ABSTRACT
Here we provide a comprehensive meta-analysis to summarize and appraise the quality of the current evidence on the associations of tea drinking in relation to cancer risk. PubMed, Embase, and the Cochrane Database of Systematic Reviews were searched up to June 2020. We reanalyzed the individual prospective studies focused on associations between tea drinking and cancer risk in humans. We conducted a meta-analysis of prospective studies and provided the highest- versus lowest-category analyses, dose-response analyses, and test of nonlinearity of each association by modeling restricted cubic spline regression for each type of tea. We graded the evidence based on the summary effect size, its 95% confidence interval, 95% prediction interval, the extent of heterogeneity, evidence of small-study effects, and excess significance bias. We identified 113 individual studies investigating the associations between tea drinking and 26 cancer sites including 153,598 cancer cases. We assessed 12 associations for the intake of black tea with cancer risk and 26 associations each for the intake of green tea and total tea with cancer risk. Except for an association between lymphoid neoplasms with green tea, we did not find consistent associations for the highest versus lowest categories and dose-response analyses for any cancer. When grading current evidence for each association (number of studies ≥2), weak evidence was detected for lymphoid neoplasm (green tea), glioma (total tea, per 1 cup), bladder cancer (total tea, per 1 cup), and gastric and esophageal cancer (tea, per 1 cup). This review of prospective studies provides little evidence to support the hypothesis that tea drinking is associated with cancer risk. More well-designed studies are still needed to identify associations between tea intake and rare cancers.
Keywords: tea, cancer, meta-analysis, grading evidence, prospective studies
Introduction
Tea, produced from the leaves of the plant Camellia sinensis, is the most widely consumed beverage, besides water, worldwide (1). Studies on the possible health benefits of tea extend back many decades (2, 3). In laboratory and animal studies, the potential health benefits of tea have been partially attributed to tea polyphenols [mainly epigallocatechin-3-gallate (EGCG)], which has been shown to possess antioxidative, anti-inflammatory, and anticancer properties (4). Other bioactive constituents including thearubigins and caffeine have also been linked to potential anticancer effects (5). Therefore, tea drinking has been considered as a healthful life habit for the prevention of cardiovascular diseases and cancers.
Numerous studies have been conducted to test the hypothesis that tea drinking may prevent chronic diseases in humans (4). Epidemiological studies, however, have not yielded clear conclusions concerning the potential protective effects of tea drinking against cancer development (6). Numerous meta-analyses of epidemiological studies of tea and cancer outcomes have been extensively performed in the last decade (7–10) with 3 major limitations. First, most meta-analyses included case-control studies as well as cohort studies. Considering that data from case-control studies are more likely subject to recall bias, these analyses might lead to confounded results for the association of tea intake on cancer risk. Second, most studies did not separate the type of tea, such as green tea and black tea intake, and the difference in the degree of fermentation may produce different health effects (11). Nonfermented green tea is rich in catechins but contains low amounts of theaflavins, while fully fermented black tea is depleted of catechins but rich in theaflavins. Last, previous meta-analyses did not comprehensively evaluate the strength of the current evidence and extent of potential biases across different cancers, which restricts the ability to make public health recommendations based on existing evidence.
Because of the evidence from experimental studies, we hypothesized that tea drinking is inversely associated with cancer risk. To advance understanding on the association of tea drinking and cancer risk in humans and provide evidence for future health policy, we comprehensively evaluated the associations between black tea, green tea, and total tea intake and the risk of cancer incidence using meta-analytical methodology and conducted evaluation of the evidence.
Methods
The current study was registered in PROSPERO (registration number: CRD42017065975).
Literature research
Our meta-analysis was conducted in 2 parts. First, we selected the prospective studies based on the current published meta-analyses with the largest number of cohorts included. Second, we additionally searched the database to retrieve new studies that were published after the meta-analyses for each tea-cancer association. We followed this approach because there are a large number of individual studies focused on tea drinking and cancer risk. Our approach is efficient and effective in finding all related prospective studies on this topic. We considered 1 association as 1 specific exposure (tea, green tea, or black tea) and 1 outcome (any type of cancer).
Two researchers (Z-YL and G-SF) independently searched PubMed, Embase, and the Cochrane Database of Systematic Reviews from their respective inceptions to June 2020 for meta-analyses of epidemiological studies investigating associations between tea drinking and the risk of developing any cancer. We did not apply any year, language, or publication status restrictions to the selection of articles for inclusion. The search was based on the following terms: (tea) AND (cancer OR carcinoma OR neoplasia OR tumor OR neoplasm) AND ("systematic review" OR "meta-analysis").
For each tea-cancer association, we again searched PubMed, Embase, and the China National Knowledge Infrastructure (CNKI) database to obtain omitted or new cohort studies that were not included in the meta-analyses we derived.
Study selection and data abstraction
We first obtained systematic reviews and meta-analyses that examined associations between tea drinking and the risk of developing cancers. We excluded randomized controlled trials, meta-analyses, and systematic reviews that did not include study-specific data [such as Relative Risks (RRs), 95% Confident Intervals (CIs), etc.]. All selection and data-extraction procedures were performed by 2 researchers independently, with disagreements resolved by discussion. First, 2 researchers conducted the literature search and screened the titles and abstracts of all articles. Second, the potentially eligible articles were examined in detail and screened for applicability as full texts. When we found >1 meta-analysis on the same association between the exposure and outcome, we included only the meta-analysis with the largest number of cohort studies to avoid duplication. We obtained the information for each individual study that was included in the obtained meta-analysis. The individual study was eligible if it reported the association between tea drinking and cancer risk using a prospective design in humans. Third, we further included the related cohort studies that were published after the obtained meta-analyses to supplement each tea-cancer analysis.
Two independent authors carried out the data extraction on the meta-analyses and individual studies identified for inclusion, and also conducted a quality assessment using the Newcastle-Ottawa Scale (NOS) (12), with any discrepancies being resolved through consensus between the 2 authors. We developed a standard data-extraction table in order to collect the following information for each tea-cancer association: first author's last name, the year of publication, the country in which the study was conducted, cohort name, cohort size, baseline age of the population, duration of follow-up, gender, dose category, number of cases and total population in each category, and maximally adjusted RRs and 95% CIs.
Statistical analyses
Pooled results
We provided 2 categories of analyses: associations for the highest versus lowest categories and dose-response analyses. First, we pooled the risk estimates by combining the multivariable-adjusted RRs of the highest compared with the lowest intake of tea category based on a random-effects model (13). Second, we estimated the RR and 95% CI for each increment of 1 cup of tea intake and explored the nonlinear association. For each study, the trend from the correlated RRs across categories of tea intake was calculated using the method proposed by Greenland and Longnecker (14) and Orsini et al. (15). When tea intake was not presented in cups/day, we transformed it into a standard measure of tea intake according to the information provided for the same population. For instance, 2.5 g dry tea or 125 mL tea was equal to 1 cup of tea. Black tea, green tea, and total tea were analyzed separately. The method requires >2 exposure categories and the following information for each category should be available: 1) the number of cases and total number of participants or person-years, 2) the RR and corresponding 95% CI, and 3) the mean or median tea consumption. When the number of cases or total number of participants or person-years was not reported, we estimated the distribution using the methods described by Aune et al. (16). We assigned the median or mean tea intake of each category to the corresponding risk estimates of each study. If the upper bound in the highest category was not available, we assumed that it had the same amplitude as the preceding one. However, there were still some studies that did not have enough data to be included in the dose-response analyses. Therefore, we only included them in the highest- versus lowest-category analyses. For nonlinear associations, we used a 2-stage, random-effects, dose-response meta-analysis by modeling tea consumption using restricted cubic splines with 3 knots at fixed percentiles (10%, 50%, and 90%) of the distribution (17). We first fitted a restricted cubic spline model into each set of RRs within a specific study and then combined the 2 regression coefficients and the variance/covariance matrices for each study using a multivariate random-effects model. A P value for nonlinearity was calculated by testing whether the coefficient of the second spline was equal to zero. The data from each systematic review or meta-analysis and updated supplemental research were used to build evidence tables.
