ABSTRACT
This systematic review and meta-analysis aimed to explore the association between the Mediterranean dietary pattern and inflammation in older adults. The Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines were followed. A search of the literature was conducted up to June 2020 in 7 electronic databases, namely PubMed, Embase, Web of Science, Scopus, Cochrane Library, Cumulative Index to Nursing and Allied Health Literature (CINAHL), and ProQuest. The Joanna Briggs Institute Critical Appraisal Checklists and the Newcastle-Ottawa Scale were used to assess the methodological quality. The overall standardized mean difference (SMD) and 95% CIs were estimated in random-effects meta-analyses. Thirteen studies were identified as having acceptable quality and were included in this systematic review: 3 randomized controlled trials (RCTs), 1 quasi-experimental study, 1 cohort study, and 8 cross-sectional studies. The circulating C-reactive protein (CRP) concentration was the most common inflammation indicator used. Results of the meta-analysis on 5 cross-sectional studies revealed a significant inverse association between the Mediterranean dietary pattern and inflammation as assessed by CRP (SMD = −0.26; 95% CI: −0.41, −0.11; P < 0.001). Other studies that investigated a variety of inflammation indicators other than CRP showed mixed results with regard to the relation between the Mediterranean dietary pattern and inflammation in older adults. Our findings suggest that the Mediterranean dietary pattern may be associated with lower inflammation in older adults. However, more long-term RCTs are required to demonstrate the effects of the Mediterranean dietary pattern on multiple inflammation parameters in older adults. The study has been registered on PROSPERO (#CRD42020140145).
Keywords: C-reactive protein, dietary pattern, inflammatory indicator, Mediterranean dietary pattern, older people
C-reactive protein was the most popular inflammation indicator among the eligible studies. The results revealed a significant inverse association between the Mediterranean dietary pattern and inflammation with C-reactive protein.
Introduction
During the past decade, the percentage of population aged ≥65 y has increased and will continue to increase in the next 10 y (1). It has been predicted that the percentage of older adults worldwide will increase from 11% in 2000 to 22% in 2050 (1). Age-related diseases, such as sarcopenia, frailty, cardiovascular diseases, and neurodegenerative diseases, increase the risk of disability in older adults, resulting in higher health care burden (2, 3). Inflammation plays a key role in developing age-related diseases, especially chronic low-grade inflammation (4–6). Chronic low-grade inflammation is defined as chronically producing steady and low-level inflammation that can be indicated by biomarkers of C-reactive protein (CRP), interleukin (IL-6), and adipokines such as Tumor Necrosis Factor (TNF-α). Age and dietary patterns both are crucial factors for inflammation (7, 8), but unlike age, dietary patterns could be modified to reduce inflammation.
Since the early 1960s, dietary patterns of Mediterranean countries have been found to have a beneficial influence on health (9). Mortality and morbidity are lower in Mediterranean countries than in the United States and Northern European countries (10, 11). Recently, the recommendations from the WHO (12) and other scientific experts and institutions (9) strongly support the concept that the Mediterranean dietary pattern may reduce the risk of chronic diseases. Although a Mediterranean dietary pattern varies from one country to another, its main components are as follows: 1) high consumption of MUFAs, vegetables, fruits, legumes, nuts, and fish; 2) moderate consumption of dairy products, meats, and wine; and 3) limited consumption of SFAs. The Mediterranean dietary pattern is healthy, and it reduces the risk of age-related diseases such as cancer and cardiovascular diseases, and promotes better mental health and quality of life in older adults (13–16).
Most previous studies investigating anti-inflammatory effects of the Mediterranean dietary pattern have mainly focused on adults and the results were inconsistent (17–21). The association between the Mediterranean dietary pattern and inflammation was different for different age groups (22). Therefore, the results of studies focusing on adults may not be applicable to the older population. Although several studies that discussed the association between the Mediterranean dietary pattern and inflammation in older adults have been published recently (23–25), the association between the Mediterranean dietary pattern and inflammation in older adults is still not clearly understood. Moreover, previous systematic reviews and meta-analyses regarding the effects of a Mediterranean dietary pattern have included a paucity of studies involving older adults (16, 26–28). In view of these literature gaps, the purpose of this systematic review and meta-analysis was to explore the association between the Mediterranean dietary pattern and inflammation in older adults.
Methods
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (29). The protocol for this review was registered at PROSPERO (registration no. CRD42020140145).
Selection criteria and search strategy
Inclusion criteria were as follows: 1) studies involving adults aged ≥65 y, including the mean age (30) regardless of study settings; 2) studies comparing data on recognized inflammatory cytokines and adipokines among individuals with a Mediterranean-type diet defined by the original article authors; 3) studies with a quantitative study design; and 4) studies written in English and published in peer-reviewed journals. Articles were excluded if they were conference abstracts, commentaries, editorials, letters to the editor, protocols, and review articles, or if information about age was missing.
The search strategy aimed to identify published articles with full texts up to June 2020. Articles were searched in the following 7 electronic databases: PubMed (NCBI), Embase (OVID), Scopus, Web of Science (Thomson Reuters), Cochrane Library, Cumulative Index to Nursing and Allied Health Literature (CINAHL, EBSCO), and ProQuest. Keywords and MeSH (medical subject heading) terms used to search were as follows: Mediterranean diet, inflammation (inflammatory OR inflammat* OR cytokine* OR CRP OR “C reactive protein” OR interleukin OR “tumor necrosis factor” OR leukocyte), and older adults (elderly OR aged OR senior OR geriatric OR “late life” OR elder* OR “older people” OR “older person” OR old* OR eld*). Reference lists of the retrieved articles were checked to identify additional studies.
