ABSTRACT
Several clinical interventions report that consuming nuts will not cause weight gain. However, it is unclear if the type of instructions provided for how to incorporate nuts into the diet impacts weight outcomes. We performed a systematic review and meta-analysis of published nut-feeding trials with and without dietary substitution instructions to determine if there are changes in body weight (BW) or composition. PubMed and Web of Science were searched through 31 December 2019 for clinical trials involving the daily consumption of nuts or nut-based snacks/meals by adults (≥18 y) for >3 wk that reported BW, BMI, waist circumference (WC), or total body fat percentage (BF%). Each study was categorized by whether or not it contained dietary substitution instructions. Within these 2 categories, an aggregated mean effect size and 95% CI was produced using a fixed-effects model. Quality of studies was assessed through the Cochrane risk-of-bias tool. Fifty-five studies were included in the meta-analysis. In studies without dietary substitution instructions, there was no change in BW [standardized mean difference (SMD): 0.01 kg; 95% CI: −0.07, 0.08; I2 = 0%] or BF% (SMD: −0.05%; 95% CI: −0.19, 0.09; I2 = 0%). In studies with dietary substitution instructions, there was no change in BW (SMD: −0.01 kg; 95% CI: −0.11, 0.09; I2 = 0%); however, there was a significant decrease in BF% (SMD: −0.32%; 95% CI: −0.61%, −0.03%; I2 = 35.4%; P < 0.05). There was no change in BMI or WC for either category of studies. Nut-enriched diet interventions did not result in changes in BW, BMI, or WC in studies either with or without substitution instructions. Slight decreases in BF% may occur if substitution instructions are used, but more research is needed. Limitations included varying methodologies between included studies and the frequency of unreported outcome variables in excluded studies.
Keywords: nuts, tree nuts, energy compensation, energy substitution, body weight, body composition, weight maintenance
Nut trials employ varying types of dietary or energy substitution instructions. Our results indicate that nut consumption does not result in weight change, independent of the type of instruction provided.
Introduction
According to the NHANES, >40% of Americans are overweight or obese (1). Elevated adiposity increases risk for chronic diseases such as type 2 diabetes (T2D), hypertension, joint problems, and cardiovascular disease (2). Unfortunately, obesity interventions often result in quick weight loss followed by weight regain (3), highlighting the need for effective obesity-prevention methods. To maintain body weight (BW), the 2015–2020 Dietary Guidelines for Americans recommends consuming a healthy eating pattern in addition to achieving an appropriate energy intake (4). Methods of following a healthy eating pattern include consuming a variety of nutrient-dense foods and to limit foods with excess sugar, sodium, and saturated fat.
Nuts and nut products, although energy dense, are rich sources of fiber, protein, and unsaturated fatty acids, which promote satiety and reduced energy intake (5–7). Numerous randomized controlled trials (RCTs) also report that regular nut consumption, even in large quantities, does not cause weight gain (8–26). However, these studies manipulate the diet or provide instructions on how to incorporate nuts into one's diet to varying degrees, and it is unclear if the type of diet instructions provided to subjects impacts weight and body-composition outcomes. Those different methods include providing no dietary instructions with the nut consumption (8, 10, 11, 13, 21, 24, 25, 27), instructions to substitute energy-equivalent foods or specific macronutrients in their typical diet for the nuts provided (14–20, 28–31), or the provision of all meals in an outpatient feeding setting designed to keep participants in energy balance (32–37).
To date, only 2 long-term nut trials (>8 wk) have compared adiposity measures in participants who received diet substitution instructions (isocaloric substitution) versus those who did not receive instructions, and BW or body composition was significantly influenced by the type of dietary instruction that was provided (38, 39). A recent meta-analytic review of weight change with nut-enriched clinical trials did not consider the effect of varying dietary substitution instructions (40). Therefore, we systematically reviewed and performed a meta-analysis of clinical trials with parallel or crossover designs to examine the impact of no dietary substitution instructions or some type of dietary substitution instructions on BW and body composition during interventions lasting >3 wk in adults. We hypothesized that studies without substitution instructions would result in a significant increase in BW, BMI, waist circumference (WC), and total body fat percentage (BF%), while studies with energy- or fat-substitution instructions would not result in changes in these aforementioned outcome variables.
Methods
Search strategy
This work was completed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (41). A systematic search identified clinical trials measuring changes in BW or body composition during nut interventions. Eligible studies were identified by searching PubMed and Web of Science databases from inception through 31 December 2019. The following search terms were used: (“nut” OR “walnut” OR “peanut” OR “hazelnut” OR “almond” OR “pistachio” OR “cashew” OR “macadamia” OR “pecan” OR “pine nut” OR “brazil nut” OR “tree nut” OR “mixed nut”) AND (“metabolic syndrome” OR “Mets” OR “overweight” OR “weight gain” OR “obesity” OR “obese” OR “adiposity” OR “adipose” OR “body weight” OR “body mass index” OR “BMI” OR “waist circumference” OR “hypertension” OR “blood pressure” OR “hypercholesterolemia” OR “dyslipidemia” OR “cholesterol” OR “triglycerides” OR “diabetes” OR “glucose” OR “glycemia” OR “WHR” OR “weight loss” OR “body fat”). Full-text studies that were published in English were considered.
Inclusion and exclusion criteria
To be included in the meta-analysis, the studies had to be peer-reviewed, published in English, and containing a parallel or crossover design. In addition, the studies needed to compare a nut-containing diet with a control diet in adults and report 1 of the following outcomes: BW, BMI, WC, or BF%. More specifically, interventions that involved the consumption of nuts alone, within snack bars, or part of a meal were included. All studies in adult populations were included regardless of disease state, gender, or age range. The minimum duration and dosage of the intervention were ≥3 wk and ≥10 g/d for 5 d/wk, respectively. When there were multiple published manuscripts on the same dataset, the paper with the longest follow-up period was selected. If the outcome data were not published in the manuscript, researchers contacted the corresponding author to obtain required data. Exclusion criteria were the following: 1) reviews, editorials, nonresearch letters; 2) BW data not available; 3) BW was measured frequently and highly controlled, such as daily self-weighing; 4) lack of comparator diet; 5) comparator diet consumed another nut or nut butter; 6) controlled-feeding trials in which all meals were provided; 7) studies using animal models; and 8) intentional weight loss or gain was used. The study selection process is illustrated in Figure 1.
FIGURE 1.
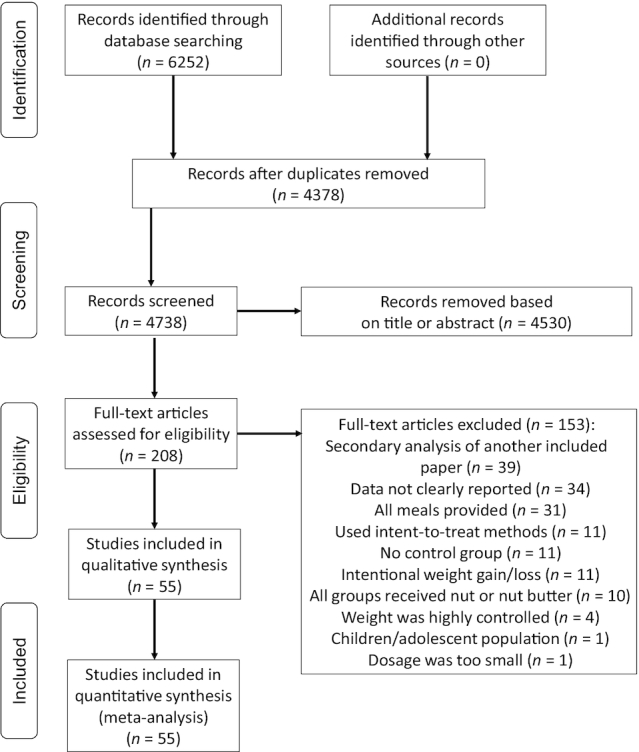
PRISMA flowchart. PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Data collection
Following the systematic search of PubMed and Web of Science, individual studies were screened based on the title and abstracts for inclusion or exclusion criteria. Next, studies entered full-text review. Studies that met the inclusion criteria during the full-text review were included in the systematic review and meta-analysis. Two reviewers collected the study details (study author, publication year, study design, baseline age, baseline BMI, dosage of nuts, type of comparator diet, type of diet instructions, length of intervention, whether or not weight loss/gain was the purpose of the study). Interclass correlation coefficients (ICCs) for absolute agreement were calculated to examine interrater reliability for BW, BMI, WC, and BF%, and any discrepancies between reviewers were resolved. Additionally, the quality of each study was assessed using the Cochrane risk-of-bias tool for RCTs (42).
All studies were categorized as either containing substitution instructions or not containing substitution instructions. A study was categorized as containing no substitution instructions if the nut intervention group did not receive specific instructions on how to incorporate the provided nuts into their usual diet. A study was categorized as containing substitution instructions if the nut intervention group received specific instructions on how to incorporate the nuts into the diet. For example, if subjects were instructed to substitute energy-equivalent foods habitually consumed in the diet for the added nuts (i.e., isocaloric substitution), the study was categorized as containing substitution instructions.
Statistical methods
The primary outcome was the standardized mean difference (SMD) in BW, BMI, WC, and BF% between subjects consuming nut-containing and control diets. For parallel studies, Cohen's d effect sizes (ESs) were calculated by subtracting the mean change in weight during the control group from the mean change in weight during the nut intervention; then, this difference was divided by the pooled pre-test SD (43–45). A positive ES value indicated a less favorable change occurred in the intervention group compared with the control group. For crossover studies, all measurements from the intervention period and all measurements from the control period were incorporated into the ES calculation, similar to the calculation for parallel trials. This methodology is a conservative estimate and reduces the statistical power of crossover studies to show an effect (46). Furthermore, to avoid unit-of-analysis error, in studies that contained >1 nut intervention group and a control group, the intervention groups were combined to develop 1 ES for the study. For example, if an intervention contained almond, walnut, and control groups, then the sample size, mean, and SDs of the almond and walnut groups were combined using the formulas recommended by the Cochrane Collaboration Handbook (47). Finally, the new calculations for the sample size, mean, and SD of the intervention group were incorporated into the ES calculation for the study.