Heterogeneity and 95% prediction interval
We evaluated heterogeneity by estimating the variance between studies using Cochran's Q test and the I2 statistic (18, 19), and we also estimated the 95% prediction interval (PI), which further accounts for between-study heterogeneity. We evaluated the effect that would be expected in a new observational study addressing that same association (20).
Publication bias and test of excessive significance
Indication of small-study effects was evaluated based on the Egger's regression asymmetry test (P = 0.10) (21). We assessed the excess significance bias by evaluating whether the observed number of studies with nominally statistically significant results (“positive” studies, P < 0.05) in the published literature was different from the expected number of studies with statistically significant results (22). The actual size of the true effect in each meta-analysis was assumed to be the effect of the largest study in each meta-analysis, which was defined based on the smallest SE. Sensitivity analysis was performed using the summary fixed- and random-effects estimates as alternative plausible effect sizes. Excess significance for a single meta-analysis was defined as P < 0.10.
Sensitivity analysis
We explored the potential sources of heterogeneity by subgroup or meta-regression analyses according to baseline characteristics and methodological factors, such as gender (men or women), follow-up years (≤median or higher), number of cases (≥1000 or less), region (Asia-Pacific, North America, Europe, and Australia), cancer subsite (only for colon cancer), and adjustment for confounders (age, sex, or other factors for each type of cancer). We only included meta-analyses with >5 individual studies.
Grading the evidence
For each individual study, we used the NOS to assess the potential bias (12). For meta-data, we categorized the associations between measures of tea drinking and cancers into strong, highly suggestive, suggestive, weak, or no association depending on the strength and validity of the evidence, such as P value of the random-effects model, total cases, I2 statistic, small-study effects, and excess significance bias (23–25). A strong association was claimed when the P value of the random-effects meta-analysis was <0.001, if cases in the meta-analysis were >1000, if there was little evidence of heterogeneity between studies (I2 ≤ 50%) and 95% PI excluded the null value, and if no evidence of small-study effects or excess significance bias was indicated. A highly suggestive association was defined when the P value of the random-effects meta-analysis was <0.001, if cases in the meta-analysis were >1000, and if moderate heterogeneity between studies was observed (I2 ≤ 75%). A suggestive association was claimed when the P value of the random-effects meta-analysis was <0.001 and if cases in the meta-analysis were >500. A weak association was claimed when the P value of the random-effects meta-analysis was <0.05. No association was claimed when the significance threshold exceeded P > 0.05.
All statistical analyses were performed using R (version 3.5.0; R Core Team, Vienna, Austria). A 2-sided P value <0.05 was considered statistically significant if not specified.
Results
Basic characteristics
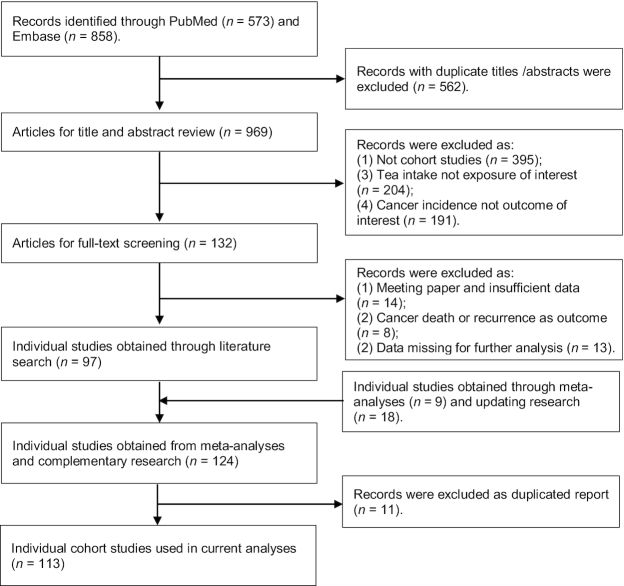
The study screening procedure is provided in Figure 1. We first selected 97 papers that reported results of meta-analyses of tea drinking and cancer incidence. In an additional literature search, we identified a further 27 publications of individual studies. After carefully screening these studies, 11 articles were removed as they represented duplicate reports.
FIGURE 1.
Systematic identification of the published literature on tea intake and risk of cancer.
We re-analyzed the data of meta-analyses on associations of tea drinking and the incidence risk of 26 specific cancers. After careful screening, 113 studies were included in the current review that included data on 153,598 cancer cases. The details are provided in Supplemental Table 1. An exclusion list for studies in full text of eligibility was provided in Supplemental Table 2. For the studies that did not specify the type of tea in the primary studies, we derived 26 associations for the highest versus lowest categories. For the intakes of green tea and black tea, we obtained associations with 12 and 26 cancer sites, respectively.
Highest versus lowest intake analyses
When we combined the risk estimates for studies examining the highest versus the lowest intake of tea, we did not find any significant associations between a higher intake of black tea and risk of cancer at any site. There was an inverse association between green tea drinking and biliary tract cancer risk (RR = 0.67; 95% CI: 0.46, 0.97; number of studies, n = 1). However, only 1 study was found for this association. There was an inverse association between green tea intake and lymphoid neoplasm (RR = 0.72; 95% CI: 0.52, 0.98; n = 3). A higher intake of tea was also associated with decreased risk of glioma (RR = 0.81; 95% CI: 0.70, 0.95; n = 6). No other significant associations were found for other cancer types. Details are presented in Figure 2.
FIGURE 2.
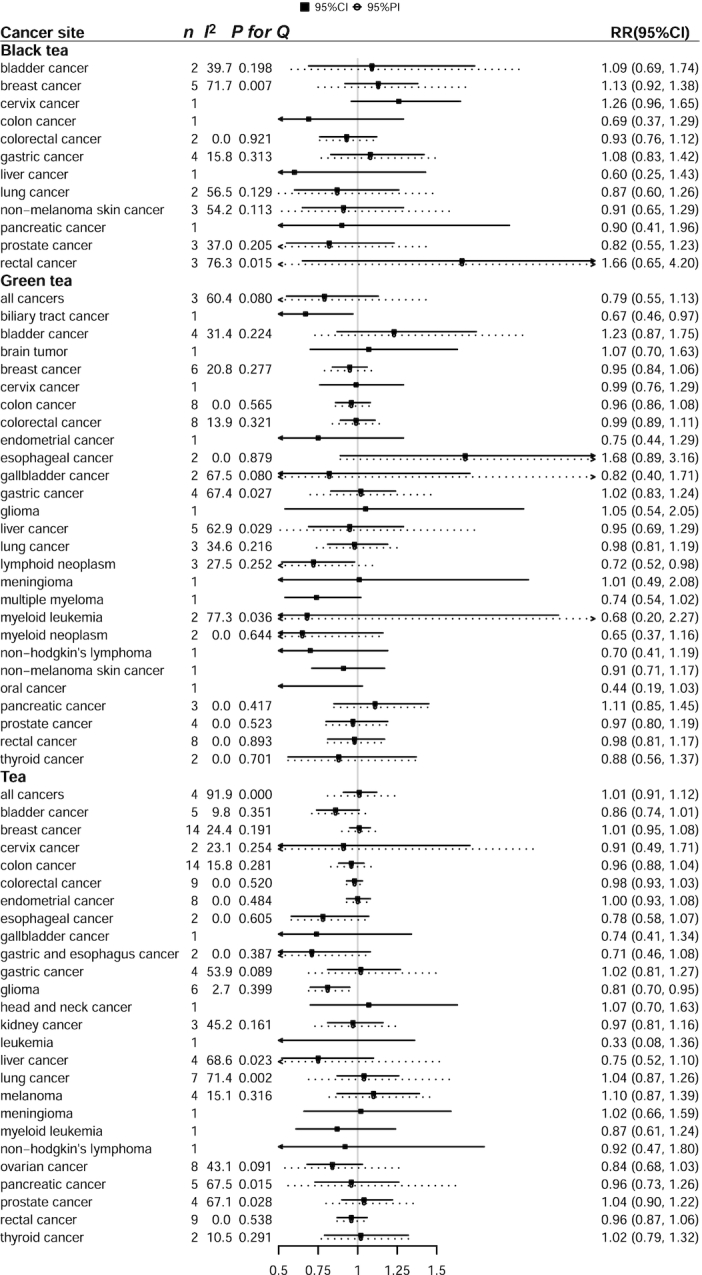
Prospective associations of tea intake with risk of cancer for the highest versus lowest intake of tea intake. I2 for heterogeneity and 95% PIs were calculated for associations with more than 1 study. n indicates number of studies included. P for Q, P value for heterogeneity, Cochran's Q test; PI, prediction interval.