Study selection and quality assessment
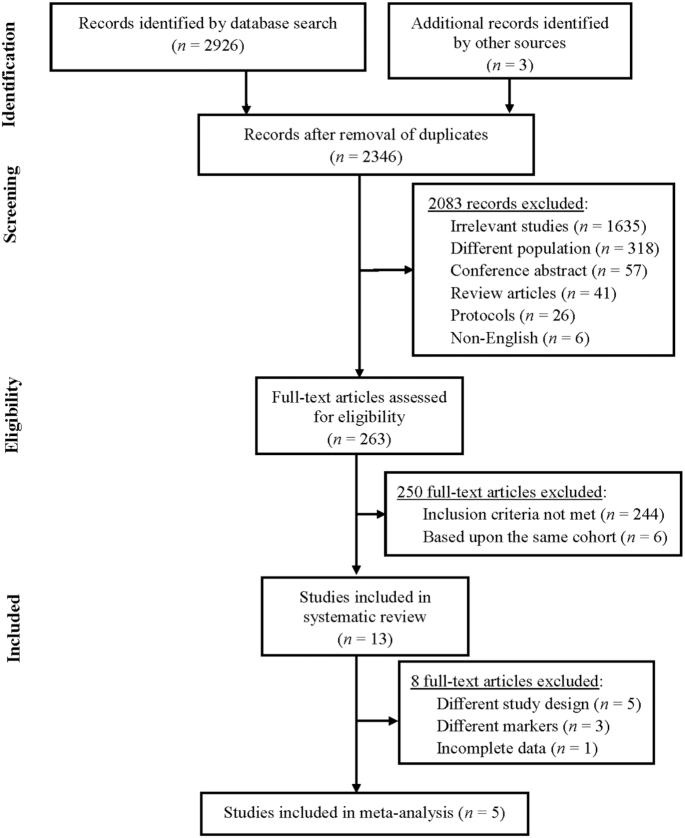
A total of 2926 papers were initially identified. After excluding duplicates and after the title and abstract screening, 263 potentially eligible full-text papers were independently assessed for complete eligibility by 2 reviewers (P-YW and K-MC) using a 3-point scale (0 = no relevance, 1 = unsure, and 2 = relevant). Any discrepancies in judgment were resolved through discussion with a third reviewer (W-CT). As shown in Figure 1, the most common reason for excluding studies was relevancy. One of the reasons was that this review defined older adults as those who were ≥65 y old. However, some initially retrieved studies had an average age of <65 y. Those studies were excluded from this systematic review. Another reason was that, although some initially retrieved studies mentioned Mediterranean diets or inflammation in abstracts, the focus of these studies was not on the Mediterranean dietary pattern or inflammation. Those studies, therefore, were excluded from this systematic review. Endnote software (version x8.0.1; Thomson Reuters, NY) was used to group results of the selected studies. If multiple publications originated from the same study cohort, only the publication with the most detailed information for outcomes was included. Finally, a total of 13 studies were identified and included in this systematic review.
FIGURE 1.
Flow diagram of the study selection process.
A standardized data-extraction tool suggested by the Cochrane Collaboration was used. One reviewer (P-YW) extracted the following data and information from the eligible studies: last name of the first author, year of publication, country, study design, number and characteristics of study population, components of the Mediterranean dietary pattern, and inflammatory biomarkers. The accuracy of extracted data was verified by all reviewers. In case of incomplete or additional information for meta-analysis calculation, such as the SE or 95% CIs of the results, the study authors were contacted. However, no reply was received. Therefore, studies with incomplete or missing information were not included in the meta-analysis, but they were discussed in the systematic review.
The Newcastle-Ottawa Scale (NOS) for cohort studies (31) and cross-sectional studies (32) and Joanna Briggs Institute (JBI) Critical Appraisal Checklists for randomized controlled trials (RCTs) and quasi-experimental studies were used for the quality assessment of the included studies (33). The NOS consisted of 8 questions assessing 3 main domains: selection of participants, comparability of participants, and assessment of outcome/exposure. The total score of each cohort study was 9 (31). The version adjusted for cross-sectional studies consisted of 7 questions, and the total score was 10 (32). In the NOS, studies obtaining 1 to 3 points were identified as having low quality, 4 to 6 points as medium quality, and ≥7 points as high quality (34, 35). Studies with medium and high quality were considered as having acceptable quality and were included in this systematic review. JBI checklists contain 9 and 13 questions for quasi-experimental studies and RCTs, respectively. Studies that answered at least half of the questions with “yes” answers—for example, 5 of 9 items in quasi-experimental studies and 7 of 13 items in RCTs—were considered as having acceptable quality to be included in this systematic review (36). Two reviewers (P-YW and K-MC) independently assessed the quality of eligible studies, and consensus between reviewers was reached. Overall, all the observational studies were of medium or high quality, and all RCTs and quasi-experimental studies had “yes” answers for more than half of the questions on the JBI checklists. Therefore, all of the 13 studies were considered as having appropriate quality and acceptable reporting bias (Supplemental Table 1).
All of the 13 studies listed inflammation indicators, and the number of indicators ranged from 1 to 7. Heterogeneity was observed among the inflammatory indicators, and CRP was the most commonly used inflammation marker. Therefore, this meta-analysis used serum CRP concentration as an indicator of inflammation. Of the 13 studies, 6 cross-sectional studies were included in the meta-analysis. However, one of these studies had incomplete data; therefore, 5 cross-sectional studies were included in the meta-analysis.
Statistical methods
The meta-analysis was conducted using Comprehensive Meta-Analysis version 2.0 (Englewood, NJ). The overall standardized mean difference (SMD) and 95% CIs were estimated in random-effects meta-analysis to determine the association between adherence to the Mediterranean dietary pattern and concentrations of CRP. Forest plots were generated to illustrate the study-specific effect sizes along with the 95% CI. If the 2-tailed P value was <0.05, the result was considered significant.
Of studies included in the meta-analysis, the heterogeneity between studies was assessed using the Cochran Q test and the I2 statistic (37). A Q test with α = 0.1, P < 0.1, indicated that heterogeneity exists. I2 values of 25%, 50%, and 75% indicated a low, medium, and high level of heterogeneity, respectively. Publication bias was evaluated using Begg's rank correlation test and Egger's intercept test, with the significance level setting at P < 0.05. Sensitivity analyses were conducted by excluding the study that reported the largest effect size (38).
