp SR, Haring B, Beresford SAA, Snetselaar L, Wassertheil-Smoller S. Artificially sweetened beverages and stroke, coronary heart disease, and all-cause mortality in the Women's Health Initiative. Stroke. 2019;50(3):555–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Odegaard AO, Koh WP, Yuan JM, Pereira MA. Beverage habits and mortality in Chinese adults. J Nutr. 2015;145(3):595–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vyas A, Rubenstein L, Robinson J, Seguin RA, Vitolins MZ, Kazlauskaite R, Shikany JM, Johnson KC, Snetselaar L, Wallace R. Diet drink consumption and the risk of cardiovascular events: a report from the Women's Health Initiative. J Gen Intern Med. 2015;30(4):462–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Liu ZM, Tse LA, Chan D, Wong C, Wong SYS. Dietary sugar intake was associated with increased body fatness but decreased cardiovascular mortality in Chinese elderly: an 11-year prospective study of Mr and Ms OS of Hong Kong. Int J Obes. 2018;42(4):808–16. [DOI] [PubMed] [Google Scholar]
- 21. Yang Q, Zhang Z, Gregg EW, Flanders D, Merritt R, Hu FB. Added sugar intake and cardiovascular diseases mortality among US adults. JAMA Intern Med. 2014;174(4):516–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Johnson RK, Lichtenstein AH, Anderson CAM, Carson JA, Despres J-P, Hu FB, Kris-Etherton PM, Otten JJ, Towfighi A, Wylie-Rosett Jet al. Low-calorie sweetened beverages and cardiometabolic health: a science advisory from the American Heart Association. Circulation. 2018;138(9):E126–E40. [DOI] [PubMed] [Google Scholar]
- 23. Miller PE, Perez V. Low-calorie sweeteners and body weight and composition: a meta-analysis of randomized controlled trials and prospective cohort studies. Am J Clin Nutr. 2014;100(3):765–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Imamura F, O'Connor L, Ye Z, Mursu J, Hayashino Y, Bhupathiraju SN, Forouhi NG. Consumption of sugar sweetened beverages, artificially sweetened beverages, and fruit juice and incidence of type 2 diabetes: systematic review, meta-analysis, and estimation of population attributable fraction. BMJ. 2015;351:h3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Narain A, Kwok CS, Mamas MA. Soft drinks and sweetened beverages and the risk of cardiovascular disease and mortality: a systematic review and meta-analysis. Int J Clin Pract. 2016;70(10):791–805. [DOI] [PubMed] [Google Scholar]
- 26. Ruanpeng D, Thongprayoon C, Cheungpasitporn W, Harindhanavudhi T. Sugar and artificially sweetened beverages linked to obesity: a systematic review and meta-analysis. QJM. 2017;110(8):513–20. [DOI] [PubMed] [Google Scholar]
- 27. Lohner S, Toews I, Meerpohl JJ. Health outcomes of non-nutritive sweeteners: analysis of the research landscape. Nutr J. 2017;16(1):55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Guercio BJ, Zhang S, Niedzwiecki D, Li Y, Babic A, Morales-Oyarvide V, Saltz LB, Mayer RJ, Mowat RB, Whittom Ret al. Associations of artificially sweetened beverage intake with disease recurrence and mortality in stage III colon cancer: results from CALGB 89803 (Alliance). PLoS One. 2018;13(7):e0199244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. J Clin Epidemiol. 2009;62(10):1006–12. [DOI] [PubMed] [Google Scholar]
- 30. Cook DA, Reed DA. Appraising the quality of medical education research methods: the Medical Education Research Study Quality Instrument and the Newcastle-Ottawa Scale–Education. Acad Med. 2015;90(8):1067–76. [DOI] [PubMed] [Google Scholar]
- 31. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. [DOI] [PubMed] [Google Scholar]
- 32. Reynolds A, Mann J, Cummings J, Winter N, Mete E, Te Morenga L. Carbohydrate quality and human health: a series of systematic reviews and meta-analyses. Lancet. 2019;393(10170):434–45. [DOI] [PubMed] [Google Scholar]
- 33. Keller A, O'Reilly EJ, Malik V, Buring JE, Andersen I, Steffen L, Robien K, Mannisto S, Rimm EB, Willett Wet al. Substitution of sugar-sweetened beverages for other beverages and the risk of developing coronary heart disease: results from the Harvard Pooling Project of Diet and Coronary Disease. Prev Med. 2020;131:105970. [DOI] [PubMed] [Google Scholar]
- 34. Borenstein M, Hedges LV, Higgins JPT, Rothstein HR. Introduction to meta-analysis. Chichester (UK): John Wiley & Sons, Ltd; 2009. [Google Scholar]
- 35. Schwingshackl L, Knuppel S, Schwedhelm C, Hoffmann G, Missbach B, Stelmach-Mardas M, Dietrich S, Eichelmann F, Kontopantelis E, Iqbal Ket al. Perspective: NutriGrade: a scoring system to assess and judge the meta-evidence of randomized controlled trials and cohort studies in nutrition research. Adv Nutr. 2016;7(6):994–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Qin P, Li Q, Zhao Y, Chen Q, Sun X, Liu Y, Li H, Wang T, Chen X, Zhou Qet al. Sugar and artificially sweetened beverages and risk of obesity, type 2 diabetes mellitus, hypertension, and all-cause mortality: a dose-response meta-analysis of prospective cohort studies. Eur J Epidemiol. 2020;35(7):655–71. [DOI] [PubMed] [Google Scholar]
- 37. Liu ZM, Tse SLA, Chen B, Chan D, Wong C, Woo J, Wong SYS. Dietary sugar intake does not pose any risk of bone loss and non-traumatic fracture and is associated with a decrease in all-cause mortality among Chinese elderly: finding from an 11-year longitudinal study of Mr. and Ms. OS Hong Kong. Bone. 2018;116:154–61. [DOI] [PubMed] [Google Scholar]
- 38. Huang C, Huang J, Tian Y, Yang X, Gu D. Sugar sweetened beverages consumption and risk of coronary heart disease: a meta-analysis of prospective studies. Atherosclerosis. 2014;234(1):11–6. [DOI] [PubMed] [Google Scholar]
- 39. Wiebe N, Padwal R, Field C, Marks S, Jacobs R, Tonelli M. A systematic review on the effect of sweeteners on glycemic response and clinically relevant outcomes. BMC Med. 2011;9:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Swithers SE. Artificial sweeteners are not the answer to childhood obesity. Appetite. 2015;93:85–90. [DOI] [PubMed] [Google Scholar]
- 41. Merkel PE, Ditto EK, Robien K, Sylvetsky AC. Perspective: chaos in a bottle—a critical evaluation of beverage categorization in nutrition research. Adv Nutr. 2020, doi: 10.1093/advances/nmaa068. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.