Dose-response analyses
In addition, we calculated the RRs per an extra 1 cup of tea and summarized the evidence. As shown in Figure 3, we also observed an increased risk of rectal cancer in relation to a higher intake of black tea in 1 cohort study (RR = 2.15; 95% CI: 1.51, 3.07; n = 1). Higher green tea intake was associated with a lower risk of lymphoid neoplasm (RR = 0.95; 95% CI: 0.92, 0.99; n = 3). Tea drinking was related to a lower risk of gastric and esophagus cancers when RRs of 2 cohorts were combined (RR = 0.85; 95% CI: 0.73, 0.99; I2 = 0%; n = 2). A lower risk for bladder cancer was also observed with an increase of 1 cup of tea (RR = 0.95; 95% CI: 0.91, 0.99; I2 = 3.3%; n = 5). We found a borderline significant association between an increase of 1 cup of tea and liver cancer risk (RR = 0.92; 95% CI: 0.85, 1.00; I2 = 30.7%; n = 4). No statistically significant associations were observed for the other cancer types.
FIGURE 3.
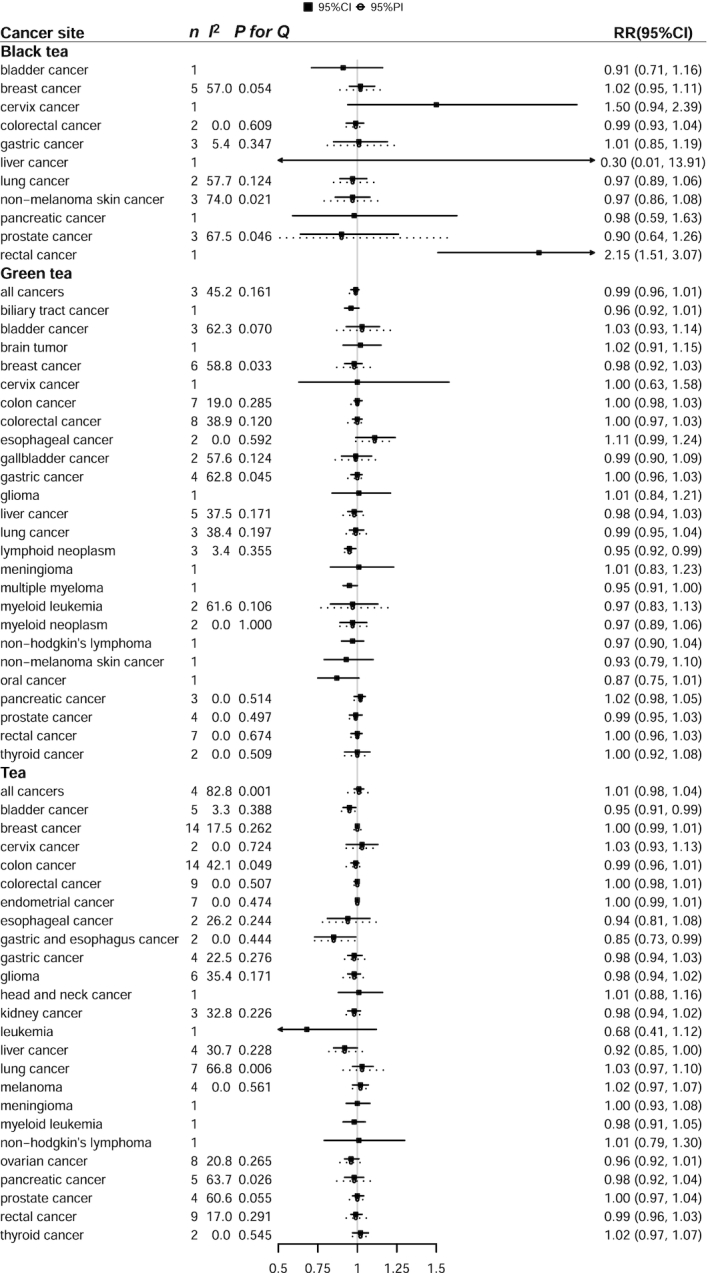
Prospective associations of tea intake with risk of cancer for per 1 cup of tea intake. I2 for heterogeneity and 95% PIs were calculated for associations with more than 1 study. n indicates number of studies included. P for Q, P value for heterogeneity, Cochran's Q test; PI, prediction interval.
We also tested for nonlinearity in the association between tea drinking and cancer incidence when the number of studies for each association was >2. Details are provided in Supplemental Figures 1–3. With the exception of lymphoid neoplasm with green tea intake and myeloid leukemia with total tea drinking, the P values for nonlinearity did not reach significance. Forest plots for each association discussed above are provided in Supplemental Figures 4–64.
Sensitivity analyses
When the number of studies was >5 for each tea-cancer association, we performed subgroup analyses according to baseline characteristics and methodological factors. In general, the results between these subgroups were consistent with our main analyses. There were no associations between tea drinking and risks of bladder, breast, colon, colorectal, endometrial, glioma, liver, lung, ovarian, pancreatic, prostate, and rectal cancers (data not shown).
Appraising the evidence
Nearly 80% (90/113) of these individual studies has an NOS score >8 (maximum = 9) (Supplemental Table 3). We appraised the association between tea drinking and cancer incidence risk for the highest versus lowest categories and dose-response analyses (number of studies >2). Based on the heterogeneity, 95% PI, Egger's test, and test of excessive significance, only 2 associations were classified as having weak evidence for the highest versus lowest intake of tea, as shown in Table 1 (green tea with lymphoid neoplasm, tea with glioma). For dose-response analyses shown in Table 2, 3 associations were deemed as having weak evidence (green tea with lymphoid neoplasm, tea with bladder cancer, and tea with gastric and esophagus cancer). We also compared our results with previous meta-analysis in Supplemental Table 4.
TABLE 1.
