ABSTRACT
There is considerable heterogeneity across the findings of systematic reviews of oral nutritional supplement (ONS) interventions, presenting difficulties for healthcare decision-makers and patients alike. It is not known whether heterogeneity arises from differences in patient populations or relates to methodological rigor. This overview aimed to collate and compare findings from systematic reviews of ONSs compared with routine care in adult patients who were malnourished or at risk of malnutrition with any clinical condition and to examine their methodological quality. Three electronic databases were searched to July 2019, supplemented with hand-searching. Data on all outcomes were extracted and review methodological quality assessed using A MeaSurement Tool for Assessment of systematic Reviews (AMSTAR). Twenty-two reviews were included, 11 in groups from mixed clinical backgrounds and 11 in specific clinical conditions. Ninety-one meta-analyses were identified for 12 different outcomes but there was discordance between results. Significant benefits of ONSs were reported in 4 of 4 analyses of energy intake, 7 of 11 analyses of body weight, 7 of 22 analyses of mortality, 10 of 17 analyses of complications (total and infectious), 1 of 3 analyses of muscle strength, 4 of 9 analyses of body composition/nutritional status, 2 of 14 analyses of length of stay, and 2 of 5 analyses of hospital readmissions. Ten reviews were high quality (AMSTAR scores 8–11), 9 moderate (AMSTAR scores 3–8), and 3 poor (AMSTAR scores 0–3). Methodological deficiencies were limitations to searches, poor reporting of heterogeneity, and failure to incorporate quality of evidence into any recommendations. Discordance between reviews was not markedly reduced when only high-quality reviews were considered. Evidence for the effects of ONS in malnourished patients or those who are at risk of malnutrition is uncertain, and discordance in results can arise from differences in clinical background of patients or the etiological basis of malnutrition.
Keywords: oral nutritional supplement, oral nutritional support, systematic review, nutritional risk, malnourished, malnutrition
Oral nutritional supplements are a key management strategy in malnutrition. The findings of systematic reviews are discordant and might result from differences in patients and interventions rather than methodological quality.
Introduction
Malnutrition is a state of nutrition in which a deficiency of energy, protein, and other nutrients causes measurable adverse effects on tissue/body form, body function, and clinical outcome (1). Malnutrition can occur as a consequence of disease or result from a range of other physiological and social conditions that can then act as cofactors in the development of, or exacerbation of, ill health. It is estimated that ∼3 million people in the United Kingdom are either malnourished or at risk of malnutrition, with >90% of these living in the community (2). In the United States data on nutritional risk in the community are limited but nutritional screening on admission to hospital suggests that 1 in 3 people are at risk of malnutrition (3). Malnutrition is associated with considerable morbidity and mortality and is a substantial economic burden to healthcare services, with the most recent estimates suggesting malnutrition costs £19.6 billion in England (4) and $157 billion in the United States (3). Oral nutritional supplements (ONSs) are proprietary liquids, semisolids, or powders that provide macro- and micronutrients, many of which are available on prescription. ONSs should be provided with instruction to maximize use but this element is likely to vary. In clinical practice ONSs are frequently used to support patients identified as malnourished or at risk of malnutrition; however, their use in some healthcare systems is controversial because of rapid increases in costs and perceived inappropriate usage (2, 5).
With the rise of evidence-based practice, decisions about healthcare interventions are frequently based on guidelines and protocols informed by clinical trials and systematic reviews of trials. Systematic reviews are recognized as the strongest source of evidence on a topic and are important for their ability to summarize large amounts of literature (6). Recently there has been a disproportionate increase in the publication rate of systematic reviews compared with clinical trials, with estimates suggesting an increase of 2728% from 1991 to 2014 in systematic reviews compared with an increase of just 153% for other study types (7). This has resulted in publication of reviews on the same or similar subjects and yet there appear to be disagreements between their findings, making decisions for healthcare planners and staff more difficult (8) and raising questions about their validity and value in informing practice (9).
Differences in the findings of systematic reviews on the same topic can result from choices made during the conduct of the review. Bias can be introduced at several points in the review process: during searching, study selection, combination of studies, and decisions about study quality (8) or equally can result from decisions made about interpretation of findings (10).
An increasing number of systematic reviews of ONSs in the management of malnourished patients have been published. An overview of 13 systematic reviews conducted in 2007 highlighted consistent benefits to nutritional intake and weight, which were associated with reductions in mortality and complications (11). The effects on length of stay and hospital admissions, which are associated with the greatest costs to healthcare, were not considered. More reviews have been published since then, with results that appear discordant with this optimistic interpretation.
In an increasingly pressured national healthcare system it is important to understand which patient groups are most likely to benefit, or conversely are unlikely to benefit from ONSs. From an academic perspective it is important to focus research endeavors into areas of most uncertainty. The first aim of this overview was to identify and compare the findings from systematic reviews of ONSs, compared with routine care, on clinical and healthcare outcomes in patients who were malnourished or at risk of malnutrition. Our second aim was to evaluate any discordance between reviews by critical appraisal of their methodological conduct.
Methods
A systematic review of systematic reviews (overview) was undertaken following the methods suggested by Smith et al. (12) and guided by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) statement (13).
Study eligibility
Systematic reviews of randomized controlled trials (RCTs) evaluating the effect of ONSs compared with routine care in nutritionally adults who were malnourished or at risk of malnutrition were considered for inclusion. Only reviews in which meta-analysis was attempted were included.
All reviews in adults who were malnourished or at risk of malnutrition from any clinical background, were eligible for inclusion. Where the nutritional status of participants was not reported, participants were judged to have been at risk of malnutrition because of their underlying clinical diagnosis. Reviews in children, pregnant women, people with eating disorders, and healthy participants were excluded.
Reviews of ONSs that were given with the intention of improving nutritional intake compared with routine care, in adults with malnutrition or considered to be at risk of malnutrition, were considered for inclusion. Reviews that included mixtures of interventions (ONSs with enteral and/or parenteral feeding) were included if either the results were analyzed separately according to intervention or <10% of the analyses contained interventions other than ONSs. Reviews that summarized outcomes in a narrative synthesis only, reviews of micronutrient supplements, and of enteral and parenteral nutrition were excluded.
Outcomes of interest were mortality, indicators of healthcare usage [hospital readmissions, length of stay (LOS), institutionalization], clinical function (complications), physical function, nutritional intake, and nutritional status.
There were no restrictions on language of publication, and unpublished reviews, abstracts, conference posters, and dissertations were considered for inclusion.
Search methods of identification of reviews
A systematic search strategy was used to search Medical Literature Analysis and Retrieval Systems Online (MEDLINE) and the Cochrane Database. Searches were undertaken to August 2014 and updated on July 23, 2019 using the search strategy (Supplemental Figure 1). Hand-searching of abstracts of meetings in the online publication of the Proceedings of the Nutrition Society was undertaken from 2003 to March 2014.
Bibliographic references of all relevant reviews were hand-searched to identify reports of additional reviews not identified through electronic searching. The related links function in PubMed was used to generate a list of articles related to each included review. The list of related articles was scrutinized for additional eligible reviews. Authors were contacted to seek full publication of work presented as conference abstracts or posters. Experts in the field were contacted to identify any unpublished work or additional reviews. The searches to March 2013 were conducted by 1 author (RS) and updated to August 2014 by a second author (MG); the final update was undertaken by a third author (CB).
Study selection and data extraction
The titles and abstracts from searches were examined on screen against the eligibility criteria by 2 authors. Full-text articles of any potentially relevant reviews were obtained. Uncertainties were resolved by discussion with a third author (CB). Reviews that did not meet the inclusion criteria were excluded and the reasons documented.
The following descriptive data were extracted by 2 authors working independently (RS and CB, and MG and CB) for each included review: first author and year of publication, the stated aim of the review, patient population, healthcare setting, number of included trials and number of participants, details of the intervention and control, nutritional status of participants, and outcomes assessed. In addition, an identifier for each eligible RCT included in the systematic reviews was tabulated to allow comparison of included studies across included reviews. Discussion between authors was used to resolve any disagreements.
Assessment of methodological quality of included reviews
The methodological quality of all included reviews was assessed using the MeaSurement Tool to Assess systematic Reviews (AMSTAR) (6). The AMSTAR tool assesses the decisions made about study identification, selection, data extraction, study quality, and the interpretation of data within the context of quality, methods to combine data, assessment of publication bias, and declaration of conflicts of interest in the conduct of a systematic review. Data relevant to the 11 components of the tool were extracted by 3 authors working independently (RS and CB, and MG and CB, and subsequently reviewed by PWE). The overall quality was scored by awarding 1 point for “yes” and 0 for “no” and summing the overall scores. For the purposes of this overview, reviews scoring 8–11 were considered to be high quality, 4–7 moderate quality, and 0–3 poor quality, as suggested by a previous overview (14).
Data synthesis
Data were extracted for all outcomes of interest and entered into a data extraction spreadsheet in Microsoft Excel. Statistical summary results reported by reviews were extracted as RRs or ORs, with the 95% CI for dichotomous outcomes, whereas data for continuous outcomes were extracted as the mean difference (MD) or standardized mean difference (SMD) with the 95% CI. Data on the statistical model used (fixed or random effects), statistical significance, and assessment of heterogeneity were also extracted, together with the number of studies included in the analysis and total number of participants represented. [Heterogeneity describes any kind of variability among studies in a systematic review related to features of participants, interventions and comparisons (clinical), or within meta-analyses (statistical).]