Results
Characteristics of studies and participants
The main characteristics and findings of included studies are summarized in Table 1. The 13 included studies were published from 2006 to 2019. Eight were cross-sectional studies (23, 24, 39–44), 3 were RCTs (25, 45, 46), 1 was a quasi-experimental study (47), and 1 was a cohort study (48). Five of the cross-sectional studies were included in the meta-analysis (23, 24, 41, 43, 44). Six effect sizes were reported in this meta-analysis because 1 study performed gender-stratified analyses and provided separate results for men and women (23). The 13 studies were conducted in the following countries: China (23), Spain (42, 45, 47), Italy (24), Germany (44), Norway (46), United Kingdom (25, 39, 48), and the United States (41, 43). One of the cross-sectional studies (40) was conducted across cities in multiple European countries: France, Greece, Italy, Germany, and Poland.
TABLE 1.
Descriptive characteristics of the included studies1
| Study (ref) | Study design | Country | Study participants | Sample size, n | Age, y | Participants, % female | Diet assessment tools | Dietary pattern for study | Inflammatory cytokines | Quality score2 |
|---|---|---|---|---|---|---|---|---|---|---|
| Camargo et al. (47) | Quasi-experimental study | Spain | Community-dwelling older adults | 20 | 67.1 | 50 | 3-d dietary records and FFQ | Intervention group: Mediterranean diet | IL-6 ↑ | 8/9 |
| SFA-rich diet | TNF-α – | |||||||||
| Low-fat, high-carbohydrate diet enriched in n–3 PUFAs | MCP-1 – | |||||||||
| Control group: nil | ||||||||||
| Chan et al. (23) | Cross-sectional studies | China | Community-dwelling older adults | 2646 | ≥65 | 50 | FFQ | Mediterranean diet: high intakes of vegetables, legumes, fruits and nuts, cereal, fish, and MUFA to SFA ratio; low intakes of meat, poultry, and dairy products | CRP – | 7/10 |
| Corley et al. (39) | Cross-sectional studies | United Kingdom | Community-dwelling older adults | 792 | ≥70 | 52 | FFQ | Mediterranean diet: high intakes of vegetables, fish, poultry, pasta, rice, water, tomato-based sauces, oil and vinegar dressing, and beans | CRP –Fibrinogen ↓ | 8/10 |
| Dedoussis et al. (40) | Cross-sectional studies | France | Noninstitutionalized old adults | 957 | 68.4- 79.93 | 56 | FFQ | Mediterranean diet: high intakes of nonrefined cereals, fruit, vegetables, potatoes, legumes, fish, and olive oil; low intakes of red meat and red meat products, poultry, full-fat dairy products, and wine | IL-8 ↓ | 5/10 |
| Germany | ||||||||||
| Greece | ||||||||||
| Italy | ||||||||||
| Poland | ||||||||||
| Estruch et al. (45) | RCT | Spain | Older adults with CVD high-risk from primary care centers | 772 | 68.5–69.54 | 56 | FFQ | Intervention group: Mediterranean diet + 50 mL extra-virgin olive oil | CRP ↓IL-6 ↓ICAM-1 ↓VCAM-1 ↓ | 11/13 |
| Mediterranean diet + 30 g mixed nuts | ||||||||||
| Control group: low-fat diet | ||||||||||
| Gu et al. (41) | Cross-sectional studies | USA | Community-dwelling older adults | 1219 | ≥65 | 67 | FFQ | Mediterranean diet: high intakes of fruit, vegetables, legumes, cereals, fish, and MUFA to SFA ratio; low intakes of meat and dairy products; moderate alcohol intake | CRP ↓ | 7/10 |
| Lo Buglio et al. (24) | Cross-sectional studies | Italy | Community-dwelling older adults with hospitalized less than 30 days | 194 | ≥65 | 56 | Data not shown | Mediterranean diet: high intakes of pasta, typical Mediterranean vegetables, fruit, legumes, olive oil, and fish; low intakes of soft drinks, butter, red meat, and potatoes; moderate alcohol intake | CRP ↓IL-1β –IL-6 ↓IL-8 –TNF-α ↑ | 5/10 |
| Luciano et al. (48) | Cohort study | United Kingdom | Community-dwelling older adults | 456–669 | 69.5 | Roughly equal | FFQ | Mediterranean diet: high intakes of vegetables, fruit and fish, poultry, pasta, rice, water, tomato-based sauces, oil and vinegar dressing, and beans (follow-up for 3 y) | CRP ↓IL-6 – | 8/9 |
| Maijo et al. (25) | RCT | United Kingdom | Community-dwelling older adults | 73 | 65–79 | 60 | 7-d dietary records | Intervention group: Mediterranean diet + 10 μg cholecalciferol supplements | IL-2 –IL-22 – | 7/13 |
| Control group: national dietary guidelines | ||||||||||
| Salas-Salvadó et al. (42) | Cross-sectional studies | Spain | Older adults from primary care centers | 772 | M: 67.6; F: 69.8 | 56 | FFQ | Mediterranean diet: high intakes of olive oil, vegetables, legumes, fruits, nuts, fish and seafood, white meats instead of red meats, homemade sauces, and red wine; low intakes of red meats, fat-rich dairy products, commercial pastries and snacks, and artificially sweetened beverages | IL-6 –ICAM-1 – | 9/10 |
| Shahar et al. (43) | Cross-sectional studies | USA | Community-dwelling older adults | 2225 | 70–79 | 50 | FFQ | Mediterranean diet: high intakes of ratio of MUFAs to SFAs, legumes, grains, fruit and nuts, vegetable, and fish; moderate intakes of alcohol and dairy products; low intakes of meat and meat products | CRP ↓IL-6 ↓ | 7/10 |
| Trøseid et al. (46) | RCT | Norway | Community-dwelling older men with high CVD risk | 563 | 64–76 | 0 | FFQ | Intervention group: Mediterranean-type diet + placebo | CRP ↓IL-6 ↓IL-18 ↓TNF-α ↓MCP-1 ↓ | 7/13 |
| Mediterranean-type diet + n–3 fatty acids supplement | ||||||||||
| Habitual diet + n–3 fatty acids supplement | ||||||||||
| Control group: habitual diet + placebo | ||||||||||
| Waldeyer et al. (44) | Cross-sectional studies | Germany | CAD patients | 1121 | 70.7 | 27 | FFQ | Mediterranean diet: high intakes of whole-grain products, fish, vegetables, fruit, nuts and legumes; low intakes of meat; moderate intakes of alcohol | CRP ↓ | 6/10 |
CAD, coronary artery disease; CRP, C-reactive protein; CVD, cardiovascular disease; FFQ, food-frequency questionnaire; ICAM-1, intercellular adhesion molecule 1; MCP-1, monocyte chemoattractant protein 1; MDS, Mediterranean Diet Score; ref, reference; RCT, randomized controlled trial; VCAM-1, vascular cell adhesion molecule 1; ↑, significant increase (P < 0.05); ↓, significant decrease (P < 0.05); –, nonsignificant (P ≥ 0.05).