Robustness of evidence grading for the highest versus lowest categories of meta-analyses of cohort studies associating tea intake and risk of cancer1
| Studies, n | Cases, n | Summary RR (95% CI) | Random-effects, P value | Egger's test3 | Excess significance4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer type | Fixed effects | Random effects | Largest study | I 2 (95% CI)2 | P for Q | (95% PI) | O | E | P 5 | Evidence | ||||
| Black tea | ||||||||||||||
| Bladder cancer | 2 | 209 | 1.13 (0.80, 1.60) | 1.09 (0.69, 1.74) | 1.32 (0.87, 2.00) | 0.7101 | 39.7 | 0.198 | (0.58, 2.05) | 0.198 | 0 | 0.392 | NA | NS |
| Breast cancer | 5 | 7289 | 1.04 (0.95, 1.14) | 1.13 (0.92, 1.38) | 0.88 (0.78, 1.00) | 0.2453 | 71.7 (0, 84.7) | 0.007 | (0.75, 1.70) | 0.024 | 2 | 2.355 | NA | NS |
| Colorectal cancer | 2 | 1495 | 0.93 (0.76, 1.12) | 0.93 (0.76, 1.12) | 0.92 (0.73, 1.16) | 0.4365 | 0 | 0.921 | (0.76, 1.12) | 0.921 | 0 | 0.102 | NA | NS |
| Gastric cancer | 4 | 1167 | 1.10 (0.87, 1.40) | 1.08 (0.83, 1.42) | 1.41 (0.98, 2.03) | 0.5493 | 15.8 (0, 87.1) | 0.313 | (0.77, 1.53) | 0.115 | 0 | 1.083 | NA | NS |
| Nonmelanoma skin cancer | 3 | 1128 | 0.86 (0.69, 1.07) | 0.91 (0.65, 1.29) | 0.70 (0.52, 0.94) | 0.6104 | 54.2 (0, 86.9) | 0.113 | (0.52, 1.59) | 0.05 | 1 | 1.063 | NA | NS |
| Prostate cancer | 3 | 921 | 0.81 (0.59, 1.11) | 0.82 (0.55, 1.23) | 0.60 (0.36, 0.94) | 0.3435 | 37 (0, 80) | 0.205 | (0.46, 1.47) | 0.188 | 1 | 0.903 | 0.903 | NS |
| Rectal cancer | 3 | 640 | 1.67 (1.07, 2.60) | 1.66 (0.65, 4.20) | 1.49 (0.78, 2.85) | 0.288 | 76.3 (22.3, 92.8) | 0.015 | (0.31, 8.93) | 0.875 | 1 | 1.111 | NA | NS |
| Green tea | ||||||||||||||
| All cancers | 3 | 4453 | 0.95 (0.86, 1.05) | 0.79 (0.55, 1.13) | 0.98 (0.89, 1.1) | 0.1989 | 60.4 (0, 88.7) | 0.08 | (0.42, 1.46) | 0.028 | 1 | 0.791 | 0.784 | NS |
| Bladder cancer | 4 | 424 | 1.20 (0.90, 1.59) | 1.23 (0.87, 1.75) | 0.9 (0.56, 1.45) | 0.2454 | 31.4 (0, 75.4) | 0.224 | (0.73, 2.08) | 0.077 | 1 | 1.127 | NA | NS |
| Breast cancer | 6 | 4887 | 0.93 (0.84, 1.03) | 0.95 (0.84, 1.06) | 0.82 (0.70, 0.95) | 0.3406 | 0 (0, 79.2) | 0.277 | (0.79, 1.12) | 0.191 | 1 | 1.780 | NA | NS |
| Colon cancer | 8 | 3548 | 0.96 (0.86, 1.08) | 0.96 (0.86, 1.08) | 1.07 (0.86, 1.33) | 0.5316 | 0 (0, 67.6) | 0.565 | (0.86, 1.08) | 0.362 | 1 | 1.386 | NA | NS |
| Colorectal cancer | 8 | 4676 | 1.00 (0.90, 1.11) | 0.99 (0.89, 1.11) | 1.01 (0.83, 1.22) | 0.8791 | 13.9 (0, 56.7) | 0.321 | (0.84, 1.17) | 0.035 | 1 | 1.363 | NA | NS |
| Esophageal cancer | 2 | 78 | 1.68 (0.89, 3.16) | 1.68 (0.89, 3.16) | 1.61 (0.71, 3.66) | 0.1093 | 0 | 0.879 | (0.89, 3.16) | 0.879 | 0 | 0.104 | NA | NS |
| Gallbladder cancer | 2 | 238 | 0.81 (0.54, 1.23) | 0.82 (0.40, 1.71) | 0.57 (0.32, 1.01) | 0.5994 | 67.5 | 0.08 | (0.27, 2.52) | 0.08 | 0 | 0.729 | NA | NS |
| Gastric cancer | 4 | 4965 | 0.99 (0.89, 1.10) | 1.02 (0.83, 1.24) | 1.06 (0.89, 1.25) | 0.8684 | 67.4 (5, 88.8) | 0.027 | (0.70, 1.48) | 0.12 | 1 | 1.246 | NA | NS |
| Liver cancer | 5 | 1064 | 0.90 (0.75, 1.08) | 0.95 (0.69, 1.29) | 0.95 (0.69, 1.30) | 0.7215 | 62.9 (1.9, 86) | 0.029 | (0.51, 1.76) | 0.045 | 1 | 1.417 | NA | NS |
| Lung cancer | 3 | 2224 | 0.98 (0.84, 1.14) | 0.98 (0.81, 1.19) | 1.00 (0.82, 1.23) | 0.8158 | 34.6 (0.0, 98.7) | 0.216 | (0.74, 1.29) | 0.836 | 0 | 0.528 | NA | NS |
| Lymphoid neoplasm | 3 | 749 | 0.73 (0.56, 0.95) | 0.72 (0.52, 0.98) | 0.89 (0.61, 1.29) | 0.0378 | 27.5 (0.0, 98.0) | 0.252 | (0.47, 1.10) | 0.162 | 1 | 0.726 | 0.711 | Weak |
| Pancreatic cancer | 3 | 647 | 1.11 (0.85, 1.45) | 1.11 (0.85, 1.45) | 1.23 (0.84, 1.80) | 0.4554 | 0 (0, 89.6) | 0.417 | (0.85, 1.45) | 0.29 | 0 | 0.435 | NA | NS |
| Prostate cancer | 4 | 1008 | 0.97 (0.80, 1.19) | 0.97 (0.80, 1.19) | 0.89 (0.65, 1.21) | 0.7852 | 0 (0, 84.7) | 0.523 | (0.80, 1.19) | 0.751 | 0 | 0.56 | NA | NS |
| Rectal cancer | 8 | 1940 | 0.98 (0.81, 1.17) | 0.98 (0.81, 1.17) | 0.85 (0.58, 1.23) | 0.8047 | 0 (0, 67.6) | 0.893 | (0.81, 1.17) | 0.81 | 0 | 1.023 | NA | NS |
| Thyroid cancer | 2 | 159 | 0.88 (0.56, 1.37) | 0.88 (0.56, 1.37) | 0.91 (0.56, 1.48) | 0.5676 | 0 | 0.701 | (0.56, 1.37) | 0.701 | 0 | 0.12 | NA | NS |
| Tea | ||||||||||||||
| All cancers | 4 | 39,221 | 0.96 (0.95, 0.97) | 1.01 (0.91, 1.12) | 0.95 (0.94, 0.96) | 0.9138 | 91.9 (82.3, 96.2) | <0.001 | (0.81, 1.25) | 0 | 2 | 1.533 | 0.631 | NS |
| Bladder cancer | 5 | 1805 | 0.87 (0.75, 1.01) | 0.86 (0.74, 1.01) | 0.91 (0.72, 1.14) | 0.0746 | 9.8 (0, 81.2) | 0.351 | (0.71, 1.05) | 0.057 | 0 | 0.826 | NA | NS |
| Breast cancer | 14 | 49,698 | 1.01 (0.96, 1.05) | 1.01 (0.95, 1.08) | 0.96 (0.89, 1.03) | 0.6748 | 24.4 (0, 59.9) | 0.191 | (0.90, 1.14) | 0.98 | 1 | 3.17 | NA | NS |
| Cervical cancer | 2 | 22,704 | 1.00 (0.70, 1.42) | 0.91 (0.49, 1.71) | 1.05 (0.73, 1.50) | 0.7765 | 23.1 | 0.254 | (0.39, 2.14) | 0.254 | 0 | 0.267 | NA | NS |
| Colon cancer | 14 | 19,384 | 0.97 (0.91, 1.03) | 0.96 (0.88, 1.04) | 0.99 (0.91, 1.08) | 0.2748 | 15.8 (0, 54) | 0.281 | (0.83, 1.10) | 0.144 | 2 | 2.578 | NA | NS |
| Colorectal cancer | 9 | 38,603 | 0.98 (0.93, 1.03) | 0.98 (0.93, 1.03) | 0.97 (0.9, 1.05) | 0.3968 | 0 (0, 64.8) | 0.52 | (0.93, 1.03) | 0.51 | 0 | 1.316 | NA | NS |