Meta-analysis data were only extracted from reviews if results were based on data from studies of RCT design and for ONS interventions. Where reviews combined data from studies of different designs (e.g., RCTs and nonrandomized trials) or interventions (e.g., ONS and enteral feeding) in meta-analysis, data were only extracted if the meta-analysis consisted solely of RCTs that were relevant to this review question or if the analyses were split according to intervention or study design. Additionally, where data were reported in different formats for an outcome, data on change from baseline were extracted in preference to other forms to take into consideration differences at baseline. Results reported in a narrative form only were not extracted.
Where possible, data reported as OR (95% CI) were converted to an RR and its 95% CI to enable comparison of data across reviews using the following equation, where ACR is the assumed control risk:
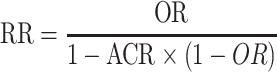 |
(1) |
The ACR was obtained from the median control group risk reported by reviews as part of the meta-analysis. Only when an ACR was not reported and therefore the RR could not be calculated were authors contacted to obtain the original data as RR (95% CI). If it was not possible to convert OR to RR and authors did not respond to a request for data, ORs were extracted. Where data for an outcome were not combined in a meta-analysis due to heterogeneity, summary measures for individual RCTs were extracted instead, if available. All calculations and analyses described were performed by 2 investigators (RS and CB) with the assistance of a statistician (Peter Milligan).
Summary data and information on statistical tests and heterogeneity for all outcomes of interest were summarized in tables.
To enable comparison of similarities and differences, reviews were categorized according to the clinical background of included patients: “mixed clinical populations” for reviews combining patients from different clinical backgrounds, or “single clinical conditions” for reviews in specific patient groups. The synthesis of results was undertaken by CB and CEW. The numbers of reviews in each category reporting statistically significant differences and reporting no significant difference between groups were tabulated. Within each category, the numbers of reviews reporting heterogeneity and the numbers reporting statistically significant heterogeneity were noted. Findings from reviews of high methodological quality were tabulated in a similar way, and the clinical subgroups associated with benefit and no benefit from ONSs highlighted.
Discordance
The results of meta-analyses were judged to be discordant if either 1) the overall effects for an outcome were in different directions (i.e., ≥1 was in the direction of benefit and ≥1 was in the direction of adverse effect), or 2) the overall effects were in the same direction but ≥1 was statistically significant and others were not (15).
Results
Results of the search strategy
Figure 1 shows the PRISMA flowchart for identification of studies. Searches resulted in retrieval of 4620 references, and 22 systematic reviews were identified for inclusion in this overview. Exclusion of 4539 reviews was on the basis of title alone, and a further 59 were excluded after reading the full-text article. All reviews were published in English. Of the 22 included reviews, 1 was published in a book (16) and includes an update of a previous review of ONSs in patients in the community (17), and 1 was part of a guideline from the National Institute of Clinical Excellence (18). The oldest review (19) was partially updated in 2001 only for the outcome mortality (20). The RR was calculated from an OR in the case of 2 reviews (20, 21).
FIGURE 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram indicating the searching and study identification process for systematic reviews of oral nutritional supplement interventions in participants who were malnourished or at nutritional risk. RCT, randomized controlled trial.
Scope and characteristics of included reviews
All reviews were of ONS compared with routine care, but the characteristics of the populations included in reviews varied (Table 1). Eleven reviews included trials of patients from a range of clinical backgrounds combined (16–25). Eleven reviews focused on patients with single diagnoses: liver disease (n = 3) (26–28), pressure ulcers (n = 2) (29, 30), preoperative patients (n = 1) (31), chronic obstructive pulmonary disease (n = 1) (32), hip fracture (n = 1) (33), stroke (n = 1) (34), cancer (n = 1) (35), and 1 review (36) included patients from mixed clinical backgrounds but reported the results according to individual clinical conditions.
TABLE 1.
Main characteristics and outcomes reported of identified systematic reviews of oral nutritional supplement interventions in participants who were malnourished or at nutritional risk. Reviews are sorted by clinical background of patients (mixed clinical background or single clinical conditions) and by year of publication1
| Reference (first author) and year | Review aim | Search strategy | Number of included studies of ONS (n participants) | Healthcare setting | Nutritional status of participants | Outcomes reported | Outcomes reported in meta-analyses |
|---|---|---|---|---|---|---|---|
| Reviews in patients with mixed clinical backgrounds | |||||||
| Potter 1998 (19) | To assess the effectiveness of routinely prescribed oral or enteral protein energy supplements in improving body weight, anthropometry and survival of adults | MEDLINE only + contact with colleagues and manufacturers No language restrictions | 20 RCTs (1310) | Any setting | Not specified as an inclusion criterion. | Mortality, % change in weight, and anthropometry | Mortality, weight, anthropometry |
| Potter 2001 (20) (partialupdate of Potter1998) | To summarize the evidence available for nutritional supplementation | Not described | 30 RCTs (2132) | Any setting | Not specified as an inclusion criterion | Mortality, % change in weight, and anthropometry, LOS, institutionalization | Mortality |
| Stratton 1999 (17) | To assess the effects of oral nutritional supplementation in different groups of patients living in the community | MEDLINE, BIDS, NHS Research and Development Evidence-based Medicine, NHS Centre for Reviews and Dissemination, Cochrane Library + contact with experts and reference lists of included studies Language limits not specified | 45 RCTs (1728) 39 non-RCTs (842) | Community | Not specified as an inclusion criterion | Food intake, appetite, body weight, body structure, functional outcomes | % weight change |
| Stratton 2003 (16)[partial update ofStratton 1999 (17)] | To assess the effects of ONSs in patients in hospital and living in the community | MEDLINE, BIDS, NHS Research and Development Evidence-based Medicine, NHS Centre for Reviews and Dissemination, Cochrane Library + contact with experts and reference lists of included studies Language limits not specified | Acute: 34 RCTs (2475), 24 non-RCTs (1408) Community: 44 RCTs (2194) 64 non-RCTs (1553) | Acute and community | Not specified as an inclusion criterion | Food intake, total energy intake, weight, body composition, functional outcome, clinical outcomes, costs | Mortality, weight, complications |
| NICE 2006 (18) | To investigate the clinical and nutritional effects of oral nutritional interventions | Cochrane, MEDLINE, EMBASE, CINAHL, Allied and Complimentary Medicine, British Nursing Index + searching reference lists, guidelines, and reports Papers not in English excluded | 32 RCTs of ONSs (no dietary advice in either arm; 7200 participants) | Acute, community, and hospital outpatients | Included patients were malnourished or at risk of malnutrition (judged that >50% of participants would meet the definition of malnourished) in any healthcare setting, all clinical diagnoses | Mortality, complications, LOS, weight | Mortality, complications, LOS, weight |
| Vanderkroft 2007 (22) | To identify the best available practices in hospital that reduce or minimize the risk of undernutrition, especially for the older patient | MEDLINE, PREMEDLINE, CINAHL, Australasian Medical Index, AustHEALTH, EMBASE, Science Citation Index + Dissertation abstracts. All searches from 1980 only + searching reference lists and hand-searching of abstracts Studies not in English excluded | 13 RCTs (1957) and 2 non-RCTs (64) | Acute | Not specified as an inclusion criterion | Dietary intake, anthropometry, body composition (weight, BMI, skinfolds, AMC, and MAC), biochemical markers, mortality, LOS, prevalence or incidence of malnutrition, functional outcomes (grip strength and ADL) | Weight, TSF, AMC, MAC, albumin, mortality, LOS, prevalence or incidence of malnutrition, handgrip strength |
| Milne 2009 (23) | To assess the effects and acceptability of oral dietary supplements in elderly people (>65 y) | Cochrane, MEDLINE, EMBASE, Healthstar, CINAHL, BIOSIS, CAB, current controlled trials + contact with experts, hand-searching and reference lists of included studies Language limits not specified | 62 RCTs (10,187) | Any setting | Not specified as an inclusion criterion | Nutritional status, dietary intake, mortality, morbidity, complications, LOS, functional status, QoL, cost | Mortality, weight, complications, AMC, LOS, and grip strength |
| Cawood 2012 (21) | To examine whether high-protein ONSs have beneficial effects in clinical practice and the extent to which these are associated with high protein intake | PubMed, Cochrane, Clinical Evidence Database, National Electronics Library for Health guidelines finder, TRIP, CINAHL National Service Frameworks + contact with experts, searching reference lists Papers not in English excluded | 36 RCTs (3790) | Any setting | Malnourished and well nourished | Mortality, LOS, complications, readmissions, strength, QoL, ADL, dyspnea, mobility, intake, weight, appetite, body composition | Mortality, LOS, complications, readmissions, strength, weight, MAMC, energy and protein intake |
| Beck 2013 (24) | To estimate the effectiveness of oral nutritional support compared with placebo or usual care in improving readmissions, survival, nutritional status, functional status, quality of life and morbidity of older (≥65 y) medical and surgical patients after discharge from hospital | MEDLINE, Embase, Web of Science hand-searching of 3 relevant systematic reviews, searching of reference lists and related citations + publication status of any previously ongoing studies checked Papers not in English excluded | 6 RCTs (716) | On discharge from hospital | Malnourished or at nutritional risk | Primary: readmissions and death Secondary: energy and protein intake, nutritional status, functional status, quality of life and morbidity, compliance, and adverse events | Mortality and hospital readmissions |