Score/total score.
The authors did not provide the mean value of age in overall participants but for different genders and countries: France (men/women): 68.4 ± 6.2/68.4 ± 6.1 y; Germany (men/women): 69.7 ± 4.8/70.3 ± 4.9 y; Greece (men/women): 73.3 ± 6.2/71.0 ± 6.3 y; Italy (men/women): 77.2 ± 9.1/79.9 ± 10.0 y; Poland (men/women): 72.7 ± 6.2/71.0 ± 7.2 y.
The authors did not provide the mean value of age in overall participants but for different dietary groups: Mediterranean diet with extra-virgin olive oil (68.5 ± 6.2 y), Mediterranean diet with mixed nuts (68.6 ± 6.9 y), and low-fat diet (69.5 ± 6.1 y).
The reviewed articles reported results from 10,936 older adults in cross-sectional studies, 1408 older adults in intervention studies, and between 456 and 669 older adults in the cohort study. The smallest sample size was 20 participants in the quasi-experimental study (47), followed by 73 participants in one of the RCTs (25), and 194 participants in one of the cross-sectional studies (24). One study included only male participants (46), and the remaining studies included both genders. Most of the participants in the included studies were ≥65 y of age, but participants in 1 study were ≥64 y old (46) and in another study participants were >60 y old (40). One study focused on adults aged 55–80 y (45) and another did not define older adults (47). Most studies recruited healthy community-dwelling older adults, 2 studies included participants with risk of cardiovascular diseases (42, 45), 1 study included patients with acute diseases (24), and 1 study focused on patients with coronary artery disease (44).
Dietary components of a Mediterranean dietary pattern in the studies included in this review were higher intakes of vegetables, fruits, fish, legumes, and nuts and lower intakes of SFAs. MUFA was also the main component in some studies (23–25, 40–43, 47, 48). In all of the 3 RCTs, dietary recommendations were provided for both the intervention group and control group. The dietary recommendations for the control group were a low-fat diet according to the American Heart Association guidelines, a healthy diet based on national dietary guidelines, and a habitual diet. The quasi-experimental study used a crossover design in which a Mediterranean diet, a low-fat and high-carbohydrate diet enriched in n–3 PUFAs, and an SFA-rich diet were provided to all participants in different periods. In 2 RCTs, the cohort study, and the 8 cross-sectional studies, a food-frequency questionnaire (FFQ) was used to measure dietary intakes. The remaining RCT used 7-d dietary records (25), and the quasi-experimental study used both 3-d dietary records and an FFQ to measure dietary intakes. However, one of the cross-sectional studies did not report how the dietary pattern was assessed (24).
Inflammation biomarkers examined in the included studies varied, and the frequently assessed indicators were CRP, IL-6, and TNF-α, which were assessed most commonly by ELISA (23, 41–43, 45–48). Other inflammation indicators assessed were fibrinogen, IL-1β, IL-2, IL-18, IL-22, IL-23, monocyte chemoattractant protein 1 (MCP-1), intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and myeloperoxidase.
Mediterranean dietary pattern and inflammation
Two of the RCTs (45, 46) reported that higher adherence to the Mediterranean diet showed decreased concentrations of CRP (−0.54 and −0.34 mg/L, respectively) and IL-6 (−1.6 and −0.2 pg/mL, respectively) in older adults. TNF-α was assessed in 1 RCT (46), and it was decreased. Other reported inflammation markers were MCP-1 (45, 46), IL-2 (25), IL-18 (46), IL-22 (25), ICAM-1 (42, 45), and VCAM-1 (45). Mixed effects of the Mediterranean dietary pattern on inflammation were found.
The participants in the quasi-experimental study followed a Mediterranean dietary pattern with virgin olive oil for 3 wk, and it was found that the concentration of postprandial plasma IL-6 (P < 0.001) was increased. However, while compared with 2 other types of dietary patterns, a statistically significant difference was not found in postprandial plasma IL-6 (P = 0.933) and TNF-α (P = 0.723) with the Mediterranean dietary pattern.
After a follow-up period of 3 y, Luciano et al. (48) in a cohort study found that participants with higher adherence to the Mediterranean dietary pattern had significantly decreased CRP (r = −0.10, P = 0.030) concentrations. However, no association was found between the Mediterranean dietary pattern and serum concentration of IL-6 (r = −0.07, P = 0.070) after 3 y.
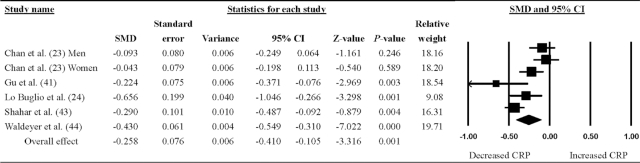
As shown in Figure 2, the meta-analysis pooled the outcomes for 5 cross-sectional studies reporting SMDs to show the association between the Mediterranean dietary pattern and CRP concentrations in older adults. The Mediterranean dietary pattern was significantly associated with a greater reduction in CRP (SMD = −0.26; 95% CI: −0.41, −0.11; P < 0.001).
FIGURE 2.
Random-effects meta-analysis of studies quantifying the association between the Mediterranean dietary pattern and circulating CRP concentration in older adults. SMDs and 95% CIs (lower limit, upper limit) are shown. An SMD <0 indicates decreased CRP concentration. A larger forest plot point reflects a study with heavier relative weight. CRP, C-reactive protein; SMD, standardized mean difference.
The Cochrane Q and I2 tests were 23.8 and 79.0%, respectively, indicating high heterogeneity among the included studies. Sensitivity analysis was conducted to examine the robustness of results by excluding the study with the largest effect size, resulting in an SMD of −0.20 (95% CI: −0.35, −0.06; P = 0.004). According to Egger's regression test and Begg's rank test, publication bias was absent in the analyses (P = 0.80 and 1.00, respectively).