Aggregated mean ESs and 95% CIs were calculated for studies with and without substitution instructions using a fixed-effects model because homogeneity was shown. The DerSimonian and Laird estimator was used to quantify heterogeneity (48), which was indicated if the Q total reached a significance level of P < 0.05 (43). Heterogeneity was also measured using the I2 statistic (49). Low, moderate, or high heterogeneity was categorized as values of 25%, 50%, and 75% for the I2 statistic, respectively. Possible bias was evaluated using Egger's test (it assesses whether the variation in the ES is due to publication bias) and funnel plots that plotted the SE against the ES. Fail-Safe Number (Fail-Safe N+), which estimates the number of additional null effects of average sample size needed to overturn the observed significant effect, was calculated for all significant effects using Rosenberg's method (50). Statistical analyses were performed with R version 3.6.2 (The R Foundation, Vienna, Austria). A couple of sensitivity analyses were used to determine if the meta-analysis findings are robust to the decisions made when determining the inclusion and exclusion criteria (47). The first sensitivity analysis removed all effects in which the control group received a dietary intervention (e.g., a biscuit containing the same energy as provided in the nut group). These studies were originally categorized based on whether or not the intervention group received dietary substitution instructions; thus, studies were removed from both categories during the sensitivity analysis. The second sensitivity analysis removed all crossover studies.
Results
Study selection
The database search yielded 6252 results, and 1874 duplicates were removed. We excluded 4530 studies based on titles and abstracts and 153 after a full-text review. Fifty-five clinical trials published between 1997 and 31 December 2019 were included in the final meta-analysis (9, 11, 13, 17, 18, 24, 25, 32, 51–97) (Table 1). There were 52 effects for BW, 36 effects for BMI, 27 effects for WC, and 19 effects for BF%. Seven studies contained ≥1 nut intervention groups (9, 13, 25, 69, 73, 86, 89), which were combined to create 1 overall ES for the study. There were 3811 subjects represented in interventions that maintained a mean duration of 13.8 ± 21.5 wk and dosage of 48.2 ± 20.8 g nuts/d. Study populations included individuals with normal weight/healthy, overweight/obese, hypercholesterolemia, metabolic syndrome, prediabetes, T2D, and elevated cardiovascular disease or T2D risk. The nut interventions included almonds, cashews, hazelnuts, macadamia nut, mixed nut, peanut, pecan, pistachio, walnut, and a nut-based snack bar. Table 2 describes the characteristics of studies with and without substitution instructions. In studies where participants received substitution instructions, those instructions included the following: substituting energy (kilocalories) from habitual diet or prescribed background diet (17, 53, 63, 67, 75, 76, 81, 89, 90, 96), substituting fat energy (18, 60, 61, 66, 93), substituting starchy foods (69, 87) or meat sources (79), substituting a combination of foods or macronutrients (71, 78), substituting specific food items recommended in a background diet (9, 80), or substituting specific foods based on individualized advice or exchange lists (58, 85).
TABLE 1.
Characteristics of included clinical trials involving nut consumption with and without substitution instructions in adults1
| First author, year (ref), country | Study design | Subjects in analysis, n | Population | Average age, y | Diet period, wk | Type of nut | Dose of nut | Nut diet | Instructions? | Control diet | Risk of bias |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Abbaspour et al., 2019 (51), USA | Parallel | 48 | Overweight/obese | Not reported | 8 | Mixed nuts | 42.5 g/d | Habitual diet | No | Habitual diet + pretzels | Some concerns |
| Agebratt et al., 2016 (52), Sweden | Parallel | 30 | Healthy | 24 | 8 | Mixed nuts | 7 kcal/kg body weight | Habitual diet | No | Habitual diet + isocaloric fruit | Some concerns |
| Bashan et al., 2018 (53), Turkey | Parallel | 49 | Dyslipidemia | 41 | 12 | Walnut | 45 g/d | AHA recommendations | Yes | AHA recommendations | Some concerns |
| Bento et al., 2014 (24), Brazil | Crossover | 20 | Mildly high cholesterol | 35 | 6 | Almond | 20 g/d | Habitual diet | No | Habitual diet + placebo | Some concerns |
| Bitok et al., 2018 (54), USA and Spain | Parallel | 307 | Healthy | 69 | 24 | Walnuts | ∼15% of caloric needs | Habitual diet | No | Habitual diet | Low |
| Burns-Whitmore et al., 2014 (91), USA | Crossover | 21 | Healthy | 38 | 42 | Walnut | 28.4 g/d | Habitual diet | No | Habitual diet + standard egg | Low |
| Canudas et al., 2019 (55), Spain | Crossover | 49 | Prediabetes | 56 | 16 | Pistachio | 57 g/d | Habitual diet | No | Habitual diet + energy intake was adjusted to compensate for lack of pistachios | Low |
| Carughi et al., 2019 (56), France | Parallel | 60 | Healthy women | 35 | 4 | Pistachio | 56 g/d | Habitual diet + pistachios as afternoon snack | No | Habitual diet + biscuit as afternoon snack | Some concerns |
| Casas-Agustench et al., 2011 (57), Spain | Parallel | 50 | Metabolic syndrome | 52 | 12 | Mixed nuts | 30 g/d | General health diet instructions | No | General healthy diet instructions | Some concerns |
| Chisholm et al., 2005 (58), New Zealand | Crossover | 28 | Healthy | 48 | 6 | Mixed nuts | 30 g/d | Low-fat background diet + self-selected nuts | Yes | Low-fat background diet + canola oil containing cereal | Low |
| Cohen and Johnston, 2011 (59), USA | Parallel | 13 | T2D | 66 | 12 | Almonds | 28 g/d | Habitual diet | No | Habitual diet + cheese | Some concerns |
| Damasceno et al., 2011 (9), Spain2 | Crossover | 36 | Mildly high cholesterol | 56 | 4 | Walnuts or almonds | 22% of energy intake | Background Mediterranean diet | Yes | Background Mediterranean diet + olive oil | High |
| Damavandi et al., 2013 (60), Iran | Parallel | 48 | T2D | 56 | 8 | Hazelnut | 10% of energy intake | Habitual diet | Yes | Habitual diet | Some concerns |
| Damavandi et al., 2019 (61), Iran | Parallel | 43 | T2D | 54 | 8 | Cashews | 10% of energy intake | Habitual diet | Yes | Habitual diet | Some concerns |
| Davidi et al., 2011 (62), USA | Parallel | 94 | Overweight | 54 | 8 | KIND (KIND, LLC) fruit and nut bars | 2 bars/d | Habitual diet | No | Habitual diet | Some concerns |
| de Souza et al., 2018, (63), Brazil | Parallel | 46 | Overweight/obese women | Not reported | 8 | Almond | 20 g/d | Prescribed individualized normocaloric diet | Yes | Prescribed individualized normocaloric diet | Some concerns |
| Dhillon et al., 2018 (64), USA | Parallel | 73 | Healthy college freshmen | 18 | 8 | Almond | 56.7 g/d | Habitual diet | No | Habitual diet + isocaloric graham cracker | Some concerns |
| Eastman and Clayshulte, 2005 (94), USA | Parallel | 44 | Hyperlipidemia | 46 | 8 | Pecan | 68 g/d | Habitual diet | No | Habitual diet | High |
| Fantino et al., 2019 (65), France | Parallel | 58 | Premenopausal women | 32 | 12 | Pistachio | 44 g/d | Habitual diet + pistachios as midmorning snack | No | Habitual diet | Some concerns |
| Gulati et al., 2014 (66), India | Parallel | 68 | Metabolic syndrome | 42 | 24 | Pistachio | 20% of energy intake | General healthy diet instructions | Yes | General healthy diet instructions | Some concerns |
| Hernández-Alonso et al., 2014 (67), Spain | Crossover | 54 | Prediabetes | 55 | 12 | Pistachio | 57 g/d | Prescribed diet designed to meet energy needs | Yes | Prescribed diet designed to meet energy needs + instructions to eat extra foods containing fat to account for lack of nuts | High |
| Hiraoka-Yamamoto et al., 2004 (68), Japan | Parallel | 47 | Healthy | 19 | 3 | Macadamia nut | 10 g/d | Habitual diet + bread containing macadamia nuts | No | Habitual diet + bread containing butter | Some concerns |
| Hollis and Mattes, 2007 (32), USA | Crossover | 20 | Healthy | 24 | 10 | Almonds | 58 g/d | Habitual diet | No | Habitual diet | Some concerns |