| Endometrial cancer | 8 | 6286 | 1.00 (0.93, 1.08) | 1.00 (0.93, 1.08) | 0.97 (0.89, 1.06) | 0.9625 | 0 (0, 67.6) | 0.484 | (0.93, 1.08) | 0.253 | 0 | 1.245 | NA | NS |
| Esophageal cancer | 2 | 438 | 0.78 (0.58, 1.07) | 0.78 (0.58, 1.07) | 0.74 (0.51, 1.08) | 0.1195 | 0 | 0.605 | (0.58, 1.07) | 0.605 | 0 | 0.147 | NA | NS |
| Gastric and esophageal cancer | 2 | 391 | 0.71 (0.46, 1.08) | 0.71 (0.46, 1.08) | 0.76 (0.48, 1.19) | 0.1063 | 0 | 0.387 | (0.46, 1.08) | 0.387 | 0 | 0.204 | NA | NS |
| Gastric cancer | 4 | 23,764 | 1.09 (0.96, 1.23) | 1.02 (0.81, 1.27) | 1.18 (1.02, 1.37) | 0.8763 | 53.9 (0, 84.8) | 0.089 | (0.69, 1.51) | 0.106 | 1 | 1.123 | NA | NS |
| Glioma | 6 | 2383 | 0.81 (0.70, 0.95) | 0.81 (0.70, 0.95) | 0.75 (0.57, 1.00) | 0.0097 | 2.7 (0.0, 84.0) | 0.399 | (0.69, 0.96) | 0.371 | 2 | 1.039 | 0.300 | Weak |
| Kidney cancer | 3 | 2384 | 0.95 (0.83, 1.08) | 0.97 (0.81, 1.16) | 0.85 (0.71, 1.02) | 0.7181 | 45.2 (0, 83.7) | 0.161 | (0.73, 1.27) | 0.07 | 0 | 0.876 | NA | NS |
| Liver cancer | 4 | 23,168 | 0.87 (0.75, 1.02) | 0.75 (0.52, 1.10) | 0.9 (0.75, 1.09) | 0.1424 | 68.6 (9.2, 89.2) | 0.023 | (0.37, 1.52) | 0.029 | 1 | 1.288 | NA | NS |
| Lung cancer | 7 | 25,837 | 1.14 (1.05, 1.23) | 1.04 (0.87, 1.26) | 1.31 (1.17, 1.46) | 0.657 | 71.4 (37.8, 86.8) | 0.002 | (0.69, 1.58) | 0.011 | 2 | 3.086 | NA | NS |
| Melanoma | 4 | 2932 | 1.11 (0.91, 1.36) | 1.10 (0.87, 1.39) | 1.30 (0.97, 1.75) | 0.4094 | 15.1 (0, 87) | 0.316 | (0.82, 1.48) | 0.677 | 0 | 0.834 | NA | NS |
| Ovarian cancer | 8 | 3041 | 0.83 (0.72, 0.97) | 0.84 (0.68, 1.03) | 0.69 (0.52, 0.93) | 0.0983 | 43.1 (0, 74.8) | 0.091 | (0.54, 1.29) | 0.581 | 3 | 2.404 | 0.646 | NS |
| Pancreatic cancer | 5 | 3837 | 1.00 (0.88, 1.14) | 0.96 (0.73, 1.26) | 0.96 (0.78, 1.16) | 0.7756 | 67.5 (15.7, 87.4) | 0.015 | (0.56, 1.64) | 0.135 | 1 | 1.53 | NA | NS |
| Prostate cancer | 4 | 10,404 | 1.00 (0.94, 1.07) | 1.04 (0.90, 1.22) | 1.05 (0.96, 1.16) | 0.5719 | 67.1 (4, 88.7) | 0.028 | (0.80, 1.37) | 0.113 | 1 | 1.384 | NA | NS |
| Rectal cancer | 9 | 6346 | 0.96 (0.87, 1.06) | 0.96 (0.87, 1.06) | 0.92 (0.80, 1.07) | 0.4569 | 0 (0, 64.8) | 0.538 | (0.87, 1.06) | 0.674 | 0 | 1.369 | NA | NS |
| Thyroid cancer | 2 | 854 | 1.01 (0.80, 1.29) | 1.02 (0.79, 1.32) | 0.93 (0.70, 1.25) | 0.8773 | 10.5 | 0.291 | (0.77, 1.36) | 0.291 | 0 | 0.297 | NA | NS |
NS, not statistically significant, P > 0.05. NA, not available; P for Q, P value for heterogeneity, Cochran's Q test; PI, prediction interval.
The 95% CI for I2 cannot be calculated when the number of studies is <2.
From Egger's regression asymmetry test.
Observed number (O) and expected number (E) of significant studies (P < 0.05) using effect of largest study (smallest SE) of each meta-analysis as a plausible effect size.
Estimated expected number (E) is larger than observed number (O) and there is no evidence of excess significance. P value of excess significance test. All statistical tests are 2-sided.
TABLE 2.
Robustness of evidence grading for the dose–response meta-analyses of cohort studies associating tea intake and risk of cancer1
| Studies, n | Cases, n | Summary RR (95% CI) | Random-effects, P value | Egger's test3 | Excess significance4 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cancer type | Fixed effects | Random effects | Largest study | I 2 (95% CI)2 | P for Q | (95% PI) | O | E | P 5 | Evidence | ||||
| Black tea | ||||||||||||||
| Breast cancer | 5 | 7289 | 1.04 (1.00, 1.08) | 1.02 (0.95, 1.11) | 1.08 (1.02, 1.15) | 0.56 | 57.0 (0, 99.8) | 0.054 | (0.89, 1.18) | 0.841 | 1 | 1.426 | NA | NS |
| Colorectal cancer | 2 | 1495 | 0.99 (0.93, 1.04) | 0.99 (0.93, 1.04) | 0.99 (0.94, 1.05) | 0.626 | 0 | 0.609 | (0.93, 1.04) | 0.609 | 0 | 0.132 | NA | NS |
| Gastric cancer | 3 | 1059 | 0.99 (0.90, 1.09) | 1.01 (0.85, 1.19) | 0.98 (0.89, 1.08) | 0.939 | 5.4 (0, 97.7) | 0.347 | (0.81, 1.25) | 0.35 | 0 | 0.411 | NA | NS |
| Lung cancer | 2 | 2250 | 0.99 (0.94, 1.04) | 0.97 (0.89, 1.06) | 1.01 (0.95, 1.07) | 0.556 | 57.7 | 0.124 | (0.85, 1.11) | 0.124 | 0 | 0.477 | NA | NS |
| Nonmelanoma skin cancer | 3 | 1128 | 0.99 (0.95, 1.04) | 0.97 (0.86, 1.08) | 0.99 (0.93, 1.05) | 0.535 | 74.0 (9.4, 99.8) | 0.021 | (0.79, 1.17) | 0.048 | 1 | 1 | 1 | NS |
| Prostate cancer | 3 | 921 | 0.98 (0.84, 1.15) | 0.90 (0.64, 1.26) | 1.13 (0.93, 1.38) | 0.546 | 67.5 (0, 98.7) | 0.046 | (0.51, 1.61) | 0.086 | 1 | 1.04 | NA | NS |
| Green tea | ||||||||||||||
| All cancers | 3 | 4453 | 0.99 (0.98, 1.01) | 0.99 (0.96, 1.01) | 1.00 (0.98, 1.01) | 0.278 | 45.2 (0, 98.2) | 0.161 | (0.95, 1.03) | 0.083 | 1 | 0.671 | 0.649 | NS |
| Bladder cancer | 3 | 328 | 1.01 (0.95, 1.07) | 1.03 (0.93, 1.14) | 0.96 (0.88, 1.04) | 0.597 | 62.3 (0, 99.2) | 0.07 | (0.87, 1.22) | 0.022 | 1 | 0.979 | 0.979 | NS |
| Breast cancer | 6 | 4887 | 0.99 (0.96, 1.02) | 0.98 (0.92, 1.03) | 1.01 (0.96, 1.05) | 0.374 | 58.8 (0, 97.0) | 0.033 | (0.87, 1.09) | 0.087 | 1 | 1.413 | NA | NS |
| Colon cancer | 7 | 3415 | 1.00 (0.98, 1.03) | 1.00 (0.98, 1.03) | 1.00 (0.95, 1.05) | 0.802 | 19.0 (0, 88.7) | 0.285 | (0.96, 1.04) | 0.908 | 1 | 1.233 | NA | NS |