| Stratton 2013 (37) | To critically review and synthesize the literature to assess the impact of ONSs used in the community setting across all patient groups on hospital admissions and readmissions | MEDLINE, EMBASE, Cochrane Library, DARE, NHSEED, Clinicaltrials.gov, ISRCTN, trial TROVE, hand-searching of reference lists, related systematic reviews and conference proceedings. No language limits specified | 9 RCTs (1316) | Patients living in the community | Any nutritional status | LOS, handgrip strength, overall healthcare use and expenditure, mortality, overall disability | Hospital readmissions |
| Feinberg 2017 (25) | To assess the benefits and harms of nutrition support vs. no intervention, treatment as usual, or placebo in hospitalized adults at nutritional risk | CENTRAL, MEDLINE, Embase, LILACS, BIOSIS, Web of Science, Clinitrials, TRIP, and Google scholar. Hand-searching of bibliographies of reviews and identified trials + conference proceedings and contact with companies making ONSs. No limits to search on publication type, status, or language | 55 RCTs (not reported) | Acute (hospitalized at the start of the intervention) | At nutritional risk according to specific criteria or characterized by trialists | Primary: all-cause mortality, serious adverse events, QoL Secondary: time to death, morbidity (defined by trialists), BMI, weight, hand-grip strength, 6-min walking distance | All-cause mortality, serious adverse events, BMI, weight |
| Reviews in patients with specific clinical backgrounds | |||||||
| Patients with cancer | |||||||
| Elia 2006 (35) | To determine the extent to which nutritional support vs. routine care improves the outcome of cancer patients receiving treatment or palliative care | PubMed, Cochrane, TRIP, Clinical Evidence, NELH, National Service Frameworks, checking of reference lists and contact with experts Full papers only included, abstracts excluded. No language limits | Not clearly reported. Results appear to be based on 7 RCTs (329) | Acute and community | Malnourished and well nourished | Energy and protein intake, weight, mortality, response to treatment | Mortality, energy intake |
| Patients with COPD | |||||||
| Ferreira 2012 (32) | To assess the impact of nutritional support on anthropometric measures, pulmonary function, respiratory and peripheral muscle strength, endurance, functional exercise capacity, and HRQoL in COPD | Cochrane, MEDLINE, Embase, CINAHL, AMED, psychINFO, hand-searching of previously published reviews, respiratory journals and abstracts, and contact with authors. No language limits | 17 RCTs (632) | Any setting | Not specified as an inclusion criterion | Weight, FFM, sum of skinfold measures, functional exercise capacity, pulmonary function, respiratory muscle strength, peripheral muscle strength, HRQoL | Weight, FFM, fat mass index, MAMC, skinfold measures and skinfolds + TSF combined, functional exercise capacity (12-min walk), pulmonary function (FEV1), respiratory muscle strength (MIP, MEP), peripheral muscle strength, HRQoL |
| Patients with hip fracture | |||||||
| Avenell 2016 (33) | To review the effects (benefits and harms) of nutritional interventions in older people recovering from hip fracture | Cochrane group specialized register, CENTRAL, MEDLINE, MEDLINE-in process, Embase, CAB Abstracts, CINAHL. Trial registers (ISRCTN, Clinicaltrials.gov, and UK Clinical Research), reference lists of articles and books, contact with colleagues and investigators. Results limited to 2008 because this is an update of a previous review No language restriction | 18 RCTs (1190) | Any setting | Both malnourished and well-nourished. Subgroup analysis specified "malnourished targeted vs. malnourished not targeted." | Primary: all-cause mortality, morbidity (postop complications); unfavorable outcome (number of participants who died + number of survivors with complications) Secondary: LOS, postop function, level of care required, QoL, fracture healing, putative side effects of treatment Other: patient tolerance/compliance, carer burden, economic | Mortality and number of people with complications |
| Patients with liver disease | |||||||
| Koretz 2012 (26) | To assess whether the nutritional interventions (ONS, enteral, parenteral) favorably impacted on the morbidity or mortality of patients with liver disease other than those that have undergone transplantation | Cochrane, MEDLINE, Embase, Science Citation Index expanded, clinicaltrials.gov, hand-searching, contact with experts and manufacturers. No language limits | 14 RCTs (987) | Any setting | Not specified as an inclusion criterion | Mortality, hepatic morbidity (appearance or failure of resolution of ascites or hepatic encephalopathy, GI bleed), QoL, adverse events, serum bilirubin, infection, postop complications, LOS, costs, nutritional status | Mortality, appearance of ascites, resolution of ascites, GI bleeding, encephalopathy (appearance and resolution), infections, serum bilirubin, albumin |
| Langer 2012 (27) | To assess the effect of enteral and parenteral nutrition as well as mono- and multinutrient supplements on morbidity and mortality of patients | Cochrane, MEDLINE, Embase, Science Citation Index, and Social Sciences citations index, contact with experts and manufacturers, checking reference | 2 RCTs (144) | Any setting | Not specified as an inclusion criterion | Acute rejections, new onset of diabetes, readmissions, occurrence of infections, changes in grade of hepatic encephalopathy, encephalopathy- | Rehospitalization, infections, acute rejections, new-onset diabetes, encephalopathy-related hospitalization, |
| before and after liver transplantation. For transplanted patients also to assess the effects of food on morbidity and mortality, which is commonly known for food–drug interactions | lists and hand-searching. No language limits | related admissions, mortality, time on waiting list | changes in grade of hepatic encephalopathy | ||||
| Ney 2013 (28) | To provide an up-to-date systematic review and meta-analysis of RCTs of oral or enteral nutritional supplementation on nutritional and clinical outcomes in adult patients with liver cirrhosis | MEDLINE, SCOPUS, Embase, and PubMed and searches of bibliographies of review articles Studies in English only | 4 RCTs (259) | Any setting | Not specified as an inclusion criterion | For ONS only mortality and weight. | Mortality and weight |
| Patients with pressure ulcers | |||||||
| Stratton 2005 (30) | To determine the effect of enteral nutritional support on pressure ulcer incidence, pressure ulcer healing, quality of life, complications, mortality, nutritional status (dietary intake, body weight), and any other clinically relevant outcome measures | PubMed, Cochrane, TRIP, Clinical Evidence, NELH, NSF, checking reference lists, and contact with experts. No language limits | 5 RCTs (1345) | Any setting | Malnourished and well nourished | Prevention of pressure ulcers, healing of pressure ulcers, complications, QoL, mortality, dietary intake, nutritional status | Prevention of pressure ulcers |
| Langer 2014 (29) | To evaluate the effect of enteral and parenteral nutrition on the prevention and treatment of pressure ulcers | Cochrane (CENTRAL + group specialist register), DARE, HTA, Cochrane methodology register, NHS Economic Evaluation Database, MEDLINE, Embase, CINAHL + 15 clinical trials registries (international) | 9 RCTs (6169) | Any setting | Not specified as an inclusion criterion | Development of new pressure ulcers (prevention), time to healing (treatment), acceptability, side effects, costs, rate of healing, rate of change in size of ulcer, QoL | Proportion of participants developing new pressure ulcers |
| Patients presurgery | |||||||
| Burden 2012 (31) | To evaluate if nutritional support intervention by any route prior to surgery improves clinical outcomes for elective GI surgical patients and to determine if nutritional support interventions provide any benefit to nutritional intake or nutritional status prior to elective GI surgery | EBM reviews, MEDLINE, Embase, AMED, British Nursing Index Archive, and reference lists of articles + author contact for abstracts. No limits to search specified | 3 RCTs (404) | Community/OPD prior to surgery | Not specified as an inclusion criterion | Total complications, infectious complications, LOS | Total complications, infectious complications, LOS |
| Patients poststroke | |||||||
| Geeganage 2012 (34) | To determine 1) if swallowing therapy improves clinical outcome, 2) the optimal administration of feeding and fluid administration, 3) if food supplementation improves clinical outcome. Nutritional supplementation with ONSs only considered for this overview | Cochrane, MEDLINE, Embase, CINAHL, conference proceedings citation index and Current Controlled Trials register, checking reference lists and review articles, contact with researchers. No language limits | 8 RCTs (4391) | Any settings | Malnourished or undernourished | Pressure sores, energy intake, protein intake, case fatality (end of trial), institutionalization, death/dependency at end of trial, LOS, albumin | Pressure sores, energy and protein intake, mortality (end of trial), institutionalization, LOS, albumin |
| Mixed conditions, reported according to individual clinical conditions | |||||||
| Koretz 2007 (36) | To evaluate the clinical efficacy (in different disease states) of medical interventions that deliver nutrients to the gut (ONS and EN). Includes: relevant data on perioperative period, nonsurgical cancer treatment, liver | 54 RCTs (not reported) | Any setting | Not specified as an inclusion criterion | Mortality, complications, LOS, costs, interventional complications, and disease-specific outcomes | Varied according to disease: Perioperative: mortality, total and infectious complications, LOS, nonsurgical cancer mortality Chronic liver disease: mortality, infectious | |
| disease, geriatrics, hip fracture | complications, encephalopathy Geriatrics: mortality, total and infectious complications Hip fracture: mortality, total and infectious complications | ||||||
1ADL, activities of daily living; AMC, arm muscle circumference; AMED, Allied and Complementary Medicine Database; BIDS, Bath Information & Data Services; CAB, Commonwealth Agricultural Bureau; COPD, chronic obstructive pulmonary disease; DARE, Database of Abstracts of Reviews of Effects; EBM, Evidence-based Medicine; EN, enteral nutrition; FEV1, forced expiratory volume; FFM, fat-free mass; GI, gastrointestinal; HRQoL, health-related quality of life; HTA, Health Technology Assessment; ISRCTN, International Standard Registered Clinical/soCial sTudy Number; LILACS, Latin American and Caribbean Health Sciences Literature; LOS, length of stay; MAC, mid-arm circumference; MAMC, mid-arm muscle circumference; MEP, maximal expiratory pressure; MIP, maximal inspiratory pressure; NELH, National Electronic Library for Health; NHS, National Health Service; NHSEED, NHS Economic Evaluation Database; NSF, National Science Foundatation; ONS: oral nutritional supplement; OPD: outpatient department; postop, postoperative; QoL, quality of life; RCT, randomized controlled trial; TRIP, Turning Research into Practice; TSF, triceps skinfold.