| Hwang et al., 2019 (95), South Korea | Crossover | 84 | Metabolic syndrome | 39 | 16 | Walnuts | 45 g/d | Habitual diet | No | Habitual diet | Low |
| Jamshed et al., 2015 (25), Pakistan2 | Parallel | 150 | CAD | 60 | 12 | Pakistani or American almond | 10 g/d | Habitual diet | No | Habitual diet | Some concerns |
| Jenkins et al., 2018 (89), Canada2 | Parallel | 117 | T2D | 33 | 12 | Mixed nut (half or full dose) | 474 kcal per 2000-kcal diet needs provided as half dose of mixed nut and half muffin or full dose of mixed nut | Background NCEP ATP III diet and ADA dietary advice | Yes | Background NCEP ATP III and ADA dietary advice + muffin with no nuts | Some concerns |
| Jenkins et al., 2002 (69), Canada2 | Crossover | 81 | Dyslipidemia | 64 | 4 | Almonds | 73 g/d or 37 g/d in muffin | Background NCEP Step 2 diet | Yes | Background NCEP Step 2 diet + muffin with no nuts | Some concerns |
| Jung et al., 2018 (70), Korea | Crossover | 84 | Overweight | 52 | 4 | Almonds | 56 g/d | Habitual diet | No | Habitual diet + isocaloric cookie | Low |
| Kasliwal et al., 2015 (71), India | Parallel | 42 | Dyslipidemia | 39 | 12 | Pistachio | 40 g/d | ADA exercise and diet counseling | Yes | ADA exercise and diet counseling | Some concerns |
| Katz et al., 2012 (96), USA | Crossover | 46 | Overweight adults | 57 | 8 | Walnuts | 56 g/d | Habitual diet | Yes | Habitual diet | Low |
| Kocyigit et al., 2006 (93), Turkey | Parallel | 44 | Healthy | 33 | 3 | Pistachio | 20% of energy intake | Habitual diet | Yes | Habitual diet | Some concerns |
| Lee et al., 2014 (72), South Korea | Parallel | 60 | Metabolic syndrome | Not reported | 6 | Mixed nuts | 30 g/d | Habitual Diet | No | Habitual diet | Some concerns |
| Liu et al., 2017 (73), Korea2 | Parallel | 169 | Healthy | 26 | 16 | Almonds consumed as premeal snack or between meals | 56 g/d | Habitual diet | No | Habitual diet + isocaloric cookie | High |
| Ma et al., 2010 (17), USA | Crossover | 24 | T2D | 58 | 8 | Walnuts | 56 g/d | Habitual diet | Yes | Habitual diet | Low |
| Mercanligil et al., 2007 (74), Turkey | Crossover | 15 | Hypercholesterolemic men | 48 | 4 | Hazelnut | 40 g/d | Low-fat, low-cholesterol, and high-carbohydrate diet | No | Low-fat, low-cholesterol, and high-carbohydrate diet | High |
| Mohan et al., 2018 (75), India | Parallel | 269 | T2D | 21 | 12 | Cashew | 30 g/d | Diabetic diet | Yes | Diabetic diet | Some concerns |
| Morgan and Clayshulte, 2000 (11), USA | Parallel | 19 | Healthy | 41 | 8 | Pecan | 68 g/d | Habitual diet | No | Habitual diet | Some concerns |
| Njike et al., 2017 (76), USA | Parallel | 32 | Overweight | 58 | 12 | KIND bars | 1–4 bars/d | Habitual diet | Yes | Habitual diet | Some concerns |
| Nouran et al., 2010 (77), Iran | Crossover | 54 | Hypercholesterolemic men | 43 | 4 | Peanuts | 20% of energy | Habitual diet | No | Habitual diet | Some concerns |
| O'Byrne et al., 1997 (78), USA | Parallel (quasi-experimental) | 25 | Hypercholesterolemic women | Not reported | 24 | Peanut | 35–68 g/d | Prescribed low-fat diet | Yes | Habitual low-fat diet | High |
| Olmedilla-Alonso et al., 2008 (79), Spain | Crossover | 25 | High CVD risk | 54 | 5 | Walnut-enriched restructured meats | 19.4 g/d | Consumed walnut-enriched meats + avoided all other meats | Yes | Consumed restructured meats without walnuts + avoided all other meats | Low |
| Palacios et al., 2019 (90), USA | Crossover | 33 | Prediabetes | 48 | 6 | Almond | 84 g/d | Habitual diet | Yes | Habitual diet | High |
| Razquin et al., 2010 (92), Spain | Parallel | 435 | High CVD risk | 68 | 156 | Mixed nuts | 30 g/d | Mediterranean diet | No | Low-fat diet | High |
| Ros et al., 2004 (80), Spain | Crossover | 21 | Moderate hypercholesterolemia | 55 | 4 | Walnut | 18% of energy intake | Background Mediterranean diet | Yes | Background Mediterranean diet | High |
| Ruisinger et al., 2015 (81), USA | Parallel | 48 | On statin therapy | 60 | 4 | Almonds | 100 g/d | Background TLC diet | Yes | Background TLC diet | Some concerns |
| Salas-Huetos et al., 2018 (82), Spain | Parallel | 98 | Healthy men | 25 | 14 | Mixed nuts | 60 g/d | Habitual diet | No | Habitual diet | Some concerns |
| Spiller et al., 1998 (83), USA | Parallel | 30 | Hypercholesterolemia | 53 | 4 | Almonds | 100 g/d | Prescribed background diet of whole and unrefined foods | No | Prescribed background diet of whole and unrefined foods + Cheddar cheese | Some concerns |
| Sweazea et al., 2014 (84), USA | Parallel | 21 | T2D | 56 | 12 | Almond | 43 g (5–7 times/wk) | Habitual diet | No | Habitual diet | Some concerns |
| Tan et al., 2013 (13), Australia2 | Parallel | 137 | Increased T2D risk | 29 | 4 | Almond | 43 g/d | No instructions or instructions to consume almonds at breakfast, lunch, morning snack, or afternoon snack | No | Habitual diet | Some concerns |
| Tapsell et al., 2004 (85), Australia | Parallel | 38 | T2D | 60 | 24 | Walnut | 30 g/d | Low-fat diet | Yes | Low-fat diet | Some concerns |
| Tapsell et al., 2009 (18), Australia | Parallel | 35 | Non–insulin-dependent T2D | 54 | 52 | Walnut | 30 g/d | Low-fat diet | Yes | Low-fat diet | Some concerns |
| Tey et al., 2013 (86), New Zealand2 | Parallel | 107 | Overweight/obese | 42 | 12 | Hazelnut | 30 or 60 g/d | Habitual diet | No | Habitual diet | Some concerns |
| Tey et al., 2011 (97), New Zealand | Parallel | 61 | Healthy adults | 38 | 12 | Hazelnut | 42 g/d | Habitual diet | No | Habitual diet | Some concerns |
| Wu et al., 2010 (87), China | Parallel | 189 | Metabolic syndrome | 48 | 12 | Almond | 30 g/d | AHA diet instructions | Yes | AHA diet instructions | Some concerns |
| Zaveri et al., 2009 (88), UK | Parallel | 23 | Healthy men | 41 | 12 | Almond | 56 g/d | General healthy advice | No | Prescribed American background diet | High |
1ADA, American Diabetes Association; AHA, American Heart Association; ATP III, Adult Treatment Program III; CAD, coronary artery disease; CVD, cardiovascular disease; NCEP, National Cholesterol Education Program; ref, reference; T2D, type 2 diabetes; TLC, therapeutic lifestyle changes.
2Denotes study in which ≥2 intervention groups were combined to calculate the overall effect size for the study.
TABLE 2.
Characteristics of nut trials included in meta-analysis with and without substitution instructions in adults1
| Effect sizes from studies without instructions (n = 36) | Effect sizes from studies with instructions (n = 28) | |
|---|---|---|
| Age, y | 41.7 ± 14.4 | 49.8 ± 10.8 |
| Nut dose, g/d | 47.6 ± 19.7 | 49.1 ± 22.6 |
| Intervention duration, wk | 15.4 ± 27.1 | 11.8 ± 10.6 |
| Effect sizes from studies with dietary intervention in control group, % (n) | 32.3 (10) | 25.0 (6) |
| Almond, % (n) | 35.5 (11) | 20.8 (5) |
| Cashew, % (n) | 0 (0) | 8.3 (2) |
| Hazelnut, % (n) | 9.7 (3) | 4.2 (1) |
| Nut-based bar, % (n) | 3.2 (1) | 4.2 (1) |
| Macadamia nut, % (n) | 3.2 (1) | 0 (0) |
| Mixed nut, % (n) | 19.4 (6) | 8.3 (2) |
| Peanut, % (n) | 3.2 (1) | 4.2 (1) |
| Pecan, % (n) | 6.5 (2) | 0 (0) |
| Pistachio, % (n) | 9.7 (3) | 16.7 (4) |
| Walnut, % (n) | 9.7 (3) | 33.3 (8) |
1All values are means ± SDs unless otherwise indicated.
Meta-analysis of nut intake and changes in adiposity
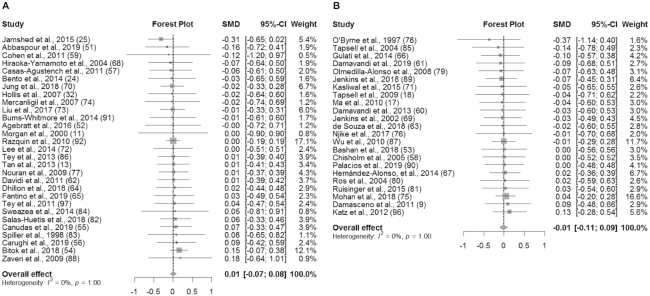
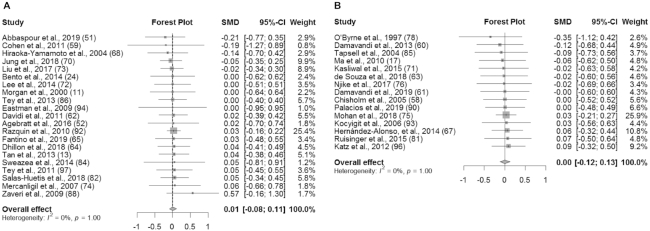
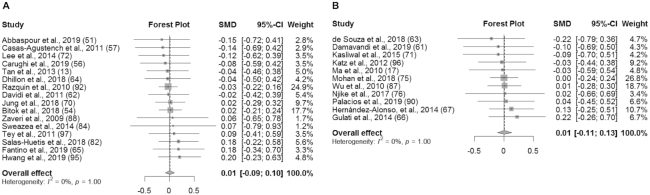
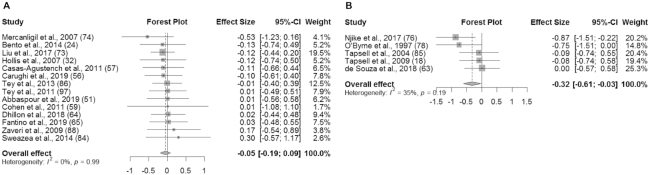
An overview of the meta-analysis results is presented in Table 3. In studies without substitution instructions, there was no significant effect of the intervention on BW (SMD: 0.01 kg; 95% CI: −0.07, 0.08; I2 = 0%), BMI (in kg/m2) (SMD: 0.01; 95% CI: −0.08, 0.11; I2 = 0%), WC (SMD: 0.01 cm; 95% CI: −0.09, 0.10; I2 = 0%), or BF% (SMD: −0.05%; 95% CI: −0.19%, 0.09%; I2 = 0%) (Figures 2A, 3A, 4A, and 5A). Similarly, in studies with substitution instructions, there was no significant effect of the intervention on BW (SMD: −0.01 kg; 95% CI: −0.11, 0.09; I2 = 0%), BMI (SMD: 0.00; 95% CI: −0.12, 0.13; I2 = 0%), or WC (SMD: 0.01 cm; 95% CI: −0.11, 0.13; I2 = 0%) (Figures 2B, 3B, 4B). There was a significant effect of the intervention on BF% in studies that used substitution instructions (SMD: −0.32%; 95% CI: −0.61%, −0.03%; I2 = 35.4%; P < 0.05) (Figure 5B).
TABLE 3.