| Colorectal cancer | 8 | 4676 | 1.00 (0.98, 1.02) | 1.00 (0.97, 1.03) | 1.01 (0.97, 1.05) | 0.921 | 38.9 (0, 97.3) | 0.12 | (0.94, 1.06) | 0.412 | 1 | 1.744 | NA | NS |
| Esophageal cancer | 2 | 78 | 1.11 (0.99, 1.24) | 1.11 (0.99, 1.24) | 1.08 (0.93, 1.26) | 0.073 | 0 | 0.592 | (0.99, 1.24) | 0.592 | 0 | 0.16 | NA | NS |
| Gallbladder cancer | 2 | 238 | 0.98 (0.92, 1.04) | 0.99 (0.90, 1.09) | 0.95 (0.88, 1.02) | 0.867 | 57.6 | 0.124 | (0.86, 1.14) | 0.124 | 0 | 0.52 | NA | NS |
| Gastric cancer | 4 | 4965 | 1.00 (0.98, 1.01) | 1.00 (0.96, 1.03) | 1.01 (0.98, 1.04) | 0.83 | 62.8 (0, 99.6) | 0.045 | (0.94, 1.06) | 0.166 | 1 | 1.309 | NA | NS |
| Liver cancer | 5 | 1064 | 0.98 (0.95, 1.01) | 0.98 (0.94, 1.03) | 0.98 (0.93, 1.04) | 0.451 | 37.5 (0, 93.3) | 0.171 | (0.91, 1.06) | 0.179 | 1 | 1.011 | NA | NS |
| Lung cancer | 3 | 2224 | 0.99 (0.96, 1.03) | 0.99 (0.95, 1.04) | 1.03 (0.97, 1.08) | 0.716 | 38.4 (0, 98.1) | 0.197 | (0.93, 1.06) | 0.498 | 0 | 0.975 | NA | NS |
| Lymphoid neoplasm | 3 | 749 | 0.95 (0.92, 0.99) | 0.95 (0.92, 0.99) | 0.97 (0.92, 1.02) | 0.017 | 3.4 (0, 98.0) | 0.355 | (0.92, 0.99) | 0.207 | 1 | 0.486 | 0.421 | Weak |
| Myeloid leukemia | 2 | 133 | 0.98 (0.90, 1.08) | 0.97 (0.83, 1.13) | 1.04 (0.93, 1.17) | 0.691 | 61.6 | 0.106 | (0.77, 1.22) | 0.106 | 0 | 0.578 | NA | NS |
| Myeloid neoplasm | 2 | 131 | 0.97 (0.89, 1.06) | 0.97 (0.89, 1.06) | 0.97 (0.87, 1.09) | 0.506 | 0 | 1 | (0.89, 1.06) | 1 | 0 | 0.1 | NA | NS |
| Pancreatic cancer | 3 | 647 | 1.02 (0.98, 1.05) | 1.02 (0.98, 1.05) | 1.02 (0.97, 1.07) | 0.432 | 0 (0, 97.8) | 0.514 | (0.98, 1.05) | 0.37 | 0 | 0.319 | NA | NS |
| Prostate cancer | 4 | 1008 | 0.99 (0.95, 1.03) | 0.99 (0.95, 1.03) | 0.98 (0.93, 1.03) | 0.536 | 0 (0, 99.2) | 0.497 | (0.95, 1.03) | 0.178 | 0 | 0.499 | NA | NS |
| Rectal cancer | 7 | 1830 | 1.00 (0.96, 1.03) | 1.00 (0.96, 1.03) | 0.97 (0.91, 1.04) | 0.848 | 0 (0, 77.1) | 0.674 | (0.96, 1.03) | 0.417 | 0 | 1.123 | NA | NS |
| Thyroid cancer | 2 | 159 | 1.00 (0.92, 1.08) | 1.00 (0.92, 1.08) | 1.01 (0.93, 1.10) | 0.976 | 0 | 0.509 | (0.92, 1.08) | 0.509 | 0 | 0.161 | NA | NS |
| Tea | ||||||||||||||
| All cancers | 4 | 28,928 | 1.02 (1.01, 1.03) | 1.01 (0.98, 1.04) | 1.05 (1.03, 1.07) | 0.603 | 82.8 (44.3, 99.0) | 0.001 | (0.94, 1.08) | 0.016 | 1 | 2.678 | NA | NS |
| Bladder cancer | 5 | 1513 | 0.95 (0.91, 0.98) | 0.95 (0.91, 0.99) | 0.94 (0.89, 0.99) | 0.009 | 3.3 (0, 98.4) | 0.388 | (0.90, 0.99) | 0.139 | 1 | 0.76 | 0.765 | Weak |
| Breast cancer | 14 | 48,106 | 1.00 (0.99, 1.01) | 1.00 (0.99, 1.01) | 0.99 (0.98, 1.01) | 0.831 | 17.5 (0, 79.5) | 0.262 | (0.98, 1.03) | 0.167 | 2 | 2.739 | NA | NS |
| Cervical cancer | 2 | 22,704 | 1.03 (0.93, 1.13) | 1.03 (0.93, 1.13) | 1.03 (0.93, 1.14) | 0.611 | 0 (0, 99.2) | 0.724 | (0.93, 1.13) | 0.724 | 0 | 0.115 | NA | NS |
| Colon cancer | 14 | 19,384 | 1.00 (0.99, 1.01) | 0.99 (0.96, 1.01) | 1.02 (1.00, 1.04) | 0.357 | 42.1 (0, 95.3) | 0.049 | (0.93, 1.05) | 0.005 | 2 | 3.569 | NA | NS |
| Colorectal cancer | 9 | 38,603 | 1.00 (0.98, 1.01) | 1.00 (0.98, 1.01) | 1.00 (0.98, 1.02) | 0.624 | 0 (0, 87.1) | 0.507 | (0.98, 1.01) | 0.54 | 0 | 1.356 | NA | NS |
| Endometrial cancer | 7 | 5711 | 1.00 (0.99, 1.01) | 1.00 (0.99, 1.01) | 1.00 (0.99, 1.01) | 0.822 | 0 (0, 88.8) | 0.474 | (0.99, 1.01) | 0.548 | 1 | 1.012 | NA | NS |
| Esophageal cancer | 2 | 445 | 0.94 (0.83, 1.06) | 0.94 (0.81, 1.08) | 0.87 (0.73, 1.04) | 0.383 | 26.2 | 0.244 | (0.78, 1.12) | 0.244 | 0 | 0.425 | NA | NS |
| Gastric and esophageal cancer | 2 | 391 | 0.85 (0.73, 0.99) | 0.85 (0.73, 0.99) | 0.87 (0.74, 1.02) | 0.034 | 0 | 0.444 | (0.73, 0.99) | 0.444 | 0 | 0.18 | NA | Weak |
| Gastric cancer | 4 | 23,734 | 0.99 (0.95, 1.02) | 0.98 (0.94, 1.03) | 1.02 (0.96, 1.07) | 0.456 | 22.5 (0, 95.9) | 0.276 | (0.92, 1.05) | 0.332 | 0 | 1.036 | NA | NS |
| Glioma | 6 | 2386 | 0.98 (0.95, 1.01) | 0.98 (0.94, 1.02) | 0.95 (0.9, 1.00) | 0.399 | 35.4 (0, 93.2) | 0.171 | (0.91, 1.06) | 0.88 | 2 | 1.643 | 0.744 | NS |
| Kidney cancer | 3 | 2172 | 0.99 (0.96, 1.01) | 0.98 (0.94, 1.02) | 1.00 (0.97, 1.03) | 0.338 | 32.8 (0, 97.6) | 0.226 | (0.93, 1.04) | 0.237 | 1 | 0.593 | 0.555 | NS |
| Liver cancer | 4 | 23,168 | 0.93 (0.89, 0.98) | 0.92 (0.85, 1.00) | 0.96 (0.91, 1.03) | 0.052 | 30.7 (0, 97.8) | 0.228 | (0.82, 1.04) | 0.85 | 1 | 0.831 | 0.835 | NS |
| Lung cancer | 7 | 24,806 | 1.05 (1.03, 1.08) | 1.03 (0.97, 1.10) | 1.10 (1.06, 1.14) | 0.301 | 66.8 (8.0, 97.7) | 0.006 | (0.91, 1.18) | 0.05 | 2 | 2.192 | NA | NS |
| Melanoma | 4 | 2932 | 1.02 (0.97, 1.07) | 1.02 (0.97, 1.07) | 0.99 (0.92, 1.06) | 0.482 | 0 (0, 87.2) | 0.561 | (0.97, 1.07) | 0.722 | 0 | 0.597 | NA | NS |
| Ovarian cancer | 8 | 2985 | 0.97 (0.93, 1.01) | 0.96 (0.92, 1.01) | 1.00 (0.93, 1.07) | 0.129 | 20.8 (0, 88.3) | 0.265 | (0.89, 1.04) | 0.328 | 1 | 1.681 | NA | NS |
| Pancreatic cancer | 5 | 3837 | 1.01 (0.98, 1.03) | 0.98 (0.92, 1.04) | 1.04 (1.00, 1.08) | 0.537 | 63.7 (0, 99.4) | 0.026 | (0.87, 1.10) | 0.009 | 2 | 2.041 | NA | NS |
NS, not statistically significant, P > 0.05. NA, not available; P for Q, P value for heterogeneity, Cochran's Q test; PI, prediction interval.