Sixteen reviews included patients from different healthcare settings combined (hospital, community, residential care homes, and hospital outpatients). Four reviews only included patients living in the community (living in their own homes or care homes) (17, 24, 31, 37), including 1 that focused on patients being discharged from hospital (24), and 2 reviews were of patients in hospital (22, 25), with the review by Feinberg et al. (25) focusing on interventions that were started in hospital.
Four reviews examined the efficacy of ONSs alone (17, 21, 23, 37); the remaining 18 reviews included interventions other than ONSs, such as enteral and parenteral nutrition. Nutritional status was not specified as an inclusion criterion in 13 of 22 (59%) reviews. Five reviews specified that both malnourished and well-nourished patients were eligible for inclusion (21, 30, 31, 35, 37), and 4 reviews specified the inclusion criterion as patients who were malnourished or at risk of malnutrition (18, 24, 25, 34).
Reviews varied considerably in reporting of the specified outcomes. For some outcomes data were presented for all studies combined and then according to specific clinical groups, and data have been extracted in both formats where relevant for this overview.
Appraisal of the methodological quality of included reviews
The methodological quality of included reviews was assessed using the AMSTAR tool and the scores with some explanatory information are provided in Table 2. Ten of the 22 (45%) reviews were judged to be of high methodological quality. Four reviews achieved the highest possible score of 11, all of which were published in the Cochrane Library (25, 27, 31, 33). Six reviews scored 8–10 and were judged to be of high quality (23, 26, 29, 32, 34, 36). Five of these 6 reviews were also published in the Cochrane Library (23, 26, 29, 32, 34). The factors that limited methodological quality in this group of studies most frequently related to aspects of the review that could not be carried out because of the small number of studies, for example, absence of a funnel plot. For 2 studies (23, 26), reporting of duplicate study selection was only partial and duplicate data extraction was not described. No authors were contacted to attempt to verify this information.
TABLE 2.
Judgment made against each quality criterion of the AMSTAR methodological checklist and final score for systematic reviews of oral nutritional supplement interventions in participants who were malnourished or at nutritional risk. Reviews are sorted by clinical background of patients (mixed clinical background or single clinical conditions) and by year of publication1
| Reference (first author) and year | Evidence of an a priori design | Duplicate study selection and data extraction | Comprehensive literature search | Publication status used as an inclusion criterion | List of included and excluded studies available | Characteristics of included studies provided | Assessment and documentation of study quality | Study quality noted in analysis and formulating conclusions | Appropriate methods used to combine studies | Assessment of publication bias | Statement of conflict of interests | AMSTAR 1 score out of 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Reviews of studies of patients with mixed clinical backgrounds | ||||||||||||
| Potter 1998 (19) | N | N | N | N | N | Y | Y | Y | Y | Y | Y | 6 |
| Potter 2001 (20) | N | N | N | N | N | N | N | N | Y | N | Y | 2 |
| Stratton 1999 (17) | N | N | Y | N | N | Y | Y | N | N | N | N | 3 |
| Stratton 2003 (16) | N | N | Y | N | N | Y | Y | Y | N | Y | Y | 6 |
| NICE 2006 (18) | Y | N | Y | N | N | Y | Y | Y | Y | N | Y | 7 |
| Vanderkroft 2007 (22) | N | Y | Y | N | Y | Y | Y | N | Y | N | Y | 7 |
| Milne 2009 (23) | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | 10 |
| Cawood 2012 (21) | N | N | Y | N | Y | Y | Y | N | N | N | Y | 5 |
| Beck 2013 (24) | N | N | Y | N | Y | Y | Y | N | Y | N | Y | 6 |
| Stratton 2013 (37) | N | N | Y | N | N | Y | Y | Y | N | N | Y | 5 |
| Feinberg 2017 (25) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 11 |
| Column totals (number ofreviews scoring yes forthe item of 11 includedreviews) | 3 | 2 | 9 | 2 | 5 | 10 | 10 | 6 | 7 | 4 | 10 | |
| Reviews of studies of patients with specific clinical backgrounds | ||||||||||||
| Patients with cancer | ||||||||||||
| Elia 2006 (35) | N | N | Y | N | N | N | Y | N | Y | n/a | N | 3 |
| Patients with COPD | ||||||||||||
| Ferreira 2012 (32) | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | 10 |
| Patients with hip fracture | ||||||||||||
| Avenell 2016 (33) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 11 |
| Patients with liver disease | ||||||||||||
| Koretz 2012 (26) | Y | N | Y | Y | Y | Y | Y | Y | Y | Y | Y | 10 |
| Langer 2012 (27) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 11 |
| Ney 2013 (28) | N | N | Y | N | N | Y | Y | Y | Y | N | Y | 6 |
| Patients with pressure ulcers | ||||||||||||
| Stratton 2005 (30) | N | N | Y | Y | Y | Y | Y | N | Y | n/a | N | 6 |
| Langer 2014 (29) | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | 10 |
| Patients presurgery | ||||||||||||
| Burden 2012 (31) | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | Y | 11 |
| Patients poststroke | ||||||||||||
| Geeganage 2012 (34) | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | 10 |
| Mixed conditions, reported according to individual clinical conditions | ||||||||||||
| Koretz 2007 (36) | Y | Y | Y | Y | Y | Y | Y | Y | Y | N | Y | 10 |
| Column totals (numberof reviews scoringyes for the item of 11included reviews) | 8 | 7 | 11 | 9 | 9 | 10 | 11 | 9 | 11 | 3 | 9 | |
AMSTAR, Assessment of Multiple Systematic Reviews measurement tool; COPD, chronic obstructive pulmonary disease; N, no (item judged to have not been carried out); n/a, insufficient studies to assess this element; Y, yes (item judged to have been carried out completely). Score 8–11: high quality; score 4–7 moderate quality; score 0–3: poor quality. Boxes shaded in black indicate a “Yes” rating; boxes shaded in white indicate a “no” rating; boxes shaded in grey indicate a “not applicable” rating.
Nine reviews (41%) were judged to be of moderate methodological quality, scoring between 4 and 7 (Table 2). Three reviews (14%) were judged to be of poor quality, scoring 0–3. The factors contributing to the judgment of reduced quality of reporting varied across reviews but in general few reviews provided evidence of a priori consideration of the research question and methodology of the review, most usually described in a review protocol. Few authors searched for both published and unpublished literature, with 11 reviews reporting either no hand-searching and/or restriction of searching to English language only, and few reviews searched ongoing trial databases (Table 2). Most reviews failed to consider or assess the likelihood of publication bias, and few reviews reported their findings in the context of the quality of the evidence within the review. Most included reviews had assessed the methodological quality of primary studies, which were frequently noted to be poor, with significant heterogeneity between studies, and an absence of blinding (either to group allocation and/or outcome assessment) in the primary research, but these limitations were frequently not reflected when presenting the overall findings of the reviews. In addition, many reviews did not describe duplicate study selection and data extraction, and only 6 described contact with authors. In general conflicts of interest were well reported across reviews, but it is notable that 8 (36%) of the included reviews had links with companies producing ONSs.
Reporting of heterogeneity
Reporting of heterogeneity varied considerably, with 0% to 100% of analyses for any 1 outcome reporting the amount of heterogeneity (Table 3). Amount of heterogeneity was reported more frequently for analyses where there was no significant difference in the outcome of interest between groups compared with analyses reporting statistically significant benefits of intervention [40/49 (82%) analyses and 23/42 (55%) analyses, respectively]. Heterogeneity was statistically significant for many of the analyses of LOS, infectious complications, and body weight. Most other analyses reported no statistically significant heterogeneity between findings.
TABLE 3.