Discussion
To our knowledge, this is the first systematic review and meta-analysis to focus on the relation between a Mediterranean dietary pattern and inflammation in older adults. The results of the current meta-analysis indicated an inverse association between the Mediterranean dietary pattern and inflammation with CRP concentrations. The included RCTs also revealed that a higher intake of a Mediterranean dietary pattern leads to a reduction in both CRP and IL-6 concentrations.
In this review, studies using MUFAs or olive oil as one of the components of a Mediterranean dietary pattern showed a significant reduction in CRP. Olive oil is rich in MUFAs and polyphenols, which may possess anti-inflammatory properties (49). The anti-inflammatory mechanism of MUFAs, however, is still unknown and requires more investigation.
In addition to MUFAs or olive oil, other components of the Mediterranean dietary pattern may also contribute to the decrease in CRP. Long-chain n–3 fatty acids may be associated with improved serum CRP and IL-6 concentrations. Fish is a major source of the long-chain n–3 fatty acids DHA and EPA, which are associated with a reduction in serum CRP in older adults (50). Long-chain n–3 fatty acids inhibit the activation of proinflammatory transcription factors to lower the expression of inflammation-associated genes, such as cyclooxygenase-2 (COX2), and to increase the activation of anti-inflammatory transcription factors, such as peroxisome proliferator-activated receptor γ (51, 52). In addition, legumes, nuts, vegetables, and fruits also reduce concentrations of inflammatory markers. Legumes and nuts have high amounts of plant proteins, dietary fiber, phytochemicals, and ɑ-linolenic acid to protect against inflammation (53, 54). Vegetables and fruits are rich sources of polyphenols (55) and are also high in antioxidants, including carotenoid, vitamin C, phytochemicals, and dietary fiber, to decrease CRP and other inflammatory cytokines (56). One of the possible mechanisms is that these antioxidants suppress the production of reactive oxygen species (ROS) and remove ROS from cells, which inhibits the translocation of NF-κB to decrease cyclooxygenase-2, which is related to the production and release of CRP and IL-6 (57). Moreover, red meat and its products are the major sources of SFAs. A lower intake of SFAs is related to lower inflammatory cytokines due to decreased induction of inflammation-associated genes (58).
Not all dietary components of a Mediterranean diet pattern were the same in the included studies in this systematic review. However, the most essential dietary components of a Mediterranean dietary pattern, such as higher intakes of vegetables, fruits, fish, legumes, and nuts and lower intakes of SFAs, were used in all of the studies that were included in this review. Those essential dietary components may have anti-inflammation properties. However, complex interactions and synergistic effects occur among the aforementioned components and nutrients in diet. Therefore, to reduce inflammation, a dietary pattern is better than a single food group or nutrient (59).
Limitations
This systematic review and meta-analysis has some limitations. First, few articles were included in the meta-analysis; hence, the statistical power may be limited. Second, this review included 1 article from the Prevención con Dieta Mediterránea (PREDIMED) study, which had the proposed characteristics of a well-designed RCT such as effective randomization (60). However, the quality of other included RCTs in this systematic review varied. The study design of the PREDIMED study was notably better than that of other RCTs. Therefore, although the effect of a Mediterranean dietary pattern on decreased concentrations of CRP was reported in the RCTs in this review, more RCTs with larger sample sizes would be needed to demonstrate the effect of a Mediterranean dietary pattern on inflammation markers. Third, heterogeneity in this meta-analysis was significantly high. Differences in the sample population, methods used to evaluate the adherence to a Mediterranean dietary pattern, and the limited number of included studies may have contributed to the observed heterogeneity. Fourth, factors other than the Mediterranean dietary pattern may influence inflammation. Only 1 cross-sectional study adjusted for confounding factors. Although this study was included in the meta-analysis and had results similar to the RCTs and the cohort study, the effects of confounding factors could not be excluded, which may affect the power to report the effect of Mediterranean dietary patterns. In addition, this review was limited to cytokines and adipokines as markers of inflammation. Finally, most participants in the included studies were community-dwelling older adults, which may limit the generalizability. The results of this review may not be applicable to older adults living in institutions. Therefore, more studies are needed to identify the association between a Mediterranean dietary pattern and inflammation in the older population in different settings.
Clinical implications
The results of this review suggest that the Mediterranean dietary pattern is related to a reduction in chronic, low-grade inflammation in older adults. Inflammation contributes to the process of aging and age-related diseases (5). The anti-inflammatory effect of the Mediterranean dietary pattern may lead to healthy aging in older adults (61) and a decrease in health care costs.
Conclusions
In this meta-analysis, an inverse association between the Mediterranean dietary pattern and inflammation was identified in older adults. The results reported in the RCTs and the cohort study also supported the association of the Mediterranean dietary pattern with lower CRP concentrations in older adults. Few studies were reviewed in this systematic review, and additional long-term RCTs are needed to gain insight into the effects of a Mediterranean dietary pattern on older adults. Moreover, further research could examine “how” the Mediterranean dietary pattern relates to inflammation and the “dose-response” in-depth.
Supplementary Material
ACKNOWLEDGEMENTS
The authors’ responsibilities were as follows—P-YW and K-MC: designed the study and conducted the literature search and data extraction; P-YW: performed the statistical analysis and interpreted the data; P-YW, K-MC, and W-CT: drafted and critically revised the manuscript; and all authors: met the criteria for authorship and read and approved the final manuscript.
Notes
This study was partially supported by the Kaohsiung Medical University Research Center (grant KMU-TC108B02).
Author disclosures: The authors report no conflicts of interests.
The funding source was not involved in the study design, data collection, analysis and interpretation of data, writing of the report, or decision to submit the manuscript for publication.
Supplemental Table 1 is available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances.
Abbreviations used: CRP, C-reactive protein; FFQ, food-frequency questionnaire; ICAM-1, intercellular adhesion molecule 1; IL, interleukin; JBI, Joanna Briggs Institute; MCP-1, monocyte chemoattractant protein 1; NOS, Newcastle-Ottawa Scale; PREDIMED, Prevención con Dieta Mediterránea; RCT, randomized controlled trial; ROS, reactive oxygen species; SMD, standardized mean difference; TNF, Tumor Necrosis Factor; VCAM-1, vascular cell adhesion molecule 1.