The 95% CI for I2 cannot be calculated when the number of studies is <2.
From Egger's regression asymmetry test.
Observed number (O) and expected number (E) of significant studies (P < 0.05) using effect of largest study (smallest SE) of each meta-analysis as a plausible effect size.
Estimated expected number (E) is larger than observed number (O) and there is no evidence of excess significance. P value of excess significance test. All statistical tests are 2-sided.
Discussion
Main findings and possible explanations
In this comprehensive meta-analysis, we did not find strong evidence to support the hypothesis that the intake of any type of tea is associated with a lower risk of cancer. Tea drinking may be weakly associated with several types of cancer in a dose-response manner, such as bladder cancer, lymphoid neoplasm, and liver cancer, with low evidence. In general, almost all of the evidence was classified as weak or not significant.
Numerous epidemiological studies have investigated the associations between tea drinking and cancer risk with inconsistent and conflicting results observed (7–10). Meta-analyses including data from both cohort and case-control studies have indicated that tea drinking was inversely associated with the risk of breast, endometrial, and lung cancers (26–28). However, when only results from the prospective studies were combined, the inverse associations between tea consumption and the relative risk of liver, stomach, breast, prostate, or colorectal cancers were not evident (29). A previous meta-analysis that incorporated 57 articles that investigated the associations between tea drinking and incidence of cancer in prospective cohort studies reported that, except for oral cancer, higher intakes of tea were not significantly associated with the risk of common cancers (30). The results of our updated meta-analysis are generally consistent with this. The World Cancer Research Fund recently published its report on the evidence for a link between cancer and nonalcoholic drink consumption (31). After a systematic review of the global scientific literature, the report concluded that the evidence was too limited in amount, consistency, and quality to draw any conclusions on tea drinking and cancer development. It found limited evidence that higher tea intake might decrease the risk of bladder cancer (RR per 1 cup = 0.94; 95% CI: 0.89, 0.98; I2 = 0%; n = 4), but evidence was deemed too limited to reach a conclusion. In our analysis, we included results from 1 additional cohort study on associations of tea drinking and bladder cancer and found a significant inverse association (RR per 1 cup = 0.95; 95% CI: 0.91, 0.99; I2 = 3.3%; n = 5). The Cochrane systematic review of 51 studies with >1.6 million participants also concluded that no firm recommendations could be made with regard to tea, especially green tea, for cancer prevention (32).
Tea drinking has been considered to be part of a healthy lifestyle due to its extraordinary performance in experimental studies and biological plausibility. Tea contains numerous bioactive compounds that may be relevant for chronic disease prevention and has been studied in many different animal models of carcinogenesis, including lung cancer, oral-digestive tract cancer, and prostate cancer (33). Based on previous studies, the polyphenols in tea have been hypothesized to prevent cancer by modulating epigenetic aberrations that occurred in DNA methylation, histone modifications, and micro‐RNA formation (34). Most experiments have focused on green tea and its most active compound, the catechin EGCG, some of which indicate that EGCG is a stronger antioxidant than vitamins C or E. Cell and animal studies show that EGCG tea polyphenols directly inhibit the development of various types of cancer. Other polyphenols in tea include thearubigins and theaflavins, which also show antioxidant properties in laboratory studies (35). Evidence from the experimental and animal studies provide a promising prospect for the prevention of cancer risk by tea drinking.
Given the extensive experimental evidence for a potential anticancer effect of tea and its constituent compounds, the relative lack of robust data from epidemiological studies is notable and possible reasons for this inconsistency deserve some discussion. First, the inconsistent results may reflect variations in our understanding of the types of tea consumed and differences in how it is prepared, genetic differences, and the impact of other lifestyle factors, such as obesity, dietary habits, smoking, alcohol drinking, and exercising. Second, bias due to measurement error, including the tea drinking and cancer case ascertainment, will also introduce methodological heterogeneity between studies. Some studies used the self-reported outcome rather than medical records, and tea drinking habits were obtained from self-report, which may also be subject to misclassification bias. Third, biological interaction with other food components and host metabolism could also be an important factor and this cannot be easily accounted for in epidemiological studies where data on tea drinking habits are obtained from questionnaires. Fourth, the high degree of heterogeneity between populations, including race, sex, and age, may also have an important modifying effect on the association of tea intake and cancer development. Finally, some of the discrepancies between the results from experimental studies and human studies may be due to the lower doses of tea that humans are typically exposed to compared with those in, for example, animal models. Even though we did not find any evidence of a dose–response relation between tea drinking and cancer risk based on the current evidence, we cannot exclude that the potential health effects of tea drinking on cancer risk may be weak and easily masked by other risk factors. All of these factors could impact on the potential effects of tea drinking on human health, as well as against cancer.
Given the weak and inconsistent associations between tea drinking and cancer risks, a recommendation for tea consumption for cancer prevention is premature. The inconsistency between cohort studies and case-control studies can be partly explained by the inherent bias within retrospective studies, such as the misclassification of tea drinking among cases and controls. Further studies should also focus on a well-defined exposure of tea drinking by considering the degree of fermentation, the sources, and the water temperature of tea. Prospective studies with a large sample size and a longer follow-up duration are also needed to clarify the delicate associations between tea drinking and cancer risk. However, from another perspective, daily tea intake is still deemed to contribute to a healthy lifestyle, which may decrease the risk of diabetes and all-cause mortality (36).
Strengths and limitations
Our study has several strengths. First, our updated meta-analysis included all published cohort studies that investigated the association between tea drinking and the risk of cancer. Our analyses encompassed 26 different cancer types, which afforded an overview of all major cancers. Second, we only included prospective cohort studies and excluded case-control and cross-sectional studies, which might mitigate selection bias, information bias, and reverse causality to some extent. It is likely that the inverse associations between tea drinking and cancer incidence observed in previous meta-analyses are subject to these biases.
We acknowledge, however, that our study also has some limitations. First, as an observational study, we cannot infer a causal relation between the exposure and the outcome. Therefore, although we did not find associations between tea intake and cancer risk, this does not mean there is no negative or positive association between tea drinking and cancer. Second, as a meta-analysis, the reliability of our study was partly dependent on the quality of the original studies. However, most of the studies included in the current meta-analysis were well-designed cohort studies, which might provide less-biased results. Third, we did not make full use of the data from these cohorts. Future studies should be conducted using more comprehensive approaches, such as pooled analyses or analyzing biomarkers to indicate the exposure of tea consumption and its constituents. Fourth, for some specific cancers, there is only a limited number of study or cases to draw a robust conclusion. More prospective studies are needed to clarify these types of cancers, including cervix cancer, esophageal cancer, and hematologic malignancy.
Conclusions
This systematic review and updated meta-analysis revealed that, based on the current evidence from epidemiological cohort studies, there may be no association between tea drinking and cancer development in humans. The current evidence from prospective cohort databases was classified as weak or not significant. More well-designed studies with less measurement error and bias are needed to illuminate associations between tea intake and rare cancers.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—Y-BX: obtained the funding; Y-BX and L-GZ: conceptualized the review and interpreted the data and results; L-GZ, Z-YL, and G-SF: conducted the research and performed the statistical analysis; L-GZ: drafted the initial manuscript; Y-BX: had final responsibility for the decision to submit the manuscript for publication; and all authors: read, reviewed, and approved the final manuscript.
Notes
This work was supported by grants from the National Key Project of Research and Development Program of China (2016YFC1302503).
Author disclosures: The authors report no conflicts of interest
None of the funders had any influence on the study design; on the collection, analysis, and interpretation of data; on the writing of the report; or on the decision to submit the article for publication.
Supplemental Tables 1–4 and Supplemental Figures 1–64 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances.
No additional data are available, and all data are from the references included in this review.
Abbreviations used: EGCG, epigallocatechin-3-gallate; NOS, Newcastle-Ottawa Scale; PI, prediction interval.