Overview of meta-analysis results of nut trials with and without dietary substitution instructions in adults1
| Outcome variable | Substitution instruction? | n | SMD (95% CI) | I 2, % | P | Egger's test | ICC | Mean difference |
|---|---|---|---|---|---|---|---|---|
| Weight, kg | No | 29 | 0.01 (−0.07, 0.08) | 0 | 0.88 | 0.56 | 0.94 | 0.13 |
| Yes | 23 | −0.01 (−0.11, 0.09) | 0 | 0.82 | 0.03 | — | −0.26 | |
| BMI, kg/m2 | No | 21 | 0.01 (−0.08, 0.11) | 0 | 0.80 | 0.84 | 0.94 | 0.04 |
| Yes | 15 | 0.00 (−0.12, 0.13) | 0 | 0.95 | 0.03 | — | 0.00 | |
| Waist circumference, cm | No | 16 | 0.01 (−0.09, 0.10) | 0 | 0.85 | 0.74 | 0.99 | 0.16 |
| Yes | 11 | 0.01 (−0.11, 0.13) | 0 | 0.86 | 0.56 | — | −0.22 | |
| Total body fat, % | No | 14 | −0.05 (−0.19, 0.09) | 0 | 0.45 | 0.63 | 0.85 | −0.31 |
| Yes | 5 | −0.32 (−0.61, −0.03) | 35.4 | 0.03 | 0.28 | — | −1.86 |
1ICC, interclass correlation coefficient; SMD, standardized mean difference.
FIGURE 2.
Forest plot of BW (kg) for studies without dietary substitution instructions (A) and BW for studies with dietary substitution instructions (B). For each individual study, the square represents the SMD in BW between the intervention and control groups. The area of the square is proportional to the weight. Horizontal lines represent 95% CIs. BW, body weight; SMD, standardized mean difference.
FIGURE 3.
Forest plot of BMI (kg/m2) for studies without dietary substitution instructions (A) and BMI for studies with dietary substitution instructions (B). For each individual study, the square represents the SMD in BMI between the intervention and control groups. The area of the square is proportional to the weight. Horizontal lines represent 95% CIs. SMD, standardized mean difference.
FIGURE 4.
Forest plot of WC (cm) for studies without dietary substitution instructions (A) and WC for studies with dietary substitution instructions (B). For each individual study, the square represents the SMD in WC between the intervention and control groups. The area of the square is proportional to the weight. Horizontal lines represent 95% CIs. SMD, standardized mean difference.
FIGURE 5.
Forest plot of total BF% for studies without dietary substitution instructions (A) and BF% for studies with dietary substitution instructions (B). For each individual study, the square represents the standardized mean difference (SMD) in BF% between the intervention and control groups. The area of the square is proportional to the weight. Horizontal lines represent 95% confidence intervals. BF%, body fat percentage; SMD, standardized mean difference.
Rater agreement
The ICC for all effects was ≥0.85 (Table 3). The ICC increased to 100% after adjusting for discrepancies between reviewers.
Homogeneity of results
For studies without substitution instructions, there was no heterogeneity for BW (Q = 6.24; P = 1.00; I2 = 0.0%), BMI (Q = 3.58; P = 1.00; I2 = 0.0%), WC (Q = 3.28; P = 1.00; I2 = 0.0%), and BF% (Q = 3.58; P = 1.00; I2 = 0.0%) (Table 3). Similarly, for studies with substitution instructions, there was no or moderate heterogeneity for BW (Q = 1.87; P = 1.00; I2 = 0.0%), BMI (Q = 1.52; P = 1.00; I2 = 0.0%), WC (Q = 2.04; P = 1.00; I2 = 0.0%), and BF% (Q = 6.19; P = 0.19; I2 = 35.4%) (Table 3). Due to the lack of significant heterogeneity among all outcome variables, a fixed-effects model was used in all analyses.
Fail safe N and publication bias
The fail-safe N for effects with substitution instructions that reported BF% was N+ = 5 using the Rosenberg method (50). For studies with and without diet instructions, visual inspections of the funnel plots showed reasonable symmetry (Supplemental Figures 1 and 2). Additionally, in studies without substitution instructions, Egger's tests indicated no publication bias for all outcomes (BW: P = .56; BMI: P = 0.84; WC: 0.74; and BF%: P = 0.63) (Table 3). Egger's tests also indicated no publication bias in studies with substitution instructions for all outcomes except for BW and BMI (BW: P = 0.03; BMI: 0.03; WC: 0.56; BF%: 0.28).
Sensitivity analysis
In the first sensitivity analysis, all effects in which the control group received a dietary intervention were removed (9, 51, 52, 56, 58, 59, 64, 69, 70, 76, 79, 83, 90, 95, 98) (such as the isocaloric equivalent food described above in Methods), and the results are presented in Table 4. For studies with substitution instructions that reported on BF%, there was no longer a significant effect of the interventions and there was no heterogeneity (SMD: −0.18%; 95% CI: −0.51%, 0.14%; I2 = 0%). All other outcomes remained nonsignificant and homogeneous.
TABLE 4.
Overview of results from sensitivity analysis in clinical trials involving nut consumption in adults1
| Outcome variable | Substitution instruction? | n | SMD (95% CI) | I 2, % | P | Egger's test |
|---|---|---|---|---|---|---|
| Weight, kg | No | 20 | 0.01 (−0.08, 0.10) | 0 | 0.78 | 0.68 |
| Yes | 17 | −0.01 (−0.12, 0.10) | 0 | 0.82 | 0.03 | |
| BMI, kg/m2 | No | 14 | 0.04 (−0.07, 0.16) | 0 | 0.48 | 0.37 |
| Yes | 12 | 0.01 (−0.13, 0.14) | 0 | 0.94 | 0.05 | |
| Waist circumference, cm | No | 11 | 0.01 (−0.10, 0.12) | 0 | 0.88 | 0.50 |
| Yes | 9 | 0.01 (−0.12, 0.14) | 0 | 0.89 | 0.53 | |
| Total body fat, % | No | 9 | −0.04 (−0.23, 0.14) | 0 | 0.64 | 0.94 |
| Yes | 4 | −0.18 (−0.51, 0.14) | 0 | 0.28 | 0.09 |
1All studies with control groups that received an intervention were removed. ICC, interclass correlation coefficient; SMD, standardized mean difference.
In the second sensitivity analysis, all effects from studies with crossover designs were removed (9, 17, 24, 32, 55, 58, 67, 69, 70, 74, 77, 79, 80, 90, 91, 95, 96), and the results are presented in Table 5. The results of this sensitivity analysis did not significantly differ from the main analysis. All outcomes remained nonsignificant and homogeneous, except for the aggregated effect of the intervention on BF% in studies with substitution instructions (SMD: −0.32%; 95% CI: −0.61%, −0.03%; I2 = 35.4%), which was also significant with moderate heterogeneity in the main analysis.
TABLE 5.
Overview of results from sensitivity analysis in clinical trials involving nut consumption in adults1
| Outcome variable | Substitution instruction? | n | SMD (95% CI) | I 2, % | P | Egger's test |
|---|---|---|---|---|---|---|
| Weight, kg | No | 22 | 0.01 (−0.08, 0.10) | 0 | 0.87 | 0.63 |
| Yes | 14 | −0.03 (−0.15, 0.09) | 0 | 0.65 | 0.02 | |
| BMI, kg/m2 | No | 18 | 0.02 (−0.08, 0.12) | 0 | 0.73 | 1.00 |
| Yes | 10 | −0.01 (−0.17, 0.14) | 0 | 0.87 | 0.10 | |
| Waist circumference, cm | No | 14 | 0.00 (−0.10, 0.10) | 0 | 0.96 | 0.90 |
| Yes | 7 | 0.00 (−0.15, 0.15) | 0 | 0.98 | 0.62 | |
| Total body fat, % | No | 11 | −0.02 (−0.17, 0.13) | 0 | 0.77 | 0.03 |
| Yes | 5 | −0.32 (−0.61, −0.03) | 35.4 | 0.03 | 0.28 |
1All crossover studies were removed. ICC, interclass correlation coefficient; SMD, standardized mean difference.
Discussion
This was the first meta-analysis to separately investigate the impact of nut-enriched diets with and without dietary substitution instructions on BW and body-composition outcomes. Based on the studies included in the meta-analysis, nut consumption does not result in changes in BW, BMI, or WC, independent of whether or not substitution instructions are provided. Conversely, nut consumption may result in decreased BF% when substitution instructions are used, but these results should be interpreted with caution as described in more detail below. Our analysis did not directly compare whether or not providing dietary substitution instructions is more or less favorable for BW or body-composition outcomes because each category of studies was analyzed in a separate model. The decision to maintain separate models for the 2 categories of studies was made a priori in an effort to most effectively answer the research question.
As mentioned above, one interesting finding from the present meta-analyses is that daily nut consumption may result in decreases in BF% if substitution instructions are used; however, these results should be interpreted with caution. Only 5 studies were included in the meta-analysis of studies involving substitution instructions that reported BF%, and a variety of methods were used to measure BF%, including DXA, bioelectrical impedance, and skinfold-thickness measurements. The variability in precision and accuracy among these methods for measuring body composition should be considered when interpreting these results. In addition, the significant effect may have been driven by 1 study in particular (76). In the study by Njike et al. (76), the intervention group ate a nut-based snack bar and the control group ate a conventional snack food for 12 wk. The intervention group lost 1.7% of body fat and the control group gained 6.2% of body fat, resulting in a negative ES that was driven by the increase in adiposity in the control group. During the first sensitivity analysis, this study was removed since the control group had also received a dietary intervention. As a result, the significant effect of the interventions on BF% was lost, and the heterogeneity was reduced from 35.4% to 0.0%.
Finding no change in outcome measures for studies with or without substitution instructions was somewhat surprising and contrary to our hypothesis. We had originally thought that studies without some type of dietary substitution instruction for the daily nut consumption would result in increased BW, BMI, WC, or BF%. This hypothesis was based on 2 previous clinical nut trials that directly compared the impact of varying dietary substitution instructions on weight and body-composition outcomes (38, 39). In those studies, weight or body composition was significantly influenced by the type of dietary instructions provided; yet, our meta-analysis results indicate that weight change does not occur with or without dietary substitution instructions. For the studies without substitution instructions in our analysis, the mean duration was 15.4 wk and the mean dosage was ∼47.8 g/d, providing ∼300 calories (99). According to the NIH body weight planner (100), an individual with a BMI of 30 would gain 3.3 kg without dietary compensation when consuming 47.8 g/d of mixed nuts for 16 wk. Since the meta-analysis showed that weight gain does not occur in studies with or without substitution instructions, there must be physiological mechanisms in place that prevent changes in weight during nut interventions without substitution instruction. Previous studies have explored possible mechanisms that allow weight maintenance with nut consumption, and they include increased satiety (101, 102) and subsequent reduced energy intake (13, 103), decreased bioavailability (104–107), increased diet-induced thermogenesis (108–110), and/or increased resting metabolic rate (39, 52). More clinical studies that directly compare the impact of interventions with and without dietary substitution instructions, and studies that investigate the mechanisms for weight maintenance with tree nut consumption, are needed to fully elucidate our hypothesis.