Number of analyses reporting statistically significant benefits or no significant difference between groups for all outcomes from identified systematic reviews of oral nutritional supplement interventions in participants who were malnourished or at nutritional risk according to clinical background of included participants and review quality assessed using AMSTAR1
| Analyses with statistically significant difference between groups | Analyses with no significant difference between groups | |||||||
|---|---|---|---|---|---|---|---|---|
| Outcome measure | Median (range) number of participants per analysis | Total number of analyses | Total number | AMSTAR score ≥8 | Number reporting heterogeneity (number with statistically significant heterogeneity) | Total number | AMSTAR score ≥8 | Number reporting heterogeneity (number with statistically significant heterogeneity) |
| Clinical and functional | ||||||||
| Mortality | ||||||||
| Mixed clinical populations | 2174 (532–8529) | 12 | 6 | 1 | 2 (0) | 6 | 2 | 5 (0) |
| Single clinical conditions2 | 710 (169–4343) | 10 | 1 | 1 | 1 (0) | 9 | 7 | 9 (0) |
| Total complications | ||||||||
| Mixed clinical populations | 1162 (384–6225) | 5 | 5 | 1 | 3 (0) | 0 | — | — |
| Single clinical conditions | 495 (216–789) | 4 | 2 | 2 | 2 (1) | 2 | 2 | 2 (1) |
| Infectious complications | ||||||||
| Mixed clinical populations | — | 0 | 0 | — | — | 0 | — | |
| Single clinical conditions | 259 (22–637) | 8 | 3 | 3 | 3 (2) | 5 | 5 | 3 (1) |
| Pressure ulcers | ||||||||
| Mixed clinical populations | — | 0 | — | — | — | 0 | — | — |
| Single clinical conditions | 4125 (1224–6062) | 3 | 2 | 1 | 2 (0) | 1 | 1 | 1 (0) |
| Muscle strength | ||||||||
| Mixed clinical populations | 174 (61–535) | 3 | 1 | 0 | 0 (0) | 2 | 1 | 2 (0) |
| Single clinical conditions | 0 | 0 | — | — | 0 | — | — | |
| Healthcare-related | ||||||||
| Length of stay | ||||||||
| Mixed clinical populations | 1097 (100–5735) | 10 | 1 | 0 | 0 (0) | 9 | 5 | 7 (5) |
| Single clinical conditions | 362 (36–4114) | 4 | 1 | 1 | 1 (0) | 3 | 3 | 2 (0) |
| Readmissions | ||||||||
| Mixed clinical populations | 546 (478–999) | 3 | 2 | 0 | 1 (0) | 1 | 0 | 1 (0) |
| Single clinical conditions | 23 (22–24) | 2 | 0 | 0 | n/a | 2 | 2 | n/a |
| Nutritional | ||||||||
| Energy intake | ||||||||
| Mixed clinical populations | 958 (672–1244) | 2 | 2 | 0 | 0 | 0 | — | — |
| Single clinical conditions | 169 (164–174) | 2 | 2 | 1 | 2 (1) | 0 | — | — |
| Protein intake | ||||||||
| Mixed clinical populations | 816 (480–1152) | 2 | 2 | 0 | 0 | 0 | — | — |
| Single clinical conditions | 177 | 1 | 1 | 1 | 1 (1) | 0 | — | — |
| Body weight | ||||||||
| Mixed clinical populations2 | 1184 (417–3058) | 10 | 7 | 1 | 3 (2) | 3 | 1 | 2 (1) |
| Two analyses reporting “considerable heterogeneity” in narrative | ||||||||
| Single clinical conditions | 177 | 1 | 0 | — | — | 1 | 0 | 1 (0) |
| Body composition/nutritional status | ||||||||
| Mixed clinical populations | ||||||||
| Mid-arm circumference | 453 (61–1382) | 3 | 2 | 1 | 2 (0) | 1 | 0 | 1 (1) |
| Mid-arm muscle circumference | 1279 (118–2439) | 2 | 2 | 0 | 0 (—) | 0 | — | — |
| Serum albumin | 112 | 1 | 0 | — | — | 1 | 0 | 1 (1) |
| Single clinical conditions | ||||||||
| Mid-arm muscle circumference | 246 | 1 | 0 | — | — | 1 | 1 (0) | |
| Serum albumin | 311 (144–477) | 2 | 0 | — | — | 1 | 2 | 2 (0) |
1Dash(—) indicates no analyses for this outcome. AMSTAR, Assessment of Multiple Systematic Reviews measurement tool; n/a, not applicable because data are reported for separate studies.
Number of participants not reported for 1 analysis.
Analyses demonstrating a significant difference between groups were slightly more likely to report significant heterogeneity than those reporting no statistically significant benefits [7/23 (30%) analyses and 10/40 (25%) analyses, respectively].
Comparison of outcomes reported in the meta-analyses
Ninety-one eligible analyses for 12 different outcomes were reported in the 22 reviews (Supplemental Tables 1–7). Mortality was reported in 15 reviews (22 analyses), length of hospital stay in 8 reviews (14 analyses), hospital readmissions in 4 reviews (5 analyses), total complications in 7 reviews (9 analyses), infectious complications in 4 reviews (8 analyses), treatment of pressure ulcers in 3 reviews (3 analyses), limb/muscle strength in 3 reviews (3 analyses), body composition in 5 reviews (6 analyses), serum albumin in 3 reviews (3 analyses), body weight in 9 reviews (11 analyses), energy intake in 3 reviews (4 analyses), and protein intake in 2 reviews (3 analyses).
There were fewer analyses from reviews in patients with mixed clinical backgrounds compared with reviews of patients with single clinical conditions (53 compared with 38). Forty-two of 91 (46%) analyses reported a statistically significant benefit to receiving ONSs, and 49 (54%) reported no significant difference between groups. Of the analyses reporting statistically significant benefits to receiving ONSs, 14 of 42 (33%) were from high-quality reviews (AMSTAR score ≥8). In contrast, 32 of 49 (65%) analyses reporting no significant difference between groups were from high-quality studies.
Overall there was discordance in the results of meta-analyses for many outcomes (Table 3). All discordance resulted from ≥1 reviews reporting statistically significant benefits for an outcome and other reviews reporting no significant difference between groups. For analyses in mixed clinical populations, concordance between the results occurred for 4 of 12 outcomes [total complications, energy intake, protein intake, and measurements of body composition (mid-arm muscle circumference)], with all reviews reporting these outcomes suggesting benefits from receiving ONSs. For analyses in single clinical conditions, concordance occurred for 3 of 12 outcomes (hospital readmissions, energy intake, and serum albumin), with 2 reviews (1 in cancer and 1 in stroke) reporting energy intake suggesting benefits to groups receiving ONSs (34, 35) and all analyses of hospital readmissions (both in liver-transplanted patients) (27) and serum albumin in stroke patients and liver-transplanted patients suggesting no difference between groups (27, 34) (Table 3).
Other outcomes
Data on a range of disease-specific outcomes were reported but there were too few data on any 1 outcome to enable comparison across reviews.
Evidence from reviews of high methodological quality
Ten reviews (46 meta-analyses representing all 12 outcomes) were judged to be of high methodological quality, 2 in mixed clinical populations (23, 25) and 8 in single clinical conditions (26, 27, 29, 31–34, 36). The number of participants in the included analyses varied from 22 (27) to 8529 (25). Only 5 of the 10 reviews included >1000 participants in analyses. Fifteen (33%) of the analyses reported statistically significant benefits to participants receiving ONSs (Table 4).
TABLE 4.
Reviews with AMSTAR score ≥8 reporting statistically significant benefits of oral nutritional supplements (ONSs) or no significant difference between groups for all outcomes from identified systematic reviews of ONS interventions in participants who were malnourished or at nutritional risk according to clinical background of included participants1
| Statistically significant difference between groups | No significant difference between groups | ||||
|---|---|---|---|---|---|
| Outcome measure | Total number of meta-analyses | n | Subgroups in which effects reported | n | Subgroups in which effects reported |
| Clinical and functional | |||||
| Mortality | |||||
| Mixed clinical populations | 3 | 1 | Malnourished older people (>65 y) | 2 | People >65 y (mixed nutritional status), hospitalized adults at nutritional risk |
| Single clinical conditions | 8 | 1 | Geriatric patients | 7 | Hip fracture, stroke, liver disease, cancer, and perioperative patients |
| Total complications | |||||
| Mixed clinical populations | 1 | 1 | People >65 y (mixed nutritional status) | 0 | |
| Single clinical conditions | 4 | 2 | Perioperative patients, hip fracture | 2 | Hip fracture, preoperative (GI surgery) |
| Infectious complications | |||||
| Mixed clinical populations | 0 | — | — | 0 | |
| Single clinical conditions | 8 | 3 | Perioperative patients, chronic liver disease | 5 | Hip fracture, geriatrics, post liver transplant, preoperative (GI surgery) |
| Pressure ulcers | |||||
| Mixed clinical populations | 0 | 0 | — | 0 | Hip fracture |
| Single clinical conditions | 2 | 1 | Stroke | 1 | Mixed clinical backgrounds |
| Muscle strength | |||||
| Mixed clinical populations | 1 | 0 | — | 1 | People >65 y (mixed nutritional status) |
| Single clinical conditions | 0 | 0 | — | 0 | |
| Healthcare-related | |||||
| Length of stay | |||||
| Mixed clinical populations | 5 | 0 | — | 5 | People (>65 y) with geriatric conditions, hip fracture, stroke, hospitalized patients, malnourished or patients at nutritional risk |
| Single clinical conditions | 4 | 1 | Perioperative patients | 3 | Stroke, chronic liver disease, preoperative (GI surgery) |
| Readmissions | |||||
| Mixed clinical populations | 0 | 0 | — | 0 | — |
| Single clinical conditions | 2 | 0 | — | 2 | Post liver transplant |
| Nutritional | |||||
| Energy intake | |||||
| Mixed clinical populations | 0 | 0 | — | 0 | — |
| Single clinical conditions | 1 | 1 | Stroke | 0 | — |
| Protein intake | |||||
| Mixed clinical populations | 0 | 0 | — | 0 | — |
| Single clinical conditions | 1 | 1 | Stroke | 0 | — |
| Body weight | |||||
| Mixed clinical populations | 1 | 1 | People >65 y (mixed nutritional status) | 0 | |
| Single clinical conditions | 1 | 1 | COPD | 0 | — |
| Body composition/nutritional status | |||||
| Mixed clinical populations | |||||
| Mid-arm muscle circumference | 1 | 1 | People >65 y (mixed nutritional status) | — | |
| Single clinical conditions | — | COPD | |||
| Mid-arm muscle circumference | 1 | 0 | — | 1 | Stroke, liver disease |
| Serum albumin | 2 | 0 | — | 2 | |
AMSTAR, Assessment of Multiple Systematic Reviews measurement tool; COPD, chronic obstructive pulmonary disease; GI, gastrointestinal.