Contributor Information
Pei-Yu Wu, Center for Long-term Care Research, Kaohsiung Medical University, Kaohsiung, Taiwan.
Kuei-Min Chen, Center for Long-term Care Research, Kaohsiung Medical University, Kaohsiung, Taiwan; College of Nursing, Kaohsiung Medical University, Kaohsiung, Taiwan; Department of Medical Research, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan.
Wan-Chi Tsai, Department of Medical Laboratory Science and Biotechnology, Kaohsiung Medical University, Kaohsiung, Taiwan; Department of Laboratory Medicine, Kaohsiung Medical University Hospital, Kaohsiung, Taiwan.
References
- 1. World Health Organization . Facts about ageing. 2014.; [cited 2019 Sep 2] [Internet]. Available from: http://www.who.int/ageing/about/facts/en. [Google Scholar]
- 2. Franceschi C, Garagnani P, Morsiani C, Conte M, Santoro A, Grignolio A, Monti D, Capri M, Salvioli S. The continuum of aging and age-related diseases: common mechanisms but different rates. Front Med. 2018;5(61):1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Prince MJ, Wu F, Guo Y, Gutierrez Robledo LM, O'Donnell M, Sullivan R, Yusuf S. The burden of disease in older people and implications for health policy and practice. Lancet. 2015;385(9967):549–62. [DOI] [PubMed] [Google Scholar]
- 4. Jensen GL. Inflammation: roles in aging and sarcopenia. J Parenter Enteral Nutr. 2008;32(6): 656–9. [DOI] [PubMed] [Google Scholar]
- 5. Michaud M, Balardy L, Moulis G, Gaudin C, Peyrot C, Vellas B, Cesari M, Nourhashemi F. Proinflammatory cytokines, aging, and age-related diseases. J Am Med Dir Assoc. 2013;14(12):877–82. [DOI] [PubMed] [Google Scholar]
- 6. Ryall JG, Schertzer JD, Lynch GS. Cellular and molecular mechanisms underlying age-related skeletal muscle wasting and weakness. Biogerontology. 2008;9(4):213–28. [DOI] [PubMed] [Google Scholar]
- 7. Krabbe KS, Pedersen M, Bruunsgaard H. Inflammatory mediators in the elderly. Exp Gerontol. 2004;39(5):687–99. [DOI] [PubMed] [Google Scholar]
- 8. Minihane AM, Vinoy S, Russell WR, Baka A, Roche HM, Tuohy KM, Teeling JL, Blaak EE, Fenech M, Vauzour Det al. Low-grade inflammation, diet composition and health: current research evidence and its translation. Br J Nutr. 2015;114(7):999–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sofi F, Abbate R, Gensini GF, Casini A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: an updated systematic review and meta-analysis. Am J Clin Nutr. 2010;92(5):1189–96. [DOI] [PubMed] [Google Scholar]
- 10. Keys A, Mienotti A, Karvonen MJ, Aravanis C, Blackburn H, Buzina R, Djordjevic BS, Dontas AS, Fidanza F, Keys MHet al. The diet and 15-year death rate in the Seven Countries Study. Am J Epidemiol. 1986;124(6):903–15. [DOI] [PubMed] [Google Scholar]
- 11. Tunstall-Pedoe H, Kuulasmaa K, Mähönen M, Tolonen H, Ruokokoski E, Amouyel P. Contribution of trends in survival and coronary-event rates to changes in coronary heart disease mortality: 10-year results from 37 WHO MONICA project populations. Monitoring Trends and Determinants in Cardiovascular Disease. Lancet. 1999;353(9164):1547–57. [DOI] [PubMed] [Google Scholar]
- 12. Renzella J, Townsend N, Jewell J, Breda J, Roberts N, Rayner M, Wickramasinghe K. What national and subnational interventions and policies based on Mediterranean and Nordic diets are recommended or implemented in the WHO European region, and is there evidence of effectiveness in reducing noncommunicable diseases?. Copenhagen (Denmark): WHO Regional Office for Europe; 2018. [PubMed] [Google Scholar]
- 13. Bollwein J, Diekmann R, Kaiser MJ, Bauer JM, Uter W, Sieber CC, Volkert D. Dietary quality is related to frailty in community-dwelling older adults. J Gerontol A Biol Sci Med Sci. 2012;68(4):483–9. [DOI] [PubMed] [Google Scholar]
- 14. Roman B, Carta L, Martínez-González MA, Serra-Majem L. Effectiveness of the Mediterranean diet in the elderly. Clin Interv Aging. 2008;3(1):97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rosato V, Temple NJ, La Vecchia C, Castellan G, Tavani A, Guercio V. Mediterranean diet and cardiovascular disease: a systematic review and meta-analysis of observational studies. Eur J Nutr. 2019;58(1):173–91. [DOI] [PubMed] [Google Scholar]
- 16. Schwingshackl L, Hoffmann G. Mediterranean dietary pattern, inflammation and endothelial function: a systematic review and meta-analysis of intervention trials. Nutr Metab Cardiovasc Dis. 2014;24(9):929–39. [DOI] [PubMed] [Google Scholar]
- 17. Blüher M, Rudich A, Klöting N, Golan R, Henkin Y, Rubin E, Schwarzfuchs D, Gepner Y, Stampfer MJ, Fiedler Met al. Two patterns of adipokine and other biomarker dynamics in a long-term weight loss intervention. Diabetes Care. 2012;35(2):342–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Esposito K, Marfella R, Ciotola M, Di Palo C, Giugliano F, Giugliano G, D'Armiento M, D'Andrea F, Giugliano D. Effect of a Mediterranean-style diet on endothelial dysfunction and markers of vascular inflammation in the metabolic syndrome: a randomized trial. JAMA. 2004;292(12):1440–6. [DOI] [PubMed] [Google Scholar]
- 19. Mayr HL, Thomas CJ, Tierney AC, Kucianski T, George ES, Ruiz-Canela M, Hebert JR, Shivappa N, Itsiopoulos C. Randomization to 6-month Mediterranean diet compared with a low-fat diet leads to improvement in Dietary Inflammatory Index scores in patients with coronary heart disease: the AUSMED Heart Trial. Nutr Res. 2018;55:94–107. [DOI] [PubMed] [Google Scholar]