In 2018, Li et al. (40) published a meta-analysis reporting that nut consumption resulted in a significant reduction in BW, BMI, and WC. This is contrary to our findings, but there are a few possible reasons for this discrepancy. First, since the previous meta-analysis was not focused on substitution instructions, all included studies were analyzed together for each outcome variable while ours were separated out based on the presence or absence of dietary substitution instructions. Second, our inclusion criteria were stricter than the previous meta-analysis. We excluded 30 studies due to controlled-feeding methodology, while the publication by Li et al. included 11 controlled-feeding trials. Likewise, we excluded weight-loss trials, but Li et al. included these trials. Finally, our weighted ESs were also standardized, while theirs was not, and Li et al. (40) did not report on BF%. Together, there are sufficient differences between the types of studies used, and the categorization of studies, in our meta-analysis compared with Li et al. (40) that likely contributed to differences in overall conclusions. This demonstrates the importance of carefully examining study parameters and the overall design and intent of each meta-analysis. Unfortunately, it can make overall interpretations or messages to the general public challenging. Based on their conclusions and ours, we can, with some confidence, state that frequent tree nut consumption does not lead to weight gain in clinical trial studies.
The overall risk of bias, assessed by the Cochrane risk-of-bias tool for RCTs, was “some concern” for 37 of 55 studies. This assessment for RCTs is conservative, and a rating of “some concern” does not indicate that the study maintained poor quality. Many studies were rated as “some concern” in domain 5 of the assessment tool because a priori statistical analysis plans were not available in the study's clinical trial registration. Notably, this lack of information does not necessarily mean that the statistical methods were not planned a priori. If a study received a rating of “some concern” in 1 domain, the study's overall score was automatically also “some concern.” Nine studies were rated as low risk of bias and 9 studies were rated as high risk of bias. Table 6 details the ratings that each study received in each domain of the tool. Visual inspection of funnel plots and most Egger's tests indicated no publication bias. However, the Egger's tests for effects with dietary substitution instructions that reported BW and BMI were significant, so results should be interpreted with caution.
TABLE 6.
Results from the Cochrane risk-of-bias tool for randomized controlled trials assessment for included clinical trials involving nut consumption in adults1
| Study, year (reference) | D1 | D2 | D3 | D4 | D5 | Overall |
|---|---|---|---|---|---|---|
| Abbaspour et al., 2019 (51) | Low | Low | Low | Low | SC | SC |
| Agebratt et al., 2016 (52) | Low | Low | Low | Low | SC | SC |
| Bashan et al., 2018 (53) | SC | Low | Low | Low | SC | SC |
| Bento et al., 2014 (24) | Low | Low | SC | Low | Low | SC |
| Bitok et al., 2018 (54) | Low | Low | Low | Low | Low | Low |
| Burns-Whitmore et al., 2014 (91) | Low | Low | Low | Low | Low | Low |
| Canudas et al., 2019 (55) | Low | Low | Low | Low | Low | Low |
| Carughi et al., 2019 (56) | Low | Low | Low | Low | SC | SC |
| Casas-Agustench et al., 2011 (57) | Low | Low | Low | Low | SC | SC |
| Chisholm et al., 2005 (58) | Low | Low | Low | Low | Low | Low |
| Cohen and Johnston, 2011 (59) | Low | Low | Low | Low | SC | SC |
| Damasceno et al., 2011 (9) | Low | High | Low | Low | Low | High |
| Damavandi et al., 2019 (61) | Low | Low | Low | Low | SC | SC |
| Damavandi et al., 2013 (60) | Low | Low | Low | Low | SC | SC |
| Davidi et al., 2011 (62) | Low | Low | Low | Low | SC | SC |
| de Souza et al., 2018 (63) | Low | Low | Low | Low | SC | SC |
| Dhillon et al., 2018 (64) | Low | Low | Low | Low | SC | SC |
| Fantino et al., 2019 (65) | Low | Low | Low | Low | SC | SC |
| Gulati et al., 2014 (66) | Low | Low | Low | Low | SC | SC |
| Hernández-Alonso, et al., 2014 (67) | Low | Low | High | Low | Low | High |
| Hiraoka-Yamamoto et al., 2004 (68) | Low | Low | Low | Low | SC | SC |
| Hollis et al., 2007 (32) | Low | Low | SC | Low | Low | SC |
| Jamshed et al., 2015 (25) | Low | Low | High | Low | Low | SC |
| Jenkins et al., 2018 (89) | Low | Low | Low | Low | SC | SC |
| Jenkins et al., 2002 (69) | Low | Low | SC | Low | Low | SC |
| Jung et al., 2018 (70) | Low | Low | Low | Low | Low | Low |
| Katz et al., 2012 (96) | Low | Low | Low | Low | Low | Low |
| Kasliwal et al., 2015 (71) | Low | Low | Low | Low | SC | SC |
| Lee et al., 2014 (72) | Low | Low | Low | Low | SC | SC |
| Liu et al., 2017 (73) | Low | Low | High | Low | SC | SC |
| Ma et al., 2010 (17) | Low | Low | Low | Low | Low | Low |
| Mercanligil et al., 2007 (74) | Low | High | Low | Low | Low | High |
| Mohan et al., 2018 (75) | Low | Low | Low | Low | SC | SC |
| Morgan et al., 2000 (11) | Low | Low | SC | Low | SC | SC |
| Njike et al., 2017 (76) | Low | Low | Low | Low | SC | SC |
| Nouran et al., 2010 (77) | Low | Low | SC | Low | Low | SC |
| O'Byrne et al., 1997 (78) | SC | Low | SC | Low | SC | High |
| Olmedilla-Alonso et al., 2008 (79) | Low | Low | Low | Low | Low | Low |
| Palacios et al., 2019 (90) | Low | Low | High | Low | Low | High |
| Razquin et al., 2010 (92) | SC | High | Low | Low | SC | High |
| Ros et al., 2004 (80) | Low | High | Low | Low | Low | High |
| Ruisinger et al., 2015 (81) | Low | Low | Low | Low | SC | SC |
| Salas-Huetos et al., 2018 (82) | Low | Low | Low | Low | SC | SC |
| Spiller et al., 1998 (83) | Low | Low | Low | Low | SC | SC |
| Sweazea et al., 2014 (84) | SC | Low | Low | Low | SC | SC |
| Tan et al., 2013 (13) | Low | Low | Low | Low | SC | SC |
| Tapsell et al., 2009 (18) | Low | Low | Low | Low | SC | SC |
| Tapsell et al., 2004 (85) | Low | Low | Low | Low | SC | SC |
| Tey et al., 2013 (86) | Low | Low | Low | Low | SC | SC |
| Tey et al., 2011 (97) | Low | Low | Low | Low | SC | SC |
| Wu et al., 2010 (87) | Low | Low | Low | Low | SC | SC |
| Zaveri et al., 2009 (88) | High | Low | High | Low | SC | High |
| Kocyigit et al., 2006 (93) | Low | Low | Low | Low | SC | SC |
| Eastman et al., 2005 (94) | High | Low | High | Low | SC | High |
| Hwang et al., 2019 (95) | Low | Low | Low | Low | Low | Low |
1D1, bias due to randomization; D2, bias due to deviations from intended intervention; D3, bias due to missing data; D4, bias due to outcome measurement; D5, bias due to selection of reported result; SC, some concerns.
This meta-analysis is not free of limitations. For example, within the category of studies that contained dietary substitution instructions, the type of substitution instruction and the degree of contact with research personnel varied across studies. Likewise, a variety of nuts and dosages of nuts were used in the studies included in the meta-analysis, which could have influenced the results. For example, walnuts are rich in PUFAs while pecans are rich in MUFAs (99). The differences in fatty acid profiles between nuts may alter each nut's potential for promoting weight maintenance, possibly through different effects on appetite (111) and/or metabolism (112). However, despite the variations in substitution instructions, types of nut, and dosage of nut, the meta-analysis resulted in low heterogeneity between studies.
Another possible limitation is that the inclusion of crossover designs introduces possible unit-of-analysis error (47). This error due to crossover designs is often considered to be conservative because these studies may be underweighted in the analysis (47). Therefore, this limitation did not outweigh the potential loss of sample size if all crossover studies were excluded. However, to further confirm that the inclusion of crossover studies in the main analysis did not impact the overall results, we removed all crossover studies in the second sensitivity analysis (Table 5). The results of this sensitivity analysis did not significantly change from the main analysis. Finally, weight and body-composition outcomes were not reported in 34 studies that were entered into the full-text review. Corresponding authors were always contacted when data were missing, but responses were often not received or the data were not collected.
In conclusion, based on the studies included in this meta-analysis, nut consumption does not result in changes in BW, BMI, or WC in studies with or without substitution instructions. Nut consumption may result in decreased BF% when substitution instructions are used. These results suggest that nuts may be consumed, even in large quantities, without changes in BW or body composition.
Supplementary Material
ACKNOWLEDGEMENTS
We thank Mary Catherine Prater and Alexis Marquardt from the Human Nutrition Laboratory at the University of Georgia (UGA) for assistance with data collection and organization and the Statistical Consulting Center at UGA for assistance with the data analysis. The authors’ responsibilities were as follows—LLG and JAC: conceived and designed the analysis; LLG: collected the data, performed the analysis, synthesized the data, and wrote the manuscript, with key revisions by JAC; and all authors: read and approved the final manuscript.
Notes
Funds for this project were provided by the University of Georgia Obesity Initiative.
Author disclosures: The authors report no conflicts of interest.
Supplemental Figures 1 and 2 are available from the “Supplementary data” link in the online posting of the article and from the same link in the online table of contents at https://academic.oup.com/advances.
Abbreviations used: BF%, total body fat percentage; BW, body weight; ES, effect size; ICC, interclass correlation coefficient; Fail-Safe N, Fail-Safe Number; RCT, randomized controlled trial; SMD, standardized mean difference; T2D, type 2 diabetes; WC, waist circumference.