Discordance was apparent for all outcomes apart from LOS [reported in 5 subgroup analyses from 1 review (23)], hospital readmissions, and serum albumin. All analyses of LOS, hospital readmissions, and serum albumin reported no significant differences between groups, but the number of participants in the analyses of serum albumin and hospital readmissions was small (n = 144 and 477 for serum albumin, and n = 22 and 24 for hospital readmissions).
When examining data on outcomes from high-quality reviews only, no clinical group emerged as experiencing significant benefits from supplementation with ONSs for all outcomes. Meta-analyses in perioperative patients suggested ONSs were associated with significant reductions in LOS, infectious and total complications, and, in older patients (>65 y), benefits to mortality (malnourished patients only), total complications, body weight, and body composition. In contrast, no benefit from supplementation with ONSs for the majority of outcomes was suggested for patients with hip fracture, because no beneficial effects were observed on mortality, total or infectious complications, pressure ulcers, or LOS. The lack of benefits to total complications in patients with hip fracture was discordant with a larger (n = 727 participants) and more recent analysis (33) suggesting significant reductions on supplementation with ONSs compared with an earlier analysis (36) with fewer participants (n = 113) suggesting no benefits of ONSs. Overall the numbers of patients in all analyses for patients with hip fracture were low (113 to 968 patients).
Discussion
The aims of this overview were to compare the findings from systematic reviews of ONSs in adult patients who were malnourished or at risk of malnutrition and to evaluate any discordance between reviews by critical examination of their methodological conduct. We identified 22 systematic reviews that met our inclusion criteria, published between 1998 and 2017, with 12 different outcomes (91 meta-analyses) reported consistently across reviews. There was discordance in findings from meta-analyses among the reviews for all outcomes other than total complications in mixed clinical populations, hospital readmissions (single conditions), energy and protein intake, some measures of change in body composition (mid-arm muscle circumference in mixed clinical populations), and serum albumin, which were reported in few reviews. ONSs were associated with improvements in total complications (mixed populations), nutritional intake, and mid-arm muscle circumference (mixed populations) in all analyses reported but there were no differences between groups for hospital readmissions (patients with liver disease) and serum albumin. There was no consistent evidence of adverse effects of ONSs on any outcome here. Methodological quality of conduct of the reviews was poor, with only 45% of reviews identified being judged as high quality. The quality of conduct of reviews did not explain all of the discordance between reported outcomes. Comparison of findings from reviews of high methodological quality revealed similar discordance in reported outcomes with no clinical group being identified to derive consistent benefits from supplementation with ONSs.
Comparison with previous overviews
To our knowledge, there is only 1 previous overview of ONSs, which identified 13 systematic reviews and concluded that ONS interventions in nourished and malnourished patients from a range of clinical backgrounds were associated with significant improvements in nutritional intake, nutritional status, mortality, and complications (11). The present overview has identified a larger number and more recent reviews. The findings on nutritional intake from both overviews are similar in suggesting that ONSs are associated with statistically significant improvements in energy intake. However, the 2 overviews differ in their findings on nutritional status and clinical outcomes. The overview by Stratton and Elia (11) identified 4 systematic reviews reporting meta-analyses of weight change, all of which reported statistically significant benefits associated with ONSs. The present overview identified 11 meta-analyses of weight, 7 of which demonstrated statistically significant benefits in favor of ONSs. Despite the majority of meta-analyses reporting significant benefits, heterogeneity was either not reported (n = 6) or high (n = 3) for 9 of the 11 analyses, making the apparently positive findings on body weight less certain.
The previous overview reported benefits to mortality, particularly in the acute setting, and lower complication rates. The findings of the present overview were less optimistic, with only 7 of 22 analyses of mortality and 12 of 20 analyses of complications (total, infectious, and pressure ulcers) suggesting benefits associated with ONSs. For complications, the analyses that identified significant benefits tended to be for total complications in mixed clinical populations (5 analyses), and the analyses that failed to find significant reductions tended to be of infectious complications, in single clinical conditions, and to include smaller numbers of patients (n = 22–503) compared with the ones that reported positive findings (n = 384–6225), which might partly explain the discordance. Few trends were noted for analyses of mortality.
Methodological quality of the reviews failed to account for the discordance between outcomes. When only the reviews of high methodological quality were taken into consideration no clinical groups emerged as being more likely to benefit consistently from ONSs. A surprising finding was the lack of evidence for an effect of ONSs on several outcomes in patients with hip fracture. This contrasts with the findings of the previous overview in this area (11), which found benefits of ONSs to intake and unfavorable outcome (mortality and complications combined). The 2007 overview (11) identified an earlier version of the review by Avenell et al. published in 2005 which included 8 RCTs of mainly low quality and drew tentative conclusions on the possible benefits of ONSs (38). The more recent updates of this review published in 2010 (39) and 2016 (33), which included 14 and 18 RCTs of ONSs, respectively, failed (with the exception of infectious complications) to confirm these earlier tentative conclusions. A possible explanation could be that length of hospital stay for patients with hip fracture, averaging 12 d (40), is too short to allow any benefits to be observed.
The previous overview (11) did not include readmissions or LOS, both of which have substantial economic implications for healthcare providers and are influenced, at least in part, by factors such as mortality and complications (41). This overview identified 5 analyses of hospital readmissions, 2 of which suggested benefits associated with ONSs (21, 37) and 3 (2 analyses reported in 1 review) that found no difference between groups (24, 27). All analyses were small (n = 22–999). Hospital LOS was reported in 14 analyses, with the majority (12/14) finding no difference between groups. Despite the evidence of potential benefits to nutritional intake, nutritional status, and complications in some patients, these frequently failed to translate into reductions in LOS.
The included reviews varied in both how and whether they defined malnutrition as an inclusion criterion for the review. In addition, there was limited reporting of nutritional status within trials included in the reviews, and many reviews combined data for both well-nourished (but at risk of malnutrition) and malnourished patients. Malnutrition is a complex condition and can arise from a variety of different causes, both disease-related and precipitated by any combination of social, psychological, and economic circumstances. The complex etiology of the underlying condition is likely to influence how a patient responds to nutritional intervention. The reviews identified for this overview and individual studies within the reviews have included patients with both acute and chronic diseases as well as participants whose nutritional vulnerability arises from frailty and older age. The complex nature of malnutrition and likely variable response to similar interventions together with variations in the nutritional interventions identified across individual studies and reviews adds to the potential for heterogeneity among the outcomes reported and can explain some of the discordance. Without access to original study data, meaningful subgroup analyses that separate out the effects of the different background causes of malnutrition are impossible.
Methodological quality
In contrast to the overview published by Stratton and Elia (11), the present overview assessed the methodological quality of the identified reviews and considered findings in relation to review quality. Less than half of the reviews met the highest methodological quality standards, with 55% of reviews identified as being of moderate or low quality. The methodological elements that were most usually omitted were those most likely to introduce publication bias. Authors searched multiple electronic databases but restricted their searches to English language only, frequently failed to search for unpublished literature, and included published full-text publications only. Publication bias is a well-recognized phenomenon arising from the submission or acceptance of manuscripts based on the strength or direction of their findings (42). Manuscripts with positive results are more likely to be submitted for publication and more likely to be accepted than those with negative findings (43). In addition, there is empirical evidence that researchers selectively report positive findings over negative findings, which can reflect the fact that these are more likely to be accepted for publication (42, 44). It has also been demonstrated that researchers who publish in both English language and non-English journals are more likely to submit manuscripts with negative findings to non–English language journals (43). The difficulties inherent in getting negative results to publication can also mean that these trials are only available in abstract form. In this overview, we have identified evidence across many of the included reviews of decisions made at the search stage that could result in bias in favor of identification of studies with positive findings and failure to identify the harder-to-find negative studies, which can lead to overall spurious positive results. It is notable that the use of funnel plots, a method used to assess publication bias, was absent from the majority of identified reviews, with only 7 of the 22 identified reviews using them.