- 20. Thomazella MCD, Góes MF, Andrade CR, Debbas V, Barbeiro DF, Correia RL, Marie SK, Cardounel AJ, daLuz PL, Laurindo FR. Effects of high adherence to Mediterranean or low-fat diets in medicated secondary prevention patients. Am J Cardiol. 2011;108(11):1523–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Jennings A, Berendsen AM, de Groot L, Feskens EJM, Brzozowska A, Sicinska E, Pietruszka B, Meunier N, Caumon E, Malpuech-Brugère Cet al. Mediterranean-style diet improves systolic blood pressure and arterial stiffness in older adults. Hypertension. 2019;73(3):578–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Whalen KA, McCullough ML, Flanders WD, Hartman TJ, Judd S, Bostick RM. Paleolithic and Mediterranean diet pattern scores are inversely associated with biomarkers of inflammation and oxidative balance in adults. J Nutr. 2016;146(6):1217–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chan R, Yu B, Leung J, Lee JS, Woo J. Association of dietary patterns with serum high-sensitivity C-reactive protein level in community-dwelling older adults. Clin Nutr ESPEN. 2019;31:38–47. [DOI] [PubMed] [Google Scholar]
- 24. Lo Buglio A, Bellanti F, Capurso C, Paglia A, Vendemiale G. Adherence to Mediterranean diet, malnutrition, length of stay and mortality in elderly patients hospitalized in internal medicine wards. Nutrients. 2019;11(4):790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maijo M, Ivory K, Clements SJ, Dainty JR, Jennings A, Gillings R, Fairweather-Tait S, Gulisano M, Santoro A, Franceschi Cet al. One-year consumption of a Mediterranean-like dietary pattern with vitamin D3 supplements induced small scale but extensive changes of immune cell phenotype, co-receptor expression and innate immune responses in healthy elderly subjects: results from the United Kingdom arm of the NU-AGE trial. Front Physiol. 2018;9(997):1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Barbaresko J, Koch M, Schulze MB, Nöthlings U. Dietary pattern analysis and biomarkers of low-grade inflammation: a systematic literature review. Nutr Rev. 2013;71(8):511–27. [DOI] [PubMed] [Google Scholar]
- 27. Mayr HL, Tierney AC, Thomas CJ, Ruiz-Canela M, Radcliffe J, Itsiopoulos C. Mediterranean-type diets and inflammatory markers in patients with coronary heart disease: a systematic review and meta-analysis. Nutr Res. 2018;50:10–24. [DOI] [PubMed] [Google Scholar]
- 28. Neale E, Batterham M, Tapsell LC. Consumption of a healthy dietary pattern results in significant reductions in C-reactive protein levels in adults: a meta-analysis. Nutr Res. 2016;36(5):391–401. [DOI] [PubMed] [Google Scholar]
- 29. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: the PRISMA statement. PLoS Med. 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O'Keeffe M, Kelly M, O'Herlihy E, O'Toole PW, Kearney PM, Timmons S, O'Shea E, Stanton C, Hickson M, Rolland Yet al. Potentially modifiable determinants of malnutrition in older adults: a systematic review. Clin Nutr. 2019;38(6):2477–98. [DOI] [PubMed] [Google Scholar]
- 31. Wells G, Shea B, O'Connell D, Peterson J, Welch V, Losos M. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in metaanalyses. 2019.; [cited Jun 5, 2020] [Internet]. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. [Google Scholar]
- 32. Herzog R, Álvarez-Pasquin MJ, Díaz C, Del Barrio JL, Estrada JM, Gil Á. Are healthcare workers' intentions to vaccinate related to their knowledge, beliefs and attitudes? A systematic review. BMC Public Health. 2013;13:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Joanna Briggs Institute . Critical appraisal tools. 2017.; [cited Jun 10, 2019] [Internet]. Available from: https://joannabriggs.org/critical_appraisal_tools. [Google Scholar]
- 34. Hofmeister M, Memedovich A, Dowsett LE, Sevick L, McCarron T, Spackman E, Stafinski T, Menon D, Noseworthy T, Clement F. Palliative care in the home: a scoping review of study quality, primary outcomes, and thematic component analysis. BMC Palliat Care. 2018;17(1):41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Strieder AP, Oliveira TM, Rios D, Cruvinel AFP, Cruvinel T. Is there a relationship of negative oral health beliefs with dental fear and anxiety regarding diverse dental patient groups? A systematic review and meta-analysis. Clin Oral Invest. 2019;23(9):3613–21. [DOI] [PubMed] [Google Scholar]
- 36. Chen SC, Jones C, Moyle W. Social robots for depression in older adults: a systematic review. J Nurs Scholarsh. 2018;50(6):612–22. [DOI] [PubMed] [Google Scholar]
- 37. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Liu JW, Tu YK, Lai YF, Lee HC, Tsai PS, Chen TJ, Huang HC, Chen YT, Chiu HY. Associations between sleep disturbances and suicidal ideation, plans, and attempts in adolescents: a systematic review and meta-analysis. Sleep. 2019;42(6):zsz054. [DOI] [PubMed] [Google Scholar]
- 39. Corley J, Kyle JAM, Starr JM, McNeill G, Deary IJ. Dietary factors and biomarkers of systemic inflammation in older people: the Lothian birth cohort 1936. Br J Nutr. 2015;114(7):1088–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Dedoussis GV, Kanoni S, Mariani E, Cattini L, Herbein G, Fulop T, Varin A, Rink L, Jajte J, Monti Det al. Mediterranean diet and plasma concentration of inflammatory markers in old and very old subjects in the ZINCAGE population study. Clin Chem Lab Med. 2008;46(7):990–6. [DOI] [PubMed] [Google Scholar]