Contributor Information
Liana L Guarneiri, Department of Foods and Nutrition, University of Georgia, Athens, GA, USA.
Jamie A Cooper, Department of Foods and Nutrition, University of Georgia, Athens, GA, USA.
References
- 1. Hales CM, Carroll MD, Fryar CD, Ogden CL. Prevalence of obesity and severe obesity among adults: United States, 2017–2018. Hyattsville (MD): National Center for Health Statistics; 2020. [Google Scholar]
- 2. Mayo Clinic. Obesity [Internet]. [Accessed 2020 Mar 1]. Available from: https://www.mayoclinic.org/diseases-conditions/obesity/symptoms-causes/syc-20375742.
- 3. Hall KD, Kahan S. Maintenance of lost weight and long-term management of obesity. Med Clin North Am. 2018;102(1):183–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. USDA . Dietary Guidelines for Americans 2015–2020. 2015[Internet]. [Cited 2019 May 13] Available from: https://health.gov/dietaryguidelines/2015/guidelines/. [Google Scholar]
- 5. Hayes D, Angove MJ, Tucci J, Dennis C. Walnuts (Juglans regia) chemical composition and research in human health. Crit Rev Food Sci Nutr. 2016;56(8):1231–41. [DOI] [PubMed] [Google Scholar]
- 6. Maguire LS, O'Sullivan SM, Galvin K, O'Connor TP, O'Brien NM. Fatty acid profile, tocopherol, squalene and phytosterol content of walnuts, almonds, peanuts, hazelnuts and the macadamia nut. Int J Food Sci Nutr. 2004;55(3):171–8. [DOI] [PubMed] [Google Scholar]
- 7. Clark M, Slavin J. The effect of fiber on satiety and food intake: a systematic review. J Am Coll Nutr. 2013;32(3):200–11. [DOI] [PubMed] [Google Scholar]
- 8. Almario RU, Vonghavaravat V, Wong R, Kasim-Karakas SE. Effects of walnut consumption on plasma fatty acids and lipoproteins in combined hyperlipidemia. Am J Clin Nutr. 2001;74(1):72–9. [DOI] [PubMed] [Google Scholar]
- 9. Damasceno NR, Pérez-Heras A, Serra M, Cofán M, Sala-Vila A, Salas-Salvadó J, Ros E. Crossover study of diets enriched with virgin olive oil, walnuts or almonds: effects on lipids and other cardiovascular risk markers. Nutr Metab Cardiovasc Dis. 2011;21(Suppl 1):S14–20. [DOI] [PubMed] [Google Scholar]
- 10. Fraser GE, Bennett HW, Jaceldo KB, Sabaté J. Effect on body weight of a free 76 kilojoule (320 calorie) daily supplement of almonds for six months. J Am Coll Nutr. 2002;21(3):275–83. [DOI] [PubMed] [Google Scholar]
- 11. Morgan WA, Clayshulte BJ. Pecans lower low-density lipoprotein cholesterol in people with normal lipid levels. J Am Diet Assoc. 2000;100(3):312–8. [DOI] [PubMed] [Google Scholar]
- 12. Ros E, Rajaram S, Sala-Vila A, Serra-Mir M, Valls-Pedret C, Cofán M, Roth I, Doménech M, Freitas T, Calvo Cet al. . Effect of a 1-year walnut supplementation on blood lipids among older individuals: findings from the Walnuts and Healthy Aging (WAHA) study. FASEB J. 2016;30(1):21343. [Google Scholar]
- 13. Tan SY, Mattes RD. Appetitive, dietary and health effects of almonds consumed with meals or as snacks: a randomized, controlled trial. Eur J Clin Nutr. 2013;67(11):1205–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Gulati S, Misra A, Pandey RM. Effect of almond supplementation on glycemia and cardiovascular risk factors in Asian Indians in north India with type 2 diabetes mellitus: a 24-week study. Metab Syndr Relat Disord. 2017;15(2):98–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wu L, Piotrowski K, Rau T, Waldmann E, Broedl UC, Demmelmair H, Koletzko B, Stark RG, Nagel JM, Mantzoros CSet al. . Walnut-enriched diet reduces fasting non-HDL-cholesterol and apolipoprotein B in healthy Caucasian subjects: a randomized controlled cross-over clinical trial. Metabolism. 2014;63(3):382–91. [DOI] [PubMed] [Google Scholar]
- 16. Kranz S, Hill AM, Fleming JA, Hartman TJ, West SG, Kris-Etherton PM. Nutrient displacement associated with walnut supplementation in men. J Hum Nutr Diet. 2014;27(Suppl 2):247–54. [DOI] [PubMed] [Google Scholar]
- 17. Ma Y, Njike VY, Millet J, Dutta S, Doughty K, Treu JA, Katz DL. Effects of walnut consumption on endothelial function in type 2 diabetic subjects: a randomized controlled crossover trial. Diabetes Care. 2010;33(2):227–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Tapsell LC, Batterham MJ, Teuss G, Tan SY, Dalton S, Quick CJ, Gillen LJ, Charlton KE. Long-term effects of increased dietary polyunsaturated fat from walnuts on metabolic parameters in type II diabetes. Eur J Clin Nutr. 2009;63(8):1008–15. [DOI] [PubMed] [Google Scholar]
- 19. Spaccarotella KJ, Kris-Etherton PM, Stone WL, Bagshaw DM, Fishell VK, West SG, Lawrence FR, Hartman TJ. The effect of walnut intake on factors related to prostate and vascular health in older men. Nutr J. 2008;7:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Zambón D, Sabaté J, Muñoz S, Campero B, Casals E, Merlos M, Laguna JC, Ros E. Substituting walnuts for monounsaturated fat improves the serum lipid profile of hypercholesterolemic men and women: a randomized crossover trial. Ann Intern Med. 2000;132(7):538–46. [DOI] [PubMed] [Google Scholar]
- 21. Fitschen PJ, Rolfhus KR, Winfrey MR, Allen BK, Manzy M, Maher MA. Cardiovascular effects of consumption of black versus English walnuts. J Med Food. 2011;14(9):890–8. [DOI] [PubMed] [Google Scholar]
- 22. Wien M, Bleich D, Raghuwanshi M, Gould-Forgerite S, Gomes J, Monahan-Couch L, Oda K. Almond consumption and cardiovascular risk factors in adults with prediabetes. J Am Coll Nutr. 2010;29(3):189–97. [DOI] [PubMed] [Google Scholar]
- 23. Wien MA, Sabaté JM, Iklé DN, Cole SE, Kandeel FR. Almonds vs complex carbohydrates in a weight reduction program. Int J Obes Relat Metab Disord. 2003;27(11):1365–72. [DOI] [PubMed] [Google Scholar]
- 24. Bento AP, Cominetti C, Simões Filho A, Naves MM. Baru almond improves lipid profile in mildly hypercholesterolemic subjects: a randomized, controlled, crossover study. Nutr Metab Cardiovasc Dis. 2014;24(12):1330–6. [DOI] [PubMed] [Google Scholar]
- 25. Jamshed H, Sultan FA, Iqbal R, Gilani AH. Dietary almonds increase serum HDL cholesterol in coronary artery disease patients in a randomized controlled trial. J Nutr. 2015;145(10):2287–92. [DOI] [PubMed] [Google Scholar]
- 26. Dhillon J, Tan SY, Mattes RD. Almond consumption during energy restriction lowers truncal fat and blood pressure in compliant overweight or obese adults. J Nutr. 2016;146(12):2513–9. [DOI] [PubMed] [Google Scholar]
- 27. Sabaté J, Cordero-Macintyre Z, Siapco G, Torabian S, Haddad E. Does regular walnut consumption lead to weight gain?. Br J Nutr. 2005;94(5):859–64. [DOI] [PubMed] [Google Scholar]
- 28. Abbey M, Noakes M, Belling GB, Nestel PJ. Partial replacement of saturated fatty acids with almonds or walnuts lowers total plasma cholesterol and low-density-lipoprotein cholesterol. Am J Clin Nutr. 1994;59(5):995–9. [DOI] [PubMed] [Google Scholar]
- 29. Bamberger C, Rossmeier A, Lechner K, Wu L, Waldmann E, Stark RG, Altenhofer J, Henze K, Parhofer KG. A walnut-enriched diet reduces lipids in healthy Caucasian subjects, independent of recommended macronutrient replacement and time point of consumption: a prospective, randomized, controlled trial. Nutrients. 2017;9(10):1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kalgaonkar S, Almario RU, Gurusinghe D, Garamendi EM, Buchan W, Kim K, Karakas SE. Differential effects of walnuts vs almonds on improving metabolic and endocrine parameters in PCOS. Eur J Clin Nutr. 2011;65(3):386–93. [DOI] [PubMed] [Google Scholar]
- 31. Neale EP, Tapsell LC, Martin A, Batterham MJ, Wibisono C, Probst YC. Impact of providing walnut samples in a lifestyle intervention for weight loss: a secondary analysis of the HealthTrack trial. Food Nutr Res. 2017;61(1):1344522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hollis J, Mattes R. Effect of chronic consumption of almonds on body weight in healthy humans. Br J Nutr. 2007;98(3):651–6. [DOI] [PubMed] [Google Scholar]
- 33. Li SC, Liu YH, Liu JF, Chang WH, Chen CM, Chen CY. Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism. 2011;60(4):474–9. [DOI] [PubMed] [Google Scholar]
- 34. Rajaram S, Burke K, Connell B, Myint T, Sabate J. A monounsaturated fatty acid-rich pecan-enriched diet favorably alters the serum lipid profile of healthy men and women. J Nutr. 2001;131(9):2275–9. [DOI] [PubMed] [Google Scholar]
- 35. Rajaram S, Haddad EH, Mejia A, Sabaté J. Walnuts and fatty fish influence different serum lipid fractions in normal to mildly hyperlipidemic individuals: a randomized controlled study. Am J Clin Nutr. 2009;89(5):1657S–63S. [DOI] [PubMed] [Google Scholar]
- 36. Richmond K, Williams S, Mann J, Brown R, Chisholm A. Markers of cardiovascular risk in postmenopausal women with type 2 diabetes are improved by the daily consumption of almonds or sunflower kernels: a feeding study. ISRN Nutr. 2013;2013:626414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Schutte AE, Van Rooyen JM, Huisman HW, Mukuddem-Petersen J, Oosthuizen W, Hanekom SM, Jerling JC. Modulation of baroreflex sensitivity by walnuts versus cashew nuts in subjects with metabolic syndrome. Am J Hypertens. 2006;19(6):629–36. [DOI] [PubMed] [Google Scholar]