Heterogeneity is a measure of the amount of variation between the results of studies identified and affects decisions about whether it is sensible to combine results in a meta-analysis. It is critical that heterogeneity is taken into consideration when planning analyses and also that it is reported because it can affect the interpretation and generalizability of results. Heterogeneity was reported in 69% of analyses, and few reviews used the recognized strategies to manage or explore heterogeneity, meaning that results of analyses could be misleading. It is interesting that heterogeneity was reported more frequently for analyses demonstrating no difference between groups, and it is possible that authors have avoided reporting heterogeneity for positive analyses, particularly when it suggests considerable inconsistency.
Assessment of the quality of included studies is a key stage in the conduct of a systematic review. The purpose of assessing study quality is to enable reviewers to present their findings within the context of the quality of evidence identified. The Cochrane Collaboration have established the use of the Grades of Recommendation, Assessment, Developments and Evaluation (GRADE) approach, which provides a framework for the summary of a body of evidence on any clinical question and informs its subsequent use in guidelines and recommendations (45). The AMSTAR tool used in this overview does not require that the GRADE approach be used, but in order to score “yes” study quality must be explicitly considered in formulating recommendations (6). Although 95% of included reviews assessed study quality, we found evidence of recognition of study quality when formulating conclusions in only 68% of included reviews. Lack of consideration of study quality when reporting overall results can lead to overconfidence about the strength of a body of evidence, which can in turn translate into inappropriately firm recommendations for the use of interventions.
A key element of study quality is the consideration of any conflicts of interest by study and review authors. AMSTAR suggests that to score “yes” for this component of the tool, review authors should declare conflicts of interest for the systematic review as well as for each included study. We found only 2 reviews that reported the source of funding for each included study (25, 33) and so based our assessment on the first element only. Conflicts of interest were well reported across identified reviews but it is of note that 36% of the included reviews had a link with manufacturers of ONSs and 9 were conducted by employees of the ONS industry or were funded by manufacturers of ONSs who might have a vested interest in publication of findings in support of their products. Ioannidis (7) comments on the use of meta-analyses related to products made by industry with vested interests being used as marketing tools, similar to support for the conduct and publication of randomized trials. In the present overview, the only reviews that reported significant improvements in hospital readmissions, LOS (in mixed clinical populations), and muscle strength were reviews that were sponsored by industry.
Strengths and limitations
The strengths of this review are that it was conducted by experienced reviewers, guided by a protocol, included duplicate study selection and data extraction, and few limitations were placed on the search strategy. Despite these strengths the findings of this review merit some caution. The search strategy was developed with advice from an information specialist but was limited to 2 electronic databases, there was limited hand-searching, and no searches of the gray literature, meaning that some eligible reviews might have been missed (46). [Gray literature refers to research that is either unpublished or has been published in a noncommercial form (organizations with commercial publishing interests) and includes government reports, policy statements, reports or briefings from nongovernmental organizations, and dissertations and conference abstracts.] The group conducting this overview have conducted several systematic reviews on similar areas, including 2 published in the Cochrane Library and so have wide experience of this area; however, we cannot rule out the possibility that we have missed some reviews.
We chose to use the original AMSTAR tool to assess methodological quality and not the updated AMSTAR-2 tool (47) (https://amstar.ca/Amstar-2.php). It is possible that the more rigorous criteria of the AMSTAR-2 tool would provide a different result on quality from the one that we report here. We chose to use the earlier tool because it is the one that was available to the majority of authors at the time of publication of their review and so might reasonably have been expected to guide methodological conduct. However, we chose to use a simple scoring system used in a previous overview (14) to summarize results and enable comparison between reviews. Caution is advised with this approach because of the potential to overestimate the importance of some elements of quality. Despite this, it is noteworthy that the aspects that accounted for poor methodological quality were similar between reviews.
Malnutrition can occur as a consequence of disease or from a range of physiological and socioeconomic factors. The included systematic reviews did not take account of this, therefore it has been impossible to separate the results of this overview according to the likely etiology of malnutrition. It is reported that ONSs are often provided to patients without advice on how to incorporate them into the diet in ways that might achieve maximum effect. Little information on differences in the amount of instruction for use and support to maximize intake of ONSs was provided in the included reviews, which might influence compliance and outcomes.
Despite being the highest grade of evidence, systematic reviews can still provide misleading results (48). A large proportion of the reviews identified for this overview were judged to be of moderate or low methodological quality. The judgments made about quality were informed by published information only and no authors were contacted to verify judgments. It is therefore possible that our judgments when applying the AMSTAR criteria are inaccurate and do not reflect the high methodological standards used in conducting the reviews. Many of the reviews identified for this overview were published in journals rather than the Cochrane Library. Word limits applied by journals could mean that important elements of methodological quality have not been reported. The AMSTAR tool also assigns no score for items that are not applicable. This could result in lower methodological ratings that don't adequately reflect the quality of the review.
Conclusions and Recommendations
The results of this overview suggest that evidence for the effects of ONSs in malnourished patients or those who are at risk of malnutrition is uncertain. Although ONSs are used routinely in clinical practice in many countries, the discordance in results means that it remains unclear which patients benefit from them most and under what circumstances.
The discordance in the outcomes of the 22 systematic reviews identified for this overview was not markedly reduced when only high-quality review were considered. This suggests that the discordance between reviews could be more to do with other issues such as heterogeneity in patient populations arising from differences in clinical background, the etiological basis for malnutrition, the influence of practice in different healthcare settings, and variation in how ONSs are provided to patients, and these factors should be taken into consideration when designing future studies or undertaking evidence syntheses.
Supplementary Material
ACKNOWLEDGEMENTS
We acknowledge the late Peter Milligan (PM) for statistical guidance and support.
The authors’ responsibilities were as follows—RS: undertook the original systematic review described in this manuscript; MG: updated the searches and data extraction to August 2014; CB: updated searches and data extraction to August 2019 and completed duplicate data extraction for all included studies; RS: made the original draft of the manuscript; CB, CEW, and PWE: completely reworked the original manuscript; and all authors: interpreted the data to represent their collective experience, thoughts, and ideas, and read and approved the final manuscript.
Notes
The authors reported no funding received for this study. CEW recently received an honorarium from Nutricia for participation in the Expert Panel of the updated COPD Malnutrition Pathway. The contribution by MG was in part fulfilment of a National Institute for Health Research–funded MRes qualification at City University, London.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figure 1 and Supplemental Tables 1–7 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances/.
Abbreviations used: ACR, assumed control risk; AMSTAR, A MeaSurement Tool to Assess systematic Reviews; GRADE, Grades of Recommendation, Assessment, Developments and Evaluation; LOS, length of stay; MD, mean difference; ONS, oral nutritional supplement; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial; SMD, standardized mean difference.
Contributor Information
Christine Baldwin, Department of Nutritional Sciences, King's College London, London, United Kingdom.
Rosemary Smith, Department of Nutritional Sciences, King's College London, London, United Kingdom.
Michelle Gibbs, Department of Nutritional Sciences, King's College London, London, United Kingdom.
C Elizabeth Weekes, Department of Nutritional Sciences, King's College London, London, United Kingdom; Department of Nutrition and Dietetics, Guy's and St Thomas’ NHS Foundation Trust, London, United Kingdom.
Peter W Emery, Department of Nutritional Sciences, King's College London, London, United Kingdom.
References
- 1. Elia M. The MUST report. Nutritional screening for adults: a multidisciplinary responsibility. development and use of the 'Malnutrition Universal Screening Tool’ (MUST) for adults [Internet]. BAPEN; 2003; [cited 2020 Jul 12]. Available from: https://www.bapen.org.uk/screening-and-must/must/must-report. [Google Scholar]
- 2. Leach RM, Brotherton A, Stroud M, Thompson R. Nutrition and fluid balance must be taken seriously. BMJ. 2013;346:f801. [DOI] [PubMed] [Google Scholar]
- 3. Snider JT, Linthicum MT, Wu Y, LaVallee C, Lakdawalla DN, Hegazi R, Matarese L. Economic burden of community-based disease-associated malnutrition in the United States. J Parenter Enteral Nutr. 2014;38(2 Suppl):77S–85S. [DOI] [PubMed] [Google Scholar]
- 4. Elia M. The cost of malnutrition in England and potential cost savings from nutritional interventions (full report). A report on the cost of disease-related malnutrition in England and a budget impact analysis of implementing the NICE clinical guidelines/quality standard on nutritional support in adults. [Internet]. 2015; [cited 2020 Apr 1]. Available from: https://www.bapen.org.uk/pds/economic-report-short.pdf.