- 41. Gu Y, Luchsinger JA, Stern Y, Scarmeas N. Mediterranean diet, inflammatory and metabolic biomarkers, and risk of Alzheimer's disease. J Alzheimers Dis. 2010;22(2):483–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Salas-Salvadó J, Garcia-Arellano A, Estruch R, Marquez-Sandoval F, Corella D, Fiol M, Gómez-Gracia E, Viñoles E, Arós F, Herrera Cet al. Components of the Mediterranean-type food pattern and serum inflammatory markers among patients at high risk for cardiovascular disease. Eur J Clin Nutr. 2008;62(5):651–9. [DOI] [PubMed] [Google Scholar]
- 43. Shahar DR, Houston DK, Hue TF, Lee JS, Sahyoun NR, Tylavsky FA, Geva D, Vardi H, Harris TB. Adherence to Mediterranean diet and decline in walking speed over 8 years in community-dwelling older adults. J Am Geriatr Soc. 2012;60(10):1881–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Waldeyer C, Brunner FJ, Braetz J, Ruebsamen N, Zyriax BC, Blaum C, Kroeger F, Kohsiack R, Schrage B, Sinning Cet al. Adherence to Mediterranean diet, high-sensitive C-reactive protein, and severity of coronary artery disease: contemporary data from the INTERCATH cohort. Atherosclerosis. 2018;275:256–61. [DOI] [PubMed] [Google Scholar]
- 45. Estruch R, Martínez-González MA, Corella D, Salas-Salvadó J, Ruiz-Gutiérrez V, Covas MI, Fiol M, Gómez-Gracia E, López-Sabater MC, Vinyoles Eet al. Effects of a Mediterranean-style diet on cardiovascular risk factors: a randomized trial. Ann Intern Med. 2006;145(1):1–11. [DOI] [PubMed] [Google Scholar]
- 46. Trøseid M, Arnesen H, Hjerkinn EM, Seljeflot I. Serum levels of interleukin-18 are reduced by diet and n-3 fatty acid intervention in elderly high-risk men. Metabolism. 2009;58(11):1543–9. [DOI] [PubMed] [Google Scholar]
- 47. Camargo A, Delgado-Lista J, Garcia-Rios A, Cruz-Teno C, Yubero-Serrano EM, Perez-Martinez P, Gutierrez-Mariscal FM, Lora-Aguilar P, Rodriguez-Cantalejo F, Fuentes-Jimenez Fet al. Expression of proinflammatory, proatherogenic genes is reduced by the Mediterranean diet in elderly people. Br J Nutr. 2012;108(3):500–8. [DOI] [PubMed] [Google Scholar]
- 48. Luciano M, Mõttus R, Starr JM, McNeill G, Jia X, Craig LCA, Deary IJ. Depressive symptoms and diet: their effects on prospective inflammation levels in the elderly. Brain Behav Immun. 2012;26(5):717–20. [DOI] [PubMed] [Google Scholar]
- 49.. In: Segura-Carretero A, Menéndez-Menéndez J, Fernández-Gutiérrez A. Preedy VR, Watson RR. Polyphenols in olive oil: the importance of phenolic compounds in the chemical composition of olive oil, editors. Olives and olive oil in health and disease prevention. San Diego (CA): Elsevier Academic Press; 2010. p. 167–75. [Google Scholar]
- 50. Baierle M, Vencato HP, Oldenburg L, Bordignon S, Zibetti M, Trentini MC, Duarte MM, Veit JC, Somacal S, Emanuelli Tet al. Fatty acid status and its relationship to cognitive decline and homocysteine levels in the elderly. Nutrients. 2014;6(9):3624–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Calder PC. Omega-3 fatty acids and inflammatory processes: from molecules to man. Biochem Soc Trans. 2017;45(5):1105–15. [DOI] [PubMed] [Google Scholar]
- 52. Lopez-Moreno J, Quintana-Navarro GM, Delgado-Lista J, Garcia-Rios A, Delgado-Casado N, Camargo A, Perez-Martinez P, Striker GE, Tinahones FJ, Perez-Jimenez Fet al. Mediterranean diet reduces serum advanced glycation end products and increases antioxidant defenses in elderly adults: a randomized controlled trial. J Am Geriatr Soc. 2016;64(4):901–4. [DOI] [PubMed] [Google Scholar]
- 53. Bouchenak M, Lamri-Senhadji M. Nutritional quality of legumes, and their role in cardiometabolic risk prevention: a review. J Med Food. 2013;16(3):185–98. [DOI] [PubMed] [Google Scholar]
- 54. Casas-Agustench P, Bulló M, Salas-Salvadó J. Nuts, inflammation and insulin resistance. Asia Pac J Clin Nutr. 2010;19(1):124–30. [PubMed] [Google Scholar]
- 55. Medina-Remón A, Casas R, Tressserra-Rimbau A, Ros E, Martínez-González MA, Fitó M, Corella D, Salas-Salvadó J, Lamuela-Raventos RM, Estruch Ret al. Polyphenol intake from a Mediterranean diet decreases inflammatory biomarkers related to atherosclerosis: a substudy of the PREDIMED trial. Br J Clin Pharmacol. 2017;83(1):114–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Wannamethee SG, Lowe GD, Rumley A, Bruckdorfer KR, Whincup PH. Associations of vitamin C status, fruit and vegetable intakes, and markers of inflammation and hemostasis. Am J Clin Nutr. 2006;83(3):567–74. [DOI] [PubMed] [Google Scholar]
- 57. Calder PC, Bosco N, Bourdet-Sicard R, Capuron L, Delzenne N, Doré J, Franceschi C, Lehtinen MJ, Recker T, Salvioli Set al. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res Rev. 2017;40:95–119. [DOI] [PubMed] [Google Scholar]
- 58. Kennedy A, Martinez K, Chuang CC, LaPoint K, McIntosh M. Saturated fatty acid-mediated inflammation and insulin resistance in adipose tissue: mechanisms of action and implications. J Nutr. 2009;139(1):1–4. [DOI] [PubMed] [Google Scholar]
- 59. Cespedes EM, Hu FB. Dietary patterns: from nutritional epidemiologic analysis to national guidelines. Am J Clin Nutr. 2015;101(5):899–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Stanley K. Design of randomized controlled trials. Circulation. 2007;115(9):1164–9. [DOI] [PubMed] [Google Scholar]
- 61. Roman-Viñas B, Serra-Majem L. Mediterranean diet to promote healthy aging. Curr Geri Rep. 2018;7(3):115–24. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.