- 38. Njike VY, Ayettey R, Petraro P, Treu JA, Katz DL. Walnut ingestion in adults at risk for diabetes: effects on body composition, diet quality, and cardiac risk measures. BMJ Open Diabetes Res Care. 2015;3(1):e000115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Alper CM, Mattes RD. Effects of chronic peanut consumption on energy balance and hedonics. Int J Obes Relat Metab Disord. 2002;26(8):1129–37. [DOI] [PubMed] [Google Scholar]
- 40. Li H, Li X, Yuan S, Jin Y, Lu J. Nut consumption and risk of metabolic syndrome and overweight/obesity: a meta-analysis of prospective cohort studies and randomized trials. Nutr Metab (Lond). 2018;15:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–94. [DOI] [PubMed] [Google Scholar]
- 42. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAet al. . The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hedges LV, Olkin I. Statistical methods for meta-analysis. 6th ed. San Diego (CA): Academic Press; 1985. pp. 79–201. [Google Scholar]
- 44. Fedewa MV, Gist NH, Evans EM, Dishman RK. Exercise and insulin resistance in youth: a meta-analysis. Pediatrics. 2014;133(1):e163. [DOI] [PubMed] [Google Scholar]
- 45. Loy BD, O'Connor PJ, Dishman RK. The effect of a single bout of exercise on energy and fatigue states: a systematic review and meta-analysis. Fatigue. 2013;1(4):223–42. [Google Scholar]
- 46. Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol. 2002;31(1):140–9. [DOI] [PubMed] [Google Scholar]
- 47. Cochrane Collaboration . Cochrane handbook for systematic reviews of interventions. Hoboken (NJ): Wiley & Sons, Inc; 2011;5(1). [Google Scholar]
- 48. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–88. [DOI] [PubMed] [Google Scholar]
- 49. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Rosenberg MS. The file-drawer problem revisited: a general weighted method for calculating fail-safe numbers in meta-analysis. Evolution. 2005;59(2):464–8. [PubMed] [Google Scholar]
- 51. Abbaspour N, Roberts T, Hooshmand S, Kern M, Hong MY. Mixed nut consumption may improve cardiovascular disease risk factors in overweight and obese adults. Nutrients. 2019;11(7):1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Agebratt C, Strom E, Romu T, Dahlqvist-Leinhard O, Borga M, Leandersson P, Nystrom FH. A randomized study of the effects of additional fruit and nuts consumption on hepatic fat content, cardiovascular risk factors and basal metabolic rate. PLoS One. 2016;11(1):e0147149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Bashan I, Bakman M. The effect of daily walnut consumption on dyslipidemia. J Food Qual. 2018;2018: 4731826. [Google Scholar]
- 54. Bitok E, Rajaram S, Jaceldo-Siegl K, Oda K, Sala-Vila A, Serra-Mir M, Ros E, Sabate J. Effects of long-term walnut supplementation on body weight in free-living elderly: results of a randomized controlled trial. Nutrients. 2018;10(9):1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Canudas S, Hernandez-Alonso P, Galie S, Muralidharan J, Morell-Azanza L, Zalba G, Garcia-Gavilan J, Marti A, Salas-Salvado J, Bullo M. Pistachio consumption modulates DNA oxidation and genes related to telomere maintenance: a crossover randomized clinical trial. Am J Clin Nutr. 2019;109(6):1738–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Carughi A, Bellisle F, Dougkas A, Giboreau A, Feeney MJ, Higgs J. A randomized controlled pilot study to assess effects of a daily pistachio (Pistacia vera) afternoon snack on next-meal energy intake, satiety, and anthropometry in French women. Nutrients. 2019;11(4):767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Casas-Agustench P, Lopez-Uriarte P, Bullo M, Ros E, Cabre-Vila JJ, Salas-Salvado J. Effects of one serving of mixed nuts on serum lipids, insulin resistance and inflammatory markers in patients with the metabolic syndrome. Nutr Metab Cardiovasc Dis. 2011;21(2):126–35. [DOI] [PubMed] [Google Scholar]
- 58. Chisholm A, Mc Auley K, Mann J, Williams S, Skeaff M. Cholesterol lowering effects of nuts compared with a Canola oil enriched cereal of similar fat composition. Nutr Metab Cardiovasc Dis. 2005;15(4):284–92. [DOI] [PubMed] [Google Scholar]
- 59. Cohen AE, Johnston CS. Almond ingestion at mealtime reduces postprandial glycemia and chronic ingestion reduces hemoglobin A(1c) in individuals with well-controlled type 2 diabetes mellitus. Metabolism. 2011;60(9):1312–7. [DOI] [PubMed] [Google Scholar]
- 60. Damavandi RD, Eghtesadi S, Shidfar F, Heydari I, Foroushani AR. Effects of hazelnuts consumption on fasting blood sugar and lipoproteins in patients with type 2 diabetes. J Res Med Sci. 2013;18(4):314–21. [PMC free article] [PubMed] [Google Scholar]
- 61. Damavandi RD, Mousavi SN, Shidfar F, Mohammadi V, Rajab A, Hosseini S, Heshmati J. Effects of daily consumption of cashews on oxidative stress and atherogenic indices in patients with type 2 diabetes: a randomized, controlled-feeding trial. Int J Endocrinol Metab. 2019;17(1):e70744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Davidi A, Reynolds J, Njike VY, Ma Y, Doughty K, Katz DL. The effect of the addition of daily fruit and nut bars to diet on weight, and cardiac risk profile, in overweight adults. J Hum Nutr Diet. 2011;24(6):543–51. [DOI] [PubMed] [Google Scholar]
- 63. de Souza RGM, Gomes AC, de Castro IA, Mota JF. A Baru almond-enriched diet reduces abdominal adiposity and improves high-density lipoprotein concentrations: a randomized, placebo-controlled trial. Nutrition. 2018;55-56:154–60. [DOI] [PubMed] [Google Scholar]
- 64. Dhillon J, Thorwald M, De La Cruz N, Vu E, Asghar SA, Kuse Q, Diaz Rios LK, Ortiz RM. Glucoregulatory and cardiometabolic profiles of almond vs. cracker snacking for 8 weeks in young adults: a randomized controlled trial. Nutrients. 2018;10(8):960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Fantino M, Bichard C, Mistretta F, Bellisle F. Daily consumption of pistachios over 12 weeks improves dietary profile without increasing body weight in healthy women: a randomized controlled intervention. Appetite. 2019;144:104483. [DOI] [PubMed] [Google Scholar]
- 66. Gulati S, Misra A, Pandey RM, Bhatt SP, Saluja S. Effects of pistachio nuts on body composition, metabolic, inflammatory and oxidative stress parameters in Asian Indians with metabolic syndrome: a 24-wk, randomized control trial. Nutrition. 2014;30(2):192–7. [DOI] [PubMed] [Google Scholar]
- 67. Hernández-Alonso P, Salas-Salvado J, Baldrich-Mora M, Juanola-Falgarona M, Bullo M. Beneficial effect of pistachio consumption on glucose metabolism, insulin resistance, inflammation, and related metabolic risk markers: a randomized clinical trial. Diabetes Care. 2014;37(11):3098–105. [DOI] [PubMed] [Google Scholar]
- 68. Hiraoka-Yamamoto J, Ikeda K, Negishi H, Mori M, Hirose A, Sawada S, Onobayashi Y, Kitamori K, Kitano S, Tashiro Met al. . Serum lipid effects of a monounsaturated (palmitoleic) fatty acid-rich diet based on macadamia nuts in healthy, young Japanese women. Clin Exp Pharmacol Physiol. 2004;31(Suppl 2):S37–8. [DOI] [PubMed] [Google Scholar]
- 69. Jenkins DJ, Kendall CW, Marchie A, Parker TL, Connelly PW, Qian W, Haight JS, Faulkner D, Vidgen E, Lapsley KGet al. . Dose response of almonds on coronary heart disease risk factors: blood lipids, oxidized low-density lipoproteins, lipoprotein(a), homocysteine, and pulmonary nitric oxide: a randomized, controlled, crossover trial. Circulation. 2002;106(11):1327–32. [DOI] [PubMed] [Google Scholar]
- 70. Jung H, Chen CO, Blumberg JB, Kwak HK. The effect of almonds on vitamin E status and cardiovascular risk factors in Korean adults: a randomized clinical trial. Eur J Nutr. 2018;57(6):2069–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kasliwal RR, Bansal M, Mehrotra R, Yeptho KP, Trehan N. Effect of pistachio nut consumption on endothelial function and arterial stiffness. Nutrition. 2015;31(5):678–85. [DOI] [PubMed] [Google Scholar]
- 72. Lee YJ, Nam GE, Seo JA, Yoon T, Seo I, Lee JH, Im D, Bahn KN, Jeong SA, Kang TSet al. . Nut consumption has favorable effects on lipid profiles of Korean women with metabolic syndrome. Nutr Res. 2014;34(9):814–20. [DOI] [PubMed] [Google Scholar]
- 73. Liu Y, Hwang HJ, Ryu H, Lee YS, Kim HS, Park H. The effects of daily intake timing of almond on the body composition and blood lipid profile of healthy adults. Nutr Res Pract. 2017;11(6):479–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Mercanligil SM, Arslan P, Alasalvar C, Okut E, Akgul E, Pinar A, Geyik PO, Tokgozoglu L, Shahidi F. Effects of hazelnut-enriched diet on plasma cholesterol and lipoprotein profiles in hypercholesterolemic adult men. Eur J Clin Nutr. 2007;61(2):212–20. [DOI] [PubMed] [Google Scholar]
- 75. Mohan V, Gayathri R, Jaacks LM, Lakshmipriya N, Anjana RM, Spiegelman D, Jeevan RG, Balasubramaniam KK, Shobana S, Jayanthan Met al. . Cashew nut consumption increases HDL cholesterol and reduces systolic blood pressure in Asian Indians with type 2 diabetes: a 12-week randomized controlled trial. J Nutr. 2018;148(1):63–9. [DOI] [PubMed] [Google Scholar]