- 5. Spence D. Bad medicine: medical nutrition. BMJ. 2012;344:e451. [DOI] [PubMed] [Google Scholar]
- 6. Shea BJ, Grimshaw JM, Wells GA, Boers M, Andersson N, Hamel C, Porter AC, Tugwell P, Moher D, Bouter LM. Development of AMSTAR: a measurement tool to assess the methodological quality of systematic reviews. BMC Med Res Methodol. 2007;7(1):10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ioannidis J. The mass production of redundant, misleading, and conflicted systematic reviews and meta‐analyses. Milbank Q. 2016;94(3):485–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Jadad AR, Cook DJ, Browman GP. A guide to interpreting discordant systematic reviews. CMAJ. 1997;156(10):1411–6. [PMC free article] [PubMed] [Google Scholar]
- 9. Hopayian K. The need for caution in interpreting high quality systematic reviews. BMJ. 2001;323(7314):681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shrier I, Boivin JF, Platt RW, Steele RJ, Brophy JM, Carnevale F, Eisenberg MJ, Furlan A, Kakuma R, Macdonald MEet al. The interpretation of systematic reviews with meta-analyses: an objective or subjective process. BMC Med Inform Decis Mak. 2008;8:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Stratton RJ, Elia M.. A review of reviews: a new look at the evidence for oral nutritional supplements in clinical practice. Clin Nutr Supplements. 2007;2(1):5–23. [Google Scholar]
- 12. Smith V, Devane D, Begley CM, Clarke M. Methodology in conducting a systematic review of systematic reviews of healthcare interventions. BMC Med Res Methodol. 2011;11(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. J Clin Epidemiol. 2009;62(10):e1–34. [DOI] [PubMed] [Google Scholar]
- 14. Flodgren G, Eccles MP, Shepperd S, Scott A, Parmelli E, Beyer FR. An overview of reviews evaluating the effectiveness of financial incentives in changing healthcare professional behaviours and patient outcomes. Cochrane Database Syst Rev. 2011;(7):CD009255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moja L, Del Rio MPF, Banzi R, Cusi C, D'Amico R, Liberati A, Lodi G, Lucenteforte E, Minozzi S, Pecoraro V. Multiple systematic reviews: methods for assessing discordances of results. Intern Emerg Med. 2012;7(6):563–8. [DOI] [PubMed] [Google Scholar]
- 16. Stratton RJ, Green CJ, Elia M. Disease-related malnutrition: an evidence-based approach to treatment. Oxfordshire (England): CABI Publishing; 2003. [Google Scholar]
- 17. Stratton RJ, Elia M. A critical, systematic analysis of the use of oral nutritional supplements in the community. Clin Nutr. 1999;18(Suppl 2):29–84.10459079 [Google Scholar]
- 18. National Collaborating Centre for Acute Care (UK) . Nutrition support for adults: oral nutrition support, enteral tube feeding and parenteral nutrition (Clinical guideline 32). [Internet]. NICE; 2006; ; updated August 2017 [cited 2015 Jun 15]. Available from: https://www.nice.org.uk/guidance/cg32. [PubMed]
- 19. Potter J, Langhorne P, Roberts M. Routine protein energy supplementation in adults: systematic review. BMJ. 1998;317(7157):495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Potter JM. Oral supplements in the elderly. Curr Opin Clin Nutr Metab Care. 2001;4(1):21–8. [DOI] [PubMed] [Google Scholar]
- 21. Cawood AL, Elia M, Stratton RJ. Systematic review and meta-analysis of the effects of high protein oral nutritional supplements. Ageing Res Rev. 2012;11(2):278–96. [DOI] [PubMed] [Google Scholar]
- 22. Vanderkroft D, Collins CE, FitzGerald M, Lewis S, Neve M, Capra S. Minimising undernutrition in the older inpatient. Int J Evid Based Healthc. 2007;5(2):110–81. [DOI] [PubMed] [Google Scholar]
- 23. Milne AC, Potter J, Vivanti A, Avenell A. Protein and energy supplementation in elderly people at risk from malnutrition. Cochrane Database Syst Rev. 2009;(2):CD003288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Beck AM, Holst M, Rasmussen HH. Oral nutritional support of older (65 years+) medical and surgical patients after discharge from hospital: systematic review and meta-analysis of randomized controlled trials. Clin Rehabil. 2013;27(1):19–27. [DOI] [PubMed] [Google Scholar]
- 25. Feinberg J, Nielsen EE, Korang SK, Halberg Engell K, Nielsen MS, Zhang K, Didriksen M, Lund L, Lindahl N, Hallum Set al. Nutrition support in hospitalised adults at nutritional risk. Cochrane Database Syst Rev. 2017;(5):CD011598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Koretz RL, Avenell A, Lipman TO. Nutritional support for liver disease. Cochrane Database Syst Rev. 2012;(5):CD008344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Langer G, Großmann K, Saal S, Grothues D, Wienke A. Nutritional interventions for liver-transplanted patients. Cochrane Database Syst Rev. 2012;(8):CD007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ney M, Vandermeer B, Van Zanten SJV, Ma MM, Gramlich L, Tandon P. Meta‐analysis: oral or enteral nutritional supplementation in cirrhosis. Aliment Pharmacol Ther. 2013;37(7):672–9. [DOI] [PubMed] [Google Scholar]
- 29. Langer G, Fink A.. Nutritional interventions for preventing and treating pressure ulcers. Cochrane Database Syst Rev. 2014;(6):CD003216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Stratton RJ, Ek A-C, Engfer M, Moore Z, Rigby P, Wolfe R, Elia M. Enteral nutritional support in prevention and treatment of pressure ulcers: a systematic review and meta-analysis. Ageing Res Rev. 2005;4(3):422–50. [DOI] [PubMed] [Google Scholar]
- 31. Burden S, Todd C, Hill J, Lal S. Pre-operative nutrition support in patients undergoing gastrointestinal surgery. Cochrane Database Syst Rev. 2012;11:CD008879. [DOI] [PubMed] [Google Scholar]
- 32. Ferreira IM, Brooks D, White J, Goldstein R. Nutritional supplementation for stable chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2012;(12):CD000998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Avenell A, Smith TO, Curtain JP, Mak JC, Myint PK. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst Rev. 2016;11:CD001880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Geeganage C, Beavan J, Ellender S, Bath PMW. Interventions for dysphagia and nutritional support in acute and subacute stroke. Cochrane Database Syst Rev. 2012;(10):CD000323. [DOI] [PubMed] [Google Scholar]
- 35. Elia M, Bokhorst-de van der Schueren V, Garvey J, Goedhart A, Lundholm K, Nitenberg G, Stratton RJ. Enteral (oral or tube administration) nutritional support and eicosapentaenoic acid in patients with cancer: a systematic review. Int J Oncol. 2006;28(1):5–23. [DOI] [PubMed] [Google Scholar]
- 36. Koretz RL, Avenell A, Lipman TO, Braunschweig CL, Milne AC. Does enteral nutrition affect clinical outcome? A systematic review of the randomized trials. Am J Gastroenterol. 2007;102(2):412. [DOI] [PubMed] [Google Scholar]
- 37. Stratton RJ, Hebuterne X, Elia M. A systematic review and meta-analysis of the impact of oral nutritional supplements on hospital readmissions. Ageing Res Rev. 2013;12(4):884–97. [DOI] [PubMed] [Google Scholar]
- 38. Avenell A, Handoll HH.. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst Rev. 2005;(2):CD001880. [DOI] [PubMed] [Google Scholar]
- 39. Avenell A, Handoll HH.. Nutritional supplementation for hip fracture aftercare in older people. Cochrane Database Syst Rev. 2010;(1):CD001880. [DOI] [PubMed] [Google Scholar]
- 40. Abbas K, Umer M, Qadir I, Zaheer J, ur Rashid H. Predictors of length of hospital stay after total hip replacement. J Orthop Surg (Hong Kong). 2011;19(3):284–7. [DOI] [PubMed] [Google Scholar]
- 41. Stratton RJ, Elia M. Encouraging appropriate, evidence-based use of oral nutritional supplements. Proc Nutr Soc. 2010;69(4):477–87. [DOI] [PubMed] [Google Scholar]
- 42. Song F, Hooper L, Loke Y. Publication bias: what is it? How do we measure it? How do we avoid it?Open Access J Clin Trials. 2013;5:71–81. [Google Scholar]
- 43. Egger M, Zellweger-Zähner T, Schneider M, Junker C, Lengeler C, Antes G. Language bias in randomised controlled trials published in English and German. Lancet. 1997;350(9074):326–9. [DOI] [PubMed] [Google Scholar]
- 44. Song F, Parekh S, Hooper L, Loke Y, Ryder J, Sutton AJ, Hing C, Kwok CS, Pang C, Harvey I. Dissemination and publication of research findings: an updated review of related biases. Health Technol Assess. 2010;14(8):iii, ix–xi, 1–193. [DOI] [PubMed] [Google Scholar]
- 45. Schünemann H, Brożek J, Guyatt G, eds. GRADE handbook . Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. [Internet]. GRADE Working Group; 2013 [cited 2015 Jan 18]. Available from: https://gdt.gradepro.org/app/handbook/handbook.html.
- 46. Avenell A, Handoll HH, Grant AM. Lessons for search strategies from a systematic review, in The Cochrane Library, of nutritional supplementation trials in patients after hip fracture. Am J Clin Nutr. 2001;73(3):505–10. [DOI] [PubMed] [Google Scholar]
- 47. Shea BJ, Reeves BC, Wells G, Thuku M, Hamel C, Moran J, Moher D, Tugwell P, Welch V, Kristjansson Eet al. AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ. 2017;358:j4008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yuan Y, Hunt RH. Systematic reviews: the good, the bad, and the ugly. Am J Gastroenterol. 2009;104(5):1086–92. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