Contributor Information
Long-Gang Zhao, State Key Laboratory of Oncogene and Related Genes and Department of Epidemiology, Shanghai Cancer Institute, Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China.
Zhuo-Ying Li, State Key Laboratory of Oncogene and Related Genes and Department of Epidemiology, Shanghai Cancer Institute, Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China.
Guo-Shan Feng, State Key Laboratory of Oncogene and Related Genes and Department of Epidemiology, Shanghai Cancer Institute, Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China.
Xiao-Wei Ji, State Key Laboratory of Oncogene and Related Genes and Department of Epidemiology, Shanghai Cancer Institute, Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China.
Yu-Ting Tan, State Key Laboratory of Oncogene and Related Genes and Department of Epidemiology, Shanghai Cancer Institute, Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China.
Hong-Lan Li, State Key Laboratory of Oncogene and Related Genes and Department of Epidemiology, Shanghai Cancer Institute, Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China.
Marc J Gunter, Section of Nutrition and Metabolism, International Agency for Research on Cancer, Lyon, France.
Yong-Bing Xiang, State Key Laboratory of Oncogene and Related Genes and Department of Epidemiology, Shanghai Cancer Institute, Renji Hospital, Shanghai Jiaotong University School of Medicine, Shanghai, China.
References
- 1. Weisburger JH. Tea and health: a historical perspective. Cancer Lett. 1997;114(1–2):315–7. [DOI] [PubMed] [Google Scholar]
- 2. Yang CS, Hong J. Prevention of chronic diseases by tea: possible mechanisms and human relevance. Annu Rev Nutr. 2013;33:161–81. [DOI] [PubMed] [Google Scholar]
- 3. Khan N, Mukhtar H. Tea and health: studies in humans. Curr Pharmaceutical Design. 2013;19(34):6141–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu XY, Zhao CN, Cao SY, Tang GY, Gan RY, Li HB. Effects and mechanisms of tea for the prevention and management of cancers: an updated review. Crit Rev Food Sci Nutr, 2020;60:(10):1693–705. [DOI] [PubMed] [Google Scholar]
- 5. Khan N, Mukhtar H. Tea polyphenols in promotion of human health. Nutrients. 2018;11(1):39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Yuan JM, Sun C, Butler LM. Tea and cancer prevention: epidemiological studies. Pharmacol Res. 2011;64(2):123–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Caini S, Cattaruzza MS, Bendinelli B, Tosti G, Masala G, Gnagnarella P, Assedi M, Stanganelli I, Palli D, Gandini S. Coffee, tea and caffeine intake and the risk of non-melanoma skin cancer: a review of the literature and meta-analysis. Eur J Nutr. 2017;56(1):1–12. [DOI] [PubMed] [Google Scholar]
- 8. Weng H, Zeng XT, Li S, Kwong JS, Liu TZ, Wang XH. Tea consumption and risk of bladder cancer: a dose-response meta-analysis. Front Physiol. 2016;7:693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yang TO, Crowe F, Cairns BJ, Reeves GK, Beral V. Tea and coffee and risk of endometrial cancer: cohort study and meta-analysis. Am J Clin Nutr. 2015;101(3):570–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Je Y, Park T. Tea consumption and endometrial cancer risk: meta-analysis of prospective cohort studies. Nutr Cancer. 2015;67(5):825–30. [DOI] [PubMed] [Google Scholar]
- 11. Yang CS, Li G, Yang Z, Guan F, Chen A, Ju J. Cancer prevention by tocopherols and tea polyphenols. Cancer Lett. 2013;334(1):79–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Wells GA, Shea B, O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses. [cited 2019 Apr 10] [Internet]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 13. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 14. Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135(11):1301–9. [DOI] [PubMed] [Google Scholar]
- 15. Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose-response data. Stata J. 2006;6(1):40–57. [Google Scholar]
- 16. Aune D, Greenwood DC, Chan DS, Vieira R, Vieira AR, Navarro Rosenblatt DA, Cade JE, Burley VJ, Norat T. Body mass index, abdominal fatness and pancreatic cancer risk: a systematic review and non-linear dose-response meta-analysis of prospective studies. Ann Oncol. 2012;23(4):843–52. [DOI] [PubMed] [Google Scholar]
- 17. Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012;175(1):66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Statist Med. 2002;21(11):1539–58. [DOI] [PubMed] [Google Scholar]
- 19. Ioannidis JP, Patsopoulos NA, Evangelou E. Uncertainty in heterogeneity estimates in meta-analyses. BMJ. 2007;335(7626):914–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta-analyses. BMJ. 2011;342:d549. [DOI] [PubMed] [Google Scholar]
- 21. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ioannidis JPA. Clarifications on the application and interpretation of the test for excess significance and its extensions. J Math Psych. 2013;57(5):184–7. [Google Scholar]
- 23. Bellou V, Belbasis L, Tzoulaki I, Evangelou E, Ioannidis JP. Environmental risk factors and Parkinson's disease: an umbrella review of meta-analyses. Parkinsonism Relat Disord. 2016;23:1–9. [DOI] [PubMed] [Google Scholar]
- 24. Bellou V, Belbasis L, Tzoulaki I, Middleton LT, Ioannidis JPA, Evangelou E. Systematic evaluation of the associations between environmental risk factors and dementia: an umbrella review of systematic reviews and meta-analyses. Alzheimers Dement. 2017;13(4):406–18. [DOI] [PubMed] [Google Scholar]
- 25. Markozannes G, Tzoulaki I, Karli D, Evangelou E, Ntzani E, Gunter MJ, Norat T, Ioannidis JP, Tsilidis KK. Diet, body size, physical activity and risk of prostate cancer: an umbrella review of the evidence. Eur J Cancer. 2016;69:61–9. [DOI] [PubMed] [Google Scholar]
- 26. Gao Y, Huang YB, Liu XO, Chen C, Dai HJ, Song FJ, Wang J, Chen KX, Wang YG. Tea consumption, alcohol drinking and physical activity associations with breast cancer risk among Chinese females: a systematic review and meta-analysis. Asian Pac J Cancer Prev. 2013;14(12):7543–50. [DOI] [PubMed] [Google Scholar]
- 27. Tang NP, Li H, Qiu YL, Zhou GM, Ma J. Tea consumption and risk of endometrial cancer: a meta-analysis. Am J Obstet Gynecol. 2009;201(6):605.e1. [DOI] [PubMed] [Google Scholar]
- 28. Wang L, Zhang X, Liu J, Shen L, Li Z. Tea consumption and lung cancer risk: a meta-analysis of case-control and cohort studies. Nutrition. 2014;30(10):1122–7. [DOI] [PubMed] [Google Scholar]
- 29. Yu F, Jin Z, Jiang H, Xiang C, Tang J, Li T, He J. Tea consumption and the risk of five major cancers: a dose-response meta-analysis of prospective studies. BMC Cancer. 2014;14:197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zhang YF, Xu Q, Lu J, Wang P, Zhang HW, Zhou L, Ma XQ, Zhou YH. Tea consumption and the incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Eur J Cancer Prev. 2015;24(4):353–62. [DOI] [PubMed] [Google Scholar]
- 31. Continuous Update Project Expert Report 2018 . Non-alcoholic drinks and the risk of cancer. World Cancer Research Fund/American Institute for Cancer Research, 2018. [Google Scholar]
- 32. Boehm K, Borrelli F, Ernst E, Habacher G, Hung SK, Milazzo S, Horneber M. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst Rev. 2009;3:CD005004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Yang CS, Wang X, Lu G, Picinich SC. Cancer prevention by tea: animal studies, molecular mechanisms and human relevance. Nat Rev Cancer. 2009;9(6):429–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bag A, Bag N. Tea polyphenols and prevention of epigenetic aberrations in cancer. J Nat Sci Biol Med. 2018;9(1):2–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Neveu V, Perez-Jimenez J, Vos F, Crespy V, du Chaffaut L, Mennen L, Knox C, Eisner R, Cruz J, Wishart Det al. Phenol-Explorer: an online comprehensive database on polyphenol contents in foods. Database (Oxford). 2010;2010:bap024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. van Dam RM, Hu FB, Willett WC. Coffee, caffeine, and health. N Engl J Med. 2020;383(4):369–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.