- 76. Njike VY, Kavak Y, Treu JA, Doughty K, Katz DL. Snacking, satiety, and weight: a randomized, controlled trial. Am J Health Promot. 2017;31(4):296–301. [DOI] [PubMed] [Google Scholar]
- 77. Nouran MG, Kimiagar M, Abadi A, Mirzazadeh M, Harrison G. Peanut consumption and cardiovascular risk. Public Health Nutr. 2010;13(10):1581–6. [DOI] [PubMed] [Google Scholar]
- 78. O'Byrne DJ, Knauft DA, Shireman RB. Low fat-monounsaturated rich diets containing high-oleic peanuts improve serum lipoprotein profiles. Lipids. 1997;32(7):687–95. [DOI] [PubMed] [Google Scholar]
- 79. Olmedilla-Alonso B, Granado-Lorencio F, Herrero-Barbudo C, Blanco-Navarro I, Blazquez-Garcia S, Perez-Sacristan B. Consumption of restructured meat products with added walnuts has a cholesterol-lowering effect in subjects at high cardiovascular risk: a randomised, crossover, placebo-controlled study. J Am Coll Nutr. 2008;27(2):342–8. [DOI] [PubMed] [Google Scholar]
- 80. Ros E, Nunez I, Perez-Heras A, Serra M, Gilabert R, Casals E, Deulofeu R. A walnut diet improves endothelial function in hypercholesterolemic subjects: a randomized crossover trial. Circulation. 2004;109(13):1609–14. [DOI] [PubMed] [Google Scholar]
- 81. Ruisinger JF, Gibson CA, Backes JM, Smith BK, Sullivan DK, Moriarty PM, Kris-Etherton P. Statins and almonds to lower lipoproteins (the STALL study). J Clin Lipidol. 2015;9(1):58–64. [DOI] [PubMed] [Google Scholar]
- 82. Salas-Huetos A, Moraleda R, Giardina S, Anton E, Blanco J, Salas-Salvado J, Bullo M. Effect of nut consumption on semen quality and functionality in healthy men consuming a Western-style diet: a randomized controlled trial. Am J Clin Nutr. 2018;108(5):953–62. [DOI] [PubMed] [Google Scholar]
- 83. Spiller GA, Jenkins DA, Bosello O, Gates JE, Cragen LN, Bruce B. Nuts and plasma lipids: an almond-based diet lowers LDL-C while preserving HDL-C. J Am Coll Nutr. 1998;17(3):285–90. [DOI] [PubMed] [Google Scholar]
- 84. Sweazea KL, Johnston CS, Ricklefs KD, Petersen KN. Almond supplementation in the absence of dietary advice significantly reduces C-reactive protein in subjects with type 2 diabetes. J Funct Foods. 2014;10:252–9. [Google Scholar]
- 85. Tapsell LC, Gillen LJ, Patch CS, Batterham M, Owen A, Baré M, Kennedy M. Including walnuts in a low-fat/modified-fat diet improves HDL cholesterol-to-total cholesterol ratios in patients with type 2 diabetes. Diabetes Care. 2004;27(12):2777–83. [DOI] [PubMed] [Google Scholar]
- 86. Tey SL, Gray AR, Chisholm AW, Delahunty CM, Brown RC. The dose of hazelnuts influences acceptance and diet quality but not inflammatory markers and body composition in overweight and obese individuals. J Nutr. 2013;143(8):1254–62. [DOI] [PubMed] [Google Scholar]
- 87. Wu HY, Pan A, Yu ZJ, Qi QB, Lu L, Zhang G, Yu DX, Zong G, Zhou YH, Chen XFet al. . Lifestyle counseling and supplementation with flaxseed or walnuts influence the management of metabolic syndrome. J Nutr. 2010;140(11):1937–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zaveri S, Drummond S. The effect of including a conventional snack (cereal bar) and a nonconventional snack (almonds) on hunger, eating frequency, dietary intake and body weight. J Hum Nutr Diet. 2009;22(5):461–8. [DOI] [PubMed] [Google Scholar]
- 89. Jenkins DJA, Kendall CWC, Lamarche B, Banach MS, Srichaikul K, Vidgen E, Mitchell S, Parker T, Nishi S, Bashyam Bet al. . Nuts as a replacement for carbohydrates in the diabetic diet: a reanalysis of a randomised controlled trial. Diabetologia. 2018;61(8):1734–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Palacios OM, Maki KC, Xiao D, Wilcox ML, Dicklin MR, Kramer M, Trivedi R, Burton-Freeman B, Edirisinghe I. Effects of consuming almonds on insulin sensitivity and other cardiometabolic health markers in adults with prediabetes. J Am Coll Nutr. 2019;39:1–10. [DOI] [PubMed] [Google Scholar]
- 91. Burns-Whitmore B, Haddad E, Sabate J, Rajaram S. Effects of supplementing n–3 fatty acid enriched eggs and walnuts on cardiovascular disease risk markers in healthy free-living lacto-ovo-vegetarians: a randomized, crossover, free-living intervention study. Nutr J. 2014;13:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Razquin C, Martinez JA, Martinez-Gonzalez MA, Salas-Salvado J, Estruch R, Marti A. A 3-year Mediterranean-style dietary intervention may modulate the association between adiponectin gene variants and body weight change. Eur J Nutr. 2010;49(5):311–9. [DOI] [PubMed] [Google Scholar]
- 93. Kocyigit A, Koylu AA, Keles H. Effects of pistachio nuts consumption on plasma lipid profile and oxidative status in healthy volunteers. Nutr Metab Cardiovasc Dis. 2006;16(3):202–9. [DOI] [PubMed] [Google Scholar]
- 94. Eastman WA, Clayshulte BJ. Pecan effects on serum lipoproteins and dietary intakes of hyperlipidemic individuals consuming self-selected diets. FCSRJ. 2009;33(3):197–207. [Google Scholar]
- 95. Hwang HJ, Liu Y, Kim HS, Lee H, Lim Y, Park H. Daily walnut intake improves metabolic syndrome status and increases circulating adiponectin levels: randomized controlled crossover trial. Nutr Res Pract. 2019;13(2):105–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Katz DL, Davidhi A, Ma Y, Kavak Y, Bifulco L, Njike VY. Effects of walnuts on endothelial function in overweight adults with visceral obesity: a randomized, controlled, crossover trial. J Am Coll Nutr. 2012;31(6):415–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Tey SL, Brown RC, Chisholm AW, Delahunty CM, Gray AR, Williams SM. Effects of different forms of hazelnuts on blood lipids and alpha-tocopherol concentrations in mildly hypercholesterolemic individuals. Eur J Clin Nutr. 2011;65(1):117–24. [DOI] [PubMed] [Google Scholar]
- 98. Liu X, Hill AM, West SG, Gabauer RM, McCrea CE, Fleming JA, Kris-Etherton PM. Acute peanut consumption alters postprandial lipids and vascular responses in healthy overweight or obese men. J Nutr. 2017;147(5):835–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. USDA . USDA Food and Nutrient Database. [Internet]. [Accessed 2020 Feb 18]. Available from: https://ndb.nal.usda.gov/ndb/. [Google Scholar]
- 100. Hall KD, Sacks G, Chandramohan D, Chow CC, Wang YC, Gortmaker SL, Swinburn BA. Quantification of the effect of energy imbalance on bodyweight. Lancet. 2011;378(9793):826–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Stevenson JL, Paton CM, Cooper JA. Hunger and satiety responses to high-fat meals after a high-polyunsaturated fat diet: a randomized trial. Nutrition. 2017;41:14–23. [DOI] [PubMed] [Google Scholar]
- 102. Kendall CW, West SG, Augustin LS, Esfahani A, Vidgen E, Bashyam B, Sauder KA, Campbell J, Chiavaroli L, Jenkins ALet al. . Acute effects of pistachio consumption on glucose and insulin, satiety hormones and endothelial function in the metabolic syndrome. Eur J Clin Nutr. 2014;68(3):370–5. [DOI] [PubMed] [Google Scholar]
- 103. Hull S, Re R, Chambers L, Echaniz A, Wickham MSJ. A mid-morning snack of almonds generates satiety and appropriate adjustment of subsequent food intake in healthy women. Eur J Nutr. 2015;54(5):803–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Baer DJ, Gebauer SK, Novotny JA. Measured energy value of pistachios in the human diet. Br J Nutr. 2012;107(1):120–5. [DOI] [PubMed] [Google Scholar]
- 105. Novotny JA, Gebauer SK, Baer DJ. Discrepancy between the Atwater factor predicted and empirically measured energy values of almonds in human diets. Am J Clin Nutr. 2012;96(2):296–301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Baer DJ, Gebauer SK, Novotny JA. Walnuts consumed by healthy adults provide less available energy than predicted by the Atwater factors. J Nutr. 2016;146(1):9–13. [DOI] [PubMed] [Google Scholar]
- 107. Baer DJ, Novotny JA. Consumption of cashew nuts does not influence blood lipids or other markers of cardiovascular disease in humans: a randomized controlled trial. Am J Clin Nutr. 2019;109(2):269–75. [DOI] [PubMed] [Google Scholar]
- 108. Barbour JA, Howe PR, Buckley JD, Bryan J, Coates AM. Effect of 12 weeks high oleic peanut consumption on cardio-metabolic risk factors and body composition. Nutrients. 2015;7(9):7381–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Duarte Moreira Alves R, Boroni Moreira AP, Silva Macedo V, Brunoro Costa NM, Goncalves Alfenas Rde C, Bressan J. High-oleic peanuts increase diet-induced thermogenesis in overweight and obese men. Nutr Hosp. 2014;29(5):1024–32. [DOI] [PubMed] [Google Scholar]
- 110. Casas-Agustench P, LÛpez-Uriarte P, BullÛ M, Ros E, GÛmez-Flores A, Salas-Salvadû J. Acute effects of three high-fat meals with different fat saturations on energy expenditure, substrate oxidation and satiety. Clin Nutr. 2009;28(1):39–45. [DOI] [PubMed] [Google Scholar]
- 111. Kaviani S, Cooper JA. Appetite responses to high-fat meals or diets of varying fatty acid composition: a comprehensive review. Eur J Clin Nutr. 2017;71(10):1154–65. [DOI] [PubMed] [Google Scholar]
- 112. Krishnan S, Cooper JA. Effect of dietary fatty acid composition on substrate utilization and body weight maintenance in humans. Eur J Nutr. 2014;53(3):691–710. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.